Abstract
Background
As support times for left ventricular assist devices (LVADs) become longer, several complications requiring device exchange may occur. To our knowledge, this is the first Canadian report regarding implantable LVAD exchange.
Methods
We retrospectively reviewed the cases of consecutive, unique patients implanted with an LVAD between June 2006 and October 2015 at Toronto General Hospital.
Results
In total, 122 patients were impanted with an LVAD during the study period. Eight patients required LVAD exchange, and 1 patient had 2 replacements (9 of 122, 7.3%). There were 7 HeartMate II (HMII), 1 HVAD and 1 DuraHeart pumps exchanged. Two of these exchanges occurred early at the time of initial implant, whereas 7 occurred late (range 8–623 d). Six exchanges were made owing to pump thrombosis. Of the 3 exchanges made for other causes, 1 HMII exchange was owing to a driveline fracture, 1 DuraHeart patient had early inflow obstruction requiring exchange to HMII at the initial implant, and the third had a suspected inflow obstruction with no evidence of thrombosis at the time of the procedure. The mean support time before exchange was 225 days, and time from exchange to transplant, death or ongoing support was 245 days. Three patients were successfully bridged to transplant, and at the time of data collection 2 were supported awaiting transplant. Three patients died after a mean duration of 394.3 days (range 78–673 d) of support postreplacement. Four cases were successfully performed using a subcostal approach.
Conclusion
Pump thrombosis is the most common cause for LVAD exchange, which can be performed with acceptable morbidity and mortality. The subcostal approach may be the preferred procedure for an HMII exchange when indicated.
Abstract
Contexte
À mesure que la durée d’utilisation des dispositifs d’assistance ventriculaire gauche (DAVG) augmente, plusieurs complications nécessitant un remplacement du dispositif peuvent survenir. À notre connaissance, il s’agit du premier rapport canadien concernant le remplacement des DAVG implantables.
Méthodes
Nous avons passé en revue de manière rétrospective les cas individuels consécutifs de patients à qui on a implanté un DAVG entre juin 2006 et octobre 2015 à l’Hôpital Général de Toronto.
Résultats
En tout, 122 patients ont reçu un DAVG pendant la période de l’étude. Huit patients ont eu besoin d’un remplacement de DAVG et 1 patient a eu besoin de 2 remplacements (9 sur 122, 7,3 %). Sept dispositifs HeartMate II (HMII), 1 dispositif HVAD et 1 dispositif DuraHeart ont été remplacés. Deux de ces remplacements sont survenus peu de temps après la pose initiale du dispositif, tandis que les 7 autres se sont produits plus tardivement (dans les 8 à 623 jours suivants). Six remplacements ont été effectués en raison d’une thrombose de la pompe. Parmi les 3 remplacements effectués pour d’autres raisons, 1 dispositif HMII a été remplacé en raison d’un bris de la ligne d’activation, 1 dispositif DuraHeart a présenté une obstruction précoce du flux entrant nécessitant la pose d’un HMII dès l’implantation initiale, et le troisième présentait une obstruction présumée du flux entrant sans signe de thrombose au moment de l’intervention. La durée moyenne d’utilisation avant le remplacement du dispositif a été de 225 jours, et l’intervalle entre le remplacement et la transplantation, le décès ou la décision de maintenir l’assistance a été de 245 jours. L’appareil a permis une transition réussie jusqu’à la transplantation chez 3 patients, et au moment de la collecte des données, 2 patients porteurs d’un DAVG étaient en attente d’une transplantation. Trois patients sont décédés après une durée moyenne de 394,3 jours (entre 78 et 673 jours) d’assistance post-remplacement. Quatre remplacements ont été effectués avec succès par une approche sous-costale.
Conclusion
La thrombose de la pompe est la cause la plus fréquente de remplacement d’un DAVG; le remplacement peut être effectué avec des taux de morbidité et de mortalité acceptables. L’approche sous-costale serait à privilégier lorsqu’un remplacement de HMII est indiqué.
Left ventricular assist devices (LVAD) have become an important tool to manage refractory heart failure. In North America, the numbers of implants have substantially increased over the past decade.1–3 With their approval for use as destination therapy (DT) and the improvement in mechanical durability of the newer devices, average support times have surpassed 12 months,1,3,4 and device exchange owing to complications will inevitably be required for a variety of indications.5,6
Although uncommon, device malfunction requiring replacement is a serious and potentially fatal complication. 2,5 Several risk factors have been identified, such as the use of first-generation devices, obesity, older age and renal failure.1,7 To our knowledge, no Canadian centre has reported on their experience with these cases, thus far. Therefore, we present our experience with device malfunction leading to exchange in a consecutive series of implantable, continuous flow LVAD recipients.
Methods
This study was reviewed and approved by the research ethics board of Toronto General Hospital, Toronto, Canada. We reviewed the clinical records of consecutive patients who underwent LVAD implantation at our institution between June 2006 and October 2015 to identify those who had required device exchange. We collected detailed information on these selected individuals, including demographic characteristics, initial device implanted, time to device failure, replacement devices, total support time and outcomes. Cases of temporary paracorporeal LVADs were not included.
Results
From June 2006 to October 2015, 122 consecutive patients underwent LVAD implantation at our instution. We identified 8 patients who required a total of 9 LVAD exchanges (1 patient required 2 device exchanges). Demographic characteristics of the patients are shown in Table 1. Two patients were women, and the overall mean age was 54 years (range 39–66 years). Mean body mass index (BMI) and body surface area (BSA) were 25 (range 21.2–29.4), and 1.91 m2 (range 1.65–2.23 m2), respectively. All patients had a previous automatic implantable cardiac defibrillator (AICD) implants, and 3 patients had prior coronary artery bypass surgery. With respect to the etiology of their heart failure syndrome, 4 patients had ischemic cardiomyopathy, 3 had dilated cardiomyopathy and 1 had hypertrophic cardiomyopathy.
Table 1.
Patient characteristics before the first LVAD implantation
| Patient | Age, yr | Sex | Height, cm | Weight, kg | BMI | BSA, m2 | DM | HTN | HL | Cr, μmol/L | Etiology | Previous cardiac surgery | Clinical presentation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 45 | M | 183 | 76 | 22.7 | 1.96 | 86 | Ischemic | CABG, AICD | DL fracture | |||
| 2 | 48 | F | 173 | 68 | 22.7 | 1.8 | 123 | DCM | AICD | Poor flows | |||
| 3 | 61 | M | 175 | 79 | 25.8 | 1.95 | + | + | + | 101 | DCM | AICD | First: poor flows; second: HF |
| 4 | 66 | M | 163 | 60 | 22.6 | 1.65 | + | + | 117 | Ischemic | CABG, AICD | Hemolysis | |
| 5 | 63 | M | 165 | 78.9 | 29 | 1.9 | + | + | + | 98 | Ischemic | CABG, AICD | Hemolysis and HF |
| 6 | 39 | M | 183 | 98.5 | 29.4 | 2.23 | 95 | DCM | AICD | RV infarct and peripheral embolism | |||
| 7 | 46 | M | 183 | 89 | 26.6 | 2.1 | 132 | HCM | Maze, AICD | Hemolysis | |||
| 8 | 61 | F | 170 | 61.4 | 21.2 | 1.7 | + | + | 147 | Ischemic | AICD | Hemolysis and AKI |
AICD = automatic implantable cardioverter-defibrillator; AKI = acute kidney injury; BMI = body mass index; BSA = body surface area; CABG = coronary artery bypass graft; Cr = creatinine; DCM = dilated cardiomyopathy; DL = driveline; DM = diabetes mellittus; F = female; HCM = hypertrophic cardiomyopathy; HF = heart failure; HL = hyperlipidemia; HTN = hypertension; LVAD = left ventricular assist device; M = male; RV = right ventricle.
Table 2 lists the initial device, time to device failure, replacement device, total support time and outcomes for all 8 patients. Excluding the 2 cases of immediate device replacement, the mean time of support to exchange was 225 days, and mean time from exchange to any outcome was 245 days. Three patients were successfully bridged to transplant, and at the time of data collection 2 were currently supported awaiting transplant. Three patients in this series died due to multisystem organ failure (day 17), sepsis (day 673) or recurrent gastrointestinal bleeding (day 432), after device replacement.
Table 2.
Perioperative and postoperative outcomes for patients undergoing LVAD exchange
| Patient | Status | Strategy | Exchange indication | Device | Support, d | Outcome | Approach | Clamp time, min | Pump time, min | ICU stay, d | Hospital stay, d |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Elective | BTC | HMII | 389 | 0 | 74 | 2 | 11 | |||
| Urgent | BTT | DL fracture | HMII | 144 | Transplant | Sternotomy | 0 | 40 | 6 | 12 | |
| 2 | Elective | BTC | HVAD | 0 | 0 | 66 | |||||
| Urgent | BTC | Pump thrombus | HVAD | 17 | Expired | Sternotomy | 0 | 22 | 17 | 17 | |
| 3 | Elective | BTC | Dura-Heart | 0 | |||||||
| BTC | Inflow obstruction | HMII | 225 | Sternotomy | 0 | 156 | 36 | 48 | |||
| Urgent | BTT | Pump thrombus | HMII | 516 | Transplant | Subcostal | 0 | 20 | 4 | 11 | |
| 4 | Elective | DT | HMII | 69 | 0 | 64 | 5 | 147 | |||
| Elective | DT | Pump thrombus | HMII | 673 | Expired | Sternotomy | 0 | 37 | 25 | 77 | |
| 5 | Elective | BTC | HMII | 136 | 0 | 76 | 15 | 38 | |||
| Urgent | BTC | Pump thrombus | HMII | 437 | Expired | Subcostal | 0 | 47 | 3 | 14 | |
| 6 | Urgent | BTT | HMII | 8 | 0 | 112 | 48 | 56 | |||
| Urgent | BTT | Pump thrombus | HMII | 37 | Transplant | Sternotomy | 0 | 85 | 40 | 48 | |
| 7 | Urgent | BTC | HMII | 126 | 0 | 82 | 41 | 92 | |||
| Elective | BTT | Pump thrombus | HMII | 119 | Supported | Subcostal | 0 | 30 | 13 | 24 | |
| 8 | Elective | BTC | HMII | 623 | 11 | 114 | 12 | 36 | |||
| Elective | BTC | Pump thrombus | HMII | 21 | Supported | Subcostal | 0 | 49 | 1 | 9 |
BTC = bridge to candidacy; BTT = bridge to transplantation; DT = destination therapy; HMII = HeartMate II ventricular assist device; HVAD = HeartWare ventricular assist device; ICU = intesive care unit; LVAD = left ventricular assist device.
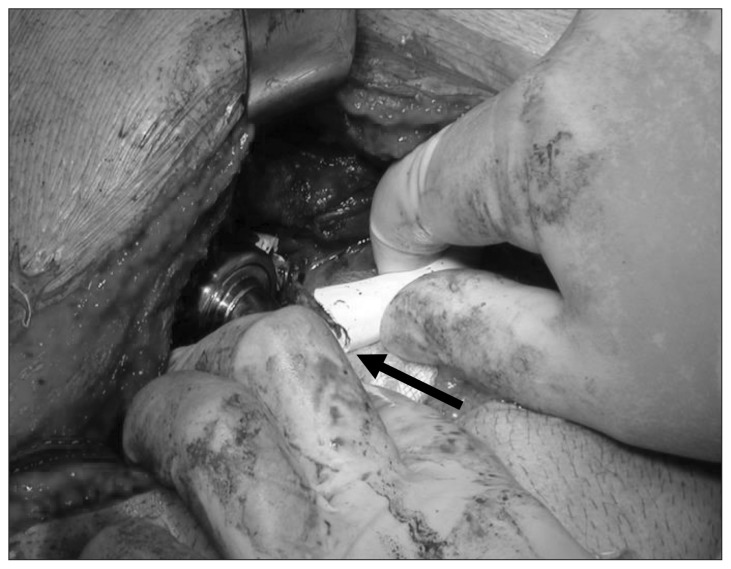
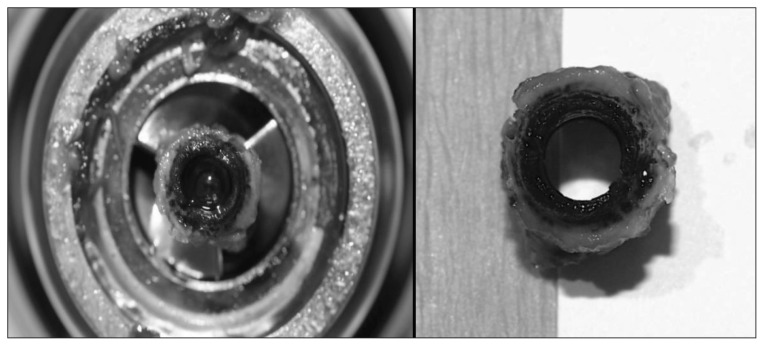
The first patient in our series presented emergently with a driveline fracture (Fig. 1) and underwent successful exchange as a bridge to heart/lung transplant, which was performed 5 months later. Two additional patients displayed early device malfunction on the day of implant, necessitating device exchange. Both presented with poor pump flow in the immediate perioperative period. The HeartWare HVAD device displayed abnormal waveforms that failed to normalize with time; therefore, mechanical entrainment of particulate matter within the pump housing was suspected. An initial DuraHeart device was explanted owing to poor flow and a suspected inflow cannula obstruction. The device was exchanged successfully for a HeartMate II (HMII) device. Interestingly, this same patient presented 225 days later with decompensated heart failure and suspected pump thrombus (Fig. 2) of his HMII and underwent successful subcostal device exchange.
Fig. 1.
Driveline fracture (arrow).
Fig. 2.
HeartMate II inlet bearing thrombus.
Four patients presented with biochemical and hematological evidence of hemolysis and pump thrombosis; 3 were subacute presentations admitted from the clinic for investigation and 1 presented to the emergency department with decompensated heart failure. They underwent ramp echocardiography studies, with 2 showing results compatible with pump obstruction, such as left ventricular dilation despite increasing pump speeds. The other 2 exams were inconclusive. All 4 patients underwent successful pump exchange. One patient presented with peripheral embolism, right ventricular infarct and biochemical evidence of hemolysis 1 week after implant, requiring exchange of the device. He was successfully transplanted 2 months later.
Table 3 outlines the parameters on the pumps at the time of exchange. There was great variability in the data, and device interrogation did not always reveal pump malfunction. A high index of suspicion, abnormal biochemistry and an echocardiogram demonstrating inadequate pump performance (e.g., poor LV decompression, persistent aortic valve opening) led to the diagnosis of pump thrombus. Indirect factors, such as patient frailty, prognosis and life expectancy, were discussed on a case-by-case basis to facilitate the decision-making process.
Table 3.
LVAD parameters at the moment of exchange
| Patient | Speed, RPM | Power, W | PI | Flow, L/min | Hemodynamics |
|---|---|---|---|---|---|
| 1 | Acute pump malfunction and stoppage | NA | NA | NA | Unstable |
| 2 | * | High | * | High flows | Unstable |
| 3 (first) | NA | NA | NA | Poor flows intra-op | Unstable |
| 3 (second) | * | * | * | Poor flows through inflow cannula and LVAD | Stable |
| 4 | 8400 | * | * | Good flows | Stable |
| 5 | 8800 | 5.1 | 7.5 | * | Stable |
| 6 | 8600 | 5.3 with intermittent drops to 1.5 | 5.4 | Good flows | Stable |
| 7 | 9000 | * | * | Good flows | Stable |
| 8 | 9900 | 6.5 | 6.1 | * | Stable |
LVAD = ventricular assist device; NA = not applicable; PI = pulsatility index; RPM = rotations per minute.
Data not recovered.
The average international normalized ratio (INR), percentage of time below target INR, platelet count, creatinine and peak lactate dehydrogenase (LDH) level between the first implant and exchange are described in Table 4. Of the 6 patients who had confirmed pump thrombosis, 2 patients had an average INR below 2. The remaining 4 patients demonstrated an average INR slightly above 2 (range 2–2.25). As per protocol, all patients received antiplatelet therapy as well. Platelet counts in the cases of thrombosis were below 200 000/mm3, except in 1 case. Notably high levels of LDH were detected in all of our cases of late pump thrombosis.
Table 4.
Mean INR, platelet count and peak LDH before LVAD exchange
| Patient | INR, mean ± SD | INR below target, % of time | Platelets, mean ± SD, billion/L | Peak LDH, U/L |
|---|---|---|---|---|
| 1 | 1.93 ± 0.49 | 79 | 276.5 ± 14.8 | 521 |
| 2 | NA | NA | 129.9 ± 14.6 | 380 |
| 3 | 2.09 ± 0.86 | 66 | 168.3 ± 23.1 | 3333 |
| 4 | 2.25 ± 0.48 | 7 | 186.3 ± 38.4 | 2926 |
| 5 | 2.24 ± 0.76 | 33 | 334.6 ± 41.1 | 2768 |
| 6 | 2 ± 0.59 | NA | 170.7 ± 64 | 539 |
| 7 | 1.62 ± 0.44 | 83 | 185 ± 19.4 | 4164 |
| 8 | 2.05 ± 0.49 | 44 | 195.6 ± 55.5 | 3179 |
INR = international normalized ratio; LDH = lactate dehydrogenase; LVAD = left ventricular assist device; NA = not applicable; SD = standard deviation.
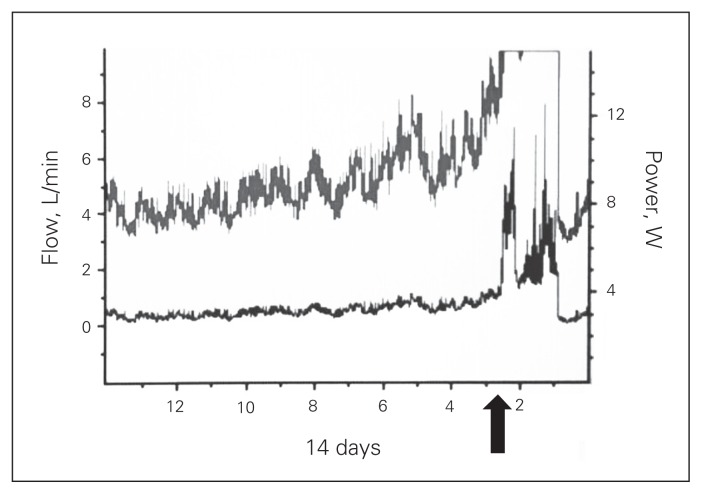
Throughout our program’s experience, there were 2 patients with established diagnosis of pump thrombosis not treated with LVAD exchange. The first patient presented after 2 years of HVAD support as a bridge to candidacy with hematuria, LVAD alarms indicative of device thrombosis and elevated LDH. She was treated with thrombolytic therapy, with dramatic improvement of pump flows (Fig. 3). Notably, the patient was on suppressive antibiotic therapy for chronic driveline infection. Early during recovery, ischemic colitis and lower gastrointestinal bleed developed, requiring a laparotomy. Afterwards, she demonstrated favourable progress and currently remains supported by her device.
Fig. 3.
HeartWare HVAD flow before and after thrombolytic therapy.
The second patient had an HMII implanted as DT. After 2 years of support, he presented with recurrent transient ischemic attacks, LDH elevation and hematuria. He was on warfarin and acetylsalicylic acid (ASA) therapy, had an INR above 2 and was extensively investigated with repeated echocardiography, laboratory and clinical assessments. Owing to the reassuring nature of the clinical scenario, the team opted for conservative treatment, increasing his target INR and adding clopidogrel to his antiplatelet regimen. He continues to be closely followed in clinic and has not presented with worsening symptoms 4 years after initial implant.
Of the 6 “late” exchanges in our series, 4 were successfully performed using a subcostal approach, avoiding resternotomy. The reasons for resternotomy were suspicion of device infection in 1 patient (case 4) and the need for concomitant right ventricular assist device implantation in another patient (case 6). This patient presented to us with right ventricular infarct and peripheral embolization. There were no significant differences in cardiopulmonary bypass time (46 ± 27.2 min v. 36.5 ± 13.9 min), length of stay in the intensive care unit (ICU; 22 ± 14.3 d v. 5.3 ± 5.3 d) or hospital stay (38.5 ± 30.2 d v. 14.5 ± 6.7 d) between patients undergoing resternotomy versus subcostal approach, respectively (Table 5).
Table 5.
Comparison between sternotomy and subcostal approaches
| Technique | Outcome, no. (%) | Outcome, mean ± SD | |||
|---|---|---|---|---|---|
|
|
|
||||
| Thrombosis | HMII exchanged | Pump time, min | ICU stay, d | Hospital stay, d | |
| Sternotomy | 4 of 5 (80) | 3 of 5 (60) | 46 ± 27.2 | 22 ± 14.3 | 38.5 ± 30.2 |
|
| |||||
| Subcostal | 4 of 4 (100) | 4 of 4 (100) | 36.5 ± 13.9 | 5.3 ± 5.3 | 14.5 ± 6.7 |
HMII = HeartMate II; ICU = intensive care unit; SD = standard deviation.
The overall mortality of our series was 33% (n = 3). Although apparently high, this may simply reflect the limited number of cases thus far. Notably, death within 30 days of pump exchange occurred in only 1 patient (case 2).
Discussion
To our knowledge, we have described the first Canadian single-centre experience with LVAD exchange. Device complication requiring exchange occurs at a relatively low frequency; our series has shown that 7.3% of patients required LVAD replacement, and this is compatible with the overall results from larger studies, such as the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS).1,6,8,9 However, the incidence of device complications requiring replacement appears to be rising, especially due to pump thrombosis. Kirklin and colleagues,2 in the HMII thrombosis substudy of the INTERMACS registry, have shown that there has been an increase in pump exchange due to thrombosis at 6 months starting in 2012. Starling and colleagues10 also found an increase in the incidence in pump thrombosis, especially in patients who underwent their first implantation more recently. Presumably, the realization of elevated LDH as a marker of pump thrombosis before pump malfunction has led to the higher detection rates.11 Our study corroborates the existing literature by showing that the main etiology for device exchange in continuous flow pumps is thrombosis. Six of our 9 exchanges were performed for suspected and subsequently confirmed pump thrombosis. Although previous publications have demonstrated that approximately 50% of replacements are secondary to this complication, 2,3,5,6,8,10,12 our higher incidence may simply be a reflection of our limited number of cases thus far. The reason for this increase in pump thrombosis in recent years is unclear. The major risk is most probably an inadequate anticoagulation strategy. A consensus target INR has not been determined for the LVAD population. 2 Slaughter and colleagues13 have shown that patients transitioned directly to warfarin and ASA therapy post-LVAD implant without heparin bridging had no increase in short-term thrombotic events. Additionally, Boyle and colleagues14 also demonstrated the long-term safety of maintaining this population’s INR between 1.5 and 2.5 in addition to ASA therapy. These 2 papers were published in 2010 and 2009, respectively. Interestingly, the peak incidence of pump thrombosis occurred in 2012, likely reflecting the delayed effect of a reduced anticoagulation strategy.
Our institution’s protocol includes oral anticoagulation therapy for all patients undergoing LVAD implantation, including an initial bridging period with heparin until adequate INR levels have been reached. Shortly after the report by Slaughter and colleagues,13 we also dispensed with heparin bridging owing to observed cases of late pericardial effusions. However, with the recent reports of pump thrombus in the HMII population, we have once again reinstituted heparin bridging starting on the first postoperative day. We reviewed the coagulation studies from our set of patients and found that we also had trouble maintaining therapeutic anticoagulation, despite a constant target INR of 2–2.5. All patients showed a very unstable INR, with results varying around 2 (above and below). This once again corroborates with the hypothesis that we may need to be more aggressive when controlling anticoagulation in this population.2
The diagnosis of pump thrombosis is not always straightforward. The typical presentation includes increased pump power, hemolysis or recurrent decompensated heart failure. Among others, LDH levels appear to be a good indicator of thrombosis, as seen in all our cases of late thrombosis. However, in the case of early thrombosis, the increase of LDH (case 6) was not significant. In this case, the patient presented with recurrent heart failure, and hemodynamic instability gradually developed. Despite having LDH levels within postoperative norms, there was a high suspicion for pump thrombosis, and thrombus was in fact detected in the outflow cannula during the second operation. Echocardiography cannot evaluate the pump directly; however, it may be beneficial to detect indirect evidence of thrombosis, such as left ventricular dilatation, consistent opening of the aortic valve during systole despite high pump speeds and a shift of the interventricular septum to the right.15 As noted previously, we routinely performed echocardiography and ramp studies in our subacute presenting patients with suspected thrombosis.
The role of computed tomography in this case is to allow the evaluation of areas not well visualized by the echocardiography, such as outflow cannula position and extracardiac features. However, this modality has the disadvantage of not being able to evaluate flow. Accordingly, this modality has proven its usefulness in cases requiring more accurate visualization, as in suspected left ventricular thrombus formation and device infection.16
Canadian health care, as a government-funded service, invariably presents funding limitations. This limits the number of devices we are able to implant, and we are required to carefully select the patients who would derive the most benefit from the intervention. Although medical therapy with intensified anticoagulation (including the use of thrombolytics) has been reported, we and others continue to believe that device exchange is the preferred management strategy for suspected and/or proven pump thrombus.11 As mentioned above, indirect factors, such as patient frailty and overall prognosis, should also be discussed in each case and considered when making therapeutic decisions.
In regard to the incidence of pump thrombosis across the different LVAD models, the present series demonstrated 6 cases of thrombosis out of 82 implanted HMII devices (7.3%) and 1 case out of 31 implanted HVAD devices (3.2%). Of the 4 DuraHeart devices implanted during the study period, 1 required exchange owing to obstruction of the inflow cannula. Despite the limited number of cases, there does not appear to be a difference in thrombosis rates among these devices. This is in accordance with the current literature on continuous flow
We have used the subcostal approach for exchanging an HMII when feasible. We do not reverse anticoagulation with fresh frozen plasma or vitamin K for this approach. It may be advantageous to keep the INR in the therapeutic range right after device exchange for patients with pump thrombosis. Ota and colleagues9 described several benefits of a subcostal approach for exchanging an HMII, including less postoperative bleeding, less blood transfusion requirement, decreased time to extubation and decreased overall length of stay in the ICU. In our experience, time on cardiopulmonary bypass, length of stay in the ICU and hospital stay were comparatively shorter with the subcostal approach than with resternotomy. However, the small number of cases precluded this finding from achieving statistical significance. Further experience may corroborate a statistically significant advantage of the subcostal approach.
Limitations
Our study has some clear limitations, starting with the number of cases we presented. We are not able at this point to proceed with an adequate statistical analysis of our findings. Further studies are needed, possibly combining data from other Canadian LVAD programs, allowing for adequate comparisons with our population of patients who did not require device replacement and exploring the long-term outcomes of these individuals. We would like to emphasize that, at this point, meaningful conclusions are difficult to make and apply to other programs. However, given the ethical considerations of pump exchange in jurisdictions where funding is limited or even absent, as in the Canadian setting, we believe our results to be clinically important and, as we have described, at least comparable to the large North American registries. Even with this relatively small series, and in accordance with previous literature, device exchange is a safe and effective method of managing suspected pump thrombus, being the preferred treatment option in our program.
Conclusion
To our knowledge, this is the first described Canadian experience with LVAD exchange. We confirm that pump thrombosis is by far the most important cause for device exchange in the new-generation devices, raising the issue of whether our current anticoagulation strategy is adequate. Also, despite the high-risk population, device exchange can be performed with acceptable morbidity and mortality. A less invasive subcostal approach has proven to be safe with reproducible results.
Footnotes
Competing interests: T. Yau is consultant with St. Jude Medical, which manufactures the HeartMate devices, and HeartWare Inc. He discloses speaker fees and education grants from these companies within the last two years. V. Rao is a consultant with St. Jude Medical and a member of the surgical advisory board of Medtronic Inc, which has recently acquired HeartWare Inc. He receives honoraria from both St. Jude Medical and Medtronic for invited lectures and attending board meetings.
Contributors: H. Tsubota, R. Ribeiro, F. Billia, T. Yau, M. Badiwala and V. Rao designed the study. H. Tsubota and R. Ribeiro acquired and analyzed the data, which R. Cusimano, W. Stansfield and V. Rao also analyzed. H. Tsubota and R. Ribeiro wrote the article, which all authors reviewed and approved for publication.
References
- 1.Kirklin JK, Naftel DC, Kormos RL, et al. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant. 2013;32:141–56. doi: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Kirklin JK, Naftel DC, Kormos RL, et al. Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) analysis of pump thrombosis in the HeartMate II left ventricular assist device. J Heart Lung Transplant. 2014;33:12–22. doi: 10.1016/j.healun.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Xie A, Phan K, Yan TD. Durability of continuous-flow left ventricular assist devices: a systematic review. Ann Cardiothorac Surg. 2014;3:547–56. doi: 10.3978/j.issn.2225-319X.2014.11.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirklin JK, Naftel DC, Pagani FD, et al. Long-term mechanical circulatory support (destination therapy): On track to compete with heart transplantation? J Thorac Cardiovasc Surg. 2012;144:584–603. doi: 10.1016/j.jtcvs.2012.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holman WL, Naftel DC, Eckert CE, et al. Durability of left ventricular assist devices: Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) 2006 to 2011. J Thorac Cardiovasc Surg. 2013;146:437–41.e1. doi: 10.1016/j.jtcvs.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 6.Moazami N, Milano CA, John R, et al. Pump replacement for left ventricular assist device failure can be done safely and is associated with low mortality. Ann Thorac Surg. 2013;95:500–5. doi: 10.1016/j.athoracsur.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 7.McCarthy FH, Kobrin D, Rame JE, et al. Increasing frequency of left ventricular assist device exchanges in the United States. Ann Thorac Surg. 2015;100:1660–5. doi: 10.1016/j.athoracsur.2015.04.072. [DOI] [PubMed] [Google Scholar]
- 8.Stulak JM, Cowger J, Haft JW, et al. Device exchange after primary left ventricular assist device implantation: indications and outcomes. Ann Thorac Surg. 2013;95:1262–7. doi: 10.1016/j.athoracsur.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 9.Ota T, Yerebakan H, Akashi H, et al. Continuous-flow left ventricular assist device exchange: clinical outcomes. J Heart Lung Transplant. 2014;33:65–70. doi: 10.1016/j.healun.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Starling RC, Moazami N, Silvestry SC, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med. 2014;370:33–40. doi: 10.1056/NEJMoa1313385. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein DJ, John R, Salerno C, et al. Algorithm for the diagnosis and management of suspected pump thrombus. J Heart Lung Transplant. 2013;32:667–70. doi: 10.1016/j.healun.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Levin AP, Uriel N, Takayama H, et al. Device exchange in HeartMate II recipients: long-term outcomes and risk of thrombosis recurrence. ASAIO J. 2015;61:144–9. doi: 10.1097/MAT.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 13.Slaughter MS, Naka Y, John R, et al. Post-operative heparin may not be required for transitioning patients with a HeartMate II left ventricular assist system to long-term warfarin therapy. J Heart Lung Transplant. 2010;29:616–24. doi: 10.1016/j.healun.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Boyle AJ, Russell SD, Teuteberg JJ, et al. Low thromboembolism and pump thrombosis with the HeartMate II left ventricular assist device: analysis of outpatient anti-coagulation. J Heart Lung Transplant. 2009;28:881–7. doi: 10.1016/j.healun.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 15.Chumnanvej S, Wood MJ, MacGillivray TE, et al. Perioperative echocardiographic examination for ventricular assist device implantation. Anesth Analg. 2007;105:583–601. doi: 10.1213/01.ane.0000278088.22952.82. [DOI] [PubMed] [Google Scholar]
- 16.Carr CM, Jacob J, Park SJ, et al. CT of left ventricular assist devices. Radiographics. 2010;30:429–44. doi: 10.1148/rg.302095734. [DOI] [PubMed] [Google Scholar]