Abstract
This report is based on the proceedings from the Inhalational Lung Injury Workshop jointly sponsored by the American Thoracic Society (ATS) and the National Institutes of Health (NIH) Countermeasures Against Chemical Threats (CounterACT) program on May 21, 2013, in Philadelphia, Pennsylvania. The CounterACT program facilitates research leading to the development of new and improved medical countermeasures for chemical threat agents. The workshop was initiated by the Terrorism and Inhalational Disasters Section of the Environmental, Occupational, and Population Health Assembly of the ATS. Participants included both domestic and international experts in the field, as well as representatives from U.S. governmental funding agencies. The meeting objectives were to (1) provide a forum to review the evidence supporting current standard medical therapies, (2) present updates on our understanding of the epidemiology and underlying pathophysiology of inhalational lung injuries, (3) discuss innovative investigative approaches to further delineating mechanisms of lung injury and identifying new specific therapeutic targets, (4) present promising novel medical countermeasures, (5) facilitate collaborative research efforts, and (6) identify challenges and future directions in the ongoing development, manufacture, and distribution of effective and specific medical countermeasures. Specific inhalational toxins discussed included irritants/pulmonary toxicants (chlorine gas, bromine, and phosgene), vesicants (sulfur mustard), chemical asphyxiants (cyanide), particulates (World Trade Center dust), and respirable nerve agents.
Contents
Overview
Introduction
Methods
Inhalational Lung Injury: Exposure Risks, Epidemiology, and Standard Therapies
The Government’s Role in Countermeasure Development: Funding, Resources, and Regulatory Environment
Challenges to Developing and Distributing Novel Pharmacologic Therapies
Methods and Approaches to Understanding Mechanisms of Lung Injury and Discovering New Therapeutic Targets
Epidemiologic Research: Graniteville, South Carolina Chlorine Gas Release and World Trade Center Disaster
Animal Models of Inhalational Lung Injury: Pathologic Mechanisms and Possible Targets
Chlorine
Bromine
Phosgene
The role of transient receptor potential (TRP) ion channels in acute airway injury
Cyanide
Sulfur mustard
Respirable nerve agents
Discussion: Ongoing Challenges and Recommendations
Overview
The American Thoracic Society (ATS) workshop on toxic inhalational lung injury was cosponsored by the ATS and National Institutes of Health (NIH) Countermeasures Against Chemical Threats (CounterACT) program. Since 2006, CounterACT has taken a leadership role in pursuing the development of new and improved medical countermeasures against chemical threat agents through basic, translational, and clinical research. Agents of highest interest are determined by the U.S. Department of Homeland Security. The 2012 Chemical Terrorism Risk Assessment list is available online (1).
The workshop took place at the 2013 ATS International Conference meeting in Philadelphia, Pennsylvania. It was initiated by members of the Terrorism and Inhalational Disasters (TID) section of the Environmental, Occupational, and Population Health Assembly (EOPH). The goals were to (1) provide a forum to review the evidence supporting current standard medical therapies, (2) present updates on our understanding of the epidemiology and underlying pathophysiology of inhalational lung injuries, (3) discuss innovative investigative approaches to further delineating mechanisms of lung injury and identifying new specific therapeutic targets, (4) discuss promising novel medical countermeasures, (5) facilitate collaborative research efforts, and (6) identify challenges and future directions in the ongoing development, manufacture, and distribution of effective and specific medical countermeasures. The principal findings and recommendations of the panel after participant presentations and group discussion were as follows:
-
•
Although general supportive therapies extrapolated from treatment of a number of different clinical syndromes including acute respiratory distress syndrome (ARDS), reactive airways dysfunction (RADS), and bronchiolitis obliterans (BO) remain the mainstay of treatment for toxic inhalational lung injuries, the field has advanced significantly. A better understanding of the underlying basic pathophysiology of lung injury after a number of different toxic exposures has resulted in the discovery of novel specific therapeutic targets leading to promising new compounds currently in the process of further development.
-
•
Novel antidotes of particular interest in preclinical development in large-animal models include R-107, a novel antioxidant countermeasure for inhalational chlorine toxicity; dimethyl trisulfide, cobinamide, and sulfanegen for the treatment of cyanide poisoning; and intratracheal tissue plasminogen factor to counter toxic airway effects of sulfur mustard (SM) inhalation.
-
•
Significant challenges to ongoing research in this field include the relative rarity and unpredictability of toxic inhalational events as well as the inherent ethical constraints restricting research and testing in humans. The following key priorities were thus delineated:
○ Continued development of appropriate animal models, realistic exposure mechanisms, and incorporation of standard therapies into both the control and therapeutic intervention groups
○ Development of U.S. Food and Drug Administration (FDA)–qualified animal models facilitating collaborative use, head-to-head comparisons of efficacy, and validation of results under identical experimental conditions
○ Extrapolation of pathophysiological mechanisms from other models of human lung injury and fostering of collaboration with scientists investigating these similar patterns of lung injury
○ Further development of global infrastructure for both acute and long-term cohort studies in exposed populations after toxic inhalational exposures
○ Linking lessons learned in the field to improved therapeutic strategies and identification of additional areas for basic scientific and translational investigation
-
•
Despite significant governmental support for ongoing basic scientific research, preclinical trials, and development of novel agents, this funding stream is subject to changes in policy based on ever-changing intelligence information and public and governmental perception of need.
-
•
Additional challenges to the development and distribution of effective, specific targeted therapies include the time and expense required to obtain FDA approval; development of therapeutic formulations that will enable rapid administration in the field, or that will still be effective despite a significant time delay; and the need to obtain the significant financial investment required for manufacture, marketing, and distribution from the pharmaceutical industry.
Introduction
To respond effectively to inhalational emergencies, clinicians and public health personnel must first have an understanding of mechanisms of lung injury, clinical sequelae, and appropriate medical management. Accidental or intentional release of respiratory toxins within a civilian population may cause mass casualties, rapidly overwhelming regional health care systems. With a growing understanding of exposure risk and increased funding and support from governmental agencies, the field of inhalational toxicology is significantly advancing. This workshop provided a venue for researchers and leaders in the field to review our current understanding of the biology of inhalational lung injury, discuss advances in the management of specific inhalational injuries, and define future goals and research priorities.
Within this broad field, we focused primarily on (1) inhaled irritants/pulmonary toxicants including chlorine gas, bromine, and phosgene; (2) vesicants, or blistering agents, such as mustards and lewisite; (3) the chemical asphyxiant hydrogen cyanide; (4) particulates in the form of World Trade Center (WTC) dust; and (5) respirable nerve agents such as insecticides, sarin, V-agents, soman, and tabun. Despite the Chemical Weapons Convention of 1993, these agents remain a significant threat. Stockpiling and use by some nations have continued, manufacture of certain agents is relatively easy, and many of these compounds are common industrial agents subject to accidental release.
Methods
Fourteen invited panelists participated in the workshop at the ATS International Conference in 2013. Two additional panelists provided materials for the final report. The panel included both domestic and international experts in the fields of inhalational toxicology, epidemiology, and pulmonary and critical care medicine, as well as representatives from U.S. governmental research funding agencies. Twelve of the participants are members of the TID section of the EOPH Assembly of the ATS. Six are funded by the NIH CounterACT program. Participants were selected on the basis of their knowledge, research interests, and/or track record of publication in the content areas. After the workshop, each participant submitted a summary of their presented material. These contributions, workshop discussions, and interval updates are summarized here. Follow-up meetings of the writing committee occurred during ATS Conferences in 2014, 2015, and 2016. All participants disclosed potential conflicts of interest before the workshop, which were managed according to the policies and procedures of the ATS. Before publication, all authors updated their conflict of interest disclosures and also provided updated content and references.
Inhalational Lung Injury: Exposure Risks, Epidemiology, and Standard Therapies
Inhalational lung injury may occur through the accidental or intentional release of chemical vapors, particulates, and/or incomplete products of combustion. Clinical manifestations of these agents are related not only to dose and proximity of exposure, but also to compound solubility or particulate size. Highly soluble compounds (ammonia) and larger particles (>10 μm) primarily affect the upper airway with rapid symptom onset. Intermediate-solubility agents such as chlorine and bromine and intermediate-sized particulates (5–10 μm) cause greatest injury between the larynx and segmental bronchi, but may also cause parenchymal injury, particularly in high concentrations. The smallest bronchi, bronchioles, and alveoli are targeted by poorly soluble compounds such as phosgene (CG) and particulate matter less than 5 μm in size (2–5). The role of neuronal sensory fiber transient receptor potential (TRP) ion channels in acute airway injury related to lung damaging agents was also discussed.
Acute manifestations of respiratory injury include upper airway obstruction, vocal cord dysfunction, acute respiratory distress syndrome (ARDS), and reactive airways dysfunction (RADS), an asthma-like syndrome characterized by airway hyperresponsiveness after a single massive inhalational event, which may persist for months or longer. Chronic syndromes may include chronic rhinosinusitis, or reactive upper airways syndrome (RUDS), irritant-induced asthma caused by multiple low-level exposures over time, bronchiolitis obliterans (BO), chronic obstructive pulmonary disease (COPD), sarcoidosis, eosinophilic pneumonia (EP), and other interstitial lung diseases. Finally, accelerated decline in lung function may occur (5–7).
Immediate treatment is based on the general precepts of personal protective equipment for first responders, removal of victims from the area, decontamination, and supportive care. Certain compounds, such as nerve agents and cyanide, which are not primarily lung-damaging agents, do have specific antidotes, although further optimization is needed (2, 5, 8). General supportive respiratory care includes warm, humidified oxygen, antitussives, and bronchodilators. Endotracheal intubation and low tidal volume mechanical ventilation may be necessary for airway compromise, copious secretions, and/or hypoxemic respiratory failure secondary to ARDS. Injuries caused by intermediate-solubility agents are similar to those caused by smoke and thermal inhalational sequelae. Bronchoscopic lavage may be necessary to maintain airway patency because of epithelial injury and sloughing. Inhaled and/or systemic corticosteroids have been used as treatments for irritant and vesicant injuries. These management strategies have been extrapolated from treatments for other forms of airway injury or pulmonary disease, and supported in humans only through anecdotal reports and small, uncontrolled studies (2, 5, 8–10). The unpredictability of inhalational events and ethical constraints of studying human populations remain among the largest ongoing challenges faced by researchers. For these reasons, advances in the field have often been driven by findings in rodent and large-animal models.
The Government’s Role in Countermeasure Development: Funding, Resources, and Regulatory Environment
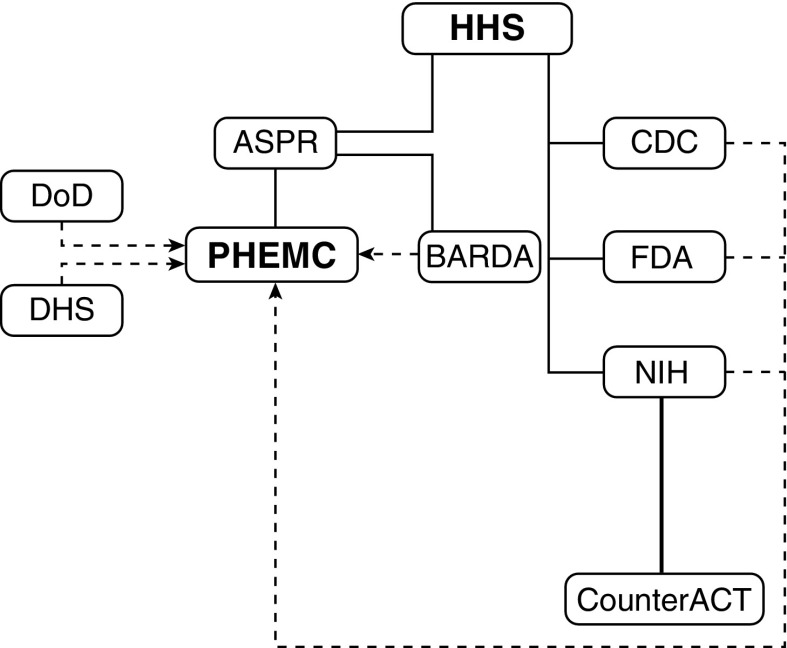
In the United States, development and distribution of medical countermeasures are coordinated by the Assistant Secretary for Preparedness and Response (ASPR) in the Department of Health and Human Services (HHS) through the Public Health Emergency Medical Countermeasures Enterprise (PHEMCE) (Figure 1). As part of the PHEMCE, the NIH supports basic and preclinical research to identify mechanisms and targets of chemical threats, design animal exposure models, and develop novel therapeutic strategies through the Countermeasures Against Chemical Threats (CounterACT) program.
Figure 1.
Organizational chart for U.S. governmental agencies directing and supporting countermeasure development. Solid lines indicate agency relationships under the Department of Health and Human Services; dotted lines indicate partnerships. ASPR = Office of the Assistant Secretary for Preparedness and Response; BARDA = Biomedical Advanced Research and Development Authority; CDC = Centers for Disease Control and Prevention; CounterACT = Countermeasures Against Chemical Threats; DHS = Department of Homeland Security; DoD = Department of Defense; FDA = Food and Drug Administration; HHS = Department of Health and Human Services; NIH = National Institutes of Health; PHEMC = Public Health Emergency Medical Countermeasures Enterprise.
Currently funded research focuses on countermeasures development against chemical warfare agents (e.g., sulfur mustard, sarin), toxic industrial chemicals (e.g., chlorine, cyanide), pesticides (e.g., parathion), and other chemicals. Important research collaborations exist with Contract Research Organizations (CROs) and the Department of Defense (DOD), including the U.S. Army Medical Research Institute of Chemical Defense (USAMRICD, Aberdeen Proving Ground, MD). Under HHS, the Biomedical Advanced Research and Development Authority (BARDA) supports ongoing development and regulatory approval of novel countermeasures, providing infrastructure for manufacture and distribution.
The U.S. Food and Drug Administration (FDA) has issued regulatory guidelines for countermeasures research, summarized under the “Animal Rule,” which guides the drug approval process when human efficacy studies are unethical (11). In this case, the FDA approves drugs on the basis of animal efficacy studies alone, generally with two animal models, with human safety evaluation under existing requirements. Examples of countermeasures approved under the Animal Rule include the Cyanokit (hydroxocobalamin) for treatment of cyanide poisoning; levofloxacin for plague; raxibacumab, a monoclonal antibody directed against inhalational anthrax; and botulinum antitoxin.
Challenges to Developing and Distributing Novel Pharmacologic Therapies
Despite this support, major challenges remain in accelerating countermeasures development and improving collaborative efforts between basic and translational scientists, industry, and government stakeholders. Research priorities are ever-changing. Limited human data, often involving a single individual or small cohort, are often the only information available for animal model development and extrapolation of mass causality injuries. Therefore, ongoing population studies after large-scale disasters remain essential.
Methods and Approaches to Understanding Mechanisms of Lung Injury and Discovering New Therapeutic Targets
Epidemiologic Research: Graniteville, South Carolina Chlorine Gas Release and World Trade Center Disaster
In January 2005, an early morning train accident released approximately 54,000 kg of chlorine gas in the small town of Graniteville, South Carolina. Other than 180 millworkers on-shift, Graniteville was asleep. Because of topography and weather, the gas plume initially settled within a bowl-shaped depression (12–14). Millworkers and most residents were told to shelter-in-place. Many fled anyway, either driving quickly through or becoming trapped in the plume. Three exposure durations were therefore defined: (1) very short-term (1–5 min), (2) short-term (∼10–15 min), and (3) long-term exposures of those who sheltered in place (∼30–60 min). Exposure doses varied from lethal (>1,000 ppm) to the harmless odor threshold (0.2 ppm). Emergency callers frequently reported bleaching of their own clothes or surrounding objects. The plume was about 1.5 km wide at severe concentrations (≥20 ppm) (14–16).
Eight of nine immediate fatalities occurred on site. All died of asphyxiation or acute respiratory failure, with pulmonary edema found on autopsy (17). The most severely injured (n = 71) were admitted, with one death several weeks after hospital discharge. All presented with pulmonary complaints, some also with cardiac, ocular, otorhinolaryngeal, gastrointestinal, and dermal. A clinical review of the patients who received both spirometry and chest X-ray in the hospital found that patients presented with a predominantly restrictive lung function pattern (18). Patients were treated with a variety of therapies, including β-agonists; ipratropium bromide; inhaled, oral, or intravenous corticosteroids; antibiotics; and nebulized or intravenous sodium bicarbonate in decreasing frequency. Five hundred and twenty-nine people were treated and released from emergency departments, and 331 more sought medical care at physician’s offices (17). The horror of the event affected the emotional health of the community as well (19, 20). Long-term follow-up of the known 1,384 victims is on-going (21–24). Preliminary evidence demonstrates significantly reduced lung volumes in the severely exposed (25).
After the September 11, 2001 WTC attacks in New York City, the subsequent fire and collapse of the twin towers and nearby buildings resulted in massive release of products of combustion and pulverized building materials throughout lower Manhattan. Aerosolized toxins included polycyclic aromatic hydrocarbons, dioxins, and volatile organic compounds. The dust was composed of numerous materials including crushed concrete, gypsum, asbestos, polychlorinated biphenyls, hydrochloric acid, silica, and heavy metals. These materials combined to make the dust highly alkaline (pH > 10). Approximately 5% of the dust particles were respirable (26).
The majority of first responders, including Fire Department of New York (FDNY) firefighters, were not wearing protective face masks. Epidemiologic studies of downstream pulmonary effects were greatly facilitated by the fact that firefighters were already being closely monitored with serial pulmonary function testing by the FDNY Bureau of Health Services. Multiple studies of sequelae in firefighters have been published, and summarized by Yip and colleagues (27). A syndrome that became known as WTC cough was described, most prevalent in those with the highest levels of exposure. Sixty-three percent of those with WTC cough had significant bronchodilator response and 24% had bronchial hyperresponsiveness (28). A high proportion demonstrated prolonged evidence of bronchial hyperresponsiveness consistent with RADS (29). This has persisted 12–13 years later (30). Another study demonstrated that exposure to WTC dust caused an average 372-ml decrease in FEV1 during the year after September 11, corresponding to an age-related decline of 12 years (31, 32). These findings have persisted over 13 years (30, 33). Other pulmonary effects included eosinophilic pneumonia, sarcoidosis, tracheobronchomalacia, and possibly BO (34, 35). Nonpulmonary toxicities included reactive upper airways syndrome, vocal cord polyps, gastroesophageal reflux disease, obstructive sleep apnea, cancers, and systemic autoimmune diseases (28, 34–38).
Animal Models of Inhalational Lung Injury: Pathologic Mechanisms and Possible Targets
Chlorine
Chlorine gas (Cl2) is one of the most common toxic inhalational injuries. It is manufactured in large quantities for a variety of industrial uses, putting populations at risk through industrial accidents and spills during transport. Exposures are also encountered in the paper industry, swimming pools, and through mixing of domestic cleaning products (10, 39). Although most exposures are accidental, chlorine has been used as a warfare agent since World War I, and most recently on civilian populations in the Syrian civil war. Chlorine is highly toxic by inhalation, leading to dyspnea, hypoxemia, airway obstruction, pneumonitis, and pulmonary edema (39). Chlorine is an intermediately water-soluble agent, causing damage from the upper airways to the alveolar compartment. Acute exposures may lead to airway mucosal sloughing, RADS, acute lung injury (ALI), and ARDS (40). Factors such as respiratory syncytial viral infection have been shown to potentiate Cl2-induced lung injury (41).
Animal models in multiple species including mice, rats, rabbits, pigs, sheep, and dogs (42–49) have been developed to study mechanisms of chlorine toxicity and response to potential therapies. Acute effects of chlorine inhalation documented in animal models include dyspnea or altered breathing patterns (50, 51), respiratory cell death (42, 43, 45, 52), inflammation and mitochondrial damage (42, 43, 51, 53), impaired surfactant function (45, 54), hypoxemia (45, 47, 48), fluid leak and pulmonary edema (45, 47, 55, 56), lipid peroxidation (51, 57), changes in gene expression (58), induction of unfolded protein response (59), pulmonary hypertension (47), and airway hyperreactivity (AHR) (42, 44, 60–62). Later effects of chlorine inhalation include abnormal epithelial repair (60), airway fibrosis (49, 52, 63, 64), mucus overproduction (60), and altered lung function including AHR and fixed obstruction (44, 60, 63, 65, 66). In addition, exposure to chlorine has been shown to cause significant cardiotoxicity (67, 68).
Multiple therapeutic strategies have shown success in treating acute chlorine injury in animal models: antioxidants including dimethylthiourea (57), AEOL 10150 (69), N-acetylcysteine (70), and ascorbic acid plus deferoxamine (71); cAMP-elevating agents such as R-formoterol (62) and rolipram (72); antiinflammatory agents such as corticosteroids (66, 73–75) and triptolide (60); nitric oxide–modulating agents including nitrite (51, 76) and 1400W (42), a potent, selective nitric oxide inhibitor; neutrophil depletion (77); aerosolized heparin (78); and high molecular weight hyaluronan (79). The role of TRP channel inhibitors in acute lung injury secondary to chlorine and other toxic inhalational agents is becoming more clear, and TRP inhibitors have been shown to suppress pulmonary inflammation in this setting (80, 81). Current challenges include translating promising findings in preclinical models into countermeasures for human use and identifying interventions to reverse or prevent long-term effects of chlorine inhalation. R-107, a novel nitric oxide donor, peroxynitrite modulator, and superoxide scavenger, was shown to ameliorate Cl2-induced ARDS in an ovine model via the intramuscular route (82). The work was initially funded by CounterACT and is now moving forward in a collaborative effort between BARDA and industry.
Bromine
World production of bromine (Br2) exceeds 300,000 tons per year. A reddish liquid at room temperature, highly volatile bromine must be transported in specifically designed noncorrosive containers. As with chlorine, large populations may be exposed after transportation and industrial accidents. On September 1, 2011, in Chelyabinsk, Russia 42 people were hospitalized and more than 200 sought medical attention after a train derailment. Facilities producing Br2 are considered high risk for terrorist attacks by the Department of Homeland Security. One such attack was attempted in Israel in 2004 (83).
Bromine inhalation results in bronchospasm, AHR, ALI/ARDS, and death from respiratory failure. Long-term effects include RADS and pulmonary fibrosis (84). At present, only standard supportive care is available for treatment. Clearly, there is a need for additional research to develop specific therapies that target the basic mechanisms by which Br2 and its reactive intermediates damage the lungs.
Free hemoglobin and heme catalyze the generation of reactive intermediates that cause extensive injury to proteins, lipids, and DNA. Significant levels of free heme were detected in bronchoalveolar lavage (BAL) fluid, lung tissues, and plasma of mice exposed to Br2 gas, originating most likely from ruptured red blood cells. Postexposure administration of hemopexin, a heme-scavenging protein, decreased lung injury and improved survival (84). Further studies demonstrated that humanized transgenic mice overexpressing heme oxygenase-1 (HO-1), the first and rate-limiting step in heme degradation into iron, carbon monoxide (CO), and biliverdin (85), were significantly protected from exposure to Br2 (84). Conversely, mice lacking HO-1 were sensitive to Br2 and died much earlier of respiratory failure compared with wild-type controls (84). These are the first studies delineating the role of heme in ARDS caused by Br2. On the basis of these findings, attenuating heme may prove useful in treating ARDS secondary to Br2 inhalation.
Phosgene
Phosgene (CCl2O) caused the greatest number of chemical deaths during World War I. Those exposed develop ALI/ARDS after an initial asymptomatic latent period. Phosgene (CG) is an important industrial chemical produced globally in large quantities. Because of the amount produced, ease of manufacture, and high toxicity, accidental or deliberate (terrorist-related) release resulting in mass casualties is of concern. Histologic damage occurs at doses greater than 30 ppm⋅min, and clinical pulmonary edema becomes evident above 150 ppm⋅min. The estimated human LD50 is 500 ppm⋅min (86).
The mechanisms of action of CG are not fully understood. There are no proven therapeutic strategies or evidence-based guidelines for the management of CG-induced injury. Continuing research is therefore necessary. Two mechanisms of action have been proposed. The first postulates hydrolysis to hydrochloric acid (HCl). However, as insufficient HCl is produced within the lower airways to overcome the natural buffering capacity of lung tissue, this mechanism appears unlikely. Alternatively, as a highly electrophilic, hydrophobic gas, CG penetrates readily into the lower respiratory tract, directly affecting the respiratory epithelium. It reacts with a wide variety of nucleophilic biological tissue components through acylation reactions with primary and secondary amines, hydroxyl, and sulfhydryl groups. This causes the downstream release of arachidonic acid mediators such as leukotrienes and up-regulation of oxidative response enzymes. Damage to the respiratory epithelium causes disruption of the alveolar–capillary interface, moving fluid and inflammatory cells into the alveoli. CG also causes lipid peroxidation and surfactant damage (86).
Development of therapeutic approaches has traditionally been based on hypothetical pathways elucidated through in vivo research. Examples include supportive care modalities such as lung-protective ventilation (87, 88) and pharmacologic interventions such as steroids and bronchodilators (89, 90). This approach has been used in the terminally anesthetized pig model of CG-induced ALI (91). More recently, genomics analysis has identified specific cellular and molecular events that can be targeted by novel therapeutic interventions (92–94). Using Ingenuity pathway analysis of microarray data, Holmes and colleagues proposed a new putative mechanism of CG injury based on direct free radical attack on neuronal, endothelial, and epithelial cells resulting in tissue destruction and mediator release (95). Such approaches have led to experimental therapeutic strategies targeting neuronal signaling, receptors involved in the endothelin pathway, and receptors/proteins involved in Ca2+ signaling. Thus, an improved understanding of CG mechanisms of action at the cellular level may lead to the identification of novel therapeutic compounds. Although small animals have been used to screen candidate therapies, such as the antiinflammatory agents angiopoietin-1, which suppresses NF-κB and p38 mitogen-activated protein kinase (MAPK) pathways (96); 1400 W, a selective nitric oxide synthase-2 (NOS-2) inhibitor (97); ulinastatin, a urinary trypsin inhibitor (98); and mesenchymal stem cell transplantation (99, 100); and have demonstrated efficacy, further testing in large-animal models more relevant to human exposures is still needed.
The role of transient receptor potential (TRP) ion channels in acute airway injury
Noxious airborne chemicals are detected by the trigeminal and vagal sensory nerves of the airways, eyes, and skin. These neurons initiate respiratory reflexes including cough, sneezing, and decreased respiratory rate; sensations of irritation and pain; and glandular secretions (80). These responses may be protective, and help diminish the toxic effects of chemical threats. However, neuronal activation can lead to severe pain and respiratory distress resulting in incapacitation (101), further exposure, and injury. In particular, chlorine gas, capsaicin (in pepper spray), acrolein (in smoke), and tear gas agents (chlorobenzylidene malononitrile [CS] and chloroacetophenone [CN]) trigger strong neuronal irritant responses (80).
TRP ion channels are key airway noxious chemical receptors (102). TRP channels conduct calcium ions and other cations, initiating neuronal excitation and subsequent physiological responses. These channels are expressed in the chemosensory fibers of the trigeminal nerve, innervating the upper airways, and in vagal sensory fibers innervating the larynx and lower airways. TRPV1, the receptor for capsaicin, is expressed in the majority of chemosensory fibers. TRPV1 is also activated by acidic stimuli and noxious heat. TRPA1 is expressed in a smaller subset of chemosensory fibers and detects a wide range of chemical threats (102). TRPA1 was initially identified through its sensitivity to mustard oil (allyl isothiocyanate), the pungent ingredient in mustard, horseradish, and wasabi (103). Later studies found that TRPA1 is the neuronal target of acrolein, a major irritant in cigarette smoke; methyl isocyanate, the toxic and highly irritating chemical released in the Bhopal, India incident in 1984; and chlorine gas (104–106). Tear gas agents (CN, CS, and dibenzoxazepine [CR]) are the most potent activators of TRPA1. TRPA1-deficient mice are insensitive to the acute irritant effects, underscoring the important role of this ion channel. TRPA1 inhibitors attenuated behavioral pain responses to CN and CS, and reduced the inflammatory response, likely through inhibition of neurogenic inflammatory mechanisms (105).
TRPV4 expressed in pulmonary tissue has been shown to be a crucial mediator of ventilator and heart failure–associated lung injury (107–109). The effects of two novel TRPV4 inhibitors were examined in mice exposed to chlorine and hydrochloric acid, the first modeling acid fume exposure and the second acid aspiration injury. In both models, postexposure treatment with TRPV4 inhibitors reduced pulmonary inflammation by diminishing neutrophils, macrophages, and associated chemokines and cytokines, while decreasing histopathologic evidence of injury. TRPV4 inhibitors reduced vascular and epithelial leakage, airway hyperreactivity, and airway elastance, while improving blood oxygen saturation. Chlorine-exposed TRPV4-deficient mice showed diminished inflammation (81).
These studies have firmly established TRP ion channels as molecular targets of noxious chemical exposures and revealed novel TRP channel–mediated mechanisms of tissue injury. On the basis of this rapidly advancing understanding, the health risks of chemical exposures to tear gas and capsaicin-based riot control agents need to be reevaluated.
Cyanide
Cyanide in the form of hydrogen cyanide (HCN), or cyanide anion salts, has been an archetypal poison through the ages. Exposure to HCN gas at concentrations of 200 to 500 ppm for 30 minutes or an ingestion of less than 1.0 mg/kg is usually fatal. Human exposures typically result from improperly prepared cassava, smoke inhalation, industrial accidents, and clinical use of nitroprusside. Animal models for antidote efficacy testing and advanced preclinical antidotes are discussed here.
Current countermeasures approved for use in the United States include a combination of nitrites, sodium thiosulfate, and hydroxocobalamin. These require intravenous administration, which is suboptimal in a mass casualty setting. This has fueled efforts to develop novel cyanide countermeasures suitable for rapid administration, such as intramuscular injection. Three antidotes in preclinical development include dimethyl trisulfide (110–114), cobinamide (115–118), and sulfanegen (119–123). Animal models have included the Nagasawa sublethal mouse (119), the Boss lethal mouse (115), the Brenner sublethal rabbit (116, 120), and lethal models developed by Borron and colleagues (121, 124, 125).
Dimethyl trisulfide is a natural product that converts cyanide to thiocyanate. It has been successfully administered as an intramuscular formulation in murine and rabbit models (110–114). Cobinamide is a biosynthetic precursor to hydroxocobalamin. Cobinamide detoxifies cyanide, forming a tight, stable complex with 2 moles of cyanide, which is then excreted in urine. The efficacy of cobinamide has been demonstrated in Drosophila, murine, and rabbit models (115–118). Sulfanegen is a prodrug of 3-mercaptopyruvate (3-MP), formed by cysteine transamination. Release of 3-MP from sulfanegen detoxifies cyanide via transfer of the 3-MP sulfur to 3-MP sulfur transferase as a persulfide. The resulting enzyme-bound persulfide reacts with cyanide, generating the less toxic thiocyanate, excreted in urine. Intramuscular sulfanegen efficacy has been demonstrated in pig and rabbit models (119–123). In addition, the combination of sulfanegen and cobinamide shows promise (126, 127). Key developments in the above-mentioned antidotes are the development of novel formulations (113, 127, 128) that improve solubility, stability, and bioavailability.
Sulfur mustard
The vesicant and cytotoxic agent sulfur mustard (bis-2-chloroethyl sulfide; SM) is the most widely used chemical weapon in history (129, 130). Because of remaining stockpiles and ease of manufacture, SM continues to be a significant threat, and has been used by Islamic State militants (131–133). After acute inhalation, airway injury may lead to rapid deposition of fibrin plugs or casts, obstructing the airways. ALI/ARDS also occur. These toxic effects can lead to acute respiratory failure and death, and have been documented in both humans and animal models (134–137). Long-term complications include airway and parenchymal fibrosis.
Decontamination in the field aimed at dermal and ocular injury are initial priorities. There are no specific therapies currently approved for the treatment of pulmonary inhalational injuries. As definitive mass casualty treatment may be delayed, development of medical countermeasures for acute and chronic pulmonary sequelae with effectiveness hours or even days after exposure are a high priority. For example, during the Iran–Iraq War, victims of SM inhalation often did not reach tertiary care centers for days to weeks (137).
Studies have shown that in the setting of acute inhalational lung injury, the coagulation system is activated in the airways and alveoli, leading to increased procoagulant and decreased fibrinolytic effects. Activation of the coagulation system in extravascular sites such as the airways requires local expression of tissue factor, a process that occurs mainly in the microparticle fraction of airway fluids (138). Tissue factor pathway inhibitor inhibits these actions (139). In addition to local factors favoring coagulation after SM inhalation, antifibrinolytic systems, including plasminogen activator inhibitor-1, thrombin-activatable fibrinolysis inhibitor, and α2-antiplasmin, are also activated in the injured airway (140), leading to persistence of casts. Direct administration of tissue plasminogen factor into airways can eliminate or reverse airways obstruction by fibrin, and prevent gas exchange abnormalities and respiratory failure (135, 136). These therapies for the acute process might be complemented by catalytic antioxidants (141), flavonones (142), and/or cell-based therapies (143).
Human survivors of acute SM inhalation may later develop chronic fibrotic processes of the airways and lung parenchyma, including BO (144) and interstitial fibrosis (145–147). Research in the field is currently underway to study whether more recently developed antifibrotic therapies will be beneficial in preventing these crippling outcomes (148, 149). Effective treatment of SM pulmonary injury may require therapies directed at the acute processes leading to airway coagulation and ALI/ARDS as well as chronically applied therapies directed at airway and parenchymal fibrosis.
Respirable nerve agents
Nerve agents are organophosphate compounds that block acetylcholinesterase (AChE) at the neuronal junction. Organophosphates are widely used as insecticides, resulting in accidental poisonings in many parts of the world. Production and stockpiling for weaponized use have been forbidden in multiple treaties, most recently the Chemical Weapons Convention of 1993. Nevertheless, there have been multiple instances of use in civilian populations over the past few decades, most recently in Syria (150).
Traditional nerve agents (TNAs) bind and functionally disable AChE. This leads to overstimulation of muscarinic and nicotinic receptors. This overstimulation causes multiple pulmonary, cardiac, and neurologic effects resulting in acute respiratory failure due to bronchoconstriction, bronchorrhea, and respiratory muscle weakness; neurologic effects including muscle weakness, seizures, and coma; cardiovascular instability; and death. After decontamination, standard combination autoinjectable treatment kits used by the U.S. military (MARK-1) include the anticholinergic agent atropine, which competitively binds to the post-synaptic muscarinic receptor, and pralidoxime chloride, which reactivates AChE (151).
Nerve agent exposure may occur through the skin or via inhalation of vapor. Highly volatile sarin (GB) and soman (GD) are G-agents. Inhaled G-agents cause significant respiratory problems within minutes of exposure. V-agents VX and VR are classified as nonvapor hazards. This may not be appropriate, as these agents can penetrate airways by attaching to inhaled dust, mist, and fog particles.
In the past, TNA studies focused on seizure activity after subcutaneous injection. More recent models include other, more realistic exposure routes, and have demonstrated additional forms of pulmonary toxicity. GB is distributed to the brain, kidney, liver, and blood plasma of mice (152, 153). Inhaled GB causes weight loss, pulmonary edema, hypoxemia, increased airway resistance, and increased minute ventilation in guinea pigs (154), with immediate inhibition of AChE activity noted in BAL fluid (155). Another study showed that the respiratory toxicity of GB, VX, and GD was independent of AChE activity (156). Exposure to G-agents affects transcriptional pathways, and causes perturbations in phosphorylation levels of cAMP response element–binding protein, c-Jun, and NF-κB (157). In addition, GD modulates p38 MAPK and extracellular receptor kinase signaling pathways (158).
Inhaled VX decreased AChE activity in both blood and lung tissue (159). Inhalational exposure to VR, which is nearly twice as toxic as VX, caused up-regulation of pulmonary AChE mRNA by 19–30% (160). Evidence of increased oxidative stress and lung injury included positive immunohistochemical staining for induced NOS, decreased surfactant D staining, increased BAL glutathione concentrations, and superoxide dismutase activity (161). In addition, Western blot analyses suggested inflammatory mediator stimulation, with up-regulation of xanthine oxidoreductase, IL-1, and IL-6 expression, and phosphorylation of p38 and protein kinase-B (Akt). Thus, new models of inhaled TNA reveal significant effects on pathways associated with oxidative stress and inflammation in the lungs, independent of neuromuscular effects. This may lead to new therapeutic targets in the future.
Discussion: Ongoing Challenges and Recommendations
The field of inhalational toxicology and the development of specific countermeasures have moved forward considerably with the support of government, military, and private funding. Nevertheless, significant challenges remain. Ongoing priorities include (1) continued development of small- and large-animal models mimicking human exposure and inhalational lung injury, (2) extrapolation from human models of similar injury, (3) the development of effective novel agents that can be administered in a feasible manner postexposure, (4) addressing the time and expense required to obtain FDA approval, (5) working with industry to facilitate bringing new devices and pharmaceutical agents to market, including improved personal protective equipment that does not require fit-testing, provides protection for extended time periods, allows for easy decontamination, and increases user comfort and communication ability, and (6) further developing appropriate infrastructure for epidemiologic research in the setting of mass casualty events on national and international levels.
Selection of appropriate animal species and methods of exposure to accurately model human lung injury in the field remains challenging. This is due to differences in airway structure and anatomy between species, minute ventilation at the time of injury, and the need for pain and sedative medications and alternative routes of administration to ensure humane treatment. These necessary interventions have the potential to alter sites of injury, inflammatory mediators, antioxidant production, and physiological parameters. Standard treatments likely to be used by clinicians must be incorporated into efficacy trials as well. Animal models can now undergo an FDA qualification process, providing a resource for collaborative use between multiple centers, allowing comparison of efficacy in identical settings, and independent validation of findings. As results of animal studies are sometimes not reproducible, these head-to-head comparisons and validation studies will be key across the NIH and BARDA. Genomic approaches may lead to an improved understanding of underlying pathophysiological processes and lead to the discovery of novel therapeutic targets. Computational modeling of lung injury is being used in the study of blast injury and ARDS, and there may be role for computational modeling in the study of inhalational toxin injury as well. Enhanced fostering of collaborative efforts between researchers in the field with scientists investigating patterns of lung injury such as ARDS, RADS, and BO from other etiologies is also imperative, as many pathways of injury overlap, and therapeutic targets may be similar.
Novel therapies require up to 10 years or more to achieve FDA approval, with more recent cost estimates in excess of $800 million (162). Expenses for chemical threat countermeasures may be somewhat less, as efficacy trials are conducted primarily in animals. However, large-animal studies, which more closely model humans, are costly. If the drug is already approved for another disease and/or route of administration, that may also contain costs. However, because use for chemical threats is a relatively low-probability event, as compared with blockbuster indications, pharmaceutical companies may not be motivated to sponsor FDA approval for this additional use. Getting novel compounds to market has been facilitated in some cases not only by BARDA, but by smaller pharmaceutical companies and nonprofit institutions.
Finally, linking lessons learned in the field from the DoD and nongovernmental organizations to knowledge learned in the laboratory setting will continue to be of the utmost importance. Investment in epidemiologic infrastructure and protocols for cohort studies, registries, and tissue banking for “fast on feet” study of both short- and long-term consequences of acute mass chemical exposures will be key factors in helping to improve future response (163). The NIH Disaster Research Response Program (DR2) was initiated in 2013 under the auspices of the National Institute of Environmental Health Sciences (NIEHS) and the National Library of Medicine (NLM) in 2013. Goals include developing a roster of subject experts who can be contacted after a disaster; advance development of research infrastructure and data collection tools; integrating research into preparedness, response, and recovery systems; and facilitating expedited institutional review board approval and funding for epidemiologic research after a disaster (164).
Because inhalation disasters and chemical threats remain real, a continued awareness of public need is necessary. Even with the combined resources of the NIH, HHS, and the DoD, the costs of countermeasure development and FDA approval are challenging. It requires tenacity and commitment to negotiate collaborations, funding sources, and intellectual property and other concerns. Current chemical stores worldwide, and modern geopolitical realities, tell us that chemical threats are real. For that reason, it remains imperative that priorities and solutions be carefully and clearly communicated between these relevant branches of government, the public, and the private sector.
Acknowledgments
Acknowledgment
S.M. would like to acknowledge the contributions of Saurabh Aggarwal, Adam Lam, and Nilam Vetal, L.A.V. and C.W.W. would like to acknowledge the contributions of Raymond D. Rancout.
This official summary of an ATS Workshop was prepared by an ad hoc subcommittee of the Terrorism and Inhalational Disasters Section of the Environmental, Occupational and Public Health Assembly.
Members of the writing committee are as follows
Eleanor M. Summerhill, M.D. (Co-Chair)
Livia A. Veress, M.D. (Co-Chair)
Gary W. Hoyle, Ph.D.
Sven-Eric Jordt, Ph.D.
Bronwen J. Jugg, Ph.D.
James G. Martin, M.D.
Sadis Matalon, Ph.D.
Steven E. Patterson, Ph.D.
David J. Prezant, M.D.
Alfred M. Sciuto, Ph.D.
Erik R. Svendsen, Ph.D.
Carl W. White, M.D.
Workshop Participants
Eleanor M. Summerhill, M.D. (Co-Chair); Livia A. Veress, M.D. (Co-Chair); Michael Gunn, M.D.; Gary W. Hoyle, Ph.D.; Sven-Eric Jordt, Ph.D.; Bronwen J. Jugg, Ph.D.; George Leikauf, Ph.D.; James G. Martin, M.D.; Srikanth Nadadur, Ph.D.; Steven E. Patterson, Ph.D.; David J. Prezant, M.D.; Daniel Salerno, M.D.
Author disclosures
S.-E.J. served on an advisory committee for Hydra Biosciences and received research support from GlaxoSmithKline and Hydra Biosciences. C.W.W. had a patent pending for use of human recombinant tissue plasminogen activator delivered into the airway for treatment of toxic chemical inhalation. E.M.S., L.A.V., G.W.H., B.J.J., J.G.M., S.M., S.E.P., D.J.P., A.M.S., E.R.S., and reported no relationships with relevant commercial interests.
Footnotes
This official Workshop Report of the American Thoracic Society (ATS) was approved by the ATS, April 2017
The research and study results presented here were supported by the CounterACT program, National Institutes of Health (NIH), Office of the Director, and the National Institute of Environmental Health Sciences (NIEHS) under grants U01ES015673 and U01ES022564 (G.W.H.), U01ES015674 and R21ES022875 (S.-E.J.), U01ES026458 and U01ES027697 (S.M.), U01NS58087 (S.E.P.), U54ES015678 and U54ES027698 (C.W.W. & L.A.V.), and R21ES026830 to L.A.V. E.R.S. was supported by NIH NIEHS grant R01ES015532.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the CounterACT program, NIH, NIEHS, or National Institute of Neurological Disease and Stroke (NINDS).
Dr. Bronwen Jugg is employed by a United Kingdom government agency, the Defence Science and Technology Laboratory; as a result, her contribution to this workshop report is covered by Crown copyright and has been licensed to the American Thoracic Society under the terms of a UK Open Government License. The workshop report is subject to American Thoracic Society copyright.
Originally Published in Press as DOI: 10.1513/AnnalsATS.201704-297WS on April 18, 2017
Contributor Information
Collaborators: on behalf of the ATS Terrorism and Inhalational Disasters Section of the Environmental, Occupational, and Population Health Assembly
References
- 1.U.S. Department of Homeland Security. Report on the Toxic Chemical Syndrome: Definitions and Nomenclature Workshop. May 2012 [accessed 2017 Feb 13]. Available from: https://chemm.nlm.nih.gov/Report_from_Toxic_Syndrome_Workshop_final.pdf.
- 2.Nelson LS, Hoffman RS. Inhaled toxins. In: Marx JA, Hockberger RS, Walls RM, editors. Rosen’s emergency medicine: concepts and clinical practice. 8th ed. Philadelphia: Elsevier/Saunders; 2014. pp. 2036–2043. [Google Scholar]
- 3.Parkes WR.Aerosols: their deposition and clearance Parkes WR.editor. Occupational lung disorders, 3rd ed Oxford: Butterworth-Heinemann; 199435 [Google Scholar]
- 4.Chen LC, Thurston G. World Trade Center cough. Lancet. 2002;360:s37–s38. doi: 10.1016/s0140-6736(02)11814-9. [DOI] [PubMed] [Google Scholar]
- 5.Muskat PC. Mass casualty chemical exposure and implications for respiratory failure. Respir Care. 2008;53:58–63, discussion 63–66. [PubMed] [Google Scholar]
- 6.Prezant DJ, Levin S, Kelly KJ, Aldrich TK. Upper and lower respiratory diseases after occupational and environmental disasters. Mt Sinai J Med. 2008;75:89–100. doi: 10.1002/msj.20028. [DOI] [PubMed] [Google Scholar]
- 7.Brooks SM, Bernstein IL. Irritant-induced airway disorders. Immunol Allergy Clin North Am. 2011;31:747–768, vi. doi: 10.1016/j.iac.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Walker PF, Buehner MF, Wood LA, Boyer NL, Driscoll IR, Lundy JB, Cancio LC, Chung KK. Diagnosis and management of inhalation injury: an updated review. Crit Care. 2015;19:351–370. doi: 10.1186/s13054-015-1077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodgers GC, Jr, Condurache CT. Antidotes and treatments for chemical warfare/terrorism agents: an evidence-based review. Clin Pharmacol Ther. 2010;88:318–327. doi: 10.1038/clpt.2010.152. [DOI] [PubMed] [Google Scholar]
- 10.White CW, Martin JG. Chlorine gas inhalation: human clinical evidence of toxicity and experience in animal models. Proc Am Thorac Soc. 2010;7:257–263. doi: 10.1513/pats.201001-008SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Department of Health and Human Services. doi: 10.3109/15360288.2015.1037530. Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Product development under the animal rule: guidance for industry. October 2015 [accessed 2017 Feb 19]. Available from: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm399217.pdf. [DOI] [PubMed]
- 12.Svendsen ER, Runkle JR, Dhara VR, Lin S, Naboka M, Mousseau TA, Bennett C. Epidemiologic methods lessons learned from environmental public health disasters: Chernobyl, the World Trade Center, Bhopal, and Graniteville, South Carolina. Int J Environ Res Public Health. 2012;9:2894–2909. doi: 10.3390/ijerph9082894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanna S, Dharmavaram S, Zhang J, Sykes I, Witlox H, Khajehnajafi S, Koslan K. Comparison of six widely-used dense gas dispersion models for three recent chlorine railcar accidents. Process Saf Prog. 2008;27:248–259. [Google Scholar]
- 14.Jani DD, Reed D, Feigley CE, Svendsen ER. Modeling an irritant gas plume for epidemiologic study. Int J Environ Health Res. 2016;26:58–74. doi: 10.1080/09603123.2015.1020414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buckley RL, Hunter CH, Werth DW, Whiteside MT, Chen KF, Mazzola CA. A case study of chlorine transport and fate following a large accidental release. Atmos Environ. 2012;62:184–198. [Google Scholar]
- 16.Buckley RL, Hunter CH, Addis RP, Parker MJ. Modeling dispersion from toxic gas released after a train collision in Graniteville, SC. J Air Waste Manag Assoc. 2007;57:268–278. doi: 10.1080/10473289.2007.10465329. [DOI] [PubMed] [Google Scholar]
- 17.Van Sickle D, Wenck MA, Belflower A, Drociuk D, Ferdinands J, Holguin F, Svendsen E, Bretous L, Jankelevich S, Gibson JJ, et al. Acute health effects after exposure to chlorine gas released after a train derailment. Am J Emerg Med. 2009;27:1–7. doi: 10.1016/j.ajem.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balte PP, Clark KA, Mohr LC, Karmaus WJ, Van Sickle D, Svendsen ER. The immediate pulmonary disease pattern following exposure to high concentrations of chlorine gas. Pulm Med. 2013;2013:325869. doi: 10.1155/2013/325869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark KA, Chanda D, Balte P, Karmaus WJ, Cai B, Vena J, Lawson AB, Mohr LC, Gibson JJ, Svendsen ER. Respiratory symptoms and lung function 8–10 months after community exposure to chlorine gas: a public health intervention and cross-sectional analysis. BMC Public Health. 2013;13:945. doi: 10.1186/1471-2458-13-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ginsberg JP, Holbrook JR, Chanda D, Bao H, Svendsen ER. Posttraumatic stress and tendency to panic in the aftermath of the chlorine gas disaster in Graniteville, South Carolina. Soc Psychiatry Psychiatr Epidemiol. 2012;47:1441–1448. doi: 10.1007/s00127-011-0449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Annang L, Wilson S, Tinago C, Wright Sanders L, Bevington T, Carlos B, Cornelius E, Svendsen E. Photovoice: assessing the long-term impact of a disaster on a community’s quality of life. Qual Health Res. 2016;26:241–251. doi: 10.1177/1049732315576495. [DOI] [PubMed] [Google Scholar]
- 22.Hoyle GW, Svendsen ER. Persistent effects of chlorine inhalation on respiratory health. Ann N Y Acad Sci. 2016;1378:33–40. doi: 10.1111/nyas.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carlisle M, Lam A, Svendsen ER, Aggarwal S, Matalon S. Chlorine-induced cardiopulmonary injury. Ann N Y Acad Sci. 2016;1374:159–167. doi: 10.1111/nyas.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Annang Ingram L, Tinago CB, Estrada R, Wilson S, Wright Sanders L, Bevington T, Carlos B, Cornelius E, Svendsen ER, Ball J. Off the rails in rural South Carolina: a qualitative study of healthcare provider perspectives on the long-term health impact of the Graniteville train disaster. Rural Remote Health. 2016;16:3906. [PubMed] [Google Scholar]
- 25.Clark KA, Karmaus WJ, Mohr LC, Cai B, Balte P, Gibson JJ, Ownby D, Lawson AB, Vena JE, Svendsen ER. Lung function before and after a large chlorine gas release in Graniteville, South Carolina. Ann Am Thorac Soc. 2016;13:356–363. doi: 10.1513/AnnalsATS.201508-525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landrigan PJ, Lioy PJ, Thurston G, Berkowitz G, Chen LC, Chillrud SN, Gavett SH, Georgopoulos PG, Geyh AS, Levin S, et al. NIEHS World Trade Center Working Group. Health and environmental consequences of the world trade center disaster. Environ Health Perspect. 2004;112:731–739. doi: 10.1289/ehp.6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yip J, Webber MP, Zeig-Owens R, Vossbrinck M, Singh A, Kelly K, Prezant DJ. FDNY and 9/11: clinical services and health outcomes in World Trade Center–exposed firefighters and EMS workers from 2001 to 2016. Am J Ind Med. 2016;59:695–708. doi: 10.1002/ajim.22631. [DOI] [PubMed] [Google Scholar]
- 28.Prezant DJ, Weiden M, Banauch GI, McGuinness G, Rom WN, Aldrich TK, Kelly KJ. Cough and bronchial responsiveness in firefighters at the World Trade Center site. N Engl J Med. 2002;347:806–815. doi: 10.1056/NEJMoa021300. [DOI] [PubMed] [Google Scholar]
- 29.Banauch GI, Alleyne D, Sanchez R, Olender K, Cohen HW, Weiden M, Kelly KJ, Prezant DJ. Persistent hyperreactivity and reactive airway dysfunction in firefighters at the World Trade Center. Am J Respir Crit Care Med. 2003;168:54–62. doi: 10.1164/rccm.200211-1329OC. [DOI] [PubMed] [Google Scholar]
- 30.Aldrich TK, Weakley J, Dhar S, Hall CB, Crosse T, Banauch GI, Weiden MD, Izbicki G, Cohen HW, Gupta A, et al. Bronchial reactivity and the course of lung function 13 years after World Trade Center exposure. Chest. 2016;150:1333–1340. doi: 10.1016/j.chest.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banauch GI, Hall C, Weiden M, Cohen HW, Aldrich TK, Christodoulou V, Arcentales N, Kelly KJ, Prezant DJ. Pulmonary function after exposure to the World Trade Center collapse in the New York City Fire Department. Am J Respir Crit Care Med. 2006;174:312–319. doi: 10.1164/rccm.200511-1736OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aldrich TK, Gustave J, Hall CB, Cohen HW, Webber MP, Zeig-Owens R, Cosenza K, Christodoulou V, Glass L, Al-Othman F, et al. Lung function in rescue workers at the World Trade Center after 7 years. N Engl J Med. 2010;362:1263–1272. doi: 10.1056/NEJMoa0910087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aldrich TK, Vossbrinck M, Zeig-Owens R, Hall CB, Schwartz TM, Moir W, Webber MP, Cohen HW, Nolan A, Weiden MD, et al. Lung function trajectories in World Trade Center–exposed New York City firefighters over 13 years: the roles of smoking and smoking cessation. Chest. 2016;149:1419–1427. doi: 10.1016/j.chest.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banauch GI, Dhala A, Prezant DJ. Pulmonary disease in rescue workers at the World Trade Center site. Curr Opin Pulm Med. 2005;11:160–168. doi: 10.1097/01.mcp.0000151716.96241.0a. [DOI] [PubMed] [Google Scholar]
- 35.Ghanei M, Harandi AA, Tazelaar HD. Isolated bronchiolitis obliterans: high incidence and diagnosis following terrorist attacks. Inhal Toxicol. 2012;24:340–341. doi: 10.3109/08958378.2012.667005. [DOI] [PubMed] [Google Scholar]
- 36.Glaser MS, Shah N, Webber MP, Zeig-Owens R, Jaber N, Appel DW, Hall CB, Weakley J, Cohen HW, Shulman L, et al. Obstructive sleep apnea and World Trade Center exposure. J Occup Environ Med. 2014;56:S30–S34. doi: 10.1097/JOM.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 37.Zeig-Owens R, Webber MP, Hall CB, Schwartz T, Jaber N, Weakley J, Rohan TE, Cohen HW, Derman O, Aldrich TK, et al. Early assessment of cancer outcomes in New York City firefighters after the 9/11 attacks: an observational cohort study. Lancet. 2011;378:898–905. doi: 10.1016/S0140-6736(11)60989-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Webber MP, Moir W, Zeig-Owens R, Glaser MS, Jaber N, Hall C, Berman J, Qayyum B, Loupasakis K, Kelly K, et al. Nested case–control study of selected systemic autoimmune diseases in World Trade Center rescue/recovery workers. Arthritis Rheumatol. 2015;67:1369–1376. doi: 10.1002/art.39059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Evans RB. Chlorine: state of the art. Lung. 2005;183:151–167. doi: 10.1007/s00408-004-2530-3. [DOI] [PubMed] [Google Scholar]
- 40.Malo JL, L’archevêque J, Castellanos L, Lavoie K, Ghezzo H, Maghni K. Long-term outcomes of acute irritant-induced asthma. Am J Respir Crit Care Med. 2009;179:923–928. doi: 10.1164/rccm.200810-1550OC. [DOI] [PubMed] [Google Scholar]
- 41.Song W, Yu Z, Doran SF, Ambalavanan N, Steele C, Garantziotis S, Matalon S. Respiratory syncytial virus infection increases chlorine-induced airway hyperresponsiveness. Am J Physiol Lung Cell Mol Physiol. 2015;309:L205–L210. doi: 10.1152/ajplung.00159.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin JG, Campbell HR, Iijima H, Gautrin D, Malo JL, Eidelman DH, Hamid Q, Maghni K. Chlorine-induced injury to the airways in mice. Am J Respir Crit Care Med. 2003;168:568–574. doi: 10.1164/rccm.200201-021OC. [DOI] [PubMed] [Google Scholar]
- 43.Tian X, Tao H, Brisolara J, Chen J, Rando RJ, Hoyle GW. Acute lung injury induced by chlorine inhalation in C57BL/6 and FVB/N mice. Inhal Toxicol. 2008;20:783–793. doi: 10.1080/08958370802007841. [DOI] [PubMed] [Google Scholar]
- 44.Demnati R, Fraser R, Ghezzo H, Martin JG, Plaa G, Malo JL. Time-course of functional and pathological changes after a single high acute inhalation of chlorine in rats. Eur Respir J. 1998;11:922–928. doi: 10.1183/09031936.98.11040922. [DOI] [PubMed] [Google Scholar]
- 45.Leustik M, Doran S, Bracher A, Williams S, Squadrito GL, Schoeb TR, Postlethwait E, Matalon S. Mitigation of chlorine-induced lung injury by low-molecular-weight antioxidants. Am J Physiol Lung Cell Mol Physiol. 2008;295:L733–L743. doi: 10.1152/ajplung.90240.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Musah S, Schlueter CF, Humphrey DM, Jr, Powell KS, Roberts AM, Hoyle GW. Acute lung injury and persistent small airway disease in a rabbit model of chlorine inhalation. Toxicol Appl Pharmacol. 2017;315:1–11. doi: 10.1016/j.taap.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gunnarsson M, Walther SM, Seidal T, Bloom GD, Lennquist S. Exposure to chlorine gas: effects on pulmonary function and morphology in anaesthetised and mechanically ventilated pigs. J Appl Toxicol. 1998;18:249–255. doi: 10.1002/(sici)1099-1263(199807/08)18:4<249::aid-jat507>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 48.Batchinsky AI, Martini DK, Jordan BS, Dick EJ, Fudge J, Baird CA, Hardin DE, Cancio LC. Acute respiratory distress syndrome secondary to inhalation of chlorine gas in sheep. J Trauma. 2006;60:944–956, discussion 956–957. doi: 10.1097/01.ta.0000205862.57701.48. [DOI] [PubMed] [Google Scholar]
- 49.Winternitz MC, Lambert RA, Jackson L, Smith GH. The pathology of chlorine poisoning. In: Winternitz MC, editor. Collected studies on the pathology of war gas poisoning. New Haven, CT: Yale University School of Medicine; 1920. pp. 1–32. [Google Scholar]
- 50.Barrow CS, Alarie Y, Warrick JC, Stock MF. Comparison of the sensory irritation response in mice to chlorine and hydrogen chloride. Arch Environ Health. 1977;32:68–76. doi: 10.1080/00039896.1977.10667258. [DOI] [PubMed] [Google Scholar]
- 51.Yadav AK, Doran SF, Samal AA, Sharma R, Vedagiri K, Postlethwait EM, Squadrito GL, Fanucchi MV, Roberts LJ, II, Patel RP, et al. Mitigation of chlorine gas lung injury in rats by postexposure administration of sodium nitrite. Am J Physiol Lung Cell Mol Physiol. 2011;300:L362–L369. doi: 10.1152/ajplung.00278.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Koren EG, Hogan BL, Gunn MD. Loss of basal cells precedes bronchiolitis obliterans–like pathological changes in a murine model of chlorine gas inhalation. Am J Respir Cell Mol Biol. 2013;49:788–797. doi: 10.1165/rcmb.2012-0369OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jurkuvenaite A, Benavides GA, Komarova S, Doran SF, Johnson M, Aggarwal S, Zhang J, Darley-Usmar VM, Matalon S. Upregulation of autophagy decreases chlorine-induced mitochondrial injury and lung inflammation. Free Radic Biol Med. 2015;85:83–94. doi: 10.1016/j.freeradbiomed.2015.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Massa CB, Scott P, Abramova E, Gardner C, Laskin DL, Gow AJ. Acute chlorine gas exposure produces transient inflammation and a progressive alteration in surfactant composition with accompanying mechanical dysfunction. Toxicol Appl Pharmacol. 2014;278:53–64. doi: 10.1016/j.taap.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoyle GW, Chang W, Chen J, Schlueter CF, Rando RJ. Deviations from Haber’s Law for multiple measures of acute lung injury in chlorine-exposed mice. Toxicol Sci. 2010;118:696–703. doi: 10.1093/toxsci/kfq264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang J, Zhang L, Walther SM. Inhaled budesonide in experimental chlorine gas lung injury: influence of time interval between injury and treatment. Intensive Care Med. 2002;28:352–357. doi: 10.1007/s00134-001-1175-4. [DOI] [PubMed] [Google Scholar]
- 57.McGovern TK, Powell WS, Day BJ, White CW, Govindaraju K, Karmouty-Quintana H, Lavoie N, Tan JJ, Martin JG. Dimethylthiourea protects against chlorine induced changes in airway function in a murine model of irritant induced asthma. Respir Res. 2010;11:138. doi: 10.1186/1465-9921-11-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leikauf GD, Pope-Varsalona H, Concel VJ, Liu P, Bein K, Berndt A, Martin TM, Ganguly K, Jang AS, Brant KA, et al. Integrative assessment of chlorine-induced acute lung injury in mice. Am J Respir Cell Mol Biol. 2012;47:234–244. doi: 10.1165/rcmb.2012-0026OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li C, Weng Z, Doran SF, Srivastava RK, Afaq F, Matalon S, Athar M. Chlorine induces the unfolded protein response in murine lungs and skin. Am J Respir Cell Mol Biol. 2013;49:197–203. doi: 10.1165/rcmb.2012-0488RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fanucchi MV, Bracher A, Doran SF, Squadrito GL, Fernandez S, Postlethwait EM, Bowen L, Matalon S. Post-exposure antioxidant treatment in rats decreases airway hyperplasia and hyperreactivity due to chlorine inhalation. Am J Respir Cell Mol Biol. 2012;46:599–606. doi: 10.1165/rcmb.2011-0196OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoyle GW, Hoyle CI, Chen J, Chang W, Williams RW, Rando RJ. Identification of triptolide, a natural diterpenoid compound, as an inhibitor of lung inflammation. Am J Physiol Lung Cell Mol Physiol. 2010;298:L830–L836. doi: 10.1152/ajplung.00014.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Song W, Wei S, Liu G, Yu Z, Estell K, Yadav AK, Schwiebert LM, Matalon S. Postexposure administration of a β2-agonist decreases chlorine-induced airway hyperreactivity in mice. Am J Respir Cell Mol Biol. 2011;45:88–94. doi: 10.1165/rcmb.2010-0226OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mo Y, Chen J, Schlueter CF, Hoyle GW. Differential susceptibility of inbred mouse strains to chlorine-induced airway fibrosis. Am J Physiol Lung Cell Mol Physiol. 2013;304:L92–L102. doi: 10.1152/ajplung.00272.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Musah S, Chen J, Hoyle GW. Repair of tracheal epithelium by basal cells after chlorine-induced injury. Respir Res. 2012;13:107. doi: 10.1186/1465-9921-13-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jonasson S, Koch B, Bucht A. Inhalation of chlorine causes long-standing lung inflammation and airway hyperresponsiveness in a murine model of chemical-induced lung injury. Toxicology. 2013;303:34–42. doi: 10.1016/j.tox.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 66.Jonasson S, Wigenstam E, Koch B, Bucht A. Early treatment of chlorine-induced airway hyperresponsiveness and inflammation with corticosteroids. Toxicol Appl Pharmacol. 2013;271:168–174. doi: 10.1016/j.taap.2013.04.037. [DOI] [PubMed] [Google Scholar]
- 67.Zaky A, Bradley WE, Lazrak A, Zafar I, Doran S, Ahmad A, White CW, Dell’Italia LJ, Matalon S, Ahmad S. Chlorine inhalation–induced myocardial depression and failure. Physiol Rep. 2015;3:e12439. doi: 10.14814/phy2.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ahmad S, Ahmad A, Hendry-Hofer TB, Loader JE, Claycomb WC, Mozziconacci O, Schöneich C, Reisdorph N, Powell RL, Chandler JD, et al. Sarcoendoplasmic reticulum Ca2+ ATPase: a critical target in chlorine inhalation–induced cardiotoxicity. Am J Respir Cell Mol Biol. 2015;52:492–502. doi: 10.1165/rcmb.2014-0005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McGovern T, Day BJ, White CW, Powell WS, Martin JG. AEOL10150: a novel therapeutic for rescue treatment after toxic gas lung injury. Free Radic Biol Med. 2011;50:602–608. doi: 10.1016/j.freeradbiomed.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wigenstam E, Koch B, Bucht A, Jonasson S. N-Acetyl cysteine improves the effects of corticosteroids in a mouse model of chlorine-induced acute lung injury. Toxicology. 2015;328:40–47. doi: 10.1016/j.tox.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 71.Zarogiannis SG, Jurkuvenaite A, Fernandez S, Doran SF, Yadav AK, Squadrito GL, Postlethwait EM, Bowen L, Matalon S. Ascorbate and deferoxamine administration after chlorine exposure decrease mortality and lung injury in mice. Am J Respir Cell Mol Biol. 2011;45:386–392. doi: 10.1165/rcmb.2010-0432OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chang W, Chen J, Schlueter CF, Rando RJ, Pathak YV, Hoyle GW. Inhibition of chlorine-induced lung injury by the type 4 phosphodiesterase inhibitor rolipram. Toxicol Appl Pharmacol. 2012;263:251–258. doi: 10.1016/j.taap.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen J, Mo Y, Schlueter CF, Hoyle GW. Inhibition of chlorine-induced pulmonary inflammation and edema by mometasone and budesonide. Toxicol Appl Pharmacol. 2013;272:408–413. doi: 10.1016/j.taap.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoyle GW, Chen J, Schlueter CF, Mo Y, Humphrey DM, Jr, Rawson G, Niño JA, Carson KH. Development and assessment of countermeasure formulations for treatment of lung injury induced by chlorine inhalation. Toxicol Appl Pharmacol. 2016;298:9–18. doi: 10.1016/j.taap.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang J, Zhang L, Walther SM. Administration of aerosolized terbutaline and budesonide reduces chlorine gas–induced acute lung injury. J Trauma. 2004;56:850–862. doi: 10.1097/01.ta.0000078689.45384.8b. [DOI] [PubMed] [Google Scholar]
- 76.Honavar J, Doran S, Oh JY, Steele C, Matalon S, Patel RP. Nitrite therapy improves survival postexposure to chlorine gas. Am J Physiol Lung Cell Mol Physiol. 2014;307:L888–L894. doi: 10.1152/ajplung.00079.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McGovern TK, Goldberger M, Allard B, Farahnak S, Hamamoto Y, O’Sullivan M, Hirota N, Martel G, Rousseau S, Martin JG. Neutrophils mediate airway hyperresponsiveness after chlorine-induced airway injury in the mouse. Am J Respir Cell Mol Biol. 2015;52:513–522. doi: 10.1165/rcmb.2013-0430OC. [DOI] [PubMed] [Google Scholar]
- 78.Zarogiannis SG, Wagener BM, Basappa S, Doran S, Rodriguez CA, Jurkuvenaite A, Pittet JF, Matalon S. Postexposure aerosolized heparin reduces lung injury in chlorine-exposed mice. Am J Physiol Lung Cell Mol Physiol. 2014;307:L347–L354. doi: 10.1152/ajplung.00152.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lazrak A, Creighton J, Yu Z, Komarova S, Doran SF, Aggarwal S, Emala CW, Sr, Stober VP, Trempus CS, Garantziotis S, et al. Hyaluronan mediates airway hyperresponsiveness in oxidative lung injury. Am J Physiol Lung Cell Mol Physiol. 2015;308:L891–L903. doi: 10.1152/ajplung.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bessac BF, Jordt SE. Sensory detection and responses to toxic gases: mechanisms, health effects, and countermeasures. Proc Am Thorac Soc. 2010;7:269–277. doi: 10.1513/pats.201001-004SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Balakrishna S, Song W, Achanta S, Doran SF, Liu B, Kaelberer MM, Yu Z, Sui A, Cheung M, Leishman E, et al. TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2014;307:L158–L172. doi: 10.1152/ajplung.00065.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fukuda S, Ihara K, Lopez E, Cox R, Southan G, Salzman A, Salsbury JR, Prough DS, Enkhbaatar P. R-107 attenuates severity of acute respiratory distress syndrome induced by chlorine gas inhalation in ovine model [abstract] Am J Respir Crit Care Med. 2016;193:A6299. [Google Scholar]
- 83.Makarovsky I, Markel G, Hoffman A, Schein O, Brosh-Nissimov TM, Finkelstien A, Tashma Z, Dushnitsky T, Eisenkraft A. Bromine: the red cloud approaching. Isr Med Assoc J. 2007;9:677–679. [PubMed] [Google Scholar]
- 84.Fredenburgh LE, Perrella MA, Mitsialis SA. The role of heme oxygenase-1 in pulmonary disease. Am J Respir Cell Mol Biol. 2007;36:158–165. doi: 10.1165/rcmb.2006-0331TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aggarwal S, Lam A, Bolisetty S, Carlisle MA, Traylor A, Agarwal A, Matalon S. Heme attenuation ameliorates irritant gas inhalation–induced acute lung injury. Antioxid Redox Signal. 2016;24:99–112. doi: 10.1089/ars.2015.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jugg B. Chemical warfare toxicology, Vol. 1: Fundamental aspects. Cambridge: RSC Publishing; 2016. Toxicology and treatment of phosgene induced lung injury; pp. 154–175. [Google Scholar]
- 87.Parkhouse DA, Brown RF, Jugg BJ, Harban FM, Platt J, Kenward CE, Jenner J, Rice P, Smith AJ. Protective ventilation strategies in the management of phosgene-induced acute lung injury. Mil Med. 2007;172:295–300. doi: 10.7205/milmed.172.3.295. [DOI] [PubMed] [Google Scholar]
- 88.Grainge C, Jugg BJ, Smith AJ, Brown RF, Jenner J, Parkhouse DA, Rice P. Delayed low-dose supplemental oxygen improves survival following phosgene-induced acute lung injury. Inhal Toxicol. 2010;22:552–560. doi: 10.3109/08958370903571831. [DOI] [PubMed] [Google Scholar]
- 89.Smith A, Brown R, Jugg B, Platt J, Mann T, Masey C, Jenner J, Rice P. The effect of steroid treatment with inhaled budesonide or intravenous methylprednisolone on phosgene-induced acute lung injury in a porcine model. Mil Med. 2009;174:1287–1294. doi: 10.7205/milmed-d-09-00050. [DOI] [PubMed] [Google Scholar]
- 90.Grainge C, Brown R, Jugg BJ, Smith AJ, Mann TM, Jenner J, Rice P, Parkhouse DA. Early treatment with nebulised salbutamol worsens physiological measures and does not improve survival following phosgene induced acute lung injury. J R Army Med Corps. 2009;155:105–109. doi: 10.1136/jramc-155-02-05. [DOI] [PubMed] [Google Scholar]
- 91.Grainge C, Rice P. Management of phosgene-induced acute lung injury. Clin Toxicol (Phila) 2010;48:497–508. doi: 10.3109/15563650.2010.506877. [DOI] [PubMed] [Google Scholar]
- 92.Leikauf GD, Concel VJ, Bein K, Liu P, Berndt A, Martin TM, Ganguly K, Jang AS, Brant KA, Dopico RA, Jr, et al. Functional genomic assessment of phosgene-induced acute lung injury in mice. Am J Respir Cell Mol Biol. 2013;49:368–383. doi: 10.1165/rcmb.2012-0337OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Everley PA, Dillman JF., III Genomics and proteomics in chemical warfare agent research: recent studies and future applications. Toxicol Lett. 2010;198:297–303. doi: 10.1016/j.toxlet.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 94.Sciuto AM, Phillips CS, Orzolek LD, Hege AI, Moran TS, Dillman JF., III Genomic analysis of murine pulmonary tissue following carbonyl chloride inhalation. Chem Res Toxicol. 2005;18:1654–1660. doi: 10.1021/tx050126f. [DOI] [PubMed] [Google Scholar]
- 95.Holmes WW, Keyser BM, Paradiso DC, Ray R, Andres DK, Benton BJ, Rothwell CC, Hoard-Fruchey HM, Dillman JF, Sciuto AM, et al. Conceptual approaches for treatment of phosgene inhalation–induced lung injury. Toxicol Lett. 2016;244:8–20. doi: 10.1016/j.toxlet.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.He DK, Shao YR, Zhang L, Shen J, Zhong ZY, Wang J, Xu G. Adenovirus-delivered angiopoietin-1 suppresses NF-κB and p38 MAPK and attenuates inflammatory responses in phosgene-induced acute lung injury. Inhal Toxicol. 2014;26:185–192. doi: 10.3109/08958378.2013.872213. [DOI] [PubMed] [Google Scholar]
- 97.Filipczak PT, Senft AP, Seagrave J, Weber W, Kuehl PJ, Fredenburgh LE, McDonald JD, Baron RM. NOS-2 inhibition in phosgene-induced acute lung injury. Toxicol Sci. 2015;146:89–100. doi: 10.1093/toxsci/kfv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shen J, Gan Z, Zhao J, Zhang L, Xu G. Ulinastatin reduces pathogenesis of phosgene-induced acute lung injury in rats. Toxicol Ind Health. 2014;30:785–793. doi: 10.1177/0748233712463776. [DOI] [PubMed] [Google Scholar]
- 99.Chen J, Shao Y, Xu G, Lim C, Li J, Xu D, Shen J. Bone marrow–derived mesenchymal stem cells attenuate phosgene-induced acute lung injury in rats. Inhal Toxicol. 2015;27:254–261. doi: 10.3109/08958378.2015.1037029. [DOI] [PubMed] [Google Scholar]
- 100.Zhang J, Shao Y, He D, Zhang L, Xu G, Shen J. Evidence that bone marrow–derived mesenchymal stem cells reduce epithelial permeability following phosgene-induced acute lung injury via activation of Wnt3a protein–induced canonical Wnt/β-catenin signaling. Inhal Toxicol. 2016;28:572–579. doi: 10.1080/08958378.2016.1228720. [DOI] [PubMed] [Google Scholar]
- 101.Filippidis AS, Zarogiannis SG, Randich A, Ness TJ, Matalon S. Assessment of locomotion in chlorine exposed mice by computer vision and neural networks. J Appl Physiol (1985) 2012;112:1064–1072. doi: 10.1152/japplphysiol.01023.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bessac BF, Jordt SE. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology (Bethesda) 2008;23:360–370. doi: 10.1152/physiol.00026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Högestätt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 104.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 105.Bessac BF, Sivula M, von Hehn CA, Caceres AI, Escalera J, Jordt SE. Transient receptor potential ankyrin 1 antagonists block the noxious effects of toxic industrial isocyanates and tear gases. FASEB J. 2009;23:1102–1114. doi: 10.1096/fj.08-117812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, Jordt SE. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest. 2008;118:1899–1910. doi: 10.1172/JCI34192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Goldenberg NM, Ravindran K, Kuebler WM. TRPV4: physiological role and therapeutic potential in respiratory diseases. Naunyn Schmiedebergs Arch Pharmacol. 2015;388:421–436. doi: 10.1007/s00210-014-1058-1. [DOI] [PubMed] [Google Scholar]
- 108.Hamanaka K, Jian MY, Weber DS, Alvarez DF, Townsley MI, Al-Mehdi AB, King JA, Liedtke W, Parker JC. TRPV4 initiates the acute calcium-dependent permeability increase during ventilator-induced lung injury in isolated mouse lungs. Am J Physiol Lung Cell Mol Physiol. 2007;293:L923–L932. doi: 10.1152/ajplung.00221.2007. [DOI] [PubMed] [Google Scholar]
- 109.Thorneloe KS, Cheung M, Bao W, Alsaid H, Lenhard S, Jian MY, Costell M, Maniscalco-Hauk K, Krawiec JA, Olzinski A, et al. An orally active TRPV4 channel blocker prevents and resolves pulmonary edema induced by heart failure. Sci Transl Med. 2012;4:159ra148. doi: 10.1126/scitranslmed.3004276. [DOI] [PubMed] [Google Scholar]
- 110.Kiss L, Holmes S, Chou CE, Dong X, Ross J, Brown D, Mendenhall B, Coronado V, De Silva D, Rockwood GA, et al. Method development for detecting the novel cyanide antidote dimethyl trisulfide from blood and brain, and its interaction with blood. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1044-1045:149–157. doi: 10.1016/j.jchromb.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 111.Manandhar E, Maslamani N, Petrikovics I, Rockwood GA, Logue BA. Determination of dimethyl trisulfide in rabbit blood using stir bar sorptive extraction gas chromatography–mass spectrometry. J Chromatogr A. 2016;1461:10–17. doi: 10.1016/j.chroma.2016.07.046. [DOI] [PubMed] [Google Scholar]
- 112.Rockwood GA, Thompson DE, Petrikovics I. Dimethyl trisulfide: a novel cyanide countermeasure. Toxicol Ind Health. 2016;32:2009–2016. doi: 10.1177/0748233715622713. [DOI] [PubMed] [Google Scholar]
- 113.Bartling CM, Andre JC, Howland CA, Hester ME, Cafmeyer JT, Kerr A, Petrel T, Petrikovics I, Rockwood GA. Stability characterization of a polysorbate 80-dimethyl trisulfide formulation, a cyanide antidote candidate. Drugs R D. 2016;16:109–127. doi: 10.1007/s40268-016-0122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kovacs K, Duke AC, Shifflet M, Winner B, Lee SA, Rockwood GA, Petrikovics I. Parenteral dosage form development and testing of dimethyl trisulfide, as an antidote candidate to combat cyanide intoxication. Pharm Dev Technol . In press doi: 10.3109/10837450.2015.1125923. [DOI] [PubMed] [Google Scholar]
- 115.Chan A, Balasubramanian M, Blackledge W, Mohammad OM, Alvarez L, Boss GR, Bigby TD. Cobinamide is superior to other treatments in a mouse model of cyanide poisoning. Clin Toxicol (Phila) 2010;48:709–717. doi: 10.3109/15563650.2010.505197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brenner M, Kim JG, Mahon SB, Lee J, Kreuter KA, Blackledge W, Mukai D, Patterson S, Mohammad O, Sharma VS, et al. Intramuscular cobinamide sulfite in a rabbit model of sublethal cyanide toxicity. Ann Emerg Med. 2010;55:352–363. doi: 10.1016/j.annemergmed.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Broderick KE, Potluri P, Zhuang S, Scheffler IE, Sharma VS, Pilz RB, Boss GR. Cyanide detoxification by the cobalamin precursor cobinamide. Exp Biol Med (Maywood) 2006;231:641–649. doi: 10.1177/153537020623100519. [DOI] [PubMed] [Google Scholar]
- 118.Broderick KE, Balasubramanian M, Chan A, Potluri P, Feala J, Belke DD, McCulloch A, Sharma VS, Pilz RB, Bigby TD, et al. The cobalamin precursor cobinamide detoxifies nitroprusside-generated cyanide. Exp Biol Med (Maywood) 2007;232:789–798. [PubMed] [Google Scholar]
- 119.Crankshaw DL, Goon DJ, Briggs JE, DeLong D, Kuskowski M, Patterson SE, Nagasawa HT. A novel paradigm for assessing efficacies of potential antidotes against neurotoxins in mice. Toxicol Lett. 2007;175:111–117. doi: 10.1016/j.toxlet.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brenner M, Kim JG, Lee J, Mahon SB, Lemor D, Ahdout R, Boss GR, Blackledge W, Jann L, Nagasawa HT, et al. Sulfanegen sodium treatment in a rabbit model of sub-lethal cyanide toxicity. Toxicol Appl Pharmacol. 2010;248:269–276. doi: 10.1016/j.taap.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Belani KG, Singh H, Beebe DS, George P, Patterson SE, Nagasawa HT, Vince R. Cyanide toxicity in juvenile pigs and its reversal by a new prodrug, sulfanegen sodium. Anesth Analg. 2012;114:956–961. doi: 10.1213/ANE.0b013e31824c4eb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Patterson SE, Monteil AR, Cohen JF, Crankshaw DL, Vince R, Nagasawa HT. Cyanide antidotes for mass casualties: water-soluble salts of the dithiane (sulfanegen) from 3-mercaptopyruvate for intramuscular administration. J Med Chem. 2013;56:1346–1349. doi: 10.1021/jm301633x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nagasawa HT, Goon DJ, Crankshaw DL, Vince R, Patterson SE. Novel, orally effective cyanide antidotes. J Med Chem. 2007;50:6462–6464. doi: 10.1021/jm7011497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Borron SW, Stonerook M, Reid F. Efficacy of hydroxocobalamin for the treatment of acute cyanide poisoning in adult beagle dogs. Clin Toxicol (Phila) 2006;44:5–15. doi: 10.1080/15563650600811672. [DOI] [PubMed] [Google Scholar]
- 125.Brenner M, Mahon SB, Lee J, Kim J, Mukai D, Goodman S, Kreuter KA, Ahdout R, Mohammad O, Sharma VS, et al. Comparison of cobinamide to hydroxocobalamin in reversing cyanide physiologic effects in rabbits using diffuse optical spectroscopy monitoring. J Biomed Opt. 2010;15:017001, 017001–017008. doi: 10.1117/1.3290816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yadav PK, Yamada K, Chiku T, Koutmos M, Banerjee R. Structure and kinetic analysis of H2S production by human mercaptopyruvate sulfurtransferase. J Biol Chem. 2013;288:20002–20013. doi: 10.1074/jbc.M113.466177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Patterson SE, Moeller B, Nagasawa HT, Vince R, Crankshaw DL, Briggs J, Stutelberg MW, Vinnakota CV, Logue BA. Development of sulfanegen for mass cyanide casualties. Ann N Y Acad Sci. 2016;1374:202–209. doi: 10.1111/nyas.13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chan A, Jiang J, Fridman A, Guo LT, Shelton GD, Liu MT, Green C, Haushalter KJ, Patel HH, Lee J, et al. Nitrocobinamide, a new cyanide antidote that can be administered by intramuscular injection. J Med Chem. 2015;58:1750–1759. doi: 10.1021/jm501565k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ghanei M, Harandi AA. Long term consequences from exposure to sulfur mustard: a review. Inhal Toxicol. 2007;19:451–456. doi: 10.1080/08958370601174990. [DOI] [PubMed] [Google Scholar]
- 130.Ghanei M, Naderi M, Kosar AM, Harandi AA, Hopkinson NS, Poursaleh Z. Long-term pulmonary complications of chemical warfare agent exposure in Iraqi Kurdish civilians. Inhal Toxicol. 2010;22:719–724. doi: 10.3109/08958371003686016. [DOI] [PubMed] [Google Scholar]
- 131.White CW, Rancourt RC, Veress LA. Sulfur mustard inhalation: mechanisms of injury, alteration of coagulation, and fibrinolytic therapy. Ann N Y Acad Sci. 2016;1378:87–95. doi: 10.1111/nyas.13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kohnavard N. Iraqi town Taza “hit in IS chemical attack” appeals for help. BBC News; March 25, 2016 [accessed 2017 March 1]. Available from: http://www.bbc.com/news/world-middle-east-35898990.
- 133.Nichols M. Inquiry finds Syrian government forces responsible for third chemical attack. Reuters World News; October 22, 2016 [accessed 2017 March 1]. Available from: http://www.reuters.com/article/us-mideast-crisis-syria-chemicalweapons-idUSKCN12M007.
- 134.Veress LA, O’Neill HC, Hendry-Hofer TB, Loader JE, Rancourt RC, White CW. Airway obstruction due to bronchial vascular injury after sulfur mustard analog inhalation. Am J Respir Crit Care Med. 2010;182:1352–1361. doi: 10.1164/rccm.200910-1618OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Veress LA, Hendry-Hofer TB, Loader JE, Rioux JS, Garlick RB, White CW. Tissue plasminogen activator prevents mortality from sulfur mustard analog–induced airway obstruction. Am J Respir Cell Mol Biol. 2013;48:439–447. doi: 10.1165/rcmb.2012-0177OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Veress LA, Anderson DR, Hendry-Hofer TB, Houin PR, Rioux JS, Garlick RB, Loader JE, Paradiso DC, Smith RW, Rancourt RC, et al. Airway tissue plasminogen activator prevents acute mortality due to lethal sulfur mustard inhalation. Toxicol Sci. 2015;143:178–184. doi: 10.1093/toxsci/kfu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Willems J. Clinical management of mustard gas casualties. Annales Medicinae Militaris Belgicae. 1989;3:1–61. [Google Scholar]
- 138.Rancourt RC, Veress LA, Guo X, Jones TN, Hendry-Hofer TB, White CW. Airway tissue factor–dependent coagulation activity in response to sulfur mustard analog 2-chloroethyl ethyl sulfide. Am J Physiol Lung Cell Mol Physiol. 2012;302:L82–L92. doi: 10.1152/ajplung.00306.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rancourt RC, Veress LA, Ahmad A, Hendry-Hofer TB, Rioux JS, Garlick RB, White CW. Tissue factor pathway inhibitor prevents airway obstruction, respiratory failure and death due to sulfur mustard analog inhalation. Toxicol Appl Pharmacol. 2013;272:86–95. doi: 10.1016/j.taap.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rancourt RC, Ahmad A, Veress LA, Rioux JS, Garlick RB, White CW. Antifibrinolytic mechanisms in acute airway injury after sulfur mustard analog inhalation. Am J Respir Cell Mol Biol. 2014;51:559–567. doi: 10.1165/rcmb.2014-0012OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.O’Neill HC, White CW, Veress LA, Hendry-Hofer TB, Loader JE, Min E, Huang J, Rancourt RC, Day BJ. Treatment with the catalytic metalloporphyrin AEOL 10150 reduces inflammation and oxidative stress due to inhalation of the sulfur mustard analog 2-chloroethyl ethyl sulfide. Free Radic Biol Med. 2010;48:1188–1196. doi: 10.1016/j.freeradbiomed.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.McElroy CS, Min E, Huang J, Loader JE, Hendry-Hofer TB, Garlick RB, Rioux JS, Veress LA, Smith R, Osborne C, et al. From the cover: catalytic antioxidant rescue of inhaled sulfur mustard toxicity. Toxicol Sci. 2016;154:341–353. doi: 10.1093/toxsci/kfw170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ghosh M, Ahmad S, White CW, Reynolds SD. Transplantation of airway epithelial stem/progenitor cells: a future for cell-based therapy. Am J Respir Cell Mol Biol. 2017;56:1–10. doi: 10.1165/rcmb.2016-0181MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Saber H, Saburi A, Ghanei M. Clinical and paraclinical guidelines for management of sulfur mustard induced bronchiolitis obliterans: from bench to bedside. Inhal Toxicol. 2012;24:900–906. doi: 10.3109/08958378.2012.725783. [DOI] [PubMed] [Google Scholar]
- 145.Emad A, Rezaian GR. Immunoglobulins and cellular constituents of the BAL fluid of patients with sulfur mustard gas–induced pulmonary fibrosis. Chest. 1999;115:1346–1351. doi: 10.1378/chest.115.5.1346. [DOI] [PubMed] [Google Scholar]
- 146.Aghanouri R, Ghanei M, Aslani J, Keivani-Amine H, Rastegar F, Karkhane A. Fibrogenic cytokine levels in bronchoalveolar lavage aspirates 15 years after exposure to sulfur mustard. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1160–L1164. doi: 10.1152/ajplung.00169.2003. [DOI] [PubMed] [Google Scholar]
- 147.Graham JS, Schoneboom BA. Historical perspective on effects and treatment of sulfur mustard injuries. Chem Biol Interact. 2013;206:512–522. doi: 10.1016/j.cbi.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 148.Malaviya R, Sunil VR, Venosa A, Verissimo VL, Cervelli JA, Vayas KN, Hall L, Laskin JD, Laskin DL. Attenuation of nitrogen mustard–induced pulmonary injury and fibrosis by anti–tumor necrosis factor-α antibody. Toxicol Sci. 2015;148:71–88. doi: 10.1093/toxsci/kfv161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Weinberger B, Malaviya R, Sunil VR, Venosa A, Heck DE, Laskin JD, Laskin DL. Mustard vesicant–induced lung injury: advances in therapy. Toxicol Appl Pharmacol. 2016;305:1–11. doi: 10.1016/j.taap.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.BBC News Services. Syria chemical attack: what we know. September 24, 2013 [accessed 2017 March 1]. Available from: http://www.bbc.com/news/world-middle-east-23927399.
- 151.Niven AS, Roop SA. Inhalational exposure to nerve agents. Respir Care Clin N Am. 2004;10:59–74. doi: 10.1016/S1078-5337(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 152.Little PJ, Reynolds ML, Bowman ER, Martin BR. Tissue disposition of [3H]sarin and its metabolites in mice. Toxicol Appl Pharmacol. 1986;83:412–419. doi: 10.1016/0041-008x(86)90223-1. [DOI] [PubMed] [Google Scholar]
- 153.Wolthuis OL, Vanwersch RA, Van Helden HP. Residual behavioral incapacitation after therapy of soman intoxication: the effect of a soman simulator. Neurobehav Toxicol Teratol. 1986;8:127–130. [PubMed] [Google Scholar]
- 154.Conti ML, Che MM, Boylan M, Sciuto AM, Gordon RK, Nambiar MP. Acute microinstillation inhalation exposure to sarin induces changes in respiratory dynamics and functions in guinea pigs. Int J Toxicol. 2009;28:436–447. doi: 10.1177/1091581809344879. [DOI] [PubMed] [Google Scholar]
- 155.Che MM, Conti M, Boylan M, Sciuto AM, Gordon RK, Nambiar MP. Blood and bronchoalveolar lavage fluid acetylcholinesterase levels following microinstillation inhalation exposure to sarin in Guinea pigs. Inhal Toxicol. 2008;20:821–828. doi: 10.1080/08958370802050957. [DOI] [PubMed] [Google Scholar]
- 156.Fawcett WP, Aracava Y, Adler M, Pereira EFR, Albuquerque EX. Acute toxicity of organophosphorus compounds in guinea pigs is sex- and age-dependent and cannot be solely accounted for by acetylcholinesterase inhibition. J Pharmacol Exp Ther. 2009;328:516–524. doi: 10.1124/jpet.108.146639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.RamaRao G, Bhattacharya BK. Multiple signal transduction pathways alterations during nerve agent toxicity. Toxicol Lett. 2012;208:16–22. doi: 10.1016/j.toxlet.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 158.Dillman JF, III, Phillips CS, Kniffin DM, Tompkins CP, Hamilton TA, Kan RK. Gene expression profiling of rat hippocampus following exposure to the acetylcholinesterase inhibitor soman. Chem Res Toxicol. 2009;22:633–638. doi: 10.1021/tx800466v. [DOI] [PubMed] [Google Scholar]
- 159.Collins JL, Peng X, Lee R, Witriol A, Pierre Z, Sciuto AM. Determination of LCt50s in anesthetized rats exposed to aerosolized nerve agents. Toxicol Mech Methods. 2013;23:127–133. doi: 10.3109/15376516.2012.730560. [DOI] [PubMed] [Google Scholar]
- 160.Peng X, Perkins MW, Simons J, Witriol AM, Rodriguez AM, Benjamin BM, Devorak J, Sciuto AM. Acute pulmonary toxicity following inhalation exposure to aerosolized VX in anesthetized rats. Inhal Toxicol. 2014;26:371–379. doi: 10.3109/08958378.2014.899410. [DOI] [PubMed] [Google Scholar]
- 161.Peng X, Rodriguez AM, Witriol AM, Bowens CM, Simons JB, Benjamin BM, Nordmark BA, Collins JL, Hamilton TA, Kan RK, et al. Oxidative stress may contribute to the obstructive pulmonary dysfunction from inhalation exposure to nerve agent VX in rats [abstract] Am J Respir Crit Care Med. 2011;183:A3251. [Google Scholar]
- 162.Adelman B. Opening up drug development to everyone. Hematology Am Soc Hematol Educ Program. 2013;2013:311–315. doi: 10.1182/asheducation-2013.1.311. [DOI] [PubMed] [Google Scholar]
- 163.Lurie N, Manolio T, Patterson AP, Collins F, Frieden T. Research as a part of public health emergency response. N Engl J Med. 2013;368:1251–1255. doi: 10.1056/NEJMsb1209510. [DOI] [PubMed] [Google Scholar]
- 164.Miller A, Yeskey K, Garantziotis S, Arnesen S, Bennett A, O’Fallon L, Thompson C, Reinlib L, Masten S, Remington J, et al. Integrating health research into disaster response: the new NIH disaster research response program. Int J Environ Res Public Health. 2016;13:676. doi: 10.3390/ijerph13070676. [DOI] [PMC free article] [PubMed] [Google Scholar]