Abstract
Introduction
There is a significant interest in developing inexpensive portable biosensing platforms for various applications including disease diagnostics, environmental monitoring, food safety, and water testing at the point-of-care (POC) settings. Current diagnostic assays available in the developed world require sophisticated laboratory infrastructure and expensive reagents. Hence, they are not suitable for resource-constrained settings with limited financial resources, basic health infrastructure, and few trained technicians. Cellulose and flexible transparency paper-based analytical devices have demonstrated enormous potential for developing robust, inexpensive and portable devices for disease diagnostics. These devices offer promising solutions to disease management in resource-constrained settings where the vast majority of the population cannot afford expensive and highly sophisticated treatment options.
Areas covered
In this review, the authors describe currently developed cellulose and flexible transparency paper-based microfluidic devices, device fabrication techniques, and sensing technologies that are integrated with these devices. The authors also discuss the limitations and challenges associated with these devices and their potential in clinical settings.
Expert commentary
In recent years, cellulose and flexible transparency paper-based microfluidic devices have demonstrated the potential to become future healthcare options despite a few limitations such as low sensitivity and reproducibility.
Keywords: Point-of-care diagnostics, cellulose paper-based analytical devices, flexible transparency paper-based analytical devices, microfluidics, biosensors, device fabrication
1. Introduction
Clinical diagnostics makes up the first and most important step in the treatment of a disease. Around 5.8 billion people worldwide live in settings categorized as low and middle income [1] and do not have access to the expensive medical facilities required for current immunoassays and other disease detection technologies [2,3]. Therefore, it is very important to develop inexpensive and portable point-of-care (POC) devices that can provide rapid and accurate results. The World Health Organization (WHO) has provided essential guidelines for future diagnostic devices using the ASSURED acronym, which stands for affordable, sensitive, specific, user-friendly, rapid and robust, equipment-free, and deliverable [4].
POC diagnostics provide a promising solution that can meet these set parameters with numerous advantages, including affordability, sustainability, portability, disposability, simplicity, and ability to handle very small volumes of unprocessed samples, such as blood, urine, and saliva [5]. The field of microfluidics has witnessed great developments during the last decade and these advancements have played a key role to design various POC devices for resource-constrained settings [6–12].
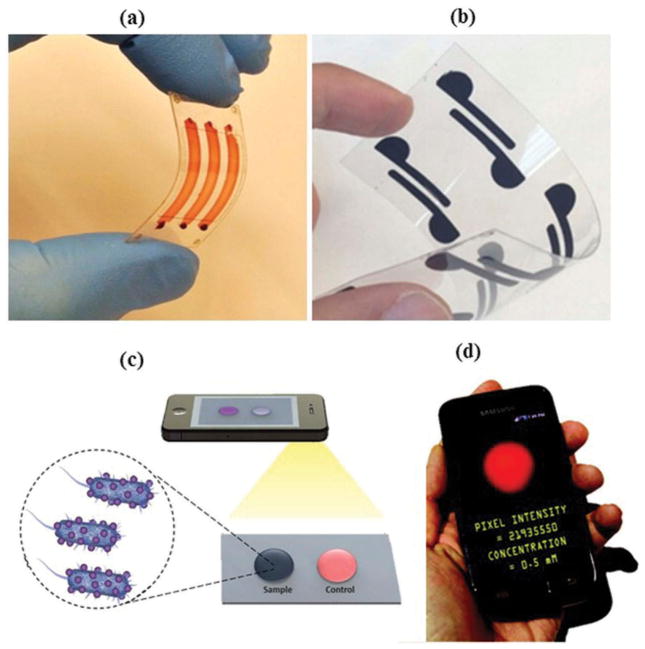
Microfluidic paper-based analytical devices (μPADs) are ideal candidates for POC diagnostic purposes, especially in personalized health care (Figure 1(a,b)). These devices are also well-suited for the food industry, security, and environmental monitoring applications [13,14]. A μPAD consists of paper, which is hydrophilic in nature and allows hydrophobic demarcations to be made with various polymers. A camera-enabled phone can be utilized with μPADs (Figure 1(c,d)), and collected data and images can then be transmitted using the wireless communications networks to centralized laboratories for analyses and obtaining results in real-time [15,16].
Figure 1.
(a) (Adapted from [16]) Microchip composed of flexible polymer. (b) (Adapted from [16]) Polyester transparency-based biosensing platform. (c) (Adapted from [16]) Image acquisition of the sample spot using the cell phone camera. (d) (Adapted with permission from Delaney et al. [17]. Copyright (2011) American Chemical Society) Image capture and analysis using cell phone.
Paper is one of the promising materials for making bioanalytical devices because of its characteristics like affordability, wide availability, and hydrophilic nature, which allow various solutions to flow through its porous structure via capillary action. In this review, we discuss various device fabrication and development methods currently employed for making μPADs. We also describe the sensing technologies that are integrated with μPADs. The challenges associated with paper-based diagnostic devices that limit their clinical applicability are also considered and future directions are highlighted.
2. Paper-based devices
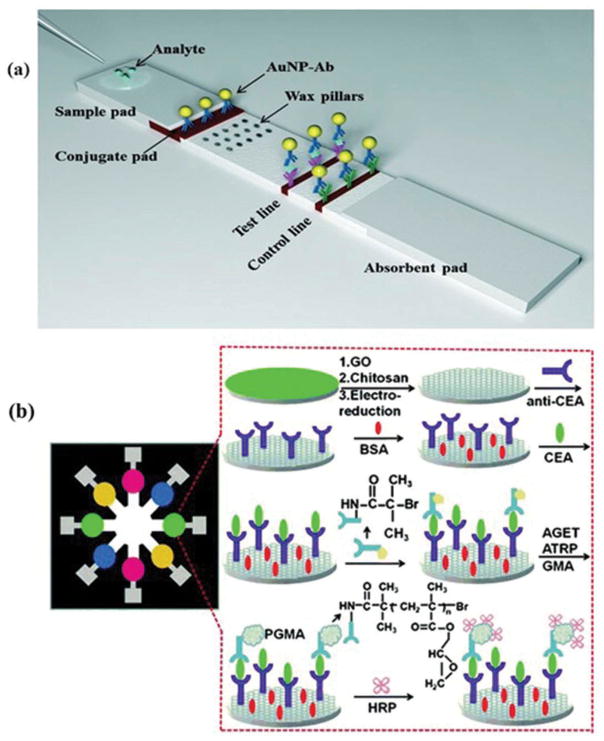
The paper-based dipstick assays are well-known; used to detect glucose in urine [18]. Urine is introduced to dipstick and the resulting color change is compared with standard chart to estimate the glucose levels [19]. Latex agglutination and radio-immunoassays were also developed [20], followed by the introduction of lateral flow technology that was utilized in human pregnancy tests. With the introduction of this home diagnostic tests, lateral flow assays (LFA) gained wide popularity [21]. Serological immunoassays based on lateral flow mechanisms are also developed and find applications in many areas including food safety, environmental monitoring, and veterinary diagnostics [19,22]. LFAs show poor detection limits and low sensitivities that confine their applications. To overcome these limitations, efforts are being made. For an instance, wax pillars have been used as delay barriers to improve the sensitivity of LFA (Figure 2(a)) [23]. The fabricated device was utilized for the detection of Human Immunoglobin G (HIgG) and improved the sensitivity by three times as compared to the conventional LFA devices.
Figure 2.
(a) (Adapted from [23] with permission from the Royal Society of Chemistry) Schematic design lateral flow assay device modified with wax fabricated pillars for the detection of protein using dual gold nanoparticles AuNP. (b) (Adapted from [24] with permission from Elsevier) Pictorial illustration of the electrochemical immunoassay procedure using CEA.
3. Paper-based microfluidic devices
Paper has been recently utilized as a substrate to fabricate various microfluidic devices [25]. Paper is inexpensive, lightweight, easily available everywhere, and disposable. It is biocompatible with various biological samples [26]. Table 1 provides detailed information about various paper substrates, listing their important characteristics and applications. In one example, chromatography paper was utilized for the detection of proteins and glucose in artificial urine samples by creating hydrophobic lines with photoresist, on hydrophilic paper (Figure 3) [27]. A single μPAD can also be used to conduct various bioassays simultaneously [28]. Examples include the detection of uric acid and nitrate in the saliva [29].
Table 1.
Paper substrates, their characteristics, and applications.
| Paper substrate | Characteristics | Applications |
|---|---|---|
| Whatman paper # 1 | It is composed of cotton cellulose. The size of pores is 11 μm [30]. Its thickness is 180 μm. It has medium retention and flow rate | It is the most widely used filter paper due to its compatibility with the majority of fabrication methods in μPADS. It is not ideal for all applications [28]. Apilux et. al. have used Whatman paper #1 for creating a device for the determination of gold and iron [31] |
| Whatman paper # 4 | It is made up of cotton cellulose. The size of pores is 20–25 μm. It is a very fast filtering paper [32]. It has an excellent retention rate. The thickness is 210 μm | Li et. al. have used Whatman paper #4 as a substrate for fabricating paper-based microfluidic sensors [33]. Its properties such as larger pore size and higher retention rate were utilized in making paper-based devices |
| Nitrocellulose membrane | Nitrocellulose membranes have very smooth and uniform pore size, normally 0.45 μm [28] | Lu et. al. have demonstrated a μPAD using nitrocellulose membrane for protein immobilization applications [34]. Nitrocellulose membranes demonstrate a high degree of nonspecific binding toward biomolecules [28] |
| Bioactive paper | It is obtained by the modification of paper matrix with biomolecules. The biggest advantage of μPADs using bioactive paper is that they do not require sophisticated equipment to operate and are quite simple [35] | Bioactive paper is used in many analytical applications. Pathogen detection is one of them [26] |
| Cellulose glossy paper | Glossy paper is composed of cellulose but it is blended with certain inorganic fillers. It is non-degradable and a better alternative to filter paper when it is necessary to have the surface modified by the nanoparticles | Arena et. al. have used glossy paper for the fabrication of flexible ethanol sensors at the room temperature [36] |
Figure 3.
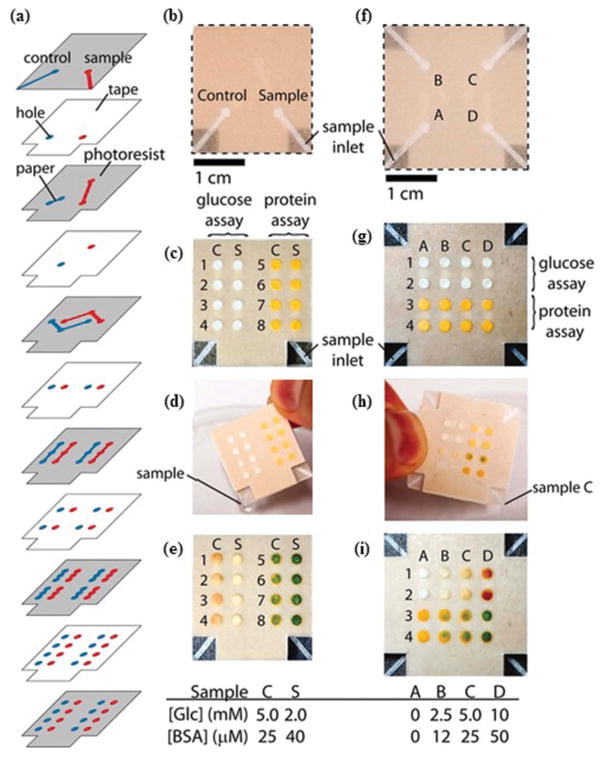
(Adapted from [27] with permission from John Wiley and Sons) Chromatography paper having patterns made by photoresist (a) device after absorbing Waterman red ink (5 mL). (b) Reagents added to perform glucose and protein assays. (c) Negative assay for glucose and urine using artificial urine (5 mL) (d) Positive assay of glucose and urine using artificial urine solution containing 550 mm glucose and 75 mm BSA. (e) Results of glucose and BSA detection assays with their varying concentrations. Full color available online.
4. Fabrication techniques of μPADS
Various μPAD device fabrication methods, such as photolithography [27,37], inkjet printing [33,38], polydimethylsiloxane (PDMS) plotting [39], wax printing [40–42], wax dipping [43], wax screen printing [42,44], and plasma treatment [45], have been proposed for a cost-effective, simple, and portable product [25]. The pros and cons of each fabrication method are listed in Table 2.
Table 2.
Advantages and limitations of different fabrication techniques for paper-based microfluidic devices.
| Fabrication methods | Advantages | Limitations |
|---|---|---|
| Photolithography [46,47] | High resolution of channel with sharp barriers, suitable for large-scale production | Requires expensive instruments and reagents, involves complex steps, fragile while bending |
| Inkjet printing [28,48] | Able to rapidly fabricate devices on a large scale | Requires a customized inkjet printer and an extra heating step for curing purposes |
| PDMS plotting [43,45] | Inexpensive technique to fabricate flexible devices | Low resolution, demands modification of the plotter, inconsistent control over the penetration of PDMS due to the nonuniform porous nature of paper |
| Laser cutting [2,3,16,49–53] | Simple and inexpensive technique to cut specific patterns and assemble devices | Requires a laser cutter/engraver, graphics software, and DSA |
| Laser printing [54] | Simple and inexpensive method to fabricate microfluidic devices | Requires laser printer, graphics software, laminator, and paper driller |
| Wax printing [19] | Fast and simple fabrication technique, suitable for mass production | Low resolution, uses expensive wax printer, not resistant to high temperatures |
| Wax dipping [43,55] | Simple and fast fabrication technique with better reproducibility, suitable for mass production | Low resolution, heating requirement |
| Screen printing [56] | Cost-effective and simple process, well-suited for mass production | Low resolution, each pattern requires an individual screen |
| Plasma treatment [45,48] | Inexpensive process, the flexibility of paper is maintained | Each pattern requires a specific photomask |
| Flexographic printing [19,47,57] | Enables fast, commercial roll-to-roll production of paper-based microfluidic devices | Multi-step process that requires complex reagents, and specialized commercial printers, requires frequent cleaning to avoid contamination, roughness of the paper substrate affects the final quality of printing |
4.1. Photolithography
Photolithography utilizes a photoresist to fabricate paper-based microfluidic devices. The masks required for photolithography can be prepared using an inkjet printer, a photocopying machine, or even hand drawn. The exposure can be accomplished using a UV lamp [37]. Although photolithography is often used in making microfluidic devices, photolithography is still quite expensive and requires access to clean room facilities [58]. A more convenient way of fabricating gold patterns on silicon substrates has been reported [59]. Using this method, patterning and etching of gold particles is quite simple, once a rubber stamp is fabricated. This technique does not require access to cleanroom facilities and other photolithography equipment [59]. Various soft lithography techniques also were developed for the cost-effective fabrication of microfluidic devices such as maskless lithography and phase shift photolithography [60–63]. Soft lithography uses elastomeric materials to fabricate pattern transfer elements and it can be used to pattern complex biochemicals based on embossing, molding, and printing [64].
4.2. Inkjet printing
Inkjet printing method has been utilized for microfluidic device fabrication for simultaneous detection of glucose, proteins, and pH [38]. Micropatterns were fabricated on paper surfaces using 10 repeated toluene printing cycles. The flow channels were 550 μm wide while the sensing areas were 1.5 mm × 1.5 mm squares. The distinct feature of inkjet printing is that the entire fabrication process relies only on the inkjet printing equipment. A new concept has been demonstrated to create paper-based sensors by generating the hydrophilic-hydrophobic contrast on paper. This technique is quite useful for the large-scale production of paper-based sensors [33], as the use of polymers to define channels is costly and expensive for large-scale fabrication.
4.3. PDMS plotting
PDMS has been utilized over its less flexible counterparts (SU-8) and poly(methyl methacrylate) (PMMA) [39]. A modified plotter is able to fabricate channels of minimum width 1 mm using PDMS dissolved in hexanes. Although this process does not require access to cleanroom, organic reagents, photolithography equipment, and expensive photoresist, it uses custom-built plotters and cannot produce structures at resolutions as high as photolithography.
4.4. Laser cutting
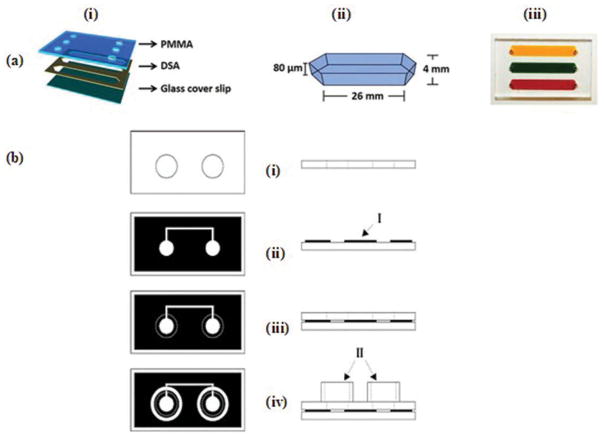
The CO2 laser cutter has been widely used to cut the paper, PMMA, and double-sided adhesive (DSA) to fabricate microfluidic devices [2,3,16,49–53]. A simple and inexpensive method was presented for the fabrication of microfluidic devices (Figure 4(a)) [3]. Laser cutter was used to cut the PMMA and DSA as per specific patterns. It is a faster, inexpensive, and efficient process to assemble microfluidic devices. The fabricated device was used to capture CD4 cells from whole blood samples.
Figure 4.
(a) (Adapted from [3] under the Creative Commons Attribution 4.0 International Public License https://creativecommons.org/licenses/by/4.0/legalcode) 3D Microfluidic device (i) Schematic of device consisting of PMMA, DSA and glass cover slip. (ii) Dimensions of microfluidic channel. (iii) Image of device where channels are filled with blood and food dyes. (b) (Adapted with permission from Lucio do Lago et al. [54]. Copyright 2003 American Chemical Society) Schematic representation of laser printing and laminating processes for the fabrication of microfluidic devices (i) Perforated transparency film. (ii) Printed polyester base (I, toner layer) (iii) Lamination of cover sheet and base and (iv) Final microfluidic device. (II, liquid reservoirs).
In another study, a μPAD was developed using laser cutting process [65]. Chromatography paper backed by aluminum foil was used to create small features. The performance of the fabricated device was demonstrated by conducting a glucose test using 2 μL of artificial urine sample. Recently, laser cutting process was used to fabricate a microchip for the diagnosis of tuberculosis (TB) [66]. This proposed microchip-based TB Enzyme-Linked Immunosorbent Assay (ELISA) (MTBE) is a rapid, inexpensive, flow-less, and magnetic actuated platform. It was demonstrated that the assay time can be reduced to almost 15 min while keeping the detection efficiency comparable to standard classical ELISA.
4.5. Laser printing
Microfluidic devices can be fabricated on polyester sheets by combining laser printing and laminating processes (Figure 4(b)) [54]. The laser printer deposits toner layers on transparency sheets, with white regions representing the microfluidic channels. The laminating process then seals the microfluidic channels. This process can be utilized to produce multiple devices on a single sheet using a laser printer, paper driller, and laminator. A technique was presented for the fabrication of polyester-toner microfluidic device [67]. The proposed device was utilized for the dynamic solid-phase extraction of DNA. The microfluidic devices prepared using laser printing process have shown great potential for various clinical assays including dengue diagnosis [68], detection and quantification of glucose in biological samples [69], and PCR amplification of DNA [70]. A toner-based microfluidic device was demonstrated to accurately perform simultaneous detection and quantification of multiple analytes including total proteins, glucose, cholesterol, and triglycerides in biological fluids [71].
4.6. Wax printing
Wax printing for the fabrication of μPADs offers numerous advantages such as lower production costs, disposability, non-toxicity, ease of fabrication, and few steps for mass production. Wax channels can be printed on paper that can confine and direct samples and reagents, so simple paper-based microfluidic devices can be formed. Therefore, this method serves as a better approach for creating paper-based micro-fluidic devices [34]. This process can be accomplished either using a wax pen or printers for large-scale production. However, the utilization of expensive wax printer and heating equipment, along with lower resolution of fabricated devices, are the main drawbacks and limitations of this wax printing technique [43]. A simple and inexpensive process of wax printing was demonstrated utilizing hot plate and printer for the large-scale production of paper-based microfluidic devices [41]. The smallest microfluidic channel with average width of 561 ± 45 μm was fabricated using this technique.
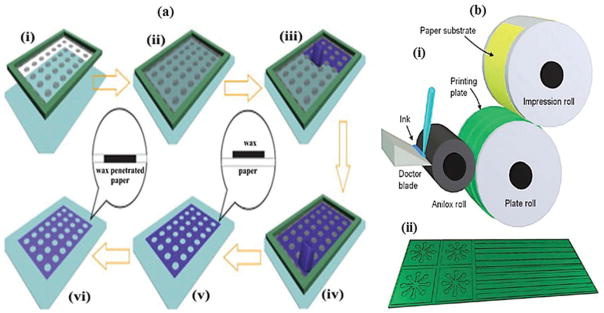
A simpler, faster, and more cost-effective wax-patterning method was demonstrated for microfluidic device fabrication [40]. It consists of printing and baking. A nitrocellulose (NC) membrane was used as a paper substrate, which is the most suitable for protein immobilization applications. The distinct features of a NC membrane, such as its smaller and uniform pore size, make the wax penetration process slower and more precisely controllable during baking. This allows preparation of up to 100-μm-wide microchannels, at a resolution comparable to that of photolithography, without the use of harmful organic reagents. The manufactured device was tested for purification of samples and protein immobilization applications. The results proved that wax-patterned NC membrane can be utilized for the purification of micrometer size impurities, such as beads and cells. The fabricated device using this method was also used in dot immunoassay.
Recently, wax-ink valves were printed onto cellulose and nitrocellulose membranes using wax-printing technique (Figure 5) [72]. These valves were thermally actuated using thin-film heater to control the release of fluids. The incorporation of thermally actuated wax-ink valves transformed current existing Lateral Flow Immunoassays (LFIA) into tunable, semiautomated, and multistep LFIA with improved limit of detection (LOD). The enhanced LFIA strips were tested for Escherichia coli (E. coli) assay. The results indicated that both 5.5×105 and 5.5 ×106 E. coli cells/mL were detectable by human eye with 6 times greater intensity than the standard LFIA strip. In one other example, micro-a-fluidics ELISA (m-ELISA) platform was developed using mineral oil to make valves [52]. The fabricated microfluidic chip was purposely designed to have five circular chambers connected with five elliptical chambers. Elliptical chambers were filled with mineral oil to physically separate stationary liquids/reagents in circular chambers. A droplet of blood along with antibody-functionalized magnetic nanoparticles was loaded onto the microfluidic chip. The device was placed on a permanent magnet fixed on a motorized stage. The movement of stage was controlled using a software program to perform m-ELISA. The device was used for rapid CD4 cell count at POC settings. Recently, a similar kind of device was proposed for the detection of tuberculosis [66].
Figure 5.
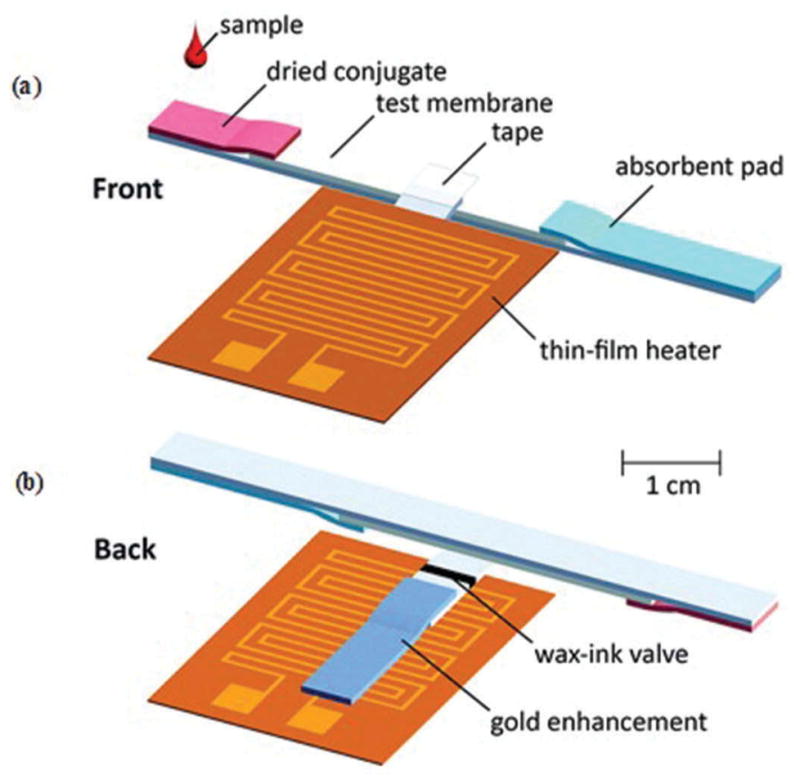
(Adapted from [72] with permission of The Royal Society of Chemistry) Schematic representation of modification process of a conventional LFIA pad into multi-step LIFA using thermally actuated wax-ink valve. (a) Front view. (b) Back view.
4.7. Wax dipping
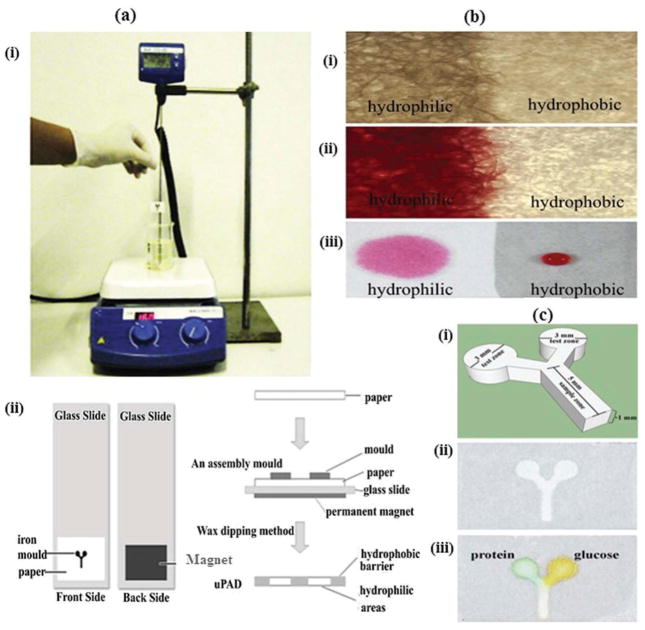
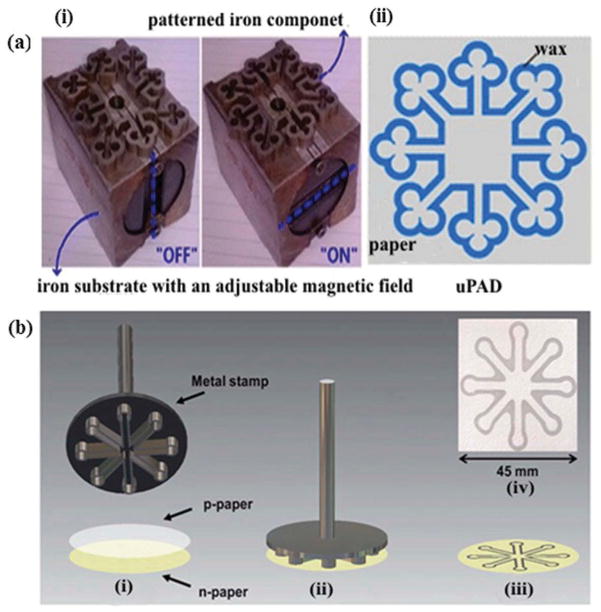
Wax dipping is also a faster and less expensive technique compared to photolithography for making microfluidic channels on paper. It consists of only wax dipping and channels can be fabricated in less than a minute via successive dipping and ordinary heating arrangements (Figure 6) [43]. The melted wax is utilized to coat hydrophobic barriers, and an iron mold is used to protect the hydrophilic channel. The iron mold is temporarily placed onto the paper using magnetic field of a permanent magnet. When this assembly is dipped into molten wax, it absorbs the wax while the iron mold prevents its penetration. The actual width of the fabricated microfluidic channel is determined by the width of iron mold utilized.
Figure 6.
(Adapted from [43] with permission from Elsevier) Schematic diagram of wax dipping process for the fabrication of μPADs (a) Method of microfluidic channel fabrication using wax dipping: (i) wax dipping apparatus. (ii) Method of patterning paper by wax dipping process in top view (left side) and lateral view (right side). (b) Photographs of fabricated paper using wax dipping method (i) hydrophilic and hydrophobic areas captured under microscope (40x). (ii) Hydrophilic area soaked with food dye color. (iii) Comparison of hydrophobic and hydrophilic zones using a drop of colored food dye. (c) μPAD fabricated by wax dipping technique: (i) basic structure and size and shape measurements of iron mold. (ii) Top view of final paper-based microfluidic device. (iii) Device after protein and glucose detection.
4.8. Movable type wax printing method
An equipment free wax printing method was presented for the fabrication of μPADs using a hot plate and movable parts to make various patterns on paper [73]. The proposed method does not require any technical expertise to fabricate a micro-fluidic device with repeatable accuracy and resolution. The process consists of three steps. Initially, moveable parts are assembled in the specific pattern on an iron supporting substrate. This is done when the support’s magnetic field is turned off. Second, these patterned parts are coated with molten wax heated via hot plate. This process is carried out in the presence of the support’s magnetic field. In the third step, this hot stamp is utilized to print wax patterns on the substrate. The molten wax eventually penetrates into the paper, and desired patterns are printed (Figure 7(a)).
Figure 7.
(a) (Adapted from Zhang et al. [73]. Copyright (2014) American Chemical Society) Schematic representation of movable-type wax printing (MTWP) technique for the fabrication of μPADs: (i) a set of iron parts assembled into specific pattern. (ii) Wax patterned μPADs. (b) (Adapted from [74] permission of The Royal Society of Chemistry) Schematic illustration of microfluidic device printing method based on stamping: (i) placement of paraffined paper (p-paper) on native paper (n-paper) (ii) preheated metal stamp brought in contact with layered papers. (iii) Final microfluidic device manufactured by handheld stamping process. (iv) Optical micrograph of manufactured device.
A hand-held and lightweight stamp has been developed for device fabrication [74]. Initially, the surface of native paper (n-paper) was oxidized. Then, paraffined paper (p-paper) was placed over the surface of n-paper. The final step involved the placement of a preheated stainless steel stamp on p-paper to thermally transfer paraffin to n-paper, and thus 3 mm micro-fluidic channels were formed on n-paper (Figure 7(b)).
4.9. Wax screen printing
Wax screen printing is also a simple, cost-effective, and fast method for the fabrication of μPADs. Initially, a mask is designed using computer software programs and printed on a transparency film. This transparency sheet is converted to various screens [44]. Wax is rubbed through the screen, onto the filter paper. Wax can be melted using a hot plate. The molten wax is absorbed by the paper and thus the hydrophobic barriers are printed. After cooling for about 10 s at room temperature, the fabricated device is ready to use. This method forgoes the need for expensive wax printers (~$2500 US) by using very inexpensive wax screens (<$5US), making it appropriate for resource-constrained settings. A paper-based device has been proposed to combine the advantages of μPADs and Chemiluminescence ELISA (CL-ELISA) [42]. The schematic illustration of the wax-screen printing process to fabricate paper-based microzone plate is shown in Figure 8(a). They correctly determined the biomarkers alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), and cancer antigen 125 (CA-125) in real human serum. The paper-based CL-ELISA showed the lower LOD and wider linear range.
Figure 8.
(a) (Adapted from [42] with permission from Elsevier) Schematic representation of wax screen-printing process (i) Paper and screen. (ii) Screen placed on the surface of paper. (iii), (iv) Solid wax utilized as a squeegee and rubbed through the screen. (v) Wax patterns formed on the surface of paper. (vi) Screen-printed paper placed in an oven for wax penetration into paper substrate and formation of the paper microzone plate. (b) (Adapted from Olkkonen et al. [57]. Copyright (2010) American Chemical Society) Illustration of flexographic printing process: (i) Schematic representation of flexographic printing equipment (ii) Final printed device using flexography.
4.10. Plasma treatment
Plasma treatment has been utilized for the channel fabrication on paper surfaces [45]. The filter paper was initially made hydrophobic by dipping it into the alkyl ketene dimer (AKD)-heptane solution and then it was immediately placed in a fume hood to facilitate evaporation of the heptane. Then these paper samples were baked in an oven at 100°C for 45 min to cure the AKD. Hydrophilic patterns can be made on this baked paper using plasma treatment. This process of plasma treatment does not change the flexibility and surface topography of paper. It is possible to print different patterns and functional components such as control switches, microreactors, and microfilters using this technique. However, this process requires customized masks, vacuum plasma reactors, and hot plate that restrict wide applicability of this technique [19].
4.11. Flexographically printed fluidic structures in paper
Flexographic printing is another important technique to fabricate μPADs on a large scale in a roll-to-roll process (Figure 8(b)) [57]. Microfluidic channels of width 500 μm were produced using this method. Flexography utilizes commercial printing press equipment and chromatography paper as a substrate. Paper-based reaction arrays were fabricated using both flexographic and inkjet printing [75]. They printed patterned PDMS ink layers on various paper substrates. The use of coated paper with good barrier properties results in a smooth uniform film and the PDMS ink remains on the surface of paper. A glucose sensor was printed on the reaction plate to verify the functionality of this printing method. The response of sensor was fairly linear with a detection limit of 0.1 mg/mL. Although flexographic printing is the fastest method, it does have a few limitations. It is a multistep process which requires a specialized flexographic printer and can only print one reagent at a time [76].
5. Sensing technologies used in μPADs
Various sensing mechanisms such as colorimetric, electrochemical, chemiluminescence (CL), electro-chemiluminescence (ECL), and fluorescence detection have been developed to quantify the results of diagnostic tests. In this section, we review these sensing mechanisms and techniques, and how these sensing methods can be integrated with the cellulose and flexible transparency paper-based devices.
5.1. Colorimetric detection
The ability to provide a semiquantitative or ‘yes/no’ answer makes colorimetric detection extensively applicable for μPADs [77]. This detection method is based on reactions between the target substances and chemical reagents. The chemical reagents include acid–base indicators, dyes, or enzymes. Litmus paper, utilized to determine the pH value of any solution, is a common example of this method.
The results of this technique can be obtained either with direct visualization or computer software. Computer software is the preferred method because direct visualization is often affected by dissimilar lighting environments, variations in color perception, and the different colors of dry and wet papers [77]. A three-dimensional microfluidic device was fabricated using paper and tape (Figure 9). Colorimetric analyses were used to measure the levels of glucose and protein in artificial urine [78]. Recently, a μPAD was utilized for colorimetric detection of human papillomavirus (HPV)16 DNA from cervical sample in less than an hour [79]. The proposed device was fabricated using paper and pressure sensitive adhesive sheets to extract, amplify, and detect nucleic acids from clinical samples. This portable, inexpensive, and disposable device can be used for rapid detection of cervical cancer in resource-constrained settings. Additionally, other biosensing platforms have been developed to detect Human Immunodeficiency Virus-1 (HIV-1), E. coli, and Staphylococcus aureus pathogens [16]. They modified gold nanoparticles with specific recognition elements. The modified gold nanoparticles solution is transferred to a cellulose paper, where bacteria samples cause nanoparticles aggregation. The resulting color change in nanoparticles can be detected by the naked eye. A cellular phone camera was used to acquire the image of nanoparticles aggregate spot. A customized image analysis tool was developed in MATLAB to quantify the color intensities of the image. The LOD was 8 CFUs/mL.
Figure 9.
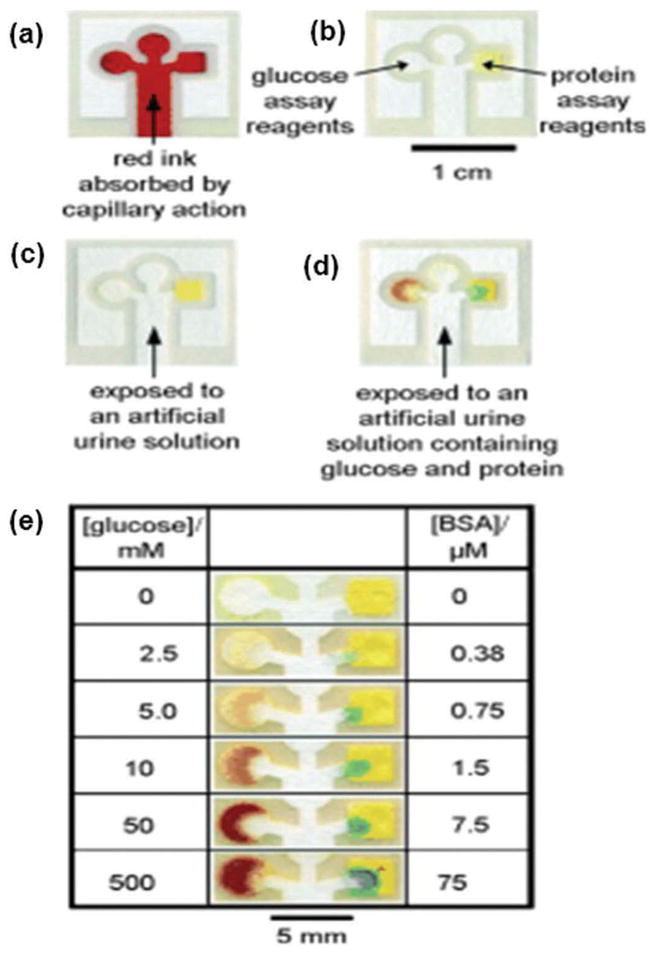
(Adapted from [78] Copyright (2008) National Academy of Sciences, U.S.A.) A three dimensional μPAD (a) Schematic representation of layers of tape and paper in a 3D microfluidic device. (b) Photograph showing the front of the dual-assay device. (c) Back of the device containing reagents for colorimetric detection of glucose and proteins. (d) Device containing a sample of artificial urine with 2-mM glucose and 40 μM BSA. 25 μL of sample is poured into device in 2 min. (e) Pictorial representation of the results of the assays for control and sample. (f) Top of the four-assay device. (g) Back of the three dimensional paper-based microfluidic device. (h) Device containing samples. Each corner of the device was dipped into a specific artificial urine sample. (i) Picture showing the results of the assays.
The μPADs often employ colorimetric detection for urine analysis. In one study, colorimetric detection was utilized for the simultaneous detection of proteins and glucose in 5 μL of artificial urine [27]. In the glucose assay, a positive test result was observed by a color change from clear to brown, i.e. from oxidation of iodide to iodine. In the adjacent protein assay, a tetrabromophenol blue color change from yellow to blue demonstrated a positive result. Urinalysis test strips, such as Chemstrip, AimStick, and Multistix, use the colorimetric method [19]. In μPADs, normally a single indicator dye is used to detect a corresponding analyte but a more accurate method has been demonstrated to measure the concentration of a single analyte using multiple indicators [80]. The use of various different indicators for a single analyte results in the generation of multiple colors corresponding to various analyte concentrations. This technique also improves the capability to visually distinguish different analyte concentrations.
The color intensity and uniformity depend on the type of paper substrates and the volume of reagents used [81]. The selection of paper is of utmost importance in color development. It has been observed that thicker substrates offer higher resistance to the flow of solution and as result demonstrate poor color readings. It is possible to obtain faster transfers of solutions and better color yield with thinner substrates. The use of flexible transparent materials along with colorimetric detection process can significantly enhance the sensitivity for POC assays [82].
In an attempt to significantly improve the color intensity and uniformity, researchers have modified the surface of μPAD with silica nanoparticles [83]. These nanoparticles were trapped within the cellulose structure and immobilized the enzymes responsible for the coloration. Later on, Fe2O3 magnetic nanoparticles, multi-walled carbon nanotubes and graphene oxide were utilized to modify the surface of μPADs [84]. The analytical performance was improved and the signal for colorimetric detection of glucose was significantly enhanced. The LOD values for the detection of glucose were 43, 18, and 62 μM for Fe2O3 magnetic nanoparticles, graphene oxide, and multiwalled carbon nanotubes, respectively.
Recently, chitosan has been used to modify the surface of μPADs [85]. The fabricated device has demonstrated lower LOD values, i.e. 37 and 23 μM for uric acid and glucose, respectively, using 4-AAP/DHBS chromogenic agent. The pixel intensity and color uniformity were significantly improved. These enhancements have resulted in colorimetric detection of glucose in human tear samples.
5.2. Electrochemical detection
Electrochemical detection is suitable for μPADs due to its low cost, portability, high selectivity, sensitivity, low electrical power consumption, and minimal instrumentation [86]. This technique has been used to simultaneously measure the concentrations of glucose, lactate, and uric acid. When an electric current passes through the electrodes, the chemical reaction is enhanced and electrodes exhibit a specific behavior. Electrochemical sensors consist of three electrodes: a working electrode, a reference electrode, and a counter electrode [20]. These sensors can be formed by depositing conductive ink on a paper matrix. Silver and graphene inks are widely used for the screen printing of electrodes and connecting wires [16].
In electrolytic cells, the target analyte is detected at the working electrode. A counter electrode is used to collect the current flowing through the circuit. The counter electrode also limits the current flowing thorough the reference electrode [87]. The reference electrode is composed of AgCl/Ag and placed away from the reaction place to maintain the known constant potential. The working electrode behaves as a transduction element. The counter electrode is responsible for making a connection with the electrolytic solution so that electric current can be applied to the working electrode [88]. A cost-effective and simple device has been demonstrated where electrochemical detection was achieved, using a mobile phone for resource-constrained settings [89]. A paper-based microfluidic device was fabricated using electrochemical detection for the quantification of glucose from the whole blood samples [90]. A paper-based electrochemical immunodevice was demonstrated for detection of four cancer biomarkers, namely carbohydrate antigen 153 (CA153), CEA, CA-125, and AFP [24]. The process of electrochemical detection is indicated in Figure 2(b). Electrochemical sensors are attractive for POC applications as these do not require a light source and are label-free.
5.3. Chemiluminescence detection
CL is a phenomenon of light generation due to chemical reaction, i.e. the conversion of chemical energy into light energy as electrons move from an excited state to a lower energy level.
Various compounds react with hydrogen peroxide or oxygen, which results in the compound decomposing and light being emitted [91]. For example, an organic compound luminol (5-amino-2, 3-dihydro-1, 4-phthalazinedione, or 3-aminophthalhy-drazide) and hydrogen peroxide react to produce light. CL detection has various advantages, such as less-expensive instrumentation, a wide dynamic range, and lower limits of detection. A device was fabricated for uric acid detection based on an enzymatic reaction, which produced hydrogen peroxide during decomposition of the substrate. The generated peroxide and rho-danine derivative reacted in acidic medium to yield CL [92]. The performance of fabricated device was verified by using a 20 μL sample solution of uric acid at various concentrations in tris-buffered saline (TBS) under optimal conditions. The LOD was 1.9 mmol/L and the graph was linear over the range of 2.6–49.0 mmol/L. Sandwich CL-ELISA was performed on μPADs for simultaneous determination of various tumor markers [42]. In order to verify the performance of the device, 4 μL samples of AFP, CA125, and CEA at different concentrations in phosphate-buffered saline (PBS) were applied under optimal conditions. The limits of detection for these three tumor markers are 0.06 ng/mL, 0.33 U/mL, and 0.05 ng/mL, respectively. The CL intensity varies linearly with the increasing concentrations of AFP, CA-125, and CEA with dynamic ranges of 0.1–35.0 ng/mL, 0.5–80.0 U/mL, and 0.1–70.0 ng/mL, respectively.
5.4. Electro-Chemiluminescence detection
ECL detection is the combination of CL and electrochemical techniques. Electrochemical reactions result in generation of light. ECL has numerous advantages such as better sensitivity and increased dynamic concentration response range [93]. It also has some prominent features such as smaller sample volumes, lack of a light source requirement, and simple instrumentation [91]. ECL is most widely used in clinical diagnosis. More than 150 different immunoassays are available on the market for detecting tumor markers and treating thyroid disease and various infectious diseases [94].
ECL has been integrated with paper-based microfluidics devices [17]. A paper-based ECL immunodevice was demonstrated to detect the presence of CEA [95]. They successfully quantified the levels of CEA in human serum. The performance of the device was analyzed using 2.0 μL of standard human CEA solutions at different concentrations in PBS under the most optimal conditions. The dynamic range of this method was 0.005–50 ng/mL while the LOD was 0.001 ng/mL at a signal-to-noise ratio of 3 for human CEA.
5.5. Fluorescence
The fluorescence method of sensing is based on a signal detection, which occurs during the interaction of target molecules and fluorescent dyes known as fluorophores [96,97]. It was first used in paper microzone plates. The fluorescence detection process consists of three stages: (i) excitation, (ii) excited-state lifetime, and (iii) fluorescence emission. The sensing process consists of (i) a source of light at a certain wavelength induces luminescence in a fluorophore, (ii) the light is filtered and emission photons isolated from excitation photons, and (iii) emission photons are detected, producing an electrical signal as an indicator.
Paper can also fluoresce with the addition of several fluorescent brightening agents. However, these materials may enhance the auto-fluorescence and result in false positives. DNA has been successfully detected using strips of paper immobilized with synthetic DNA oligonucleotides [98]. It was demonstrated that paper can serve as a future option for simple, cost-effective DNA detection. In this study, poly (N-isopropylacrylamide) microgels (MGs) coupled with an oligonucleotide was spotted onto a paper strip. This paper strip was used for the ligation–rolling circle amplification (RCA)–hybridization-based detection. The target DNA concentrations ranging from 10 pM to 10 nM were used in the experiment. The relative fluorescence intensities of paper with DNA target and without DNA target were measured. The results demonstrated quantitative DNA analysis with a LOD of 100 pM. In another study, a microfluidic device was fabricated for the quantification of lactoferrin in the human tear samples [99]. They utilized the fluorescence signal emitted by the lactoferrin–terbium complexes and achieved antibody-free sensing system. The LOD of 0.30 mg/mL was achieved.
6. Future of paper-based microfluidic devices
Microfluidics have truly changed the modern era of diagnostics, whether it is for biomedical research [8,11,46,100–109], fertility [9,10,12,110], DNA analysis [111–116], environmental monitoring [116], or food safety [118]. POC diagnostics using paper-based microfluidics is also the area of interest for many large pharmaceutical companies and non-profit organizations, who strive to develop POC diagnostic devices for resource-limited settings. Although many POC diagnostic devices have been fabricated and tested, a transition from academia to industry is mandatory for their commercialization. Generally, these μPADs have the following limitations: (i) sample retention and evaporation issues can cause serious problems with the transportation of sample [119], (ii) variation in the specificity and sensitivity of μPADs is the main concern of the present era because it plays a crucial role in avoiding false-positive results [120], and (iii) reagents used in these μPADs, such as enzymes, antigens, and antibodies, must withstand the harsh environmental conditions met during shipping and storage processes [3,121].
The combination of digital microfluidics (DMF) with paper is another flourishing area to implement complex and multistep assays. DMF can manipulate micro-to-nanoliter-sized liquid drops on an array of electrodes in two dimensions using electric fields [122]. The phenomenon of electrowetting is used for the movement of droplets from one electrode to other. The electrostatic forces can be utilized to mix, dispense, split, and merge various liquid drops. There is an unmet demand to fabricate paper-based DMF devices using cost-effective and scalable methods for multitude of applications such as sandwich ELISA. DMF instrumentation is quite complex and costly. It is necessary to efficiently manage the switching of hundreds to thousands of signals for the drop movement. Paper-based DMF can truly revolutionize the field of POC disease diagnostics in future.
Smartphone and lensless imaging-based disease detection and quantification is another area of immense interest [16,123,124]. The smartphone market is growing at an unprecedented pace. It is predicted that the number of worldwide smartphone users will increase to around 2.6 billion by 2020 [125]. Smartphones are equipped with high-resolution digital cameras and have advanced computing capabilities. They can easily be coupled with paper-based microfluidic devices for resource-constrained settings. They can provide a viable solution for data acquisition and results analysis. Although a few efforts have been made in the recent past to integrate camera enable phones with μPADs, more research should be performed in the near future to fully utilize their benefits in both resource-rich and resource-constrained settings for POC diagnostics. Smartphones can be used for disease diagnostics using instant on-site quantification and/or later expert opinion by the physicians/skilled technicians to expedite the clinical decision-making process. Paper is a nonhomogenous medium of cellulose fibers making the detection of complex analytes challenging [126]. However, smartphones can solve this non-homogeneity problem associated with paper. It is possible to average out the nonuniform optical signals arising from non-homogenous cellulose fibers over a substantial area. Furthermore, the use of white LEDs to maintain constant illumination conditions can also average out the signal variations over a range of wavelengths. Lensless imaging has emerged as an alternative option for POC diagnostic devices [127]. This technology is suitable for developing imaging platforms with high resolution and provides wide field-of-view that is suitable to image the whole chip surface in few seconds. Lensless imaging requires a light source and complementary metal-oxide semiconductor image sensor. This technology is compatible with cell-phone platforms. Novel microfluidics and nanoelectronics, coupled with smartphones, offer a bright future for technology-driven disease diagnostics [128]. The complexity of the paper-based microfluidic devices can be substantially reduced when various tasks like detection, data processing, and power are handled by smartphones [129]. Flexible materials such as transparency paper can be a good alternative to be integrated with optical sensors.
The clinical validations of various already proposed μPADs are required before their adoption in POC settings. It is very important to keep these devices as simple as possible in terms of cost and operation as per guidelines of WHO. Their user interfaces should be simplified. The improvement in their lower limits of detection is the most challenging task of present times. Currently, there are tremendous efforts being carried out to address these challenges. Joint efforts from different fields like material science, chemistry, computer science, nanotechnology, and bioengineering are of utmost importance in this regard [130]. The collaboration between the industry and academia can contribute to broaden the impact of paper-based microfluidic devices. This can be accomplished by initiating bilateral projects, contractual services, consulting arrangements, and public–private partnerships in the field of paper-based microfluidics [131].
7. Conclusion
Cellulose and flexible transparency paper-based assays can provide a promising direction for disease management in resource-constrained settings where current expensive diagnostic assays are not suitable. These portable devices can be very useful in POC testing even in the developed countries. Paper-based devices are inexpensive, easily fabricated, and environmentally friendly. These characteristics make them a desirable option for clinical applications.
This review presents the recent advancements related to the fabrication and sensing mechanisms of μPADs. The challenges associated with these devices are also discussed in detail and future directions are provided. Although various fabrication and sensing techniques have been proposed and tested in laboratories, there is still room for further improvements in specificity, sensitivity, and reliability before the commercialization of such devices and their widespread usage in real-world clinical applications.
8. Expert commentary
μPADs have emerged as a cost-effective solution for disease diagnostics in resource-constrained settings lacking basic health-care facilities [130]. These devices have demonstrated potential to become future health-care options despite a few limitations such as low sensitivity and reproducibility. The performance of paper-based devices must be enhanced to be on par with that of existing health-care technologies. Although various paper-based devices have been proposed, fabricated, and tested by researchers and scientists worldwide, the task of improving sensitivity remains a challenging barrier to commercialization. Further, current paper-based microfluidic devices have limited multiplex detection capabilities. To correctly diagnose a disease, more than one biomarker is often necessary, so it is imperative to improve the multiple detection features of paper-based devices [132].
The integration of smart-phone and lensless imaging with paper-based analytic devices is an invaluable tool for POC disease diagnostics. Smartphones offer many promising features that can help aid rapid disease detection such as data acquisition, processing, analysis, and transmission. Smartphone app development can potentially simplify the whole detection process and make it user-friendly.
Efforts to translate laboratory prototypes of paper-based microfluidic devices to final end products would benefit society as these devices may be used in real-world clinical settings. Industry and academic collaborations to explore new avenues in this field and to commercialize final products would have significant impact in disease diagnostics at the POC settings. The emerging infectious diseases like Ebola and Zika pose a threat to human health. It is necessary to develop new paper-based microfluidic devices to detect and monitor these diseases rapidly from a drop of whole blood, saliva, or urine.
9. Five-year view
We foresee great advances in the field of paper-based micro-fluidics in next five years. These devices will become simpler, cost-effective, easy-to-use, and more efficient as per guidelines of WHO [4]. Efforts will be made to improve the limit-of-detection and sensitivity of these devices. Different kinds of chemically modified electrodes could be fabricated and tested for this purpose.
The integration process of digital microfluidics (DMF) with paper will also potentially accelerate in near future. Further, integration of these devices with smartphones or lensless imaging will enable rapid disease diagnostics in both resource-rich and resource-constrained settings. Clinical validations of already proposed paper devices will be carried out in coming years. μPADs will be a game changer in the field of technology-driven diagnostics.
Key issues.
Cellulose and flexible transparency paper based microfluidic devices have limitations like low limit-of-detection and sensitivity.
Sample retention and evaporation can cause serious issues with the transportation of samples.
Multiplexed detection is another key challenge, which requires detection of multiple analytes in a single assay.
Reagents utilized in paper-based devices should withstand the harsh conditions met during the shipping and transportation process.
Variation in the specificity and sensitivity of these devices needs to be addressed.
Integration of smart phones and lensless imaging with paper-based devices has resulted in easier detection and analysis but it is suggested to further simplify the user interface.
Clinical validations of these paper-based devices is required before their commercialization and widespread adoption in POC settings.
Acknowledgments
The authors would like to thank Chad Coarsey and Benjamin Coleman for reviewing the manuscript and providing invaluable comments and suggestions.
Funding
This manuscript has been supported by research support from NIH R15AI127214 and a start-up research support from College of Engineering and Computer Science, Florida Atlantic University, Boca Raton, FL. Dr. Demirci would like to acknowledge R01 AI093282, R01 GM108584, R01 DE02497101, R01 AI081534, R21 Al113117, R21 Al110277, U54 EB015408, DOD LC150650 11976867 and Canary Center seed grant. This work was funded in part by an NCI-NIH grant to the Center for Cancer Nanotechnology Excellence for Translational Diagnostics (CCNE-TD) at Stanford University.
Footnotes
Declaration of interest
U. Demirci is a founder of, and has an equity interest in: (i) DxNow Inc., a company that is developing microfluidic and imaging technologies for point-of-care diagnostic solutions, and (ii) Koek Biotech, a company that is developing microfluidic IVF technologies for clinical solutions. U. Demerci’s interests were viewed and managed in accordance with the conflict of interest policies. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.World Bank. World development indicators database. Washington, DC: World Bank; 2014. Dec 16, [Google Scholar]
- 2.Safavieh M, Kanakasabapathy MK, Tarlan F, et al. Emerging loop-mediated isothermal amplification-based microchip and microdevice technologies for nucleic acid detection. ACS Biomater Sci Eng. 2016;2:278–294. doi: 10.1021/acsbiomaterials.5b00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asghar W, Yuksekkaya M, Shafiee H, et al. Engineering long shelf life multi-layer biologically active surfaces on microfluidic devices for point of care applications. Sci Rep. 2016;6:21163. doi: 10.1038/srep21163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4•.Mabey D, Peeling RW, Ustianowski A, et al. Tropical infectious diseases: diagnostics for the developing world. Nat Rev Microbiol. 2004;2(3):231–240. doi: 10.1038/nrmicro841. Comprehensive review on infectious disease diagnostic assays in resource-constrained settings. [DOI] [PubMed] [Google Scholar]
- 5•.Lee WG, Kim Y-G, Chung BG, et al. Nano/Microfluidics for diagnosis of infectious diseases in developing countries. Adv Drug Deliv Rev. 2010;62(4–5):449–457. doi: 10.1016/j.addr.2009.11.016. Comprehensive review on the recent advancements of nano/microfluidic technologies for POC disease diagnostics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novak M, Kotanen C, Carrara S, et al. Diagnostic tools and technologies for infectious and non-communicable diseases in low-and-middle-income countries. Health Technol. 2013;3(4):271–281. [Google Scholar]
- 7.Chin CD, Chin SY, Laksanasopin T, et al. Low-cost microdevices for point-of-care testing. In: Issadore D, Westervelt RM, editors. Point-of-care diagnostics on a chip. Berlin, Heidelberg: Springer; 2013. pp. 3–21. [Google Scholar]
- 8.Safavieh M, Coarsey C, Esiobu N, et al. Advances in Candida detection platforms for clinical and point-of-care applications. Crit Rev Biotechnol. 2016:1–18. doi: 10.3109/07388551.2016.1167667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rappa KL, Rodriguez HF, Hakkarainen GC, et al. Sperm processing for advanced reproductive technologies: where are we today? Biotechnol Adv. 2016;34:578–587. doi: 10.1016/j.biotechadv.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Asghar W, El Assal R, Shafiee H, et al. Preserving human cells for regenerative, reproductive, and transfusion medicine. Biotechnol J. 2014;9(7):895–903. doi: 10.1002/biot.201300074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ilyas A, Asghar W, Ahmed S, et al. Electrophysiological analysis of biopsy samples using elasticity as an inherent cell marker for cancer detection. Anal Methods. 2014;6(18):7166–7174. [Google Scholar]
- 12.Asghar W, Velasco V, Kingsley JL, et al. Selection of functional human sperm with higher DNA integrity and fewer reactive oxygen species. Adv Healthc Mater. 2014;3(10):1671–1679. doi: 10.1002/adhm.201400058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.López-Marzo AM, Merkoçi A. Paper-based sensors and assays: a success of the engineering design and the convergence of knowledge areas. Lab Chip. 2016;16(17):3150–3176. doi: 10.1039/c6lc00737f. [DOI] [PubMed] [Google Scholar]
- 14.Meredith NA, Quinn C, Cate DM, et al. Paper-based analytical devices for environmental analysis. Analyst. 2016;141(6):1874–1887. doi: 10.1039/c5an02572a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15••.Martinez AW, Phillips ST, Carrilho E, et al. Simple telemedicine for developing regions: camera phones and paper-based microfluidic devices for real-time, off-site diagnosis. Anal Chem. 2008;80(10):3699–3707. doi: 10.1021/ac800112r. Article presents a prototype for the quantification of assays at POC settings. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16••.Shafiee H, Asghar W, Inci F, et al. Paper and flexible substrates as materials for biosensing platforms to detect multiple biotargets. Sci Rep. 2015;5:8719. doi: 10.1038/srep08719. Article presents paper and various other flexible materials-based platforms with biosensing applications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delaney JL, Hogan CF, Tian J, et al. Electrogenerated chemiluminescence detection in paper-based microfluidic sensors. Anal Chem. 2011;83(4):1300–1306. doi: 10.1021/ac102392t. [DOI] [PubMed] [Google Scholar]
- 18.Free AH, Adams EC, Kercher ML, et al. Simple specific test for urine glucose. Clin Chem. 1957;3(3):163–168. [PubMed] [Google Scholar]
- 19•.Yetisen AK, Akram MS, Lowe CR. Paper-based microfluidic point-of-care diagnostic devices. Lab Chip. 2013;13(12):2210–2251. doi: 10.1039/c3lc50169h. Comprehensive review on paper-based microfluidic devices. [DOI] [PubMed] [Google Scholar]
- 20.Yetisen AK. Holographic sensors. Switzerland: Springer; 2015. Point-of-care diagnostics; pp. 1–25. [Google Scholar]
- 21.Valcárcel M, López-Lorente ÁI. Gold nanoparticles in analytical chemistry. Amsterdam, Netherlands: Elsevier; 2014. [Google Scholar]
- 22.Ngom B, Guo Y, Wang X, et al. Development and application of lateral flow test strip technology for detection of infectious agents and chemical contaminants: a review. Anal Bioanal Chem. 2010;397(3):1113–1135. doi: 10.1007/s00216-010-3661-4. [DOI] [PubMed] [Google Scholar]
- 23.Rivas L, Medina-Sánchez M, De La Escosura-Muñiz A, et al. Improving sensitivity of gold nanoparticle-based lateral flow assays by using wax-printed pillars as delay barriers of microfluidics. Lab Chip. 2014;14(22):4406–4414. doi: 10.1039/c4lc00972j. [DOI] [PubMed] [Google Scholar]
- 24.Wu Y, Xue P, Hui KM, et al. A paper-based microfluidic electrochemical immunodevice integrated with amplification-by-polymerization for the ultrasensitive multiplexed detection of cancer biomarkers. Biosens Bioelectron. 2014;52:180–187. doi: 10.1016/j.bios.2013.08.039. [DOI] [PubMed] [Google Scholar]
- 25.Martinez AW, Phillips ST, Whitesides GM, et al. Diagnostics for the developing world: microfluidic paper-based analytical devices. Anal Chem. 2010;82(1):3–10. doi: 10.1021/ac9013989. [DOI] [PubMed] [Google Scholar]
- 26.Pelton R. Bioactive paper provides a low-cost platform for diagnostics. Trac Trends Anal Chem. 2009;28(8):925–942. doi: 10.1016/j.trac.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez AW, Phillips ST, Butte MJ, et al. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew Chem Int Ed. 2007;46(8):1318–1320. doi: 10.1002/anie.200603817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li XJ, Zhou Y. Microfluidic devices for biomedical applications. Sawston, Cambridge: Elsevier; 2013. [Google Scholar]
- 29.Li X, Tian J, Shen W. Progress in patterned paper sizing for fabrication of paper-based microfluidic sensors. Cellulose. 2010;17(3):649–659. [Google Scholar]
- 30.Whatman® qualitative filter paper, Grade 1. 2016 [cited 2016 Aug 1]. Available from: http://pubs.rsc.org/en/content/articlehtml/2011/lc/c1lc20479c.
- 31.Apilux A, Dungchai W, Siangproh W, et al. Lab-on-paper with dual electrochemical/colorimetric detection for simultaneous determination of gold and iron. Anal Chem. 2010;82(5):1727–1732. doi: 10.1021/ac9022555. [DOI] [PubMed] [Google Scholar]
- 32.Whatman® qualitative filter paper, Grade 4. [cited 2016 Aug 2]. Available from: http://pubs.rsc.org/en/content/articlehtml/2013/lc/c3lc50976a.
- 33•.Li X, Tian J, Garnier G, et al. Fabrication of paper-based microfluidic sensors by printing. Colloids Surf B. 2010;76(2):564–570. doi: 10.1016/j.colsurfb.2009.12.023. Article demonstrates a novel technique for fabrication of μPADs using printing process. [DOI] [PubMed] [Google Scholar]
- 34.Lu Y, Shi W, Jiang L, et al. Rapid prototyping of paper-based microfluidics with wax for low-cost, portable bioassay. Electrophoresis. 2009;30(9):1497–1500. doi: 10.1002/elps.200800563. [DOI] [PubMed] [Google Scholar]
- 35.Kavruk M, Özalp VC, Öktem HA. Portable bioactive paper-based sensor for quantification of pesticides. J Anal Methods Chem. 2013;2013:1–8. doi: 10.1155/2013/932946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arena A, Donato N, Saitta G, et al. Flexible ethanol sensors on glossy paper substrates operating at room temperature. Sens Actuators B Chem. 2010;145(1):488–494. [Google Scholar]
- 37.Martinez AW, Phillips ST, Wiley BJ, et al. FLASH: a rapid method for prototyping paper-based microfluidic devices. Lab Chip. 2008;8(12):2146–2150. doi: 10.1039/b811135a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abe K, Suzuki K, Citterio D. Inkjet-printed microfluidic multianalyte chemical sensing paper. Anal Chem. 2008;80(18):6928–6934. doi: 10.1021/ac800604v. [DOI] [PubMed] [Google Scholar]
- 39.Bruzewicz DA, Reches M, Whitesides GM. Low-cost printing of poly (dimethylsiloxane) barriers to define microchannels in paper. Anal Chem. 2008;80(9):3387–3392. doi: 10.1021/ac702605a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu Y, Shi W, Qin J, et al. Fabrication and characterization of paper-based microfluidics prepared in nitrocellulose membrane by wax printing. Anal Chem. 2010;82(1):329–335. doi: 10.1021/ac9020193. [DOI] [PubMed] [Google Scholar]
- 41.Carrilho E, Martinez AW, Whitesides GM. Understanding wax printing: a simple micropatterning process for paper-based microfluidics. Anal Chem. 2009;81(16):7091–7095. doi: 10.1021/ac901071p. [DOI] [PubMed] [Google Scholar]
- 42.Wang S, Ge L, Song X, et al. Paper-based chemiluminescence ELISA: lab-on-paper based on chitosan modified paper device and wax-screen-printing. Biosens Bioelectron. 2012;31(1):212–218. doi: 10.1016/j.bios.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 43.Songjaroen T, Dungchai W, Chailapakul O, et al. Novel, simple and low-cost alternative method for fabrication of paper-based micro-fluidics by wax dipping. Talanta. 2011;85(5):2587–2593. doi: 10.1016/j.talanta.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 44.Dungchai W, Chailapakul O, Henry CS. A low-cost, simple, and rapid fabrication method for paper-based microfluidics using wax screen-printing. Analyst. 2011;136(1):77–82. doi: 10.1039/c0an00406e. [DOI] [PubMed] [Google Scholar]
- 45.Li X, Tian J, Nguyen T, et al. Paper-based microfluidic devices by plasma treatment. Anal Chem. 2008;80(23):9131–9134. doi: 10.1021/ac801729t. [DOI] [PubMed] [Google Scholar]
- 46.Asghar W, Ramachandran PP, Adewumi A, et al. Rapid nanomanufacturing of metallic break junctions using focused ion beam scratching and electromigration. J Manuf Sci Eng. 2010;132(3):030911. [Google Scholar]
- 47.Li X, Ballerini DR, Shen W. A perspective on paper-based microfluidics: current status and future trends. Biomicrofluidics. 2012;6(1):011301. doi: 10.1063/1.3687398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahmed S, Bui M-PN, Abbas A. Paper-based chemical and biological sensors: engineering aspects. Biosens Bioelectron. 2016;77:249–263. doi: 10.1016/j.bios.2015.09.038. [DOI] [PubMed] [Google Scholar]
- 49.Shafiee H, Kanakasabapathy MK, Juillard F, et al. Printed flexible plastic microchip for viral load measurement through quantitative detection of viruses in plasma and saliva. Sci Rep. 2015;5:9919. doi: 10.1038/srep09919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tokel O, Yildiz UH, Inci F, et al. Portable microfluidic integrated plasmonic platform for pathogen detection. Sci Rep. 2015;5:9152. doi: 10.1038/srep09152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shafiee H, Jahangir M, Inci F, et al. Acute on-chip HIV detection through label-free electrical sensing of viral nanolysate. Small. 2013;9(15):2553–2563. doi: 10.1002/smll.201202195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang S, Tasoglu S, Chen PZ, et al. Micro-a-fluidics ELISA for rapid CD4 cell count at the point-of-care. Sci Rep. 2014;4:3796. doi: 10.1038/srep03796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tasoglu S, Khoory JA, Tekin HC, et al. Levitational image cytometry with temporal resolution. Adv Mater. 2015;27(26):3901–3908. doi: 10.1002/adma.201405660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lucio Do Lago C, Da Silva HDT, Neves CA, et al. A dry process for production of microfluidic devices based on the lamination of laser-printed polyester films. Anal Chem. 2003;75(15):3853–3858. doi: 10.1021/ac034437b. [DOI] [PubMed] [Google Scholar]
- 55.He Y, Wu Y, Fu J-Z, et al. Fabrication of paper-based microfluidic analysis devices: a review. RSC Adv. 2015;5(95):78109–78127. [Google Scholar]
- 56.Kong F-Y, Gu S-X, Li W-W, et al. A paper disk equipped with graphene/polyaniline/Au nanoparticles/glucose oxidase biocompo-site modified screen-printed electrode: toward whole blood glucose determination. Biosens Bioelectron. 2014;56:77–82. doi: 10.1016/j.bios.2013.12.067. [DOI] [PubMed] [Google Scholar]
- 57.Olkkonen J, Lehtinen K, Erho T. Flexographically printed fluidic structures in paper. Anal Chem. 2010;82(24):10246–10250. doi: 10.1021/ac1027066. [DOI] [PubMed] [Google Scholar]
- 58.Kane RS, Takayama S, Ostuni E, et al. Patterning proteins and cells using soft lithography. Biomaterials. 1999;20(23–24):2363–2376. doi: 10.1016/s0142-9612(99)00165-9. [DOI] [PubMed] [Google Scholar]
- 59.Kumar A, Whitesides GM. Features of gold having micrometer to centimeter dimensions can be formed through a combination of stamping with an elastomeric stamp and an alkanethiol “ink” followed by chemical etching. Appl Phys Lett. 1993;63(14):2002–2004. [Google Scholar]
- 60.Xia Y, Whitesides GM. Soft lithography. Annu Rev Mater Sci. 1998;28(1):153–184. [Google Scholar]
- 61.Whitesides GM, Ostuni E, Takayama S, et al. Soft lithography in biology and biochemistry. Annu Rev Biomed Eng. 2001;3(1):335–373. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- 62.Xiang N, Yi H, Chen K, et al. Investigation of the maskless lithography technique for the rapid and cost-effective prototyping of microfluidic devices in laboratories. J Micromech Microeng. 2013;23(2):025016. [Google Scholar]
- 63.Lipomi D, Martinez R, Cademartiri L, et al. 7.11: soft lithographic approaches to nanofabrication. Polym Sci Compr Ref. 2012;10:211–231. [Google Scholar]
- 64.Weibel DB, DiLuzio WR, Whitesides GM. Microfabrication meets microbiology. Nat Rev Microbiol. 2007;5(3):209–218. doi: 10.1038/nrmicro1616. [DOI] [PubMed] [Google Scholar]
- 65.Mahmud MA, Blondeel EJM, Kaddoura M, et al. Creating compact and microscale features in paper-based devices by laser cutting. Analyst. 2016;141(23):6449–6454. doi: 10.1039/c6an02208a. [DOI] [PubMed] [Google Scholar]
- 66.Mani V, Paleja B, Larbi K, et al. Microchip-based ultrafast serodiagnostic assay for tuberculosis. Sci Rep. 2016;6:35845. doi: 10.1038/srep35845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duarte GRM, Price CW, Augustine BH, et al. Dynamic solid phase DNA extraction and PCR amplification in polyester-toner based microchip. Anal Chem. 2011;83(13):5182–5189. doi: 10.1021/ac200292m. [DOI] [PubMed] [Google Scholar]
- 68.Oliveira KA, Damasceno D, De Oliveira CR, et al. Dengue diagnosis on laser printed microzones using smartphone-based detection and multivariate image analysis. Anal Methods. 2016;8(35):6506–6511. [Google Scholar]
- 69.Silva PBM, Oliveiraa KA, Coltro WKT. Colorimetric detection of glucose in biological fluids using toner-based microzone plates. J Braz Chem Soc. 2016;28(1):197–201. [Google Scholar]
- 70.DuVall JA, Le Roux D, Tsuei A-C, et al. A rotationally-driven poly-ethylene terephthalate microdevice with integrated reagent mixing for multiplexed PCR amplification of DNA. Anal Methods. 2016;8(40):7331–7340. [Google Scholar]
- 71.Oliveira KA, De Souza FR, De Oliveira CR, et al. Microfluidic toner-based analytical devices: disposable, lightweight, and portable platforms for point-of-care diagnostics with colorimetric detection. In: Rasooly A, Herold KE, editors. Mobile health technologies: methods and protocols. New York: Springer; 2015. pp. 85–98. [DOI] [PubMed] [Google Scholar]
- 72.Phillips EA, Shen R, Zhao S, et al. Thermally actuated wax valves for paper-fluidic diagnostics. Lab Chip. 2016;16(21):4230–4236. doi: 10.1039/c6lc00945j. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Y, Zhou C, Nie J, et al. Equipment-free quantitative measurement for microfluidic paper-based analytical devices fabricated using the principles of movable-type printing. Anal Chem. 2014;86(4):2005–2012. doi: 10.1021/ac403026c. [DOI] [PubMed] [Google Scholar]
- 74.De Tarso Garcia P, Cardoso TMG, Garcia CD, et al. A handheld stamping process to fabricate microfluidic paper-based analytical devices with chemically modified surface for clinical assays. RSC Adv. 2014;4(71):37637–37644. [Google Scholar]
- 75.Määttänen A, Fors D, Wang S, et al. Paper-based planar reaction arrays for printed diagnostics. Sens Actuators B Chem. 2011;160(1):1404–1412. [Google Scholar]
- 76.Cate DM, Adkins JA, Mettakoonpitak J, et al. Recent developments in paper-based microfluidic devices. Anal Chem. 2015;87(1):19–41. doi: 10.1021/ac503968p. [DOI] [PubMed] [Google Scholar]
- 77.Liana DD, Raguse B, Gooding JJ, et al. Recent advances in paper-based sensors. Sensors. 2012;12(9):11505–11526. doi: 10.3390/s120911505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78••.Martinez AW, Phillips ST, Whitesides GM. Three-dimensional micro-fluidic devices fabricated in layered paper and tape. Proc Natl Acad Sci. 2008;105(50):19606–19611. doi: 10.1073/pnas.0810903105. Article presents fabrication method of a 3-dimensional micro-fluidic device. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rodriguez NM, Wong WS, Liu L, et al. A fully integrated paperfluidic molecular diagnostic chip for the extraction, amplification, and detection of nucleic acids from clinical samples. Lab Chip. 2016;16(4):753–763. doi: 10.1039/c5lc01392e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dungchai W, Chailapakul O, Henry CS. Use of multiple colorimetric indicators for paper-based microfluidic devices. Anal Chim Acta. 2010;674(2):227–233. doi: 10.1016/j.aca.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 81.Evans E, Gabriel EFM, Coltro WKT, et al. Rational selection of substrates to improve color intensity and uniformity on microfluidic paper-based analytical devices. Analyst. 2014;139(9):2127–2132. doi: 10.1039/c4an00230j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang S, Chinnasamy T, Lifson MA, et al. Flexible substrate-based devices for point-of-care diagnostics. Trends Biotechnol. 2016;34(11):909–921. doi: 10.1016/j.tibtech.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Evans E, Gabriel EFM, Benavidez TE, et al. Modification of micro-fluidic paper-based devices with silica nanoparticles. Analyst. 2014;139(21):5560–5567. doi: 10.1039/c4an01147c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Figueredo F, Garcia PT, Cortón E, et al. Enhanced analytical performance of paper microfluidic devices by using Fe3O4 nanoparticles, MWCNT, and graphene oxide. ACS Appl Mater Interfaces. 2016;8(1):11–15. doi: 10.1021/acsami.5b10027. [DOI] [PubMed] [Google Scholar]
- 85.Gabriel EFM, Garcia PT, Cardoso TMG, et al. Highly sensitive colorimetric detection of glucose and uric acid in biological fluids using chitosan-modified paper microfluidic devices. Analyst. 2016;141:4749–4756. doi: 10.1039/c6an00430j. [DOI] [PubMed] [Google Scholar]
- 86.Dungchai W, Chailapakul O, Henry CS. Electrochemical detection for paper-based microfluidics. Anal Chem. 2009;81(14):5821–5826. doi: 10.1021/ac9007573. [DOI] [PubMed] [Google Scholar]
- 87.Flanagan RJ, Perrett D. RSC chromatography monographs, volume 10: electrochemical detection in HPLC: analysis of drugs and poisons. Cambridge (UK): Royal Society of Chemistry; 2005. [Google Scholar]
- 88.Grieshaber D, MacKenzie R, Voeroes J, et al. Electrochemical biosensors-sensor principles and architectures. Sensors. 2008;8(3):1400–1458. doi: 10.3390/s80314000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nemiroski A, Christodouleas DC, Hennek JW, et al. Universal mobile electrochemical detector designed for use in resource-limited applications. Proc Natl Acad Sci. 2014;111(33):11984–11989. doi: 10.1073/pnas.1405679111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Noiphung J, Songjaroen T, Dungchai W, et al. Electrochemical detection of glucose from whole blood using paper-based micro-fluidic devices. Anal Chim Acta. 2013;788:39–45. doi: 10.1016/j.aca.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 91.Parveen S, Aslam MS, Hu L, et al. Electrogenerated chemiluminescence: protocols and applications. Berlin, Heidelberg: Springer; 2013. [Google Scholar]
- 92.Yu J, Wang S, Ge L, et al. A novel chemiluminescence paper microfluidic biosensor based on enzymatic reaction for uric acid determination. Biosens Bioelectron. 2011;26(7):3284–3289. doi: 10.1016/j.bios.2010.12.044. [DOI] [PubMed] [Google Scholar]
- 93.Ge L, Yan J, Song X, et al. Three-dimensional paper-based electro-chemiluminescence immunodevice for multiplexed measurement of biomarkers and point-of-care testing. Biomaterials. 2012;33(4):1024–1031. doi: 10.1016/j.biomaterials.2011.10.065. [DOI] [PubMed] [Google Scholar]
- 94.Parveen S, Aslam MS, Hu L, et al. Electrogenerated chemiluminescence. Berlin Heidelberg: Springer; 2013. Applications of electrochemiluminescence; pp. 123–152. [Google Scholar]
- 95.Yan J, Ge L, Song X, et al. Paper-based electrochemiluminescent 3D immunodevice for lab-on-paper, specific, and sensitive point-of-care testing. Chem Eur J. 2012;18(16):4938–4945. doi: 10.1002/chem.201102855. [DOI] [PubMed] [Google Scholar]
- 96.Pires NMM, Dong T, Hanke U, et al. Recent developments in optical detection technologies in lab-on-a-chip devices for biosensing applications. Sensors. 2014;14(8):15458–15479. doi: 10.3390/s140815458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Terai T, Nagano T. Small-molecule fluorophores and fluorescent probes for bioimaging. Pflugers Arch. 2013;465(3):347–359. doi: 10.1007/s00424-013-1234-z. [DOI] [PubMed] [Google Scholar]
- 98.Ali MM, Aguirre SD, Xu Y, et al. Detection of DNA using bioactive paper strips. Chem Commun. 2009;(43):6640–6642. doi: 10.1039/b911559e. [DOI] [PubMed] [Google Scholar]
- 99.Yamada K, Takaki S, Suzuki K, et al. Microfluidic paper-based analytical device for fluorescence detection of lactoferrin in tear fluid. Chemical and biological microsystems society; 17th International Conference on Miniaturized Systems for Chemistry and Life Sciences; 2013 Oct 27–31; Freiburg, German. [Google Scholar]
- 100.Islam M, Asghar W, Young-Tae K, et al. Cell elasticity-based micro-fluidic label-free isolation of metastatic tumor cells. Br J Med Med Res. 2014;4(11):2129. [Google Scholar]
- 101.Asghar W, Shafiee H, Chen P, et al. In vitro three-dimensional cancer culture models. In: Bae YH, Mrsny RJ, Park K, editors. Cancer targeted drug delivery. New York: Springer; 2013. pp. 635–665. [Google Scholar]
- 102.Iqbal SM, Asghar W, Vidyala SD. Porous organic nanolayers for coating of solid-state devices. J Nanobiotechnology. 2011;9(1):18. doi: 10.1186/1477-3155-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ilyas A, Asghar W, Kim Y-T, et al. Parallel recognition of cancer cells using an addressable array of solid-state micropores. Biosens Bioelectron. 2014;62:343–349. doi: 10.1016/j.bios.2014.06.048. [DOI] [PubMed] [Google Scholar]
- 104.Asghar W, El Assal R, Shafiee H, et al. Engineering cancer micro-environments for in vitro 3-D tumor models. Mater Today. 2015;18(10):539–553. doi: 10.1016/j.mattod.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ilyas A, Asghar W, Allen PB, et al. Electrical detection of cancer biomarker using aptamers with nanogap break-junctions. Nanotechnology. 2012;23(27):275502. doi: 10.1088/0957-4484/23/27/275502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Asghar W, Wan Y, Ilyas A, et al. Electrical fingerprinting, 3D profiling and detection of tumor cells with solid-state micropores. Lab Chip. 2012;12(13):2345–2352. doi: 10.1039/c2lc21012f. [DOI] [PubMed] [Google Scholar]
- 107.Wang L, Asghar W, Demirci U, et al. Nanostructured substrates for isolation of circulating tumor cells. Nano Today. 2013;8(4):374–387. doi: 10.1016/j.nantod.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wan Y, Tan J, Asghar W, et al. Velocity effect on aptamer-based circulating tumor cell isolation in microfluidic devices. J Phys Chem B. 2011;115(47):13891–13896. doi: 10.1021/jp205511m. [DOI] [PubMed] [Google Scholar]
- 109.Tasoglu S, Gurkan UA, Wang S, et al. Manipulating biological agents and cells in micro-scale volumes for applications in medicine. Chem Soc Rev. 2013;42(13):5788–5808. doi: 10.1039/c3cs60042d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Asghar W, Shafiee H, Velasco V, et al. Toxicology study of single-walled carbon nanotubes and reduced graphene oxide in human sperm. Sci Rep. 2016;6:30270. doi: 10.1038/srep30270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Asghar W, Ilyas A, Billo JA, et al. Shrinking of solid-state nanopores by direct thermal heating. Nanoscale Res Lett. 2011;6(1):1–6. doi: 10.1186/1556-276X-6-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ramachandran A, Liu Y, Asghar W, et al. Characterization of DNA-nanopore interactions by molecular dynamics. Am J Biomed Sci. 2009;1(4):344–351. [Google Scholar]
- 113.Asghar W, Ilyas A, Deshmukh RR, et al. Pulsed plasma polymerization for controlling shrinkage and surface composition of nano-pores. Nanotechnology. 2011;22(28):285304. doi: 10.1088/0957-4484/22/28/285304. [DOI] [PubMed] [Google Scholar]
- 114.Hafeez A, Asghar W, Rafique MM, et al. GPU-based real-time detection and analysis of biological targets using solid-state nanopores. Med Biol Eng Comput. 2012;50(6):605–615. doi: 10.1007/s11517-012-0893-9. [DOI] [PubMed] [Google Scholar]
- 115.Billo JA, Asghar W, Iqbal SM. SPIE defense, security, and sensing. International Society for Optics and Photonics; 2011. An implementation for the detection and analysis of negative peaks in an applied current signal across a silicon nanopore; pp. 80312T–80312T–80317. [Google Scholar]
- 116.Billo JA, Jones J, Asghar W, et al. Viscosity and surface-free energy effects in thermal shrinking of solid-state nanopores. Appl Phys Lett. 2012;100(23):233107. [Google Scholar]
- 117.Marle L, Greenway GM. Microfluidic devices for environmental monitoring. Trac Trends Anal Chem. 2005;24(9):795–802. [Google Scholar]
- 118.Skurtys O, Aguilera J. Applications of microfluidic devices in food engineering. Food Biophys. 2008;3(1):1–15. [Google Scholar]
- 119.Xia Y, Si J, Li Z. Fabrication techniques for microfluidic paper-based analytical devices and their applications for biological testing: a review. Biosens Bioelectron. 2016;77:774–789. doi: 10.1016/j.bios.2015.10.032. [DOI] [PubMed] [Google Scholar]
- 120.Walji N. Characterization of fluid flow in paper-based microfluidic devices. Ontario, Canada: University of Ontario Institute of Technology; 2015. [Google Scholar]
- 121.Hu J, Wang S, Wang L, et al. Advances in paper-based point-of-care diagnostics. Biosens Bioelectron. 2014;54:585–597. doi: 10.1016/j.bios.2013.10.075. [DOI] [PubMed] [Google Scholar]
- 122.Fobel R, Kirby AE, Ng AHC, et al. Paper microfluidics goes digital. Adv Mater. 2014;26(18):2838–2843. doi: 10.1002/adma.201305168. [DOI] [PubMed] [Google Scholar]
- 123.Sobieranski AC, Inci F, Tekin HC, et al. Portable lensless wide-field microscopy imaging platform based on digital inline holography and multi-frame pixel super-resolution. Light: Sci Appl. 2015;4(10):e346. doi: 10.1038/lsa.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang S, Zhao X, Khimji I, et al. Integration of cell phone imaging with microchip ELISA to detect ovarian cancer HE4 biomarker in urine at the point-of-care. Lab Chip. 2011;11(20):3411–3418. doi: 10.1039/c1lc20479c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.6.1B smartphone users globally by 2020, overtaking basic fixed phone subscriptions. [cited 2016 Apr 15]. Available from: http://aip.scitation.org/doi/abs/10.1063/1.4725515.
- 126.San Park T, Li W, McCracken KE, et al. Smartphone quantifies Salmonella from paper microfluidics. Lab Chip. 2013;13(24):4832–4840. doi: 10.1039/c3lc50976a. [DOI] [PubMed] [Google Scholar]
- 127.Ozcan A, Demirci U. Ultra wide-field lens-free monitoring of cells on-chip. Lab Chip. 2008;8(1):98–106. doi: 10.1039/b713695a. [DOI] [PubMed] [Google Scholar]
- 128.Li M, Diamandis EP. Technology-driven diagnostics: from smart doctor to smartphone. Crit Rev Clin Lab Sci. 2016;53(4):1–35. doi: 10.3109/10408363.2016.1149689. [DOI] [PubMed] [Google Scholar]
- 129.Ng AHC, Wheeler AR. Next-generation microfluidic point-of-care diagnostics. Clin Chem. 2015;61(10):1233–1234. doi: 10.1373/clinchem.2015.240226. [DOI] [PubMed] [Google Scholar]
- 130••.Wang S, Lifson MA, Inci F, et al. Advances in addressing technical challenges of point-of-care diagnostics in resource-limited settings. Expert Rev Mol Diagn. 2016;16(4):449–459. doi: 10.1586/14737159.2016.1142877. Article presents technical challenges associated with POC diagnostic devices in resource-constrained settings. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pronk JT, Lee SY, Lievense J, et al. How to set up collaborations between academia and industrial biotech companies. Nat Biotechnol. 2015;33(3):237–240. doi: 10.1038/nbt.3171. [DOI] [PubMed] [Google Scholar]
- 132.Shah P, Zhu X, Li C-Z. Development of paper-based analytical kit for point-of-care testing. Expert Rev Mol Diagn. 2013;13(1):83–91. doi: 10.1586/erm.12.130. [DOI] [PubMed] [Google Scholar]