Abstract
Study Objectives:
As lung volume decreases radial traction on the upper airway is reduced, making it more collapsible. The purpose of this study was to measure change in end-expiratory lung volume (EELV) following sleep onset and to evaluate the relationship between change in EELV and sleep-disordered breathing.
Methods:
Twenty subjects underwent overnight polysomnography, of whom 14 (70%) had obstructive sleep apnea (OSA). Change in EELV was measured throughout the night using magnetometry. Sleep was staged and respiratory events scored using American Academy of Sleep Medicine criteria. An additional 10 subjects had change in EELV measured simultaneously by magnetometer and spirometer while awake.
Results:
In the subjects studied while awake, change in EELV calculated from magnetometer data correlated very strongly (r = 0.89, P < .001) with that obtained by spirometry. In the 20 subjects who underwent polysomnography, there was a decline in EELV for sleep stages N1, N2, N3, and R (REM sleep); 17.9 ± 121.0 mL (mean ± standard deviation), 228.5 ± 151.8 mL, 198.1 ± 122.1 mL, and 316.7 ± 131.9 mL, respectively. Mean EELV reduction during stage R sleep doubled that noted during non-stage R sleep (316.7 ± 131.9 mL versus 150.9 ± 89.7 mL, respectively) (P < .001). The difference in EELV between non-stage R and stage R sleep inversely correlated with mean oxygen saturation (r = −0.56, P = .06). EELV reduction in individuals with moderate and severe OSA was greater than in those with mild SDB but did not reach statistical significance.
Conclusions:
Magnetometry provides a precise, unobtrusive, and continuous means to study lung volume changes during sleep. EELV declines from sleep onset, reaching its nadir during stage R sleep. The reduction in EELV in stage R sleep was associated with lower mean oxygen saturation but was not associated with greater sleep-disordered breathing.
Citation:
Koo P, Gartman EJ, Sethi JM, Kawar E, McCool FD. Change in end-expiratory lung volume during sleep in patients at risk for obstructive sleep apnea. J Clin Sleep Med. 2017;13(8):941–947.
Keywords: end-expiratory lung volume, magnetometry, REM sleep, sleep-disordered breathing
INTRODUCTION
Obstructive sleep apnea (OSA) is a common cause of disrupted sleep, affecting approximately 15% of males and 10% of females.1–2 It is characterized by repetitive narrowing and opening of the upper airway resulting in reduced or absent airflow, oxyhemoglobin desaturation, and sleep fragmentation. Risk factors for developing OSA include obesity,3 male sex,4 older age,5 and the postmenopausal state.6 The pathophysiology of OSA can be related to craniofacial abnormalities, loss of upper airway muscle tone, or factors that increase upper airways resistance such as enlarged tonsils or redundant pharyngeal tissue.7 Sleep-disordered breathing (SDB) may be aggravated during sleep stages when upper airway muscle activation is reduced or absent.8 The loss of upper airway dilator muscle function makes the airway more likely to collapse when intraluminal airway pressure becomes subatmospheric during inspiration. Lung volume also affects upper airway collapsibility.9 As lung volume increases, there is greater caudal traction on the upper airway, which serves to stiffen the upper airway and thereby making it less collapsible and less likely to limit inspiratory airflow.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Sleep-disordered breathing (SDB) may be worsened by reductions in end-expiratory lung volume (EELV). However, there are few reports examining change in EELV following sleep onset and during stage R (REM sleep). Previous studies incorporated invasive means to study lung volume changes. These methods may have interfered with sleep or induced change in EELV. The current study investigated whether noninvasive measures of chest wall motion using magnetometry could be used to measure change in EELV during sleep and if changes were related to indices of SDB.
Study Impact: Measurements of change in EELV with magnetometry can be used during sleep and provide a precise, noninvasive, continuous alternative to more invasive methods used to measure change in EELV. The significant reduction in EELV during stage R sleep compared to non-stage R sleep may be one mechanism accounting for the predominance of SDB during stage R sleep noted in some individuals.
Typically, end-expiratory lung volume (EELV) decreases following sleep onset. This reduction in EELV may be due to cephalad displacement of the diaphragm when an individual assumes the recumbent position or because of inspiratory muscle hypotonia or atonia. As previously discussed, any reduction in EELV during sleep may promote SDB by decreasing caudal traction of the lung on the upper airway.10 In addition, a reduction in EELV would promote SDB by reducing pharyngeal cross-sectional area and increasing airway resistance. The purpose of this study was to quantitate the change in EELV (ΔEELV) following sleep onset in subjects referred for diagnostic polysomnography (PSG) and to evaluate the relationship between ΔEELV and SDB. We postulated that SDB would be greatest (1) in individuals with the most pronounced reductions in EELV and (2) during stage R (REM sleep) when muscle hypotonia or atonia is greatest.
METHODS
Subjects
We enrolled 30 adult subjects. All subjects were referred for baseline PSG between November 2011 and August 2013. Subjects were selected at random during the night of PSG to participate in the study. They were excluded if they were referred for positive airway pressure titration. Twenty subjects underwent overnight PSG with magnetometry. Eleven subjects participated in the magnetometer validation protocol, which was performed while the subjects were awake. Of the subjects who participated in the magnetometer validation protocol, 10 did not participate in the main study. Prior to enrollment, written informed consent was obtained. The Institutional Review Board at the Memorial Hospital of Rhode Island approved the study.
Measurements
The subjects arrived at the sleep laboratory 2 hours prior to the start of the study for evaluation, overview, and preparation. The sleep technologist attached all PSG sensors according to standard American Academy of Sleep Medicine (AASM) protocols. A custom-made magnetometer system was used to measure ΔEELV as previously reported.11 Briefly, two pairs of magnetic coils were attached to microfoam tape, which then was adhered to the skin to create a stable platform. One pair of coils was attached anteriorly over the manubrium just above the xiphoid process and posteriorly to the mid-upper back at the same level. The other pair of coils was attached anteriorly 2 cm above the umbilicus and posteriorly to the mid-lower back at the same level. The coils were secured with hypoallergenic silk tape. The magnetometer system was calibrated using a 3-L calibration syringe. The subject then breathed through a flow-meter while lying in each of the following body positions: supine, right lateral, and left lateral decubitus positions (5 breaths at varied tidal volumes totaling 15 to 20 breaths per body position). The magnetometer signals were sampled at a rate of 15 Hz. These data were used to calculate coefficients for the following equation:
where α, β, and γ are coefficients for the rib cage (RC), the abdomen (Ab), and the axial displacement of the chest wall (Xi), respectively. Regression coefficients for Equation 1 were determined for the supine, left, and right lateral decubitus positions. Magnetometer readings acquired during the overnight PSG were used postacquisition to construct a spirogram of VT versus time. Because the regression coefficients for each variable are weighted to give the best fit for measured tidal volume, their absolute values are highly variable and have little physiologic meaning on their own.
The acquisition of PSG data started shortly after completion of the magnetometer calibration process. Data recording continued until the completion of the PSG in the morning. The PSG was scored for sleep stage and respiratory events using the following AASM criteria: apneas were defined as a greater than 90% decrease in the peak thermal sensor excursion lasting 10 or more seconds. Hypopneas were defined as a greater than 30% decrease from baseline in nasal pressure lasting 10 or more seconds associated with a 4% or greater oxygen desaturation from pre-event baseline. Epochs of sleep with acceptable magnetometer signals were selected for each subject. Position-specific regression coefficients were applied to the matching magnetometer segment, and magnetometer derived values of VT were used to construct a spirogram. ΔEELV for each breath was calculated as the difference between the EELV at sleep onset and the EELV for subsequent breaths. The total number of observations (breaths) varied across subjects and sleep stage. The range of observations per subject for stage N1 was 5 to 50; for stage N2 was 10 to 1,200; for stage N3 was 20 to 500; and for stage R was 20 to 600. The mean value of ΔEELV for all breaths was determined for each sleep stage for each subject.
Validation of Magnetometer-Derived ΔEELV in Subjects Presenting for Polysomnography
We previously demonstrated that ΔEELV could be accurately measured using magnetometers.12 Because the precision of magnetometry-derived values of ΔEELV with overweight and obese subjects presenting for PSG is unknown, we performed a validation study on 11 subjects who were referred for PSG. A computer-based, handheld spirometer (HDpft 1000, nSpire Health, Longmont, Colorado, United States) was brought to the subject's room in the sleep center. The spirometer was calibrated using a 3-L calibrating syringe. The magnetometer coils were attached to the subject and calibrated as previously described. The subject wore nose clips and was asked to lie supine and breathe through a mouthpiece and filter connected to a spirometer at various levels of EELV for 15 to 20 breaths. The ΔEELV was determined from the spirometer and magnetometer data by measuring the difference between EELV of the initial breath (set as zero) and EELV of the subsequent breaths. Pearson correlation was used to measure the linear relationship between the magnetometer- and spirometer-derived ΔEELV. A Bland-Altman plot was constructed to assess agreement between the two measurements.13
Comparison of ΔEELV Between Non-Stage R and Stage R Sleep
Pearson correlation was used to analyze the association between ΔEELV for non-stage R or stage R sleep and the following measurements: body mass index (BMI), apnea-hypopnea index (AHI), mean oxygen saturation, nadir oxygen saturation, and absolute time spent with oxygen saturation less than 90% during stage R sleep. ΔEELV in stage R sleep, the sleep stage with the greatest reduction in EELV that would most affect airway collapsibility, was correlated with the previously mentioned measurements.
Welch analysis of variance was used to compare individual mean ΔEELV across the sleep stages. Subsequently, post hoc pairwise analysis accounting for multiple comparisons was performed using Tukey-Kramer adjustment. Unpaired two-sample t test with equal variance was used to compare mean ΔEELV between non-stage R and stage R sleep, mean ΔEELV during stage R sleep between mild and severe OSA, and mean ΔEELV during stage R sleep between sexes.
All statistical analyses and generation of plots were performed using Stata 13.0 SE (StataCorp LP, College Station, Texas, United States).
RESULTS
The baseline characteristics of the 11 subjects studied for validation of measurements of ΔEELV are shown in Table 1. Eight of the subjects were overweight or obese. An example of the magnetometer and spirometer data for one subject is shown in Figure 1. The ΔEELV calculated from magnetometer data correlated very strongly (r = 0.89, P < .001) with those obtained from a spirometer. Using Bland-Altman analysis, we found that the agreement between magnetometry-derived and spirometry-derived ΔEELV was mainly within the 95% confidence interval, and therefore, the average bias is low (Figure 2).
Table 1.
Baseline characteristics subjects recruited to validate the use of magnetometry to measure lung volume.

Figure 1. Magnetometer and spirometer volumes.
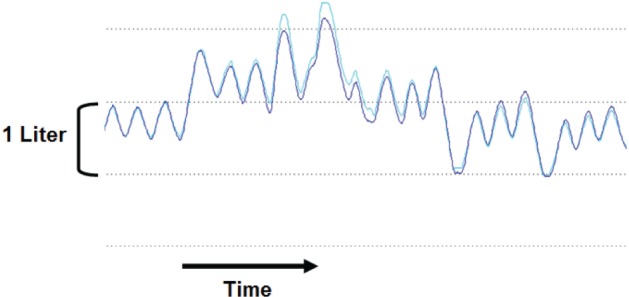
Spirogram for spirometer (dark blue) and magnetometer (light blue) derived volumes in one subject who was asked to increase and then decrease end-expiratory lung volume (EELV) during a validation study. The change in EELV (ΔEELV) for both magnetometer and spirometer is measured relative to the subjects relaxed EELV (first three breaths).
Figure 2. Bland-Altman plot of spirometer- and magnetometer-derived ΔEELV to examine the agreement between the two tests.
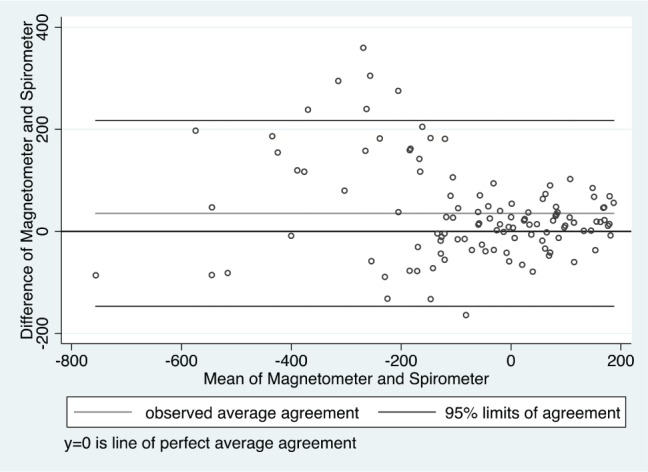
The units for the y-axis and x-axis are expressed in milliliters. Most of the comparisons lay within the 95% confidence limits with only a few outliers. Therefore, the average discrepancy between the spirometer-derived ΔEELV and magnetometer-derived ΔEELV is small and clinically insignificant. ΔEELV = change in end-expiratory lung volume.
The baseline characteristics of the 20 subjects (10 males and 10 females) who underwent overnight PSG are shown in Table 2. Of the 20 subjects, 14 (70%) had OSA, ranging from mild to severe. An example of the three magnetomer-derived chest wall signals obtained during sleep with the derived tidal volume is presented in Figure 3. Individual mean ΔEELV distributed by sleep stages are presented in Table 3. In general, following sleep onset, there was a small decline in EELV in stage N1, a moderate decline in stages N2 and N3, and the greatest decline in stage R sleep (Figure 4). The mean decrease in ΔEELV from wakefulness for stages N1, N2, N3, and R sleep was 17.9 ± 121.0 mL (mean ± standard deviation), 228.5 ± 151.8 mL, 198.1 ± 122.1 mL, and 316.7 ± 131.9 mL, respectively. When partitioning sleep into stage R and non-stage R, the reduction in ΔEELV during stage R sleep was significantly greater than that during non-stage R sleep (316.7 ± 131.9 mL versus 150.9 ± 89.7 mL, respectively) (P < .001).
Table 2.
Baseline characteristics of subjects recruited during the night of polysomnography.
Figure 3. Chest wall dimensions and magnetometer volume in stage N2 sleep.
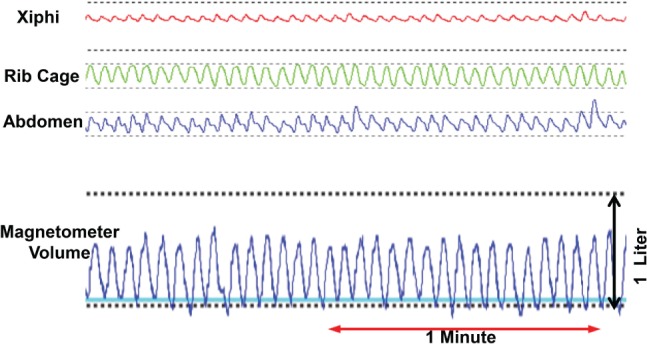
Sample magnetometer signals (xiphi-umbilical, rib cage, and abdomen) and derived tidal volume tracings during a stage N2 sleep segment. For the volume tracing, the y-axis is volume and x-axis is time.
Table 3.
End-expiratory lung volume of each subject distributed by sleep stages.
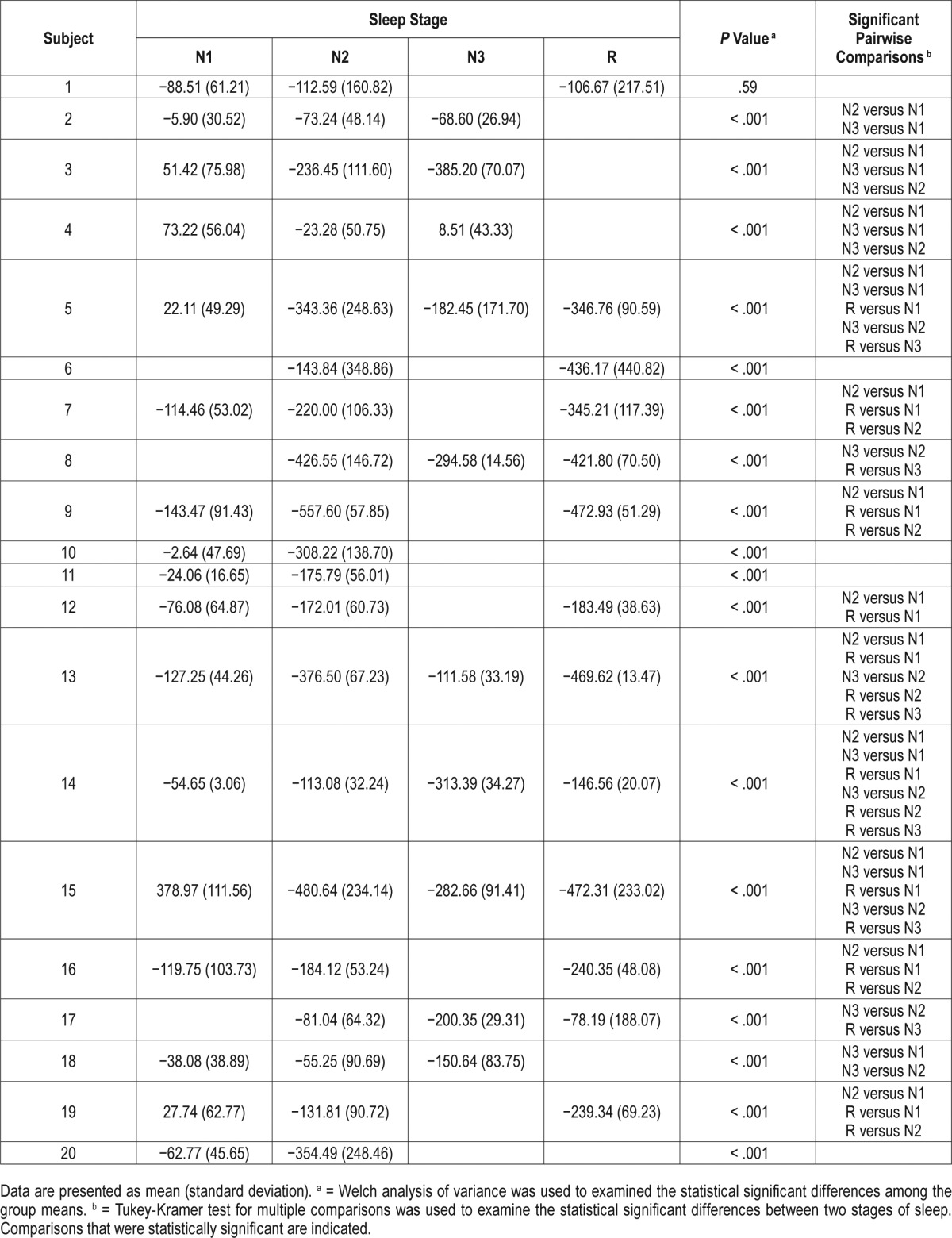
Figure 4. Mean ΔEELV between stages of sleep.
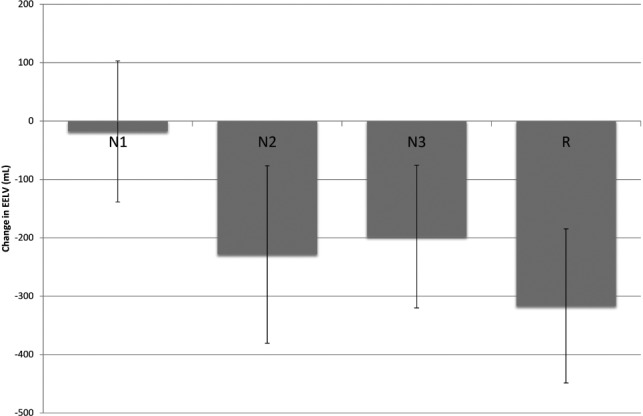
Bar graph depicting ΔEELV with standard deviations through different stages of sleep: N1, N2, N3, and R. The difference in variance of ΔEELV between each stage of sleep is similar. Mean ΔEELV between non-stage R and stage R sleep was statistically significant. See Table 3 for significant changes for each subject. ΔEELV = change in end-expiratory lung volume.
The absolute reduction in EELV during either non-stage R or stage R sleep did not significantly correlate with AHI, BMI, mean and nadir oxygen saturation, or absolute time spent with oxygen saturation less than 90%. There was a better association between the decline in EELV between non-stage R and stage R sleep and mean oxygen saturation (r = −0.56, P = .06) and with absolute time spent with oxygen saturation less than 90% (r = 0.50, P = .10), but this also did not reach statistical significance.
When evaluating only the 14 subjects with OSA, the reduction in EELV during stage R sleep was greater in those with moderate to severe OSA compared to mild OSA (372.3 ± 106.2 mL versus 271.6 ± 119.8 mL, respectively), but the difference was not statistically significant (P = .21). There were no significant associations between ΔEELV during stage R sleep and AHI, BMI, mean and nadir oxygen saturation, and absolute time spent with oxygen saturation < 90% for this cohort. ΔEELV during stage R sleep did not differ between females and males (377.7 ± 155.8 mL versus 289.6 ± 119.5 mL, respectively; P = .28).
DISCUSSION
We found that magnetometry-derived ΔEELV had a strong linear relationship and agreement with spirometry-derived ΔEELV. In addition, we noted that (1) EELV declined from sleep onset to later stages of sleep; (2) there was a significant reduction in EELV during stage R sleep when compared to non-stage R sleep; (3) individuals with moderate to severe OSA had greater reductions in EELV than those with no or mild OSA; and (4) ΔEELV as one transitions from non-stage R to stage R sleep was weakly associated with characteristics of OSA, such as lower nocturnal oxygenation and greater time spent with oxygen saturation less than 90%. These observed trends were consistent with the notion that greater reductions in EELV during sleep will decrease radial traction of the upper airway and worsen SDB.
The relationship between ΔEELV and sleep stage is not well described. Hudgel and Devadatta used helium dilution in 10 normal-weight subjects and found that EELV was reduced by 190 mL from wakefulness to stage N2 and by as much as 310 mL in stage R sleep, when muscle atonia is present. The reduction in EELV reported by Hudgel and Devadatta was similar to what we report.10 However, they did not study subjects with SDB and their use of tight-fitting facemasks and the need to frequently awaken their subjects would affect breathing pattern and limit reproducibility.
Stadler et al.14 extended the observations of Hudgel and Devadatta10 to patients with OSA. They used magnetometry to measure ΔEELV following sleep onset in 8 subjects with OSA and 8 healthy controls and found that EELV decreased by 61 mL in OSA patients and by 34 mL in controls following sleep onset. There were no data for stage N3 or stage R sleep. In the patients with OSA, the reduction in EELV during stage N1 was greater than that in our findings. Their study was limited by subject instrumentation with esophageal catheters, which may have influenced breathing pattern and lowered EELV. In addition, sleep was recorded only for 5 minutes. Our study extended these finding to progressive stages of sleep (N2, N3, and R sleep) over longer durations of time.
In contrast to lowering EELV, increasing lung volume during sleep improves OSA. Heinzer et al. increased EELV in subjects with sleep apnea by applying negative extrathoracic pressure to the thorax.15 EELV was increased to the same volume associated with a given level of continuous positive airway pressure (CPAP). This design allowed the investigators to isolate the effects of lung volume from the effects of CPAP on the upper airway. They found that increasing lung volume alone significantly reduced AHI, 3% desaturation index, and arousal index. Furthermore, the same group later demonstrated that increasing EELV in combination with stimulation of the genioglossus did not significantly affect pharyngeal critical pressure (Pcrit) compared to just increasing EELV alone.16 These studies confirmed that EELV significantly affects pharyngeal collapsibility. The increase in EELV that Heinzer et al. induced with negative extrathoracic pressure to eliminate SDB was greater in magnitude (a mean increase in EELV of 770 ± 165 mL) than the reduction in EELV that naturally occurred during stage R sleep in our study (316.7 ± 131.9 mL). This may partly explain why Heinzer et al. found a significant association between ΔEELV and AHI, and we did not.
As EELV decreases, Pcrit increases (becomes less negative), leading to airway narrowing and eventual airway collapse during inspiration.17 Because the greatest reduction in EELV is during stage R sleep, SDB may be more severe in this stage of sleep.18 This may account, in part, for the clinical observation that SDB is predominant during stage R sleep in some individuals. Obese individuals may be more susceptible to reduction in EELV with sleep. We noted greater reductions in ΔEELV during sleep and desaturation time during stage R sleep as BMI increased. Similarly, in individuals who are awake, there are reductions in functional residual capacity and expiratory reserve volume with obesity.19,20
The prior studies evaluating ΔEELV had methodological limitations, such as placing esophageal and epiglottis catheters, arousing subjects frequently throughout the night, and not allowing the subject to engage in the natural course of sleep. These issues were avoided in the current study as magnetometry provided a noninvasive means for measuring changes in lung volume and allowed the subject to sleep for the complete duration of the study. Being able to sleep for complete overnight testing allows for capturing of information from the natural sleep process. Another difference between our study and those that previously evaluated ΔEELV was the greater inclusion of women in our study. Women are underrepresented in many OSA studies because they underreport or have atypical symptoms compared to men.21 Our study had equal sex representation demonstrating no differences in ΔEELV between men and women.
Limitations
Our study was limited because stage N3 and R sleep were not recorded in all individuals (only 10 had stage N3 and 13 subjects had stage R sleep recorded). This was due to poor sleep quality and frequent arousals, which is not uncommonly encountered in patients with OSA. Consequently, our study was likely underpowered for some of the secondary comparisons. In addition, the mean AHI of the subjects in our study was in the mild category. ΔEELV from our sample may not be applicable to subjects with greater OSA severity. Given that ΔEELV reduction was inversely related to the severity of OSA, subjects with moderate to severe OSA may have even greater reductions in EELV than we report. With a lower threshold of airway collapse, subjects with more severe OSA would have difficulty achieving adequate stage R sleep. This would pose a challenge to obtain a large sample with moderate to severe OSA that has analyzable stage R sleep subsegments. In our sample, six subjects had an AHI in the moderate to severe range (> 15 events/h). Of the six subjects, four had analyzable stage R sleep subsegments.
CONCLUSIONS
Chest wall magnetometry provides a precise, unobtrusive, and continuous means to study lung volume changes during sleep. Using this technology, we demonstrated that EELV is reduced during sleep with the most pronounced reduction during stage R sleep. This finding suggests that reductions in EELV may be one of the mechanisms whereby SDB is more prominent in some individuals during stage R sleep.
DISCLOSURE STATEMENT
Work for this study was performed at Alpert Medical School of Brown University, Memorial Hospital of Rhode Island. This study was performed with no financial support, and all authors have no conflicts of interest to disclose.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- Ab
abdomen
- AHI
apnea-hypopnea index
- ANOVA
analysis of variance
- BMI
body mass index
- CPAP
continuous positive airway pressure
- ΔEELV
change in end-expiratory lung volume
- EELV
end-expiratory lung volume
- ERV
expiratory reserve volume
- FRC
functional residual capacity
- Non-stage R
non-REM sleep
- OSA
obstructive sleep apnea
- Pcrit
pharyngeal critical pressure
- PSG
polysomnography
- RC
rib cage
- REM
rapid eye movement
- SDB
sleep-disordered breathing
- Stage R
REM sleep
- VT
tidal volume
- Xi
axial displacement
REFERENCES
- 1.Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90(1):47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz AR, Patil SP, Laffan AM, Polotsky V, Schneider H, Smith PL. Obesity and obstructive sleep apnea: pathogenic mechanisms and therapeutic approaches. Proc Am Thorac Soc. 2008;5(2):185–192. doi: 10.1513/pats.200708-137MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 5.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14(6):486–495. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilhout SC, Suratt PM. Obstructive sleep apnea in pre-menopausal women. A comparison with men and postmenopausal women. Chest. 1987;91(5):654–657. doi: 10.1378/chest.91.5.654. [DOI] [PubMed] [Google Scholar]
- 7.Sforza E, Petiau C, Weiss T, Thibault A, Krieger J. Pharyngeal critical pressure in patients with obstructive sleep apnea syndrome. Clinical implications. Am J Respir Crit Care Med. 1999;159(1):149–157. doi: 10.1164/ajrccm.159.1.9804140. [DOI] [PubMed] [Google Scholar]
- 8.Worsnop C, Kay A, Pierce R, Kim Y, Trinder J. Activity of respiratory pump and upper airway muscles during sleep onset. J Appl Physiol. 1998;85(3):908–920. doi: 10.1152/jappl.1998.85.3.908. [DOI] [PubMed] [Google Scholar]
- 9.Owens RL, Malhotra A, Eckert DJ, White DP, Jordan AS. The influence of end-expiratory lung volume on measurements of pharyngeal collapsibility. J Appl Physiol. 2010;108(2):445–451. doi: 10.1152/japplphysiol.00755.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hudgel DW, Devadatta P. Decrease in functional residual capacity during sleep in normal humans. J Appl Physiol. 1984;57(5):1319–1322. doi: 10.1152/jappl.1984.57.5.1319. [DOI] [PubMed] [Google Scholar]
- 11.McCool FD, Wang J, Ebi KL. Tidal volume and respiratory timing derived from a portable ventilation monitor. Chest. 2002;122(2):684–691. doi: 10.1378/chest.122.2.684. [DOI] [PubMed] [Google Scholar]
- 12.Casserly B, McCool FD, Sethi JM, Kawar E, Read R, Levy MM. A method for determining optimal mean airway pressure in high-frequency oscillatory ventilation. Lung. 2013;191(1):69–76. doi: 10.1007/s00408-012-9434-4. [DOI] [PubMed] [Google Scholar]
- 13.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- 14.Stadler DL, McEvoy RD, Bradley J, Paul D, Catcheside PG. Changes in lung volume and diaphragm muscle activity at sleep onset in obese obstructive sleep apnea patients vs. healthy-weight controls. J Appl Physiol. 2010;109(4):1027–1036. doi: 10.1152/japplphysiol.01397.2009. [DOI] [PubMed] [Google Scholar]
- 15.Heinzer RC, Stanchina ML, Malhotra A, et al. Effect of increased lung volume on sleep disordered breathing in patients with sleep apnoea. Thorax. 2006;61(5):435–439. doi: 10.1136/thx.2005.052084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jordan AS, White DP, Owens RL, et al. The effect of increased genioglossus activity and end-expiratory lung volume on pharyngeal collapse. J Appl Physiol. 2010;109(2):469–475. doi: 10.1152/japplphysiol.00373.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Squier SB, Patil SP, Schneider H, Kirkness P, Smith PL, Schwartz AR. Effect of end-expiratory lung volume on upper airway collapsibility in sleeping men and women. J Appl Physiol. 2010;109(4):977–985. doi: 10.1152/japplphysiol.00080.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neilly JB, Gaipa EA, Maislin G, Pack AI. Ventilation during early and late rapid-eye-movement sleep in normal humans. J Appl Physiol. 1991;71(4):1201–1215. doi: 10.1152/jappl.1991.71.4.1201. [DOI] [PubMed] [Google Scholar]
- 19.Conwell W, Patel B, Doeing D, et al. Prevalence, clinical features, and CPAP adherence in REM-related sleep-disordered breathing: a cross-sectional analysis of a large clinical population. Sleep Breath. 2012;16(2):519–526. doi: 10.1007/s11325-011-0537-6. [DOI] [PubMed] [Google Scholar]
- 20.Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest. 2006;130(3):827–833. doi: 10.1378/chest.130.3.827. [DOI] [PubMed] [Google Scholar]
- 21.Redline S, Kump K, Tishler PV, Browner I, Ferrette V. Gender differences in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med. 1994;149(3 Pt 1):722–726. doi: 10.1164/ajrccm.149.3.8118642. [DOI] [PubMed] [Google Scholar]


