Abstract
In order to develop safe vaccines for effective mucosal immunity to major pulmonary bacterial infections, one must consider appropriate vaccine antigens (Ags), delivery systems and nontoxic molecular adjuvants. Such vaccine constructs can induce Ag-specific immune responses which provide effective protection from mucosal infections. In particular, it has been shown that adjuvant-based mucosal vaccine preparations are relatively easy to construct by simply mixing the adjuvant with the bacterial Ag, and the resulting vaccine can elicit protective immunity. We have studied DNA-based nasal adjuvants targeting mucosal dendritic cells (DCs) in order to induce Ag-specific mucosal and systemic immune responses that provide essential protection against microbial pathogens which invade our mucosal surfaces. In this review, we initially introduce a plasmid encoding the cDNA of Flt3 ligand (pFL), a molecule which is a growth factor for DCs as an effective adjuvant for mucosal immunity to pneumococcal infections. Next, we discuss the potential of adding unmethylated CpG oligodeoxynucleotide together with pFL together with a pneumococcal Ag for protection from pneumococcal infections. To do this, we have used pneumococcal surface protein A as vaccine for the restoration of mucosal immunity in aging. Further, we have also used our nasal pFL adjuvant system with phosphorylcholine-keyhole limpet hemocyanin (PC-KLH) in pneumococcal vaccine development, to successfully induce complete protection from nasal carriage by Streptococcus pneumoniae. Finally, we discuss the possibility that anti-PC antibodies induced by nasal delivery of pFL plus PC-KLH may play a protective role for prevention of atherogenesis and thus block the subsequent development of cardiovascular disease.
Keywords: Dendritic cells, DNA-based adjuvants, Nasal vaccination, Streptococcus pneumoniae
INTRODUCTION
The pneumococcus is the major pathogen of bacterial pneumonia, and gives rise to other airway and systemic infections ranging from otitis media to sepsis, bacteremia, and meningitis. In developing countries, it is estimated that 14.5 million infants suffer from severe pneumococcal diseases each year, resulting in over 800,000 deaths (1). Thus, pneumococcal infections cause major problems in both men and women of all ages worldwide. The current 7-valent polysaccharide conjugate vaccine (PCV) for children provides effective protection against invasive disease and colonization. However, the use of this vaccine has resulted in strain replacement in both colonization and disease (2, 3). The current licensed PCV and pneumococcal polysaccharide vaccine (PPV) for adults are effective and composed with capsular polysaccharides derived from 13 and 23 serotypes, respectively (PCV13; PREVNAR13® and PPV23; PNEUMOVAX NP®). However, these vaccines fail to induce immunity at mucosal surfaces where this pathogen normally resides and often invades the host. To this end, it is important to consider developing next generation of vaccines that could elicit anti-pneumococcal mucosal secretory IgA (SIgA) antibodies (Abs) in the upper respiratory tract since it has been shown that these Abs are the central player in immune defense and control the dissemination of respiratory pathogenic bacteria such as Streptococcus pneumoniae (S. pneumoniae) (4). Effective mucosal vaccines provide two layers of protective immunity in both mucosal and systemic tissue compartments. Thus, it has been shown that vaccination via the nasal route most effectively induces Ag-specific SIgA and IgG Ab responses in the upper respiratory tract (4). Since this adjuvant is one of the essential elements for the development of nasal vaccines, we have focused our studies on more effective nasal adjuvants which would enhance S. pneumoniae-specific SIgA Ab responses. In this review, we will emphasize novel DNA-based adjuvants which target mucosal DCs for the development of nasal vaccines to prevent pneumococcal infections.
THE MUCOSAL IMMUNE NETWORK
The mucosal surfaces of the respiratory, digestive, urinary and reproductive tracts of mammals remain healthy despite constantly receiving various stress signals from foods, allergens, and pathogenic microorganisms. In this regard, a functional mucosal immune system is required and it is composed of a unified network of tissues, lymphoid and mucosa-associated cells, and effector innate (e.g., mucins and defensins) and acquired (e.g., cytokine, chemokine and Ab) molecules. Among these innate and acquired molecules, polymeric IgA Abs play a central role in mucosal immune system. In order to elicit protective mucosal immune responses, one could appreciate the common mucosal immune system, which provides the essential concept of defined mucosal inductive and effector lymphoid tissues (Fig. 1) (5, 6). The inductive lymphoid tissues, including Waldeyer’s ring of tonsils and adenoids as nasopharyngeal-associated lymphoid tissue (NALT) and the Peyer’s patches as major gut-associated lymphoid tissue (GALT), consist a mucosa-associated lymphoid tissue network, which constantly provides Ag-specific and memory T and B cells to diffused-type of mucosal effector tissues like the respiratory, digestive, and other sites where IgA Abs are produced by localized plasma cells. The secretory form of IgA results following transport of polymeric (mainly dimeric) IgA coupled to secretory component across epithelial cells (7). The migration of lymphocytes from inductive to mucosal effector tissues is the cellular basis for the common mucosal immune system, a process directed by expression of adhesion molecules (e.g., integrin α4β7) or chemokine receptors (e. g., CCR9, CCR10) (8, 9). In summary, oral or nasal vaccination can elicit mucosal immunity in distant multiple effector sites (Fig. 1).
Fig. 1. Induction of Ag-specific mucosal SIgA Ab responses via the Common Mucosal Immune System.
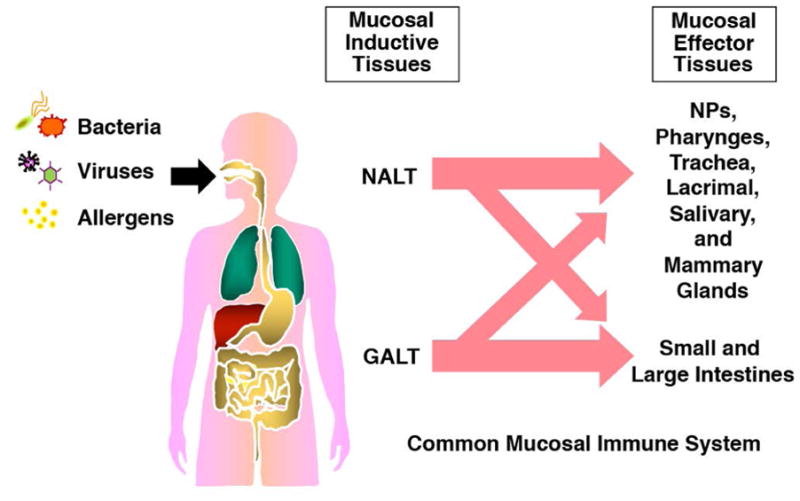
Ag derived from foreign compounds is presented by APCs beneath mucosal surfaces to naïve lymphocytes within inductive sites of organized mucosal lymphoid tissues, e.g., NALT and GALT. Activated lymphocytes leave these tissues via draining lymph nodes and reenter mucosal effector tissue sites throughout the body from the circulation, and subsequently establish mucosal immune responses.
The mucosa-associated lymphoid tissue generally consists of three well-organized regions including a subepithelium which contains Ag-presenting cells (APCs), most notably dendritic cells (DCs), a B-cell zone with germinal centers including follicular DCs, and adjacent T cell areas containing an equal distribution of naïve and memory T cell phenotypes (1, 6). Covering the mucosa-associated lymphoid tissue is a follicle-associated epithelium that includes fully-differentiated epithelial cells, termed microfold (M) cells, lymphoid cells and columnar epithelial cells within the epithelium (10). The M cells are crucial players in the initiation of mucosal immune responses by ingesting luminal Ags from the nasal and intestinal mucosa. Thus, M cells transports the intact form of Ag to the underlying APCs for initial induction of Ag-specific immune responses. Subsequently, Ag-stimulated and memory T and B cell populations egress from the mucosal inductive sites via lymphatic drainage, circulate through the bloodstream and finally migrate into mucosal effector tissues including nasal and intestinal lamina propria regions, and glandular tissues (e.g., the salivary, lacrimal and other glands). These mucosal effector sites are featured as more diffused connective tissues, which reside the Ag-specific CD4+ T helper (Th) 1 and Th2 cells as well as CD8+ cytotoxic T lymphocytes (CTLs) responsible for cell-mediated immunity (CMI) / CTL functions and IgA-committed B lymphocytes responsible for IgA Ab responses (11). Further, it has been shown that regulatory T cells and Th17 cells were identified in the lamina propria of small intestine which play central roles for protection and/or inflammation within the gut immune system (12–14). Due to this integrated mucosal immune network, appropriate nasal or oral vaccination can induce Ag-specific mucosal immunity in multiple distant effector sites (Fig. 1).
DNA-BASED ADJUVANTS WHICH TARGET MUCOSAL DCs
DCs possess the immune surveillance role and provide key functions in activation of naïve CD4+ Th cells by the uptake and processing of Ags. Thus, DCs induce naïve CD4+ T cells to differentiate into Th1- or Th2-type cells in the presence of IFN-γ and IL-12p70 or IL-4. Further, DCs are also involved in regulatory T cell induction with TGF-β, and expansion of thymus-derived Foxp3+ natural regulatory T cells in the presence of IL-2 (15, 16). Recent studies showed that a balanced IL-2 and IL-23 response by pulmonary CD103+ DCs regulate Th17 cell function in inflammatory responses (17). DC activation, maturation and expansion is regulated by an array of molecules including viral products, bacterial-derived Ags and growth factors, as well as cytokines (18, 19). For example, Flt3 ligand (FL), a growth factor binds to the fms-like tyrosine kinase receptor Flt3 / Flk2 and dramatically induces the division of DCs in vivo without inducing their activation (20, 21). It has been shown that systemic injection of FL induced marked increases in the numbers of DCs in both mucosal lymphoid tissues (i.e., the intestinal lamina propria, Peyer’s patches and mesenteric lymph nodes) and systemic (i.e., spleen) of mice (22), resulting in oral tolerance induction (23). In contrast, it has been shown that FL administration facilitates the induction of immune responses after mucosal (23), systemic (24), or cutaneous (25) delivery of vaccine Ags. Of interest, the adjuvant activity of FL protein was confirmed at both the level of Ab production and enhanced CMI responses when plasmid DNA encoding FL gene was co-administered with plasmids encoding protein Ags or linked to the Ag itself (26, 27). This would indicate that usage of costly FL protein may now be replaced by the application of plasmid FL as adjuvant.
Based upon these findings, we hypothesized that FL would be a good candidate as a new-generation mucosal adjuvant which could stimulate DCs in mucosal inductive tissues. To test this idea, we have employed the pFL as a nasal DC-targeting adjuvant to elicit Ag-specific protective mucosal immunity. Our previous studies showed that young adult mice given the weak Ag ovalbumin (OVA) plus pFL nasally induced OVA-specific mucosal SIgA and systemic IgG and IgA Ab responses (28). Of interest, nasal immunization with OVA plus pFL as a mucosal adjuvant preferentially expanded CD8+ DCs in NALT and subsequently provoked Ag-specific, Th2-type immune responses mediated by IL-4-producing CD4+ T cells (Fig. 2). The highest expression of this pFL-specific, ampicillin resistant gene was detected in NALT of mice given nasal pFL as mucosal adjuvant. In this regard, the actual FL protein product was significantly increased in nasal washes (NWs) when compared with those from mice given Ag alone or Ag plus empty plasmid (pORF) as controls. These results suggest that immune cells in NALT are the targets of pFL where initiation of FL adjuvant function most likely occurs. Although FL levels in plasma were also increased, we speculate that high levels of FL in plasma were primarily due to exudation from the nasal mucosa since the spleen as well as other lymph nodes did not express this plasmid-specific gene. Taken together, these findings show that nasally delivered pFL was mainly taken up by NALT which leads local FL protein production in these tissues, which resulted in the subsequent expansion and activation of DCs in this mucosal inductive site (28).
Fig. 2. Nasal dendritic cell-targeting adjuvant systems using pFL and/or CpG ODN for induction of protective mucosal immunity.
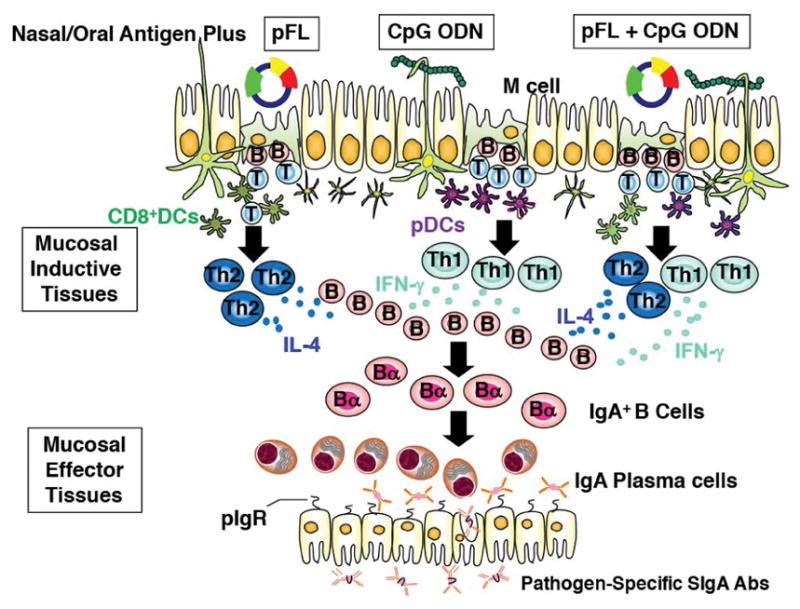
Nasal pFL as mucosal adjuvant preferentially expands the CD8+ DCs and subsequently elicits Th2-type cytokine-mediated Ag-specific Ab responses. In contrast, CpG ODN activates B220+ pDCs for the induction of Th1-type, CMI, CTLs and Ag-specific SIgA Ab responses. Thus, a combined nasal adjuvant consisting of both pFL and CpG ODN stimulates both CD8+ DCs and pDCs and successfully induces Th1- and Th2- directed Ag-specific SIgA Ab responses.
The innate immune system is essential in subsequent induction of acquired immunity. Thus, TLRs expressed by innate immune cells, including DCs, specifically recognize pathogen-associated molecular patterns (e.g., LPS, CpG DNA, and flagellin among others) for initiation of innate immunity. It has been shown that pathogen-associated molecular patterns and bacterial/viral DNA imply a significantly high frequency of unmethylated CpG motifs (29, 30). The unmethylated CpG motifs are perceived by the innate immune system via TLR9, which is mainly expressed by plasmacytoid DCs (pDCs) and B cells (Fig. 2) (31). Thus, unmethylated CpG oligodeoxynucleotide (CpG ODN) elicits professional pDCs stimulation and maturation followed by Ag-specific, CD4+ Th1 and CD8+ CTL responses (32, 33). It has been shown that protective immune responses similar to those triggered by bacterial DNA were induced by synthetic CpG ODN (34–37). Since CpG ODN is an effective adjuvant, capable of targeting malignancies and of reducing allergic responses, CpG motifs are recognized to be of central importance in TLR9-mediated innate immune responses (38, 39). Additionally, CpG ODN functions as a potent adjuvant for the induction of Ag-specific immunity (40). Indeed, CpG ODN up-regulated both OVA-specific Ab and CMI responses in mice (41). Further, toxoid and viral vaccines given with CpG ODN significantly elevated levels of Ag-specific Ab and CTL responses (42–47). Mucosal immunization of CpG ODN plus hepatitis B virus or formalin-inactivated influenza virus surface Ag successfully evoked virus-specific Ab responses in both plasma and external secretions of mice (46, 47). This CpG ODN motif is also an effective oral adjuvant for both hepatitis B Ag and tetanus toxoid (48). Further, we have previously shown that nasal immunization with recombinant protective Ag of the anthrax lethal toxin plus CpG ODN revealed high levels of Ag-specific mucosal SIgA and plasma IgG2a Ab responses (49). This CpG ODN adjuvant is quite potent and thus, can elicit a shift from a predominant Th2- to a Th1-type cytokine response (50). It has been shown that CpG ODN-induced p38 MAP kinase activity which subsequently promotes activation of NF-κB, AP-1 and CREB, which collectively contribute to the production of TNF-α, IL-10 and IL-12p70 in a murine macrophage-like cell line (RAW 264.7 cells) (51). In this regard, the postulated mechanisms for CpG ODN adjuvanticity suggest upregulation of MAP kinases associated with IL-12 production by APCs.
It is ideally important that novel mucosal vaccines elicit both CD4+ Th1- and Th2-type responses, since these responses are closely associated with Ag-specific Ab and CTL responses which provide protective immunity against bacterial and viral infections. Further, a balanced Th1- and Th2-type cytokine response would evade exaggerated Th1-cytokine mediated inflammation or hyper Th2-cytokine related allergic responses. Since CpG ODN preferentially provokes a CD4+ Th1-type, cytokine-mediated immunity (49) whereas pFL is favor to induce Th2-type cytokine responses (28), it was intriguing to assess whether a combination of pFL and CpG ODN as a mucosal DC targeting adjuvant would induce an enhanced but more balanced immune response. Indeed, our previous study shown that nasal immunization with OVA plus a combination of pFL and CpG ODN upregulated anti-OVA mucosal and systemic Ab responses similar to those elicited by a single nasal adjuvant regimen (either pFL or CpG ODN given alone) (Fig. 2) (52). Notably, pFL and CpG ODN as a combined nasal adjuvant supported prolonged (more than 25 weeks) and high levels of OVA-specific Ab responses (52). Of importance, when aged (2-year-old) mice were nasally immunized with OVA plus combined adjuvant, significantly elevated levels of OVA-specific SIgA Ab responses were noted in mucosal secretions, which were essentially equivalent to those seen in identically immunized, young adult mice (52).
PROTECTIVE IMMUNE RESPONSES TO PNEUMOCOCCAL SURFACE PROTEIN A (PspA) WHICH EMPLOY THE pFL/CpG ODN ADJUVANT
S. pneumoniae retains more than 90 capsular serotypes based upon the structure of the polysaccharide capsule which serves as a major virulence factor (53). It is known that the capsule enables S. pneumoniae to avoid entrapment by external secretions in the nasal cavity and the upper respiratory tract, and also to protect pneumococci from opsonisation and phagocytosis (54). A PPV23 (PNEUMOVAX NP®) for adults and PCV7, 10, 13 (PREVNAR 13®) for children have been developed for public use and are delivered by intramascular injection (55–57). It has been shown that the polysaccharide vaccine PNEUMOVAX NP® fails to elicit adequate T cell-dependent immune responses. Further, infants have immature B cells and thus respond poorly to this T cell-independent vaccine (58). On the other hand, PREVNAR 13® is capable of inducing both memory and active T cell immune responses, since this vaccine has diphtheria toxoid conjugated to the 13-valent polysaccharide which leads to the induction of a sufficient immune response in both infants and the elderly (59). However, these polysaccharide serotype-based vaccines provide little or no protection against initial colonization by pneumococci (60). Further, both PCV or PPV have the potential risk that pneumococcal strains of non-vaccine capsular types may replace the more common types and result in extensive carriage (57, 60). On the other hand, it has been shown that the protein-based vaccines provide better coverage for all strains and protect against colonization by all strains (61).
Pneumococci also possess various surface associated proteins that contribute to its virulence. One protein, PspA can elicit measurable protective immunity in mice when employed as a vaccine Ag (62, 63). In this regard, we have shown that nasal delivery of pFL as a mucosal adjuvant enhanced anti-PspA SIgA Ab responses in the lungs (64) as well as the nasal cavity (4) of young adult mice which inhibited S. pneumoniae colonization (Fig. 3). IgA deficient (IgA−/−) mouse model provided additional direct evidence which showing the importance of anti-PspA SIgA Abs for the prevention of bacterial colonization of the nasal cavity. revealed significantly Despite of high levels of PspA-specific IgG Ab responses, significantly high numbers of S. pneumoniae colony-forming units (CFUs) were detected in the nasal cavity of IgA−/− mice given nasal PspA plus pFL. In contrast, wild type IgA+/+ mice given nasal PspA plus pFL harbored essentially no S. pneumoniae in the nasal mucosa. Finally, a PspA-based vaccine containing pFL as nasal adjuvant effectively diminished pre-existing S. pneumoniae in the nasal mucosa (4). These findings suggest that a PspA-based nasal vaccine to induce specific SIgA Abs could play an indispensable role in the regulation of S. pneumoniae colonization in the upper respiratory tract.
Fig. 3. Comparison of protective effects against S. pneumoniae infection with a nasal pFL vaccine.
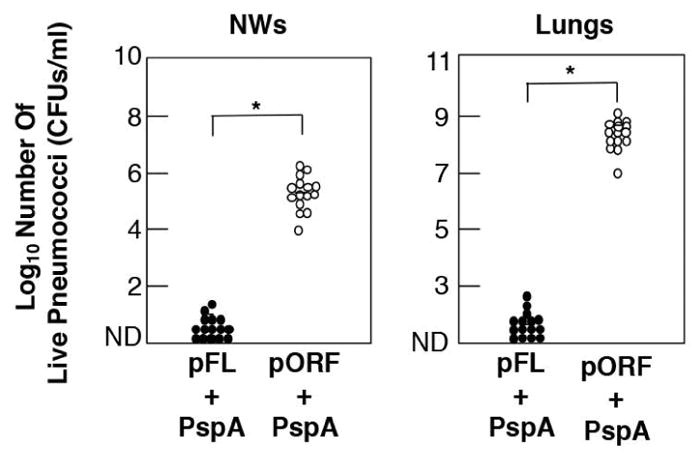
One weeks after the last immunization with PspA plus pFL (closed circle) or pORF (open circle), mice were challenged with 1.8 X 107 CFUs of live S. pneumoniae in suspension. Forty eight hr after bacterial challenge, NWs were harvested aseptically by flushing with 1 ml of sterile PBS and cultured on agar medium. The lungs were removed aseptically and homogenized in 9 ml of sterile saline per gram of lung tissue for culture. After overnight incubation at 37° C in 5 % CO2, the numbers of individual bacterial colonies were counted. The concentration of S. pneumoniae was expressed as CFUs per ml. The closed circles represent CFUs from mice given nasal PspA plus pFL, while the open circles indicate CFUs from mice nasally immunized with PspA plus pORF. Each line represents the median Log10 CFU per mouse. A 95 percent confidence interval (*) was used to determine statistical significance between the test group and the control group given PspA and pORF.
We first established that combined pFL and CpG ODN as nasal adjuvant was a potent strategy when used with the weak Ag OVA in aged mice (52). Thus, this double adjuvant system could facilitate development of practical vaccines for the elderly since the current licensed vaccines often fail to induce protective vaccine-Ag-specific immunity in this immunocompromised population. In this regard, we examined whether this double adjuvant system could successfully support PspA-specific SIgA Ab responses in the upper respiratory tract for prevention of nasal S. pneumoniae infection. Nasal vaccination with PspA plus a combination of pFL and CpG ODN induced increased levels of PspA-specific mucosal SIgA and plasma IgG Ab responses in both young adult and aged mice (Fig. 4). Of importance, although young adult mice administered nasally with PspA and pFL or CpG ODN displayed increased levels of PspA-specific Ab responses, the same single adjuvant strategies failed to elicit anti-PspA mucosal SIgA and plasma IgG Ab responses (65). When CD4+ T cell responses were assessed, significantly increased levels of PspA-induced Th1- and Th2-type cytokine responses were observed in NALT and cervical lymph nodes of aged mice given nasal PspA plus pFL and CpG ODN. Further, mucosal tissues of these aged mice contained elevated numbers of mature-type CD11b- or CD8-expressing DCs. Of importance, aged mice nasally immunized with PspA plus pFL and CpG ODN showed protection against nasal S. pneumoniae colonization. In contrast, both young adult and aged mice given nasal PspA alone failed to show sufficient protection after nasal infections with S pneumoniae. Similarly, aged mice given PspA-based vaccine containing either pFL or CpG ODN only also failed to clear bacterial colonization in NPs and NWs. These results clearly showed that nasal PspA-based vaccine containing a combined DNA adjuvant offers significant potential for protection against S. pneumoniae in the elderly.
Fig. 4. Comparison of PspA-specific IgA Ab responses in external secretions and plasma of aged mice.
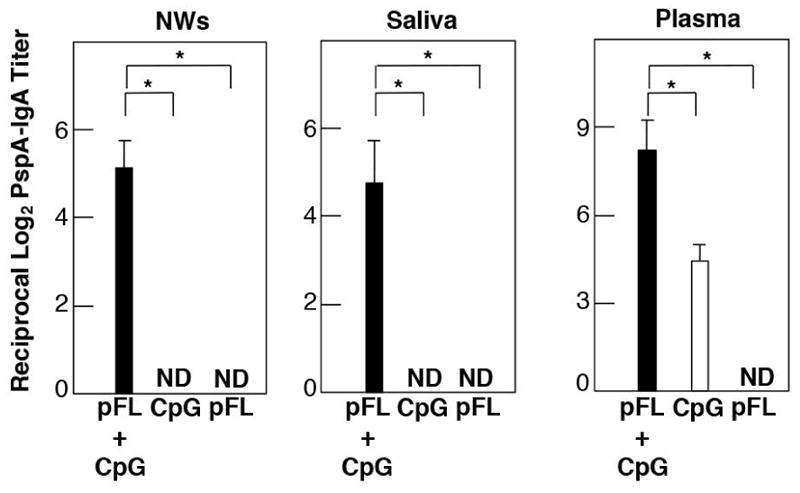
Aged mice were nasally immunized with 5 μg of PspA and 50 μg of pFL or 10 μg of CpG ODN (open column), or 5 μg of PspA and 50 μg of pFL plus 10 μg of CpG ODN (closed column) three times at weekly intervals. Seven days after the final immunization, levels of anti-PspA SIgA Abs in NWs, saliva and IgA in plasma were determined by PspA-specific ELISA. ND: optical density values were not detected. The values shown are the mean ± SEM. A 95 percent confidence interval is indicated (*).
A NOVEL S. PNEUMONIAE MUCOSAL VACCINE CONSISTING OF pFL PLUS PHOSPHORYLCHOLINE (PC) CONJUGATED WITH PROTEIN
Choline is known to be one of the nutritional requirements for a wide variety of pathogenic agents including the pneumococcus. In pneumococci, PC is biosynthesized from choline and adenosine triphosphate is incorporated into teichoic acid and lipoteicoic acid as a cell wall component (66). In this regard, PC is recognized as an immunodominant epitope of a structural cell wall component of S. pneumoniae (67, 68). Based upon these findings, we have postulated that PC conjugated with a carrier protein such as KLH (PC-KLH) could be used as a candidate Ag for the development of potential protective vaccines against pneumococcal infections. Young adult mice given nasal PC-KLH plus pFL exhibited increased levels of PC-specific IgM and IgA Abs in airway secretions and plasma (Fig. 5) (69). In addition, S. pneumoniae colonization was significantly inhibited in the nasal cavity and lungs of mice nasally immunized with pFL plus PC-KLH (Fig. 6) (69). In contrast, mice given an empty plasmid (pORF) as a control adjuvant or no adjuvant (PC-KLH alone) showed low levels of anti-PC Ab responses and failed to provide protection against S. pneumoniae colonization (69). Although it has been reported that hapten conjugated with KLH induced significant anti-hapten Ab responses without adjuvant (70), our studies show that a nasal adjuvant is required for induction of protective immunity to S. pneumoniae infection.
Fig. 5. Nasal pFL as mucosal adjuvant enhances anti-PC Ab responses in both mucosal and systemic immune compartments.
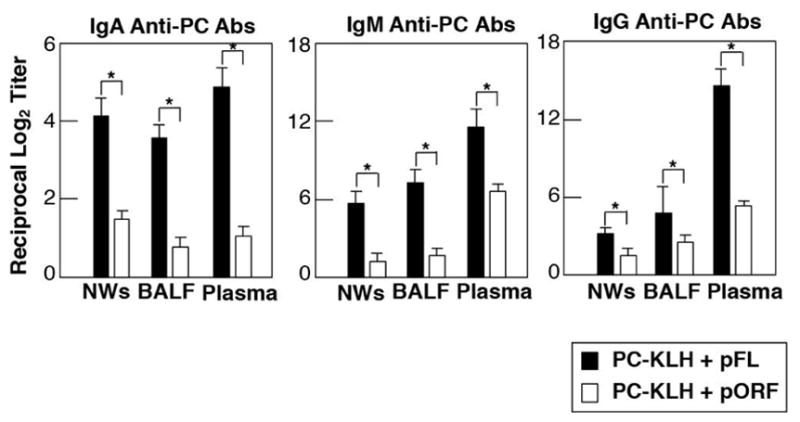
C57BL/6 mice were nasally immunized with PC-KLH plus pFL (closed column) or control pORF (open column) as mucosal adjuvant weekly for three consecutive weeks. Seven days after the final immunization, the levels of anti-PC IgA, IgM and IgG Abs in NWs, BALF and plasma were determined by PC-specific ELISA. The values are the mean ± SEM. A 95 percent confidence interval is indicated (*). BALF, denotes bronchoalveolar lavage fluids.
Fig. 6. The inhibition of S. pneumoniae colonization in the upper respiratory tract by a nasal dendritic cell-targeting adjuvant system for prevention of pneumococcal infection.
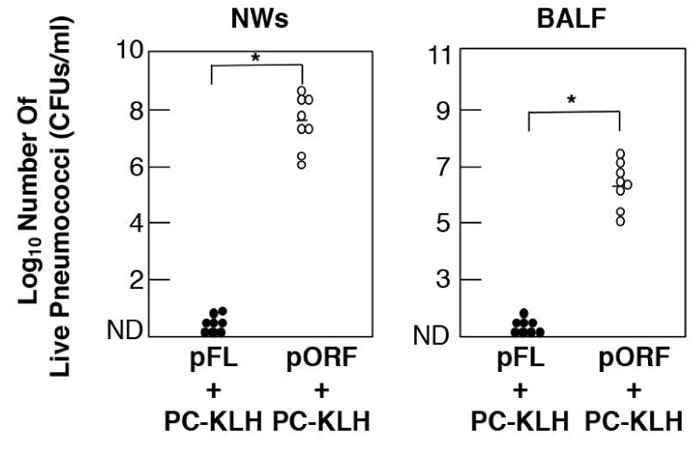
C57BL/6 mice were nasally immunized with PC-KLH plus pFL (closed column) or control pORF (open column) as mucosal adjuvant weekly for three consecutive weeks. Aliquots of 2.0 × 107 CFUs of live S. pneumoniae in suspension were nasally administered seven days after the final immunization. Twelve hr later, mice were sacrificed and BALF and NWs were obtained. The samples were diluted in sterile PBS and spread on blood agar plates. After overnight incubation at 37° C in 5 % CO2, the numbers of individual bacterial colonies were counted. The concentration of S. pneumoniae was expressed as CFUs per ml. The closed circles represent CFUs from mice given nasal PC-KLH plus pFL, while the open circles indicate CFUs from mice nasally immunized with PC-KLH plus pORF. Each line represents the median Log10 CFU per mouse. A 95 percent confidence interval is indicated (*) and was used to determine statistical significance between the test group and the control group given PC-KLH and pORF. BALF, denotes bronchoalveolar lavage fluids.
It has been shown that TEPC15 myeloma protein (T15) Ab, which is a mouse anti-PC monoclonal Ab, specifically reacts with AB1–2 monoclonal Ab (71). In this regard, this T15 idiotype Ab exhibited the most effective Ab for the prevention of pneumococcal infection (72). When the specificity of IgA and IgM Abs elicited by nasal pFL with PC-KLH for PC-binding was determined, Ab-binding to PC was significantly blocked in the presence of free hapten PC in a dose-dependent manner (69). Further, these PC-specific Abs bound to AB1–2 monoclonal Ab in the same fashion (69). Based upon these findings, mucosal and systemic PC-specific Abs induced by nasal pFL plus PC-KLH vaccination are most likely T15 idiotype Abs. T15 idiotype Abs are mainly produced by peritoneal CD5+ B (B-1 B) cells (73). Additionally, B-1 B cells have been shown to produce Abs in response to PC, LPS, phosphatidylcholine, as well as to undefined determinants expressed by both E. coli and Salmonella (74). It has been shown that B-1 B cells are an important source of IgA Abs against cell wall protein Ags of commensal bacteria occurring in the enteric mucosal tissues (75, 76). Indeed, we have shown that nasal delivery of TNP-LPS and cholera toxin as adjuvant enhanced mucosal IgA Ab production by B-1 B cells in submandibular glands and NPs (77). Since nasal PC-KLH plus pFL vaccine resulted in increased numbers of B-1 B cells in the lungs and NPs, it is possible that anti-PC IgA and IgM Abs are produced by these mucosal B-1 B cells.
Taken together, nasal vaccination with PC-KLH and pFL induced mostly T15-like PC-specific IgA and IgM Abs in the upper respiratory tract and this response was associated with reduced S. pneumoniae colonization of these tissues. We are currently assessing the amino acid sequence of PC-specific IgA and IgM Abs in the nasal washes induced by nasal delivery of PC-KLH plus pFL in order to confirm that these Abs are of the T-15 idiotype. Use of our nasal adjuvant system may facilitate the development of a more effective PC-based nasal vaccine for prevention of S. pneumoniae infection.
PC-based vaccines possess a major additional benefit since it has been indicated that PC-specific Abs are central players for protection against atherogenesis as well as the development of cardiovascular disease (78–81). Thus, upregulation of natural Abs to PC by active vaccination with PC or PC-associated bacteria as Ag would reduce atherosclerosis development by a mechanism that includes the inhibition of foam-cell formation and an anti-inflammatory effect against inflammatory reactions in atherosclerotic lesions. Further, it was reported that anti-PC IgG Abs exhibited atheroprotective effects by inhibiting platelet-activating factor-induced adhesion molecule (e.g., ICAM-1 and VCAM-1) expression on endothelial cells in vitro (81). Based upon these findings, anti-PC plasma IgG Abs induced by nasal delivery of PC-KLH plus pFL most likely play a protective role in the development of atherosclerosis in addition to preventing pneumococcal infection. We are currently investigating the levels of intra-aortic plaque accumulation by using an atherosclerosis mouse model (apolipoprotein E-deficient mice) in order to examine atheroprotective outcomes by nasal PC-KLH plus pFL vaccination. Since PC-based pneumococcal vaccine provides atheroprotective effects in addition to pneumococcal immunity, it would be of interest to whether this vaccine system could provide effective protection in the aged population which is more susceptible to infection. It has been shown that the classical mucosal adjuvants, i.e., cholera toxin as well as other nasal adjuvant including pFL and CpG ODN alone failed to induce protective mucosal immunity in aged mice (52). In this regard, it would be important to test whether a combination of pFL and CpG ODN could enhance anti-pneumococcal immunity in the elderly when employed as nasal adjuvant for PC-based vaccines.
CONCLUSIONS
S. pneumoniae is a potent bacterial pathogen which colonizes the upper respiratory tract of the host, resulting in significant morbidity and death. In order to prevent pneumococcal infections, the most ideal vaccines should prevent both colonization and growth of this pathogen at mucosal surfaces. In this regard, we have shown that nasal delivery of S. pneumoniae-derived Ags (PspA, PC-KLH) with the innate adjuvant molecules pFL and/or CpG ODN enhance Ab responses which prevent pneumococcal infection (Fig. 7). Further, we have provided evidence that a nasal PC-KLH plus pFL vaccine could have anti-atherosclerotic effects (Fig. 7).
Fig. 7. A novel type of pneumococcal vaccine with dendritic cell-targeting DNA-based nasal adjuvant.
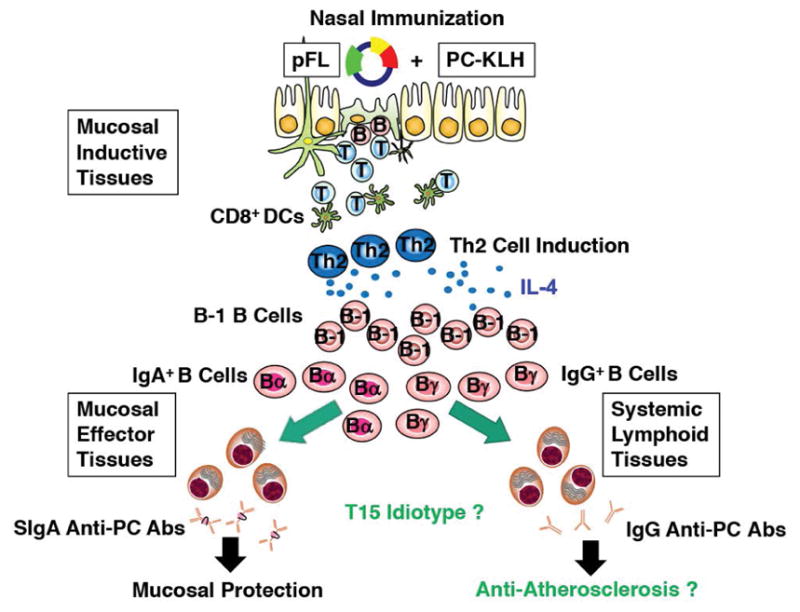
Nasal delivery of PC-KLH plus pFL resulted in inhibition of S. pneumoniae colonization in the upper and lower respiratory tracts, concurrent with the expansion of mucosal DCs and enhancement of anti-PC Ab production. In addition, this nasal vaccination increased the numbers of B-1 B cells producing the T15 idiotype in mucosal effector tissues. It should now be possible to consider use of a nasal PC-KLH plus pFL vaccine which could also exhibit anti-atherosclerotic effects.
For future PC-based vaccine development, it would be better to use other protein carriers instead of KLH, since pFL as a mucosal adjuvant also induces carrier protein (KLH)-specific Ab responses which could be a potential problem for human use, especially, as a nasal vaccine component. Indeed, it has been shown that KLH could induce Ag-non-specific, cell-mediated and humoral Ab responses (82). In this, regard, it was also reported that KLH immunization elicited mild to moderate adverse effects (82). In the future, it would be interesting to employ PspA as a carrier protein for PC in order to elicit both T cell-dependent and independent Ab responses to S. pneumoniae. Taken together, it should become possible to employ a strategy of using appropriate mucosal DC-targeting adjuvants, such as DNA-based nasal adjuvants, as a way to develop more safe and effective mucosal vaccines to bacterial and viral pathogens.
Acknowledgments
We are grateful to Dr. Jerry R. McGhee for scientific discussion, critiques in the preparation of this review as well as to our collaborators at the University of Alabama at Birmingham, Osaka University, Tokushima University and Osaka Dental University. We also thank the Japanese Society of Microbiology for providing the opportunity to write this review article. This work was supported by National Institutes of Health (NIH) grant AG025873 to K. F. as well as Grants-in-Aids C-1792179, C-19592403 and B-23390481 from the Japan Society for the promotion of Science/Ministry of Education, Culture, Sports, Science and Technology to K. K.
List of Abbreviations
- Abs
antibodies
- Ags
antigens
- APCs
antigen-presenting cells
- CFUs
colony-forming units
- CpG ODN
unmethylated CpG oligodeoxynucleotide
- CMI
cell-mediated immunity
- CTLs
cytotoxic T lymphocytes
- DCs
dendritic cells
- FL
Flt3 ligand
- GALT
gut-associated lymphoid tissue
- M cells
microfold cells
- NALT
nasopharyngeal-associated lymphoid tissue
- NPs
nasal passages
- NWs
nasal washes
- OVA
ovalbumin
- PC
phosporylcholine
- PC-KLH
PC conjugated to keyhole limpet hemocyanin
- PCV
polysaccharide conjugate vaccine
- pDCs
plasmacytoid DCs
- pFL
plasmid encoding the DNA of Flt3 ligand
- pORF
empty plasmid
- PPV
pneumococcal polysaccharide vaccine
- PspA
pneumococcal surface protein A
- SIgA
secretory IgA
- S. pneumoniae
Streptococcus pneumoniae
- Th
T helper
Footnotes
DISCLOSURE
The authors declare that they have no conflicts of interest for this article.
References
- 1.O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Levine OS, Hajjeh R, Mulholland K, Cherian T. Hib and pneumococcal global burden of disease study team. Burden of diseases caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 2.Croney CM, Coats MT, Nahm MH, Briles DE, Crain MJ. PspA family distribution, unlike capsular serotype, remains unaltered following introduction of the heptavalent pneumococcal conjugate vaccine. Clin Vaccine Immunol. 2012;19:891–896. doi: 10.1128/CVI.05671-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, Reingold A, Thomas A, Schaffner W, Craig AS, Smith PJ, Beall BW, Whitney CG, Moore MR. Active Bacterial Core Surveillance/Emerging Infections Program Network. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41. doi: 10.1086/648593. [DOI] [PubMed] [Google Scholar]
- 4.Fukuyama Y, King JD, Kataoka K, Kobayashi R, Gilbert RS, Oishi K, Hollingshead SK, Briles DE, Fujihashi K. Secretory-IgA antibodies play an important role in the immunity to Streptococcus pneumoniae. J Immunol. 2010;185:1755–1762. doi: 10.4049/jimmunol.1000831. [DOI] [PubMed] [Google Scholar]
- 5.Fujihashi K, Boyaka PN, McGhee JR. Host defenses at mucosal surfaces. In: Rich RT, Fleisher TA, Shearer WT, Schroeder HW, Frew AJ, Weyand CM, editors. Clinical Immunology. Philadelphia, PA: Elsevier; 2013. pp. 239–251. [Google Scholar]
- 6.Kiyono H, Kunisawa J, McGhee JR, Mestecky J. Fundamental Immunology. 5. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. The mucosal immune system; pp. 983–1030. [Google Scholar]
- 7.Kaetzel CS, Bruno MEC. Epithelial transport of IgA the polymeric immunoglobulin receptor. In: Kaetzel CS, editor. Mucosal Immune Defence: Immunoglobulin A. Springer; New York: 2007. pp. 43–89. [Google Scholar]
- 8.Williams IR. Chemokine receptors and leukocyte trafficking in the mucosal immune system. Immunol Res. 2004;29:283–292. doi: 10.1385/IR:29:1-3:283. [DOI] [PubMed] [Google Scholar]
- 9.Mora JR, von Andrian UH. Differentiation and homing of IgA-secreting cells. Mucosal Immunol. 2008;1:96–109. doi: 10.1038/mi.2007.14. [DOI] [PubMed] [Google Scholar]
- 10.Kunisawa J, Nochi T, Kiyono H. Immunological commonalities and distinctions between airway and digestive immunity. Trends Immunol. 2008;29:505–513. doi: 10.1016/j.it.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Kiyono H, Fukuyama S. NALT- versus Peyer’s-patch-mediated mucosal immunity. Nature Rev Immunol. 2004;4:699–710. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubin PJ, Kolls JK. Th17 cytokines and mucosal immunity. Immunol Rev. 2008;226:160–171. doi: 10.1111/j.1600-065X.2008.00703.x. [DOI] [PubMed] [Google Scholar]
- 13.Kitani A, Xu L. Regulatory Y cells and the induction of IL-17. Mucosal Immunol. 2008;1(Supple. 1):S43–S46. doi: 10.1038/mi.2008.51. [DOI] [PubMed] [Google Scholar]
- 14.Maloy KJ, Kullberg MC. IL-23 and Th17 cytokines in intestinal homeostasis. Mucosal Immunol. 2008;1:339–349. doi: 10.1038/mi.2008.28. [DOI] [PubMed] [Google Scholar]
- 15.Yamazaki S, Iyoda T, Tarbell K, Olson K, Velinzon K, Inaba K, Steinman RM. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J Exp Med. 2003;198:235–247. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamazaki S, Bonito AJ, Spisek R, Dhodapkar M, Inaba K, Steinman RM. Dendritic cells are specialized accessory cells along with TGF- for the differentiation of Foxp3+ CD4+ regulatory T cells from peripheral Foxp3 precursors. Blood. 2007;110:4293–4302. doi: 10.1182/blood-2007-05-088831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zalente T, Wong AY, Ping TJ, Chen J, Sumatoh HR, Viganò E, Hong Bing Y, Lee B, Zolezzi F, Fric J, Newell EW, Mortellaro A, Poidinger M, Puccetti P, Ricciardi-Castagnoli P. CD103(+) dendritic cells control Th17 cell function in the lung. Cell Reports. 2015;12:239–248. doi: 10.1016/j.celrep.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 18.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 19.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 20.Brasel K, McKenna HJ, Morrissey PJ, Charrier K, Morris AE, Lee CC, Williams DE, Lyman SD. Hematologic effects of flt3 ligand in vivo in mice. Blood. 1996;88:2004–2012. [PubMed] [Google Scholar]
- 21.Maraskovsky E, Brasel K, Teepe M, Roux ER, Lyman SD, Shortman K, McKenna HJ. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184:1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viney JL, Mowat AM, O’Malley JM, Williamson E, Fanger NA. Expanding dendritic cells in vivo enhances the induction of oral tolerance. J Immunol. 1998;160:5815–5825. [PubMed] [Google Scholar]
- 23.Williamson E, Westrich GM, Viney JL. Modulating dendritic cells to optimize mucosal immunization protocols. J Immunol. 1999;163:3668–3675. [PubMed] [Google Scholar]
- 24.Pisarev VM, Parajuli P, Mosley RL, Sublet J, Kelsey L, Sarin PS, Zimmerman DH, Winship MD, Talmadge JE. Flt3 ligand enhances the immunogenicity of a gag-based HIV-1 vaccine. Int J Immunopharmacol. 2000;22:865–876. doi: 10.1016/s0192-0561(00)00048-5. [DOI] [PubMed] [Google Scholar]
- 25.Baca-Estrada ME, Ewen C, Mahony D, Babiuk LA, Wilkie D, Foldvari M. The haemopoietic growth factor, Flt3L, alters the immune response induced by transcutaneous immunization. Immunology. 2002;107:69–76. doi: 10.1046/j.1365-2567.2002.01488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hung CF, Hsu KF, Cheng WF, Chai CY, He L, Ling M, Wu TC. Enhancement of DNA vaccine potency by linkage of antigen gene to a gene encoding the extracellular domain of Fms-like tyrosine kinase 3-ligand. Cancer Res. 2001;61:1080–1088. [PubMed] [Google Scholar]
- 27.Moore AC, Kong WP, Chakrabarti BK, Nabel GJ. Effects of antigen and genetic adjuvants on immune responses to human immunodeficiency virus DNA vaccines in mice. J Virol. 2002;76:243–250. doi: 10.1128/JVI.76.1.243-250.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kataoka K, McGhee JR, Kobayashi R, Fujihashi K, Shizukuishi S, Fujihashi K. Nasal Flt3 ligand cDNA elicits CD11c+ CD8+ dendritic cells for enhanced mucosal immunity. J Immunol. 2004;172:3612–3619. doi: 10.4049/jimmunol.172.6.3612. [DOI] [PubMed] [Google Scholar]
- 29.Cardon LR, Burge C, Clayton DA, Karlin S. Pervasive CpG suppression in animal mitochondrial genomes. Proc Natl Acad Sci U S A. 1994;91:3799–3803. doi: 10.1073/pnas.91.9.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Razin A, Friedman J. DNA methylation and its possible biological roles. Prog Nucleic Acid Res Mol Biol. 1981;25:33–52. doi: 10.1016/s0079-6603(08)60482-1. [DOI] [PubMed] [Google Scholar]
- 31.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wanger H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 32.Klinman DM. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat Rev Immunol. 2004;4:249–258. doi: 10.1038/nri1329. [DOI] [PubMed] [Google Scholar]
- 33.Wagner H. Bacterial CpG DNA activates immune cells to signal infectious danger. Adv Immunol. 1999;73:329–368. doi: 10.1016/s0065-2776(08)60790-7. [DOI] [PubMed] [Google Scholar]
- 34.Klinman DM, Yi AK, Beaucage SL, Conover J, Krieg AM. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci U S A. 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto S, Yamamoto T, Kataoka T, Kuramoto E, Yano O, Tokunaga T. Unique palindromic sequences in synthetic oligonucleotides are required to induce IFN and augment IFN-mediated natural killer activity. J Immunol. 1992;148:4072–4076. [PubMed] [Google Scholar]
- 37.Zimmermann S, Egeter O, Hausmann S, Lipford GB, Rocken M, Wanger H, Heeg K. CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine leishmaniasis. J Immunol. 1998;160:3627–3630. [PubMed] [Google Scholar]
- 38.Jahrsdorfer B, Weiner GJ. CpG oligodeoxynucleotides for immune stimulation in cancer immunotherapy. Curr Opin Investig Drugs. 2003;4:686–690. [PubMed] [Google Scholar]
- 39.Kline JN, Waldschmidt TJ, Businga TR, Lemish JE, Weinstock JV, Thorne PS, Kreig AM. Modulation of airway inflammation by CpG oligodeoxynucleotides in a murine model of asthma. J Immunol. 1998;160:2555–2559. [PubMed] [Google Scholar]
- 40.Klinman DM, Barnhart KM, Conover J. CpG motifs as immune adjuvants. Vaccine. 1999;17:19–25. doi: 10.1016/s0264-410x(98)00151-0. [DOI] [PubMed] [Google Scholar]
- 41.Klinman DM. Therapeutic applications of CpG-containing oligodeoxynucleotides. Antisense Nucleic Acid Drug Dev. 1998;8:181–184. doi: 10.1089/oli.1.1998.8.181. [DOI] [PubMed] [Google Scholar]
- 42.Brazolot Millan CL, Weeratna R, Krieg AM, Siegrist CA, Davis HL. CpG DNA can induce strong Th1 humoral and cell-mediated immune responses against hepatitis B surface antigen in young mice. Proc Natl Acad Sci U S A. 1998;95:15553–15558. doi: 10.1073/pnas.95.26.15553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davis HL, Weeratna R, Waldschmidt TJ, Tygrett L, Schorr J, Krieg AM. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J Immunol. 1998;160:870–876. [PubMed] [Google Scholar]
- 44.Eastcott JW, Holmberg CJ, Dewhirst FE, Esch TR, Smith DJ, Taubman MA. Oligonucleotide containing CpG motifs enhances immune response to mucosally or systemically administered tetanus toxoid. Vaccine. 2001;19:1636–1642. doi: 10.1016/s0264-410x(00)00422-9. [DOI] [PubMed] [Google Scholar]
- 45.Kovarik J, Bozzotti P, Love-Homan L, Pihlgren M, Davis HL, Lambert PH, Kreig AM, Siegrist CA. CpG oligodeoxynucleotides can circumvent the Th2 polarization of neonatal responses to vaccines but may fail to fully redirect Th2 responses established by neonatal priming. J Immunol. 1999;162:1611–1617. [PubMed] [Google Scholar]
- 46.McCluskie MJ, Davis HL. CpG DNA is a potent enhancer of systemic and mucosal immune responses against hepatitis B surface antigen with intranasal administration to mice. J Immunol. 1998;161:4463–4466. [PubMed] [Google Scholar]
- 47.Moldoveanu Z, Love-Homan L, Huang WQ, Krieg AM. CpG DNA, a novel immune enhancer for systemic and mucosal immunization with influenza virus. Vaccine. 1998;16:1216–1624. doi: 10.1016/s0264-410x(98)80122-9. [DOI] [PubMed] [Google Scholar]
- 48.McCluskie MJ, Weeratna RD, Krieg AM, Davis HL. CpG DNA is an effective oral adjuvant to protein antigens in mice. Vaccine. 2000;19:950–957. doi: 10.1016/s0264-410x(00)00215-2. [DOI] [PubMed] [Google Scholar]
- 49.Boyaka PN, Tafaro A, Fischer R, Leppla SH, Fujihashi K, McGhee JR. Effective mucosal immunity to anthrax: neutralizing antibodies and Th cell responses following nasal immunization with protective antigen. J Immunol. 2003;170:5636–5643. doi: 10.4049/jimmunol.170.11.5636. [DOI] [PubMed] [Google Scholar]
- 50.Weeratna RD, Brazolot Millan CL, McCluskie MJ, Davis HL. CpG ODN can re-direct the Th bias of established Th2 immune responses in adult and young mice. FEMS Immunol Med Microbiol. 2001;32:65–71. doi: 10.1111/j.1574-695X.2001.tb00535.x. [DOI] [PubMed] [Google Scholar]
- 51.Yi AK, Yoon JG, Yeo SJ, Hong SC, English BK, Krieg AM. Role of mitogen-activated protein kinases in CpG DNA-mediated IL-10 and IL-12 production: central role of extracellular signal-regulated kinase in the negative feedback loop of the CpG DNA-mediated Th1 response. J Immunol. 2001;168:4711–4720. doi: 10.4049/jimmunol.168.9.4711. [DOI] [PubMed] [Google Scholar]
- 52.Fukuiwa T, Sekine S, Kobayashi R, Suzuki H, Kataoka K, Gilbert RS, Kurono Y, Boyaka PN, Kreig AM, McGhee JR, Fujihashi K. A combination of Flt3 ligand cDNA and CpG ODN as nasal adjuvant elicits NALT dendritic cells for prolonged mucosal immunity. Vaccine. 2008;26:4849–4859. doi: 10.1016/j.vaccine.2008.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park IH, Pritchard DG, Cartee R, Brandao A, Nahm MH. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J Clin Microbiol. 2007;45:1225–1233. doi: 10.1128/JCM.02199-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abeyta M, Hardy GG, Yother J. Genetic alteration of capsule type but not PspA type affects accessibility of surface-bound complement and surface antigens of Streptococcus pneumoniae. Infect Immun. 2003;71:218–225. doi: 10.1128/IAI.71.1.218-225.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jackson LA, Janoff EN. Pneumococcal vaccination of elderly adults: new paradigms for protection. Clin Infect Dis. 2008;47:1328–1338. doi: 10.1086/592691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nuorti JP, Whiteney CG. Prevention of pneumococcal disease among infants and children-use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine-recommendations of the Advisory Committee on Immunization Practices (ACIP) MWR Recomm Rep. 2010;59:1–18. [PubMed] [Google Scholar]
- 57.Oosterhuis-Kafeja F, Beutels P, Van Damme P. Immunogenecity, efficacy, safety and effectiveness of pneumococcal conjugate vaccines (1998–2006) Vaccine. 2007;25:2194–2212. doi: 10.1016/j.vaccine.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 58.Spratt BG, Greenwood BM. Prevention of pneumococcal disease by vaccination: does serotype replacement matter? Lancet. 2000;356:1210–1211. doi: 10.1016/S0140-6736(00)02779-3. [DOI] [PubMed] [Google Scholar]
- 59.Namkoong H, Ishii M, Funatsu Y, Kimizuka Y, Yagi K, Asami T, Asakura T, Suzuki S, Kamo T, Fujisawa H, Tasaka S, Betsuyaku T, Hasegawa N. Theory and strategy for Pneumococcal vaccines in the elderly. Hum Vaccine Immunother. 2016;12:336–343. doi: 10.1080/21645515.2015.1075678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dagan R, Patterson S, Juergens C, Greenberg D, Givon-Lavi N, Porat N, Gurtman A, Gruber WC, Scott DA. Comparative immunogenicity and efficacy of 13-valent and 7-valent pneumococcal conjugate vaccines in reducing nasopharyngeal colonization: a randomized double-blind trial. Clin Infect Dis. 2013;57:952–962. doi: 10.1093/cid/cit428. [DOI] [PubMed] [Google Scholar]
- 61.Cao J, Li D, Gong Y, Tin N, Chen T, Wong CK, Xu W, Luo J, Zhang X, Lam CWK, Yin Y. Caseinolytic protease: a protein vaccine which could elicit serotype-independent protection against invasive pneumococcal infection. Clin Exp Immunol. 2009;156:52–60. doi: 10.1111/j.1365-2249.2008.03866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kadioglu A, Weiser JN, Paton JC, Andrew PW. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol. 2008;6:288–301. doi: 10.1038/nrmicro1871. [DOI] [PubMed] [Google Scholar]
- 63.Ginsburg AS, Nahm MH, Khambaty FM, Alderson MR. Issues and challenges in the development of pneumococcal protein vaccines. Expert Rev Vaccines. 2012;11:279–285. doi: 10.1586/erv.12.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kataoka K, Fujihashi K, Oma K, Fukuyama Y, Hollingshead SK, Sekine S, Kawabata S, Ito HO, Briles DE, Oishi K. The nasal dendritic cell-targeting Flt3 ligand as a safe adjuvant elicits effective protection against fatal pneumococcal pneumonia. Infect Immun. 2011;79:2819–2828. doi: 10.1128/IAI.01360-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fukuyama Y, King JD, Kataoka K, Kobayashi R, Gilbert RS, Hollingshead SK, Briles DE, Fujihashi K. A combination of Flt3 ligand cDNA and CpG oligodeoxynucleotide as nasal adjuvant elicits protective secretory-IgA immunity to Streptococcus pneumoniae in aged mice. J Immunol. 2011;186:2454–2461. doi: 10.4049/jimmunol.1002837. [DOI] [PubMed] [Google Scholar]
- 66.Fischer W, Behr T, Hartmann R, Peter-Katalini J, Egge H. Teichoic acid and lipoteichoic acid of Streptococcus pneumoniae possess identical chain structures. A reinvestigation of teichoic acid (C polysaccharide) Eur J Biochem. 1993;215:851–857. doi: 10.1111/j.1432-1033.1993.tb18102.x. [DOI] [PubMed] [Google Scholar]
- 67.Briles DE, Nahm M, Shroer K, Davie J, Baker P, Kearney J, Barletta R. Antiphosphorylcholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 Streptococcus pneumoniae. J Exp Med. 1981;153:694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harnett W, Harnett MM. Phosphorylcholine: friend or foe of the immune system ? Immunol Today. 1999;20:125–129. doi: 10.1016/s0167-5699(98)01419-4. [DOI] [PubMed] [Google Scholar]
- 69.Baatarjav T, Kataoka K, Gilbert RS, Terao Y, Fukui M, Goto M, Kawabata S, Yamamoto M, Fujihashi K, Ito HO. Mucosal immune features to phosphorylcoline by nasal Flt3 ligand cDNA-based vaccination. Vaccine. 2011;29:5747–5757. doi: 10.1016/j.vaccine.2011.05.097. [DOI] [PubMed] [Google Scholar]
- 70.Peng Y, Zhang Y, Mitchell WJ, Zhang G. Development of a lipopolysaccharide-targeted peptide mimic vaccine against Q fever. J Immunol. 2012;189:4909–4920. doi: 10.4049/jimmunol.1201622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cerny J, Wallich R, Hammerling GJ. Analysis of T15 idiotopes by monoclonal antibodies: variability of idiotopic expression on phosphorylcholine-specific lymphocytes from individual inbred mice. J Immunol. 1982;128:1885–1891. [PubMed] [Google Scholar]
- 72.Briles DE, Forman C, Hudak S, Claflin JL. Anti-phosphorylcholine antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae. J Exp Med. 1982;156:1177–1185. doi: 10.1084/jem.156.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Masmoudi H, Mota-Santos T, Huetz F, Coutinho A, Cazenave PA. All T15 Id-positive antibodies (but not the majority of VHT15+ antibodies) are produced by peritoneal CD5+ B lymphocytes. Int Immunol. 1990;2:515–520. doi: 10.1093/intimm/2.6.515. [DOI] [PubMed] [Google Scholar]
- 74.Kantor AB, Herzenberg LA. Origin of murine B cell lineages. Annu Rev Immunol. 1993;11:501–538. doi: 10.1146/annurev.iy.11.040193.002441. [DOI] [PubMed] [Google Scholar]
- 75.Fagarasan S, Honjo T. T-independent immune response: new aspects of B cell biology. Science. 2000;290:89–92. doi: 10.1126/science.290.5489.89. [DOI] [PubMed] [Google Scholar]
- 76.Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000;288:2222–2226. doi: 10.1126/science.288.5474.2222. [DOI] [PubMed] [Google Scholar]
- 77.Kataoka K, Fujihashi K, Sekine S, Fukuiwa T, Kobayashi R, Suzuki H, Nagata H, Takatsu K, Shizukuishi S, McGhee JR, Fujihashi K. Nasal cholera toxin elicits IL-5 and IL-5 receptor alpha-chain expressing B-1a B cells for innate mucosal IgA antibody responses. J Immunol. 2007;178:6058–6065. doi: 10.4049/jimmunol.178.10.6058. [DOI] [PubMed] [Google Scholar]
- 78.Binder CJ, Horkko S, Dewan A, Chang MK, Kieu EP, Goodyear CS, Shaw PX, Palinski W, Witztum JL, Silverman GJ. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nature Med. 2003;9:736–743. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- 79.Caligiuri G, Khallou-Laschet J, Vandaele M, Gaston AT, Delignat S, Mander C, Kohler HV, Kaveri SV, Nicoletti A. Phosphorylcholine-targeting immunization reduces atherosclerosis. J Am Coll Cardiol. 2007;50:540–546. doi: 10.1016/j.jacc.2006.11.054. [DOI] [PubMed] [Google Scholar]
- 80.de Faire U, Frostegard J. Natural antibodies against phosphorylcholine in cardiovascular disease. Ann N Y Acad Sci. 2009;1173:292–300. doi: 10.1111/j.1749-6632.2009.04748.x. [DOI] [PubMed] [Google Scholar]
- 81.Su J, Hua X, Concha H, Svenungsson E, Cederholm A, Frostegard J. Natural antibodies against phosphorylcholine as potential protective factors in SLE. Rheumatology. 2008;47:1144–1150. doi: 10.1093/rheumatology/ken120. [DOI] [PubMed] [Google Scholar]
- 82.Swaminathan A, Lucas RM, Dear K, McMichael AJ. Keyhole limpet haemocyanin- a model antigen for human immunotoxicological studies. British J Clin Pharmacol. 2014;78:1135–1142. doi: 10.1111/bcp.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]


