Abstract
Purpose
To estimate the discriminative value of serum P1NP/βCTX ratio and albumin levels in hospitalized orthogeriatric patients with and without nonvertebral fractures.
Methods
In 1,239 orthogeriatric patients (mean age 78.1±9.52 years, 69.1% women) including 854 (68.9%) with osteoporotic nonvertebral fractures (455 [36.7%] with hip fracture [HF]) and 385 (31.1%) without fractures, markers of bone formation (procollagen type 1 N-terminal propeptide [P1NP], osteocalcin [OC], and bone resorption (beta-C-terminal cross-linking telopeptide of type 1 collagen [βCTX]), indices of mineral metabolism, and parameters of liver and renal functions were assessed; data on clinical and laboratory characteristics were collected prospectively.
Results
Both lower serum P1NP/βCTX ratio and albumin concentration (as continuous or categorical variables) were independently associated with fracture presence in multivariate logistic regressions. Compared with the highest P1NP/βCTX tertile, the prevalence of HF, after adjustment for multiple covariates, was 3-fold higher in the lowest tertile and 1.5 times higher in the middle tertile; presence of any fracture was 2.3- and 1.6-fold higher, respectively; patients with albumin levels in the lowest tertile had multivariate odds ratio (OR) of 4.6 for HF and 2.8 for any fracture, in the middle tertile the ORs were 2.2 and 1.3, respectively. The P1NP/βCTX <100.0 (median) and hypoalbuminemia (<33 g/L) demonstrated area under the curve values for HF of 0.802 and 0.806, respectively, and for any fractures of 0.711 and 0.706, respectively. When both characteristics were combined, the ORs for HF or any fracture, compared with the nonfractured group, were 7.8 and 3.2, respectively, with an accuracy of 79.6% and 71.6%, respectively.
Conclusions
In orthogeriatric patients, both serum P1NP/βCTX ratio and albumin levels demonstrated an inverse dose–effect relationship with the prevalence of nonvertebral fractures and independently indicated fracture presence with acceptable discriminatory power. Lower P1NP/βCTX (<100) and hypoalbuminemia could be useful simple additive prognostic tools for fracture risk stratification in the elderly.
Keywords: nonvertebral fractures, P1NP/βCTX ratio, albumin, elderly, orthopedic patients
Introduction
The ability to predict and prevent fragility fractures is limited.1–3 Currently, the predicting is largely based on bone mineral density (BMD) testing and several clinical risk factors (the World Health Organization’s fracture risk assessment tool FRAX,4,5 Garvan and QFracture).1 However, BMD indicated osteoporosis only in 30%–50% of patients with major fragility fracture6 and in 4% of women with a distal radial fracture.7 The prognostic value of clinical risk factors alone in FRAX is comparable to that of BMD alone.8 There is an obvious need of identifying additional fracture risk factors not included in currently available strategies.
Numerous studies on the prognostic value of bone turnover markers (BTMs) yielded conflicting results.9–17 BTMs display significant analytical and biological variability18,19 and currently are recommended only for monitoring the efficacy of osteoporosis treatment and compliance.11,20,21 Bone formation and resorption are coupled but not always remain in balance: in the elderly bone is lost because remodeling becomes unbalanced.22 However, most of the reports focused on separate BTMs. One way to overcome the existing discrepancies between studies would be to characterize the balance between total bone formation and resorption. In the only prognostic study, which assessed such index, the ratio of a urinary resorption (N-telopeptide of type 1 collagen [u-NTx]) to serum formation marker (osteocalcin, OC) (u-NTx/OC) predicted fractures independently of FRAX, but did not significantly improve the accuracy of fracture risk prediction in addition to FRAX.23 Recently the joint international working group proposed serum procollagen type 1 N-terminal propeptide (P1NP) and beta-C-terminal cross-linking telopeptide of type 1 collagen (βCTX) as the reference BTMs to evaluate bone formation and bone resorption, respectively.11 To our knowledge, no studies of P1NP/βCTX ratio measurements have been undertaken.
Albumin, one of the major proteins synthesized in the liver and the most abundant protein in the circulatory system, has pleotropic physiologic effects.24,25 Hypoalbuminemia is strongly associated with inflammation–malnutrition complex25 and various systemic disorders (liver, kidneys, cardiovascular, diabetes, and malignancy), many of which are particularly common in the elderly, increases the risk of falls and fractures,26,27 and is linked to poor prognosis and mortality in the general population28,29 as well as in the orthopedic patients.30,31 Only limited information with conflicting results is available regarding the role of hypoalbuminemia in osteoporosis,32,33 and its value in predicting fractures has not been sufficiently investigated.34,35
We hypothesized that in orthogeriatric patients lower serum P1NP/βCTX ratio and/or hypoalbuminemia, two indices that current algorithms do not take into consideration, may be associated with the presence of fracture, indicating a greater osteoporosis-related fracture risk. In this study, we aimed to investigate the relationship between serum P1NP/βCTX ratio, an index of bone turnover balance, and hypoalbuminemia with the presence of osteoporotic hip or other nonvertebral fractures in a cohort of hospitalized orthogeriatric patients, which reflects the real world in regard to the prevalence of major fractures.
Methods
Patients
This was an observational study using prospectively collected data on 1,899 consecutive patients >60 years of age who were admitted to the Department of Orthopedic Surgery at the Canberra Hospital (a 500-bed university-affiliated tertiary care center, Australian Capital Territory, Australia) between 1 January 2012 and 31 December 2014. After excluding patients with high-trauma fractures, vertebral and periprostetic fractures, primary hyperparathyroidism, Paget’s disease, metastatic cancer to bone, or who lacked adequate laboratory data, 1,239 patients were evaluated for the study. Of these 1,239 hospitalized patients (mean age 78.1±9.52 years, 69.1% women), 854 (68.9%) had a low-energy trauma (falls from standing height) which resulted in a nonvertebral bone fracture: 455 (36.7%) had a hip fracture (HF) (52.0% cervical and 48.0% trochanteric) and 399 (32.2%) had other nonvertebral fractures (humerus −79, femur −75, ankle −68, tibia or/and fibula −27, knee −16, wrist −16, forearm −15, others −103). There were 385 (31.1%) patients without fractures (elective hip or knee replacement −343, suspected surgical site infections not confirmed by further investigation −15, and 27 patients with a prosthetic joint infection following total hip [n=20] or knee [n=7] arthroplasty). Data were collected on demographics, orthopedic and medical diagnoses, procedures performed, laboratory characteristics, medication used, and outcomes.
The study was approved by the Australian Capital Territory Ethical Review Board and performed in accordance with the principles of the Helsinki declaration. All study patients or the legally authorized carers gave their informed consent.
Validation dataset
A retrospective analysis of a second cohort included data obtained from electronic medical and administrative records from 417 consecutive orthogeriatric patients (mean age 78.9±8.7 years, 68.2% women) admitted to the same department between October 2011 and August 2012. Among these patients, there were 152 (36.5%) subjects with an HF, 103 (24.7%) with other nonvertebral fractures, and 162 (38.8%) without fractures.
Laboratory evaluation
In each patient, fasting venous blood samples were collected within 24 hours of admission and the following tests were performed: serum concentrations of P1NP, OC, and βCTX using an automated electrochemiluminescent immunoassay (Elecsys 2010, Roche Diagnostics, Ltd Corp., Indianapolis, IN, USA), 25 (OH) vitamin D [25(OH)D] by a radioimmunoassay kit (DiaSorin, Stillwater, MN, USA), intact PTH by 2-site chemiluminescent enzyme-linked immunoassay on DPC Immulite 2000 (Diagnostic Products, Los Angeles, CA, USA), total calcium, phosphate and magnesium, as well as routine laboratory investigations, including complete blood count, electrolytes, renal (creatinine, urea), liver (alanine aminotransferase [ALT], gamma-glutamyltransferase [GGT], alkaline phosphatase [ALP], albumin, and total bilirubin) and thyroid function tests (thyroid-stimulating hormone [TSH]; free thyroxine [fT4]), by standard automated laboratory methods. Intra- and inter-assay coefficients of variation (CV) for P1NP were 2.6% and 4.1%, respectively, for OC 3.6% and 6.6%, respectively, and for βCTX 3.2% and 6.5%, respectively; for 25(OH)D and PTH, the intra- and interassay CV ranged from 2.1% to 12.7%. Calcium concentrations were corrected for serum albumin. Vitamin D status was defined as deficient for circulating 25(OH)D concentration <25 nmol/L and as insufficient for 25–50 nmol/L. Secondary hyperparathyroidism (SHPT) was defined as elevated serum PTH (>6.8 pmol/L, the upper limit of the laboratory reference range). The glomerular filtration rate (GFR) was estimated36 and chronic kidney disease (CKD ≥stage 3) was defined as GFR <60 mL/min/1.73 m2. Anemia was defined as hemoglobin <120 g/L. Similar laboratory tests, equipment, methods, and definitions were used in the validation cohort.
Statistical analyses
The statistical analysis was performed with Stata software version 10 (StataCorp, College Station, TX, USA). Continuous variables are expressed as mean ± SD and compared using analysis of variance. Categorical variables are presented as numeral/percentages and compared by chi-square and Fisher exact tests. The correlations between the variables were determined by Pearson’s coefficients. For Pearson correlations and regressions, values of all continuous laboratory parameters were logarithmically transformed to account for the skewed nature of most of these variables. The admission P1NP/βCTX ratio was analyzed as both a continuous and a categorical variable; in the latter, P1NP/βCTX ratio was categorized into three groups (tertiles) or as the median value. Univariate and multivariate logistic analyses were performed to identify factors associated with the presence of HF or of any nonvertebral fracture. Multivariate forward stepwise procedures (covariates with P≤0.100 in univariate analysis were selected for entry) were performed. To quantify the significance of multicollinearity phenomena in regression analyses, the variance inflation factor was calculated. To quantify the discriminative utility for serum P1NP/βCTX ratio, albumin concentration, other parameters of interest, and their combination receiver operating characteristic (ROC), analysis was used and the predictive accuracy was expressed as area under curve (AUC). All statistical tests were two tailed and P-values <0.05 was considered statistically significant.
Results
Patient characteristics
In the entire cohort, patients averaged 2.7 chronic diseases per person, and the most common were hypertension (60.0%), osteoarthritis (42.5%), abnormal gait with the use of an assistive device (42.0%), diabetes mellitus type 2 (DM, 22.0%), CKD (21.3%), coronary artery disease (CAD, 17.1%), chronic obstructive airway disease (COPD, 15.4%), atrial fibrillation (AF, 14.8%), dementia (14.4%), cerebrovascular disease (12.2%), malignancy (10.4%), and chronic heart failure (7.8%). Four and more chronic conditions were identified in total in 28.5% of patients with the greatest burden, as expected, among the HF patients (36.0% vs 29.4% in the nonfracture group, P=0.040). Prior to admission, osteoporosis has been diagnosed in 239 (19.3%) patients and 182 (14.7%) subjects were receiving antiresorptive treatment.
Regarding laboratory parameters, both groups with fracture (HF and other nonvertebral fractures), compared to the nonfracture group, had significantly higher mean levels of βCTX, lower P1NP/βCTX ratios, and concentrations of calcium and higher prevalence of hyperparathyroidism (Table 1). Subjects with HF in addition exhibited higher levels of PTH, lower OC/βCTX ratios, phosphate, magnesium, hemoglobin, albumin concentrations and GFR, as well as a higher prevalence of hypoalbuminemia, vitamin D deficiency, and anemia. On admission, hypoalbuminemia (<33 g/L) was observed in 688 (55.6%) patients including 342 (75.2%) with HF, 185 (46.4%) with other fractures, and 161 (42.2%) without fractures. Of note, mean serum levels of P1NP, OC, P1NP/OC ratio, 25(OH)D, as well as creatinine, ALP, TSH, and fT4 did not differ between the three groups.
Table 1.
Demographic, clinical, and laboratory characteristics of orthogeriatric patients by fracture status
| Variables | Total (n=1,239) | HF (n=455) | Non-HF (n=399) | No fracture (n=385) |
|---|---|---|---|---|
| Age, years | 78.1±9.52 | 83.0±8.48c,f | 76.6±9.49c | 73.9±8.06 |
| Females, n (%) | 855 (69.1) | 331 (73.2)c | 292 (72.8)c | 232 (60.6) |
| RCF resident, n (%) | 190 (15.4) | 126 (27.7)c,f | 42 (10.5)a | 22 (5.7) |
| Osteoporosis, n (%) | 239 (19.3) | 120 (26.4)c,e | 73 (18.3)a | 46 (12.0) |
| Dementia, n (%) | 178 (14.4) | 125 (27.5)c,f | 33 (8.3) | 20 (5.2) |
| P1NP, µg/L | 58.7±89.3 | 56.5±101.6 | 58.0±71.2 | 62.0±90.8 |
| OC, ng/mL | 6.8±4.7 | 6.5±4.8 | 7.1±4.6 | 6.9±4.7 |
| βCTX, µg/L | 0.50±0.35 | 0.56±0.36c,e | 0.49±0.36a | 0.43±0.32 |
| P1NP/βCTX | 123.3±101.9 | 103.4±93.9c,f | 127.1±83.8b | 147.2±104.9 |
| OC/βCTX | 16.2±11.1 | 13.6±9.4c,f | 18.1±12.5 | 19.4±12.2 |
| 25(OH)D, nmol/L | 62.9±26.3 | 61.6±27.6 | 64.8±26.5 | 62.4±24 |
| <25 nmol/L, n (%) | 96 (7.8) | 49 (10.8)a,e | 22 (5.6) | 25 (6.7) |
| <50 nmol/L, n (%) | 394 (31.9) | 155 (34.1) | 122 (30.8) | 117 (31.2) |
| PTH, pmol/L | 7.4±5.36 | 8.1±5.99c,e | 7.0±4.96 | 6.8±4.84 |
| >6.8 pmol/L, n (%) | 472 (38.2) | 206 (45.6)c,d | 147 (37.3)a | 119 (31.5) |
| Calcium,* mmol/L | 2.41±0.13 | 2.39±0.14c,d | 2.41±0.13b | 2.43±0.13 |
| Phosphate, mmol/L | 0.91±0.25 | 0.87±0.24c,f | 0.93±0.25 | 0.94±0.24 |
| Magnesium, mmol/L | 0.76±0.10 | 0.74±0.10c,f | 0.78±0.09 | 0.77±0.10 |
| Albumin, g/L | 32.1±4.41 | 30.2±3.90c,f | 32.8±4.29 | 33.5±4.35 |
| <33 g/L, n (%) | 688 (55.6) | 342 (75.2)c,f | 185 (46.4) | 161 (42.2) |
| Hemoglobin, g/L | 109.5±17.9 | 104.8±17.2c,f | 112.5±17.6 | 118.1±17.9 |
| <120 g/L, n (%) | 876 (70.8) | 366 (80.4)c,f | 254 (63.7) | 256 (66.8) |
| GFR, mL/min/1.73 m2 | 72.6±18.9 | 70.7±19.8e | 74.7±17.4 | 72.5±19.0 |
Notes: Data expressed as mean ± SD or number (percentage). Comparison with no fracture group:
P<0.05,
P<0.01, and
P<0.001. Comparison patients with hip fracture and other nonvertebral fractures:
P<0.05,
P<0.01, and
P<0.001.
Calcium corrected for albumin. Of note, the proportion of patients with hypertension, chronic heart failure (CHF), diabetes mellitus (DM), chronic obstructive airway disease (COPD), chronic kidney disease (CKD, GFR <60 mL/min/1.73 m2), history of malignancy, as well as current smokers and warfarin users were similar in the three groups; the mean serum levels of alkaline phosphatase (ALP), thyroid-stimulating hormone (TSH), and free thyroxine (fT4) did not differ between the three groups (data not shown).
Abbreviations: βCTX, β C-terminal βcross-linked telopeptide of type I collagen; HF, hip fracture; OC, osteocalcin; P1NP, N-terminal propeptide of type I procollagen; PTH, parathyroid hormone; RCF, residential care facility.
Patients receiving antiosteoporotic therapy (a bisphosphonate plus vitamin D and calcium supplements) at least for 3 months prior to hospital admission (n=182) compared to those who were not treated (n=1,057) had significantly higher mean levels of serum P1NP/βCTX ratio (+16.7%: 141.8± 120.7 vs 121.5±90.8) and 25(OH)D (+19.6%: 73.1±24.4 vs 61.1±26.3 nmol/L) and lower βCTX (−7.3%: 0.38±0.27 vs 0.52±0.36 µg/mL) (all P<0.01), whereas the P1NP, PTH, and albumin levels were not different.
Osteoporotic fractures and laboratory parameters (correlation analyses)
These relationships have been evaluated for laboratory parameters expressed as both continuous and categorical variables. Among 13 studied laboratory parameters (P1NP, OC, βCTX, P1NP/βCTX ratio, OC/βCTX ratio, PTH, 25(OH)D, calcium, phosphate, magnesium, ALP, albumin, and hemoglobin), analyzed as continuous log-transformed variables adjusted for age and gender, the highest Pearson correlation coefficients in relation to the presence of HF or any fracture demonstrated the PINP/βCTX ratio and albumin: −0.3015 and −0.3667, respectively, for HF, and −0.1971 and −0.2133, respectively, for any fracture (all P=0.000). Only age showed higher correlation coefficients (r=0.4736, P=0.000 for HF; r=0.2902, P=0.000 for any fracture). Other clinical (female, dementia, AF, CAD, anemia, arthritis, osteoporosis, history of stroke or transient ischemic attack (TIA), use of walking aid) and laboratory (P1NP, OC, βCTX, OC/βCTX ratio, PTH, calcium, phosphate, magnesium) parameters were also significantly but weaker associated with the presence of fracture (r ranged between 0.2930 for dementia and HF, and 0.075 for stroke and any fracture).
Multivariate logistic regressions performed with HF or any fracture as a dependent variable and all clinical and laboratory characteristics with P≤0.100 in univariate analysis as independent variables after adjusting for age and gender revealed that both lower serum P1NP/βCTX ratio and lower albumin concentration (as continuous variables) are independent and significant factors associated with these conditions (β coefficients 0.897 and 0.869, respectively, P=0.000 for both variables). These models explained 28.5% and 22.3% of variance among patients with an HF or any fracture, respectively, correctly classifying 77.4% and 70.5% of cases, respectively. For HF, the model’s sensitivity was 79.4%, specificity 74.9%, positive predictive value (PPV) 79.2% and negative predictive value (NPV) 75.1%, and for any fracture, 89.9%, 26.7%, 73.5%, and 53.8%, respectively. For the presence of HF, the AUC was 0.799 for P1NP/βCTX and 0.816 for albumin, for the presence of any fracture, the AUC was 0.701 and 0.713, respectively.
Because of practical considerations, we further examined the impact and clinical usefulness of the P1NP/βCTX ratio and albumin level as categorical variables. First, we examined the association of fracture presence and the serum P1NP/βCTX ratio divided into tertiles. Table 2 shows the tertile groupings and the percentage of individuals in each tertile, as well as the ORs for fracture presence. Proportion of patients with fractures decreased sharply from tertile 1 (lowest) to tertile 3 (highest). With tertile 3 (P1NP/βCTX >129.2, mean 219.3±112.6) used as the reference, the odds of fracture were significantly higher in tertiles 2 (P1NP/βCTX 78.6–129.2, mean 100.7±14.3) and 1 (P1NP/βCTX <78.6, mean 53.9±15.8). After adjusting for age and gender, patients in tertile 2 had a 1.6-fold higher risk of HF or any fracture, while for those in tertile 1, the risk of HF was 3.4-fold higher and the risk of any fracture was 2.5-fold higher. In other words, the ORs for the presence of fracture linearly increased across decreasing P1NP/βCTX ratio tertiles, indicating a dose–response effect.
Table 2.
Presence of fracture in hospitalized orthogeriatric patients according to serum P1NP/βCTX ratio tertiles
| P1NP/βCTX ratio | Model 1a
|
Model 2b
|
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Hip fracture | ||||||
| Tertile 1 (<78.6), n=221 (48.1%) | 3.44 | 2.32–5.12 | 0.000 | 3.05 | 2.02–4.63 | 0.000 |
| Tertile 2 (78.6–129.2), n=140 (30.8%) | 1.62 | 1.10–2.38 | 0.016 | 1.54 | 1.03–2.31 | 0.036 |
| Any fracture | ||||||
| Tertile 1 (<78.6), n=334 (39.1%) | 2.51 | 1.80–3.48 | 0.000 | 2.30 | 1.63–3.23 | 0.000 |
| Tertile 2 (78.6–129.2), n=287 (33.9%) | 1.61 | 1.19–2.18 | 0.002 | 1.56 | 1.14–2.13 | 0.005 |
Notes: Logistic regression models for fracture presence by tertiles of serum P1NP/βCTX ratio; as the reference used tertile 3 (P1NP/βCTX >129.2, the highest), n=94 (20.4%) in the hip fracture group and n=230 (27.0%) among all patients with fractures.
Adjustment only for age and gender.
Adjustment for age, gender, 25(OH)D, PTH, calcium (corrected for albumin), phosphate, magnesium, osteocalcin, albumin, alkaline phosphatase, GFR, presence of dementia, cardiovascular diseases (coronary artery disease, atrial fibrillation, and chronic heart failure), cerebrovascular diseases, diabetes mellitus, history of smoking, and alcohol consumption status.
Abbreviations: P1NP, N-terminal propeptide of type 1 procollagen; βCTX, cross-linked carboxy-terminal telopeptide of type 1 collagen; OR, odds ratio; CI, confidence interval.
We further performed multivariate forward stepwise logistic regression analyses for the presence of HF or of any fracture according to serum P1NP/βCTX ratio tertiles including in the models the following variables: age, gender, 25(OH)D, PTH, calcium (corrected for albumin), phosphate, magnesium, OC, albumin, alkaline phosphatase, presence of dementia, cardiovascular diseases (CAD, AF, CVA, and CHF), diabetes mellitus, history of smoking, and alcohol consumption status. The adjustment for all these confounding factors did not significantly alter the results. Lower P1NP/βCTX ratio remained an independent and powerful indicator for fracture presence. The ORs for HF and any fracture demonstrated a similar linearly increased pattern across decreasing P1NP/βCTX ratio tertiles (Table 2, model 2). Compared with the highest tertile, the presence of HF among patients in the lowest tertile was more than 3-fold higher and among patients in the middle tertile 1.5 times higher, whereas the presence of any fracture was 2.3-fold and 1.6-fold higher, respectively.
Albumin levels on admission analyzed in tertiles and adjusted for age and gender also demonstrated a dose–response relationship with fracture presence. Compared to tertile 3 (the highest: >34 g/L, mean 37.3±2.2 g/L), the HF patients in tertile 2 (16–34 g/L, mean 32.4±1.04 g/L) had an OR of 2.2 (95% CI: 1.4–3.3, P=0.000) and in tertile 1 (the lowest: <31 g/L, mean 27.6±2.5 g/L), an OR of 4.6 (95% CI: 3.0–7.1, P=0.000); similarly, patients with any fracture had ORs of 1.3 (95% CI: 0.98–1.8, P=0.065) and 2.8 (95% CI: 2.0–3.8, P=0.000), respectively.
Next, we dichotomized subjects using the median value of the P1NP/βCTX ratio (100.0) in our cohort. Of 612 orthogeriatric patients with the P1NP/βCTX <100.0 (under median level) on admission, 484 (79.1%) presented with a fracture. In stepwise multiple linear regression analyses which included all laboratory indices along with sociodemographic and clinical characteristics, P1NP/βCTX <100.0 and hypoalbuminemia (<33 g/L) were independent indicators of an HF (OR 2.8, 95% CI: 2.0–3.8, P=0.000 and OR 3.1, 95% CI: 2.2–4.3, P=0.000, respectively) or any fracture (OR 2.1, 95% CI: 1.6–2.8, P=0.000 and OR 1.7, 95% CI: 1.3–2.3, P=0.000, respectively).
Determinants of lower PINP/βCTX ratio and hypoalbuminemia
In multivariate forward stepwise regression analyses, which included all univariate clinical (dementia, CAD, AF, history of stroke, TIA, CKD, osteoporosis, antiosteoporotic medications use, smoking status), sociodemographic (age, gender, residence type, and use of walking aids), laboratory parameters with P≤0.100 and the presence of an HF or any fracture (separate analyses), the PINP/βCTX ratio as a continuous variable was independently predicted by the presence of HF (β=−28.602, P=0.000) or of any fracture (β=−23.4845, P=0.000), age (β=−1.3032, P=0.000), OC (β=2.574, P=0.000), GFR (β=0.482, P=0.002), and the use of antiosteoporotic medications (β=29.761, P=0.000). These data indicate that lower PINP/βCTX ratio is largely determined by increasing age, lower OC, and decline of renal function, and is strongly associated with any nonvertebral fracture and the nonuse of antiosteoporotic therapy.
Independent determinants of P1NP/βCTX <100.0 (under median level) were also assessed in a similar stepwise multiple linear regression analyses which included the laboratory, sociodemographic, and clinical characteristics. The probability of P1NP/βCTX <100.0 increased with increment in each year of age by 4% (OR 1.04, 95% CI: 1.02–1.06, P=0.000), the presence of HF by 2.8-fold (OR 2.8, 95% CI: 2.0–3.8, P=0.000), and the presence of any fracture by 2.1-fold (OR 2.1, 95% CI: 1.6–2.8, P=0.000), and decreased with the use of antiosteoporotic medications by 34.6% for HF (OR 0.65, 95% CI: 0.43–1.00, P=0.050) and 32.4% for any fracture (OR 0.68, 95% CI: 0.48–0.96, P=0.027).
With regard to hypoalbuminemia (<33 g/L), a similar multiple regression revealed that the presence of HF (OR 2.8, 95% CI: 2.0–3.9, P=0.000) or of any fracture (OR 1.5, 95% CI: 1.2–2.0, P=0.002) is an independent determinant of this condition and its probability with each year of age increases by 3% in HF patients (OR 1.03, 95% CI: 1.01–1.05, P=0.005) and by 4% in the group with any fracture (OR 1.04, 95% CI: 1.02–1.05, P=0.000).
Taken together, these data suggest that among the studied laboratory parameters both lower serum PINP/βCTX ratio and hypoalbuminemia are strongly associated with and are the best to indicate a nonvertebral osteoporotic fracture.
Informative/predictive values of lower P1NP/βCTX ratio and hypoalbuminemia
In the attempt to give to practicing physicians a simple tool, we focused on the median P1NP/βCTX ratio (<100.0) and low albumin (<33 g/L) as cutoff values. We additionally evaluated the discriminative values of recently recommended treatment targets for antiosteoporotic therapies: P1NP >62 µg/L for bone-forming agents37 and βCTX <0.250 µg/L for antiresorptive therapy.20,38 In our cohort, there were only nine (0.73%) patients (including five without fractures, one with an HF, and three with other fractures) in whom both these markers were within the targeted zone; none of them had P1NP/βCTX <100.0 and eight subjects (including all four with fractures) have been receiving antiosteoporotic treatment. In other words, both markers were in the desired zone only in 1.3% of patients without fractures and in 0.47% of patients with any fracture (0.22% among HF). However, P1NP <62 µg/L was found in 942 (76.6%) patients (in 368 without fracture, 304 with HF and 270 with other fractures), and βCTX >0.250 µg/L in 955 (77.7%) patients (in 376, 302 and 277, respectively).
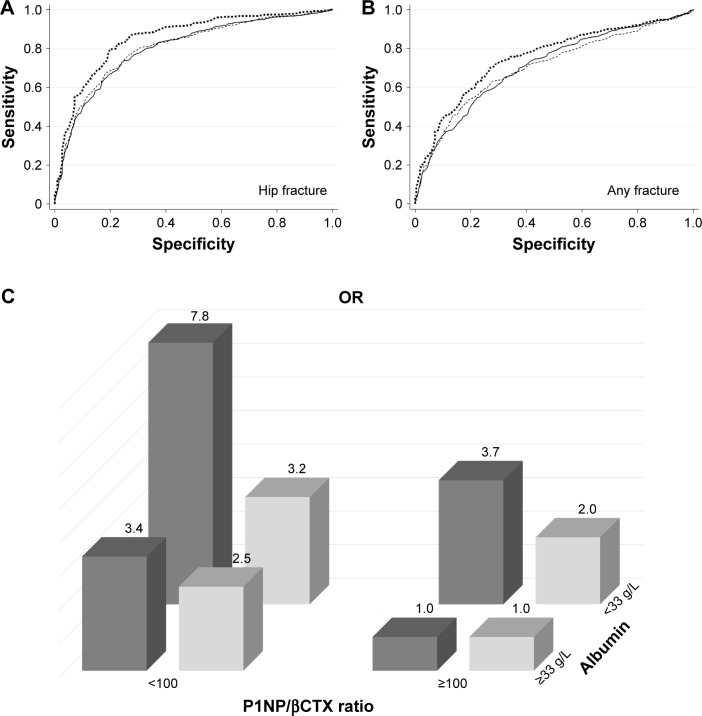
The results of the analyses performed with five explanatory variables are displayed in Table 3 and Figure 1A and B. AUC values after adjustment for age and gender for all markers were between 0.691 and 0.855, indicating a mild-to-moderate discriminatory ability. The P1NP/βCTX ratio <100.0 and albumin <33 g/L yielded the best AUC measures for HF (0.802 and 0.806, respectively) or for any fractures (0.711 and 0.706, respectively).
Table 3.
Bone turnover markers and hypoalbuminemia as indicators of nonvertebral osteoporotic fractures in orthogeriatric patients
| Variables | OR (95% CI) | AUC | Sensitivity, % | Specificity, % | PPV, % | NPV, % | Accuracy, % |
|---|---|---|---|---|---|---|---|
| Hip fracture | |||||||
| P1NP/βCTX <100 | 2.8 (2.0; 3.8)a | 0.802 | 78.5 | 68.9 | 75.2 | 72.8 | 74.1 |
| P1NP <62.0 µg/L | 1.7 (1.2; 2.4)b | 0.785 | 76.3 | 65.2 | 72.3 | 69.8 | 71.2 |
| βCTX >0.250 µg/L | 1.3 (0.9; 1.9)c | 0.781 | 77.0 | 65.5 | 72.9 | 70.4 | 71.8 |
| Albumin <33 g/L | 3.1 (2.2; 4.3)a | 0.806 | 78.7 | 71.5 | 76.7 | 73.8 | 75.4 |
| P1NP/βCTX <100 + albumin <33 g/L | 7.8 (4.9–12.4)a | 0.855 | 83.0 | 75.6 | 80.4 | 78.7 | 79.6 |
| Any fracture | |||||||
| P1NP/βCTX <100 | 2.1 (1.6; 2.8)a | 0.711 | 90.1 | 27.1 | 73.5 | 54.8 | 70.7 |
| P1NP <62.0 µg/L | 1.5 (1.1; 2.0)b | 0.695 | 90.3 | 18.7 | 71.3 | 46.4 | 68.2 |
| βCTX >0.250 µg/L | 1.2 (0.9; 1.6)c | 0.691 | 91.3 | 17.2 | 71.3 | 46.8 | 68.5 |
| Albumin <33 g/L | 2.2 (1.3; 2.7)a | 0.706 | 89.3 | 19.9 | 71.4 | 45.5 | 67.9 |
| P1NP/βCTX <100 + albumin <33 g/L | 3.2 (2.2–4.6)a | 0.754 | 85.7 | 41.8 | 75.7 | 58.0 | 71.6 |
Notes: Data adjusted for age and gender;
P<0.001;
P<0.01;
P>0.200.
Abbreviations: P1NP, N-terminal propeptide of type 1 procollagen; βCTX, cross-linked carboxy-terminal telopeptide of type 1 collagen; OR, odds ratio; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value.
Figure 1.
Discriminative information on nonvertebral fracture presence according to serum P1NP/βCTX ratio and albumin concentrations in orthogeriatric patients.
Notes: (A, B) Receiver operating characteristic curves (ROC) adjusted for age and gender for P1NP/βCTX <100 (solid line), albumin <33 g/L (thin dashed line), and their combination (thick dashed line) as prognostic tests for HF (A) or for any fracture (B). (C) odds ratios (ORs) adjusted for age and gender for the presence of an HF or of any nonvertebral fracture. The group with P1NP/βCTX >100.0 and albumin >33 g/L on admission used as the reference one. Among patients with only P1NP/βCTX <100.0, the ORs are 3.4- and 2.5-fold higher in subjects with an HF or any nonvertebral fracture, respectively, and among patients with only albumin <33 g/L, the ORs are 3.7- and 2.0-fold higher, respectively; if both conditions are present (combined), the ORs are 7.8- and 3.2-fold higher, respectively.
Abbreviations: βCTX, cross-linked carboxy-terminal telopeptide of type 1 collagen; HF, heart failure; P1NP, amino-terminal propeptide of type 1 procollagen.
Interestingly, among patients with only P1NP/βCTX <100.0, the ORs were 3.4-fold (95% CI: 2.0–5.7, P=0.000) and 2.5-fold (95% CI: 1.7–3.7, P=0.000) higher in subjects with HF or any nonvertebral fracture, respectively, and among patients with only albumin <33 g/L, the ORs were 3.7-fold (95% CI: 2.7–6.0, P=0.000) and 2.0-fold (95% CI: 1.4–2.8, P=0.000) higher, respectively (Figure 1C). The data further suggest the independent and strong association of each of these two factors with nonvertebral fractures.
When P1NP/βCTX ratio <100.0 and albumin <33 g/L were combined, the ORs for HF or for any fracture, compared with the nonfractured group, were 7.8 and 3.2, respectively, and the AUC improved (0.855 and 0.754, respectively). The presence of both these characteristics was a more sensitive (83.0% for HF and 85.7% for any fracture) and accurate indicator of fracture presence (79.6% and 71.6%, respectively) compared to other variables.
Validation of serum P1NP/βCTX ratio and albumin levels as indicators of nonvertebral fracture
Patients in the validation dataset comparing to those in the test dataset did not show significant differences in sociodemographics, comorbidities, and antiresorptive medication use. When the P1NP/βCTX ratio cutoff of <100.0 and albumin <33 g/L were applied to the validation dataset, they showed significant and similar discriminative values. P1NP/βCTX <100: for HF AUC 0.810 (sensitivity 78.7%, PPV 75.9%), for any fracture AUC 0.710 (sensitivity 89.3%, PPV 73.8%); albumin <33 g/L: for HF AUC 0.811 (sensitivity 78.3%, PPV 71.7%), for any fracture AUC 0.705 (sensitivity 88.3%, PPV 71.7%); both factors combined: for HF AUC 0.861 (sensitivity 86.2%, PPV 81.1%), for any fracture AUC 0.765 (sensitivity 88.0%, PPV 76.7%).
Discussion
In this study, in a large cohort of consecutive hospitalized orthogeriatric patients, lower levels of serum P1NP/βCTX ratio and albumin concentration were 1) strong independent indicators of HF or of any nonvertebral fracture, 2) showed a dose-dependent relationship with the prevalence of fractures, and 3) demonstrated a discrimination ability of acceptable precision that exceeded the discrimination ability of other studied laboratory parameters. To the best of our knowledge, this is the first study to demonstrate the clinical utility of lower serum P1NP/βCTX ratio and hypoalbuminemia as promising biomarkers for predicting osteoporotic fractures in older adults.
The prevalence of both lower P1NP/βCTX ratio (reflects an imbalance between total bone formation and resorption in favour of the latter) and hypoalbuminaemia increase with age, and both factors are independently associated with osteoporotic fractures; lower PINP/βCTX ratio is also largely determined by decline of renal function and the nonuse of antiosteoporotic therapy. It appears that these two characteristics – serum PINP/βCTX ratio and albumin – accumulate key determinants of fracture risk incorporating the effects of multiple clinical and metabolic abnormalities reported in the literature and observed in our univariate analysis. Although there are no prior studies using P1NP/βCTX ratio, our results are consistent with the only previous report showing that the ratio of a urinary resorption to serum formation marker (u-NTx/OC) was predictive of fractures independent of FRAX.23 A number of reports, but not all,11,20,39,40 indicated a link between abnormalities in BTMs, bone loss, and increased risk of fracture independent of BMD.10,12,13,41–44
The pathophysiological mechanism(s) underlying the relationship between altered albumin homeostasis and osteoporotic fractures is not fully understood. Hypoalbuminemia could be caused and/or aggravated by numerous chronic diseases associated with increased risk of falls and fractures.26,27 Hypoalbuminemia may directly and indirectly influence bone status, shifting the balance toward bone resorption via its effects on the nuclear factor-kB, disturbed inflammatory and antioxidant responses, reduced flux of minerals to and from the bone, decreased formation of calcium phosphate apatite crystals, as well as affecting the metabolism of PTH, vitamin D binding protein, and Gla-protein.32,45
Our data showed that among hospitalized orthogeriatric patients, the serum P1NP/βCTX <100 or/and albumin <33 g/L at admission outweighed other laboratory parameters in its discriminatory ability of fracture presence, especially for HF, and the combination of both signs doubles the ORs. The fact that near equal proportions of patients admitted with a fracture had only one of these characteristics (Figure 1C) reflects the complexity, multifactorial nature, and heterogeneity of metabolic mechanisms underlying osteoporotic fractures and indicates the usefulness to include in the screening strategy measuring of both parameters, each of which demonstrated a strong independent association with fractures.
Taken together, in older patients, serum BTMs and albumin may perhaps help distinguish subgroups with different prognoses for osteoporotic fracture: 1) high risk if P1NP/βCTX <100.0 and albumin <33 g/L (OR 7.8 for HF and 3.2 for any fracture), 2) intermediate risk if P1NP/βCTX <100.0 (OR 3.4 and 2.5, respectively) or albumin <33 g/L (OR 3.7 and 2.0), and 3) low risk (<0.5%) in older adults with βCTX <0.250 µg/L and P1NP >62.0 µg/L. Although the discriminative ability of these markers is only moderate (but higher when compared with other currently available indices), they may be particularly useful in persons who have negative BMD test. It should be, however, emphasized that the fracture risk remains substantial in subjects with P1NP/βCTX >100.0 and albumin >33 g/L; such characteristics demonstrated 58.1% of orthogeriatric patients without fracture but also 18.7% of all fracture patients including 9.0% with HF, indicating that in near 1/5 of subjects with fragility fractures other factors rather than the total balance between bone formation and resorption and/or albumin homeostasis are important in the development of fractures.
Limitations of the study include: 1) cross-sectional design (results describe associations rather than causation), 2) comparison with nonfractured elderly orthopedic patients (not a healthy control group), a significant proportion of which may have undiagnosed/undocumented osteoporosis, 3) reliance on single measurement, and 4) data from one medical center, mostly on white older adults, limiting the generalizability of the results. Of note, within 26 hours after fracture, BTMs are not altered from the preinjury levels,46 but both bone formation and bone resorption markers significantly increase within 6 weeks to 6 months after fracture, reflecting the fracture healing process, and these changes may persist for up to a year.46–49 As in all our patient, fasting venous blood samples were collected within 24 hours of admission to the hospital it is unlikely that the fracture per se contributed to the observed changes in BTMs.
This study also has several strengths: the relatively large number of patients, adjustment for a wide range of confounding factors, and the use of validation cohort. In multivariate regression analyses, the variance inflation factor was between 1.07 and 1.18, indicating that the amount of multicollinearity was not significant.
Conclusion
In an unselected cohort of hospitalized consecutive orthogeriatric patients, both serum P1NP/βCTX ratio and albumin levels demonstrated an inverse dose–effect relationship with the prevalence of nonvertebral fractures and independently indicated fracture presence with acceptable discriminatory power. Lower P1NP/βCTX (<100) and hypoalbuminemia (<33 g/L) could be useful simple and inexpensive tools to obtain additive prognostic information on fracture risk in the elderly. However, confirmation in other cohorts is needed to further support the applicability of these characteristics to the total population.
Summary
In a cohort of unselected orthogeriatric patients (n=1,239), serum P1NP/βCTX ratio and albumin levels demonstrated an inverse dose–effect relationship with the prevalence of nonvertebral fractures. P1NP/βCTX <100 and hypoalbuminemia (<33 g/L) could be useful additive prognostic tools for fracture risk stratification in the elderly.
Footnotes
Author contributions
AF was the coordinator of the study and together with LF participated in the study design, data collection, analysis, interpretation, and article writing. PS operated on the patients and contributed to data interpretation. WS performed statistical analysis and took part in interpretation of data. The final version of the manuscript was approved by all authors. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Marques A, Ferreira RJ, Santos E, Loza E, Carmona L, da Silva JA. The accuracy of osteoporotic fracture risk prediction tools: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74(11):1958–1967. doi: 10.1136/annrheumdis-2015-207907. [DOI] [PubMed] [Google Scholar]
- 2.Leslie WD, Lix LM. Comparison between various fracture risk assessment tools. Osteoporos Int. 2014;25(1):1–21. doi: 10.1007/s00198-013-2409-3. [DOI] [PubMed] [Google Scholar]
- 3.Dagan N, Cohen-Stavi C, Leventer-Roberts M, Balicer RD. External validation and comparison of three prediction tools for risk of osteoporotic fractures using data from population based electronic health records: retrospective cohort study. BMJ. 2017;356:i6755. doi: 10.1136/bmj.i6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanis JA, Harvey NC, Cooper C, et al. Advisory Board of the National Osteoporosis Guideline Group A systematic review of intervention thresholds based on FRAX: a report prepared for the National Osteoporosis Guideline Group and the International Osteoporosis Foundation. Arch Osteoporos. 2016;11(1):25. doi: 10.1007/s11657-016-0278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanis JA, Oden A, Johansson H, Borgström F, Ström O, McCloskey E. FRAX and its applications to clinical practice. Bone. 2009;44(5):734–743. doi: 10.1016/j.bone.2009.01.373. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen ND, Eisman JA, Center JR, Nguyen TV. Risk factors for fracture in nonosteoporotic men and women. J Clin Endocrinol Metab. 2007;92(3):955–962. doi: 10.1210/jc.2006-1476. [DOI] [PubMed] [Google Scholar]
- 7.Rozental TD, Herder LM, Walley KC, et al. 25-Hydroxyvitamin-D and bone turnover marker levels in patients with distal radial fracture. J Bone Joint Surg Am. 2015;97(20):1685–1693. doi: 10.2106/JBJS.O.00313. [DOI] [PubMed] [Google Scholar]
- 8.Kanis JA, Harvey NC, Johansson H, Odén A, Leslie WD, McCloskey EV. FRAX and fracture prediction without bone mineral density. Climacteric. 2015;18(Suppl 2):2–9. doi: 10.3109/13697137.2015.1092342. [DOI] [PubMed] [Google Scholar]
- 9.Burch J, Rice S, Yang H, et al. Systematic review of the use of bone turnover markers for monitoring the response to osteoporosis treatment: the secondary prevention of fractures, and primary prevention of fractures in high-risk groups. Health Technol Assess. 2014;18(11):1–180. doi: 10.3310/hta18110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansson H, Oden A, Kanis JA, et al. A meta-analysis of reference markers of bone turnover for prediction of fracture. Calcif Tissue Int. 2014;94(5):560–567. doi: 10.1007/s00223-014-9842-y. [DOI] [PubMed] [Google Scholar]
- 11.Vasikaran S, Eastell R, Bruyere O, et al. IOF-IFCC Bone Marker Standards Working Group Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22(2):391–420. doi: 10.1007/s00198-010-1501-1. [DOI] [PubMed] [Google Scholar]
- 12.Arceo-Mendoza RM, Camacho P. Prediction of fracture risk in patients with osteoporosis: a brief review. Womens Health (Lond) 2015;11(4):477–482. doi: 10.2217/whe.15.14. [DOI] [PubMed] [Google Scholar]
- 13.Chubb SA, Byrnes E, Manning L, et al. Reference intervals for bone turnover markers and their association with incident hip fractures in older men: the Health in Men study. J Clin Endocrinol Metab. 2015;100(1):90–99. doi: 10.1210/jc.2014-2646. [DOI] [PubMed] [Google Scholar]
- 14.Shigdel R, Osima M, Ahmed LA, et al. Bone turnover markers are associated with higher cortical porosity, thinner cortices, and larger size of the proximal femur and non-vertebral fractures. Bone. 2015;81:1–6. doi: 10.1016/j.bone.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 15.Shieh A, Han W, Ishii S, Greendale GA, Crandall CJ, Karlamangla AS. Quantifying the balance between total bone formation and total bone resorption: an index of net bone formation. J Clin Endocrinol Metab. 2016;101(7):2802–2809. doi: 10.1210/jc.2015-4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hannemann A, Wallaschofski H. Reference intervals for serum concentrations of three bone turnover markers for men and women. Bone. 2016;93:216. doi: 10.1016/j.bone.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 17.Marques EA, Gudnason V, Sigurdsson G, et al. Are bone turnover markers associated with volumetric bone density, size, and strength in older men and women? The AGES-Reykjavik study. Osteoporos Int. 2016;27(5):1765–1776. doi: 10.1007/s00198-015-3442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biver E. Use of bone turnover markers in clinical practice. Curr Opin Endocrinol Diabetes Obes. 2012;19(6):468–473. doi: 10.1097/MED.0b013e3283591492. [DOI] [PubMed] [Google Scholar]
- 19.Garnero P. New developments in biological markers of bone metabolism in osteoporosis. Bone. 2014;66:46–55. doi: 10.1016/j.bone.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Vasikaran SD, Chubb SA. The use of biochemical markers of bone turnover in the clinical management of primary and secondary osteoporosis. Endocrine. 2016;52(2):222–225. doi: 10.1007/s12020-016-0900-2. [DOI] [PubMed] [Google Scholar]
- 21.Eastell R, Rogers A, Ni X, Krege JH. Effects of raloxifene and alendronate on bone turnover as assessed by procollagen type I N-terminal propeptide. Osteoporos Int. 2011;22(6):1927–1934. doi: 10.1007/s00198-010-1380-5. [DOI] [PubMed] [Google Scholar]
- 22.Seeman E, Nguyen TV. Bone remodeling markers: so easy to measure, so difficult to interpret. Osteoporos Int. 2016;27(1):33–35. doi: 10.1007/s00198-015-3374-9. [DOI] [PubMed] [Google Scholar]
- 23.Melton LJ, 3rd, Atkinson EJ, Achenbach SJ, et al. Potential extensions of the US FRAX algorithm. J Osteoporos. 2012;2012:528790. doi: 10.1155/2012/528790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ascenzi P, di Masi A, Fanali G, Fasano M. Heme-based catalytic properties of human serum albumin. Cell Death Discov. 2015;1:15025. doi: 10.1038/cddiscovery.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fanali G, di Masi A, Trezza V, Marino M, Fasano M, Ascenzi P. Human serum albumin: from bench to bedside. Mol Aspects Med. 2012;33(3):209–290. doi: 10.1016/j.mam.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Drevet S, Bioteau C, Maziere S, et al. Prevalence of protein-energy malnutrition in hospital patients over 75 years of age admitted for hip fracture. Orthop Traumatol Surg Res. 2014;100(6):669–674. doi: 10.1016/j.otsr.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Goisser S, Schrader E, Singler K, et al. Malnutrition according to mini nutritional assessment is associated with severe functional impairment in geriatric patients before and up to 6 months after hip fracture. J Am Med Dir Assoc. 2015;16(8):661–667. doi: 10.1016/j.jamda.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Cabrerizo S, Cuadras D, Gomez-Busto F, Artaza-Artabe I, Marín-Ciancas F, Malafarina V. Serum albumin and health in older people: review and meta analysis. Maturitas. 2015;81(1):17–27. doi: 10.1016/j.maturitas.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Levitt DG, Levitt MD. Human serum albumin homeostasis: a new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int J Gen Med. 2016;9:229–255. doi: 10.2147/IJGM.S102819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fisher L, Srikusalanukul W, Fisher A, Smith P. Liver function parameters in hip fracture patients: relations to age, adipokines, comorbidities and outcomes. Int J Med Sci. 2015;12(2):100–115. doi: 10.7150/ijms.10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuwen P, Chen W, Lv H, et al. Albumin and surgical site infection risk in orthopaedics: a meta-analysis. BMC Surg. 2017;17(1):7. doi: 10.1186/s12893-016-0186-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Afshinnia F, Pennathur S. Association of hypoalbuminemia with osteoporosis: analysis of the National Health and Nutrition Examination Survey. J Clin Endocrinol Metab. 2016;101(6):2468–2474. doi: 10.1210/jc.2016-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura K, Oyama M, Saito T, et al. Nutritional and biochemical parameters associated with 6-year change in bone mineral density in community-dwelling Japanese women aged 69 years and older: the Muramatsu Study. Nutrition. 2012;28(4):357–361. doi: 10.1016/j.nut.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 34.Formosa MM, Xuereb-Anastasi A. Biochemical predictors of low bone mineral density and fracture susceptibility in Maltese postmenopausal women. Calcif Tissue Int. 2016;98(1):28–41. doi: 10.1007/s00223-015-0060-z. [DOI] [PubMed] [Google Scholar]
- 35.Ma MK, Yap DY, Yip TP, Lui SL, Lo WK. Charlson co-morbidity index and albumin significantly associated with fracture risk in peritoneal dialysis patients. Nephrology (Carlton) 2013;18(5):365–368. doi: 10.1111/nep.12056. [DOI] [PubMed] [Google Scholar]
- 36.Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80(1):17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 37.Eastell R, Christiansen C, Grauer A, et al. Effects of denosumab on bone turnover markers in postmenopausal osteoporosis. J Bone Miner Res. 2011;26(3):530–537. doi: 10.1002/jbmr.251. [DOI] [PubMed] [Google Scholar]
- 38.Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res. 2003;18(6):1051–1056. doi: 10.1359/jbmr.2003.18.6.1051. [DOI] [PubMed] [Google Scholar]
- 39.Szulc P, Montella A, Delmas PD. High bone turnover is associated with accelerated bone loss but not with increased fracture risk in men aged 50 and over: the prospective MINOS study. Ann Rheum Dis. 2008;67(9):1249–1255. doi: 10.1136/ard.2007.077941. [DOI] [PubMed] [Google Scholar]
- 40.Finnes TE, Lofthus CM, Meyer HE, et al. Procollagen type 1 amino- terminal propeptide (P1NP) and risk of hip fractures in elderly Norwegian men and women. A NOREPOS study. Bone. 2014;64:1–7. doi: 10.1016/j.bone.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 41.Garnero P. Biomarkers for osteoporosis management: utility in diagnosis, fracture risk prediction and therapy monitoring. Mol Diagn Ther. 2008;12(3):157–170. doi: 10.1007/BF03256280. [DOI] [PubMed] [Google Scholar]
- 42.Ivaska KK, Gerdhem P, Vaananen HK, Akesson K, Obrant KJ. Bone turnover markers and prediction of fracture: a prospective follow-up study of 1040 elderly women for a mean of 9 years. J Bone Miner Res. 2010;25(2):393–403. doi: 10.1359/jbmr.091006. [DOI] [PubMed] [Google Scholar]
- 43.Dai Z, Wang R, Ang LW, Yuan JM, Koh WP. Bone turnover biomarkers and risk of osteoporotic hip fracture in an Asian population. Bone. 2016;83:171–177. doi: 10.1016/j.bone.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morris HA, Eastell R, Jorgensen NR, et al. IFCC-IOF Working Group for Standardisation of Bone Marker Assays (WG-BMA) Clinical usefulness of bone turnover marker concentrations in osteoporosis. Clin Chim Acta. 2017;497:34–41. doi: 10.1016/j.cca.2016.06.036. [DOI] [PubMed] [Google Scholar]
- 45.Abu-Amer Y. NF-kappaB signaling and bone resorption. Osteoporos Int. 2013;24(9):2377–2386. doi: 10.1007/s00198-013-2313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ivaska KK, Gerdhem P, Akesson K, Garnero P, Obrant KJ. Effect of fracture on bone turnover markers: a longitudinal study comparing marker levels before and after injury in 113 elderly women. J Bone Miner Res. 2007;22(8):1156–1164. doi: 10.1359/jbmr.070505. [DOI] [PubMed] [Google Scholar]
- 47.Akesson K, Kakonen SM, Josefsson PO, Karlsson MK, Obrant KJ, Petersson K. Fracture-induced changes in bone turnover: a potential confounder in the use of biochemical markers in osteoporosis. J Bone Miner Metab. 2005;23(1):30–35. doi: 10.1007/s00774-004-0537-8. [DOI] [PubMed] [Google Scholar]
- 48.Obrant KJ, Ivaska KK, Gerdhem P, Alatalo SL, Petersson K, Vaananen HK. Biochemical markers of bone turnover are influenced by recently sustained fracture. Bone. 2005;36(5):786–792. doi: 10.1016/j.bone.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 49.Ikegami S, Kamimura M, Nakagawa H, et al. Comparison in bone turnover markers during early healing of femoral neck fracture and trochanteric fracture in elderly patients. Orthop Rev (Pavia) 2009;1(2):e21. doi: 10.4081/or.2009.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]