Abstract
Objectives
Pseudoxanthoma elasticum (PXE) is an inherited metabolic disease characterized by elastic fiber fragmentation and calcification in the cutaneous, ophthalmologic, and vascular tissues. Cardiovascular manifestations such as peripheral arterial disease (PAD) are frequent in PXE. Because of the changes in the elastic properties and medial calcification of the arterial wall in PXE, the impact of the arterial remodeling on the ankle brachial index (ABI), a well-established diagnostic method for the detection and follow-up of PAD, remains to be determined in this disease.
Methods
This was a cross-sectional, comparative, open study, which took place at the PXE Consultation Center, University Hospital of Angers. The subjects were 53 patients (mean age, 49 ± 14 years; 35 females) with PXE clinically proven on the basis of established criteria (skin changes, angioid streaks, and skin biopsy). The ABI at rest, symptoms of intermittent claudication (IC), carotid intima-media thickness (IMT), carotid-femoral pulse wave velocity (c-f PWV), compliance (CC), and β stiffness index were measured in a single-center cohort.
Results
Forty-five percent of the PXE patients had an ABI ≤0.90, but only one patient had an ABI >1.40. IC was found in 23% of the patients with an ABI ≤0.90. There were no significant differences between the patients with a low and normal ABI in terms of IMT (P = .566) or β stiffness index (P = .194), but differences were significant for c-f PWV (P = .010) and CC (P = .011). Adjusted multivariate linear regression for the Framingham-Laurier score showed that patients with a low ABI had less compliant carotid arteries (B = 0.318, P = .039).
Conclusions
PAD detected by a low ABI is very frequent in PXE, although with limited prevalence of symptomatic claudication. Unexpectedly, ABI was low in such calcifying PAD and associated with lower CC, independently of atherosclerosis risk factors. These findings demonstrate that PXE represents a unique monogenic model of PAD in which the specific arterial wall remodeling could change the diagnostic value of the ABI to detect PAD.
Pseudoxanthoma elasticum (PXE; OMIM 264800) is an autosomal recessive multisystem disorder affecting connective tissue.1–3 PXE is characterized by the fragmentation and mineralization of elastic fibers in the skin, Bruch’s membrane of the retina, and the vasculature. Its prevalence is estimated at between 1/25,000 and 1/50,000. The causative mutations have been identified in the ABCC6 gene encoding an ATP-binding cassette transporter that is primarily expressed in the liver and to a lesser extent only in affected tissues.3 Consequently, PXE is now considered to be a metabolic disease of genetic origin.
Patients with PXE usually present with cutaneous and ophthalmologic involvement, the combination of which provides the diagnosis in most cases.2–4 Yellowish cutaneous papules and loss of skin elasticity are aesthetic concerns, but the complication of angioid streaks may severely impair visual acuity. Cardiovascular expression is said to be another frequent clinical manifestation of PXE, including arterial hypertension, and cerebral and coronary ischemic attacks.1,5,6 The basic arterial changes observed in PXE are the consequences of mineralization of the internal elastic laminae of the medial layer in muscular and elastic arteries, mimicking those observed in other acquired metabolic diseases such as diabetes and end-stage renal disease.7 Early and severe peripheral arterial disease (PAD) or slowly worsening lower limb claudication without obvious cardiovascular risk factors have been repeatedly reported in PXE.1,2,6,8,9 There are very few studies on PAD in PXE undertaken with a significant population and using stringent detection methods. The ankle brachial index (ABI; the ratio of the ankle systolic pressure and brachial systolic pressure) is a clinically validated method for the detection and the evaluation of the severity of PAD in atherosclerosis.10,11 In mineralizing arterial diseases, a high ABI is usually reported because of the severely reduced or noncompressible arterial wall of the distal arteries. Because of the complex remodeling affecting the arterial wall in PXE, the reliability of the ABI to detect and manage PAD needs to be clarified in this monogenic arterial disease.
The study presented here was designed to determine the specific features of PAD detected by the ABI and its relationship with the biomechanical characteristics of the arterial wall in patients with PXE.
METHODS
Patients
Fifty-three consecutive PXE patients (35 females) involved in an ongoing single-center cohort (PXE Consultation Center, University Hospital of Angers, France) were prospectively included for this cross-sectional study. The diagnosis of PXE was based on the combination of established criteria for undisputable PXE (ie, clinically suggestive skin changes, angioid streaks, and demonstration of fragmented and calcified elastic fibers on skin biopsy).4
Biometric variables and smoking habits were recorded for all patients. Blood samples were collected after overnight fasting for measurement of lipid and glucose profiles by standard techniques. Brachial systolic (SABP) and diastolic blood pressures (DABP) were measured in the supine position with an automatic sphygmomanometer (Welch Allyn Inc., Model SPOT, France) using an appropriately sized cuff. The brachial pulse pressure (PP) was calculated as SABP minus DABP. Cardiovascular risk factors were scored as follows: (1) Hypertension: systolic blood pressure (SABP) >140 mm Hg and diastolic blood pressure (DABP) >90 mm Hg and/or use of antihypertensive medication. (2) Diabetes: fasting glucose ≥7.0 mmol/L and/or use of glucose-lowering medication (including insulin). (3) Hyperlipemia: low-density lipoprotein (LDL) cholesterol >3.4 mmol/L, high-density lipoprotein (HDL) cholesterol <1 mmol/L, triglyceridemia >2 mmol/L and/or use of lipid-lowering medication. The 10-year absolute and relative risks for a cardiovascular event were calculated using the Framingham-Laurier equation modified for European populations (F-L score in %).12
Symptomatic and asymptomatic PAD
Symptoms of lower limb intermittent claudication were detected using a French-translated version of the Edinburgh Claudication Questionnaire (ECQ) administered by the investigators and scored as “asymptomatic” or “symptomatic.” The ABI was measured for all patients according to standardized methods with a bidirectional 8-MHz ultrasound Doppler velocimeter and a sphygmomanometer. SBP was measured in the posterior tibial and dorsalis pedis arteries of both legs and in the brachial artery of both arms. ABI was calculated for each leg by dividing the highest lower limb SBP value by the highest upper limb SBP value. ABI was considered normal between 0.91 and 1.39.13 The presence of PAD was assessed on an ABI ≤0.90. An ABI ≥1.40 indicated PAD with noncompressible arteries.
Screening for arterial lesions
All participants underwent complete ultrasound screening of the lower limb (ie, aorta, femoral, popliteal, and ankle) and carotid (common, internal, and external) arteries within a single day using a high-resolution B-mode scanning system (Alpha-10, Aloka, Japan). Atherosclerotic plaques were defined as a distinct focal area of high echogenicity or with an acoustic shadow in relation to the adjacent segment, and/or a focal protrusion of at least 50% of the surrounding intima-media thickness encroaching into the vessel lumen. A severity score was established by the operator as follows: 0 = absence of detectable lesion, 1 = mildly calcified in subpopliteal arteries, 2 = moderately calcified in both femoral and distal segments, 3 = severely calcified in proximal (aorta and femoral) and subpopliteal arterial segments, 4 = one or more proximal arterial segment(s) occluded (eg, femoral).
Carotid and aortic artery stiffness
The IMT of both common carotid arteries (CCA) was measured and averaged from the ultrasound scans obtained with a high-resolution 13-MHz linear array transducer. The digitized scans were analyzed off-line using an Internet-based software program (Diamed V1.0, http://www.televasc.fr, France). Carotid end-diastolic lumen diameter (D in mm), arterial wall compliance (CC = π(2D. ΔD + D2)/(4. ΔP) in mm2 kPa−1) and stiffness index (β = ln(SABP/DABP)/(ΔD/D)) were determined by an echo-tracking technique (e-Tracking, Aloka) coupled to the ultrasound system.14 Aortic stiffness was determined by measuring carotid-femoral pulse wave velocity (c-f PWV in ms−1) with a tonometric technique (Pulse Pen, Diatcne, Italy).
Statistical analyses
All normally distributed variables are expressed as mean (± SD) unless otherwise stated. The differences between the groups with normal and abnormal ABI were compared using analysis of variance. Differences between groups for categorical variables were determined using χ2 tests. Correlation coefficients were determined by Pearson’s statistics. A multivariate linear regression analysis was applied with the value of ABI as the dependent variable, and structural and functional variables (IMT, c-f PWV, β stiffness index, pulse pressure, and CC) and the presence or absence of IC as the independent variables, after adjustment for the confounding effect for the atherosclerosis risk factors (ie, F-L score). All statistics were performed with the SPSS v15.0 package (SPSS, Chicago, IL) with significance set at P ≤ .05, and adjustments were made for multiple comparisons when needed.
RESULTS
Cohort characteristics
The PXE cohort had more females (65%) than males. The mean age was 49 ± 14 years, with a body mass index (BMI) of 26 kg/m2 (one patient had a BMI >40). Five PXE patients had a history of overt cardiovascular disease (two angina pectoris, one femoral angioplasty, two strokes, and one toe amputation). One patient had experienced two events. The patient with the toe amputation also had type 1 diabetes. Hypertension was present in 23% of the patients; 23% smoked and 27% of the patients had dyslipidemia. Only one patient had type 2 diabetes. This patient was not excluded from the statistics since his diabetes was corrected by antidiabetic medication. The F-L score was not significantly different between the two groups. No patient had renal insufficiency or was dialyzed or had pulmonary disease (Tables I and II).
Table I.
Cardiovascular risk factors in the PXE patients with a low (≤0.90) and normal ABI
| ABI ≤0.90 | ABI > 0.90 | P (Khi2) | |
|---|---|---|---|
| Gender | |||
| Male | 10 (19%) | 8 (15%) | 0.243 |
| Female | 14 (27%) | 20 (39%) | |
| Diabetes | |||
| No | 23 (44%) | 28 (54%) | 0.349 |
| Yes | 1 (2%) | 0 (0%) | |
| Tobacco use | |||
| No | 19 (37%) | 21 (40%) | 0.492 |
| Yes | 5 (10%) | 7 (13%) | |
| Hypertension | |||
| No | 15 (29%) | 25 (48%) | 0.025 |
| Yes | 9 (17%) | 3 (6%) | |
| Dyslipidemia | |||
| No | 16 (31%) | 22 (42%) | 0.257 |
| Yes | 8 (15%) | 6 (12%) |
ABI, Ankle-brachial index; PXE, pseudoxanthoma elasticum.
Data are indicated as number of patients with percentage from the overall population in parentheses.
Table II.
Biometric and hemodynamic data according to the ABI
| All | ABI ≤0.90 | ABI >0.90 | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Mean | SD | Mean | SD | Mean | SD | Pa | |
| Age (years) | 49 | 14 | 54 | 12 | 45 | 15 | .019 |
| Weight (kg) | 72 | 20 | 71 | 18 | 72 | 21 | .779 |
| Height (m) | 1.66 | .09 | 1.65 | .10 | 1.67 | .09 | .447 |
| Body mass index (kg/m2) | 25.8 | 5.1 | 25.7 | 4.3 | 25.7 | 5.9 | .993 |
| Total cholesterol (g/L) | 1.89 | .23 | 1.86 | .22 | 1.90 | .24 | .502 |
| High-density lipoprotein cholesterol (g/L) | .51 | .17 | .51 | .18 | .51 | .16 | .945 |
| Low-density lipoprotein cholesterol (g/L) | .84 | .49 | .93 | .48 | .75 | .50 | .187 |
| Triglycerides (g/L) | 1.03 | .48 | 1.10 | .51 | .96 | .45 | .300 |
| Serum creatinine (mmol/L) | 68 | 12 | 69 | 15 | 67 | 9 | .535 |
| Serum albumin (g/L) | 71 | 5 | 71 | 5 | 71 | 6 | .917 |
| Brachial systolic blood pressure (mm Hg) | 127 | 16 | 128 | 15 | 125 | 17 | .462 |
| Brachial diastolic blood pressure (mm Hg) | 74 | 10 | 76 | 11 | 73 | 9 | .342 |
| MABP (mm Hg) | 92 | 11 | 93 | 12 | 90 | 11 | .360 |
| Pulse pressure (mm Hg) | 52.8 | 11.1 | 52.8 | 1.0 | 52.1 | 12.6 | .816 |
| Framingham-Laurier risk score (%) | 10% | 10% | 12 | 10 | 8 | 10 | .174 |
| ABI | .90 | .18 | .74 | .12 | 1.06 | .12 | .000 |
| Carotid intima-media thickness (mm) | .628 | .108 | .638 | .087 | .620 | .123 | .566 |
| Compliance (mm2/kPa) | .9130 | .34173 | .79 | .27 | 1.03 | .37 | .011 |
| β stiffness index (AU) | 7.1981 | 3.67159 | 7.87 | 2.94 | 6.56 | 4.11 | .194 |
| Carotid-femoral pulse wave velocity (m/s) | 8.1306 | 1.91407 | 8.98 | 1.81 | 7.41 | 1.67 | .010 |
ABI, Ankle-brachial index; AU, arbitrary unit.
Statistical comparisons performed between ABI ≤0.90 and ABI >0.90 groups.
Unadjusted statistics.
ABI and other hemodynamic data
An ABI ≤0.90 was found in 45% of patients (n = 24), and only one had an ABI >1.40. Since no statistics could be performed for only one case, this patient was excluded from the subsequent statistical analysis. Although the proportion of women with a low ABI was high (69%), there was no significant difference in gender. Unadjusted statistics showed that the patients with a low ABI were older, with lower CC, and higher c-f PWV, but higher blood pressure than the groups with a normal ABI. IC was detected by the ECQ in 28% (15/53) of the patients, and 23% (12/53) had an IC associated with an ABI ≤0.90. There were no significant differences in age between the patients with or without IC (54 ± 9 vs 47 ± 16 years, respectively; P = .111). Duplex ultrasound scans revealed arterial calcification in almost all patients. The severity score was significantly higher (2.25 ± 1.18) in patients with a low ABI compared with the patients with a normal ABI (1.28 ± 0.71, P < .001) and increased with age (r = .358, P = .009). Calcified lesions predominated in the subpopliteal arteries and distal superficial femoral arteries, whereas carotid arteries were rarely calcified.
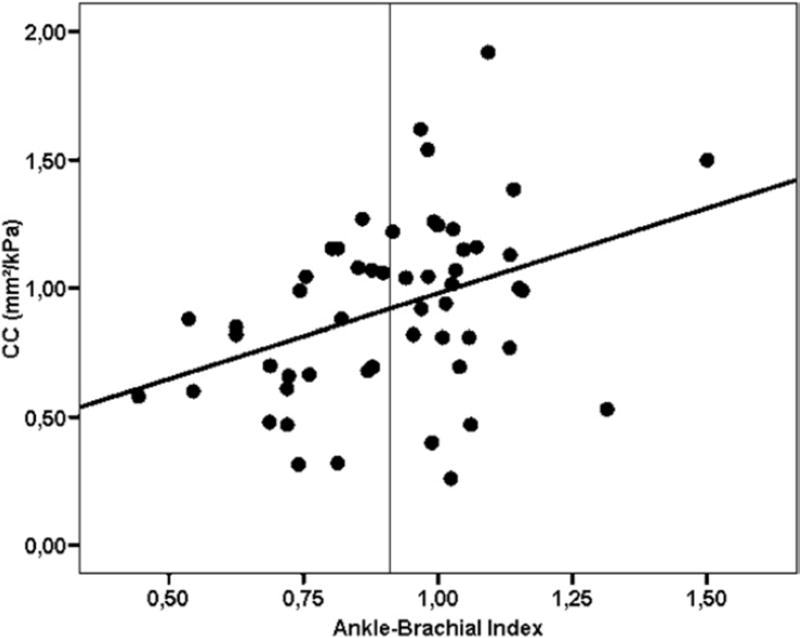
Unadjusted analysis showed a significant correlation only between the ABI and the CC (r = .379, P = .005, Fig) but not with the other variables. The multivariate linear regression analysis adjusted for the F-L score showed that the CC was an independent determinant of the ABI (Table III).
Fig.
Relationship between carotid arterial compliance and ankle-brachial index in patients with a pseudoxanthoma elasticum. The vertical line indicates the threshold for the abnormal ABI. Unadjusted R2 = 0.143; ABI = 0.216; CC (±0.074) + 0.379; P < .005.
Table III.
Adjusted (Framingham-Laurier risk score) multivariate linear regression analysis with the ankle brachial index
| Dependant variable: Ankle-brachial index |
B | SE | β | t | P value |
|---|---|---|---|---|---|
| Constant | .428 | .303 | 1.412 | .165 | |
| Carotid intima-media thickness | .717 | .363 | .318 | 1.976 | .054 |
| β stiffness index | .016 | .013 | .337 | 1.218 | .229 |
| Compliance | .318 | .150 | .493 | 2.120 | .039 |
| Carotid-femoral pulse wave velocity | −.038 | .020 | −.256 | −1.908 | .062 |
| Pulse pressure | −.002 | .003 | −.130 | −.719 | .475 |
DISCUSSION
To the best of our knowledge, this is the first study examining the impact of arterial stiffness on the ABI in pseudoxanthoma elasticum, a monogenic mineralizing vascular disease. The major findings are (1) a very high prevalence of PAD detected by a low ABI and (2) the value of the ABI is independently predicted from the IC and arterial stiffness in PXE.
The presence or absence of PAD in this study was based on validated detection methods (ECQ and ABI) and not only on self-reported information or clinical examination alone (ie, pulse detection). We found that 23% of the overall PXE patient cohort complained of symptomatic IC, although 45% of the whole cohort exhibited PAD as detected by the ABI. Our findings are in accordance with previous studies reporting a prevalence of PAD of 30% but using less stringent clinical criteria.1 In a Belgian PXE cohort (n = 42 patients), PAD was diagnosed in 22% of the patients on self-reported intermittent claudication, but in 53% by means of ultrasonographic evaluation.6 By contrast, the prevalence of PAD detected by ABI in the general population aged >55 years ranges from only 17% in adult men and 21% women15 up to 24%.16 Therefore, our findings in a large PXE cohort confirm that PAD is clearly a frequent symptom of the disease.
The reason for the high prevalence of a low ABI value in this mineralizing disease is unclear. The calcifications accumulating in the tunica media of PXE patients are responsible for extensive mineralization of almost all arteries visible by various imaging techniques.17–19 We therefore expected to find a high prevalence of ABI >1.40, a validated threshold for stiffened arteries usually reported in other mineralizing PADs.7,11 Only one patient in our cohort (2%) had an ABI value >1.40, a value within the range of mineralized arteries.20
We hypothesize that the ABI value found could be related to the specific structural and functional properties of the arterial wall reported in PXE. Rare histologic reports demonstrated elastic fiber fragmentation, mineralization, and accumulation of proteoglycans, mostly in medium and small-sized arteries.18,19 This remodeling contributes to thickening of the medial layer, reduces the lumen size of the radial artery, and increases the IMT in the carotid arteries. Functional changes are characterized by reduced stiffness, mainly in older patients (>40 years) compared with healthy matched controls.19,21,22 This has also been found to be associated with higher compliance in controls, mainly at older ages, although the slope for changes with age was less steep than in controls.
Indeed, in our study, the relationship between age and the other arterial variables could partially explain the changes observed since the PXE patients with a low ABI were also older. After adjustment for age, most of the correlations became nonsignificant except for arterial compliance. These findings suggest that age is highly associated with arterial changes in PXE and that most of the vascular complications are likely to develop after the fifth decade of life. In atherosclerosis, carotid and femoral artery compliance is reduced in patients with PAD and independently associated with a low ABI.23–25 Since the PXE patients are also exposed to the usual cardiovascular risk factors (ie, smoking, hypertension, etc) mainly integrated in the F-L score, atherosclerosis may also superimpose to PXE. Adjusted analysis for the F-L score showed an independent association of ABI with arterial compliance. We thus demonstrated for the first time that arterial compliance (but not stiffness) is an important determinant of the ABI value, independently from the atherosclerotic process.
The contribution of the larger and predominantly elastic arteries, such as the aorta, was also examined. Interestingly, we found that the c-f PWV was not a significant predictor of ABI after adjustment for the F-L score. This finding is in accordance with other studies in atherosclerosis in which the relationship with pulse wave analysis did not clearly show an effect on ABI.24,26 This finding also suggests that the aorta is differently affected in PXE compared with the smaller arteries, as already reported in microscopic examinations.18
Our PXE cohort mainly comprised women (65%), which is a common finding in PXE,1 although the reason for this gender ratio has not been explained to date. Women with PAD generally exhibit a lower prevalence of lower limb claudication, a higher prevalence of atypical ischemic symptoms, better tolerance of reduced walking distance, and a higher prevalence of PAD detected by ABI.27 In fact, the prevalence of PAD is 10% overestimated in women since they have about 0.02 lower ABI values than men.28 In our study, after adjustment of the ABI to a cut-off value of 0.88 for women, the higher prevalence of PAD (50%) in women with PXE and PAD remained unchanged compared with men.
Beside the fact that small series have intrinsic limited statistical power, this study has other limitations. First, although the present results were obtained with one of the largest PXE cohorts reported to date, genotype determination was not available in all patients. This precludes any findings in relation to genotype/phenotype correlation. Second, we did not provide specific information on the nature and structure of the arterial changes, although extended calcifications of the arterial wall, characterized by “ultrasound shadows,” clearly emerged as a common trait in our patients. As already mentioned,9 occlusion of the proximal trunks (ie, carotid, aortoiliac, or common femoral arteries) seems rare in PXE and possibly overlaps with atherosclerotic lesions, especially in older patients. Finally, as arterial stiffness in the distal leg arteries was not investigated directly due to lack of sufficient spatial resolution of our standard ultrasound techniques, we cannot dissect out the role of local structural and geometrical changes induced by PXE on the ABI.
In conclusion, in addition to the well-described cutaneous and ophthalmologic signs, our findings confirm that PAD is a major clinical feature of PXE that can be objectively identified with ABI and ECQ. Although the place of conventional diagnostic tools such as ABI in the detection and management of PAD in PXE remains to be determined, the mechanistic role of the arterial remodeling in the ABI in this monogenic mineralizing disease remains to be clarified.
Acknowledgments
The authors thank the French Society of Vascular Medicine, PXE France Patients’ Association for continuing support and G. Tew for his help in the preparation of the manuscript.
Footnotes
Competition of interest: none.
AUTHOR CONTRIBUTIONS
Conception and design: GL
Analysis and interpretation: GL
Data collection: GL, YLC, FP, LM
Writing the article: GL, PA, OLS, DH, LM
Critical revision of the article: PA, YLC, PD, OLS, DH, FP
Final approval of the article: GL, YLC, PA, OLS, DH, LM
Statistical analysis: GL
Obtained funding: French Society of Vascular Medicine
Overall responsibility: GL
References
- 1.Neldner KH. Pseudoxanthoma elasticum. Clin Dermatol. 1988;6:1–159. doi: 10.1016/0738-081x(88)90003-x. [DOI] [PubMed] [Google Scholar]
- 2.Chassaing N, Martin L, Calvas P, Le Bert M, Hovnanian A. Pseudoxanthoma elasticum: a clinical, pathophysiological and genetic update including 11 novel ABCC6 mutations. J Med Genet. 2005;42:881–92. doi: 10.1136/jmg.2004.030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q, Jiang Q, Pfendner E, Váradi A, Uitto J. Pseudoxanthoma elasticum: clinical phenotypes, molecular genetics and putative pathomechanisms. Exp Dermatol. 2009;18:1–11. doi: 10.1111/j.1600-0625.2008.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lebwohl M, Neldner K, Pope FM, De Paepe A, Christiano AM, Boyd CD, et al. Classification of pseudoxanthoma elasticum: report of a consensus conference. J Am Acad Dermatol. 1994;30:103–7. doi: 10.1016/s0190-9622(08)81894-4. [DOI] [PubMed] [Google Scholar]
- 5.Lebwohl M, Schwartz E, Lemlich G, Lovelace O, Shaikh-Bahai F, Fleischmajer R, et al. Abnormalities of connective tissue components in lesional and non-lesional tissue of patients with pseudoxanthoma elasticum. Arch Dermatol Res. 1993;285:121–6. doi: 10.1007/BF01112912. [DOI] [PubMed] [Google Scholar]
- 6.Vanakker OM, Leroy BP, Coucke P, Bercovitch LG, Uitto J, Viljoen D, et al. Novel clinico-molecular insights in pseudoxanthoma elasticum provide an efficient molecular screening method and a comprehensive diagnostic flowchart. Hum Mutat. 2008;29:205. doi: 10.1002/humu.9514. [DOI] [PubMed] [Google Scholar]
- 7.Giachelli CM. Vascular calcification mechanisms. J Am Soc Nephrol. 2004;15:2959–64. doi: 10.1097/01.ASN.0000145894.57533.C4. [DOI] [PubMed] [Google Scholar]
- 8.von Beckerath O, Gaa J, von Mohrenfels CW, von Beckerath N. Images in cardiovascular medicine. Intermittent claudication in a 28-year-old man with pseudoxanthoma elasticum. Circulation. 2008;118:102–4. doi: 10.1161/CIRCULATIONAHA.107.760355. [DOI] [PubMed] [Google Scholar]
- 9.Donas KP, Schulte S, Horsch S. Balloon angioplasty in the treatment of vascular lesions in pseudoxanthoma elasticum. J Vasc Interv Radiol. 2007;18:457–9. doi: 10.1016/j.jvir.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Ankle Brachial Index Collaboration. Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aboyans V, Lacroix P, Laskar M. Prevalence of symptomatic and asymptomatic peripheral arterial disease in primary care patients. Atherosclerosis. 2004;175:183–4. doi: 10.1016/j.atherosclerosis.2004.02.002. Author reply:5–6. [DOI] [PubMed] [Google Scholar]
- 12.Laurier D, Nguyen PC, Cazelles B, Segond P. Estimation of CHD risk in a French working population using a modified Framingham model. The PCV-METRA Group. J Clin Epidemiol. 1994;47:1353–64. doi: 10.1016/0895-4356(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 13.Bird CE, Criqui MH, Fronek A, Denenberg JO, Klauber MR, Langer RD, et al. Quantitative and qualitative progression of peripheral arterial disease by non-invasive testing. Vasc Med. 1999;4:15–21. doi: 10.1177/1358836X9900400103. [DOI] [PubMed] [Google Scholar]
- 14.Emoto M, Nishizawa Y, Kawagishi T, Maekawa K, Hiura Y, Kanda H, et al. Stiffness indexes beta of the common carotid and femoral arteries are associated with insulin resistance in NIDDM. Diabetes Care. 1998;21:1178–82. doi: 10.2337/diacare.21.7.1178. [DOI] [PubMed] [Google Scholar]
- 15.Meijer WT, Hoes AW, Rutgers D, Bots ML, Hofman A, Grobbee DE, et al. Peripheral arterial disease in the elderly: the Rotterdam Study. Arterioscler Thromb Vasc Biol. 1998;18:185–92. doi: 10.1161/01.atv.18.2.185. [DOI] [PubMed] [Google Scholar]
- 16.Boccalon H, Lehert P, Mosnier M. Assessment of the prevalence of atherosclerotic lower limb arteriopathy in France as a systolic index in a vascular risk population. J Mal Vasc. 2000;25:38–46. [PubMed] [Google Scholar]
- 17.Miwa K, Higashikata T, Mabuchi H. Intravascular ultrasound findings of coronary wall morphology in a patient with pseudoxanthoma elasticum. Heart. 2004;90:e61. doi: 10.1136/hrt.2004.040592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gheduzzi D, Sammarco R, Quaglino D, Bercovitch L, Terry S, Taylor W, et al. Extracutaneous ultrastructural alterations in pseudoxanthoma elasticum. Ultrastruct Pathol. 2003;27:375–84. [PubMed] [Google Scholar]
- 19.Kornet L, Bergen AA, Hoeks AP, Cleutjens JP, Oostra RJ, Daemen MJ, et al. In patients with pseudoxanthoma elasticum a thicker and more elastic carotid artery is associated with elastin fragmentation and proteoglycans accumulation. Ultrasound Med Biol. 2004;30:1041–8. doi: 10.1016/j.ultrasmedbio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Aboyans V, Ho E, Denenberg JO, Ho LA, Natarajan L, Criqui MH, et al. The association between elevated ankle systolic pressures and peripheral occlusive arterial disease in diabetic and nondiabetic subjects. J Vasc Surg. 2008;48:1197–203. doi: 10.1016/j.jvs.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Boutouyrie P, Germain DP, Tropeano AI, Laloux B, Carenzi F, Zidi M, et al. Compressibility of the carotid artery in patients with pseudoxanthoma elasticum. Hypertension. 2001;38:1181–4. doi: 10.1161/hy1101.096108. [DOI] [PubMed] [Google Scholar]
- 22.Germain DP, Boutouyrie P, Laloux B, Laurent S. Arterial remodeling and stiffness in patients with pseudoxanthoma elasticum. Arterioscler Thromb Vasc Biol. 2003;23:836–41. doi: 10.1161/01.ATV.0000067428.19031.28. [DOI] [PubMed] [Google Scholar]
- 23.Lind L. Arterial stiffness, but not endothelium-dependent vasodilation, is related to a low ankle-brachial index. Clin Physiol Funct Img. 2011;31:182–7. doi: 10.1111/j.1475-097X.2010.00996.x. [DOI] [PubMed] [Google Scholar]
- 24.Duprez DA, De Buyzere MM, De Bruyne L, Clement DL, Cohn JN. Small and large artery elasticity indices in peripheral arterial occlusive disease (PAOD) Vasc Med. 2001;6:211–4. doi: 10.1177/1358836x0100600402. [DOI] [PubMed] [Google Scholar]
- 25.Cheng KS, Tiwari A, Baker CR, Morris R, Hamilton G, Seifalian AM, et al. Impaired carotid and femoral viscoelastic properties and elevated intima-media thickness in peripheral vascular disease. Atherosclerosis. 2002;164:113–20. doi: 10.1016/s0021-9150(02)00042-4. [DOI] [PubMed] [Google Scholar]
- 26.Resnick HE, Lindsay RS, McDermott MM, Devereux RB, Jones KL, Fabsitz RR, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation. 2004;109:733–9. doi: 10.1161/01.CIR.0000112642.63927.54. [DOI] [PubMed] [Google Scholar]
- 27.Aboyans V, Salazar J, Lacroix P. Obliterating arterial disease of the legs in women. Presse Med. 2010;39:263–70. doi: 10.1016/j.lpm.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Aboyans V, Criqui MH, McClelland RL, Allison MA, McDermott MM, Goff DC, et al. Intrinsic contribution of gender and ethnicity to normal ankle-brachial index values: the Multi-Ethnic Study of Atherosclerosis (MESA) J Vasc Surg. 2007;45:319–27. doi: 10.1016/j.jvs.2006.10.032. [DOI] [PubMed] [Google Scholar]