Abstract
Emerging research provides strong evidence that activation of CNS glial cells occurs in neurological diseases and brain injury and results in elevated production of neuroimmune factors. These factors can contribute to pathophysiological processes that lead to altered CNS function. Recently, studies have also shown that both acute and chronic alcohol consumption can produce activation of CNS glial cells and the production of neuroimmune factors, particularly the chemokine CCL2. The consequences of alcohol-induced increases in CCL2 levels in the CNS have yet to be fully elucidated. Our studies focus on the hypothesis that increased levels of CCL2 in the CNS produce neuroadaptive changes that modify the actions of alcohol on the CNS. We utilized behavioral testing in transgenic mice that express elevated levels of CCL2 to test this hypothesis. The increased level of CCL2 in the transgenic mice involves increased astrocyte expression. Transgenic mice and their non-transgenic littermate controls were subjected to one of two alcohol exposure paradigms, a two-bottle choice alcohol drinking procedure that does not produce alcohol dependence or a chronic intermittent alcohol procedure that produces alcohol dependence. Several behavioral tests were carried out including the Barnes Maze, Y-Maze, cued and contextual conditioned fear test, light-dark transfer, and forced swim test. Comparisons between alcohol naïve, non-dependent, and alcohol dependent CCL2 transgenic and non-tg mice show that elevated levels of CCL2 in the CNS interact with alcohol in tests for alcohol drinking, spatial learning and associative learning.
Keywords: Two-bottle choice alcohol drinking, chronic intermittent alcohol exposure, spatial learning, associative learning
Introduction
The neuroimmune system of the brain, which is primarily comprised of glial cells, plays an important regulatory and protective role in the CNS through the action of a relatively large number of signaling factors, including small signaling proteins such as chemokines and cytokines. The chemokine CCL2 (chemokine ligand 2, previously known as monocyte chemoattractant protein-1 or MCP-1) is a key innate neuroimmune chemokine in the CNS. Although the roles CCL2 play in the CNS have yet to be fully elucidated, pharmacological studies indicate that CCL2 has neuronal actions, consistent with a role in CNS physiology or pathophysiology (Banisadr et al., 2005; van Gassen et al., 2005; Cho and Gruol, 2008; Jung et al., 2008; Guyon et al., 2009; You et al., 2010; Zhou et al., 2011). Increased CCL2 levels in the CNS have been reported for a number of neurologic conditions (e.g., Multiple Sclerosis (Beier et al., 2014), CNS infection (Vagenas et al., 2015), brain injury (Teng and Molina, 2014), and are thought to contribute to CNS pathophysiology associated with those conditions. Elevated levels of CCL2 in the CNS also occurs with alcohol use, a finding that has resulted in the idea that CCL2 may also play a role in alcohol use disorders.
A number of studies have demonstrated elevated levels of CCL2 mRNA or protein in the CNS of alcoholics. In these studies, higher levels of CCL2 were observed in several brain regions (e.g., hippocampus and cortex) of post-mortem human alcoholics compared to post-mortem human brains from non-alcoholics (Lewohl et al., 2000; He and Crews, 2008). Higher levels of CCL2 were also present in the cerebrospinal fluid (CSF) of alcohol-dependent human subjects compared to non-dependent subjects (Umhau et al., 2014). Studies in experimental animals confirmed that increased expression of CCL2 is produced by alcohol (ethanol) exposure/withdrawal. In these studies, significant increases in the levels of CCL2 mRNA and/or protein were observed in several brain regions following either single or repeated alcohol exposure/withdrawal (Qin et al., 2008; Knapp et al., 2011; Ehrlich et al., 2012; Freeman et al., 2012; Kane et al., 2013; Vetreno et al., 2013; Kane et al., 2014; Chang et al., 2015; Drew et al., 2015; Harper et al., 2015; Pascual et al., 2015; Knapp et al., 2016). For example, elevated levels of CCL2 mRNA were observed in the hippocampus of mice one day following a 10 day exposure to alcohol by gavage (BALs ~ 300 mg/dl) (Kane et al., 2013; Kane et al., 2014). In rats, alcohol consumption for 12 months increased mean CCL2 levels (measured by ELISA) in the cortex to 23.8±3.7 pg/mg protein compared to 16.5±1.2 pg/mg protein in the cortex of control animals (Ehrlich et al., 2012). In other studies, increased expression of CCL2 mRNA was observed after withdrawal from a liquid alcohol diet, and the level of CCL2 mRNA expression correlated with blood levels of alcohol observed in the animals prior to withdrawal (Freeman et al., 2012; Harper et al., 2015; Knapp et al., 2016). In mice subjected to repeated alcohol exposure/withdrawal cycles at a high dose of alcohol (5 gm/kg by gavage), the increase in CCL2 mRNA persisted for days after withdrawal, indicating that alcohol can have long-term effects on CCL2 mRNA levels in the CNS (Qin et al., 2008).
While these studies establish that alcohol exposure induces CNS expression of CCL2, few studies have addressed the role of CCL2 in alcohol use disorders or the consequences of CCL2/alcohol interactions relative to CNS function. Studies using gene knockout mice suggest that CCL2 plays a role in the motivational aspects of alcohol consumption (Blednov et al., 2005). In these studies, mice null for CCL2 and/or its receptor, CCR2, showed a decrease in alcohol consumption and preference for alcohol when compared to wild-type mice in a two-bottle choice test (Blednov et al., 2005). Consistent with such a role, recent studies show that chronic intracerebroventricular infusion of CCL2 increased self-administration of sweetened alcohol, but not a sucrose-only solution (Valenta and Gonzales, 2016). This effect developed over several weeks of infusion and persisted for several weeks after CCL2 infusion was terminated (Valenta and Gonzales, 2016). These findings suggest that persistent exposure to CCL2 may be required for CCL2/alcohol interactions and that CCL2 (or CCL2/alcohol interactions) can have long-term effects on alcohol consumption.
Our studies of transgenic mice that chronically express elevated levels of CCL2 in the CNS (CCL2-tg) also support a potential role for CCL2 in alcohol use disorders. The elevated level of CCL2 in the CCL2-tg mice was achieved through genetic modification of astrocyte expression using the human GFAP promoter (Huang et al., 2002). Astrocytes are an important source of CCL2 in the CNS under both normal conditions and during alcohol exposure (Farina et al., 2007; Conductier et al., 2010; Choi et al., 2014; Szabo and Lippai, 2014). Astrocytes are widely distributed throughout the CNS, with high numbers in the hippocampus (Savchenko et al., 2000). Control studies using the human GFAP promoter linked to β-galactosidase to confirm expression in astrocytes showed that β-galactosidase activity occurred in astrocytes but not other cell types in the CNS (Brenner et al., 1994). The level of GFAP varies across astrocytes, and the level of GFAP promoter activity in transgenic mice also varied across astrocytes (Brenner et al., 1994; Martin and O’Callaghan, 1995). In conjunction with increased CCL2 production by astrocytes, downstream CNS cells such as microglia may have been induced to produce CCL2 and thereby contribute to the increased CNS levels of CCL2 in the CCL2-tg mice.
The CCL2-tg mice provide a model that can be used to study the CNS consequences of elevated levels CCL2 and potential CCL2/alcohol interactions that play a role in the effects of alcohol on the CNS. Non-transgenic (non-tg) littermates serve as controls. In our previous studies of the CCL2-tg and non-tg mice, the CCL2-tg mice did not show the acute alcohol-induced impairments in contextual learning that were observed in the non-tg mice (Bray et al., 2013). These results suggest that CCL2 can produce neuroadaptive changes that alter the effects of alcohol on memory and learning. Consistent with the behavioral results, hippocampal slices from the CCL2-tg mice were resistant to the depressing effects of acute alcohol (20–60 mM) on long-term synaptic plasticity, an important cellular mechanism of learning and memory that were observed in hippocampal slices from non-tg mice (Bray et al., 2013).
Given the lack of data on the biological consequence of CCL2/alcohol interactions, in the current studies we have extended our behavioral studies of CCL2/alcohol interactions to conditions of chronic alcohol exposure. Understanding such interactions is important not only to alcohol use disorders but also to situations where excessive alcohol consumption occurs in individuals with neurologic conditions associated with increased levels of CCL2 in the CNS. In the behavioral studies, consequences of CCL2/alcohol interactions on alcohol consumption in non-dependent mice and mice made alcohol dependent by a chronic intermittent alcohol exposure paradigm were investigated. Several other alcohol-sensitive behaviors also were examined in the alcohol dependent mice. Results from these studies support the idea that CCL2/alcohol interactions can modify the behavioral actions of alcohol and that the modification can lead to either impairment or apparent protection, depending on the CNS function being measured.
Experimental Procedures
Subjects
Transgenic mice with enhanced expression of CCL2 were initially obtained from Dr. Richard Ransohoff of the Cleveland Clinic Foundation. Their generation has been described (Huang et al., 2002). The enhanced expression of CCL2 in the CCL2-tg mice was targeted to astrocytes by insertion of the murine CCL2 gene under control of the huGFAP promoter. The initial line was backcrossed onto a C57BL/6J background and has been maintained for years by breeding wild-type female C57BL/6J mice with heterozygous male CCL2-tg mice. The mice were genotyped at weaning (21–28 days old) from DNA samples obtained from cut tail tips using the Mouse Tail Quick Extraction Kit (Pioneer Inc., San Diego, CA) as previously described (Bray et al., 2013). Male and female CCL2-tg mice heterozygous for the transgene and their age matched non-transgenic (non-tg) littermates were used for all experiments. All animal procedures were carried out according to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Animal facilities and experimental protocols were in accordance with the Association for the Assessment and Accreditation of Laboratory Animal Care.
Two-bottle choice alcohol (ethanol) drinking
The protocol for alcohol drinking using two-bottle choice was adapted from the published method of Blednov and colleagues (Blednov et al., 2005). Prior to starting the protocol, mice were acclimated to individual housing for 1 week. Two drinking tubes were continuously available to each mouse on Monday – Friday, with consumption measurement made daily. One bottle of water was continuously available on weekends. Food was available at all times and mouse weight was documented on a weekly basis. Initially, mice were given 4 days of water consumption (on Monday, off Friday; water in both tubes). On the following Monday–Friday, the mice were offered 3% alcohol (v/v) versus water, which was incrementally increased over the next 4 weeks mice to 6, 9, 12, and then 15% alcohol. To control for position preferences, tube positions were changed daily. Mice were then given tubes containing 15% alcohol for 2 hr per day for 5 days to determine limited access two-bottle-choice (2BC) drinking. Alcohol consumption for both drinking protocols (g/kg body weight/24 h or 2 h) was calculated on an individual mouse basis and expressed as an average for each 4–5 day measurement period at each alcohol concentration. The mice subjected to this paradigm are not alcohol dependent and are referred to as non-dependent mice. Alcohol naïve mice served as controls.
Chronic intermittent alcohol (ethanol) (CIE/2BC)
CCL2-tg and non-tg mice were made dependent on alcohol using the CIE/2BC model, which involves chronic intermittent vapor exposure (CIE) and 2BC limited access drinking (CIE/2BC). The specific methodology used is available to the public on the INIA-West website http://www.scripps.edu/cnad/inia/ under “Methodology”, “SOP Chronic Intermittent Alcohol – Two-Bottle Choice Mouse Model PDF” (Becker and Lopez, 2004; Lopez and Becker, 2005; Finn et al., 2007; Griffin et al., 2009a; Griffin et al., 2009b). Initially, baseline drinking was determined for each mouse over a 15 day period (5 days per week for 3 weeks). Mice were placed in individual cages for two hours daily with access to two drinking tubes, one containing 15% alcohol and the other containing water (i.e. 2BC drinking). The tubes were placed in the cages 30 min before the lights turned off. Measurements were made of the alcohol and water consumption during these 2-hour periods. Based on baseline measurements, mice were divided into two balanced groups with equal baseline alcohol and water consumptions in each genotype/sex. One group was placed in alcohol vapor chambers and exposed to intermittent alcohol vapor (CIE). The second group was placed identical chambers but exposed to air. The vapor group was injected with 1.75 g/kg alcohol + 68.1 mg/kg pyrazole (alcohol dehydrogenase inhibitor; used to stabilize blood alcohol levels), placed in the vapor chambers and received intermittent alcohol vapor for 3 days (16 hours vapor on, 8 hr off). At the end of each 16 hr exposure to alcohol vapor, mice were removed from the chambers and blood alcohol levels were determined from tail blood samples. Target blood alcohol levels were 150–225 mg%. After the third day of exposure, mice were left undisturbed for 72 hours. The mice then were subjected to 5 days of 2-hour access to tubes containing 15% alcohol or water to measure alcohol drinking and preference for the alcohol solution. The control group was injected with 68.1 mg/kg pyrazole in saline, placed in chambers delivering air for three days and then was tested for two-bottle choice drinking in parallel with the vapor groups. The vapor/air exposure and 5 days of two-bottle choice testing was repeated 2 more times for a total of 3 rounds of vapor and 3 rounds of two-bottle choice drinking. This CIE plus 2BC drinking paradigm (CIE/2BC) paradigm leads to increases in alcohol drinking and other behavioral changes associated with alcohol dependence (Becker and Lopez, 2016). The mice exposed to the CIE/2BC paradigm are referred to as the dependent group, the mice exposed to the air chambers and 2BC portion of CIE/2BC paradigm are referred to as the control group. Results from the dependent mice were compared to results from the 2BC control mice of the same genotype, to determine the consequence of the CIE exposure.
Behavioral studies
Six cohorts of littermate CCL2-tg (n = 31 males, 24 females) and non-tg (n = 32 males, 28 females) mice approximately 12 weeks of age at the start of the studies were used for the behavioral analyses. The mice were subjected to a series of behavioral tests, performed 3–30 days following the termination of alcohol exposure. The mice were considered alcohol dependent, non-dependent, or naïve, depending on the alcohol treatment they were exposed to. Both females and males were used. Statistical analysis (ANOVA) showed that there were no significant sex differences or sex x genotype interaction for the behavior tests. Therefore, results have been collapsed across sex for presentation of data.
Of the six cohorts, cohorts 1 and 2 were tested for alcohol drinking behavior and then learning and memory in the Y-maze and Barnes maze tests. Cohort 3 consisted of alcohol naïve mice and provided the control data for mice subjected to 2BC drinking or the CIE/2BC paradigm. Mice in cohort 3 were assessed in the Y-maze, Barnes maze and cued and contextual fear conditioning tests. The other three cohorts were assessed for alcohol drinking induced by the CIE paradigm and the behavioral consequences of alcohol dependence. Specifically, cohorts 4–6 were subjected to the CIE/2BC paradigm followed by the light/dark transfer test and forced swim test. Additionally, cohorts 4 and 6 were tested for cued and contextual fear conditioning, and cohorts 4 was tested in the Y-maze.
Barnes maze
The Barnes maze is a spatial learning and memory test initially developed in rats (Barnes, 1979), but later adapted and used widely in mice (Bach et al., 1995; Paylor et al., 2001; Holmes et al., 2002; Chen et al., 2011). The Barnes maze is considered a direct test of spatial learning, as there are visual cues surrounding the maze that are held constant, but no cues on the maze itself. The Barnes maze used in the current study consisted of an opaque Plexiglas disc 75 cm in diameter with twenty holes, 5 cm in diameter, located 5 cm from the perimeter. The Plexiglas disc was elevated 58 cm above the floor by a tripod. A black Plexiglas box (19 × 8 × 7 cm) was positioned under one of the holes to serve as the escape box. The escape tunnel that led to the escape box was always located underneath the same hole (stable within the spatial environment), which was moved by 90 degrees for each consecutive mouse. Distinct spatial cues, which were kept constant throughout the study, were situated all around the Plexiglas disc. Videotape recordings were made of each session and used by an experimenter blind to the genotype to score mouse performance in each test. The maze was cleaned thoroughly between mice.
In trial 1, the mouse was placed in the escape box for 1 minute prior to the nine standard daily acquisition trials. At the beginning of each of these nine sessions the mouse was placed in the middle of the maze in a 10 cm high cone-shaped opaque start chamber. After 10 seconds, the start chamber was removed, and a buzzer (80 dB) and light (400 lux) were turned on. The mouse was allowed to explore the maze for the rest of the session. The session ended when the mouse entered the escape tunnel or after 3 min had elapsed. The buzzer was turned off when the mouse entered the escape tunnel, and the mouse was allowed to remain in the dark for one minute. If the mouse did not enter the escape tunnel by itself, it was gently put in the escape box for one minute. Mice were tested daily for 9 days for the acquisition phase of the study. On day 10, the probe test was carried out. For this test, the escape tunnel was removed and each mouse was free to explore the maze for 3 min. Measurements were made of the escape latencies and the number of errors per session (trials 1–9) and the time spent in each of the 4 quadrants of the maze (trial 10). The percent time spent with all 4 paws in the target quadrant (the one originally containing the escape box) was compared with the average percent time in the other three quadrants. Errors were defined as nose pokes and head deflections into holes not leading to the escape chamber.
Y-maze test for spontaneous alternations
Spontaneous alternation behavior, a measure of spatial working memory, exploratory behavior, and responsiveness to novelty (Lalonde, 2002; Hughes, 2004), was tested using a Y-maze with 34 × 8 × 14 cm arms. Each mouse was tested in a single 5 min trial. The arm choices and total numbers of arm entries during each trial were recorded. Spontaneous alternation, expressed as a percent, refers to the ratio of sets of three unique arm choices (i.e. visiting arm 3 then 1 then 2 in sequence) to the total number of arm entries. Because mice have the opportunity to do repeated entries into a single arm, there is a chance performance level of 22% (2/9) for spontaneous alternations (Holcomb et al., 1999; Pennanen et al., 2006). In our hands, healthy young C57BL6/J mice typically make 50–70% spontaneous alternations in this test.
Cued and contextual fear conditioning
This test enables an assessment of both hippocampus-dependent (contextual portion) and amygdala-dependent (cued portion) learning processes in the same mouse (Rudy et al., 2004; Kenney and Gould, 2008). During conditioning, mice learn to associate a novel environment (context) and a previously neutral stimulus (conditioned stimulus, a tone and light) with an aversive foot shock stimulus (Maren, 2001). Testing is done in the absence of the aversive stimulus. Conditioned animals, when subsequently exposed to the context or conditioned stimuli with no shock present display the conditioned response, freezing behavior. Freezing behavior in the context and cued tests (relative to the same context prior to shock and an altered context prior to tone, respectively) is indicative of the formation of an association between the particular stimulus (either the environment or the tone) and the shock, indicating that learning has occurred. Conditioning was carried out in Plexiglas Freeze Monitor chambers (Med Associates, Inc.) (26 × 26 × 17 cm) with speakers and lights mounted on two opposite walls, and shockable grid floors. The chambers were housed in sound proofed boxes. Sessions were recorded with real-time digital video and recordings were calibrated to distinguish between subtle movements, such as whisker twitches, tail flicks, and freezing behavior.
On day 1, mice were habituated to the chambers in a 5 min shock-free test. On day 2, the mice were subjected to the context and conditioned stimulus (30 seconds, 3000 Hz, 80 dB sound + white light) in association with foot shock (0.60 mA, 2 second, scrambled current). During their 5 min test, the mice received 2 shocks, each during the last 2 sec of a 30 sec tone/light exposure. On day 3, contextual conditioning (as determined by freezing behavior) was measured during a 5-minute test in the chamber where the mice were trained (context test). The next day, cued conditioning (CS+ test) was tested. The mice were placed in a novel context for 3 minutes, after which they were exposed to the conditioned stimuli (light + tone) for 3 minutes. To create a novel context, the chamber was covered with new walls (white opaque plastic creating a circular compartment in contrast to a clear plastic square compartment) and a new floor (white opaque plastic in contrast to metal grid).
Light/dark transfer test
The light/dark transfer test has been used to assess anxiety-like behavior in mice by capitalizing on the conflict between exploration of a novel environment and the avoidance of a brightly lit open space (Holmes et al., 2002). The apparatus is a rectangular box made of Plexiglas divided by a partition into two environments. One compartment (14.5×27×26.5 cm) is dark (8–16 lux) and the other compartment (28.5×27×26.5 cm) is highly illuminated (400–600 lux) by a 60 W light source located above it. The compartments are connected by an opening (7.5×7.5 cm) located at floor level in the center of the partition. Mice were placed in the dark compartment to start the 5-minute test. The time spent in the light compartment was used as a predictor of anxiety-like behavior, i.e. a greater amount of time in the light compartment would be indicative of decreased anxiety-like behavior.
Forced swim test
The forced swim test is a classic test used as a predictive animal model for antidepressant actions of drugs. We used a modification of the test originally described by Porsolt and colleagues (Porsolt, 2000) and adapted by Lucki (Lucki, 1997). Mice were individually placed into clear polypropylene cylinders containing 23–25 °C water, 15 cm deep, for 6 min. An observer blind to the mouse genotype and alcohol exposure group recorded the length of time each mouse was immobile. Immobility was measured when no activity was observed other than that required to keep the mouse’s head above the water.
Statistics
Statistical analysis was done using Statview 5 (SAS Institute, Inc., Cary, NC). Data were assessed for normality using the Kolmogorov-Smirnov (K-S) test or the F-test. Most data were normally distributed and were analyzed by ANOVA or the paired t-test. The Fisher’s PLSD or Tukey/Kramer test was used for post hoc analysis. The Mann–Whitney test was used for non-parametric data. Data are expressed as mean ± SEM. n values are the number of animals tested. A p value of 0.05 was considered significant.
Results
Alcohol drinking
Mice lacking CCL2 and/or its receptor CCR2 show a lower preference for alcohol and consume less alcohol in a 2BC test when compared to wild-type mice (Blednov et al., 2005). These results suggested that mice expressing elevated level of CCL2 in the CNS would show a higher level of alcohol consumption than mice with normal CCL2 expression. To test this possibility, CCL2-tg mice and their non-tg littermates were tested for alcohol consumption and preference in a 2BC alcohol drinking protocol. The 2BC protocol does not produce alcohol dependence. Alcohol dependence significantly increases alcohol consumption (Becker and Lopez, 2016). Therefore, increased levels of CCL2 in the CNS of the transgenic mice could impact alcohol drinking in dependent animals. To test this possibility, alcohol drinking was also studied in CCL2-tg and non-tg mice were made dependent on alcohol.
Non-dependent animals
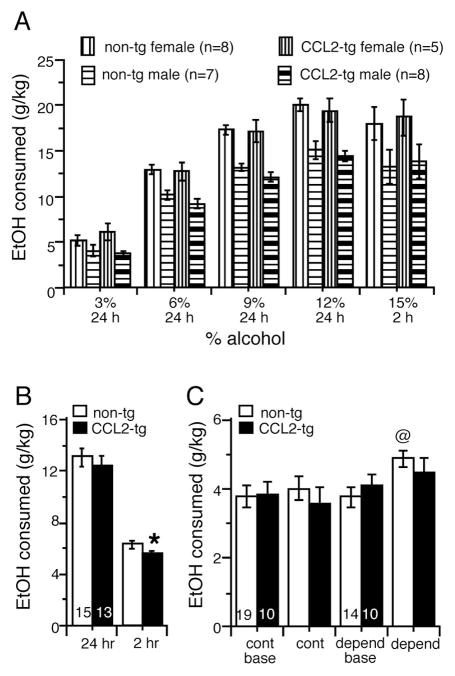
Both mouse genotypes showed increased alcohol consumption as concentrations in the drinking tubes were increased from 3% to 12% (Fig. 1A). Overall, female mice consumed more alcohol than males across all concentrations tested (Fig. 1A). However, there were no genotype x sex interactions; therefore data were collapsed on sex for presentation (Fig. 1B). There were no effects of genotype on 24 h consumption of 3%, 6%, 9%, 12%, or 15% alcohol (F(1,26) = 0.51, p = 0.48, Repeated Measures ANOVA)(Fig. 1A). When the mice were shifted to a limited access (2 hr) schedule, consumption of 15% alcohol for CCL2-tg mice was significantly lower (18%) than for the non-tg mice (F(1,26) = 6.15, p = 0.02, ANOVA)(Fig. 1A,B). There was no significant effect of genotype on alcohol preference (F(1,26) = 0.00, p = 0.99, Repeated Measures ANOVA, not shown) or daily water consumption during the initial habituation period (CCL2-tg = 1.9 ± 0.1 g, n=10; non-tg = 1.9 ± 0.1 g, n=10, p > 0.05, unpaired t-test). Also, there was no genotypic difference in weight of the mice averaged over the course of the studies (CCL2-tg = 23.8±1.0 g, n=10; non-tg = 22.6 ± 0.8 g, n=10, p > 0.05, unpaired t-test). Taken together, these data show that CCL2 over-expression does not increase alcohol drinking behavior in non-dependent mice under the choice conditions tested (15% concentration during 2 hours limited access).
Figure 1.
Alcohol consumption in non-dependent and alcohol dependent CCL2-tg and non-tg mice. (A) In non-dependent CCL2-tg and non-tg mice subjected to the 2BC drinking paradigm, females drank more than males but comparable increases in alcohol consumption were observed for CCL2-tg and non-tg mice of the same sex during the periods of 24 hr access to escalating concentrations of alcohol. (B) During the period of limited access, a small reduction in mean alcohol consumption (data from males and females combined) was observed in the CCL2-tg mice compared to non-tg mice. (C) In CCL2-tg and non-tg mice made alcohol dependent by the CIE/2BC paradigm, alcohol consumption was significantly increased in the non-tg mice but not in the CCL2-tg mice. Numbers in bars are number of mice tested. *significant genotypic difference. @significantly different from baseline values for the same group and genotype.
Dependent animals
CCL2-tg and non-tg mice were made dependent on alcohol using the CIE/2BC model, which involves intermittent vapor exposure (CIE) and 2BC limited access drinking. Under baseline conditions prior to initiating the CIE/2BC paradigm, there was no significant difference in alcohol consumption between the CCL2-tg mice made alcohol dependent compared to their respective control group (F(1,22) = 0.56, p = 0.46, ANOVA)(Fig. 1C). Alcohol consumption in the non-tg mice was significantly increased (~30%) in the dependent group compared to the non-tg control group (U = 42.5 p = 0.02, Mann Whitney test) (Fig. 1C). Blood alcohol levels achieved during the alcohol vapor exposure bouts did not differ between non-tg (203 ± 15 mg/dl, n=14) and CCL2-tg mice (210 ± 14 mg/dl, n=15). Thus, in the dependent group, the CCL2-tg mice showed lower alcohol consumption compared to the non-tg mice, a result similar to that noted above for mice subjected to alcohol drinking under non-dependent conditions (Fig. 1B). These results are consistent with a role for astrocyte produced CCL2 in the regulation of alcohol consumption, and show that elevated levels of astrocyte produced CCL2 can lower alcohol consumption.
Performance in learning tasks
Our previous studies showed that elevated levels of CCL2 in the CCL2-tg mice reduced the negative effects of acute alcohol in a behavioral task, cued and contextual fear conditioning, results that suggest a potential protective role for CCL2 against the learning impairment produced by alcohol (Bray et al., 2013). Effects of alcohol on learning and memory are thought to contribute to the development and maintenance of alcohol addiction (Lee et al., 2009). Therefore, to determine if protective effects of CCL2 were evident in non-dependent and dependent animals, we measured the performance of CCL2-tg and non-tg mice in standard learning tasks that are sensitive to alcohol exposure in mice.
Barnes maze
CCL2-tg and non-tg mice subjected to alcohol drinking under non-dependent conditions and alcohol naïve controls were tested for spatial learning and memory using the Barnes maze. The tests were carried out approximately 1 month after the termination of 2BC drinking.
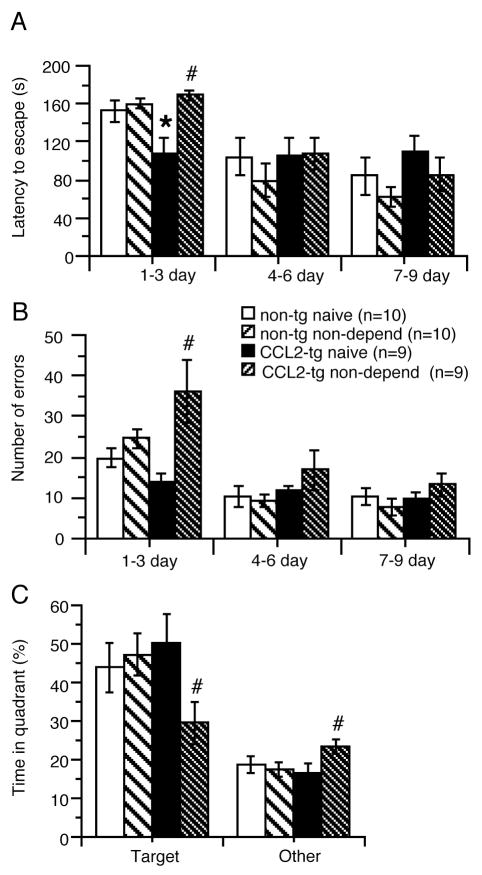
Measurements were made during a 1–9 day period, with results averaged for 1–3, 4–6 and 7–9 days. The non-dependent CCL2-tg group showed a significant increase in latency to escape compared to their respective naïve control group at 1–3 day (U = 9.5, p = 0.005, Mann Whitney test), but no significant difference in latency to escape at 4–6 (F(1,16) = 0.01, p = 0.92) and 7–9 days (F(1,16) = 0.78, p = 0.39, ANOVA)(Fig. 2A). There was no significant difference in latency to escape between the non-dependent non-tg group and their respective naïve control group for any of the time periods studied (1–3 day, F(1,18) = 0.31, p = 0.59; 4–6 day, F(1,18) = 0.87, p = 0.36; 7–9 day F(1,18) = 0.97, p = 0.34; ANOVA)(Fig. 2A).
Figure 2.
Alcohol-induced impairments in spatial learning in non-dependent CCL2-tg mice. (A–C) Non-dependent CCL2-tg mice subjected to the 2BC drinking paradigm showed impairments in spatial learning and memory in the Barnes maze test compared with the alcohol naïve CCL2-tg mice. Data were averaged over 3 day periods. During the first time period (1–3 d) non-dependent CCL2-tg mice had increased latency (A), errors to escape (B) and spent less time in the target quadrant (C). Non-dependent and alcohol naïve non-tg mice exposed to the same paradigms did not show impairments. *significant genotypic difference for the same group. # significant group difference for the same genotype.
The non-dependent CCL2-tg group also showed a significant increase in errors to escape compared to the naïve CCL2-tg group at 1–3 day (U = 6.0, p = 0.002, Mann Whitney test) (Fig. 2B), but no significant difference in latency to escape at 4–6 (F(1,16) = 1.03, p = 0.33) and 7–9 days (F(1,16) = 1.00, p = 0.33, ANOVA)(Fig. 2A). There was no significant difference in errors to escape between the non-dependent non-tg group and the naïve non-tg group for any of the time periods studied (1–3 day, F(1,18) = 2.31, p = 0.15; 4–6 day, F(1,18) = 0.12, p = 0.73; 7–9 day F(1,18) = 0.82, p = 0.19; ANOVA)(Fig. 2B).
The non-dependent CCL2-tg group spent significantly less time in the target quadrant than the CCL2-tg naïve group (p = 0.04, paired t-test), whereas there was no significant difference between the non-dependent and naïve non-tg mice in time spent in the target quadrant (p = 0.70, paired t-test) (Fig. 2C). Taken together, these results suggest that alcohol induced a spatial learning impairment in the non-dependent CCL2-tg mice but not in the non-dependent non-tg mice.
Y-maze
Alcohol dependent CCL2-tg and non-tg mice and their respective control mice were evaluated in the Y-maze test for spatial working memory. Results from the dependent group were compared to results from the control group of the same genotype, to determine the consequence of the CIE paradigm. The Y-maze test was carried out ~5 days after the termination of the CIE/2BC paradigm. There was no significant (p>0.05, ANOVA) effect of genotype or group on overall arm entries, spontaneous alternations, or percent spontaneous alternations (Fig. 3A,B). However, significant group x genotype interactions were observed for spontaneous alternations and percent spontaneous alternations (F(1, 35) = 4.57, p = 0.04, ANOVA). Post hoc tests showed that the dependent CCL2-tg group showed fewer spontaneous alternations and percent spontaneous alternations than the control CCL2-tg group (p < 0.05, Tukey/Kramer), a difference that was not observed for the dependent non-tg group compared to control non-tg group (Fig. 3B,C). Thus, the dependent CCL2-tg mice showed an alcohol-induced impairment in spatial learning, an effect that was not evident in dependent non-tg mice. In a separate cohort of alcohol naïve mice, no genotypic differences were observed for overall arm entries (F(1, 48) = 0.137, p = 0.71, spontaneous alternations (F(1, 48) = 0.08, p = 0.93), or percent spontaneous alternations (F(1, 48) = 0.06, p = 0.81) (Fig. 3A–C).
Figure 3.
Alcohol-induced impairments in spatial learning in alcohol dependent CCL2-tg mice. (A ) Alcohol dependent CCL2-tg and non-tg mice showed similar numbers of arm entries compared to their respective controls in the Y-maze test. (B,C) Alcohol dependent CCL2-tg mice showed fewer spontaneous alternations (B) and percent spontaneous alternations (C) than their control CCL2-tg mice, an effect not observed in the alcohol dependent non-tg mice. Numbers in bars are the number of mice tested. # significant group difference for the same genotype.
Cued and contextual fear conditioning
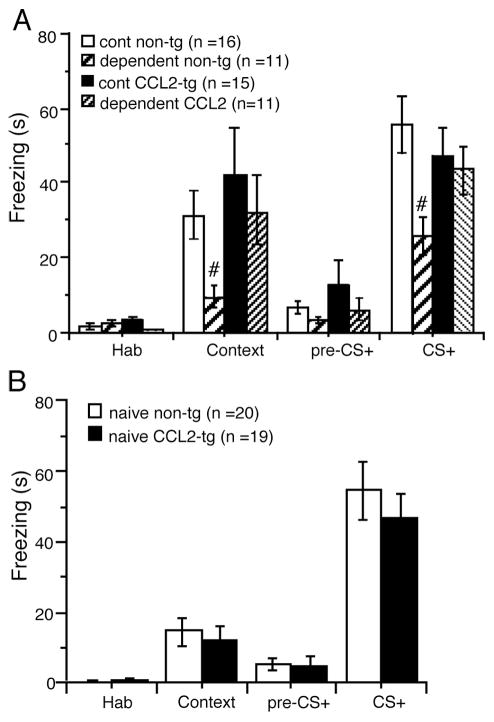
Alcohol dependent CCL2-tg and non-tg mice and their respective control mice were assessed for impairment in associative learning by the cued and contextual fear conditioning task 1–2 weeks following the termination of the CIE/2BC paradigm. Freezing was significantly reduced in the dependent non-tg group relative to control non-tg group during the context test (U = 32, p = 0.001, Mann Whitney test) (Fig. 4A). Freezing was also significantly reduced in the dependent non-tg group relative to control non-tg group during the CS+ test (t(25) = 2.99 p < 0.01, unpaired t-test) (Fig. 4A), consistent with previous reports showing a prominent negative effect of alcohol on contextual and cued learning behaviors (Ripley et al., 2003; Stephens et al., 2005). In contrast, there was no difference between the dependent CCL2-tg group and the control CCL2-tg group in the context test (t(24) = 0.55, p = 0.58, unpaired t-test) or CS+ test (t(24) = 0.36, p = 0.72, unpaired t-test (Fig. 4A). There was no significant difference between the dependent group and control group for the habituation and pre-CS testing for either genotype (Fig. 4A). These results support the idea that overexpression of CCL2 has a protective effect against alcohol induced impairments in associative learning. In a separate cohort of alcohol naïve mice, no genotypic differences were observed for the cued and contextual fear conditioning tasks (habituation, context, pre-CS+ and CS+, p > 0.05 for all, unpaired t-test)(Fig. 4B).
Figure 4.
Dependent CCL2-tg mice were resistant to alcohol-induced impairment in associative learning. Mice made alcohol dependent by the CIE/2BC paradigm were assessed for impairment in associative learning in the cued and contextual fear conditioning task. Learning was assessed by measurement of freezing behavior. Freezing was significantly reduced In the dependent non-tg mice relative to control non-tg mice during both the context test and condition stimulus (CS+) test. There was no significant effect on freezing in either test for the dependent CCL2-tg mice relative to control CCL2-tg mice. #significant group difference for the same genotype.
Tests for anxiety and depression
In addition to behavior tests of learning, we tested the dependent and control CCL2-tg and non-tg mice in two other behavioral paradigms that are known to show alcohol sensitivity in mice: (a) anxiety-like behavior as assessed by the light/dark transfer test, and (b) depressive-like behavior, as assessed by the forced swim test. Alcohol-induced anxiety and depression are well-characterized symptoms of alcohol withdrawal in dependent animals (Kliethermes, 2005; Walker et al., 2010).
Light/dark transfer
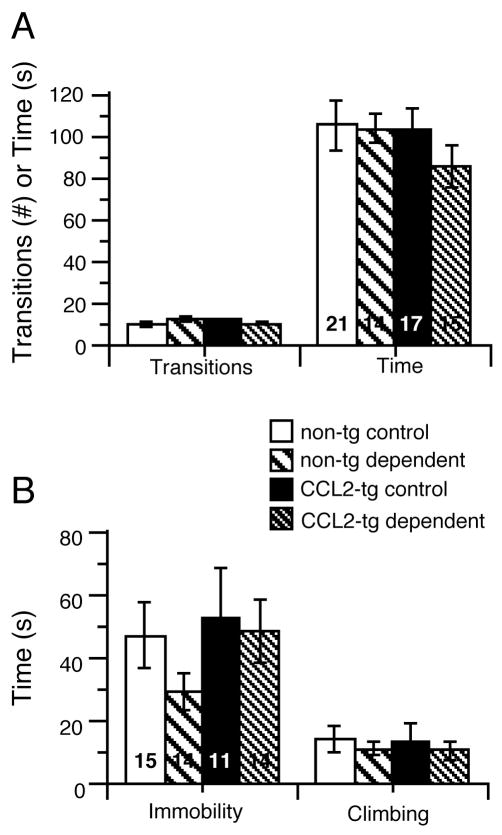
Mice were tested ~ 3 days after the termination of the CIE/2BC paradigm. There were no significant effects of genotype (F1,63) = 0.96, p = 0.33, ANOVA), group (F1,63) = 0.80, p = 0.38) or genotype x group interaction (F1,65) = 0.52, p = 0.47) on the time spent in the light compartment (Fig. 5A). There were also no significant genotype (F1,63) = 0.00, p = 0.99) or group (F1,63) = 0.31, p = 0.58) effects on the number of dark-to-light transitions (Fig. 5A). However, there was a significant genotype x group interaction (F1,63) = 4.25, p = 0.04). Post hoc analysis (Fisher’s PLSD) of this interaction revealed a trend (p = 0.08) for more dark-to-light transitions in the dependent non-tg group compared to control non-tg group (Fig. 5A).
Figure 5.
Performance of dependent and control mice was comparable in the light/dark transfer and forced swim tests. (A) No genotypic or group differences were observed for the number of transitions or time spent in the light compartment in the light/dark transfer test. (B) No genotypic or group differences were observed for total immobility time or climbing time in the forced swim test.
Forced-swim test
Mice were assessed in the forced swim test 1 week after the termination of the CIE/2BC paradigm. The dependent CCL2-tg and non-tg groups, showed no significant effects of genotype (F(1,54) = 0.33, p = 0.31, ANOVA), group (F(1,54) = 1.04, p = 0.25) or genotype x group interaction (F(1,54) = 0.4.47, p = 0.51) for total immobility time or climbing time compared to their respective control CCL2-tg and non-tg groups (Fig. 5B).
Results from the behavioral studies are summarized in Table 1. Non-dependent CCL2-tg mice showed greater learning impairment than non-dependent non-tg mice in the Barnes Maze. Similarly, alcohol dependent CCL2-tg mice showed greater impairment in spatial working memory than dependent non-tg mice in the Y Maze. These results suggest a negative interaction of alcohol and CCL2 on spatial learning. In contrast, associative learning, as measured by the conditioned fear test, was impaired in alcohol dependent non-tg mice but not in the alcohol dependent CCL2-tg mice, suggesting a protective effect of CCL2 against the negative actions of alcohol on this behavior.
Table 1.
Summary of behavioral results
| Behavioral test | naive | non-dependent | dependent | ||
|---|---|---|---|---|---|
| CCL2-tg vs non-tg | CCL2-tg 2BC vs naive | non–tg 2BC vs naive | CCL2-tg CIE vs cont | non–tg CIE vs cont | |
| Barnes Maze | |||||
| -latency | No Δ | No Δ | No Δ | - | - |
| -errors | No Δ | ↑ | No Δ | - | - |
| -% target quadrant | No Δ | ↓ | No Δ | - | - |
| Y-maze | |||||
| -arm entries | No Δ | - | - | No Δ | No Δ |
| - spont. alternations | No Δ | - | - | ↓ | No Δ |
| -% spont. alternations | No Δ | - | - | ↓ | No Δ |
| Context fear conditioning | No Δ | - | - | No Δ | ↓ |
| Cued fear conditioning | No Δ | - | - | No Δ | ↓ |
| Light/dark transfer | No Δ | - | - | No Δ | No Δ |
| Forced-swim test | No Δ | - | - | No Δ | No Δ |
Note: In these tests the functional consequence for the ↓ or ↑ is impairment.
Discussion
Elevated levels of CCL2 in the CNS have been reported for a variety of neurological conditions associated with impaired CNS function including alcohol use disorders. Studies in animal models confirmed that alcohol induces elevated expression of CCL2 in the CNS (see Introduction). CCL2 has been shown to have neuronal actions in experimental preparations (Qin et al., 2005; van Gassen et al., 2005; Cho and Gruol, 2008; Edman et al., 2008; Jung et al., 2008; Guyon et al., 2009; You et al., 2010; Belkouch et al., 2011; Kao et al., 2012; Zhou et al., 2016). These findings raise the possibility that effects of CCL2 may play a role in the CNS actions of alcohol. In the current studies, we used CCL2-tg mice to model conditions of increased CNS expression of CCL2 previously produced by alcohol or a neurological condition, and to determine if the increased levels of CCL2 affect the actions of alcohol. Results showed no difference in performance of alcohol naïve CCL2-tg and non-tg mice in a variety of behavioral tests, indicating that the elevated CNS expression of CCL2 in the CCL2-tg mice did not compromise the basic behavioral functions tested. However, alcohol exposure either by 2BC drinking or CIE/2BC paradigms revealed that interactions occurred between CCL2 and alcohol that altered the effects of alcohol on certain behaviors. Taken together, these data suggest that persistent production of CCL2 as occurs with alcohol exposure may produce long-term effects on the CNS that can significantly impact brain function.
Alcohol drinking
Mice with a deletion of genes (KO) for CCL2 or CCR2 showed a lower preference for alcohol and consumed lower amounts of alcohol in a 2BC drinking test compared to wildtype mice (Blednov et al., 2005). These findings suggested a role for CCL2 in alcohol drinking behavior. Based on these findings, we anticipated that the CCL2-tg mice would show increased alcohol drinking. However, neither the non-dependent nor the alcohol dependent CCL2-tg mice showed increased alcohol drinking compared to their respective non-dependent or alcohol dependent non-tg control mice. Rather, the non-dependent CCL2-tg mice showed a small reduction in alcohol drinking in a limited access test, and the dependent CCL2-tg mice showed no change in drinking behavior relative to their respective controls. However, because the alcohol drinking behaviors in the CCL2-tg mice differed from that in the non-tg mice, these findings support the idea that CCL2 plays a role in drinking behavior. The KO mice lack CCL2 throughout the body, whereas the CCL2-tg mice express elevated levels of CCL2 in the CNS. These differences both in levels and regions of the body affected by the altered levels of CCL2 between the KO and the CCL2-tg mice are likely to contribute to the discrepancy between our predicted and experimental results.
Relevant to this issue is a recent study by Valenta and Gonzales (Valenta and Gonzales, 2016) showing that chronic intracerebroventricular infusion of CCL2 increased self-administration of sweetened alcohol (but not a sucrose solution) in rats, results that support a role for CCL2 in alcohol drinking but appear to conflict with our results. However, a number of differences exist between the two studies that likely contribute to the differences in results, and may be relevant to the interactions between CCL2 and drinking behavior. For example, high doses of CCL2 were most effective in studies using CCL2 infusion. Our ELISA studies of hippocampus and cerebellum indicate that tissue levels of CCL2 are considerably lower (~1.5 ng/ml) (Gruol et al., 2014) than the most effective doses of CCL2 (e.g., 2 μg/d) in the infusion experiments. Differences in the brain regions exposed to CCL2, and cellular accessibility may also be important differences between the two studies. In the CCL2-tg CNS, the secretory dynamics of astrocytes or other cell types that produce CCL2, location of secretory sites on the cells, diffusion factors and catabolism are important factors that determine the characteristics of CNS exposure to CCL2. With infusion, it is likely that the CCL2 is more uniformly distributed, although concentration would vary from the source. Taken together these studies indicate that CCL2 regulation can involve an increase or decrease alcohol drinking depending conditions in the relevant brain regions. Increased drinking appears to be a consequence of CCL2 levels that are higher than in our studies, whereas lower levels observed in our studies and the KO mice (Blednov et al., 2005) appear to be associated with lower alcohol consumption. Studies of the CCL2 KO and wildtype mice showed sex differences in alcohol consumption, indicating that other factors are also involved in CCL2 regulation of alcohol drinking (Blednov et al., 2005).
Behavior testing
Tests that assess learning and memory, anxiety, and depression were carried out to determine if the elevated expression of CCL2 in the CCL2-tg mice affected behavior in alcohol naïve and alcohol exposed mice. Results showed no significant differences between alcohol naïve CCL2-tg and non-tg mice in all behaviors tested. There were also no significant differences in performance in tests for anxiety (light/dark transfer test) or depression (forced swim test) for alcohol dependent CCL2-tg and non-tg mice compared to their respective CCL2-tg and non-tg controls. In contrast, both non-dependent CCL2-tg mice (2BC drinking) and dependent CCL2-tg (CIE) showed alcohol-induced impairment in spatial learning compared to their respective CCL2-tg controls as measured by the Barnes maze or Y-maze tests. Neither the non-dependent nor dependent non-tg mice showed impairment in spatial learning relative to their respective non-tg controls. Because spatial learning is dependent on hippocampal function (Dillon et al., 2008), these results suggest that CCL2/alcohol interactions produced neuroadaptive changes that impaired hippocampal mechanisms involved in spatial learning and memory in the CCL2-tg mice. Alcohol impairment of spatial learning and memory has been shown to be transient in adult rodents (Silvers et al., 2003; Sircar and Sircar, 2005; Kuzmin et al., 2012), which could explain why impairment of spatial learning was not observed in the non-tg mice exposed to alcohol. Thus the impairment of spatial learning and memory observed in the CCL2-tg mice could reflect a prolongation of the effects of alcohol on this behavior. There was no apparent impairment of spatial learning and memory in the alcohol naïve CCL2-tg mice.
The mechanism underlying the impairment of spatial learning in the Barnes maze or Y-maze tests observed in the non-dependent or dependent CCL2-tg mice remains to be determined. However, the mechanisms may involve Group II metabotropic glutamate receptors (mGluRs), which includes mGluR2 and mGluR3. Group II mGluRs are expressed in all regions of the hippocampus (Petralia et al., 1996) and act primarily as presynaptic autoinhibitory receptors that regulate release of glutamate from synaptic terminals that innervate excitatory and inhibitory neurons. Group II mGluR regulation of long-term depression (LTD) in the hippocampus has been shown to play a key role in spatial learning and memory (Altinbilek and Manahan-Vaughan, 2007). Results from our Western blot studies using an antibody that detects both mGluR2 and mGluR3 (mGluR2/3) showed that mGluR2/3 levels were reduced in hippocampus from both 2BC drinking (non-dependent) and CIE/2BC treated (dependent) CCL2-tg mice compared to non-tg mice of the same alcohol treatment group (Gruol et al., 2014). Studies have shown that mGluR2/3 mRNA or protein is downregulated in other brain regions by CIE (Meinhardt et al., 2013; Barker et al., 2016) and that mGluR2 mRNA is downregulated in the cortex of post-mortem alcohol-dependent humans (Meinhardt et al., 2013), consistent with the ability of alcohol to affect expression of this protein. Thus, the alcohol-induced impairment in spatial learning in these behavioral tests in the CCL2-tg mice to may involve an alcohol-induced reduction of mGluR2/3 expression in the CCL2-tg mice. Further studies will be needed to test this possibility. There was no genotypic difference in the level of mGluR2/3 in the hippocampus from alcohol naïve CCL2-tg and non-tg mice (Gruol et al., 2014).
Differences between CCL2-tg and non-tg mice were also observed in the effects of alcohol on context (hippocampus-dependent) and cued fear (amygdala-dependent) conditioning. The alcohol dependent CCL2-tg mice showed no difference in context and cued fear conditioning compared to their respective control CCL2-tg mice, whereas alcohol dependent non-tg mice showed reduced contextual and cued fear conditioning compared to their respective control non-tg mice. The reduced fear conditioning in the alcohol-dependent non-tg mice is consistent with the known effects of alcohol in this behavioral paradigm (Melia et al., 1996; Gould, 2003; Stephens et al., 2005). These findings are also similar to previous data from our laboratory showing that CCL2-tg mice were resistant to the impairment in contextual and cued fear conditioning produced by acute alcohol (Bray et al., 2013). The fact that the alcohol naïve CCL2-tg mice did not show altered behavior prior to alcohol exposure suggests that the persistently elevated levels of CCL2 in the CNS produces covert neuroadaptive changes that are revealed by interactions with alcohol. Other conditions associated with elevated levels of CCL2 in the CNS such as neurological disorders or CNS injury could also involve covert neuroadaptive actions of CCL2.
Conclusion
These studies show that elevated levels of CCL2 in the CNS through increased astrocyte expression can significantly interact with the behavioral effects of alcohol. Alterations in the behavioral effects of alcohol were observed for both non-dependent mice exposed to 2BC drinking and mice made dependent by CIE and in our previous studies for acute alcohol exposure (Bray et al., 2013). These findings suggest that the persistently expressed CCL2 in the CCL2-tg mice results in neuroadaptive changes that interact with effects of alcohol, although a contribution of alcohol-induced CCL2 during alcohol exposure/withdrawal cannot be eliminated at this time. In our studies, the CCL2-tg mice were used to model the levels of CCL2 in the CNS produced by prolonged alcohol use. However, results may also be relevant to effects of alcohol in the CNS of individuals that have neurological conditions that result in increased levels of CCL2 in the CNS.
Highlights.
Increased CNS expression of CCL2 reduced alcohol consumption
Both dependent and non-dependent mice showed altered behavior
Increased CNS expression of CCL2 altered effects of alcohol on spatial learning
Increased CNS expression of CCL2 altered effects of alcohol on associative learning
Acknowledgments
This work was supported by NIAAA Grants AA019261, AA024484, and the Integrated Neuroscience Initiative on Alcoholism (INAI)-West grant AA020893. Jennifer G. Bray was supported by a National Research Service Award, FAA020441. The authors thank Khanh Vo, Tali Nadav and Lindsay Cates for technical assistance.
Abbreviations
- ANOVA
analysis of variance
- CCL2
chemokine ligand 2
- CIE
chronic intermittent vapor exposure
- CNS
central nervous system
- CSF
cerebrospinal fluid
- DNA
deoxyribonucleic acid
- ELISA
enzyme-linked immunosorbent assay
- non-tg
non-transgenic
- GFAP
glial fibrillary acidic protein
- huGFAP
human glial fibrillary acidic protein
- mRNA
messenger ribonucleic acid
- MCP-1
monocyte chemoattractant protein-1
- 2BC
two-bottle-choice
Footnotes
Conflict of interest
The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altinbilek B, Manahan-Vaughan D. Antagonism of group III metabotropic glutamate receptors results in impairment of LTD but not LTP in the hippocampal CA1 region, and prevents long-term spatial memory. The European journal of neuroscience. 2007;26:1166–1172. doi: 10.1111/j.1460-9568.2007.05742.x. [DOI] [PubMed] [Google Scholar]
- Bach ME, Hawkins RD, Osman M, Kandel ER, Mayford M. Impairment of spatial but not contextual memory in CaMKII mutant mice with a selective loss of hippocampal LTP in the range of the theta frequency. Cell. 1995;81:905–915. doi: 10.1016/0092-8674(95)90010-1. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Gosselin RD, Mechighel P, Rostene W, Kitabgi P, Melik Parsadaniantz S. Constitutive neuronal expression of CCR2 chemokine receptor and its colocalization with neurotransmitters in normal rat brain: functional effect of MCP-1/CCL2 on calcium mobilization in primary cultured neurons. The Journal of comparative neurology. 2005;492:178–192. doi: 10.1002/cne.20729. [DOI] [PubMed] [Google Scholar]
- Barker JM, Lench DH, Chandler LJ. Reversal of alcohol dependence-induced deficits in cue-guided behavior via mGluR2/3 signaling in mice. Psychopharmacology (Berl) 2016;233:235–242. doi: 10.1007/s00213-015-4101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcoholism, clinical and experimental research. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. An Animal Model of Alcohol Dependence to Screen Medications for Treating Alcoholism. International review of neurobiology. 2016;126:157–177. doi: 10.1016/bs.irn.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier M, D’Orio V, Spat J, Shuman M, Foley FW. Alcohol and substance use in multiple sclerosis. Journal of the neurological sciences. 2014;338:122–127. doi: 10.1016/j.jns.2013.12.029. [DOI] [PubMed] [Google Scholar]
- Belkouch M, Dansereau MA, Reaux-Le Goazigo A, Van Steenwinckel J, Beaudet N, Chraibi A, Melik-Parsadaniantz S, Sarret P. The chemokine CCL2 increases Nav1.8 sodium channel activity in primary sensory neurons through a Gbetagamma-dependent mechanism. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:18381–18390. doi: 10.1523/JNEUROSCI.3386-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Bergeson SE, Walker D, Ferreira VM, Kuziel WA, Harris RA. Perturbation of chemokine networks by gene deletion alters the reinforcing actions of ethanol. Behavioural brain research. 2005;165:110–125. doi: 10.1016/j.bbr.2005.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray JG, Reyes KC, Roberts AJ, Ransohoff RM, Gruol DL. Synaptic plasticity in the hippocampus shows resistance to acute ethanol exposure in transgenic mice with astrocyte-targeted enhanced CCL2 expression. Neuropharmacology. 2013;67:115–125. doi: 10.1016/j.neuropharm.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner M, Kisseberth WC, Su Y, Besnard F, Messing A. GFAP promoter directs astrocyte-specific expression in transgenic mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14:1030–1037. doi: 10.1523/JNEUROSCI.14-03-01030.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Leibowitz SF. Prenatal exposure to ethanol stimulates hypothalamic CCR2 chemokine receptor system: Possible relation to increased density of orexigenic peptide neurons and ethanol drinking in adolescent offspring. Neuroscience. 2015;310:163–175. doi: 10.1016/j.neuroscience.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Prior M, Dargusch R, Roberts A, Riek R, Eichmann C, Chiruta C, Akaishi T, Abe K, Maher P, Schubert D. A novel neurotrophic drug for cognitive enhancement and Alzheimer’s disease. PloS one. 2011;6:e27865. doi: 10.1371/journal.pone.0027865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J, Gruol DL. The chemokine CCL2 activates p38 mitogen-activated protein kinase pathway in cultured rat hippocampal cells. J Neuroimmunol. 2008;199:94–103. doi: 10.1016/j.jneuroim.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SS, Lee HJ, Lim I, Satoh J, Kim SU. Human astrocytes: secretome profiles of cytokines and chemokines. PloS one. 2014;9:e92325. doi: 10.1371/journal.pone.0092325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conductier G, Blondeau N, Guyon A, Nahon JL, Rovere C. The role of monocyte chemoattractant protein MCP1/CCL2 in neuroinflammatory diseases. J Neuroimmunol. 2010;224:93–100. doi: 10.1016/j.jneuroim.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Dillon GM, Qu X, Marcus JN, Dodart JC. Excitotoxic lesions restricted to the dorsal CA1 field of the hippocampus impair spatial memory and extinction learning in C57BL/6 mice. Neurobiol Learn Mem. 2008;90:426–433. doi: 10.1016/j.nlm.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Drew PD, Johnson JW, Douglas JC, Phelan KD, Kane CJ. Pioglitazone blocks ethanol induction of microglial activation and immune responses in the hippocampus, cerebellum, and cerebral cortex in a mouse model of fetal alcohol spectrum disorders. Alcoholism, clinical and experimental research. 2015;39:445–454. doi: 10.1111/acer.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman LC, Mira H, Arenas E. The beta-chemokines CCL2 and CCL7 are two novel differentiation factors for midbrain dopaminergic precursors and neurons. Exp Cell Res. 2008;314:2123–2130. doi: 10.1016/j.yexcr.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Ehrlich D, Pirchl M, Humpel C. Effects of long-term moderate ethanol and cholesterol on cognition, cholinergic neurons, inflammation, and vascular impairment in rats. Neuroscience. 2012;205:154–166. doi: 10.1016/j.neuroscience.2011.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends in immunology. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12–41) Alcoholism, clinical and experimental research. 2007;31:939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Freeman K, Brureau A, Vadigepalli R, Staehle MM, Brureau MM, Gonye GE, Hoek JB, Hooper DC, Schwaber JS. Temporal changes in innate immune signals in a rat model of alcohol withdrawal in emotional and cardiorespiratory homeostatic nuclei. Journal of neuroinflammation. 2012;9:97. doi: 10.1186/1742-2094-9-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ. Ethanol disrupts fear conditioning in C57BL/6 mice. J Psychopharmacol. 2003;17:77–81. doi: 10.1177/0269881103017001702. [DOI] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Becker HC. Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcoholism, clinical and experimental research. 2009a;33:1893–1900. doi: 10.1111/j.1530-0277.2009.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Yanke AB, Middaugh LD, Becker HC. Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology (Berl) 2009b;201:569–580. doi: 10.1007/s00213-008-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruol DL, Vo K, Bray JG, Roberts AJ. CCL2-ethanol interactions and hippocampal synaptic protein expression in a transgenic mouse model. Frontiers in integrative neuroscience. 2014;8:29. doi: 10.3389/fnint.2014.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyon A, Skrzydelski D, De Giry I, Rovere C, Conductier G, Trocello JM, Dauge V, Kitabgi P, Rostene W, Nahon JL, Melik Parsadaniantz S. Long term exposure to the chemokine CCL2 activates the nigrostriatal dopamine system: a novel mechanism for the control of dopamine release. Neuroscience. 2009;162:1072–1080. doi: 10.1016/j.neuroscience.2009.05.048. [DOI] [PubMed] [Google Scholar]
- Harper KM, Knapp DJ, Breese GR. Withdrawal from Chronic Alcohol Induces a Unique CCL2 mRNA Increase in Adolescent But Not Adult Brain-Relationship to Blood Alcohol Levels and Seizures. Alcoholism, clinical and experimental research. 2015 doi: 10.1111/acer.12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Experimental neurology. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb LA, Gordon MN, Jantzen P, Hsiao K, Duff K, Morgan D. Behavioral changes in transgenic mice expressing both amyloid precursor protein and presenilin-1 mutations: lack of association with amyloid deposits. Behav Genet. 1999;29:177–185. doi: 10.1023/a:1021691918517. [DOI] [PubMed] [Google Scholar]
- Holmes A, Wrenn CC, Harris AP, Thayer KE, Crawley JN. Behavioral profiles of inbred strains on novel olfactory, spatial and emotional tests for reference memory in mice. Genes Brain Behav. 2002;1:55–69. doi: 10.1046/j.1601-1848.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- Huang D, Tani M, Wang J, Han Y, He TT, Weaver J, Charo IF, Tuohy VK, Rollins BJ, Ransohoff RM. Pertussis toxin-induced reversible encephalopathy dependent on monocyte chemoattractant protein-1 overexpression in mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:10633–10642. doi: 10.1523/JNEUROSCI.22-24-10633.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RN. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neuroscience and biobehavioral reviews. 2004;28:497–505. doi: 10.1016/j.neubiorev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Jung H, Toth PT, White FA, Miller RJ. Monocyte chemoattractant protein-1 functions as a neuromodulator in dorsal root ganglia neurons. Journal of neurochemistry. 2008;104:254–263. doi: 10.1111/j.1471-4159.2007.04969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane CJ, Phelan KD, Douglas JC, Wagoner G, Johnson JW, Xu J, Drew PD. Effects of ethanol on immune response in the brain: region-specific changes in aged mice. Journal of neuroinflammation. 2013;10:66. doi: 10.1186/1742-2094-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane CJ, Phelan KD, Douglas JC, Wagoner G, Johnson JW, Xu J, Phelan PS, Drew PD. Effects of ethanol on immune response in the brain: region-specific changes in adolescent versus adult mice. Alcoholism, clinical and experimental research. 2014;38:384–391. doi: 10.1111/acer.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao DJ, Li AH, Chen JC, Luo RS, Chen YL, Lu JC, Wang HL. CC chemokine ligand 2 upregulates the current density and expression of TRPV1 channels and Nav1.8 sodium channels in dorsal root ganglion neurons. Journal of neuroinflammation. 2012;9:189. doi: 10.1186/1742-2094-9-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JW, Gould TJ. Nicotine enhances context learning but not context-shock associative learning. Behav Neurosci. 2008;122:1158–1165. doi: 10.1037/a0012807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliethermes CL. Anxiety-like behaviors following chronic ethanol exposure. Neuroscience and biobehavioral reviews. 2005;28:837–850. doi: 10.1016/j.neubiorev.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Harper KM, Whitman BA, Zimomra Z, Breese GR. Stress and Withdrawal from Chronic Ethanol Induce Selective Changes in Neuroimmune mRNAs in Differing Brain Sites. Brain sciences. 2016:6. doi: 10.3390/brainsci6030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJ, Whitman BA, Wills TA, Angel RA, Overstreet DH, Criswell HE, Ming Z, Breese GR. Cytokine involvement in stress may depend on corticotrophin releasing factor to sensitize ethanol withdrawal anxiety. Brain, behavior, and immunity. 2011;25(Suppl 1):S146–154. doi: 10.1016/j.bbi.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin A, Liljequist S, Meis J, Chefer V, Shippenberg T, Bakalkin G. Repeated moderate-dose ethanol bouts impair cognitive function in Wistar rats. Addict Biol. 2012;17:132–140. doi: 10.1111/j.1369-1600.2010.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde R. The neurobiological basis of spontaneous alternation. Neuroscience and biobehavioral reviews. 2002;26:91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- Lee H, Roh S, Kim DJ. Alcohol-induced blackout. International journal of environmental research and public health. 2009;6:2783–2792. doi: 10.3390/ijerph6112783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewohl JM, Wang L, Miles MF, Zhang L, Dodd PR, Harris RA. Gene expression in human alcoholism: microarray analysis of frontal cortex. Alcoholism, clinical and experimental research. 2000;24:1873–1882. [PubMed] [Google Scholar]
- Lopez MF, Becker HC. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology (Berl) 2005;181:688–696. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 1997;8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Martin PM, O’Callaghan JP. A direct comparison of GFAP immunocytochemistry and GFAP concentration in various regions of ethanol-fixed rat and mouse brain. Journal of neuroscience methods. 1995;58:181–192. doi: 10.1016/0165-0270(94)00175-g. [DOI] [PubMed] [Google Scholar]
- Meinhardt MW, Hansson AC, Perreau-Lenz S, Bauder-Wenz C, Stahlin O, Heilig M, Harper C, Drescher KU, Spanagel R, Sommer WH. Rescue of infralimbic mGluR2 deficit restores control over drug-seeking behavior in alcohol dependence. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:2794–2806. doi: 10.1523/JNEUROSCI.4062-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melia KR, Ryabinin AE, Corodimas KP, Wilson MC, Ledoux JE. Hippocampal-dependent learning and experience-dependent activation of the hippocampus are preferentially disrupted by ethanol. Neuroscience. 1996;74:313–322. doi: 10.1016/0306-4522(96)00138-8. [DOI] [PubMed] [Google Scholar]
- Pascual M, Balino P, Aragon CM, Guerri C. Cytokines and chemokines as biomarkers of ethanol-induced neuroinflammation and anxiety-related behavior: role of TLR4 and TLR2. Neuropharmacology. 2015;89:352–359. doi: 10.1016/j.neuropharm.2014.10.014. [DOI] [PubMed] [Google Scholar]
- Paylor R, Zhao Y, Libbey M, Westphal H, Crawley JN. Learning impairments and motor dysfunctions in adult Lhx5-deficient mice displaying hippocampal disorganization. Physiol Behav. 2001;73:781–792. doi: 10.1016/s0031-9384(01)00515-7. [DOI] [PubMed] [Google Scholar]
- Pennanen L, Wolfer DP, Nitsch RM, Gotz J. Impaired spatial reference memory and increased exploratory behavior in P301L tau transgenic mice. Genes Brain Behav. 2006;5:369–379. doi: 10.1111/j.1601-183X.2005.00165.x. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Niedzielski AS, Wenthold RJ. The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience. 1996;71:949–976. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- Porsolt RD. Animal models of depression: utility for transgenic research. Rev Neurosci. 2000;11:53–58. doi: 10.1515/revneuro.2000.11.1.53. [DOI] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. Journal of neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Wan Y, Wang X. CCL2 and CXCL1 trigger calcitonin gene-related peptide release by exciting primary nociceptive neurons. Journal of neuroscience research. 2005;82:51–62. doi: 10.1002/jnr.20612. [DOI] [PubMed] [Google Scholar]
- Ripley TL, O’Shea M, Stephens DN. Repeated withdrawal from ethanol impairs acquisition but not expression of conditioned fear. The European journal of neuroscience. 2003;18:441–448. doi: 10.1046/j.1460-9568.2003.02759.x. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: insights from a two-process model. Neuroscience and biobehavioral reviews. 2004;28:675–685. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Savchenko VL, McKanna JA, Nikonenko IR, Skibo GG. Microglia and astrocytes in the adult rat brain: comparative immunocytochemical analysis demonstrates the efficacy of lipocortin 1 immunoreactivity. Neuroscience. 2000;96:195–203. doi: 10.1016/s0306-4522(99)00538-2. [DOI] [PubMed] [Google Scholar]
- Silvers JM, Tokunaga S, Berry RB, White AM, Matthews DB. Impairments in spatial learning and memory: ethanol, allopregnanolone, and the hippocampus. Brain research Brain research reviews. 2003;43:275–284. doi: 10.1016/j.brainresrev.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Sircar R, Sircar D. Adolescent rats exposed to repeated ethanol treatment show lingering behavioral impairments. Alcoholism, clinical and experimental research. 2005;29:1402–1410. doi: 10.1097/01.alc.0000175012.77756.d9. [DOI] [PubMed] [Google Scholar]
- Stephens DN, Ripley TL, Borlikova G, Schubert M, Albrecht D, Hogarth L, Duka T. Repeated ethanol exposure and withdrawal impairs human fear conditioning and depresses long-term potentiation in rat amygdala and hippocampus. Biological psychiatry. 2005;58:392–400. doi: 10.1016/j.biopsych.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Szabo G, Lippai D. Converging actions of alcohol on liver and brain immune signaling. International review of neurobiology. 2014;118:359–380. doi: 10.1016/B978-0-12-801284-0.00011-7. [DOI] [PubMed] [Google Scholar]
- Teng SX, Molina PE. Acute alcohol intoxication prolongs neuroinflammation without exacerbating neurobehavioral dysfunction following mild traumatic brain injury. Journal of neurotrauma. 2014;31:378–386. doi: 10.1089/neu.2013.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umhau JC, Schwandt M, Solomon MG, Yuan P, Nugent A, Zarate CA, Drevets WC, Hall SD, George DT, Heilig M. Cerebrospinal fluid monocyte chemoattractant protein-1 in alcoholics: support for a neuroinflammatory model of chronic alcoholism. Alcoholism, clinical and experimental research. 2014;38:1301–1306. doi: 10.1111/acer.12367. [DOI] [PubMed] [Google Scholar]
- Vagenas P, Azar MM, Copenhaver MM, Springer SA, Molina PE, Altice FL. The Impact of Alcohol Use and Related Disorders on the HIV Continuum of Care: a Systematic Review : Alcohol and the HIV Continuum of Care. Current HIV/AIDS reports. 2015;12:421–436. doi: 10.1007/s11904-015-0285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenta JP, Gonzales RA. Chronic Intracerebroventricular Infusion of Monocyte Chemoattractant Protein-1 Leads to a Persistent Increase in Sweetened Ethanol Consumption During Operant Self-Administration But Does Not Influence Sucrose Consumption in Long-Evans Rats. Alcoholism, clinical and experimental research. 2016;40:187–195. doi: 10.1111/acer.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gassen KL, Netzeband JG, de Graan PN, Gruol DL. The chemokine CCL2 modulates Ca2+ dynamics and electrophysiological properties of cultured cerebellar Purkinje neurons. The European journal of neuroscience. 2005;21:2949–2957. doi: 10.1111/j.1460-9568.2005.04113.x. [DOI] [PubMed] [Google Scholar]
- Vetreno RP, Qin L, Crews FT. Increased receptor for advanced glycation end product expression in the human alcoholic prefrontal cortex is linked to adolescent drinking. Neurobiology of disease. 2013;59:52–62. doi: 10.1016/j.nbd.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Drimmer DA, Walker JL, Liu T, Mathe AA, Ehlers CL. Effects of prolonged ethanol vapor exposure on forced swim behavior, and neuropeptide Y and corticotropin-releasing factor levels in rat brains. Alcohol (Fayetteville, NY) 2010;44:487–493. doi: 10.1016/j.alcohol.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You H, Altier C, Zamponi GW. CCR2 receptor ligands inhibit Cav3.2 T-type calcium channels. Mol Pharmacol. 2010;77:211–217. doi: 10.1124/mol.109.059022. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Tang H, Xiong H. Chemokine CCL2 enhances NMDA receptor-mediated excitatory postsynaptic current in rat hippocampal slices-a potential mechanism for HIV-1-associated neuropathy? Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2016;11:306–315. doi: 10.1007/s11481-016-9660-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Tang H, Liu J, Dong J, Xiong H. Chemokine CCL2 modulation of neuronal excitability and synaptic transmission in rat hippocampal slices. Journal of neurochemistry. 2011;116:406–414. doi: 10.1111/j.1471-4159.2010.07121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]