Abstract
Background
Contemporary descriptions of classical Hodgkin lymphoma (cHL) are lacking from sub-Saharan Africa where human immunodeficiency virus (HIV) and Epstein–Barr virus (EBV) are prevalent.
Methods
We describe a prospective cHL cohort in Malawi enrolled from 2013 to 2015. Patients received standardized treatment and evaluation, including HIV status and EBV testing of tumors and plasma.
Results
Among 31 patients with confirmed cHL, the median age was 19 years (range, 2–51 years) and 22 (71%) were male. Sixteen patients (52%) had stage III/IV, 25 (81%) B symptoms, and 16 (52%) performance status impairment. Twenty-three patients (74%) had symptoms >6 months, and 11 of 29 (38%) had received empiric antituberculosis treatment. Anemia was common with median hemoglobin 8.2 g/dL (range, 3.1–17.1 g/dL), which improved during treatment. No children and 5 of 15 adults (33%)were HIV+. All HIV+ patients were on antiretroviral therapy for a median 15 months (range, 2–137 months), with median CD4 count 138 cells/μL (range, 23–329 cells/μL) and four (80%) having undetectable HIV. EBV was present in 18 of 24 (75%) tumor specimens, including 14 of 20 (70%) HIV− and 4 of 4 (100%) HIV+. Baseline plasma EBV DNA was detected in 25 of 28 (89%) patients, with median viral load 4.7 (range, 2.0–6.7) log10copies/mL, and subsequently declined in most patients. At 12 months, overall survival was 75% (95% confidence interval [CI], 55%–88%) and progression-free survival 65% (95% CI, 42%–81%). Baseline plasma EBV DNA and persistent viremia during treatment were associated with poorer outcomes.
Conclusion
cHL in Malawi is characterized by delayed diagnosis and advanced disease. Most cases were EBV associated and one-third of adults were HIV+. Despite resource limitations, 12-month outcomes were good.
Keywords: Epstein–Barr virus, Hodgkin lymphoma, Malawi, pediatric cancer, sub-Saharan Africa
1 | INTRODUCTION
In sub-Saharan Africa (SSA), classical Hodgkin lymphoma (cHL) is a common malignancy among children and young adults, accounting for 4%–9% of pediatric cancers.1–3 Given limited pathology infrastructure, cHL is also likely underreported since gradually progressive lymphadenopathy may be empirically treated as tuberculosis, leading to delayed or missed cHL diagnosis.3–5 In resource-rich countries, 75% of newly diagnosed patients present with early stage I/II cHL,6,7 while in resource-limited countries, more than half of patients present with advanced stage III/IV disease.2 In resource-rich settings, cHL is highly curable with greater than 90% long-term survival for stage I/II and 80% for stage III/IV.8 Although data are scarce, reported survival rates for cHL in SSA are lower ranging from 60% to 70%.3,9
In resource-rich settings, cHL is one of the commonest non-AIDS defining cancers among individuals infected with human immunodeficiency virus (HIV).10 Despite reductions in other HIV-associated lymphoma subtypes, the incidence of cHL appears stable or increasing in the current antiretroviral therapy (ART) era.9,11–17 Moreover, the risk of HIV-associated cHL may be increased in the first months after ART initiation due to immune reconstitution.12,17,18 Studies in the United States have shown that cHL incidence is 5–20 times higher among HIV+ patients, with 3%–4% of newly diagnosed cHL cases occurring in HIV-infected individuals.11–13,19 HIV-infected individuals with cHL are more likely to present with advanced stage, B symptoms, and extranodal involvement.10,11,19 However, when treated appropriately, cHL patients with HIV can receive similar regimens and experience comparable outcomes to those without HIV.12,20,21
cHL is also often associated with Epstein–Barr virus (EBV),22 an oncogenic herpesvirus that infects more than 80% of children in SSA typically before 5 years of age.23 The frequency of EBV-positive cHL differs geographically, being more common in resource-limited regions of the world including SSA where 70%–90% of cHL cases are EBV-positive. 3,22,24 EBV-positive cHL is also more common in children, males, and HIV-infected individuals.10,12,22,24
Although cHL is a common cancer frequently associated with HIV and EBV, both of which are highly prevalent in SSA, to our knowledge there are no prospective clinical studies of cHL from SSA from the contemporary era of widespread ART availability. Herein we describe clinical characteristics, treatment course, HIV and EBV associations, and survival for a longitudinal cHL cohort at Kamuzu Central Hospital (KCH), a national teaching hospital in the Malawian capital, Lilongwe. It serves approximately 8–9 million people in a country with an HIV prevalence of 10%, ART coverage of 67%,25 annual gross domestic product per capita of 314 US dollars,26 and Human Development Index rank of 173 of 188 countries.27
2 | METHODS
The KCH Lymphoma Study is a prospective cohort initiated in June 2013. Patients with pathologically confirmed lymphoproliferative disorders are eligible to participate after informed consent. We focused on patients with newly diagnosed cHL between June 1, 2013, and December 31, 2015. The study was conducted in accordance with the Helsinki Declaration, after approval by the University of North Carolina Institutional Review Board and Malawi National Health Sciences Research and Ethics Committee.
All cases were diagnosed through weekly telepathology consultation involving two to four pathologists in Malawi and the United States who rendered a consensus opinion after reviewing hematoxylin and eosin stained tissue sections.28,29 Immunohistochemistry (IHC) was performed locally at the interpreting pathologists’ discretion, including CD3, CD20, CD30, CD45, CD138, Ki-67, and terminal deoxynucleotidyl transferase. In rare pediatric cases, a cytologic diagnosis of cHL was made when clinical findings were consistent and binucleated Reed–Sternberg cells were clearly identified, only when tissue biopsy was not possible due to anatomic location and limited pediatric surgery capacity. All specimens were then shipped to the United States for secondary hematopathologist review, where diagnoses were confirmed by a larger panel of automated IHC stains, including CD3, CD15, CD20, CD30, and PAX5. For cHL subtyping, two US hematopathologists (Y.F. and N.D.M.) assigned a consensus subtype. EBV was assessed in tumors by EBV-encoded RNA (EBER) in situ hybridization (Leica Biosystems, Wetzlar, Germany) and in plasma using a proprietary real-time quantitative polymerase chain reaction (qPCR) assay performed at the University of North Carolina Vironomics Core with a linear detection range of 2.0–8.0 log10copies/mL.30 Plasma EBV qPCR was assessed at baseline, mid-treatment (cycle 3, day 21), treatment completion, and whenever possible at clinical relapse.
At cHL diagnosis, a comprehensive baseline evaluation was performed including chest radiography, abdominal ultrasound, performance status, and Ann Arbor stage. Unilateral bone marrow examination was performed routinely in adults but not children due to lack of pediatric sedation capabilities. Response was assessed using standardized criteria incorporating physical examination, chest radiography, and abdominal ultrasound. There are no formal cancer treatment guidelines or radiotherapy in Malawi. However, efforts to standardize care have been ongoing. Adult patients received ABVD (doxorubicin 25 mg/m2, bleomycin 10,000 IU/m2, vinblastine 6 mg/m2, dacarbazine 375 mg/m2 on days 1 and 15) every 4 weeks for a total of six cycles. Adults could receive up to eight cycles if they achieved a partial response after six cycles without severe adverse events related to therapy. Children received modified ABVE-PC without etoposide (doxorubicin 40 mg/m2, vincristine 2 mg/m2, bleomycin 10 units/m2, cyclophosphamide 800 mg/m2 on day 1, prednisolone 1.5 mg/kg/day on days 1–5) every 3 weeks for a total of eight cycles. Patients were not routinely screened at baseline for malaria or parasitic infections unless clinically indicated.
Laboratory studies were performed before each chemotherapy dose. If blood counts prohibited chemotherapy, they were repeated 1 week later and treatment proceeded if blood counts allowed, with standardized algorithms for cytotoxic dose adjustment. Hematopoietic growth factors were not available, and supportive care was standardized. Antiemetics included ondansetron, promethazine, and/or dexamethasone. Transfusions were provided for hemoglobin less than 7 g/dL and platelets less than 20×103/μL. Febrile neutropenia was treated empirically with a third-generation cephalosporin until a source was identified. Nutritional supplementation for malnourished children included ready-to-use therapeutic feeding packets three times per day, and high-protein diet counseling for adults. HIV-infected patients received ART concurrently with chemotherapy, typically with tenofovir–lamivudine–efavirenz regimen as per Malawi guidelines.
Interval complications were recorded prior to each cycle. Toxicities were graded using National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.31 Transportation reimbursement was provided to promote retention throughout care. Medicines were freely provided by the Ministry of Health, supplemented by external funding for back-up chemotherapy to mitigate treatment interruptions.
Baseline characteristics were summarized using descriptive statistics. Follow-up time was calculated from enrollment until progression or death, loss to follow-up, or administrative censoring on May 15, 2016. Overall survival and progression-free survival were estimated using Kaplan–Meier methods, and the log-rank test was used to assess survival differences between groups. Cox proportional hazards were used to estimate unadjusted hazard ratios for overall survival and progression-free survival. The cause of death was adjudicated by consensus review involving two study investigators (K.D.W. and S.G.). All analyses were performed using STATA SE version 12.1 (College Station, TX).
3 | RESULTS
3.1 | Baseline characteristics
Between June 1, 2013, and December 31, 2015, 31 patients (11%) with cHL were enrolled of 274 total patients with pathologically confirmed lymphoproliferative disorders in the cohort overall. During the study period, we were aware of three cases, all children, diagnosed with Hodgkin lymphoma who were not enrolled in the study for the following reasons: one child died before the pathologic diagnosis was confirmed, one child’s family elected not be participate, and one child was diagnosed during a brief enrollment pause resulting from minor administrative delay in obtaining annual Malawi regulatory renewal for the study.
Baseline characteristics for patients with cHL are shown in Table 1. The median age was 19 years (range, 2–51 years) and 22 (71%) were male. A total of 16 patients (52%) had stage III/IV disease, 25 (81%) B symptoms, and 16 (52%) significant performance status impairment. Of 26 patients with primary sites that could be accurately measured, 11 (42%) had bulky disease.32,33 Twenty-three of 31 patients (74%) had symptom durations greater than 6 months at cHL diagnosis, and 11 of 29 (38%) had received empiric antituberculosis treatment for lymphadenopathy. Eleven of 31 patients (35%) were significantly underweight. Anemia was common with median hemoglobin 8.2 g/dL (range, 3.1–17.1 g/dL) and bone marrow involvement in 6 of 12 (50%) adults assessed. Of three adults who did not undergo bone marrow evaluation, two declined and one was felt to be too sick to tolerate the procedure safely in clinic. Elevated lactate dehydrogenase (LDH) was also common with a median baseline LDH of 359 IU/L (range, 161–894 IU/L, laboratory upper limit of normal 250 IU/L). No children and five (33%) adults were HIV-infected, all of whom were on ART at cHL diagnosis for a median 15 months (range, 2–137 months), with a median CD4 count of 138 cells/μL (range, 23–329 cells/μL) and four of five patients (80%) having undetectable HIV RNA.
TABLE 1.
Baseline characteristics of classical Hodgkin lymphoma patients in Malawi, June 2013–December 2015
| Total, n | Entire cohort 31 |
HIV positive 5 |
|---|---|---|
| Demographics | ||
| Male, n (%) | 22 (71) | 4 (80) |
| Pediatrics, <18 years, n (%) | 16 (51) | 0 |
| Age, years, median (range) | 19 (2–51) | 33 (30–51) |
| Diagnostic specimen, n (%) | ||
| Cytology | 3 (10) | 0 |
| Histology | 28 (90) | 5 (100) |
| Clinical stage, n (%) | ||
| Stage I/II | 15 (48) | 0 |
| Stage III/IV | 16 (52) | 5 (100) |
| Clinical presentation | ||
| Underweighta, n (%) | 11 (35) | 1 (20) |
| B symptoms, n (%) | 25 (81) | 4 (80) |
| Symptoms >6 months, n (%) | 23 (74) | 4 (80) |
| Cervical lymph node involvement, n (%) | 22 (71) | 3 (60) |
| Bulky diseaseb, n (%) | 11/26 (42) | 0 |
| Received prior empiric anti-tuberculosis treatment for lymphadenopathyc, n (%) | 11/29 (38) | 3 (60) |
| Impaired performance statusd, n (%) | 16 (52) | 2 (40) |
| Laboratory data | ||
| White blood cells, 103/μL, median (range) | 7.6 (1.5–46.0) | 4.1 (1.5–7.8) |
| Absolute neutrophil count, 103/μL, median (range) | 4.2 (0.5–35.0) | 2.4 (0.8–5.6) |
| Hemoglobin, g/dL, median (range) | 8.2 (3.1–17.1) | 9.8 (5.7–10.7) |
| Platelets, 103/μL, median (range) | 351 (27–839) | 381 (182–764) |
| Lactate dehydrogenasee, IU/L, median (range) | 359 (161–894) | 359 (161–492) |
| Albumin, g/dL, median (range) | 2.7 (1.7–5.2) | 3.4 (2.0–3.7) |
| HIV specific | ||
| On ART at cHL diagnosis, n (%) | – | 5 (100) |
| Duration of ART prior to diagnosis, months, median (range) | – | 15 (2–137) |
| CD4 count, cells/μL, median (range) | – | 138 (23–329) |
| HIV RNA <400 copies/mL, n (%) | – | 4 (80) |
ART = antiretroviral therapy.
Underweight defined as weight-for-age z-score < −2 if <5 years, bodymass index (BMI) z-score < −2 if ≥5 years, or BMI < 16.5 kg/m2 for adults.
26 of 31 total cases had primary sites that could be accurately measured, with bulky disease defined as >10 cm in adults and >6 cm in children.
29 of 31 total cases had known tuberculosis treatment history.
Impaired performance status defined as Eastern Cooperative Oncology Group (ECOG) performance status ≥2 in adults or Lansky performance status <70 in children.
Laboratory upper limit of normal is 250 IU/L.
Table 2 depicts histologic subtypes and tumor EBV status. Of 24 cases further classified according to cHL subtype, 8 were mixed cellularity, 7 lymphocyte depleted, 6 nodular sclerosing, and 3 lymphocyte rich. Of these, 18 (75%) were EBER-positive by in situ hybridization, including 14 of 20 (70%) HIV-negative patients and 4 of 4 (100%) HIV-positive patients.
TABLE 2.
Subtypes and tumor Epstein–Barr virus association for classical Hodgkin lymphoma in Malawi
| N | EBER+ | Mixed cellularity | Lymphocyte depleted | Nodular sclerosing | Lymphocyte rich | |
|---|---|---|---|---|---|---|
| All | 24 | 18 (75%) | 8 | 7 | 6 | 3 |
| HIV+ adults | 4 | 4 (100%) | 1 | 0 | 2 | 1 |
| HIV− adults | 10 | 7 (70%) | 2 | 5 | 2 | 1 |
| Children | 10 | 7 (70%) | 5 | 2 | 2 | 1 |
EBER = Epstein–Barr virus encoded RNA in situ hybridization.
3.2 | Treatment course and toxicities
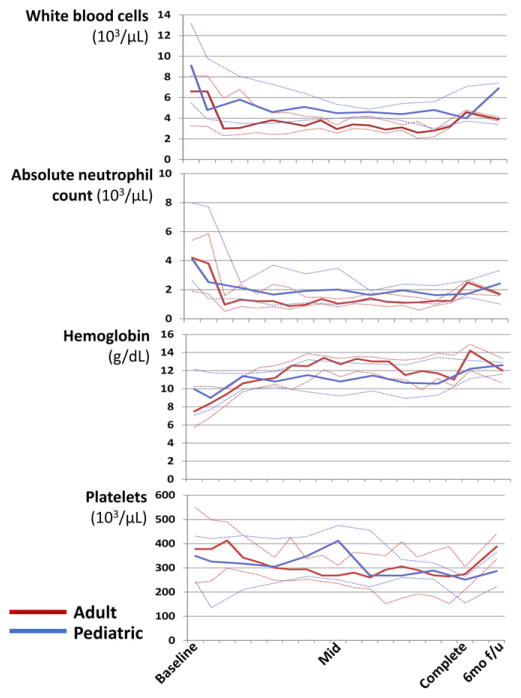
As of May 15, 2016, the median number of cycles completed for all 31 patients was 7 (range, 1–8). Twenty-one (68%) patients completed all prescribed chemotherapy, nine (29%) died before treatment completion, and one (3%) was still completing front-line treatment. Figure 1 depicts peripheral blood counts during treatment. Median ANC generally remained above 1.0×103/μL throughout treatment for both children and adults, with subsequent recovery after chemotherapy completion. In both children and adults, anemia at baseline improved over the course of treatment.
FIGURE 1.
Median peripheral blood counts with interquartile ranges during chemotherapy for classical Hodgkin lymphoma in Malawi. Mid= mid-treatment; Complete=Treatment completion; 6mo f/u=6-month follow-up
Of 81 grade 3/4 neutropenia events, 56 (69%) were grade 3 and 25 (31%) grade 4. Eleven of 15 (73%) adults and nine of 16 (56%) children experienced at least one grade 3/4 neutropenia event during treatment. Fifteen grade 3/4 anemia events occurred, with four adults and three children experiencing at least one grade 3/4 anemia event during treatment. In the entire cohort, there was only one grade 3 thrombocytopenia event, and no grade 3/4 non-hematologic toxicity recorded. Of 16 patients with at least one chemotherapy cycle delayed by more than 7 days, all delays were due to neutropenia.
Of three HIV-infected patients who had follow-up CD4 and HIV RNA measurements 6 months after enrollment, after they had completed chemotherapy, median CD4 count increased to 483 cells/μL (range, 349–517 cells/μL) from a median of 138 cells/μL (range, 102– 298 cells/μL) at baseline, with all three patients having undetectable HIV RNA at baseline and 6-month follow-up. One HIV-infected patient was still completing first-line chemotherapy as of May 15, 2016, and one patient was known to be well at home through regular telephone contact, but had not returned to clinic for repeat blood draws.
3.3 | Outcomes and associations with plasma EBV DNA
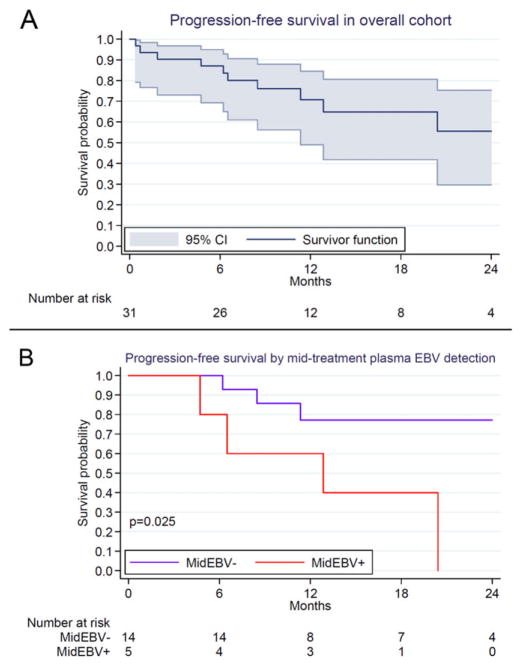
As of May 15, 2016, disease status and vital status were known for all patients after a median follow-up of 12.3 months (range, 5.9–26.8 months) among 22 patients still alive. Overall survival estimates with 95% confidence intervals (CIs) are shown in Table 3. Overall survival was 75% (95% CI 55%–88%) 12 months after enrollment, and 61% (95% CI 35%–79%) 24 months after enrollment, without significant differences between adults and children. Progression-free survival was 71% (95% CI 42%–81%) 12 months after enrollment, and 56% (95% CI 14%–68%) 24 months after enrollment. All five HIV-infected patients were alive as of the censoring date after a median follow-up of 14.5 months (range, 6.0–26.0 months). Of the patients still alive, 18 were in complete remission, 2 in partial remission, and 2 had progressed or relapsed cHL. Central adjudication of nine deaths during the study period was undertaken to attribute cause of death (Supplemental Table S1). Six deaths were attributed to progressive cHL and three to treatment-related complications.
TABLE 3.
Kaplan–Meier overall survival and progression-free survival for classical Hodgkin lymphoma patients in Malawi, stratified by age and stage
| n | 12-month | 12-month | 24-month | 24-month | |||||
|---|---|---|---|---|---|---|---|---|---|
| OS % | 95% CI | PFS % | 95% CI | OS % | 95% CI | PFS % | 95% CI | ||
| Overall | 31 | 75 | 55–88 | 71 | 49–85 | 61 | 36–79 | 56 | 30–75 |
|
| |||||||||
| Pediatric | 16 | 74 | 45–89 | 74 | 45–89 | 59 | 24–83 | 56 | 18–82 |
|
| |||||||||
| Adult | 15 | 78 | 46–92 | 69 | 36–87 | 62 | 24–85 | 57 | 24–81 |
|
| |||||||||
| Stage I/II | 15 | 72 | 42–89 | 72 | 42–89 | 72 | 42–89 | 72 | 42–89 |
|
| |||||||||
| Stage III/IV | 16 | 78 | 46–92 | 70 | 36–88 | 54 | 19–79 | 45 | 13–73 |
CI = confidence interval; OS = overall survival; PFS = progression-free survival.
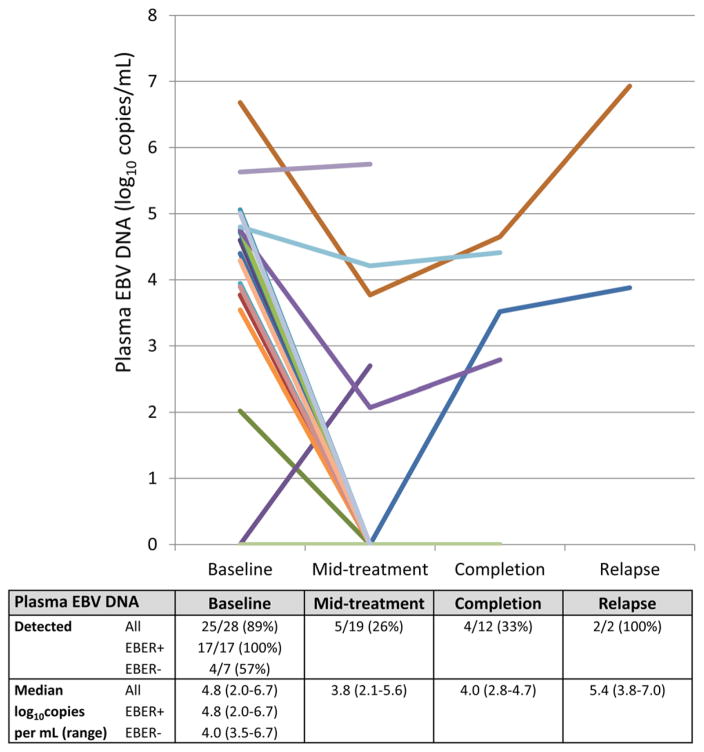
Of 28 patients with baseline plasma tested for EBV DNA, 25 (89%) had detectable viremia with a median viral load of 4.7 log10copies/mL (range, 2.0–6.7 log10copies/mL), which resolved in most patients by the mid-point of chemotherapy treatment (Fig. 2). While very high plasma EBV DNA was clearly demonstrated in two patients at the time of clinical relapse as shown, a total of six patients including those two cases had increasing plasma EBVDNA at either the chemotherapy mid-point or chemotherapy completion. Of these, five experienced relapse or death during follow-up, with only one patient alive and in remission four months after enrollment. In addition, for patients in whom both baseline plasma EBV DNA and tumor EBER were assessed, results were strongly correlated, with plasma EBV DNA being detected in 17 of 17 EBER-positive patients versus 4 of 7 EBER-negative patients (P = 0.017).
FIGURE 2.
Quantitative Epstein–Barr virus plasma DNA during classical Hodgkin lymphoma treatment in Malawi. EBV = Epstein–Barr virus; EBER = Epstein–Barr virus encoded RNA in situ hybridization
Our small cohort did not allow robust multivariate assessment of risk factors associated with outcomes. However, in exploratory unadjusted analyses, only increased LDH was associated with increased risk of progression or death in the cohort overall (unadjusted hazard ratio [HR] 1.37 per 100 IU/L increase, 95% CI 1.02–1.84, P = 0.038), while baseline plasma EBV DNA was not (unadjusted HR 1.09, 95% CI 0.73–1.63, P = 0.66). Similarly, stage and performance status were not significantly associated with outcomes. However, when restricted only to patients with detectable baseline plasma EBV DNA, both LDH (unadjusted HR 1.47, 95% CI 1.06–2.04, P = 0.022) and plasma EBV DNA (unadjusted HR 2.53, 95% CI 1.17–5.45, P = 0.018) were associated with increased risk of progression or death. In addition, among patients with plasma EBV DNA measurement at the mid-point of chemotherapy, there was a significant association between detectable virus at this time point and worse progression-free survival subsequently (Fig. 3).
FIGURE 3.
Kaplan–Meier progression-free survival for classical Hodgkin lymphoma patients in Malawi. (A) Overall cohort with 95% confidence intervals. (B) Stratified by mid-treatment plasma Epstein– Barr virus DNA detection. EBV = Epstein–Barr virus
4 | DISCUSSION
To our knowledge, this is the first prospective clinical description of cHL from SSA reflecting the current ART era for HIV. Our cohort was characterized by advanced stage, poor performance status, long symptom durations prior to cHL diagnosis, frequent empiric treatment for tuberculosis lymphadenitis, and significant anemia. However, both children and adults had reasonably good overall and progression-free survival with standardized treatment in a severely resource-limited environment, irrespective of HIV status.
Marked anemia was a notable feature of our cohort, much more so than our previous Malawi reports of adult and pediatric non-Hodgkin lymphoma.34,35 Although comprehensive evaluation to identify various causes of anemia was not routinely done unless clinically indicated, hemoglobin improved during treatment, and half of adults had demonstrated bone marrow involvement. Anemia is also commonly reported in cHL in resource-rich countries, typically in advanced disease even without bone marrow involvement, and is much less severe in published descriptions than we observed in our Malawi cHL population.36 This suggests that anemia should not be viewed as a major barrier to pursuing curative-intent treatment for cHL in SSA with appropriate cumulative dose and dose intensity, despite difficulties with reliable transfusion support in the region.
Among adults with cHL, one-third were HIV-infected. All HIV-infected adults were on ART at cHL diagnosis for a median of more than a year, and most had undetectable HIV RNA. cHL may therefore emerge as a more frequent lymphoma subtype in Malawi among HIV-infected individuals, as earlier and more widespread application of ART continues, just as in resource-rich countries.11 In high-income countries, HIV is present in 3%–4% of cHL cases with higher proportions among lymphocyte-depleted and mixed cellularity subtypes,13 and it remains unclear whether cHL incidence has changed significantly among HIV-infected individuals in the ART era.14–17 In cross-sectional studies from SSA, HIV prevalence has typically been reported as 20%–50% among adults with cHL,37–39 and <10% among children,40,41 with cHL incidence appearing to be stable thus far despite ART scale-up.42
We also found strong associations between cHL and EBV as expected, with 75% of cases being EBER-positive and 89% having elevated baseline plasma EBV viral load, similar to other reports from SSA.3,22,24 In resource-rich settings, quantitative plasma EBV DNA has been demonstrated to have potential utility in cHL for diagnosis, prognosis, and monitoring.43–46 Although our small cohort allowed only limited assessment of plasma EBV DNA for cHL prognosis and response assessment, to our knowledge, this is the first study from SSA to specifically examine serial EBV viral loads during cHL treatment. We observed markedly elevated levels at baseline, which declined in the majority of patients during chemotherapy. We also found significant associations between plasma EBVDNA levels at baseline and persistence of plasma EBV DNA at the mid-point of chemotherapy with subsequent progression-free survival. These results deserve further investigation in larger cohorts from the region. If confirmed, EBV viral load could represent an important, clinically implementable biomarker in SSA, a region where capacity for plasma HIV RNA measurement is already well established.47 Existing HIV RNA instruments in many settings could likely be readily adapted to also measure plasma EBV DNA. In addition, EBV DNA measurement in plasma has greater utility as a clinical biomarker than measurement in peripheral blood mononuclear cells for EBV-associated lymphoproliferative disorders, an approach that is far more implementable at the point of care in low-resource settings.48
Despite adverse baseline characteristics and a severely resource-constrained environment, overall survival was 75% at 12 months and 61% at 24 months for the cohort, with generally good outcomes for children and adults irrespective of HIV status. This compares favorably with other reports from SSA settings,3,9 and demonstrates that outcomes can be good even in Malawi if patients receive appropriate treatment with standard regimens and careful monitoring.
The strengths of our study include a detailed, prospective cohort with high-quality pathologic diagnoses, protocol-guided treatment, and longitudinal follow-up with standardized assessments. In addition, we had complete retention and outcome ascertainment. Patient retention in clinical cancer studies in SSA is a major challenge, and survival can be significantly overestimated when patients are lost to follow-up, since death is a major cause of loss to follow-up in this environment. Finally, all patients received standardized treatment according to institutional guidelines during the study period. The limitations include referral bias at a national teaching hospital, small sample size, lack of systematic evaluation for various causes of anemia, and assigning cause of death based on inference after centralized review. In addition, our cohort was likely understaged relative to cohorts in resource-rich settings due to absent bone marrow evaluation particularly in children and lack of advanced imaging for all patients.
To conclude, cHL occurred primarily among adolescents and young adults in Malawi, typically associated with EBV and often HIV. Despite advanced disease and impaired performance status, children and adults were successfully treated with good short-term outcomes irrespective of HIV status. Survival can likely be further improved through continued efforts to achieve earlier diagnosis and define optimal treatment approaches for cHL in this environment. Such efforts may benefit from further evaluation of plasma EBV DNA as a clinically important and implementable biomarker in Malawi and comparable settings to facilitate better risk-adapted and response-guided therapeutic strategies.
Acknowledgments
The authors would like to thank the patients and their families for agreeing to participate in the study. They also thank Dr. Peter Kazembe for his leadership in initiating pediatric cancer care in Lilongwe over the last several decades, Wiza Kumwenda for developing the study database, Toon van der Gronde for assistance with manuscript preparation, and the University of North Carolina Translational Pathology Laboratory for performing additional stains including extensive technical contributions from Michelle Mathews. They are also grateful to the leadership of Kamuzu Central Hospital, Malawi Ministry of Health, UNC Project-Malawi, Lineberger Comprehensive Cancer Center, and Baylor College of Medicine Children’s Foundation Malawi for support of this study.
FUNDING INFORMATION
This work is supported by grants from the National Institutes of Health (K01TW009488, R21CA180815, and U54CA190152 to S.G. and P01CA019014 to D.P.D.), the Medical Education Partnership Initiative (U2GPS001965), the Lineberger Comprehensive Cancer Center (P30CA016086), AIDS Malignancy Consortium (U01CA121947), and Fogarty Global Health Fellows Program (R25TW009340).
Abbreviations
- ABVD
doxorubicin, bleomycin, vincristine, dacarbazine
- ABVE-PC
doxorubicin, bleomycin, vincristine, etoposide, prednisolone, cyclophosphamide
- ART
antiretroviral therapy
- cHL
classical Hodgkin lymphoma
- CI
confidence interval
- EBER
Epstein–Barr virus-encoded RNA
- EBV
Epstein–Barr virus
- HIV
human immunodeficiency virus
- HR
hazard ratio
- IHC
immunohistochemistry
- KCH
Kamuzu Central Hospital
- LDH
lactate dehydrogenase
- qPCR
quantitative polymerase chain reaction
- SSA
sub-Saharan Africa
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare that there is no conflict of interest.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- 1.Stefan DC. Patterns of distribution of childhood cancer in Africa. J Trop Pediatr. 2015;61(3):165–173. doi: 10.1093/tropej/fmv005. [DOI] [PubMed] [Google Scholar]
- 2.Sherief LM, Elsafy UR, Abdelkhalek ER, et al. Hodgkin lymphoma in childhood: clinicopathological features and therapy outcome at 2 centers from a developing country. Medicine (Baltimore) 2015;94(15):e670. doi: 10.1097/MD.0000000000000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stefan DC. Hodgkin lymphoma in Africa: present and future. Transfus Apher Sci. 2013;49(2):144–146. doi: 10.1016/j.transci.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 4.Karakas Z, Agaoglu L, Taravari B, et al. Pulmonary tuberculosis in children with Hodgkin’s lymphoma. Hematol J. 2003;4(1):78–81. doi: 10.1038/sj.thj.6200219. [DOI] [PubMed] [Google Scholar]
- 5.Masamba LPL, Jere Y, Brown ERS, Gorman DR. Tuberculosis diagnosis delaying treatment of cancer: experience from a New Oncology Unit in Blantyre, Malawi. J Glob Oncol. 2016 doi: 10.1200/JGO.2015.000299. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schellong G, Potter R, Bramswig J, et al. High cure rates and reduced long-term toxicity in pediatric Hodgkin’s disease: the German- Austrian multicenter trial DAL-HD-90. The German-Austrian Pediatric Hodgkin’s Disease Study Group. J Clin Oncol. 1999;17(12):3736–3744. doi: 10.1200/JCO.1999.17.12.3736. [DOI] [PubMed] [Google Scholar]
- 7.Hunger SP, Link MP, Donaldson SS. ABVD/MOPP and low-dose involved-field radiotherapy in pediatric Hodgkin’s disease: the Stanford experience. J Clin Oncol. 1994;12(10):2160–2166. doi: 10.1200/JCO.1994.12.10.2160. [DOI] [PubMed] [Google Scholar]
- 8.Ansell SM. Hodgkin lymphoma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91(4):434–442. doi: 10.1002/ajh.24272. [DOI] [PubMed] [Google Scholar]
- 9.Davidson A, Wainwright RD, Stones DK, et al. Malignancies in South African children with HIV. J Pediatr Hematol Oncol. 2014;36(2):111–117. doi: 10.1097/MPH.0b013e31829cdd49. [DOI] [PubMed] [Google Scholar]
- 10.Clarke CA, Glaser SL. Epidemiologic trends in HIV-associated lymphomas. Curr Opin Oncol. 2001;13(5):354–359. doi: 10.1097/00001622-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Gopal S, Patel MR, Yanik EL, et al. Temporal trends in presentation and survival for HIV-associated lymphoma in the antiretroviral therapy era. J Natl Cancer Inst. 2013;105(16):1221–1229. doi: 10.1093/jnci/djt158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uldrick TS, Little RF. How I treat classical Hodgkin lymphoma in patients infected with human immunodeficiency virus. Blood. 2015;125(8):1226–1235. doi: 10.1182/blood-2014-08-551598. quiz 1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiels MS, Koritzinsky EH, Clarke CA, Suneja G, Morton LM, Engels EA. Prevalence of HIV Infection among U.S. Hodgkin lymphomacases. Cancer Epidemiol Biomarkers Prev. 2014;23(2):274–281. doi: 10.1158/1055-9965.EPI-13-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biggar RJ, Jaffe ES, Goedert JJ, Chaturvedi A, Pfeiffer R, Engels EA. Hodgkin lymphoma and immunodeficiency in persons with HIV/AIDS. Blood. 2006;108(12):3786–3791. doi: 10.1182/blood-2006-05-024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clifford GM, Rickenbach M, Lise M, et al. Hodgkin lymphoma in the Swiss HIV Cohort Study. Blood. 2009;113(23):5737–5742. doi: 10.1182/blood-2009-02-204172. [DOI] [PubMed] [Google Scholar]
- 16.Bohlius J, Schmidlin K, Boue F, et al. HIV-1-related Hodgkin lymphoma in the era of combination antiretroviral therapy: incidence and evolution of CD4(+) T-cell lymphocytes. Blood. 2011;117(23):6100–6108. doi: 10.1182/blood-2010-08-301531. [DOI] [PubMed] [Google Scholar]
- 17.Lanoy E, Rosenberg PS, Fily F, et al. HIV-associated Hodgkin lymphoma during the first months on combination antiretroviral therapy. Blood. 2011;118(1):44–49. doi: 10.1182/blood-2011-02-339275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gopal S, Patel MR, Achenbach CJ, et al. Lymphoma immune reconstitution inflammatory syndrome in the center for AIDS research network of integrated clinical systems cohort. Clin Infect Dis. 2014;59(2):279–286. doi: 10.1093/cid/ciu270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sissolak G, Sissolak D, Jacobs P. Human immunodeficiency and Hodgkin lymphoma. Transfus Apher Sci. 2010;42(2):131–139. doi: 10.1016/j.transci.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Montoto S, Shaw K, Okosun J, et al. HIV status does not influence outcome in patients with classical Hodgkin lymphoma treated with chemotherapy using doxorubicin, bleomycin, vinblastine, and dacarbazine in the highly active antiretroviral therapy era. J Clin Oncol. 2012;30(33):4111–4116. doi: 10.1200/JCO.2011.41.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hentrich M, Berger M, Wyen C, et al. Stage-adapted treatment of HIV-associated Hodgkin lymphoma: results of a prospective multicenter study. J Clin Oncol. 2012;30(33):4117–4123. doi: 10.1200/JCO.2012.41.8137. [DOI] [PubMed] [Google Scholar]
- 22.Lee JH, Kim Y, Choi JW, Kim YS. Prevalence and prognostic significance of Epstein-Barr virus infection in classical Hodgkin’s lymphoma: ameta-analysis. Arch Med Res. 2014;45(5):417–431. doi: 10.1016/j.arcmed.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Piriou E, Asito AS, Sumba PO, et al. Early age at time of primary Epstein-Barr virus infection results in poorly controlled viral infection in infants from Western Kenya: clues to the etiology of endemic Burkitt lymphoma. J Infect Dis. 2012;205(6):906–913. doi: 10.1093/infdis/jir872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray P, Bell A. Contribution of the Epstein-Barr Virus to the Pathogenesis of Hodgkin Lymphoma. Curr Top Microbiol Immunol. 2015;390(Pt 1):287–313. doi: 10.1007/978-3-319-22822-8_12. [DOI] [PubMed] [Google Scholar]
- 25.UNAIDS. [Accessed 22 May 2016];Malawi Progress Report for 2015. Available at: http://www.unaids.org/en/regionscountries/countries/malawi.
- 26.United Nations Statistics Division. [Accessed 22 May 2016];Malawi country profile. Available at: http://data.un.org/CountryProfile.aspx?crName=malawi.
- 27.United Nations Development Program. [Accessed 22 May 2016];Human Development Report. 2015 Available at: http://hdr.undp.org/en/2015-report.
- 28.Gopal S, Krysiak R, Liomba G. Building a pathology laboratory in Malawi. Lancet Oncol. 2013;14(4):291–292. doi: 10.1016/S1470-2045(13)70109-8. [DOI] [PubMed] [Google Scholar]
- 29.Gopal S, Krysiak R, Liomba NG, et al. Early experience after developing a pathology laboratory in Malawi, with emphasis on cancer diagnoses. PLoS One. 2013;8(8):e70361. doi: 10.1371/journal.pone.0070361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Real-time PCR arrays. University of North Carolina Vironomics Core; [Accessed 22 May 2016]. Available at: https://www.med.unc.edu/vironomics/services/real-time-pcr-arrays-available. [Google Scholar]
- 31.National Cancer Institute. [Accessed 22 May 2016];Common Terminology Criteria for Adverse Events (CTCAE) v 4.0. Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
- 32.Friedman DL, Chen L, Wolden S, et al. Dose-intensive response-based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate-risk Hodgkin lymphoma: a report from the Children’s Oncology Group Study AHOD0031. J Clin Oncol. 2014;32(32):3651–3658. doi: 10.1200/JCO.2013.52.5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Cancer Institute. Adult Hodgkin Lymphoma Treatment (PDQ(R)): Health Professional Version. [Accessed 22 May 2016];PDQ Cancer Information Summaries. Available at: http://www.ncbi.nlm.nih.gov/books/NBK66038/
- 34.Stanley CC, Westmoreland KD, Heimlich BJ, et al. Outcomes for paediatric Burkitt lymphoma treated with anthracycline-based therapy in Malawi. Br J Haematol. 2016;173(5):705–712. doi: 10.1111/bjh.13986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gopal S, Fedoriw Y, Kaimila B, et al. CHOP chemotherapy for aggressive non-Hodgkin lymphoma with and without HIV in the antiretroviral therapy era in Malawi. PLoS One. 2016;11(3):e0150445. doi: 10.1371/journal.pone.0150445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hohaus S, Massini G, Giachelia M, et al. Anemia in Hodgkin’s lymphoma: the role of interleukin-6 and hepcidin. J Clin Oncol. 2010;28(15):2538–2543. doi: 10.1200/JCO.2009.27.6873. [DOI] [PubMed] [Google Scholar]
- 37.Kaaya EE, Castanos-Velez E, Ekman M, et al. AIDS and non AIDS-related malignant lymphoma in Tanzania. Afr Health Sci. 2006;6(2):69–75. doi: 10.5555/afhs.2006.6.2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stein L, Urban MI, O’Connell D, et al. The spectrum of human immunodeficiency virus-associated cancers in a South African black population: results from a case-control study, 1995–2004. Int J Cancer. 2008;122(10):2260–2265. doi: 10.1002/ijc.23391. [DOI] [PubMed] [Google Scholar]
- 39.Wiggill TM, Mantina H, Willem P, Perner Y, Stevens WS. Changing pattern of lymphoma subgroups at a tertiary academic complex in a high-prevalence HIV setting: a South African perspective. J Acquir Immune Defic Syndr. 2011;56(5):460–466. doi: 10.1097/QAI.0b013e31820bb06a. [DOI] [PubMed] [Google Scholar]
- 40.Stefan DC, Wessels G, Poole J, et al. Infection with human immunodeficiency virus-1 (HIV) among children with cancer in South Africa. Pediatr Blood Cancer. 2011;56(1):77–79. doi: 10.1002/pbc.22672. [DOI] [PubMed] [Google Scholar]
- 41.Sinfield RL, Molyneux EM, Banda K, et al. Spectrum and presentation of pediatric malignancies in the HIV era: experience from Blantyre, Malawi, 1998–2003. Pediatr Blood Cancer. 2007;48(5):515–520. doi: 10.1002/pbc.20917. [DOI] [PubMed] [Google Scholar]
- 42.Mutyaba I, Phipps W, Krantz EM, et al. A population-level evaluation of the effect of antiretroviral therapy on cancer incidence in Kyadondo County, Uganda, 1999–2008. J Acquir Immune Defic Syndr. 2015;69(4):481–486. doi: 10.1097/QAI.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wagner HJ, Schlager F, Claviez A, Bucsky P. Detection of Epstein- Barr virus DNA in peripheral blood of paediatric patients with Hodgkin’s disease by real-time polymerase chain reaction. Eur J Cancer. 2001;37(15):1853–1857. doi: 10.1016/s0959-8049(01)00152-6. [DOI] [PubMed] [Google Scholar]
- 44.Kanakry JA, Li H, Gellert LL, et al. Plasma Epstein-Barr virus DNA predicts outcome in advanced Hodgkin lymphoma: correlative analysis from a large North American cooperative group trial. Blood. 2013;121(18):3547–3553. doi: 10.1182/blood-2012-09-454694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gandhi MK, Lambley E, Burrows J, et al. Plasma Epstein-Barr virus (EBV) DNA is a biomarker for EBV-positive Hodgkin’s lymphoma. Clin Cancer Res. 2006;12(2):460–464. doi: 10.1158/1078-0432.CCR-05-2008. [DOI] [PubMed] [Google Scholar]
- 46.Spacek M, Hubacek P, Markova J, et al. Plasma EBV-DNA monitoring in Epstein-Barr virus-positive Hodgkin lymphoma patients. Apmis. 2011;119(1):10–16. doi: 10.1111/j.1600-0463.2010.02685.x. [DOI] [PubMed] [Google Scholar]
- 47.Lecher S, Ellenberger D, Kim AA, et al. Scale-up of HIV viral load monitoring—seven sub-Saharan African countries. MMWR Morb Mortal Wkly Rep. 2015;64(46):1287–1290. doi: 10.15585/mmwr.mm6446a3. [DOI] [PubMed] [Google Scholar]
- 48.Kanakry JA, Hegde AM, Durand CM, et al. The clinical significance of EBV DNA in the plasma and peripheral blood mononuclear cells of patients with or without EBV diseases. Blood. 2016;127(16):2007–2017. doi: 10.1182/blood-2015-09-672030. [DOI] [PMC free article] [PubMed] [Google Scholar]