Abstract
The engineered expression of chimeric antigen receptors (CARs) on the surface of T cells enables the redirection of T-cell specificity. Early clinical trials using CAR T cells for the treatment of patients with cancer showed modest results, but the impressive outcomes of several trials of CD19-targeted CAR T cells in the treatment of patients with B-cell malignancies have generated an increased enthusiasm for this approach. Important lessons have been derived from clinical trials of CD19-specific CAR T cells, and ongoing clinical trials are testing CAR designs directed at novel targets involved in haematological and solid malignancies. In this Review, we discuss these trials and present strategies that can increase the antitumour efficacy and safety of CAR T-cell therapy. Given the fast-moving nature of this field, we only discuss studies with direct translational application currently or soon-to-be tested in the clinical setting.
Chimeric antigen receptors (CARs) consist of an extracellular antigen-recognition domain, which is usually an antibody single-chain variable fragment (scFv), but can also be a peptide or another protein, linked to an intracellular signalling domain – usually the CD3ζ (CD3 zeta) chain of the T-cell receptor. The extracellular portion of the CAR permits the recognition of a specific antigen by a T cell and, subsequently, the signalling domains stimulate T-cell proliferation, cytolysis and cytokine secretion to eliminate the target cell. The patients’ own T cells (or those from an allogeneic donor) are isolated, activated and genetically modified to generate CAR T cells, which are then infused into the same patient. This approach carries a very low risk of graft-versus-host disease and enables lipid, protein and carbohydrate antigens to be targeted by T cells in an MHC-unrestricted fashion. Additionally, one CAR design can be used to treat all cancers expressing the same antigen. The need to generate T cells for each patient was once considered to be a financial and technical obstruction to this therapeutic approach, but the success of CAR-T-cell therapy for the treatment of B-cell acute lymphoblastic leukaemia (B-ALL) has demonstrated that CAR T cells can be produced efficiently and for a reasonable cost.
CD19-targeted CAR T cells have been investigated clinically for the treatment of B-cell malignancies. CD19-targeted CAR-T-cell therapy has repeatedly demonstrated to produce marked antitumour responses in patients with B-ALL1–3. Following this success, much attention has been devoted to the development of CAR T cells for the successful treatment of other haematological malignancies and solid tumours. In this Review, we discuss successful CD19-targeted CAR-T-cell therapies, CAR-T-cell designs targeting other molecules for the treatment of haematological malignancies, and novel targets proposed for the treatment of solid tumours. This discussion will be limited to approaches with registered clinical trials. In our opinion, the findings from these trials will be instrumental to increase our understanding and optimize the efficacy of this promising cancer treatment.
Design features of CAR constructs
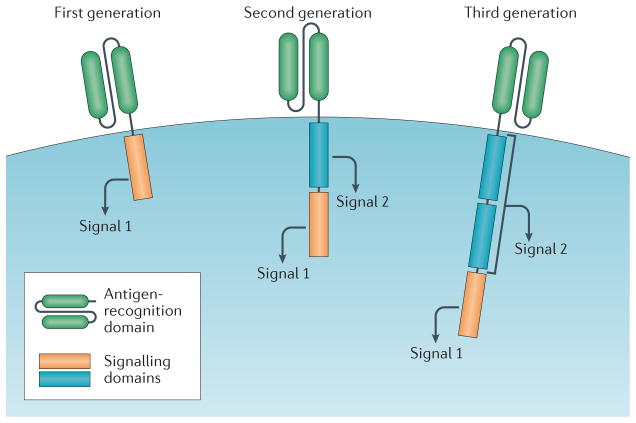
CARs contain an extracellular antigen-recognition domain, and an intracellular signalling domain (FIG. 1). The antigen-recognition domain most-commonly consists of an scFv, which is anchored to the cell with a hinge and/or transmembrane domain and binds to the tumour-associated antigen (TAA). The intracellular portion consists of signalling domains necessary for activation of T cells. First-generation CARs typically utilize the CD3ζ signalling chain, which provides an activation signal termed signal 1 First-generation CAR T cells showed limited efficacy in clinical trials, probably owing to activation-induced cell death (AICD) of the transplanted T cells, or to the lack of long-term T-cell expansion4–6. Indeed, evidence from early studies demonstrates some persistence of first-generation tumour-specific CAR T cells, however, the overall expansion and antitumour efficacy of these cells remains low7. By contrast, results observed in the context of HIV treatment indicate that CD4-specific CAR T cells can have a half-life of >16 years8.
Figure 1. CAR-T-cell design.
All chimeric antigen receptor (CAR) designs contain an antigen-recognition domain and a signalling domain that provides ‘signal 1’ to activate T cells. Only this signalling domain is present in first-generation CARs. By contrast, a co-stimulatory signalling domain that provides ‘signal 2’ is added in second-generation CARs, and in third-generation CARs two co-stimulatory signalling domains are added.
Second-generation CARs use first-generation CARs as a backbone and include an additional co-stimulatory signalling domain, termed signal 2, to provide a second signal and, therefore, the same receptor delivers signal 1 and signal 2 to optimally activate the T cell. Second-generation CAR T cells specific for CD19 that include both a CD3ζ and a CD28 signalling moiety have been demonstrated to have enhanced persistence and proliferation compared with first-generation CD19-specific CAR T cells (signalling through CD3ζ alone), when infused simultaneously into patients with non-Hodgkin lymphoma (NHL) at the Baylor College of Medicine, Texas, USA (Baylor)7. In the past 5 years, second-generation CD19-targeted CAR T cells with either CD28 or 4-1BB (CD137) co-stimulatory signalling domains have demonstrated clinical efficacy in the treatment of B-ALL, but the optimal signalling moieties to be used in second-generation CAR T cells remain to be determined1,3,9. Another question that remains to be solved, especially when comparing different CAR T-cell designs, is whether a threshold persistence and/or expansion is required for effective clinical outcomes following treatment (BOX 1).
Box 1. CAR-T-cell persistence and therapy efficacy.
Both persistence and expansion of CAR T cells are often used to compare second-generation CARs containing a CD28 signalling domain and 4-1BB signalling domain. However, impressive clinical outcomes have been observed using CAR T cells targeted to CD19 with either signalling moiety, with complete response rates of 70–90% consistently observed, although long-term persistence of CAR T cells was only observed in a few patients2–4. Therefore, at least in the setting of CD19-targeted CAR T-cell therapy of B-cell acute lymphoblastic leukaemia, cell persistence does not seem to be the only correlate for clinical efficacy. The role of persistence in the efficacy of other CAR-T-cell therapies designed to treat haematological malignancies and solid tumours remains to be established.
The signalling domain of third-generation CARs contains a CD3ζ domain and two co-stimulatory domains. The co-stimulatory signalling moieties used in third-generation CARs include CD28, 4-1BB, or OX40 (CD134). In preclinical studies, third-generation CAR T cells have demonstrated superior antitumour efficacy compared with second-generation CAR T cells10,11. A clinical trial currently recruiting patients at Baylor will compare the antitumour efficacy of second-generation versus third-generation CAR T cells (NCT01853631)12. Patients with NHL, B-ALL or chronic lymphocytic leukaemia (CLL) will be treated with a mixture of T cells expressing either a CD19-targeted second-generation CAR (CD3ζ/CD28) or a CD19-targeted third-generation CAR (CD3ζ/4-1BB/CD28). The results of this trial might provide insight into whether future CARs should be designed with one or two co-stimulatory signalling domains.
CD19-targeted CAR T-cells in the clinic
Clinical trials of CAR T-cell therapy for the treatment of cancer have mostly been conducted in patients with CD19-positive haematological diseases, such as B-ALL, CLL, follicular lymphoma (FL), diffuse large B-cell lymphoma (DLBCL), and mantle-cell lymphoma (MCL). The experience in each of these diseases is described in the following sections.
B-ALL
Promising results have been observed using CD19-specific CAR T cells to treat patients with B-ALL at several institutions; although each trial used different criteria for patient selection, preconditioning chemotherapy, and CAR design, similarly impressive results were obtained (TABLE 1). Researchers at Memorial Sloan Kettering Cancer Center in New York, USA (MSKCC) were the first to publish results using a second-generation CD19-targeted CAR (CD28/CD3ζ) for adults with relapsed or refractory B-ALL (NCT01044069)13. The initial results revealed that all patients treated on the trial had achieved minimum residual disease (MRD)-negative complete remissions1. Four out of five patients then received a standard-of-care allogeneic haematopoietic stem-cell transplant (alloHSCT)1. In an updated report of this study, an overall complete response rate of 91% in 32 evaluable patients was demonstrated14. The toxicities reported for these patients included transient B-cell aplasias, cytokine-release syndrome (CRS), and neurological toxicities14.
Table 1.
CD19-specific-CAR T-cell therapy outcomes in patients with B-ALL
| Institution | CAR design | Patient population | Outcome | Toxicities | Reference |
|---|---|---|---|---|---|
| MSKCC | CD28, CD3ζ |
|
91% CR |
|
NCT01044069 (REF. 13) |
| UPenn/CHOP | 4-1BB, CD3ζ |
|
90% CR |
|
NCT01626495 (REF. 15) |
| NCI | CD28, CD3ζ |
|
70% CR |
|
NCT01593696 (REF. 17) |
| Fred Hutchinson | 4-1BB, CD3ζ |
|
83% CR | CRS | NCT01865617 (REF. 18) |
Preconditioning chemotherapy was used in all the trials shown in this table. B-ALL, B-cell acute lymphoblastic leukaemia; chemo, chemotherapy; CHOP, Children’s Hospital of Philadelphia; CR, complete response; CRS, cytokine-release syndrome; Fred Hutchinson, Fred Hutchinson Cancer Research Center; MSKCC, Memorial Sloan Kettering Cancer Center; NCI, National Cancer Institute; R/R, relapsed and/or refractory; UPenn, The University of Pennsylvania.
CD19-targeted CAR T cells have similarly been used in clinical trials at the University of Pennsylvania (UPenn) and the Children’s Hospital of Philadelphia (CHOP), in Pennsylvania, USA (NCT01626495)15. Paediatric and adult patients with B-ALL were treated with second-generation CAR T cells (4-1BB/CD3ζ). An initial case report described that two paediatric patients had achieved MRD-negative complete remissions following CAR T-cell therapy16. One of these patients experienced disease relapse 2 months after treatment with a tumour escape variant negative for CD19 expression. In an updated report with results from 30 additional paediatric and adult patients, a 90% response rate was described, with all of the responding patients having CRS symptoms3.
An ‘intent-to-treat’ clinical trial performed in children and young adults with relapsed or refractory B-ALL or NHL was performed at the National Cancer Institute (NCI) in Maryland, USA (NCT01593696)17. A total of 20 paediatric and young adult patients were treated with CD19-specific CAR T cells (CD28/CD3ζ) with a complete response rate of 70%9. The toxicities reported included severe CRS in three out of 21 patients, and B-cell aplasias.
Researchers at the Fred Hutchinson Cancer Research Center (Fred Hutchinson) in Washington, USA, investigated the use of CAR T cells for the treatment of a wide range of CD19-positive malignancies (NCT01865617)18. Patients were treated with CD19-targeted CAR T cells (CD3ζ/41BB) that were generated from defined cell subsets comprising CD4+ and CD8+ central memory T cells19. These T-cell subsets were isolated, and then transduced and expanded for infusion. This therapeutic approach using CAR T-cell-generated with a defined subset composition yielded a 90% complete response rate, and 15 out of 18 evaluable patients with B-ALL achieved a complete bone-marrow remission20.
Low-grade B-cell malignancies
Mixed clinical responses have been observed using CD19-directed CARs to treat CLL and other indolent B-cell malignancies. In the case of low-grade B-cell malignancies, the comparison of these trials is difficult because several differences exist in the trial designs (including patient selection, preconditioning chemotherapy, and CAR design) and the results differed between institutions21–24. In a clinical trial performed at the MSKCC, CD19-specific second-generation CAR T cells (CD28/CD3ζ) were infused into patients with CLL or B-ALL who had previously received preconditioning chemotherapy. Three out of four patients experienced either stable disease or a significant reduction in lymphadenopathy, with no evidence of CRS observed21. Similar results were obtained in a trial conducted at the NCI, also with CD19-specific second-generation CAR T cells (CD28/CD3ζ)22. Out of four patients with CLL treated, one achieved a complete response, two experienced partial responses and one patient had stable disease. In the trials conducted at UPenn, investigators used a CD19-specific (4-1BB/CD3ζ) CAR design to treat patients with CLL23. Initial reports from the evaluation of 23 patients revealed that five patients (22%) achieved a complete response and four (17%) achieved a partial response23; 1 year later, an updated publication reported that the overall response rate among 14 patients was 57%, with four patients having a partial response and four patients experiencing complete responses24, although whether this group of 14 patients included the four patients analysed in the initial trial publication is not clear23. All responding patients experienced CRS23.
CAR T cells specific for CD19 have also been investigated for the treatment of FL, DLBCL and MCL. A group of researchers at the NCI used CD19-specific CAR T cells (CD28/CD3ζ) to treat patients with DLBCL, indolent lymphoma, or CLL; 22 out of 27 patients achieved either a complete response or a partial response25. Similar studies were conducted at UPenn, with CD-19-specific CAR T cells (4-1BB/CD3ζ) used to treat patients with DLBCL and FL; at 3-month follow up, two patients with DLBCL and one patient with FL had achieved a complete response, and one patient with FL had achieved a partial response26. An antitumour response was observed using CD19-targeted CAR-T-cell therapy to treat patients with aggressive B-cell NHL in the context of autologous haematopoietic stem-cell transplantation (autoHSCT) at the MSKCC (NCT01840566)27, with five out of eight patients achieving a complete response28. Of note, although some researchers believe that CRS is associated with favourable outcomes of CAR-T-cell therapy22,29,30, in this trial, only 50% of the patients with an objective response experienced CRS23. Patients with CLL were also treated in the trial conducted at Fred Hutchinson with defined-composition-generated CD19-targeted CAR T cells, with two out of three patients experiencing bone-marrow remissions20.
Multiple myeloma
An ongoing trial at UPenn will investigate CD19-specific CAR T cells for the treatment of multiple myeloma (MM), using the rationale that CD19 is expressed in a minor subclonal population of MM cells (NCT02135406)31. In this study, patients with MM are receiving autoHSCT and melphalan followed by an infusion of second-generation CD19-targeted CAR T cells (4-1BB/CD3ζ). Initial findings of this trial have been published in the form of a case-report describing one patient who experienced a complete response for 12 months following treatment32. Supplementary data in this publication demonstrated that four out of eight patients experienced partial responses (with short follow-up duration) and the remaining four patients had progressive disease32.
Novel targets in haematological cancer
Novel CAR targets are required for the effective treatment of patients with haematological malignancies that do not express CD19. Additionally, a subset of patients treated with emergence of CD19-specific CAR T cells experience disease relapse with CD19-negative tumour escape variants3,9,33. Examples of the most-promising CAR-T-cell targets for the treatment of haematological malignancies include: CD22 (REF. 34), CD20 (REF. 35), inactive tyrosine-protein kinase transmembrane receptor ROR1 (ROR1)36, and the immunoglobulin kappa chain (Igκ)37, for the treatment of B-cell malignancies; B-cell maturation antigen (BCMA)38 and CD138 (REF. 39), for plasma malignancies; and CD33 (REF. 40), CD123 (REF. 41) and Lewis Y antigen (LeY)42, for myeloid malignancies (TABLE 2).
Table 2.
CAR-T-cell targets for the treatment of haematological malignancies
| Target | CAR structure | Malignancy | Institution | Reference |
|---|---|---|---|---|
| CD22 | CD3ζ and CD28 | FL, NHL, DLBCL, B-ALL | NCI | NCT02315612 (REF. 34) |
| CD20 | CD3ζ or CD3ζ and 4-1BB | CD20-positive malignancies | PLA General Hospital | NCT01735604 (REF. 47) |
| ROR1 | CD3ζ and 4-1BB | CLL, SLL | MD Anderson | NCT02194374 (REF. 36) |
| Igκ | CD3ζ and CD28 | CLL, low-grade B-cell malignancies | Baylor | NCT00881920 (REF. 37) |
| CD30 | CD3ζ and CD28 | HL, NHL | Baylor | NCT01316146 (REF. 56) |
| CD123 | CD3ζ and CD28 | AML | City of Hope | NCT02159495 (REF. 41) |
| CD33 | CD3ζ and 4-1BB | AML | PLA General Hospital | NCT01864902 (REF. 40) |
| LeY | CD3ζ and CD28 | AML | Peter Mac | NCT01716364 (REF. 42) |
| BCMA | CD3ζ and 4-1BB | MM | NCI | NCT02215967 (REF. 38) |
| CD138 | CD3ζ and 4-1BB | MM | PLA General Hospital | NCT01886976 (REF. 39) |
Does not include CD19 targets. AML, acute myeloid leukaemia; B-ALL, B-cell acute lymphoblastic leukaemia; Baylor, Baylor College of Medicine (USA); BCMA, B-cell maturation antigen; City of Hope, City of Hope National Medical Center (USA); CLL, chronic lymphocytic leukaemia; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; HL, Hodgkin lymphoma; LeY, Lewis Y antigen; MM, multiple myeloma; MD Anderson, MD Anderson Cancer Center (USA); NCI, National Cancer Institute; NHL, non-Hodgkin lymphoma; Peter Mac, Peter MacCallum Cancer Centre (Australia); PLA General Hospital, People’s Liberation Army General Hospital (China); ROR1, inactive tyrosine-protein kinase transmembrane receptor ROR1; SLL, small lymphocytic lymphoma.
Other CAR T cells for B-cell malignancies
The expression patterns of CD22 and CD20 are similar to those of CD19 and, therefore, targeting these molecules will probably result in ‘on-target, off-tumour’ destruction of non-malignant B cells, leading to B-cell aplasias43: such toxicities have been observed with CD19-specific CAR-T-cell therapy, but have been considered acceptable because they could be managed clinically with monthly intravenous infusion of immunoglobulin (IVIg)44.
The use of CAR T cells that target CD22 for the treatment of patients with FL, NHL, ALL, and large-cell lymphoma is being investigated at the NCI34. The results from preclinical studies performed at the same institute indicate that the optimal anti-CD22 CAR was a second-generation CAR in which the scFv binds a proximal CD22 epitope45. In the ongoing phase I clinical trial, investigators aim to investigate the efficacy of this CD22-specific CAR T cells (4-1BB/CD3ζ) design for the treatment of patients with CD22-positive leukaemia or lymphoma following induction chemotherapy pretreatment consisting of fludarabine and cyclophosphamide (NCT02315612)34.
The antitumour efficacy of CD20-targeted CAR T cells was studied by a team from Fred Hutchinson (NCT00621452)35. A third-generation CAR (CD28/4-1BB/CD3ζ) T cell was used to treat two patients with MCL and one patient with FL. Two patients that had no measurable disease before treatment remained free from disease progression for 12 and 24 months, respectively, and the other patients, who had MCL, had an objective partial response46. Overall, the treatment was well-tolerated in all three patients. Another trial investigating the efficacy of CD20-specific CAR T cells (4-1BB/CD3ζ) in patients with refractory DLBCL is currently enrolling patients at the People’s Liberation Army (PLA) General Hospital, in Beijing, China (NCT01735604)47. Initial reports from this trial described that the treatment of six patients resulted in three partial responses, one complete response, one case of stable disease, and one patient had progressive disease48. The toxicities associated with this treatment included CAR-T-cell-mediated destruction of the nontransformed tissue surrounding tumour lesions, as well as a reduction in circulating CD20-positive B cells48.
Targeting ROR1 or Igκ is an attractive approach because these proteins are preferentially expressed in tumorigenic B-cells and, therefore, ‘on-target, off-tumour’ toxicity might be reduced compared with approaches targeting CD19, CD20, or CD22. ROR1 is a glycosylated type I membrane protein that is expressed on the surface of many cancer cells, including CLL and ALL cells, but not on nonmalignant B cells or haematopoietic stem cells49. Nevertheless, the expression pattern of ROR1 is controversial, with evidence reported for its expression on nontumour pancreatic and lung tissue; if so, any ‘on-target, off-tumour’ toxicity could have serious consequences50,51. Nontransformed B cells express either kappa (κ) or lambda (λ) immunoglobulin light chains and thus, targeting either of these will spare a fraction of the total B-cell population from toxicity. One concern that arises with targeting Igκ, is that its soluble form would reduce the antitumour efficacy of the CAR T cells by binding to the CAR and blocking it from recognition of the antigen on tumorigenic B cells. The results from preclinical studies, however, have demonstrated that the antitumour efficacy of Igκ-specific CAR T cells was not inhibited by soluble Igκ52.
Preclinical studies have demonstrated that ROR-1-specific CARs can redirect T-cell function to eliminate tumour cells positive for ROR1 expression53. Subsequently, a phase I clinical trial investigating the efficacy of using ROR1-specific CAR T cells in patients with CLL following preconditioning chemotherapy is planned at the MD Anderson Cancer Center (MD Anderson), Texas, USA (NCT02194374)36. Preclinical studies investigating T cells modified to express an Igκ-specific CAR demonstrated that this strategy enables the efficient lysis of tumour cells expressing Igκ52. Investigators at Baylor have designed a phase I clinical trial to examine the treatment of patients with B-cell leukaemia (including CLL), lymphoma, or MM, with CAR T cells targeted at Igκ (NCT00881920)37.
Hodgkin lymphoma and NHL
CD30 is expressed on Reed-Sternberg cells, a distinctive cell type found in patients with Hodgkin lymphoma (HL), but also on a small population of activated lymphocytes54. Similarly to the case of Igκ, the main concern with targeting CD30 is that high levels of soluble CD30 are often found in patients and these might compete for CAR binding. Findings of preclinical studies, however, have demonstrated that soluble CD30 does not inhibit CAR-T-cell cytolytic function55. Researchers at Baylor plan to administer CD30-specific CAR T cells to patients with HL and NHL (NCT01316146)56. Another trial investigating CD30-targeted CAR T-cell therapy is currently recruiting patients with relapsed or refractory HL and NHL at the PLA General Hospital (NCT02259556)57.
MM
Potential targets for CAR-T-cell therapy of MM include syndecan-1 (also known as CD138), BCMA, and CD19. CD138 is a type 1 integral membrane heparan sulfate proteoglycan expressed not only on the cell surface of plasma cells and MM cells, but also on bronchial epithelia; therefore, lung toxicity could be a potential limitation of therapies using CD138-targeted CAR T cells58. CAR T cells specific for CD138 (4-1BB/CD3ζ) are being used to treat pagtients with MM in a clinical trial at the PLA General Hospital (NCT01886976)39. A publication from this group described that, out of five patients with MM treated with CD138-specific CAR T cells, four experienced stable disease59. This study reported only grade 3 fevers and no dose-limiting toxicities; therefore, the authors concluded that treatment with CD138-specific CAR T cells was well tolerated59.
BCMA is a cell-surface receptor that belongs to the tumour necrosis superfamily and its expression is restricted to plasma cells. For this reason, BCMA-based therapies might result in eradication of plasma cells, both cancerous and nonmalignant, an ‘on-target, off-tumour’ toxicity that can be effectively managed clinically with IVIg. The expression pattern of BCMA and results of preclinical studies have indicated that BCMA is an attractive target for CAR therapy of MM60, and currently a trial is underway at the NCI to investigate a CAR-T-cell-specific for BCMA (4-1BB/CD3ζ) for the treatment of patients with MM or plasma-cell myeloma (NCT02215967)38.
Myeloid malignancies
Current targets under clinical investigation for CAR-T-cell therapy of myeloid malignancies include IL-3 receptor (IL-3R) subunit-α (also known as CD123), CD33, and the LeY antigen. Given that these molecules can be expressed on some nonmalignant cell types, as well as on malignant cells, the use of CAR T cells that recognize these antigens might be associated with a serious risk of ‘on-target, off-tumour’ toxicity. Clinical trials in which CD123 and CD33 have been targeted with monoclonal antibodies have yielded evidence of favourable safety profiles61,62; however, therapeutic approaches based on monoclonal antibodies have different effects and biological distributions than CAR T cells, and the safety of using CAR T cells to target these antigens needs to be assessed carefully.
CD123 is part of the IL-3R and is expressed on malignant myeloid cells, as well as on endothelial cells and monocytes; for this reason, some toxicity has been observed in preclinical CD123-targeted studies63–65. Treatment of patients with acute myeloid leukaemia (AML) using CD123-specific CAR T cells following preconditioning chemotherapy is currently being tested in a clinical trial at The City of Hope National Medical Center (City of Hope) in California, USA (NCT02159495)41.
CD33 is another receptor expressed in malignant myeloid cells, but targeting this protein might be problematic because some T cells and haematopoietic stem cells also express CD33 and, therefore, effective treatment might result in prolonged cytopenias and delayed haematopoietic recovery66. In addition, this therapeutic approach might also cause liver toxicity because CD33 is also expressed on Kupffer cells in this organ67. The PLA General Hospital is currently recruiting patients for a trial to investigate the clinical efficacy of CD33-specific CAR T cells in patients with AML (NCT01864902)40. A case report from this trial describes that, in one patient, the treatment with CD33-targeted CAR T cells resulted in some tumour destruction, but was followed by disease progression68. The toxicities reported in this study included fever and persistence of pre-existing pancytopenia. The authors propose that targeting CD33 might be useful as a tumour debulking strategy before HSCT.
LeY is a fucosylated carbohydrate antigen expressed on a proportion of AML cells but also on gastrointestinal and pancreatic cells69–71. A trial with LeY-specific CAR T cells (CD28/CD3ζ) in four patients with AML is ongoing at the Peter MacCallum Cancer Centre in Victoria, Australia (NCT01716364)42. CAR T cells (CD28/CD3ζ) were shown to traffic to the bone marrow (demonstrated in one patient evaluated) and to mediate some antitumour effects; however, all patients ultimately experienced relapse72. The authors concluded that targeting LeY with CAR T cells for the treatment of AML is safe, but targeting this TAA with CAR T cells as a single agent might not be optimal for the treatment of this disease because LeY is expressed on only a proportion of AML blasts.
Novel targets for solid malignancies
Several researchers are investigating the use of CAR T-cell therapy for the treatment of solid tumours. These studies require the prior identification of new antigens and the development of preclinical models of solid tumour in which to characterize therapies based on these antigens. In the next sections, we review approaches that are the focus of upcoming CAR-T-cell trials for the treatment of solid tumours (TABLE 3).
Table 3.
CAR targets for the treatment of solid malignancies
| Target | CAR structure | Malignancy | Institution | Reference |
|---|---|---|---|---|
| PSMA | CD3ζ and CD28 | Prostate cancer | MSKCC | NCT01140373 (REF. 76) |
| Roger Williams | NCT00664196 (REF. 78) | |||
| Mesothelin | CD3 and 4-1BB | Malignant pleural mesothelioma | UPenn | NCT01355965 (REF. 81) |
| Pancreatic cancer | UPenn | NCT02465983 (REF. 156) | ||
| Metastatic pancreatic (ductal) adenocarcinoma, epithelial ovarian cancer and malignant epithelial pleural mesothelioma | UPenn | NCT02159716 (REF. 84) | ||
| CD3ζ and CD28 | Mesothelioma and malignant pleural disease | MSKCC | NCT02414269 (REF. 85) | |
| CD3ζ, CD28 and 4-1BB | Mesothelioma, pancreatic and ovarian cancer | NCI | NCT01583686 (REF. 86) | |
| FAP | CD3ζ and CD28 | Mesothelioma | University of Zurich (Switzerland) | NCT01722149 (REF. 90) |
| EGFRvIII | CD3ζ and 4-1BB | Glioma | UPenn | NCT02209376 (REF. 95) |
| CD3ζ, CD28 and 4-1BB | Glioma | NCI | NCT01454596 (REF. 97) | |
| EGFR | Unknown | Malignant glioma | Renji Hospital (China) | NCT02331693 (REF. 98) |
| CEA | CD3ζ and CD28 | Liver metastases | Roger Williams | NCT02146466 (REF. 100) |
| Unknown | Lung, colorectal, gastric, breast and pancreatic cancer | Southwest Hospital (China) | NCT02349724 (REF. 103) | |
| CD171 | CD3ζ and 4-1BB or CD3ζ, CD28 and 4-1BB | Neuroblastoma | Seattle Children’s | NCT02311621 (REF. 106) |
| GD2 | CD3ζ, OX40, CD28 | Neuroblastoma, osteosarcoma and melanoma | NCI | NCT02107963 (REF. 112) |
| Neuroblastoma | Baylor | NCT01822652 (REF. 114) | ||
| CD3ζ, Ox40, CD28, virus specific | Sarcoma | Baylor | NCT01953900 (REF. 115) | |
| Glypican-3 | CD3ζ, CD28 and 4-1BB | Advanced-stage hepatocellular carcinoma | Renji Hospital (China) | NCT02395250 (REF. 117) |
| HER2 | CD3ζ and CD28 virus specific | Sarcoma | Baylor | NCT00902044 (REF. 122) |
| CD3ζ and CD28 | Glioblastoma | Baylor | NCT02442297 (REF. 126) | |
| Glioblastoma multiforme | Baylor | NCT01109095 (REF. 127) | ||
| IL-13 | RαCD3ζ and 4-1BB | Glioma | City of Hope | NCT02208362 (REF. 131) |
Baylor, Baylor College of Medicine (USA); CEA, carcinoembrionic antigen; City of Hope, City of Hope National Medical Center (USA); EGFRVII, epidermal growth factor receptor variant III; FAP, prolyl endopeptidase FAP/fibroblast activation protein alpha; MSKCC, Memorial Sloan Kettering Cancer Center (USA); NCI, National Cancer Institute (USA); PSMA, prostate-specific membrane antigen; Roger Williams, Roger Williams Medical Center (USA); Seattle Children’s, Seattle Children’s Hospital (USA); UPenn, University of Pennsylvania (USA).
Prostate-specific membrane antigen
Glutamate carboxypeptidase 2 (commonly known as prostate specific membrane antigen; PSMA) is a type II membrane protein that is highly expressed on most prostate-cancer cells and also on the tumour-associated neovasculature of many solid tumour types73,74. Given the expression pattern of PSMA, CAR T cells targeting this antigen might have antiangiogenic effects as well as direct antitumour effects. A second-generation CAR (CD28/CD3ζ) targeting PSMA has been designed at MSKCC for the treatment of prostate cancer (NCT01140373)75,76. An early report described that two out of three patients with progressive prostate cancer had stable disease for 6 months following treatment with this agent77. Another study at Roger Williams Medical Center in Rhode Island, USA, investigated second-generation (CD28/CD3ζ) PSMA-specific CAR T cells (NCT00664196)78. Early reports describe that two out of five patients had a decrease in PSA levels, and disease progression was delayed in four out of five patients79. The researchers who conducted this study propose that increasing the CAR-T-cell dose, together with that of exogenous IL-2, would improve this therapeutic approach, although updated results have not been published to date.
Mesothelin
Mesothelin is a TAA present on malignant pleural mesothelioma, as well as on ovarian, pancreatic and some lung cancers80. Mesothelin-specific CAR T cells were investigated in a first-in-man clinical trial at UPenn for patients with malignant pleural mesothelioma (NCT01355965)81. Mesothelin is also expressed on nontransformed peritoneal, pleural and pericardial mesothelial cells and, for that reason, transient CAR expression (achieved by RNA transfection of T cells) was used in this study, in order to determine the safety of this approach82. In spite of one death caused by an anaphylactic response to the mouse-derived scFv used in this construct, early results from this trial demonstrated that the treatment was otherwise well tolerated82,83. Despite the absence of preconditioning therapy, CAR-T-cell-mediated antitumour activity was demonstrated by a decrease in tumour-cell numbers in ascitic fluid and a reduction in the number of peritoneal lesions82,83. Given the observed safety profile of mesothelin-specific CAR T cells, investigators have subsequently opened a trial that will test CAR T cells transduced with lentivirus (to achieve stable CAR expression) for the treatment of metastatic pancreatic ductal adenocarcinoma, epithelial ovarian cancer, and malignant epithelial pleural mesothelioma (NCT02159716)84. Another trial opened at MSKCC in 2015 is also using second-generation (CD28/CD3ζ) CAR to target mesothelin, based on results of preclinical studies demonstrating that local administration of CAR T cells was required for optimal antitumour efficacy (NCT02414269)85. Patients with malignant pleural disease, mesothelioma, breast cancer, or lung cancers positive for mesothelin expression have been enrolled in this trial and will be treated with mesothelin-targeted CAR T cells infused via intraperitoneal injection. Additionally, the NCI is conducting a trial with mesothelin-targeted CAR T cells that contain a third-generation CAR (CD28/4-1BB/CD3ζ) for the treatment of patients with pancreatic cancer, mesothelioma, or ovarian cancer (NCT01583686)86.
Fibroblast activation protein
Investigators at the University of Zurich plan to treat patients with mesothelioma using T cells expressing a CAR targeted at prolyl endopeptidase FAP (commonly known as fibroblast activation protein alpha (FAP)), a membrane-bound gelatinase expressed on cancer-associated stromal cells present in epithelial cancers87. Preclinical studies with FAP-specific CAR T cells (CD28/CD3ζ) have generated encouraging results in mouse models of cancer therapy and, therefore, these CAR T cells will be tested in a clinical trial for patients with malignant pleural mesothelioma (NCT01722149)88–90.
EGFR and EGFR variants
CARs specific for EGFR and EGFR variant receptors are undergoing clinical evaluation in patients with glioma. EGFR overexpression is frequent in many tumour types, and correlates with tumour invasion and metastases91. The mutated form of EGFR, EGFRvIII (EGFR variant III), is also frequently overexpressed in cancer, and is commonly associated with glioblastoma92. EGFRvIII might be a suitable target for CAR-T-cell therapy, as indicated by the results of clinical studies in which monoclonal antibodies targeting EGFR variants were well tolerated93. Preclinical studies using a second-generation CAR (4-1BB/CD3ζ) specific for EGFRvIII revealed the high antitumour efficacy of this CAR, which might be suitable for the treatment of glioma94. Investigators of a trial at UPenn are currently recruiting patients with recurrent glioma or residual disease following initial resection to test the safety, feasibility, and antitumour efficacy of EGFRvIII-targeted CAR T cells (NCT02209376)95. Another trial at the NCI is testing a third-generation EGFRvIII-specific CAR that showed promising antitumour activity in preclinical studies96. Investigators of this trial are currently enrolling patients with malignant glioma, glioblastoma, and other brain cancers (NCT01454596)97. EGFR-specific CAR T cells are also being tested in a trial at Renji Hospital in Shanghai, China, in patients with advanced glioma (NCT02331693)98. Nonmutated EGFR, which is ubiquitously expressed, is the target of the CAR being used in this study, and, therefore, the safety of this approach is a concern.
Carcinoembryonic antigen
Carcinoembryonic antigen (CEA) is a glycoprotein that is expressed on many tumours of epithelial origin, but can also be found on cells of the gastrointestinal tract99. CEA-targeted CAR T cells are currently being tested to treat patients with adenocarcinoma liver metastases that are positive for CEA expression at Roger Williams Medical Center (NCT02416466)100. Previous clinical investigations found that intravenous infusion of CEA-specific TCR-transduced T cells resulted in severe inflammatory colitis in three out of three patients with refractory metastatic colorectal cancer101. In the Roger Williams Medical Center trial100, second-generation CAR T cells are being administered through percutaneous hepatic artery infusion because localized delivery has been proposed to limit CAR-T-cell-mediated extra-hepatic toxicity; a report from 2015 described that out of five evaluable patients treated with this protocol, one patient achieved stable disease and four patients had progressive disease102.
All the patients who received CAR-T-cell therapy showed necrosis of metastastic liver lesions; in addition, those who received IL-2 infusions alongside CAR-T-cell therapy had a reduction in CEA levels102. The authors of this study propose that this approach is safe and might elicit antitumour immune responses, warranting further study. CAR T cells specific for CEA will also be tested in patients with lung, colorectal, gastric, breast, or pancreatic cancer at the Southwest Hospital in Chongquing, China (NCT02349724)103.
CD171 (L1-CAM)
Neural cell adhesion molecule L1 (most commonly referred to as CD171 or L1-CAM) is expressed on a range of tumours including ovarian cancer, neuroblastoma, and malignant melanoma, and is also expressed on normal tissues including peripheral nerve bundles and kidney104. CD171 expressed on malignant cells has a different glycosylation pattern to that of CD171 expressed on nontransformed cells, and a CD171-targeted CAR has been developed that is specific for glycosylated CD171 expressed on malignant cells105. CAR T cells targeted at the form of CD171 expressed on malignant cells are being investigated for the treatment of refractory or recurrent neuroblastoma in a clinical trial at Seattle Children’s Hospital in Washington, USA (NCT02311621)106. To increase safety and enable the elimination of CAR T cells in the event of serious toxicity, the CAR T cells used in this trial will be modified to express an elimination gene, truncated EGFR (tEGFR) — this approach is discussed in the safety section of this Review107. This trial has two treatment cohorts, wherein patients will be treated with either second-generation (4-1BB/CD3ζ) or third-generation (CD28/4-1BB/CD3ζ) CARs106.
Disialoganglioside GD2
Tumours of neuro-ectodermal origin, including neuroblastoma, overexpress the disialoganglioside GD2 (REF. 108). However, this glycosphingolipid is expressed at low levels on nontransformed neural tissues109. CAR T cells targeted at GD2 have been investigated in clinical trials. In the initial clinical trial of such agents, virus-specific T cells expressing a first-generation CAR were infused into patients with neuroblastoma and induced complete tumour responses in three out of 11 patients with active disease at the time of treatment (NCT00085930)110,111. In an ongoing phase I trial, third-generation GD2-specific CAR T cells (OX40/CD28/CD3ζ) are being tested in patients with neuroblastoma, osteosarcoma, and melanoma (NCT02107963)112,113. To improve the safety of this approach, the CAR T cells are further modified to express an inducible caspase 9 (iCasp9) suicide gene (this approach is discussed in the following section on safety). A similar third-generation CAR specific for GD2 is also being tested in patients with neuroblastoma in a trial at Baylor (NCT01822652)114. Additionally, researchers at Baylor have initiated a trial to investigate this third-generation CAR in the context of virus-specific T cells for the treatment of patients with sarcoma (NCT01953900)115; T cells specific for Varicella Zoster virus will be modified to express the GD2-targeted CAR and will be administered in the context of Varicella vaccinations to enhance the antitumour efficacy and persistence of the CAR T cells.
Glypican-3
Glypican-3 is a surface proteoglycan that is overexpressed on hepatocellular carcinoma cells, whereas expression on nonmalignant cells present in gastric glands, kidney tubules, and on testicular germ cells is limited; therefore it might be an attractive target for CAR T-cell therapy116. A third-generation glypican-3 specific CAR has been tested preclinically and will be investigated for clinical efficacy in patients with hepatocellular carcinoma at Renji hospital in China (NCT02395250)116,117.
HER2
The receptor tyrosine-protein kinase erbB2 (most commonly known as HER2) is expressed on some epithelial cells in the gastrointestinal, respiratory, reproductive, and urinary tract118. The highest levels of HER2 expression, however, are detected in tumour cells, including those of breast, colon, and ovarian cancers119. Following the success of therapy with the HER2-specific monoclonal antibody trastuzumab, a clinical trial was conducted at NCI to test third-generation HER2-specific CAR T cells (CD28/4-1BB/CD3ζ) in patients with metastatic cancers (NCT00924287)120; however, the trial was terminated after a patient with colon cancer died of severe acute respiratory failure as a result of the treatment. A case report demonstrated that in this patient, immediately after infusion, CAR T cells had localized to the lung where they were stimulated to release cytokines in response to the low levels of HER2 expressed on the lung tissue121. Despite this severe toxicity, other trials with HER2-specific CAR T cells are planned. In a clinical trial by investigators at Baylor, patients with advanced-stage sarcoma will be treated with second-generation HER2-specific CAR T cells (CD28/CD3ζ) containing a different scFv to the one used in the NCI trial (NCT00902044)122. A publication has described the results from the first 19 patients treated in this trial, with no adverse events related to T-cell infusion reported123; four out of 17 evaluable patients had stable disease and, after removal of residual metastases, three patients remained in remission at 6, 12, and 16 months following treatment. The reason for the discrepancies in safety between the NCI and Baylor trials is not clear. The scFv used in the NCI trial was derived from trastuzumab, distinct from the scFv used in the Baylor study, which was derived from a different anti-HER2 monoclonal antibody, termed FRP5 (REFS 124,125). Patients enrolled in the NCI trial were pretreated with nonmyeloablative chemotherapy (60 mg/kg per day cyclophosphamide for 2 days and 25 mg/m2 per day fludarabine for 5 days). By contrast, the Baylor trial was designed with three arms to improve the safety of the trial: in the first arm, patients will receive no pretreatment before CAR-T-cell infusion; in the second arm, patients will receive fludarabine pretreatment; and in the third arm, patients will receive both cyclophosphamide and fludarabine pretreatment before CAR T cell infusion. One significant difference between the trials is the dosage of cells and the use of cytokine support. The NCI investigators treated their patient with 1010 cells infused with 300 IU/ml IL-2, whereas those at Baylor used two-log-fold lower doses of CAR T cells and performed dose-escalation, whereby the highest dose level was 1 × 108/m2, and no IL-2 was used. These discrepancies could account for the substantial differences in safety observed for both trials.
In addition to the aforementioned trial, clinicians at Baylor will conduct a different trial to determine the safety and antitumour efficacy of HER2-specific CAR T cells in patients with glioblastoma (NCT02442297)126. In an ongoing trial, Baylor investigators are also evaluating HER2-specific CAR T cells in the context of cytomegalovirus (CMV)-specific T cells for the treatment of glioblastoma multiforme (NCT01109095)127. The rational for this trial is that the CAR T cells specific for CMV will be more active than unselected T cells, as most people have been exposed to CMV and the T cells can receive activation stimuli through the endogenous TCR.
IL-13Rα
IL-13Ra is a high affinity monomer receptor that is overexpressed in 50% of glioblastoma, but is not expressed at substantial levels on normal brain tissues128. First-generation CAR T cells targeted at IL-13Rα have been tested clinically in three patients with glioblastoma (NCT00730613)129. A report has described the results of this trial indicating that intracranial delivery of this CAR is well-tolerated130. Two patients showed evidence of antiglioma responses as determined by MRI. A follow-up study is currently recruiting patients with malignant glioma who will be treated with second-generation (4-1BB/CD3ζ) IL-13Rα-targeted CAR T cells (NCT02208362)131.
Approaches to improve CAR T-cells
Several approaches have been developed to improve the antitumour efficacy of CAR T-cell therapy, and they extend beyond the choice of a target antigen. In this section we discuss the strategies that are currently being translated to the clinic (FIG. 2).
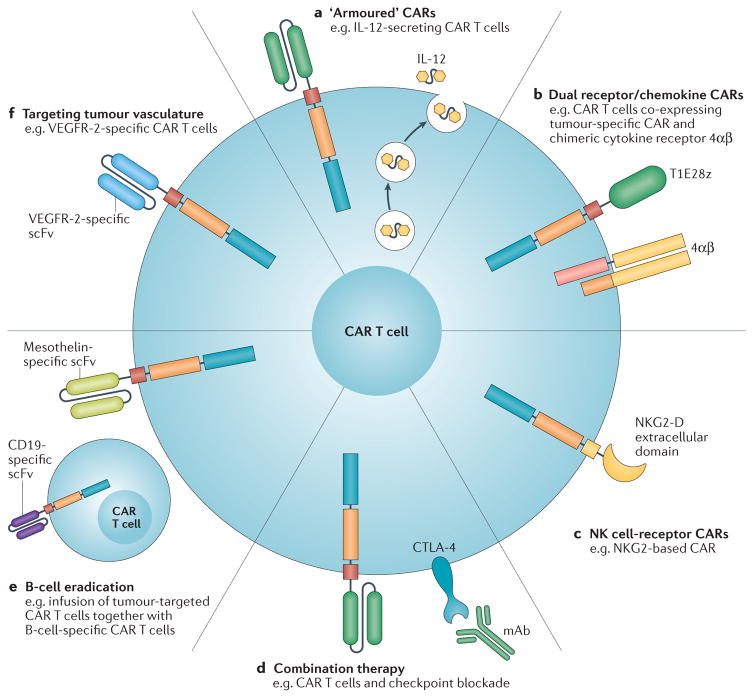
Figure 2. Approaches to improve CAR-T-cell therapy.
An overview of the improvements to chimeric antigen receptor (CAR)-T-cell therapy is shown, and clinical trials testing those strategies are indicated in the legend. a | Engineered CAR T cells that secrete pro-inflammatory cytokines (armoured CAR T cells; NCT02498912)137. b | Dual receptor expression to target tumour cells and convert tumour-derived cytokines into T-cell activators (NCT01818323)164. c | Using natural killer (NK)-cell-based recognition domains, such as NKG2-D, in CARs (NCT02203825)148. d | Combination therapy with monoclonal antibodies (mAb) targeting immune-checkpoint inhibitory receptors to relieve immunosuppression (NCT00586391)154. e | Infusion of two populations of CAR T cells to eradicate B cells and enable increased persistence of tumour-specific CAR T cells by preventing antibody responses against their foreign antigen components (NCT02465983)156. f | Targeting the tumour vasculature with CAR T cells, such as VEGFR-2-specific CAR T cells NCT01218867160. 4αβ, 4αβ chimeric cytokine receptor; CTLA-4, cytotoxic T-lymphocyte-associate antigen-4; T1E28z, T1E28z chimeric antigen receptor; VEGFR-2, vascular endothelial growth factor receptor 2.
Armoured CARs
‘Armoured’ CAR T cells are CAR T cells that are further modified to secrete pro-inflammatory cytokines to protect the CAR T cell from the inhibitory tumour microenvironment. Armoured CAR T cells modified to secrete pro-inflammatory IL-12 have yielded encouraging results in several preclinical studies132–135. The use of armoured CAR T cells led to improved antitumour efficacy, compared with T cells expressing the CAR alone, which was attributed to increased CAR-T-cell-mediated suppressive function, as well as to resistance of the transplanted T cells to immunosuppressive cells found in the tumour microenvironment, including regulatory T (TREG) cells and myeloid-derived suppressor cells (MDSCs)134,135. Previous studies investigated the treatment of patients with metastatic melanoma with tumour-infiltrating lymphocytes modified to express IL-12. Considerable toxicity was reported with high doses of these cells (0.3–3 × 109 cells), including liver toxicity, fevers, and life-threatening haemodynamic instabilities136. Nevertheless, a similar approach will be used in an upcoming trial of the treatment of ovarian cancer at MSKCC (NCT02498912)137. The second-generation CAR (CD28/CD3ζ) in this trial binds to the ectodomain of mucin-16 (MUC-16) that is retained on the cell surface following the cleavage of this protein, which is overexpressed on 70% of ovarian cancer cells138–140. The phase I trial at MSKCC will enrol patients with recurrent ovarian, fallopian, or primary peritoneal cancers that overexpress MUC-16 ectodomain, and will involve both systemic and intraperitoneal infusion of IL-12-secreting CAR T cells targeting this antigen139. To avoid toxicity issues, such as those described for previous trials, dose-escalation, beginning at 3 × 105 cells/kg, will be used in the MSKCC trial to monitor the safety and clinical efficacy of therapy with CAR–IL-12 T cells136,139.
Dual receptor and chemokine receptor CARs
To improve the persistence of CAR T cells, investigators at King’s College London, UK, have engineered T cells to express two artificial receptors, one for TAA recognition and one to provide cytokine-mediated growth stimulation to the T cell141. For example, the TAA-recognition receptor, T1E28z, is composed of second-generation CAR signalling domains (CD28/CD3ζ) ligated to T1E, a chimeric polypeptide that binds to HER1 homodimers and to HER2/HER3 heterodimers142. The signalling receptor is a chimeric cytokine receptor (4αβ), in which the IL-4 receptor ectodomain is ligated to the β-chain subunit shared by IL-2 and IL-15 receptors, enabling IL-2/IL-15-like pro-inflammatory stimulating signalling triggered by IL-4 — a cytokine that has a role in the immune microenvironment in many tumour types141. This approach, termed ‘T4-immunotherapy’, is currently being investigated in the clinical setting for the treatment of patients with head and neck cancers (NCT01818323)143,164.
Natural killer cell receptor CARs
Natural killer (NK) cells express receptors that can distinguish between tumour cells and nonmalignant cells; these receptors are being used as antigen recognition domains in CARs to improve tumour recognition by T cells. One such receptor is NKG2-D, and cognate ligands include stress-inducible proteins that are often overexpressed on many tumour types144. Linking the extracellular domain of NKG2-D to intracellular T-cell signalling domains enables NKG2-D ligands to active T cells, a strategy that has been shown to result in antitumour efficacy in preclinical studies145. A trial sponsored by Celdara Medical LLC has been designed to test chimeric NKG2-D receptor-modified T cells, and investigators are currently recruiting patients with AML, MM, and myelodysplastic syndrome (NCT02203825)146–148.
Combination with checkpoint blockade
CAR T cells are subject to inhibitory immune-checkpoint signals present within the tumour microenvironment, such as those from programmed cell-death protein 1 (PD-1) ligands or cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) ligands. These inhibitory receptor–ligand interactions can be blocked with monoclonal antibodies that abrogate suppression of T cells; although this approach has shown encouraging results in some malignancies, the success of these therapies clearly relies on the presence of a pre-existing endogenous tumour-specific T-cell response149–152. Indeed, CAR T cells are susceptible to PD-1-mediated inhibition and, therefore, the combination of CAR T-cell therapy with monoclonal antibody immune-checkpoint inhibitors is an obvious progression to protect CAR-T-cell function within the tumour microenvironment153. An ongoing clinical trial at Baylor is investigating such combination therapy in patients with B-cell lymphoma, CLL and B-ALL (NCT00586391)154. Patients will be treated with CD19-specific CAR (CD28/CD3ζ) T cells in combination with ipilumimab, a CTLA-4 blocking monoclonal antibody.
Improving CAR-T-cell persistence
A potential reason for failed CAR-T-cell therapy is reduced persistence of CAR T cells owing to immune recognition of CAR-derived foreign peptides and subsequent immune-mediated destruction of the modified T cells. Indeed, this effect has been noted in several clinical trials6,83,155. One approach to combat this immune-mediated destruction is to deplete B cells in an effort to prevent the host from developing human antimouse-peptide antibodies specific for the CAR, which would result in the elimination of CAR T cells. In a clinical trial at UPenn, investigators plan to treat patients with pancreatic cancer with both T cells expressing a mesothelin-specific CAR and T cells expressing the CD19-specific CAR (NCT02465983)156. Using this approach, the researchers predict that the CD19-specific CAR T cells will eradicate the B cells and prevent the development of antibodies to the mesothelin-specific CAR, allowing CAR T cells to persist longer. This promising approach, however, does not address the possibility of T-cell-mediated immune destruction of infused CAR T cells.
Tumour-vasculature-targeted CAR T cells
CAR T cells targeting the tumour vasculature are being investigated for the treatment of metastatic cancers, including melanoma and renal cancer. The presence of angiogenic growth factors, such as VEGF, in the tumour microenvironment and the overexpression of their receptors in tumour cells are associated with poor prognosis and metastasis157. VEGFR-2 is overexpressed on tumour stromal cells and some tumour cells158. The results of preclinical studies indicate that targeting VEGFR-2 with a CAR can redirect T-cell efficacy to target tumour stromal cells while sparing nontransformed tissues159. At present, patients with metastatic melanoma and renal cancer are being treated with VEGFR-2-specific CAR T cells in a trial at the NCI (NCT01218867)160.
Improving safety of CAR-T-cell therapy
The serious risk of toxicity associated with CAR-T-cell therapies is becoming increasingly apparent. In the following sections we discuss several factors that need to be considered in order to balance this toxicity with maximum antitumour efficacy.
CRS toxicities and their management
One of the probable causes of CRS is the release of inflammatory cytokines produced by large numbers of activated CAR T cells29. The symptoms of CRS include hypotension, fevers, and reversible neurological complications, such as delirium and seizure-like activity22,29,30 (BOX 2). Despite these adverse events, the intensity of CRS toxicity has been correlated with beneficial antitumour responses in trials of CAR T cells carried out at multiple research centres22,29,30. Currently, most CRS toxicities are manageable with high-dose steroids, vasopressors, ventilatory support, and supportive care. An increase in IL-6 levels has been associated with severe CRS, and an anti-IL-6R antibody, tocilizumab, has been used in the clinic to dampen the effects of CRS in many patients16,29, but this approach has not been effective in all cases of CRS161.
Box 2. Cytokine-release syndrome Symptoms.
Hypotension, vascular leak, coagulopathy, cytopenia
Respiratory/renal insufficiency
Myalgia, fevers
Neurological complications, including dyphasia, confusion, delirium, visual hallucinations, seizure-like activity
Diagnostics
C-reactive protein
Ferritin
Treatment and/or management
Vasopressors
Ventilatory support
Corticosteroids
Anti-IL-6 receptor antibody (tocilizumab) therapy
Supportive care
A correlation has been found between the levels of C-reactive protein in serum and CRS severity2. Thus, the levels of C-reactive protein in blood can serve as a biomarker to determine if patients are at risk of severe CRS. In addition, CRS severity can be associated with tumour burden at the time of treatment, which indicates that patients with lower tumour burden could have a reduced risk of severe CRS1.
Transient mRNA-mediated CAR expression
The safety of CAR T cells targeted at novel antigens can initially be assessed with transient genetic modification of T cells. In the first clinical trial of mesothelin-specific CAR T cells (NCT01355965)81, investigators used a transient CAR expression system to evaluate the potential for ‘on-target, off-tumour’ toxicity, given the expression of mesothelin on normal cells (peritoneal, pleural and pericardial mesothelial surfaces)81,82. In this pilot trial, no evidence of ‘on-target, off-tumour’ toxicity was observed; therefore, targeting mesothelin was deemed a safe approach. In addition, the authors concluded that future clinical trials can use stable genetic modification strategies to achieve stable expression of mesothelin-targeted CAR on T cells, an approach that will be tested clinically in patients with adenocarcinoma, ovarian cancer, and mesothelioma (NCT02159716)84. Of note, transient expression of the CAR might necessitate repeated infusions of CAR T cells, as was the case of the initial clinical trial of mesothelin-specific CAR T cells (NCT01355965)81; however, the repeated infusion of CAR T cells resulted in an anaphylactic response to the mouse-derived scFv used in the CAR in one patient83. This case was the first report of an anaphylactic response following CAR-T-cell infusion and might illustrate the necessity of adopting human-derived scFvs in CARs to be used in the setting of repeated CAR-T-cell infusion treatment strategies.
Expression of elimination genes
High doses of lymphotoxic steroids can limit CAR-T-cell-mediated toxicities, but they can also eradicate CAR T cells and adversely affect the clinical outcome of patients29. A potential improvement of this strategy would involve expressing an elimination gene that can specifically eradicate CAR T cells after the administration of a pro-drug or antibody. An inducible caspase-9 gene (iCasp9) has been used in the clinic to successfully eliminate T cells in the context of patients receiving an allogeneic transplant162. The antitumour efficacy of this suicide gene in the context of CAR-T-cell therapy will be tested in the mesothelin-specific CAR-T-cell trial at MSKCC (NCT02414269)85, and in the GD2-specific CAR-T-cell trial at the NCI (NCT02107963)112. Another elimination gene used in the clinic is a nonfunctional, truncated version of EGFR that is engineered to be expressed on the surface of CAR T cells. In this setting, in the event of serious toxicities, CAR T cells can be rapidly eliminated with the administration of cetuximab, an FDA-approved monoclonal antibody specific for EGFR that can recognize the truncated protein163. The administration of cetuximab would result in the elimination of CAR T cells through activation of antibody-dependent cellular cytotoxicity and/or the complement cascade. This approach is currently being used in the context of the CD19-targeted CAR-T-cell trial at Fred Hutchinson (NCT01865617)18, and will also be used in an upcoming trial at the MSKCC in patients with ovarian cancer (NCT02498912)137,139.
Conclusions
CAR-T-cell therapy has been established as a potentially effective treatment of B-cell malignancies. Following the success of this approach for the treatment of B-ALL, many investigators are currently designing strategies to obtain similarly successful results in the treatment of other haematological malignancies and solid tumours. Challenging aspects of this transition include the careful selection of target antigen, the management of ‘on-target, off-tumour’ toxicity, and the modulation of the immunosuppressive tumour microenvironment. The outcomes of the ongoing clinical trials discussed in this Review are eagerly awaited with the hope that they will ultimately provide guidance for the development of safer and more-effective CAR-T-cell therapy for an expanding array of malignancies.
Key points.
Chimeric antigen receptor (CAR)-T-cell therapy has shown enormous promise in the treatment of B-cell acute lymphoblastic leukaemia; at present, CD19-targeted CAR-T-cell-based treatment of other B-cell malignancies is less effective
Other targets for CAR-T-cell therapy of haematological malignancies that are undergoing clinical testing include CD20, CD22, ROR1, IgK, BCMA, CD138, CD33, CD123 and LewisY antigen
Targets for CAR-T-cell therapy of solid malignancies currently being tested in the clinic include PSMA, FAP, CEA, CD171, GD2, glypican-3, HER2 and IL-13Rα
Several strategies are under development to improve CAR-T-cell-mediated antitumour responses; these include, among others, ‘armoured’ CAR T cells, dual receptor/cytokine-based CARs, CARs based on natural-killer-cell receptors and other cell receptors
Strategies to improve the safety of CAR-T-cell therapy involve improved management of cytokine-release syndrome, as well as engineered CAR T cells that are easier to eradicate in case of adverse events
Acknowledgments
R.J.B. receives financial support from The Annual Terry Fox Run for Cancer Research (New York) organized by the Canada Club of New York, Carson Family Charitable Trust, Kate’s Team, Emerald Foundation, the Experimental Therapeutics Center of Memorial Sloan Kettering Cancer Center (Innovations in the structures, functions and targets of monoclonal antibody-based drugs for cancer), The Leukemia & Lymphoma society, The National Institutes of Health Grants (R01CA138738-05, PO1CA059350, PO1CA190174-01) and the William Lawrence and Blanche Hughes Foundation.
Footnotes
Author contributions
H.J.J., S.R. and R.J.B. researched data for the article, contributed to discussing the article’s content, wrote, reviewed and edited the manuscript before submission.
Competing interests statement
H.J.J. and S.R. declare no competing interests. R.J.B. is a co-founder, stockholder and consultant for Juno Therapeutics Inc.
References
- 1.Brentjens RJ, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra138. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee DW, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maude SL, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jensen MC, et al. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transplant. 2010;16:1245–1256. doi: 10.1016/j.bbmt.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamers CH, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24:e20–e22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 6.Till BG, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savoldo B, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121:1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scholler J, et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med. 2012;4:132ra153. doi: 10.1126/scitranslmed.3003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee DW, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2014;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carpenito C, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci USA. 2009;106:3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong XS, Matsushita M, Plotkin J, Riviere I, Sadelain M. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol Ther. 2010;18:413–420. doi: 10.1038/mt.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT01853631.
- 13.US National Library of Science. ClinicalTrials.gov [online] 2016 https://clinicaltrials.gov/ct2/show/NCT01044069.
- 14.Park JH, et al. Efficacy and safety of CD19-targeted 19-28z CAR modified T cells in adult patients with relapsed or refractory B-ALL [abstract] J Clin Oncol. 2015;33(Suppl):7010. [Google Scholar]
- 15.US National Library of Science. ClinicalTrials.gov [online] 2016 https://clinicaltrials.gov/ct2/show/NCT01626495.
- 16.Grupp SA, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT01593696.
- 18.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT01865617.
- 19.Sommermeyer D, et al. Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia. 2015;30:492–500. doi: 10.1038/leu.2015.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turtle CJ, et al. Immunotherapy with CD19-specific chimeric antigen receptor (CAR)-modified T cells of defined subset composition [abstract] J Clin Oncol. 2015;33(Suppl):3006. [Google Scholar]
- 21.Brentjens RJ, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kochenderfer JN, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porter DL, et al. Randomized, phase II dose optimization study of chimeric antigen receptor modified t cells directed against CD19 (CTL019) in patients with relapsed, refractory CLL. Presented at the 55th ASH Annual Meeting and Exposition; 2014. [Google Scholar]
- 24.Porter DL, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7:303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kochenderfer JN, et al. Anti-CD19 CAR T cells administered after low-dose chemotherapy can induce remissions of chemotherapy-refractory diffuse large B-cell lymphoma. Blood. 2014;124:550. [Google Scholar]
- 26.Schuster SJ, et al. Phase IIa trial of chimeric antigen receptor modified T cells directed against CD19 (CTL019) in patients with relapsed or refractory CD19+ lymphomas [abstract] Blood. 2014;124(Suppl):3087. [Google Scholar]
- 27.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT01840566.
- 28.Sauter CS, et al. Phase I trial of 19-28z chimeric antigen receptor modified T cells (19-28z CAR-T) post-high dose therapy and autologous stem cell transplant (HDT-ASCT) for relapsed and refractory (rel/ref) aggressive B-cell non-Hodgkin lymphoma (B-NHL) [abstract] J Clin Oncol. 2015;33(Suppl):8515. [Google Scholar]
- 29.Davila ML, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra225. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT02135406.
- 32.Garfall AL, et al. Chimeric antigen receptor T cells against CD19 for multiple myeloma. N Engl J Med. 2015;373:1040–1047. doi: 10.1056/NEJMoa1504542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sotillo E, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5:1282–1295. doi: 10.1158/2159-8290.CD-15-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT02315612.
- 35.US National Library of Science. ClinicalTrials.gov [online] 2014 https://clinicaltrials.gov/ct2/show/NCT00621452.
- 36.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT02194374.
- 37.US National Library of Science. ClinicalTrials.gov [online] 2016 https://clinicaltrials.gov/ct2/show/NCT00881920.
- 38.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT02215967.
- 39.US National Library of Science. ClinicalTrials.gov [online] 2016 https://clinicaltrials.gov/ct2/show/NCT01886976.
- 40.US National Library of Science. ClinicalTrials.gov [online] 2016 https://clinicaltrials.gov/ct2/show/NCT01864902.
- 41.US National Library of Science. ClinicalTrials.gov [online] 2016 https://clinicaltrials.gov/ct2/show/NCT02159495.
- 42.US National Library of Science. ClinicalTrials.gov [online] 2012 https://clinicaltrials.gov/ct2/show/NCT01716364.
- 43.Nitschke L. CD22 and Siglec-G: B-cell inhibitory receptors with distinct functions. Immunol Rev. 2009;230:128–143. doi: 10.1111/j.1600-065X.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- 44.Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev. 2014;257:107–126. doi: 10.1111/imr.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haso W, et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood. 2013;121:1165–1174. doi: 10.1182/blood-2012-06-438002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Till BG, et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood. 2012;119:3940–3950. doi: 10.1182/blood-2011-10-387969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT01735604.
- 48.Wang Y, et al. Effective response and delayed toxicities of refractory advanced diffuse large B-cell lymphoma treated by CD20-directed chimeric antigen receptor-modified T cells. Clin Immunol. 2014;155:160–175. doi: 10.1016/j.clim.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Zhang S, et al. The onco-embryonic antigen ROR1 is expressed by a variety of human cancers. Am J Pathol. 2012;181:1903–1910. doi: 10.1016/j.ajpath.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Al-Shawi R, Ashton SV, Underwood C, Simons JP. Expression of the Ror1 and Ror2 receptor tyrosine kinase genes during mouse development. Dev Genes Evol. 2001;211:161–171. doi: 10.1007/s004270100140. [DOI] [PubMed] [Google Scholar]
- 51.Hudecek M, et al. The B-cell tumor-associated antigen ROR1 can be targeted with T cells modified to express a ROR1-specific chimeric antigen receptor. Blood. 2010;116:4532–4541. doi: 10.1182/blood-2010-05-283309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vera J, et al. T lymphocytes redirected against the κ light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood. 2006;108:3890–3897. doi: 10.1182/blood-2006-04-017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deniger DC, et al. Sleeping Beauty transposition of chimeric antigen receptors targeting receptor tyrosine kinase-like orphan receptor-1 (ROR1) into diverse memory T-cell populations. PLoS ONE. 2015;10:e0128151. doi: 10.1371/journal.pone.0128151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ellis TM, Simms PE, Slivnick DJ, Jack HM, Fisher RI. CD30 is a signal-transducing molecule that defines a subset of human activated CD45RO+ T cells. J Immunol. 1993;151:2380–2389. [PubMed] [Google Scholar]
- 55.Hombach A, et al. An anti-CD30 chimeric receptor that mediates CD3-ζ-independent T-cell activation against Hodgkin’s lymphoma cells in the presence of soluble CD30. Cancer Res. 1998;58:1116–1119. [PubMed] [Google Scholar]
- 56.US National Library of Science. ClinicalTrials.gov [online] 2016 https://clinicaltrials.gov/ct2/show/NCT01316146.
- 57.US National Library of Science. ClinicalTrials.gov [online] 2016 https://clinicaltrials.gov/ct2/show/NCT02259556.
- 58.Zhang H, et al. Differential expression of syndecan-1 mediates cationic nanoparticle toxicity in undifferentiated versus differentiated normal human bronchial epithelial cells. ACS Nano. 2011;5:2756–2769. doi: 10.1021/nn200328m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo B, et al. CD138-directed adoptive immunotherapy of chimeric antigen receptor (CAR)-modified T cells for multiple myeloma. J Cell Immunother. 2015 http://dx.doi.org/10.1016/j.jocit.2014.11.001.
- 60.Carpenter RO, et al. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res. 2013;19:2048–2060. doi: 10.1158/1078-0432.CCR-12-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feldman E, et al. Treatment of relapsed or refractory acute myeloid leukemia with humanized anti-CD33 monoclonal antibody HuM195. Leukemia. 2003;17:314–318. doi: 10.1038/sj.leu.2402803. [DOI] [PubMed] [Google Scholar]
- 62.Roberts AW, et al. A phase I study of anti-CD123 monoclonal antibody (mAb) CSL360 targeting leukemia stem cells (LSC) in AML [abstract] J Clin Oncol. 2010;28(Suppl):e13012. [Google Scholar]
- 63.Mardiros A, et al. T cells expressing CD123-specific chimeric antigen receptors exhibit specific cytolytic effector functions and antitumor effects against human acute myeloid leukemia. Blood. 2013;122:3138–3148. doi: 10.1182/blood-2012-12-474056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shi J, et al. Identification of CD123+ myeloid dendritic cells as an early-stage immature subset with strong tumoristatic potential. Cancer Lett. 2008;270:19–29. doi: 10.1016/j.canlet.2008.04.048. [DOI] [PubMed] [Google Scholar]
- 65.Tettamanti S, et al. Targeting of acute myeloid leukaemia by cytokine-induced killer cells redirected with a novel CD123-specific chimeric antigen receptor. Br J Haematol. 2013;161:389–401. doi: 10.1111/bjh.12282. [DOI] [PubMed] [Google Scholar]
- 66.Sievers EL, et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol. 2001;19:3244–3254. doi: 10.1200/JCO.2001.19.13.3244. [DOI] [PubMed] [Google Scholar]
- 67.Hoyer JD, Grogg KL, Hanson CA, Gamez JD, Dogan A. CD33 detection by immunohistochemistry in paraffin-embedded tissues: a new antibody shows excellent specificity and sensitivity for cells of myelomonocytic lineage. Am J Clin Pathol. 2008;129:316–323. doi: 10.1309/E36008Y2H08Q1AYY. [DOI] [PubMed] [Google Scholar]
- 68.Wang QS, et al. Treatment of CD33-directed chimeric antigen receptor-modified T cells in one patient with relapsed and refractory acute myeloid leukemia. Mol Ther. 2015;23:184–191. doi: 10.1038/mt.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kobayashi K, et al. Lewis blood group-related antigen expression in normal gastric epithelium, intestinal metaplasia, gastric adenoma, and gastric carcinoma. Am J Gastroenterol. 1993;88:919–924. [PubMed] [Google Scholar]
- 70.Peinert S, et al. Gene-modified T cells as immunotherapy for multiple myeloma and acute myeloid leukemia expressing the Lewis Y antigen. Gene Ther. 2010;17:678–686. doi: 10.1038/gt.2010.21. [DOI] [PubMed] [Google Scholar]
- 71.Schuessler MH, et al. Blood group and blood-group-related antigens in normal pancreas and pancreas cancer: enhanced expression of precursor type 1, Tn and sialyl-Tn in pancreas cancer. Int J Cancer. 1991;47:180–187. doi: 10.1002/ijc.2910470204. [DOI] [PubMed] [Google Scholar]
- 72.Ritchie DS, et al. Persistence and efficacy of second generation CAR T cell against the LeY antigen in acute myeloid leukemia. Mol Ther. 2013;21:2122–2129. doi: 10.1038/mt.2013.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chang SS. Overview of prostate-specific membrane antigen. Rev Urol. 2004;6:S13–S18. [PMC free article] [PubMed] [Google Scholar]
- 74.Haffner MC, et al. Prostate-specific membrane antigen expression in the neovasculature of gastric and colorectal cancers. Hum Pathol. 2009;40:1754–1761. doi: 10.1016/j.humpath.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 75.Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRζ/CD28 receptor. Nat Biotechnol. 2002;20:70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 76.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT01140373.
- 77.Slovin SF, et al. Targeting castration resistant prostate cancer (CRPC) with autologous PSMA-directed CAR+ T cells. J Clin Oncol. 2012;30:TPS4700. [Google Scholar]
- 78.US National Library of Science. ClinicalTrials.gov [online] 2014 https://clinicaltrials.gov/ct2/show/NCT00664196.
- 79.Junghans RP, et al. Abstract C13: phase I trial of anti-PSMA designer T cells in advanced prostate cancer. Cancer Res. 2012;72:C13. [Google Scholar]
- 80.Hassan R, Ho M. Mesothelin targeted cancer immunotherapy. Eur J Cancer. 2008;44:46–53. doi: 10.1016/j.ejca.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT01355965.
- 82.Beatty GL, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res. 2014;2:112–120. doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maus MV, et al. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol Res. 2013;1:26–31. doi: 10.1158/2326-6066.CIR-13-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.US National Library of Science. ClinicalTrials.gov [online] 2016 https://clinicaltrials.gov/ct2/show/NCT02159716.
- 85.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT02414269.
- 86.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT01583686.
- 87.Mathew S, et al. The gene for fibroblast activation protein α (FAP), a putative cell surface-bound serine protease expressed in cancer stroma and wound healing, maps to chromosome band 2q23. Genomics. 1995;25:335–337. doi: 10.1016/0888-7543(95)80157-h. [DOI] [PubMed] [Google Scholar]
- 88.Petrausch U, et al. Re-directed T cells for the treatment of fibroblast activation protein (FAP)-positive malignant pleural mesothelioma (FAPME-1) BMC Cancer. 2012;12:615. doi: 10.1186/1471-2407-12-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schuberth PC, et al. Treatment of malignant pleural mesothelioma by fibroblast activation protein-specific re-directed T cells. J Transl Med. 2013;11:187. doi: 10.1186/1479-5876-11-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.US National Library of Science. ClinicalTrials.gov [online] 2016 https://clinicaltrials.gov/ct2/show/NCT01722149.
- 91.Arteaga CL. Epidermal growth factor receptor dependence in human tumors: more than just expression? Oncologist. 2002;7(Suppl 4):31–39. doi: 10.1634/theoncologist.7-suppl_4-31. [DOI] [PubMed] [Google Scholar]
- 92.Feldkamp MM, Lala P, Lau N, Roncari L, Guha A. Expression of activated epidermal growth factor receptors, Ras-guanosine triphosphate, and mitogen-activated protein kinase in human glioblastoma multiforme specimens. Neurosurgery. 1999;45:1442–1453. doi: 10.1097/00006123-199912000-00034. [DOI] [PubMed] [Google Scholar]
- 93.Thomas M. Cetuximab: adverse event profile and recommendations for toxicity management. Clin J Oncol Nurs. 2005;9:332–338. doi: 10.1188/05.CJON.332-338. [DOI] [PubMed] [Google Scholar]
- 94.Johnson LA, et al. Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci Transl Med. 2015;7:275ra222. doi: 10.1126/scitranslmed.aaa4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.US National Library of Science. ClinicalTrials.gov [online] 2016 https://clinicaltrials.gov/ct2/show/NCT02209376.
- 96.Morgan RA, et al. Recognition of glioma stem cells by genetically modified T cells targeting EGFRvIII and development of adoptive cell therapy for glioma. Hum Gene Ther. 2012;23:1043–1053. doi: 10.1089/hum.2012.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.US National Library of Science. ClinicalTrials.gov [online] 2016 https://clinicaltrials.gov/ct2/show/NCT01454596.
- 98.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT02331693.
- 99.Hammarstrom S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9:67–81. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- 100.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT02416466.
- 101.Parkhurst MR, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011;19:620–626. doi: 10.1038/mt.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Katz SC, et al. Phase I hepatic immunotherapy for metastases study of intra-arterial chimeric antigen receptor-modified T-cell therapy for CEA+ liver metastases. Clin Cancer Res. 2015;21:3149–3159. doi: 10.1158/1078-0432.CCR-14-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT02349724.
- 104.Huszar M, et al. Expression profile analysis in multiple human tumors identifies L1 (CD171) as a molecular marker for differential diagnosis and targeted therapy. Hum Pathol. 2006;37:1000–1008. doi: 10.1016/j.humpath.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 105.Meli ML, et al. Anti-neuroblastoma antibody chCE7 binds to an isoform of L1-CAM present in renal carcinoma cells. Int J Cancer. 1999;83:401–408. doi: 10.1002/(sici)1097-0215(19991029)83:3<401::aid-ijc17>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 106.US National Library of Science. ClinicalTrials.gov [online] 2014 https://clinicaltrials.gov/ct2/show/NCT02311621.
- 107.Hong H, et al. Diverse solid tumors expressing a restricted epitope of L1-CAM can be targeted by chimeric antigen receptor redirected T lymphocytes. J Immunother. 2014;37:93–104. doi: 10.1097/CJI.0000000000000018. [DOI] [PubMed] [Google Scholar]
- 108.Suzuki M, Cheung NK. Disialoganglioside GD2 as a therapeutic target for human diseases. Expert Opin Ther Targets. 2015;19:349–362. doi: 10.1517/14728222.2014.986459. [DOI] [PubMed] [Google Scholar]
- 109.Zhang S, et al. Selection of tumor antigens as targets for immune attack using immunohistochemistry: I. Focus on gangliosides. Int J Cancer. 1997;73:42–49. doi: 10.1002/(sici)1097-0215(19970926)73:1<42::aid-ijc8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 110.Louis CU, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT00085930.
- 112.US National Library of Science. ClinicalTrials.gov [online] 2016 https://clinicaltrials.gov/ct2/show/NCT02107963.
- 113.Gargett T, Brown MP. Different cytokine and stimulation conditions influence the expansion and immune phenotype of third-generation chimeric antigen receptor T cells specific for tumor antigen GD2. Cytotherapy. 2015;17:487–495. doi: 10.1016/j.jcyt.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 114.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT01822652.
- 115.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT01953900.
- 116.Baumhoer D, et al. Glypican 3 expression in human nonneoplastic, preneoplastic, and neoplastic tissues: a tissue microarray analysis of 4,387 tissue samples. Am J Clin Pathol. 2008;129:899–906. doi: 10.1309/HCQWPWD50XHD2DW6. [DOI] [PubMed] [Google Scholar]
- 117.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT02395250.
- 118.Press MF, Cordon-Cardo C, Slamon DJ. Expression of the HER-2/neu proto-oncogene in normal human adult and fetal tissues. Oncogene. 1990;5:953–962. [PubMed] [Google Scholar]
- 119.Slamon DJ, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 120.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT00924287.
- 121.Morgan RA, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT00902044.
- 123.Ahmed N, et al. Human epidermal growth factor receptor 2 (HER2) -specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J Clin Oncol. 2015;33:1688–1696. doi: 10.1200/JCO.2014.58.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ahmed N, et al. Immunotherapy for osteosarcoma: genetic modification of T cells overcomes low levels of tumor antigen expression. Mol Ther. 2009;17:1779–1787. doi: 10.1038/mt.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhao Y, et al. A herceptin-based chimeric antigen receptor with modified signaling domains leads to enhanced survival of transduced T lymphocytes and antitumor activity. J Immunol. 2009;183:5563–5574. doi: 10.4049/jimmunol.0900447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.US National Library of Science. ClinicalTrials.gov [online] 2016 https://clinicaltrials.gov/ct2/show/NCT02442297.
- 127.US National Library of Science. ClinicalTrials.gov [online] 2016 https://clinicaltrials.gov/ct2/show/NCT01109095.
- 128.Debinski W, Gibo DM, Hulet SW, Connor JR, Gillespie GY. Receptor for interleukin 13 is a marker and therapeutic target for human high-grade gliomas. Clin Cancer Res. 1999;5:985–990. [PubMed] [Google Scholar]
- 129.US National Library of Science. ClinicalTrials.gov [online] 2011 https://clinicaltrials.gov/ct2/show/NCT00730613.
- 130.Brown CE, et al. Bioactivity and safety of IL13Rα2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin Cancer Res. 2015;21:4062–4072. doi: 10.1158/1078-0432.CCR-15-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT02208362.
- 132.Chinnasamy D, et al. Local delivery of interleukin-12 using T cells targeting VEGF receptor-2 eradicates multiple vascularized tumors in mice. Clin Cancer Res. 2012;18:1672–1683. doi: 10.1158/1078-0432.CCR-11-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chmielewski M, Kopecky C, Hombach AA, Abken H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively Muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 2011;71:5697–5706. doi: 10.1158/0008-5472.CAN-11-0103. [DOI] [PubMed] [Google Scholar]
- 134.Kerkar SP, et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest. 2011;121:4746–4757. doi: 10.1172/JCI58814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pegram HJ, et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119:4133–4141. doi: 10.1182/blood-2011-12-400044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang L, et al. Tumor-infiltrating lymphocytes genetically engineered with an inducible gene encoding interleukin-12 for the immunotherapy of metastatic melanoma. Clin Cancer Res. 2015;21:2278–2288. doi: 10.1158/1078-0432.CCR-14-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT02498912.
- 138.Chekmasova AA, et al. Successful eradication of established peritoneal ovarian tumors in SCID-beige mice following adoptive transfer of T cells genetically targeted to the MUC16 antigen. Clin Cancer Res. 2010;16:3594–3606. doi: 10.1158/1078-0432.CCR-10-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Koneru M, O’Cearbhaill R, Pendharkar S, Spriggs DR, Brentjens RJ. A phase I clinical trial of adoptive T cell therapy using IL-12 secreting MUC-16ecto directed chimeric antigen receptors for recurrent ovarian cancer. J Transl Med. 2015;13:102. doi: 10.1186/s12967-015-0460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yin BW, Lloyd KO. Molecular cloning of the CA125 ovarian cancer antigen: identification as a new mucin, MUC16. J Biol Chem. 2001;276:27371–27375. doi: 10.1074/jbc.M103554200. [DOI] [PubMed] [Google Scholar]
- 141.Wilkie S, et al. Selective expansion of chimeric antigen receptor-targeted T-cells with potent effector function using interleukin-4. J Biol Chem. 2010;285:25538–25544. doi: 10.1074/jbc.M110.127951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Davies DM, et al. Flexible targeting of ErbB dimers that drive tumorigenesis by using genetically engineered T cells. Mol Med. 2012;18:565–576. doi: 10.2119/molmed.2011.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.van Schalkwyk MC, et al. Design of a phase I clinical trial to evaluate intratumoral delivery of ErbB-targeted chimeric antigen receptor T-cells in locally advanced or recurrent head and neck cancer. Hum Gene Ther Clin Dev. 2013;24:134–142. doi: 10.1089/humc.2013.144. [DOI] [PubMed] [Google Scholar]
- 144.Zhang J, Basher F, Wu JD. NKG2D ligands in tumor immunity: two sides of a coin. Front Immunol. 2015;6:97. doi: 10.3389/fimmu.2015.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Spear P, Barber A, Rynda-Apple A, Sentman CL. NKG2D CAR T-cell therapy inhibits the growth of NKG2D ligand heterogeneous tumors. Immunol Cell Biol. 2013;91:435–440. doi: 10.1038/icb.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sentman CL, Meehan KR. NKG2D CARs as cell therapy for cancer. Cancer J. 2014;20:156–159. doi: 10.1097/PPO.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang T, Barber A, Sentman CL. Chimeric NKG2D modified T cells inhibit systemic T-cell lymphoma growth in a manner involving multiple cytokines and cytotoxic pathways. Cancer Res. 2007;67:11029–11036. doi: 10.1158/0008-5472.CAN-07-2251. [DOI] [PubMed] [Google Scholar]
- 148.US National Library of Science. ClinicalTrials.gov [online] 2016 https://www.clinicaltrials.gov/ct2/show/NCT02203825.
- 149.Rizvi NA, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Robert C, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 151.Van Allen EM, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350:207–211. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Choudhury N, Nakamura Y. The importance of immunopharmacogenomics in cancer treatment: patient selection and monitoring for immune checkpoint antibodies. Cancer Sci. 2015;107:107–115. doi: 10.1111/cas.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.John LB, et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res. 2013;19:5636–5646. doi: 10.1158/1078-0432.CCR-13-0458. [DOI] [PubMed] [Google Scholar]
- 154.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT00586391.
- 155.Kershaw MH, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.US National Library of Science. ClinicalTrials.gov [online] 2016 https://clinicaltrials.gov/ct2/show/NCT02465983.
- 157.Chen JC, Chang YW, Hong CC, Yu YH, Su JL. The role of the VEGF-C/VEGFRs axis in tumor progression and therapy. Int J Mol Sci. 2012;14:88–107. doi: 10.3390/ijms14010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fukumura D, et al. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94:715–725. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 159.Chinnasamy D, et al. Gene therapy using genetically modified lymphocytes targeting VEGFR-2 inhibits the growth of vascularized syngenic tumors in mice. J Clin Invest. 2010;120:3953–3968. doi: 10.1172/JCI43490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT01218867.
- 161.Kochenderfer JN, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Di Stasi A, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang X, et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood. 2011;118:1255–1263. doi: 10.1182/blood-2011-02-337360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT01818323.