Abstract
Cancer therapy has developed around the concept of killing, or stopping the growth of, the cancer cells. Molecularly targeted therapy is the modern expression of this paradigm. Increasingly, however, the realization that the cancer has co-opted the normal cells of the stroma for its own survival has led to the concept that the tumor microenvironment (TME) could be targeted for effective therapy. In this Review we outline the importance of tumor associated macrophages (TAMs), a major component of the TME, in the response of tumors to cancer therapy. We discuss the normal role of macrophages in wound healing, the major phenotypes of TAMs and their role in blunting the efficacy to cancer treatment by radiation and anticancer drugs both by promoting tumor angiogenesis and by suppressing antitumor immunity. Finally we review the many preclinical studies that have shown that the response of tumors to irradiation and anticancer drugs can be improved, sometimes markedly so, by depleting TAMs from tumors or by suppressing their polarization from an M1 to an M2 phenotype. The data clearly support the validity of clinical testing of combining targeting TAMs with conventional therapy.
Introduction
Tumor associated macrophages (TAMs) have increasingly become recognized as an attractive target in cancer therapy. Not only do essentially all the preclinical and clinical literature demonstrate that the extent of TAM infiltration into tumors negatively affects outcome (1, 2), but also many preclinical studies have shown that the response to therapy can be potentiated by blocking macrophage entry into tumors (3, 4), or by changing their polarization from an M2 to an M1 phenotype (5). Unlike tissue resident macrophages, which are derived largely from the yolk sac in embryogenesis (6), TAMs derive from circulating monocytes (1), and are among the most abundant normal cells in the tumor microenvironment. Though their normal role is in promoting both innate and adaptive immunity and in phagocytosis of dead or dying cells and cell debris, tumors have largely re-educated them to a phenotype that promotes tumor growth and spread. These activities include suppression of adaptive immunity by T-cells, and enhancement of angiogenesis, tumor cell invasion and intravasation into blood vessels (7, 8). These distinct activities are carried out by different subsets of TAMs, which coexist in different microenvironments within the tumor (9). These macrophages form a phenotypic continuum from M1-like, or classically activated macrophages, which are pro-inflammatory, pro-immunity and anti-tumor, to M2-like, or alternatively activated macrophages, which are anti-inflammatory, immune suppressive, proangiogenic and pro-tumor. The tumor microenvironment strongly polarizes macrophages towards an M2-like phenotype and this is especially the case for tumors recovering from cancer treatment. M2 polarized macrophages have been shown to be enriched in the hypoxic areas of experimental tumors (10–12), and are associated with higher tumor grade in human gliomas (13).
Though the anti-inflammatory M2-like polarized macrophages promote tumor growth and metastasis, this is not the case in the early development of cancer. In many cases an inflammatory response promotes tumor initiation and macrophages are an essential component of an inflammatory response. Examples include the chronic infection caused by hepatitis B or C virus in the liver, which is the main cause of hepatocellular carcinoma (14), Helicobacter pylori in the stomach which is linked to gastric carcinoma (15), and the enhanced risk of colon cancer in patients with inflammatory bowel disease (16). A thorough review of this area has been published recently (1) and is outside the area of the present review. Here we will focus on strategies to enhance the treatment of existing cancers by manipulation of the TAM population.
The role of macrophages in wound healing
Cancers have aptly been described as “wounds that do not heal” to connote the similarities within their microenvironments (17, 18). Not surprisingly, considering their abundant distribution in both disease processes, macrophages are important drivers. One of the distinct differences between cancers and wounds however is that there is a distinct distribution of macrophage phenotype according to the damage and healing process, the understanding of which provides many insights into how their regulation can impact tumor growth.
Macrophages are important components of the innate immune response in mechanical and mucosal injury because of their ability to initiate and resolve inflammation and to communicate with other innate and adaptive immune cells. During inflammation, macrophages are recruited to the wound site where they display impressive plasticity in that they can express a polarization of classic and alternative activation phenotypes that are mediated by cytokines, oxidants, lipids and growth factors (19–21).
Wound macrophages exhibit complex and dynamic phenotypes that change as the wound matures (Fig. 1). As the “big eaters” of the myeloid lineage, the macrophages represents the patrolling phagocyte that is the first cell to encounter and initiate inflammation in response to infection. Later, macrophages further coordinate wound closure by secreting cytokines and growth factors including TGF-β that play a pivotal role in restructuring the wound bed with accompanying matrix reorganization and epithelial barrier repair (22). Furthermore, in addition to mediating the inflammatory phase of tissue repair, macrophages are also involved in guiding the angiogenic response that plays a key role in the proliferative phase of tissue repair. Not surprisingly, therefore, macrophage depletion in the early inflammatory phase severely reduces granulation tissue formation and re-epithelialization, whereas later depletion during granulation tissue formation results in severely disturbed neoangiogenesis and wound closure due to insufficient TGF-β1 and VEGF concentrations (22–25). Considering their diverse roles in wound repair, macrophages display variable phenotypes that range from a classically activated M1 to an alternative M2 type. M1 macrophages rapidly differentiate after migration, being activated by bacterial-derived products such as LPS as well as signals associated with infections such as IFN. They are highly inflammatory with high phagocytic and bactericidal potential. They secrete important proinflammatory cytokines such as TNF, IL-1, -6 and -12 as well as reactive oxygen species (ROS) (26–28).
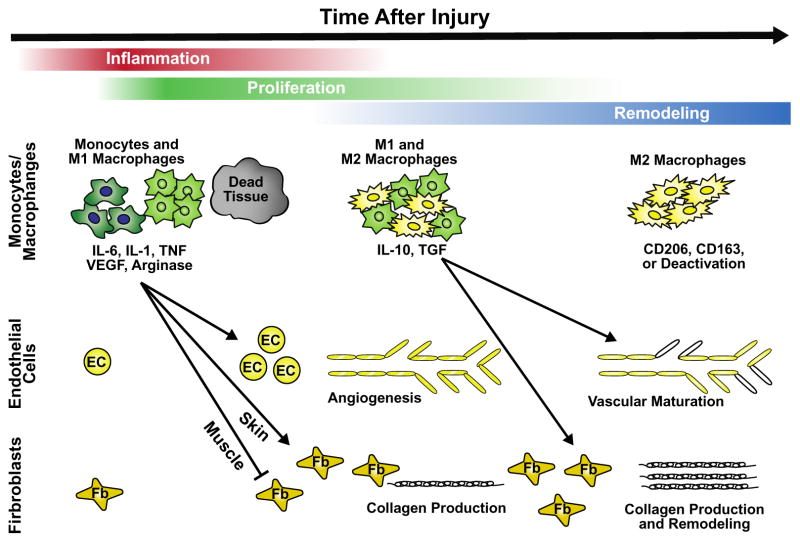
Figure 1.
Key roles of macrophages in the process of injury and repair. After wounding, repair proceeds in three phases: inflammation, proliferation and remodeling. Macrophages, which are predominantly M1 phenotype in the period of inflammation and then shift towards M2 in the remodeling phase, are influential drivers of each phase and impact endothelial, myoblast and fibroblast cellular functioning through release of a number of important cytokines and growth factors. Redrawn from Novak and Koh (32), with permission from Elsevier. EC endothelial cell, Fb fibroblast.
In contrast, M2 macrophages are present later in the healing process when granulation tissue formation occurs; they antagonize the inflammatory response, thus allowing initiation of healing. These anti inflammatory cells recruit fibroblasts and activate them to differentiate toward myofibroblasts that release proangiogenic factors to recruit endothelial progenitor cells and enable new vessel formation, a process that occurs through secretion of key anti-inflammatory cytokines IL-4, -10 and -13 (20, 21, 28–34) and are also associated with decreased production of ROS, nitric oxide and TNFα. M2 macrophages represent an important constituent of host defense, playing roles in Th2-mediated activation of humoral immune responses (35), eradication of parasitic infections and resolution of inflammation (36).
Although classically subdivided into classical and alternative, other macrophages subtypes have been described including pro-fibrotic M2-like induced in the phase of new tissue formation that produce growth factors and ECM and fibrolytic M2-like macrophages induced in the ischemic scar milieu that secrete proteases (37). Although each of these subtypes might be distinct, more recent evidence is more consistent with there being extensive overlap of these characteristics, including simultaneous expression of traits typically associated with both alternative and classical macrophage activation (38). For example during resolution of inflammation activated M1 macrophages can acquire the phenotype of tissue resident ones (39). Furthermore, the removal of apoptotic neutrophils by macrophages potently initiates a phenotypic switch from pro-inflammatory M1 to anti-inflammatory M2 phenotype (40, 41). Examples such as this strongly support a “plastic” phenotype.
It is therefore probable that at any time point during healing, wound macrophages display “hybrid” M1/M2 activation phenotypes, which may enable versatility in rapid switching between different functions. Although the switches responsible for orchestrating these different profiles at the molecular level remain largely unknown, it is clear that the role played by macrophages in wounds have several parallels with the situation in cancer.,.
Phenotypes of tumor associated macrophages
Tumor associated macrophages play three distinct roles in promoting tumor growth and spread.
TAMs facilitate the intravasation of tumor cells into the vasculature thereby promoting metastases. They do this via a paracrine loop consisting of macrophage colony stimulating factor-1, CSF-1 (M-CSF), from the tumor cells and EGF from the macrophages and their receptors (42). The net result is that tumor cells alternate with macrophages along collagen fibers until they reach blood vessels at which point the macrophages facilitate the entry of the tumor cells into the blood stream (43, 44). A recent elegant study using real-time intravital high-resolution two-photon microscopy showed that the macrophages in contact with the blood vessels are VEGF-A expressing TEMs and these produce highly localized vascular permeability thereby facilitating the extravasation of the tumor cells (45). Though inhibition of this pathway by reprogramming or depleting TAMs could have an effect in reducing metastatic spread, it is not likely to be the most productive therapeutic approach as it will not have an effect on pre-existing metastases and long-term therapy could have unwanted side effects. However, the importance of TAMs for metastatic spread is highlighted by a recent study showing that the incidence of metastases in triple negative breast cancer patients can be at least partially predicted from the gene expression profile of TAMs in the tumors (46).
-
TAMs promote tumor growth by inhibiting both adaptive and innate antitumor immunity through a variety of diverse mechanisms. M2 polarized TAMs block T cell immune responses to tumor antigens by secreting immune suppressive molecules including TGF-β, IL-10, arginase-1 and nitric oxide (47–50). TGF-β has direct blocking activity of stimulation, differentiation, proliferation, and effector function of conventional CD4+ and CD8+T cells that mediate immune responses (51, 52). In addition, TGF-β promotes the induction of CD4+CD25+FoxP3+ regulatory T cells that block the immune function of conventional CD4+ and CD8+ T cells (see below) (53–55). IL-10 also has the capacity to block the function of conventional CD4+ and CD8+ T cells so that the development of effector T cells is markedly reduced (56–58).
Arginase-1 (Arg-1) is a catabolic enzyme that depletes arginine from the environment of conventional T cells (59–61). Since the conventional T cells require arginine for activation in response to antigens, this depletion blocks their capacity to generate immune effector cells (59–61). In addition, catabolic products of arginine are immunosuppressive (59–61).
Nitric oxide (NO) and other reactive oxygen species produced by TAMs synergize with Arg-1 to interfere with conventional T cell activation such that the combination is considerably more immune suppressive than either modality alone (59–65). Since macrophages can develop either pro-inflammatory or anti-inflammatory/immunosuppressive functions, it is clear that TAMs have become polarized toward the suppressive functions. A critical molecular switch in macrophages that controls polarization is PI3-kinase gamma, since signaling via this kinase promotes immune suppression during tumor growth, and inactivation of the kinase promotes CD8+ T cell immunity and cytotoxicity (66, 67)
In addition to the suppression of antitumor immunity by TAMs their suppressive activity is enhanced by their interaction with a population of tumor infiltrating cells termed myeloid derived suppressor cells (MDSC). These are defined by their cell surface markers CD11b and Gr-1 and are considered to be a mixed population of monocytic and granulocytic cells (68). MDSCs differ from TAMS by their lack of expression of class II MHC receptors that are present on TAMs (62, 63). MDSCs are immune suppressive cells that are elevated in the bone marrow, blood and spleens of patients and mice with tumors and are associated with poor overall survival (69). In tumors they have been shown to differentiate into immune suppressive TAMs, a process that is mediated by tumor hypoxia and HIF-1α (70, 71). MDSC’s also support tumor growth through their secretion of MMP9, which acts to release VEGF from the matrix. Deletion of MM9 abolishes this activity (72), as well as the ability of tumors to grow in an irradiated site (73).
Certain populations of TAMs, particularly the Tie2 expressing macrophages (TEMs), are pro-angiogenic, thereby promoting tumor growth and recovery from cancer therapy. De Palma and colleagues demonstrated the importance of TEMs for angiogenesis by demonstrating that genetic depletion of TEMs inhibited angiogenesis and tumor growth in various subcutaneous tumor models (74), and Chen and colleagues demonstrated that Tie2 macrophages were crucial to the recovery of the tumor vasculature and recurrence of the transplanted MCA205 tumor following doxorubicin (75). Gene expression studies by Pucci and colleagues demonstrated that TEMs are a subset of TAMs and are at the extreme end of the M2 polarization spectrum (76). Consistent with their proangiogenic phenotype they are enriched in the perivascular regions of tumors (77). In addition to its expression on TEMs, Tie2 is also expressed on endothelial cells (ECs). Tie2 is the receptor for the angiopoietins Ang1, which promotes vasculature maturity, and Ang2, which destabilizes blood vessels thereby sensitizing the ECs to proliferative signals provided by VEGF and other pro-angiogenic cytokines in the tumors (78). Consistent with the importance of the Tie2/Ang2 axis, inhibitors of Ang2 show efficacy in a wide spectrum of preclinical tumor models (79, 80). For more in depth discussion of macrophage polarization and location in different tumors the reader is referred to an excellent recent review of Lahmar and colleagues (6).
Improved treatment response by manipulating TAMs in conjunction with standard therapy
A common response of tumors to cancer treatment, as demonstrated in a variety of preclinical studies, is to promote the accumulation of bone marrow derived myeloid cells, which differentiate into TAMs, in the treated tumors. This has been demonstrated following irradiation (73, 81–83), following vascular disruptive agents (VDAs) such as combretastatin A4 (84), certain chemotherapeutic drugs (4, 85–87) and anti-VEGF therapy (88, 89). More than one mechanism is responsible for this influx. In the case of radiation, VDA’s, anti-VEGF therapy and at least some chemotherapeutic agents, it is the result of increased tumor hypoxia secondary to vascular damage. This increases tumor HIF-1α that in turn promotes high levels of CXCL12 (SDF-1), the ligand for CXCR4 expressed on monocytes and ECs (81, 84) (Fig. 2). However, CXCL12 levels in treated tumors can rise in the absence of increased hypoxia (4). These high levels of CXCL12 both capture the circulating monocytes in the treated tumors and mobilize monocytes from the bone marrow. Increased TAM infiltration after therapy has also been demonstrated in patients with breast cancer (86, 87) and with glioblastoma (81). Another mechanism for the increased influx of TAMs into tumors is the treatment-induced increased expression of CSF-1 by the tumor cells (86, 90).
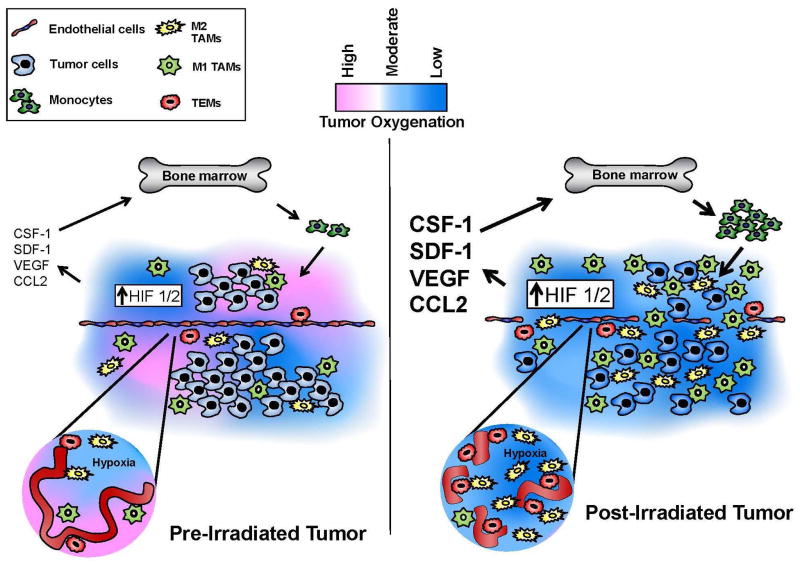
Figure 2. Representation of the tumor microenvironment before and after irradiation.
Following irradiation and other anticancer agents the vasculature of the tumor is damaged leading to reduced tumor blood flow and increased hypoxia. This produces increased expression of HIF-1 and HIF-2, which results in increased expression of a diverse spectrum of cytokines, including stromal cell-derived factor-1 (SDF-1, CXCL2), producing greater recruitment and influx into the tumor of bone marrow derived monocytes which differentiate into TAMs. TEMs commonly associate with the vasculature, while CD68+ TAMs frequently localize to areas of severe hypoxia. Redrawn from Russell and Brown (83).
There is also evidence that the CCL2/CCR2 chemokine axis is involved in monocyte recruitment into some tumors after chemotherapy (85, 91). CCR2+ monocytes are inflammatory monocytes and are likely to be the precursors of the M2 polarized TAMs. In agreement with this, Nakasone et al (85) found an increase in CCR2+ monocytes in tumors 48 hours after doxorubicin treatment but no increase in TAMs; though such an increase has been shown 7–12 days later in the same tumor model after chemotherapy (86). It is not clear at this time as to whether the CCL2/CCR2 and CXCL12/CXCR4 axes are independent pathways for recruiting monocytes into treated tumors, whether they are activated by different treatments or recruit different subsets of monocytes.
Importantly, the TAMs infiltrating tumors after therapy do not have the same phenotypic distribution as in untreated tumors: Rather, they are preferentially polarized into M2-like TAMs with high expression of Tie2 (4, 81) (Fig. 2). This M2-like polarization is driven both by tumor hypoxia (75) and by the increased expression by treated tumor cells of CSF-1 and IL-34, the ligands for the receptor CSF-1R on macrophages, thereby enhancing both their accumulation into treated tumors and polarization into an M2-like phenotype (86, 90, 92). Not only is this highly proangiogenic but also this polarization is immune suppressive. Reprogramming and selective killing of the M2 macrophages by blockade of the CSF-1/CSF-1R axis improves antitumor immunity in a mouse model of pancreatic ductal adenocarcinoma (93). A recent study of Baer and colleagues suggests that microRNAs (miRNAs) are involved in the polarization of M1 to M2 macrophages and that conditional knockout of the miRNA processing enzyme DICER in macrophages produces M1-like programming (94). This reprogramming abolished the immunosuppressive activity of the TAMs and recruited activated cytotoxic T lymphocytes (CTLs) to the tumors. In addition this functional polarization of TAMs to an M1 phenotype abolished the anti-tumor effect of CSF-1R blockade, underlying the importance of M2 TAMs to tumor response. Clinically the importance of M2 TAMs is highlighted in a recent study Sugimura and colleagues who showed in a multivariate analysis that the extent of tumor infiltration by M2 TAMs, is associated with a poor response to chemotherapy and poor prognosis of patients with esophageal cancer following surgery (95).
The dependence of the tumor after therapy for an influx of TAMs driven by the CXCL12/CXCR4 or CCL2/CCR2 axes and their polarization into an M2-like, pro-angiogenic, phenotype driven by CSF-1/CSF-1R provides multiple therapeutic opportunities, in many cases with drugs that are currently available, or close to being available, for clinical use. Tables 1 and 2 list the preclinical data from studies that show improved response of a variety of tumor models to irradiation (Table 1) and to chemotherapy (Table 2) when the influx of TAMs after treatment is either prevented (e.g. by blocking the CXCL12/CXCR4 or CCL2/CCR2 pathway), or the polarization to the M2-like phenotype is inhibited (e.g. by blocking the CSF-1/CSF-1R pathway).
Table 1.
Studies that show improved radiation response with macrophage manipulation
| IR schedule | Macrophage targeting agent | Molecular Target | Tumor model | Tumor site | Host species | Ref. |
|---|---|---|---|---|---|---|
| 20 Gy | Liposomal clodronate, Enbrel | Macrophage depletion, TNF depletion | B16.SIY Mouse melanoma |
Subcutaneous | C57BL/6 TNF−/− |
(121) |
| 12 Gy 20 Gy |
Monoclonal antibody | CD11b cells | FaDu H & N Sq Ca |
Subcutaneous | Nude mice | (108) |
| 15 Gy | NSC-134754 | HIF-1, HIF-2 | U251 human GBM | Orthotopic | Nude mice | (81) |
| 5 x 2 Gy 15 Gy |
Plerixafor (AMD3100) | CXCR4 | ||||
| 15 Gy | Carrageenan | Macrophage depletion | ||||
| 21 Gy | 6 Gy whole body irradiation | Monocyte depletion | 54A human lung tumors | Subcutaneous | Nude mice | (122) |
| MCa 8 | FVB mice | |||||
| 15 x 4 Gy | Plerixafor (AMD3100) | CXCR4 | TRAMP-C1 mouse prostate | Intramuscular | C57BL/6 | (123) |
| 7 x 5 Gy | Plerixafor (AMD3100) | CXCR4 | PC3 human prostate | Subcutaneous | Nude mice | (124) |
| 15 x 2 Gy | Plerixafor (AMD3100) | CXCR4 | Two cervix PDX tumors | Orthotopic | Nude mice | (110) |
| 12 Gy | CCX771 | CXCR7 | U251 human GBM | Orthotopic | Nude mice | (100) |
| 8 Gy 15 Gy |
siRNA | CXCL12 (SDF-1) | ALTS1C1 mouse glioma | Orthotopic | C57BL/6 | (125) |
| 5 x 3 Gy | PLX3397 | CSF-1R | RM-1 mouse prostate ca | Subcutaneous | C57BL/6 | (115) |
| 12 Gy | PLX3397 | CSF-1R | U251 and GBM12 human PDX | Orthotopic | Nude mice | (90) |
| 20 Gy | Ola-peg (NOX-A12) | CXCL12 (SDF-1) | Chem induced gliomas | Autochthonous | Sprague- Dawley rats | (99) |
| 20 Gy | CCX662 | CXCR7 | Chem induced gliomas | Autochthonous | Sprague- Dawley rats | (100) |
| 18 Gy | CCX662 | CXCR7 | C6 glioma | Orthotopic | Sprague- Dawley rats | (100) |
Table 2.
Studies that show improved response to chemotherapeutic drugs with macrophage manipulation
| Chemotherapy drug | Macrophage targeting agent | Molecular Target | Tumor model | Tumor site | Host species | Ref |
|---|---|---|---|---|---|---|
| Androgen blockage by castration | PLX3397 | CSF-1R | Myc-CaP mouse prostate ca | Subcutaneous | FVB mice | (92) |
| CWR22Rv1 human prostate ca | Prostate | SCID/Beige | ||||
| Cyclophosphamide + methotrexate, + 5-fluorouracil; | anti-CSF-1 Fab). | CSF-1 | MCF-7 breast cancer | Subcutaneous | Nude mice | (126) |
| None | BLZ945 | CSF-1R | PDGF-driven glioma | Autochthonous | Transgenic mice | (97) |
| Various human GBM | Orthotopic | NOD-SCID | ||||
| None | JNJ-28312141 | CSF-1R | H460 human lung tumor | Subcutaneous | Nude mice | (96) |
| Paclitaxel | Neutralizing mAB or PLX3397 | CSF-1R | MMTV-PyMT | Mammary fat pads | FVB/n female mice | (86) |
| Paclitaxel | Plerixafor (AMD3100) | CXCR4 | 4T1 murine mammary ca | Mammary fat pads | Female Balb/c | (4) |
| Cyclophosphamide | Lewis lung carcinoma | Subcutaneous | C57BL/6 | |||
| Paclitaxel | Plerixafor (AMD3100) | CXCR4 | Lewis lung carcinoma | Subcutaneous | C57BL/6 | (127) |
| Combretastatin | Plerixafor (AMD3100) | CXCR4 | N202 mouse mammary | Subcutaneous | C57BL/6 | (84) |
| Doxorubicin Cisplatin | ccr2 knockout | CCR2 | MMTV-PyMT cells | Subcutaneous | C57BL/6 vs. ccr2−/− mice | (85) |
| Paclitaxel | Cathepsin inhibitor JPM | Cathepsin | MMTV-PyMT mammary tumors | Spontaneous | MMTV-PyMT mice | (87) |
| Paclitaxel Carboplatin | IL-10R mAb, CSF-1 mAb | CSF-1 IL-10 |
MMTV-PyMT mammary tumors | Spontaneous | MMTV-PyMT mice | (58) |
| Doxorubicin | Tie2 knockout | Tie2+ macroph. | MCA205 | Subcutaneous | C57BL/6 | (75) |
In some studies blocking these pathways alone had an effect on tumor growth (96, 97), or in one study reversed the vascular leakage causing ascites in late stage epithelial ovarian cancer (98), but in most cases there was little or no effect of inhibition of these pathways on the growth of untreated tumors. Thus the clinical benefit of manipulation of TAMs will typically be seen when it is combined with standard therapy. Of particular relevance to translation of this strategy to the clinic is that the effect of blocking or re-educating TAMs is often large: For example blocking the CXCL12/CXCR4/7 pathway combined with irradiation of an autochthonous brain tumor in rats increased survival time from 3 weeks for radiation alone to almost 6 months for the combination of radiation with the inhibitor (99, 100). In contrast one recent report found that depletion of TAMs did not improve the radiation response of a murine tumor (101). However, timing is important: macrophages invade tumors 1–3 weeks after radiation (90) and in the negative report macrophage depletion was only performed one day prior to irradiation. Ideally following irradiation macrophage depletion or repolarization needs to continue for 4 or more weeks (81, 90, 99).
In addition to blocking entry of TAMs into tumors or preventing their M2 polarization it appears that at least in the case of myeloid cell induced resistance to anti-VEGF therapy that inhibition of the PI3K in myeloid cells can also overcome the resistance. In this study in mouse models of pancreatic neuroendocrine and mammary tumors Rivera and colleagues showed that anti-VEGF therapy produced initial tumor regression but angiogenesis and immune suppression was reinitiated by activating PI3K signaling in all CD11b+ cells rendering the tumors nonresponsive to VEGF inhibition (102). PI3K inhibition overcame this induced resistance.
More than one mechanism is responsible for the protective role of TAMs against cancer treatment. For irradiation and other agents that severely damage the tumor vasculature TAMs promote the early restoration of the vasculature and blood flow (81) in part at least by enhanced VEGFA production (4). For other agents it is the blocking of immune suppression by MDSCs and the accumulation of anti-tumor CD8+ cytotoxic T-cells that enhances the efficacy of the therapy (58, 86, 103). For the vasculature damaging agents both mechanisms are likely to be involved.
For more information on the role of myeloid cells and TAMs in cancer and the immunological aspects of the response to treatment the reader is referred to excellent recent reviews (104–107).
An important question that needs addressing with any agent or procedure that enhances tumor response is whether there is a similar enhancement of toxicity to normal tissues. This has not been determined with chemotherapy but several studies have demonstrated that the response of normal tissues to radiation is actually protected by blocking macrophage entry either by anti-CD11b antibodies (108), or by the CXCR4 antagonist plerixafor (109, 110). A particularly relevant recent study showed that the neurocognitive impairment by whole brain irradiation to mice could be prevented by post irradiation depletion of microglia (resident macrophages in the brain) using the CSF1-R inhibitor PLX5622 (111).
Another key question that has to be addressed with targeting of TAMs (or any stromal cells) is the extent to which resistance to the therapy can develop and the mechanism of this resistance. Though the well-described methods by which cancer cells, because of their genomic instability, develop mutations to targeting agents do not apply, or apply to a lesser extent, with stromal cells, it is nonetheless likely that resistance will develop. Clinical data targeting the CCL2-CCR2 pathway (described below) suggests that resistance develops by compensatory upregulation of the target, and a recent elegant study by Quail and colleagues has described the development of a novel TME mediated resistance in response to prolonged CSF-1R inhibition (112). The authors found in a mouse GBM model that prolonged treatment with the CSF-1R antagonist, BLZ945, that the TAMs produced elevated levels of IGF-1 and with high levels of IGF-1R on some of the tumor cells, this resulted in PI3K pathway activation and tumor cell survival. When the PI3K pathway was blocked in addition to CSF-1 inhibition this resistance was overcome. This is clearly an important avenue for further research to increase the power of clinical studies of TAM targeting.
Clinical data
Clinical results often do not reproduce the promise of preclinical data. There are several reasons for this discrepancy: On the preclinical side one issue is that investigators often choose models that are genetically homogeneous and respond well to treatments. However this is unlikely to be the case for macrophage depletion, as the preceding sections show that there is almost universal improvement of treatment response with many different preclinical models. On the clinical side, reasons for lack of reproducing preclinical data include a) drug doses that are insufficient to block the intended target or do not do so for a sufficiently long period, and b) using the drug in a manner not expected to yield positive results. Clinical results have to be evaluated with these issues in mind.
Plerixafor, an inhibitor of the SDF-1/CXCR4 pathway, has been used extensively and safely in the clinic. Its current use has been largely restricted to acute doses to mobilize hematopoietic stem cells from the bone marrow. However In a report of a phase 1 trial, Thomas and colleagues infused Plerixafor for 4 weeks in conjunction with standard therapy for newly diagnosed glioblastoma patients, and reported at target plasma levels no dose-limiting toxicities with promising indications of activity (113). In a phase II study with recurrent glioblastoma Butowski and colleagues reported that the CSF-1R Inhibitor PLX3397 was well tolerated but showed no efficacy (114). However, preclinical studies have shown that PLX3397 is only active when combined with standard treatment (90, 115) so these clinical results are not unexpected. Prolonged inhibition of CSF-1R using the monoclonal antibody emactuzumab (RG7155) has been reported to cause some adverse events, thought non dose limiting, notably facial edema, asthenia and pruritis in a phase 1 trial with diffuse-type tenosynovial giant cell tumor (116). This rare tumor is characterized by an overexpression of CSF-1 and the trial showed significant tumor response of CSF-1R inhibition as well as reduced macrophage numbers in the tumors.
Another potential target is the CCL2 - CCR2 signaling axis. In addition to its role as a chemo attractant for TAMs CCL2 is also expressed on the malignant cells of a number of tumors including breast, colorectal, prostate, melanoma, gastric and ovarian cancers. Carlumab (CNTO888) is a monoclonal antibody with high specificity for CCL2 thereby inhibiting binding to its receptor CCR2. In one phase 1 trial of 44 patients with advanced solid tumors CNT0888 was well tolerated but CCL2 was only transiently suppressed and there were no objective responses (117). In another phase 1 trial CNTO888 was given in combination with 4 standard of care chemotherapies in 53 patients. Inhibition of CCL2 was again transitory and there was no evidence of increased anti-tumor activity of adding the inhibitor (118). Similar transitory inhibition of CCL2 and no evidence of activity was seen in a phase 2 trial of the antibody in combination with docetaxel (119). Blocking of the CCR2 receptor has also been evaluated using the humanized antibody MLN1202, but only as monotherapy. Again, the therapy was well tolerated but there was no indication of anti-tumor activity (120). Taken together efforts to interfere with the CCL2-CCR2 axis in clinical studies have been disappointing possibly because of CCL2 expression being augmented in response to the initial CCL2 inhibition or compensation by other chemokine pathways.
Conclusion
Tumor associated macrophages (TAMs) are a common component of wounds and of experimental and human solid cancers. Whereas the normal role of macrophages is to promote immunity, phagocytosis of dead cells and cell debris, tumors have largely educated them to a phenotype (the so-called M2, or alternatively activated phenotype) that promotes tumor growth and spread. They do this by facilitating the intravasation of tumor cells into the vasculature, by inhibiting antitumor immunity, and by stimulating blood vessel growth after therapy. However, the influx of TAMs into tumors and their tumor stimulating properties (by changing TAM polarization from an M1 to an M2 phenotype) depend on just two or three signaling pathways: The CXCL12/CXCR4 and possibly the CCL2/CCR2 chemokine axes, which promote macrophage influx into the tumors after therapy and the CSF-1/CSF-1R pathway, which is responsible for the M1 to M2 polarization. Many preclinical studies using small molecules or antibodies to block each of these pathways individually have demonstrated significant improvement in the response of a wide variety of tumors to therapy, particularly to radiotherapy. Clinical results indicate that blockage of these pathways is generally well tolerated but in the case of abrogation of the CCL2-CCR2 pathway the results have been disappointing. Efficacy data when successful TAM targeting has been combined with radiation or chemotherapy using inhibitors of the SDF-1/CXR4 or CSF-1/CSF-1R pathways are not yet available.
Acknowledgments
Grant Support
The study was supported by grants R01 CA149318 (JMB and LR) and R01 CA 163441 (SS).
Footnotes
Conflict of interest: The authors declare no potential conflicts of interest.
References
- 1.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med. 2010;362:875–85. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. 2013;23:277–86. doi: 10.1016/j.ccr.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Hughes R, Qian BZ, Rowan C, Muthana M, Keklikoglou I, Olson OC, et al. Perivascular M2 Macrophages Stimulate Tumor Relapse after Chemotherapy. Cancer Res. 2015;75:3479–91. doi: 10.1158/0008-5472.CAN-14-3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu M, Liu M, Du X, Li S, Li H, Li X, et al. Intratumoral Delivery of IL-21 Overcomes Anti-Her2/Neu Resistance through Shifting Tumor-Associated Macrophages from M2 to M1 Phenotype. J Immunol. 2015;194:4997–5006. doi: 10.4049/jimmunol.1402603. [DOI] [PubMed] [Google Scholar]
- 6.Lahmar Q, Keirsse J, Laoui D, Movahedi K, Van Overmeire E, Van Ginderachter JA. Tissue-resident versus monocyte-derived macrophages in the tumor microenvironment. Biochimica et biophysica acta. 2016;1865:23–34. doi: 10.1016/j.bbcan.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–91. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–12. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 10.Laoui D, Van Overmeire E, Di Conza G, Aldeni C, Keirsse J, Morias Y, et al. Tumor hypoxia does not drive differentiation of tumor-associated macrophages but rather fine-tunes the M2-like macrophage population. Cancer Res. 2014;74:24–30. doi: 10.1158/0008-5472.CAN-13-1196. [DOI] [PubMed] [Google Scholar]
- 11.Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–39. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Cao J, Ma S, Dong R, Meng W, Ying M, et al. Tumor hypoxia enhances Non-Small Cell Lung Cancer metastasis by selectively promoting macrophage M2 polarization through the activation of ERK signaling. Oncotarget. 2014;5:9664–77. doi: 10.18632/oncotarget.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol. 2008;216:15–24. doi: 10.1002/path.2370. [DOI] [PubMed] [Google Scholar]
- 14.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–73. e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–31. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 16.Munkholm P. Review article: the incidence and prevalence of colorectal cancer in inflammatory bowel disease. Aliment Pharmacol Ther. 2003;18(Suppl 2):1–5. doi: 10.1046/j.1365-2036.18.s2.2.x. [DOI] [PubMed] [Google Scholar]
- 17.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–9. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 18.Dvorak HF. Tumors: wounds that do not heal-redux. Cancer immunology research. 2015;3:1–11. doi: 10.1158/2326-6066.CIR-14-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brancato SK, Albina JE. Wound macrophages as key regulators of repair: origin, phenotype, and function. The American journal of pathology. 2011;178:19–25. doi: 10.1016/j.ajpath.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laskin DL, Sunil VR, Gardner CR, Laskin JD. Macrophages and tissue injury: agents of defense or destruction? Annual review of pharmacology and toxicology. 2011;51:267–88. doi: 10.1146/annurev.pharmtox.010909.105812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahdavian Delavary B, van der Veer WM, van Egmond M, Niessen FB, Beelen RH. Macrophages in skin injury and repair. Immunobiology. 2011;216:753–62. doi: 10.1016/j.imbio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Muller W, et al. Differential roles of macrophages in diverse phases of skin repair. Journal of immunology (Baltimore, Md : 1950) 2010;184:3964–77. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- 23.Mirza R, DiPietro LA, Koh TJ. Selective and specific macrophage ablation is detrimental to wound healing in mice. The American journal of pathology. 2009;175:2454–62. doi: 10.2353/ajpath.2009.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagaoka T, Kaburagi Y, Hamaguchi Y, Hasegawa M, Takehara K, Steeber DA, et al. Delayed wound healing in the absence of intercellular adhesion molecule-1 or L-selectin expression. The American journal of pathology. 2000;157:237–47. doi: 10.1016/S0002-9440(10)64534-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishida Y, Gao JL, Murphy PM. Chemokine receptor CX3CR1 mediates skin wound healing by promoting macrophage and fibroblast accumulation and function. Journal of immunology (Baltimore, Md : 1950) 2008;180:569–79. doi: 10.4049/jimmunol.180.1.569. [DOI] [PubMed] [Google Scholar]
- 26.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nature reviews Immunology. 2011;11:723–37. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sindrilaru A, Scharffetter-Kochanek K. Disclosure of the Culprits: Macrophages-Versatile Regulators of Wound Healing. Advances in wound care. 2013;2:357–68. doi: 10.1089/wound.2012.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Odegaard JI, Chawla A. Alternative macrophage activation and metabolism. Annual review of pathology. 2011;6:275–97. doi: 10.1146/annurev-pathol-011110-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nature immunology. 2010;11:889–96. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 31.Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert reviews in molecular medicine. 2011;13:e23. doi: 10.1017/S1462399411001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Novak ML, Koh TJ. Phenotypic transitions of macrophages orchestrate tissue repair. Am J Pathology. 2013;183:1352–1363. doi: 10.1016/j.ajpath.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barros MH, Hauck F, Dreyer JH, Kempkes B, Niedobitek G. Macrophage polarisation: an immunohistochemical approach for identifying M1 and M2 macrophages. PLoS One. 2013;8:e80908. doi: 10.1371/journal.pone.0080908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murray PJ. The primary mechanism of the IL-10-regulated antiinflammatory response is to selectively inhibit transcription. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:8686–91. doi: 10.1073/pnas.0500419102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gordon S. Alternative activation of macrophages. Nature reviews Immunology. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 36.Biswas SK, Mantovani A. Orchestration of metabolism by macrophages. Cell Metab. 2012;15:432–7. doi: 10.1016/j.cmet.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 37.Weidenbusch M, Anders HJ. Tissue microenvironments define and get reinforced by macrophage phenotypes in homeostasis or during inflammation, repair and fibrosis. Journal of innate immunity. 2012;4:463–77. doi: 10.1159/000336717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daley JM, Brancato SK, Thomay AA, Reichner JS, Albina JE. The phenotype of murine wound macrophages. Journal of leukocyte biology. 2010;87:59–67. doi: 10.1189/jlb.0409236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao W, Hong H, Kawakami Y, Lowell CA, Kawakami T. Regulation of myeloproliferation and M2 macrophage programming in mice by Lyn/Hck, SHIP, and Stat5. The Journal of clinical investigation. 2008;118:924–34. doi: 10.1172/JCI34013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lucas M, Stuart LM, Savill J, Lacy-Hulbert A. Apoptotic cells and innate immune stimuli combine to regulate macrophage cytokine secretion. Journal of immunology (Baltimore, Md : 1950) 2003;171:2610–5. doi: 10.4049/jimmunol.171.5.2610. [DOI] [PubMed] [Google Scholar]
- 41.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. The Journal of clinical investigation. 1998;101:890–8. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–52. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–6. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 44.Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649–56. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- 45.Harney AS, Arwert EN, Entenberg D, Wang Y, Guo P, Qian BZ, et al. Real-Time Imaging Reveals Local, Transient Vascular Permeability, and Tumor Cell Intravasation Stimulated by TIE2hi Macrophage-Derived VEGFA. Cancer discovery. 2015;5:932–43. doi: 10.1158/2159-8290.CD-15-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frankenberger C, Rabe D, Bainer R, Sankarasharma D, Chada K, Krausz T, et al. Metastasis Suppressors Regulate the Tumor Microenvironment by Blocking Recruitment of Prometastatic Tumor-Associated Macrophages. Cancer Res. 2015;75:4063–73. doi: 10.1158/0008-5472.CAN-14-3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, et al. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–52. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–8. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 49.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–66. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Munder M, Eichmann K, Modolell M. Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. J Immunol. 1998;160:5347–54. [PubMed] [Google Scholar]
- 51.Sheng J, Chen W, Zhu HJ. The immune suppressive function of transforming growth factor-beta (TGF-beta) in human diseases. Growth Factors. 2015;33:92–101. doi: 10.3109/08977194.2015.1010645. [DOI] [PubMed] [Google Scholar]
- 52.Yoshimura A, Muto G. TGF-beta function in immune suppression. Curr Top Microbiol Immunol. 2011;350:127–47. doi: 10.1007/82_2010_87. [DOI] [PubMed] [Google Scholar]
- 53.Liu VC, Wong LY, Jang T, Shah AH, Park I, Yang X, et al. Tumor evasion of the immune system by converting CD4+CD25− T cells into CD4+CD25+ T regulatory cells: role of tumor-derived TGF-beta. J Immunol. 2007;178:2883–92. doi: 10.4049/jimmunol.178.5.2883. [DOI] [PubMed] [Google Scholar]
- 54.Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: the role of T regulatory cells. Immunology. 2006;117:433–42. doi: 10.1111/j.1365-2567.2006.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu L, Kitani A, Strober W. Molecular mechanisms regulating TGF-beta-induced Foxp3 expression. Mucosal immunology. 2010;3:230–8. doi: 10.1038/mi.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grutz G. New insights into the molecular mechanism of interleukin-10-mediated immunosuppression. J Leukoc Biol. 2005;77:3–15. doi: 10.1189/jlb.0904484. [DOI] [PubMed] [Google Scholar]
- 57.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 58.Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CM, Pryer N, et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26:623–37. doi: 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nature reviews Immunology. 2005;5:641–54. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 60.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–90. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rodriguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev. 2008;222:180–91. doi: 10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature reviews Immunology. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–31. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 65.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–96. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kaneda MM, Cappello P, Nguyen AV, Ralainirina N, Hardamon CR, Foubert P, et al. Macrophage PI3Kgamma Drives Pancreatic Ductal Adenocarcinoma Progression. Cancer discovery. 2016;6:870–85. doi: 10.1158/2159-8290.CD-15-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaneda MM, Messer KS, Ralainirina N, Li H, Leem CJ, Gorjestani S, et al. PI3Kgamma is a molecular switch that controls immune suppression. Nature. 2016;539:437–42. doi: 10.1038/nature19834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nature reviews Immunology. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang S, Ma X, Zhu C, Liu L, Wang G, Yuan X. The Role of Myeloid-Derived Suppressor Cells in Patients with Solid Tumors: A Meta-Analysis. PLoS One. 2016;11:e0164514. doi: 10.1371/journal.pone.0164514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–53. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Doedens AL, Stockmann C, Rubinstein MP, Liao D, Zhang N, DeNardo DG, et al. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res. 2010;70:7465–75. doi: 10.1158/0008-5472.CAN-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–21. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 73.Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell. 2008;13:193–205. doi: 10.1016/j.ccr.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Palma M, Venneri MA, Galli R, Sergi LS, Politi LS, Sampaolesi M, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–26. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 75.Chen L, Li J, Wang F, Dai C, Wu F, Liu X, et al. Tie2 Expression on Macrophages Is Required for Blood Vessel Reconstruction and Tumor Relapse after Chemotherapy. Cancer Res. 2016;76:6828–38. doi: 10.1158/0008-5472.CAN-16-1114. [DOI] [PubMed] [Google Scholar]
- 76.Pucci F, Venneri MA, Biziato D, Nonis A, Moi D, Sica A, et al. A distinguishing gene signature shared by tumor-infiltrating Tie2-expressing monocytes, blood “resident” monocytes, and embryonic macrophages suggests common functions and developmental relationships. Blood. 2009;114:901–14. doi: 10.1182/blood-2009-01-200931. [DOI] [PubMed] [Google Scholar]
- 77.Lewis CE, Harney AS, Pollard JW. The Multifaceted Role of Perivascular Macrophages in Tumors. Cancer Cell. 2016;30:365. doi: 10.1016/j.ccell.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 78.Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–8. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 79.Huang H, Bhat A, Woodnutt G, Lappe R. Targeting the ANGPT-TIE2 pathway in malignancy. Nat Rev Cancer. 2010;10:575–85. doi: 10.1038/nrc2894. [DOI] [PubMed] [Google Scholar]
- 80.Mazzieri R, Pucci F, Moi D, Zonari E, Ranghetti A, Berti A, et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell. 2011;19:512–26. doi: 10.1016/j.ccr.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 81.Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest. 2010;120:694–705. doi: 10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen FH, Chiang CS, Wang CC, Tsai CS, Jung SM, Lee CC, et al. Radiotherapy decreases vascular density and causes hypoxia with macrophage aggregation in TRAMP-C1 prostate tumors. Clin Cancer Res. 2009;15:1721–9. doi: 10.1158/1078-0432.CCR-08-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Russell JS, Brown JM. The irradiated tumor microenvironment: role of tumor-associated macrophages in vascular recovery. Frontiers in physiology. 2013;4:157. doi: 10.3389/fphys.2013.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Welford AF, Biziato D, Coffelt SB, Nucera S, Fisher M, Pucci F, et al. TIE2-expressing macrophages limit the therapeutic efficacy of the vascular-disrupting agent combretastatin A4 phosphate in mice. J Clin Invest. 2011 doi: 10.1172/JCI44562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nakasone ES, Askautrud HA, Kees T, Park JH, Plaks V, Ewald AJ, et al. Imaging tumor-stroma interactions during chemotherapy reveals contributions of the microenvironment to resistance. Cancer Cell. 2012;21:488–503. doi: 10.1016/j.ccr.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer discovery. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shree T, Olson OC, Elie BT, Kester JC, Garfall AL, Simpson K, et al. Macrophages and cathepsin proteases blunt chemotherapeutic response in breast cancer. Genes Dev. 2011;25:2465–79. doi: 10.1101/gad.180331.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deng L, Stafford JH, Liu S-C, Chernikova SB, Merchant M, Recht L, et al. SDF-1 blockade enhances anti-VEGF therapy of glioblastoma and can be monitored by MRI. Neoplasia. 2016;19:1–7. doi: 10.1016/j.neo.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ferrara N. Role of myeloid cells in vascular endothelial growth factor-independent tumor angiogenesis. Current opinion in hematology. 2010;17:219–24. doi: 10.1097/MOH.0b013e3283386660. [DOI] [PubMed] [Google Scholar]
- 90.Stafford JH, Hirai T, Deng L, Chernikova SB, Urata K, West BL, et al. Colony stimulating factor 1 receptor inhibition delays recurrence of glioblastoma after radiation by altering myeloid cell recruitment and polarization. Neuro Oncol. 2016;18:797–806. doi: 10.1093/neuonc/nov272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sanford DE, Belt BA, Panni RZ, Mayer A, Deshpande AD, Carpenter D, et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res. 2013;19:3404–15. doi: 10.1158/1078-0432.CCR-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Escamilla J, Schokrpur S, Liu C, Priceman SJ, Moughon D, Jiang Z, et al. CSF1 receptor targeting in prostate cancer reverses macrophage-mediated resistance to androgen blockade therapy. Cancer Res. 2015;75:950–62. doi: 10.1158/0008-5472.CAN-14-0992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74:5057–69. doi: 10.1158/0008-5472.CAN-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baer C, Squadrito ML, Laoui D, Thompson D, Hansen SK, Kiialainen A, et al. Suppression of microRNA activity amplifies IFN-gamma-induced macrophage activation and promotes anti-tumour immunity. Nat Cell Biol. 2016;18:790–802. doi: 10.1038/ncb3371. [DOI] [PubMed] [Google Scholar]
- 95.Sugimura K, Miyata H, Tanaka K, Takahashi T, Kurokawa Y, Yamasaki M, et al. High infiltration of tumor-associated macrophages is associated with a poor response to chemotherapy and poor prognosis of patients undergoing neoadjuvant chemotherapy for esophageal cancer. J Surg Oncol. 2015;111:752–9. doi: 10.1002/jso.23881. [DOI] [PubMed] [Google Scholar]
- 96.Manthey CL, Johnson DL, Illig CR, Tuman RW, Zhou Z, Baker JF, et al. JNJ-28312141, a novel orally active colony-stimulating factor-1 receptor/FMS-related receptor tyrosine kinase-3 receptor tyrosine kinase inhibitor with potential utility in solid tumors, bone metastases, and acute myeloid leukemia. Mol Cancer Ther. 2009;8:3151–61. doi: 10.1158/1535-7163.MCT-09-0255. [DOI] [PubMed] [Google Scholar]
- 97.Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–72. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moughon DL, He H, Schokrpur S, Jiang ZK, Yaqoob M, David J, et al. Macrophage Blockade Using CSF1R Inhibitors Reverses the Vascular Leakage Underlying Malignant Ascites in Late-Stage Epithelial Ovarian Cancer. Cancer Res. 2015;75:4742–52. doi: 10.1158/0008-5472.CAN-14-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu SC, Alomran R, Chernikova SB, Lartey F, Stafford J, Jang T, et al. Blockade of SDF-1 after irradiation inhibits tumor recurrences of autochthonous brain tumors in rats. Neuro Oncol. 2014;16:21–8. doi: 10.1093/neuonc/not149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Walters MJ, Ebsworth K, Berahovich RD, Penfold ME, Liu SC, Al Omran R, et al. Inhibition of CXCR7 extends survival following irradiation of brain tumours in mice and rats. Br J Cancer. 2014;110:1179–88. doi: 10.1038/bjc.2013.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gupta A, Probst HC, Vuong V, Landshammer A, Muth S, Yagita H, et al. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J Immunol. 2012;189:558–66. doi: 10.4049/jimmunol.1200563. [DOI] [PubMed] [Google Scholar]
- 102.Rivera LB, Meyronet D, Hervieu V, Frederick MJ, Bergsland E, Bergers G. Intratumoral myeloid cells regulate responsiveness and resistance to antiangiogenic therapy. Cell Rep. 2015;11:577–91. doi: 10.1016/j.celrep.2015.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chang AL, Miska J, Wainwright DA, Dey M, Rivetta CV, Yu D, et al. CCL2 Produced by the Glioma Microenvironment Is Essential for the Recruitment of Regulatory T Cells and Myeloid-Derived Suppressor Cells. Cancer Res. 2016;76:5671–82. doi: 10.1158/0008-5472.CAN-16-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Coffelt SB, de Visser KE. Immune-mediated mechanisms influencing the efficacy of anticancer therapies. Trends Immunol. 2015;36:198–216. doi: 10.1016/j.it.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 105.Engblom C, Pfirschke C, Pittet MJ. The role of myeloid cells in cancer therapies. Nat Rev Cancer. 2016;16:447–62. doi: 10.1038/nrc.2016.54. [DOI] [PubMed] [Google Scholar]
- 106.Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27:462–72. doi: 10.1016/j.ccell.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nature reviews Clinical oncology. 2017 doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ahn GO, Tseng D, Liao CH, Dorie MJ, Czechowicz A, Brown JM. Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc Natl Acad Sci U S A. 2010;107:8363–8. doi: 10.1073/pnas.0911378107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim JH, Kolozsvary A, Jenrow KA, Brown SL. Plerixafor, a CXCR4 antagonist, mitigates skin radiation-induced injury in mice. Radiat Res. 2012;178:202–6. doi: 10.1667/rr2886.1. [DOI] [PubMed] [Google Scholar]
- 110.Chaudary N, Pintilie M, Jelveh S, Lindsay P, Hill RP, Milosevic M. Plerixafor Improves Primary Tumor Response and Reduces Metastases in Cervical Cancer Treated with Radio-Chemotherapy. Clin Cancer Res. 2017;23(5):1242–9. doi: 10.1158/1078-0432.CCR-16-1730. [DOI] [PubMed] [Google Scholar]
- 111.Acharya MM, Christie LA, Lan ML, Donovan PJ, Cotman CW, Fike JR, et al. Rescue of radiation-induced cognitive impairment through cranial transplantation of human embryonic stem cells. Proc Natl Acad Sci U S A. 2009;106:19150–5. doi: 10.1073/pnas.0909293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Quail DF, Bowman RL, Akkari L, Quick ML, Schuhmacher AJ, Huse JT, et al. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science. 2016;352:aad3018. doi: 10.1126/science.aad3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thomas RP, Nagpal S, Michael I, Soltys SG, Corbin Z, Xu LW, et al. A phase I study of chemo-radiotherapy with plerixafor for newly diagnosed glioblastoma (GB) J Clin Oncol. 2016;34(suppl) (abstr 2068) [Google Scholar]
- 114.Butowski N, Colman H, De Groot JF, Omuro AM, Nayak L, Wen PY, et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro Oncol. 2016;18:557–64. doi: 10.1093/neuonc/nov245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xu J, Escamilla J, Mok S, David J, Priceman S, West B, et al. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73:2782–94. doi: 10.1158/0008-5472.CAN-12-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cassier PA, Italiano A, Gomez-Roca CA, Le Tourneau C, Toulmonde M, Cannarile MA, et al. CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: a dose-escalation and dose-expansion phase 1 study. Lancet Oncol. 2015;16:949–56. doi: 10.1016/S1470-2045(15)00132-1. [DOI] [PubMed] [Google Scholar]
- 117.Sandhu SK, Papadopoulos K, Fong PC, Patnaik A, Messiou C, Olmos D, et al. A first-in-human, first-in-class, phase I study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 in patients with solid tumors. Cancer chemotherapy and pharmacology. 2013;71:1041–50. doi: 10.1007/s00280-013-2099-8. [DOI] [PubMed] [Google Scholar]
- 118.Brana I, Calles A, LoRusso PM, Yee LK, Puchalski TA, Seetharam S, et al. Carlumab, an anti-C-C chemokine ligand 2 monoclonal antibody, in combination with four chemotherapy regimens for the treatment of patients with solid tumors: an open-label, multicenter phase 1b study. Target Oncol. 2015;10:111–23. doi: 10.1007/s11523-014-0320-2. [DOI] [PubMed] [Google Scholar]
- 119.Pienta KJ, Machiels JP, Schrijvers D, Alekseev B, Shkolnik M, Crabb SJ, et al. Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. Invest New Drugs. 2013;31:760–8. doi: 10.1007/s10637-012-9869-8. [DOI] [PubMed] [Google Scholar]
- 120.Vela M, Aris M, Llorente M, Garcia-Sanz JA, Kremer L. Chemokine receptor-specific antibodies in cancer immunotherapy: achievements and challenges. Front Immunol. 2015;6:12. doi: 10.3389/fimmu.2015.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Meng Y, Beckett MA, Liang H, Mauceri HJ, van Rooijen N, Cohen KS, et al. Blockade of tumor necrosis factor alpha signaling in tumor-associated macrophages as a radiosensitizing strategy. Cancer Res. 2010;70:1534–43. doi: 10.1158/0008-5472.CAN-09-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kozin SV, Kamoun WS, Huang Y, Dawson MR, Jain RK, Duda DG. Recruitment of myeloid but not endothelial precursor cells facilitates tumor regrowth after local irradiation. Cancer Res. 2010;70:5679–85. doi: 10.1158/0008-5472.CAN-09-4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen FH, Fu SY, Yang YC, Wang CC, Chiang CS, Hong JH. Combination of vessel-targeting agents and fractionated radiation therapy: the role of the SDF-1/CXCR4 pathway. Int J Radiat Oncol Biol Phys. 2013;86:777–84. doi: 10.1016/j.ijrobp.2013.02.036. [DOI] [PubMed] [Google Scholar]
- 124.Domanska UM, Boer JC, Timmer-Bosscha H, van Vugt MA, Hoving HD, Kliphuis NM, et al. CXCR4 inhibition enhances radiosensitivity, while inducing cancer cell mobilization in a prostate cancer mouse model. Clin Exp Metastasis. 2014;31:829–39. doi: 10.1007/s10585-014-9673-2. [DOI] [PubMed] [Google Scholar]
- 125.Wang SC, Yu CF, Hong JH, Tsai CS, Chiang CS. Radiation therapy-induced tumor invasiveness is associated with SDF-1-regulated macrophage mobilization and vasculogenesis. PLoS One. 2013;8:e69182. doi: 10.1371/journal.pone.0069182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Paulus P, Stanley ER, Schafer R, Abraham D, Aharinejad S. Colony-stimulating factor-1 antibody reverses chemoresistance in human MCF-7 breast cancer xenografts. Cancer Res. 2006;66:4349–56. doi: 10.1158/0008-5472.CAN-05-3523. [DOI] [PubMed] [Google Scholar]
- 127.Voloshin T, Gingis-Velitski S, Bril R, Benayoun L, Munster M, Milsom C, et al. G-CSF supplementation with chemotherapy can promote revascularization and subsequent tumor regrowth: prevention by a CXCR4 antagonist. Blood. 2011;118:3426–35. doi: 10.1182/blood-2010-11-320812. [DOI] [PMC free article] [PubMed] [Google Scholar]