Abstract
Chimaeric antigen receptor (CAR) T-cells are T-cells that have been genetically modified to express an artificial construct consisting of a synthetic T-cell receptor (TCR) targeted to a predetermined antigen expressed on a tumour. Coupling the T-cell receptor to a CD3ζ signalling domain paved the way for first generation CAR T-cells that were efficacious against cluster of differentiation (CD)19-expressing B-cell malignancies. Optimization with additional signalling domains such as CD28 or 4-1BB in addition to CD3ζ provided T-cell activation signal 2 and further improved the efficacy and persistence of these second generation CAR T-cells. Third generation CAR T-cells which utilize two tandem costimulatory domains have also been reported. In this review, we discuss a different approach to optimization of CAR T-cells. Through additional genetic modifications, these resultant armored CAR T-cells are typically modified second generation CAR T-cells that have been further optimized to inducibly or constitutively secrete active cytokines or express ligands that further armor CAR T-cells to improve efficacy and persistence. The choice of the ‘armor’ agent is based on knowledge of the tumour microenvironment and the roles of other elements of the innate and adaptive immune system. Although there are several variants of armored CAR T-cells under investigation, here we focus on three unique approaches using interleukin-12 (IL-12), CD40L and 4-1BBL. These agents have been shown to further enhance CAR T-cell efficacy and persistence in the face of a hostile tumour microenvironment via different mechanisms.
Keywords: adoptive immunotherapy, armored chimaeric antigen receptor T-cell (CAR T-cell), chimaeric antigen receptor
Introduction
Chimaeric antigen receptor (CAR) T-cell therapy represents an exciting new paradigm in the treatment of patients with cancer [1]. Adoptive transfer of CAR T-cells has shown significant promise in the treatment of haematologic malignancies and to a lesser extent, solid tumours. The CAR construct typically consists of a single chain variable fragment (scFv) directed against a known tumour antigen [2]. This is followed by a signalling domain, typically cluster of differentiation 3ζ (CD3ζ), which provides the so called ‘signal 1’, necessary for T-cell activation [2]. Engagement and signalling via the ζ chain is required for T-cell stimulation and proliferation but is not often sufficient for sustained proliferation and activity in the absence of a second signal or ‘signal 2’. Preclinical studies using first generation CAR T-cells were promising when directed against cluster of differentiation (CD)19 [3] and HER2/Neu [4]. In both cases, there was robust activation of the CAR T-cells when exposed to cells expressing the target antigen followed by effective target cell killing in vitro and in preclinical in vivo tumour models [3]. Unfortunately, anti-tumour efficacy was not seen in subsequent clinical trials. For example, in a phase I study of patients with metastatic renal cell carcinoma using first generation CAR T-cells directed against an epitope on carbonic anhydrase IX (CAIX), there were no objective clinical responses [5]. Unfortunately, patients treated on this trial developed acute liver toxicity attributed to CAR T-cell therapy [6]. Furthermore, the authors found induction of a human anti-chimaera response (HACA) and limited peripheral persistence of the infused CAR T-cells in vivo [5]. In another report, Till et al. [7] treated patients with indolent non-Hodgkin lymphoma with a first generation CAR against CD20, an antigen commonly expressed on normal and malignant B-cells. Of the eight patients treated, two patients who had already achieved a complete response (CR) after cytoreductive therapy remained in CR and only one other patient achieved a partial response. Notably, there was no host-generated immunoreactivity to the CAR T-cells in these patients. In order to address some of the shortcomings of first generation CAR T-cells, further genetic modifications were made to include a CD28 costimulatory domain that functioned independently of its ligand B7. These CD28/CD3ζ (CD28ζ) domains provide both ‘signal 1’ and ‘signal 2’ for T-cell activation upon antigen recognition on the target cell and mitigate the anergy and activation-induced cell death seen with first generation CAR T-cells [8]. To test the efficacy of second generation CAR T-cells, Brentjens et al. [9] used CAR T-cells directed against CD19-expressing NALM-6 cells in an ALL (acute lymphoblastic leukaemia) xenograft tumour model and reported significantly enhanced anti-tumour efficacy. Similarly, enhanced activation and efficacy was reported in a preclinical model of prostate cancer using PSMA (prostate specific membrane antigen) directed CD28ζ second generation CAR T-cells [10]. Savoldo et al. [11] compared first and second generation CAR T-cells (CD19ζ compared with CD19–28ζ) targeted against CD19, an antigen commonly expressed on normal and malignant B-cells in patients with non-Hodgkin lymphoma. After infusing patients with both anti-CD19ζ and anti-CD19-28ζ CAR T-cells simultaneously, anti-CD19–28ζ CAR T-cells showed vastly superior expansion, persistence and infiltration of tumour sites compared with anti-CD19ζ CAR T-cells in the same patients. Patients with relapsed B-cell ALL treated with anti-CD19-28ζ CAR T-cells had a rapid response to therapy in all five patients treated [12]. In another study, Davila et al. [13] reported an 88% CR rate in patients with relapsed/refractory B-cell malignancies treated with anti-CD19-28ζ CAR T-cell therapy [13]. Second generation CAR T-cell therapy utilizing 4-1BB, another commonly used costimulatory molecule, has also shown efficacy in the treatment of haematologic malignancies including chronic lymphocytic leukaemia (CLL) [14,15]. Further optimization has led to the development of ‘third generation’ CAR T-cells which utilize two distinct costimulatory domains (e.g. CD28/4-1BB/CD3ζ or CD28/OX-40/CD3ζ). These constructs have shown varying degrees of in vitro and in vivo levels of activation, proliferation and interleukin-2 (IL-2) production [16–18].
This review focuses on the optimization of CAR T-cell efficacy via additional genetic modifications designed to secrete cytokines, or express ligands that are known to enhance or interact with endogenous immune cells such as dendritic cells (DCs), macrophages or regulatory T-cells (Treg cells) [19]. These so-called armored CAR T-cells have been specifically designed to survive, disrupt and/or favourably modulate an otherwise immunosuppressive tumour microenvironment. In solid tumour malignancies where exciting preclinical CAR T therapy has not translated in clinical gains, these armored CAR T-cells represent a potential advancement in CAR T-cell therapy. Here we focus on three distinct armored CAR T-cell approaches utilizing IL-12, CD40L and 4-1BBL.
IL-12
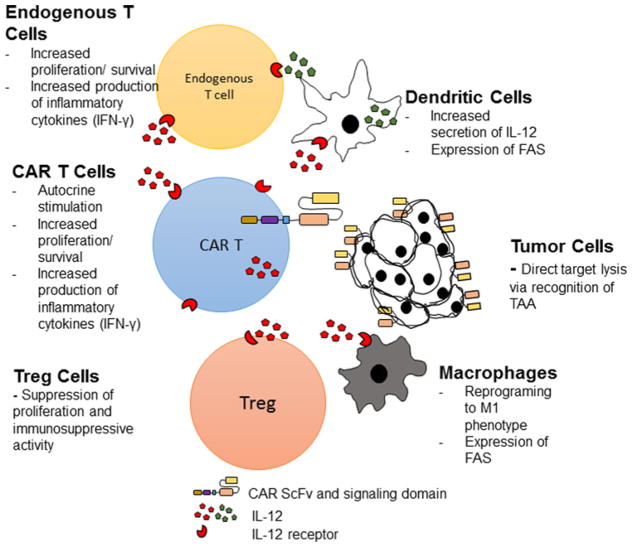
IL-12 is a potent inflammatory cytokine consisting of a heterodimeric p35 and p40 subunit which constitutes the active IL-12 p70 protein [20]. IL-12 is not secreted by T-cells but is produced by DCs, macrophages and neutrophils and has been shown to induce polarization of CD8 + T-cells to a pro-inflammatory TH1 (CD62Lhi, IL-7Rαhi, IL-2Rαhi, Sca1hi) phenotype, enhance antigen cross presentation and reprogramme myeloid derived suppressor cells [21]. Furthermore, IL-12 enhances the cytotoxic ability of CD8 + T-cells and leads to increases in interferon-γ (IFN-γ), tumour necrosis factor-α (TNF-α) and granulocyte macrophage colony stimulating factor (GM-CSF) secretion [22]. Taken together, introduction of IL-12 into the tumour microenvironment is predicted to improve CAR T-cell cytotoxic function, mitigate Treg-suppression and reprogramme tumour-associated macrophages and DCs (Figure 1). Multiple preclinical studies demonstrate anti-tumour efficacy of intra-tumoural injection of IL-12 expressing vectors [23,24] or IL-12 expressing bone marrow derived DCs [25] without any significant toxicity in mice. Pegram et al. [26] showed that treatment with anti-CD19–28ζ/IL-12 CAR T-cells in a syngeneic mouse EL4 (expressing human CD19) tumour model led to improved tumour eradication without the need for cytotoxic chemotherapy preconditioning [26]. Furthermore, IL-12 was reported to function in an autocrine manner via IL-12 receptors on the CAR T-cells and significantly mediate resistance to Treg inhibition [26]. A study by Kerkar et al. [27] reported IL-12 toxicity in mice characterized by weight loss and decreased survival. In this study, the authors speculated that this was due to the dose of adoptively transferred IL-12 producing T-cells. In clinical trials, attempts at systemic IL-12 treatment have not been as successful. For instance, systemic IL-12 treatment in patients with metastatic renal cell carcinoma led to severe side effects and treatment-related mortality [28]. The phase II trial had to be halted after a single dose of 500 mg/kg of IL-12, given the development of grade 3/4 fatigue, dyspnoea, stomatitis, leukopenia, elevated transaminases and two treatment-related mortalities. Although these toxicities were felt to be due to the schedule of IL-12 administration, regardless, there has been reluctance to further clinical development of systemic IL-12 therapy. If IL-12 is targeted to the tumour microenvironment, less IL-12 could be needed to provide anti-tumour efficacy while mitigating systemic side effects. Since CAR T-cells traffic to the tumour site, modifying CAR T-cells to secrete IL-12 could serve as a mechanism to deliver IL-12 to the tumour microenvironment. By engineering the IL-12 gene under the control of an IRES (internal ribosomal entry site) promoter, we were able to generate constitutive IL-12 producing CAR T-cells that secreted less IL-12 than would be needed for systemic administration and we did not observe any adverse effects in mice [26]. As mentioned above, we were able to improve tumour eradication and the requirement for preconditioning using this approach [26]. In a SCID Beige mouse model of ovarian cancer, we were also able to show superior efficacy of IL-12 armored CAR T-cells directed against the ectoplasmic domain of MUC16 (MUC16ecto) [29]. In a syngeneic mouse model of ovarian cancer, we observed increased CAR T-cell expansion, cytokine production, cytotoxic effect and reprogramming of tumour-associated macrophages using our MUC16ecto-directed CAR T-cells (unpublished work; Chekmasova, A. Renier Brentjens Laboratory). Most importantly, none of the previously described toxicities were seen in mice treated with our IL-12 armored CAR T-cell. However, it should be noted that mouse models do not completely recapitulate IL-12 toxicity in humans. Clinical trials utilizing IL-12 armored CAR T-cells are currently underway [30].
Figure 1. Armored CAR T-cells genetically engineered to constitutively secrete IL-12.
CAR T-cells modified to secrete IL-12 improve CAR T anti-tumour efficacy by potentially modulating the tumour microenvironment via autocrine activity on CAR T-cells, suppression of Treg cells, reprogramming of tumour-associated macrophages and enhanced IL-12 production by DCs.
CD40L
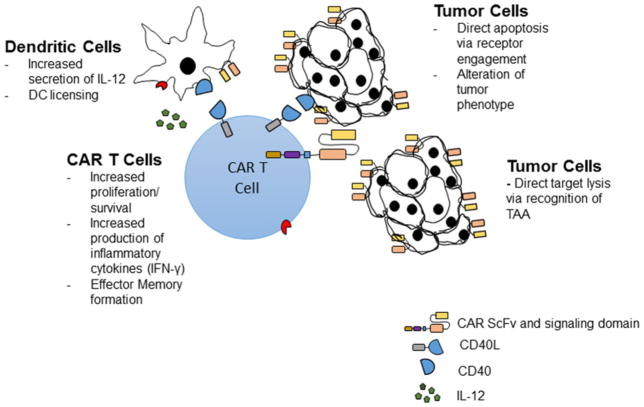
CD40L (CD154) is a type II transmembrane protein that binds to its cognate receptor, CD40, a member of the TNF-α receptor superfamily [31]. Expression of CD40L is induced upon activation of CD4 + T-lymphocytes, but its expression has also been described on DCs, macrophages and B-cells [32]. Upon binding to CD40 (CD138) on DCs, CD40L mediates CD8 + T-cell immunity via secretion of inflammatory cytokines such as IL-12 and IFN-γ, increases downstream antigen presentation and DC antigen licensing [33]. In a murine experimental model, animals lacking CD40L expression showed a deficiency in antigen-dependent T-cell priming and activation [34]. Engagement of CD40L/CD40 also increases T-cell proliferation and CD8 + T-cell immunity and memory [35]. Furthermore, CD40 engagement by CD40L on CD40-expressing tumour cells has been shown to change the tumour phenotype [36] and induce direct tumour apoptosis [37]. Due to the prominent role of immunosuppressive macrophages and DCs in the tumour microenvironment (Figure 2), several preclinical studies have attempted to utilize CD40L expression as a means of improving T-cell efficacy. In one study, infusion of adenovirus encoding murine CD40L in CLL patients led to an increase in inflammatory cytokines (IL-12 and IFN-γ) and an objective anti-tumour response [38]. Other studies have also utilized agonistic CD40 antibodies with evidence of DC activation and anti-tumour efficacy [39,40]. Curran et al. [36] generated a CAR vector with an additional CD40L gene designed to constitutively express CD40L. In vitro, CD40L-expressing CAR T-cells co-cultured with DoHH2 tumour cells altered the tumour phenotype to increase susceptibility to apoptosis and increase immunogenicity via up-regulation of costimulatory molecules (CD80, CD86), HLA molecules and Fas-death receptor on tumour cells. Furthermore, CD19-directed CAR T-cells with constitutive CD40L expression showed increased in vitro cytotoxicity compared with CD19-directed second generation CAR T-cells. This enhanced efficacy resulted in a significant survival benefit in DoHH2 tumour bearing mice treated with CD40L armored CAR T-cells compared with control second generation CAR T-cells. CD40L armored CAR T-cells represent a promising approach to improving CAR T-cell therapy and are currently under consideration for clinical trials.
Figure 2. Armored CAR T-cells genetically engineered to constitutively express CD40L.
CAR T-cells constitutively expressing CD40L improve CAR T anti-tumour efficacy by enhancing IL-12 secretion by DCs, maintaining effector T-cell memory and mediating direct tumour apoptosis upon CD40 engagement.
4-1BBL
4-1BB and its ligand, 4-1BBL, belong to the tumour necrosis factor receptor and tumour necrosis factor superfamily respectively [41]. 4-1BB is expressed on T-cells and is known to facilitate cell activation, survival and proliferation upon binding to its ligand, 4-1BBL [41]. 4-1BB has long been described as a stimulatory receptor on T-cells [42]. Additionally, 4-1BB expression is present on monocytes, DC, Tregs, neutrophils and natural killer cells (NK cells) [43]. Of note, 4-1BB expression has been reported on tumour vasculature but not on tumour cells [44]. 4-1BB signalling has also been shown to increase the expansion of CD8 + T-cells [45] and play key roles in central memory T-cell maintenance [46]. Binding of 4-1BB on DCs by 4-1BBL enhances secretion of IL-12, IL-6 and other inflammatory cytokines necessary for T-cell activation [47]. Taken together, these studies provided the rationale for the incorporation of constitutively expressed 4-1BBL on engineered CAR T-cells due to the predicted effect of enhanced CAR T-cell activity, cytotoxic function and tumour microenvironment modulation (Figure 3). Preclinical studies by Stephan et al. [48] showed T-cells co-transduced with CD80 and 4-1BBL showed robust proliferation and increased cytokine production compared with T-cells transduced with either construct alone. Furthermore, they were able to show successful eradication of disseminated prostate cancer (LNCap) cells in a SCID Beige model using PSMA directed 4-1BBL armored CAR T-cells. They also showed evidence for transcostimulation of tumour-specific bystander T-cells, opening the possibility of broadening anti-tumour activity via antigen spreading. In a recent study, Zhao et al. [49] compared the persistence and functionality of first generation, second generation and 4-1BBL armored CD-19 targeted CAR T-cells. Compared with first (19ζ1) and second (1928ζ and 19BBζ) generation CAR T-cells, 4-1BBL armored CAR T-cells showed enhanced in vitro efficacy. In vivo, mice treated with 1928ζ/4-1BBL CAR T-cells had the highest survival and tumour eradication rates. Furthermore, this approach provided a comparably increased tumoricidal profile and increased CAR T-cell persistence. The authors were further able to show that this anti-tumour efficacy was mediated by IRF7 (interferon regulatory factor 7), a transcription factor regulating the interferon type I (IFN-1)/interferon-β (IFN-β) pathway. The authors postulated that IFN-β could inhibit Treg activation [50], augment tumour antigen cross presentation [51] and disrupt tumour microvasculature [52]. All these factors are proposed to synergistically amplify the efficacy of CAR T-cells and produce ongoing anti-tumour activity even after the infused CAR T-cells are no longer detectable. The ability of 4-1BBL to not only induce intrinsic proliferation and activation of CAR T-cells, but also modify the landscape of the tumour microenvironment by recruiting endogenous T-cells has made it a promising candidate currently under clinical development.
Figure 3. Armored CAR T-cells genetically engineered to constitutively express 4-1BBL.
CAR T-cells constitutively expressing 4-1BBL improve CAR T anti-tumour efficacy by potentially modulating the tumour microenvironment via enhancement of DC IL-12 secretion, transcostimulation of endogenous T-cells and central memory maintenance.
Conclusions
CAR T-cells are an exciting new development in cancer immunotherapy. The ability to target CAR T-cells to virtually any tumour-associated antigen (TAA) provides an unparalleled flexibility that has yet to be fully exploited. With each new generation of CAR T-cells, there has been step-wise improvement in anti-tumour efficacy and CAR T-cell persistence. However, despite these improvements, solid tumour malignancies remain an unconquered frontier in CAR T-cell immunotherapy. Despite impressive preclinical results, there have not been any significant clinical gains. One plausible explanation for this discrepancy is the immunosuppressive effect of the tumour microenvironment that cannot be fully recapitulated in current in vitro and in vivo animal models.
Armored CAR T-cells represent the next generation of optimization that focuses on reinforcing modified CAR T-cells against the influences of a hostile tumour microenvironment. By carefully optimizing second or third generation CAR T-cells with additional genetic modifications such as IL-12, CD40L or 4-1BBL, CAR T-cell efficacy and persistence could be further improved, especially for hard-to-treat haematologic or solid tumour malignancies.
Although the opportunities and benefits of armored CAR T-cells are apparent, there are also unexpected on-tumour off-target side effects. In the case of IL-12, the toxicities of elevated systemic levels have been well described [27,28]. In the case of interleukin-15 (IL-15), another promising cytokine for application in armored CAR T-cells, concerns of T-cell leukemogenicity has tempered some of the excitement surrounding this candidate [53]. No such concerns have been reported for CD40L or 4-1BBL armored CAR T-cells but as previously alluded to, our murine models might not always be accurate and complete predictors of toxicity or response.
As we glean better understanding of the tumour microenvironment and how it overcomes endogenous and adoptively transferred T-cells, we can better design CAR T-cells that are armored against these inhibitory influences. Research efforts directed at understanding how armored CAR T-cells interact with endogenous T-cells and other elements of the immune system are urgently needed. Additionally, armored CAR T-cells with other cytokines, combination of cytokines or novel ligands need to be explored. It is not difficult to envision rationally designed armored CAR T-cells to different types of haematologic and solid tumour malignancies based on what is known about the nature of the inhibitory microenvironment. In fact, combination of different types of armored CAR T-cells such as cytokine secreting CAR T-cells and ligand-expressing CAR T-cells can be explored based on the niche of the tumour.
Acknowledgments
Funding
This work was supported by the National Institutes of Health [grant numbers R01CA138738-05, PO1CA059350 and PO1CA190174-01]; the Ovarian Cancer Research Fund [grant number 327501]; the Memorial Sloan Kettering T32 Investigational Therapeutics Training Program [grant number T32-CA009207]; The Annual Terry Fox Run for Cancer Research (New York, NY) [grant number 29410]; the Kate’s Team, Carson Family Charitable Trust [grant number 10171]; the William Lawrence and Blanche Hughes Foundation [grant number 10251]; the Emerald Foundation [grant number 11625]; and the Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center [grant number 13072].
Abbreviations
- ALL
acute lymphoblastic leukaemia
- CAR T-cell
chimaeric antigen receptor T-cell
- CD19ζ
cluster of differentiation 19ζ
- CD28ζ
cluster of differentiation 28ζ
- CLL
chronic lymphocytic leukaemia
- CR
complete response
- DC
dendritic cell
- IFN-β
interferon-β
- IFN-γ
interferon-γ
- IL-12
interleukin-12
- IL-15
interleukin-15
- PSMA
prostate specific membrane antigen
- TNF-α
tumour necrosis factor-α
- Treg
regulator T-cells
Footnotes
Conflicts of interest
R.J. Brentjens is a scientific co-founder of, reports receiving a commercial research grant from, has ownership interest (including patents) in and is a consultant/advisory board member for JUNO Therapeutics. No potential conflicts of interest were disclosed by the other author.
References
- 1.Davila ML, Bouhassira DCG, Park JH, Curran KJ, Smith EL, Pegram HJ, Brentjens R. Chimeric antigen receptors for the adoptive T cell therapy of hematologic malignancies. Int J Hematol. 2014;99:361–371. doi: 10.1007/s12185-013-1479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sadelain M, Brentjens R, Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3:388–398. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brentjens RJ, Latouche JB, Santos E, Marti F, Gong MC, Lyddane C, King PD, Larson S, Weiss M, Rivière I, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9:279–286. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 4.Stancovski I, Schindler DG, Waks T, Yarden Y, Sela M, Eshhar Z. Targeting of T lymphocytes to Neu/HER2-expressing cells using chimeric single chain Fv receptors. J Immunol. 1993;151:6577–6582. [PubMed] [Google Scholar]
- 5.Lamers CHJ, Willemsen R, van Elzakker P, van Steenbergen-Langeveld S, Broertjes M, Oosterwijk-Wakka J, Oosterwijk E, Sleijfer S, Debets R, Gratama JW. Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood. 2011;117:72–82. doi: 10.1182/blood-2010-07-294520. [DOI] [PubMed] [Google Scholar]
- 6.Lamers CHJ, Sleijfer S, Vulto AG, Kruit WHJ, Kliffen M, Debets R, Gratama JW, Stoter G, Oosterwijk E. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24:e20–e22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 7.Till BG, Jensen MC, Wang J, Chen EY, Wood BL, Greisman HA, Qian X, James SE, Raubitschek A, Forman SJ, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chambers CA, Allison JP. Co-stimulation in T cell responses. Curr Opin Immunol. 1997;9:396–404. doi: 10.1016/s0952-7915(97)80087-8. [DOI] [PubMed] [Google Scholar]
- 9.Brentjens RJ, Santos E, Nikhamin Y, Yeh R, Matsushita M, La Perle K, Quintas-Cardama A, Larson SM, Sadelain M. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res. 2007;13:5426–5435. doi: 10.1158/1078-0432.CCR-07-0674. [DOI] [PubMed] [Google Scholar]
- 10.Maher J, Brentjens RJ, Gunset G, Rivière I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nat Biotechnol. 2002;20:70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 11.Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, Kamble RT, Bollard CM, Gee AP, Mei Z, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121:1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska M, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Y, Wang QJ, Yang S, Kochenderfer JN, Zheng Z, Zhong X, Sadelain M, Eshhar Z, Rosenberg SA, Morgan RA. A herceptin-based chimeric antigen receptor with modified signaling domains leads to enhanced survival of transduced T lymphocytes and antitumor activity. J Immunol. 2009;183:5563–5574. doi: 10.4049/jimmunol.0900447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong XS, Matsushita M, Plotkin J, Riviere I, Sadelain M. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8 + T cell-mediated tumor eradication. Mol Ther. 2010;18:413–420. doi: 10.1038/mt.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilkie S, Picco G, Foster J, Davies DM, Julien S, Cooper L, Arif S, Mather SJ, Taylor-Papadimitriou J, Burchell JM, et al. Retargeting of human T cells to tumor-associated MUC1: the evolution of a chimeric antigen receptor. J Immunol. 2008;180:4901–4909. doi: 10.4049/jimmunol.180.7.4901. [DOI] [PubMed] [Google Scholar]
- 19.Pegram HJ, Smith EL, Rafiq S, Brentjens RJ. CAR therapy for hematological cancers: can success seen in the treatment of B-cell acute lymphoblastic leukemia be applied to other hematological malignancies? Immunotherapy. 2015;7:545–561. doi: 10.2217/imt.15.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carra G, Gerosa F, Trinchieri G. Biosynthesis and posttranslational regulation of human IL-12. J Immunol. 2000;164:4752–4761. doi: 10.4049/jimmunol.164.9.4752. [DOI] [PubMed] [Google Scholar]
- 21.Kerkar SP, Goldszmid RS, Muranski P, Chinnasamy D, Yu Z, Reger RN, Leonardi AJ, Morgan RA, Wang E, Marincola FM, et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest. 2011;121:4746–4757. doi: 10.1172/JCI58814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao J, Zhao J, Perlman S. Differential effects of IL-12 on Tregs and non-Treg T cells: roles of IFN-γ, IL-2 and IL-2R. PLoS One. 2012;7:e46241. doi: 10.1371/journal.pone.0046241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Addison CL, Bramson JL, Hitt MM, Muller WJ, Gauldie J, Graham FL. Intratumoral coinjection of adenoviral vectors expressing IL-2 and IL-12 results in enhanced frequency of regression of injected and untreated distal tumors. Gene Ther. 1998;5:1400–1409. doi: 10.1038/sj.gt.3300731. [DOI] [PubMed] [Google Scholar]
- 24.Bramson JL, Hitt M, Addison CL, Muller WJ, Gauldie J, Graham FL. Direct intratumoral injection of an adenovirus expressing interleukin-12 induces regression and long-lasting immunity that is associated with highly localized expression of interleukin-12 4937. Hum Gene Ther. 1996;16:1995–2002. doi: 10.1089/hum.1996.7.16-1995. [DOI] [PubMed] [Google Scholar]
- 25.Hu J, Yuan X, Belladonna ML, Ong JM, Wachsmann-Hogiu S, Farkas DL, Black KL, Yu JS. Induction of potent antitumor immunity by intratumoral injection of interleukin 23-transduced dendritic cells. Cancer Res. 2006;66:8887–8896. doi: 10.1158/0008-5472.CAN-05-3448. [DOI] [PubMed] [Google Scholar]
- 26.Pegram HJ, Lee JC, Hayman EG, Imperato GH, Tedder TF, Sadelain M, Brentjens RJ. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119:4133–4141. doi: 10.1182/blood-2011-12-400044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerkar SP, Muranski P, Kaiser A, Boni A, Sanchez-Perez L, Yu Z, Palmer DC, Reger RN, Borman ZA, Zhang L, et al. Tumor-specific CD8 + T cells expressing interleukin-12 eradicate established cancers in lymphodepleted hosts. Cancer Res. 2010;70:6725–6734. doi: 10.1158/0008-5472.CAN-10-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leonard JP, Sherman ML, Fisher GL, Buchanan LJ, Larsen G, Atkins MB, Sosman JA, Dutcher JP, Vogelzang NJ, Ryan JL. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood. 1997;90:2541–2548. [PubMed] [Google Scholar]
- 29.Koneru M, Purdon TJ, Spriggs D, Koneru S, Brentjens RJ. IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. Oncoimmunology. 2015;4:e994446. doi: 10.4161/2162402X.2014.994446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koneru M, O’Cearbhaill R, Pendharkar S, Spriggs DR, Brentjens RJ. A phase I clinical trial of adoptive T cell therapy using IL-12 secreting MUC-16(ecto) directed chimeric antigen receptors for recurrent ovarian cancer. J Transl Med. 2015;13:102. doi: 10.1186/s12967-015-0460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armitage RJ, Fanslow WC, Strockbine L, Sato TA, Clifford KN, Macduff BM, Anderson DM, Gimpel SD, Davis-Smith T, Maliszewski CR. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992;357:80–82. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- 32.Schönbeck U, Libby P. The CD40/CD154 receptor/ligand dyad. Cell Mol Life Sci. 2001;58:40–43. doi: 10.1007/PL00000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grewal IS, Xu J, Flavell RA. Impairment of antigen-specific T-cell priming in mice lacking CD40 ligand. Nature. 1995;378:617–620. doi: 10.1038/378617a0. [DOI] [PubMed] [Google Scholar]
- 35.Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8 + T cells in the generation of CD8 + T cell memory. Science. 2002;297:2060–2063. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 36.Curran KJ, Seinstra BA, Nikhamin Y, Yeh R, Usachenko Y, van Leeuwen DG, Purdon T, Pegram HJ, Brentjens RJ. Enhancing antitumor efficacy of chimeric antigen receptor T cells through constitutive CD40L expression. Mol Ther. 2015;23:769–778. doi: 10.1038/mt.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khong A, Nelson DJ, Nowak AK, Lake RA, Robinson BWS. The use of agonistic anti-CD40 therapy in treatments for cancer. Int Rev Immunol. 2012;31:246–266. doi: 10.3109/08830185.2012.698338. [DOI] [PubMed] [Google Scholar]
- 38.Wierda WG, Cantwell MJ, Woods SJ, Rassenti LZ, Prussak CE, Kipps TJ. CD40-ligand (CD154) gene therapy for chronic lymphocytic leukemia. Blood. 2000;96:2917–2924. [PubMed] [Google Scholar]
- 39.Mangsbo SM, Broos S, Fletcher E, Veitonmäki N, Furebring C, Dahlén E, Norlén P, Lindstedt M, Tötterman TH, Ellmark P. The human agonistic CD40 antibody ADC-1013 eradicates bladder tumors and generates T-cell-dependent tumor immunity. Clin Cancer Res. 2015;21:1115–1126. doi: 10.1158/1078-0432.CCR-14-0913. [DOI] [PubMed] [Google Scholar]
- 40.Khalil M, Vonderheide RH. Anti-CD40 agonist antibodies: preclinical and clinical experience. Update Cancer Ther. 2007;2:61–65. doi: 10.1016/j.uct.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watts TH, DeBenedette MA. T cell co-stimulatory molecules other than CD28. Curr Opin Immunol. 1999;11:286–293. doi: 10.1016/s0952-7915(99)80046-6. [DOI] [PubMed] [Google Scholar]
- 43.DeBenedette MA, Shahinian A, Mak TW, Watts TH. Costimulation of CD28-T lymphocytes by 4-1BB ligand. J Immunol. 1997;158:551–559. [PubMed] [Google Scholar]
- 44.Wang Q, Zhang P, Zhang Q, Wang X, Li J, Ma C, Sun W, Zhang L. Analysis of CD137 and CD137L expression in human primary tumor tissues. Croat Med J. 2008;49:192–200. doi: 10.3325/cmj.2008.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takahashi C, Mittler RS, Vella AT. Cutting edge: 4-1BB is a bona fide CD8 T cell survival signal. J Immunol. 1999;162:5037–5040. [PubMed] [Google Scholar]
- 46.Sabbagh L, Snell LM, Watts TH. TNF family ligands define niches for T cell memory. Trends Immunol. 2007;28:333–339. doi: 10.1016/j.it.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 47.Futagawa T, Akiba H, Kodama T, Takeda K, Hosoda Y, Yagita H, Okumura K. Expression and function of 4-1BB and 4-1BB ligand on murine dendritic cells. Int Immunol. 2002;14:275–286. doi: 10.1093/intimm/14.3.275. [DOI] [PubMed] [Google Scholar]
- 48.Stephan MT, Ponomarev V, Brentjens RJ, Chang AH, Dobrenkov KV, Heller G, Sadelain M. T cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nat Med. 2007;13:1440–1449. doi: 10.1038/nm1676. [DOI] [PubMed] [Google Scholar]
- 49.Zhao Z, Condomines M, van der Stegen SJC, Perna F, Kloss CC, Gunset G, Plotkin J, Sadelain M. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell. 2015;28:415–428. doi: 10.1016/j.ccell.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Srivastava S, Koch MA, Pepper M, Campbell DJ. Type I interferons directly inhibit regulatory T cells to allow optimal antiviral T cell responses during acute LCMV infection. J Exp Med. 2014;211:961–974. doi: 10.1084/jem.20131556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang X, Zhang X, Fu ML, Weichselbaum RR, Gajewski TF, Guo Y, Fu YX. Targeting the tumor microenvironment with interferon-β bridges innate and adaptive immune responses. Cancer Cell. 2014;25:37–48. doi: 10.1016/j.ccr.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spaapen RM, Leung MYK, Fuertes MB, Kline JP, Zhang L, Zheng Y, Fu YX, Luo X, Cohen KS, Gajewski TF. Therapeutic activity of high-dose intratumoral IFN-β requires direct effect on the tumor vasculature. J Immunol. 2014;193:4254–4260. doi: 10.4049/jimmunol.1401109. [DOI] [PubMed] [Google Scholar]
- 53.Hsu C, Hughes MS, Zheng Z, Bray RB, Rosenberg SA, Morgan RA. Primary human T lymphocytes engineered with a codon-optimized IL-15 gene resist cytokine withdrawal-induced apoptosis and persist long-term in the absence of exogenous cytokine. J Immunol. 2005;175:7226–7234. doi: 10.4049/jimmunol.175.11.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]