ABSTRACT
Although the autophagy-related (ATG) conjugation systems are thought to be important for a late step of autophagosome formation, their precise function has been poorly understood because they are also required for localization of the most important autophagosomal marker LC3. In our recent study we found that, using the autophagosomal SNARE STX17 (syntaxin 17) as an alternative marker, autophagosome-like structures were generated in ATG conjugation system-deficient cells. Those structures could fuse with lysosomes but the degradation of the inner autophagosomal membrane was significantly delayed. We suggest that the ATG conjugation-dependent closure of autophagosomes causes the inner autophagosomal membrane to become sensitive to lysosomal degradation.
KEYWORDS: ATG-conjugation systems, autolysosome, autophagosome inner-membrane degradation, autophagosome maturation, LC3–PE, syntaxin 17
Although the molecular mechanisms of autophagosome formation have been well studied, those of autophagosome maturation processes such as completion of autophagosome formation and fusion with lysosomes have been studied less extensively. To precisely describe autophagosome maturation processes, we conducted live-cell imaging using the autophagosomal SNARE protein STX17 (syntaxin17) as an autophagosome marker, LAMP1 as a lysosome membrane marker, and LysoTracker Red (LTR) as an acidic compartment marker. What we found in wild-type cells are:
-
(1)
Approximately 2 min after the recruitment of STX17, an autophagosomal SNARE protein, to autophagosomes, several small lysosomes start to fuse with autophagosomes, leading to the appearance of ring-shaped LTR signals, which suggests acidification of the space between the outer autophagosomal membrane (OAM) and inner autophagosomal membrane (IAM).
-
(2)
Approximately 7 min later, the matrix of autophagosomes is entirely acidified, suggesting that the IAM is finally degraded.
-
(3)
The morphology of elongating phagophores is relatively oblate, but is changed into spherical immediately before or after STX17 recruitment.
To our knowledge, this was the first demonstration of IAM breakdown by live-cell imaging, and our data suggest that the IAM is not immediately degraded after lysosomal fusion. We also speculate that closure of the phagophore edge triggers a morphological change into spherical autophagosomes.
Having these useful markers, we reassessed the functions of autophagy-related (ATG) proteins. The Atg8 and Atg12 conjugation systems have been thought to be required for a late step, most likely for the closure step, of autophagosome formation in mammals because elongated phagophores and autophagosome-like structures but not autolysosomes accumulate in ATG conjugation-deficient cells. However, the fate of these elongated phagophores and autophagosome-like structures was unknown; if any of the components in these conjugation systems is lacking, conjugation of mammalian ATG8 homologs (MAP1LC3/LC3 [microtubule-associated protein 1 light chain 3], etc.) to phosphatidylethanolamine (PE) does not occur, taking the most important autophagosome marker protein out of our hands. To overcome this limitation, we used STX17 as a marker instead of LC3. As a result, we obtained the following unexpected findings in ATG conjugation-deficient cells.
-
(1)
A number of STX17-positive autophagosome-like structures are generated in ATG conjugation-deficient cells such as Atg3 knockout (KO), Atg5 KO, and Atg7 KO MEFs (but not in Rb1cc1 KO and Atg14 KO cells).
-
(2)
Nonselective and selective autophagic activities are profoundly suppressed in our biochemical analysis.
-
(3)
The transition rate of ATG5-positive phagophores to STX17-positive autophagosomes is reduced to approximately 30% of that in wild-type cells (suggesting that the efficiency of autophagosome formation itself is significantly reduced).
-
(4)
The morphology of the STX17-positive autophagosome-like structures in Atg3 KO cells is more oblate compared with those in wild-type cells (suggesting that the edge closure is defective).
-
(5)
These STX17-positive structures become ring-shaped LTR structures with a comparable time course to wild-type cells, and positive for SNAP29 and LAMP1 (suggesting that STX17 structures fuse with lysosomes normally).
-
(6)
The lifetime of STX17-positive LTR-ring autophagosome-like structures was significantly prolonged (longer than 30–60 min) (suggesting that the IMA is resistant to lysosomal degradation).
Taken together, we propose that the ATG conjugation systems are important for the closure of phagophores by separation of the IAM from the OAM, which leads to a spherical morphology, and also for subsequent degradation of the IAM by lysosomal enzymes (Fig. 1). By contrast, the conjugation systems are not absolutely essential for the formation of autophagosome-like structures (likely with a remaining open edge) and fusion with lysosomes.
Figure 1.
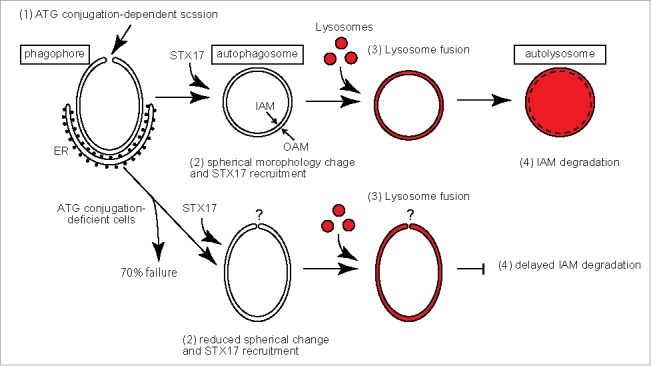
A model of autophagosome maturation in wild-type and ATG conjugation-deficient cells. (1) Scission between the IAM and OAM in an ATG conjugation-dependent manner. (2) An oblate-to-spherical morphological change of the autophagosome and STX17 recruitment (immediately before or after closure). (3) Fusion of lysosomes with the OAM and introduction of lysosomal enzymes into the space between the OAM and IAM. (4) Degradation of the IAM. The steps that are affected in ATG conjugation-deficient cells are indicated.
Our data suggest that the IAM might become sensitive to lysosomal enzymes only after fission from the OAM in an ATG conjugation system-dependent manner. The inward membrane scission could also be a mechanism for efficient degradation by vacuolar/lysosomal enzymes during microautophagy and formation of multivesicular bodies. Although the precise mechanism is unknown, there must be a common mechanism (e.g., different physical or chemical properties and opposite membrane curvature of the inner membrane) that makes inner membranes sensitive to vacuolar/lysosomal enzymes.
These observations do not represent so-called “ATG5- and ATG7-independent alternative autophagy,” in which autophagosomes are generated from the Golgi in a RAB9-dependent manner and typically induced by etoposide treatment. Autophagosome-like structures that we observed in ATG conjugation-deficient cells are likely generated in a canonical pathway; these structures are induced upon amino-acid starvation, and generated throughout the cytoplasm not only from the Golgi area in the absence of RAB9A and RAB9B (unpublished observation).
Our findings lead to several new questions regarding autophagosome maturation:
-
(1)
What is the exact molecular function of LC3–PE?
-
(2)
What machinery is involved in the scission/closure of the autophagosomal edge?
-
(3)
What biochemical or physical difference exists between the IAM and OAM?
-
(4)
How does the oblate-to-spherical morphological transition occur?
-
(5)
What are the mechanisms of the recruitment and dissociation of STX17?
The door to new exploration for the mechanisms of autophagosome maturation has just opened.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by JSPS KAKENHI Grant-in-Aid for Scientific Research on Innovative Areas (Grant Number 25111001 and 25111005) (to N.M.).


