Abstract
Background
Robotic surgery was introduced as a platform for minimally invasive lung resection in Canada in October 2011. We present the first Canadian series of robotic pulmonary resection for lung cancer.
Methods
Prospective databases at 2 institutions were queried for patients who underwent robotic resection for lung cancer between October 2011 and June 2015. To examine the effect of learning curves on patient and process outcomes, data were organized into 3 temporal tertiles, stratified by surgeon.
Results
A total of 167 consecutive patients were included in the study. Median age was 66 (range 27–88) years, and 46.1% (n = 77) of patients were men. The majority of patients (n = 141, 84%) underwent robotic lobectomy. Median duration of surgery was 270 (interquartile range [IQR] 233–326) minutes, and median length of stay (LOS) was 4 (IQR 3–6) days. Twelve patients (7%) were converted to thoracotomy. Total duration of surgery and console time decreased significantly (p < 0.001) across tertiles, with a steady decline until case 20, followed by a plateau effect. Across tertiles, there was no significant difference in LOS, number of lymph node stations removed, or perioperative complications.
Conclusion
The results of this case series are comparable to those reported in the literature. A prospective study to examine the outcomes and cost of robotic pulmonary resection compared with video-assisted thoracoscopic surgery should be done in the context of the Canadian health care system. We have presented the first consecutive case series of robotic lobectomy in Canada. Outcomes compare favourably to other series in the literature.
Abstract
Contexte
C’est en octobre 2011 que la chirurgie robotisée a fait son entrée au Canada comme approche à la résection pulmonaire minimalement effractive. Nous présentons à notre connaissance la première série canadienne sur la résection pulmonaire robotisée pour le cancer du poumon.
Méthodes
Nous avons interrogé les bases de données prospectives de 2 établissements pour recenser les patients ayant subi une résection robotisée pour un cancer du poumon entre octobre 2011 et juin 2015. Nous avons organisé les données en 3 tertiles temporels et nous les avons stratifiées par chirurgien pour mesurer l’effet des courbes d’apprentissage sur les résultats enregistrés chez les patients et du point de vue des procédés.
Résultats
En tout, 167 patients consécutifs ont été inclus dans l’étude. L’âge médian était de 66 ans (entre 27 et 88 ans) et 46,1 % (n = 77) étaient des hommes. La majorité des patients (n = 141, 84 %) ont subi une lobectomie robotisée. La durée moyenne des interventions a été de 270 minutes (intervalle interquartile [IIQ] 96) et la durée médiane des séjours a été de 4 jours (IIQ 3). L’intervention s’est transformée en thoracotomie chez 12 patients (7 %). La durée totale de la chirurgie et le temps passé à la console ont diminué significativement (p < 0,001) selon les différents tertiles, avec un déclin constant jusqu’au cas no 20, suivi d’un effet de plateau. Entre les tertiles, on n’a noté aucune différence significative pour ce qui est de la durée des séjours hospitaliers, du nombre de chaînes ganglionnaires excisées ou des complications périopératoires.
Conclusion
Les résultats de cette série de cas sont comparables à ceux qui sont rapportés dans la documentation. Une étude prospective, dans le but de comparer les résultats et le coût des résections pulmonaires robotisées à ceux des chirurgies thoracoscopiques vidéo-assistées, s’imposerait dans le contexte des soins de santé canadiens. Nous avons présenté la première série de cas consécutifs de lobectomies robotisées au Canada. Les résultats se comparent favorablement à ceux d’autres séries décrites dans la documentation.
Minimally invasive surgery (MIS) has demonstrated substantial benefits compared with thoracotomy for lung cancer resection, including lower complication rates, shorter hospital length of stay (LOS) and faster recovery.1 Robotic-assisted thoracic surgery (RATS) is the most recent MIS platform for lung resection. Compared with other MIS platforms, RATS provides a number of advantages, such as 3-dimensional visualization, 7 degrees of freedom of motion and improved visual haptics. The first RATS pulmonary lobectomy for lung cancer in Canada was performed in October 2011 by surgeons at the Toronto General Hospital (K.Y. and T.K.W.). This multicentre study presents the results of the first consecutive case series of RATS lobectomy in Canada.
Methods
This is a retrospective review of all RATS resections for non–small cell lung cancer (NSCLC) performed between October 2011 and June 2015. The data were retrieved from 2 prospectively entered robotic databases at 2 tertiary care centres for thoracic surgery (the University of Toronto’s Toronto General Hospital and McMaster University’s St. Joseph’s Healthcare Hamilton). Four surgeons (K.Y., W.C.H., T.K.W. and Y.S.) performed the operations.
Surgical technique
The operations were completed with the patients under general anesthesia and lung isolation, using either a 3-arm or 4-arm robotic technique, as described by Cerfolio and colleagues.2 Mediastinal lymph node sampling or dissection was performed at the discretion of the operating surgeon. Postoperative analgesia was delivered through epidural catheter and/or intravenous patient-controlled analgesia at the discretion of the treating anesthesiologist. Early on, 2 attending surgeons performed the robotic cases; however, the role of the assisting attending was quickly transitioned to a surgical trainee.
Data collection
Institutional review board approval was granted at both study sites. Data were abstracted from prospective databases, and when required, patient medical records were reviewed. Preoperative variables collected included age, sex, smoking status, previous cancer, tumour location and size, resection type and pulmonary function. Intraoperative variables included date of operation, operating surgeon, tumour location, type of resection, rate of conversion to thoracotomy, total time in the operating room, operative time on the robotic console and intraoperative complications. Postoperative variables included hospital LOS, duration of chest tube drainage and postoperative complications.
Statistical analysis
We used descriptive statistics to present the data. Data were stratified by surgeon and divided into temporal tertiles. The first tertile comprised the first 20 cases for each surgeon. Remaining cases were divided evenly by surgeon for tertiles 2 and 3. Temporal tertiles were chosen to keep this series in line with previously published data in this field.3–5 Surgeons included in the tertile analysis were required to have performed more than 20 robotic resections, the minimum number required to achieve proficiency on the robotic platform. 3–5 Surgeons included in the tertile analysis were all proficient in video-assisted thoracoscopic surgery (VATS) before using the robot. As such, there was no need to stratify by proficiency in VATS versus open approaches. We used the Kruskal–Wallis test to compare differences between tertiles for nonparametric data and 1-way analysis of variance for parametric data. Nonparametric data were analyzed using χ2 and Fisher exact tests. Statistical tests were 2-sided, with results considered significant at p < 0.05. We conducted post hoc tests using the standard residual method for nonparametric data and Bonferroni correction for parametric data. Data analysis was performed using SPSS software version 22. A scatterplot with locally weighted scatter-plot smoothing (LOWESS) was used to explore the effect of the learning curve, where case number (X axis) was plotted against console time and total duration of surgery (Y axis). This method was chosen over the cumulative sum control (CUSUM) method, as we did not hold a clear idea of the expected values associated with the learning curve against which to plot the realized values. Though historical values from published studies could have been used to predict a Y axis on the CUSUM, we chose to instead perform a tertile analysis to remain consistent with previously published literature in this field.5,6 We used univariate linear regression to determine statistical differences between durations of surgery in each tertile. These models were generated using Stata software version 14.0.
Results
A total of 167 consecutive patients were included in this series (Table 1). The median age was 66 (interquartile range [IQR] 60–74) years, and 46.1% (n = 77) were men. Of this sample, 22.2% (n = 37) were smokers, 44.9% (n = 75) were former smokers and 30.5% (n = 51) were nonsmokers. History of a previous cancer was reported in 28% (n = 47) of patients, and 2.4% (n = 4) had a previous lung resection. Median tumour size was 2.5 (IQR 1.5–3.2) cm. The majority of patients (n = 85, 50.9%) had T1 stage tumours and presented with stage 1 NSCLC (n = 111, 67%). Eighty-four percent (n = 141) of patients underwent a robotic lobectomy, and the remainder received segmental (n = 13, 7.8%) or nonanatomic resection (n = 8, 4.8%). Tumour location was most prevalent in the right upper lobe (n = 65, 38.9%), followed by the right lower lobe (n = 32, 19.2%; Table 1). Most patients (n = 101, 60.5%) presented with adenocarcinoma, 10.2% (n = 17) with squamous cell carcinoma, 12% (n = 20) with neuroendocrine tumours and 17.3% (n = 29) with other tumours.
Table 1.
Demographic and clinical characteristics of patients undergoing RATS (n = 167)
| Characteristic | No. (%) or median [IQR] |
|---|---|
| Age, yr | 66 [60–74] |
| Male sex | 77 (46.1) |
| Smoking status | |
| Smoker | 37 (22.2) |
| Former smoker | 75 (44.9) |
| Nonsmoker | 51 (30.5) |
| Unknown | 4 (2.4) |
| Previous cancer | 47 (28.1) |
| Previous lung resection | 4 (2.4) |
| Tumour location | |
| Right upper lobe | 65 (38.9) |
| Right middle lobe | 19 (11.4) |
| Right lower lobe | 32 (19.2) |
| Left upper lobe | 27 (16.2) |
| Left lower lobe | 24 (14.4) |
| Tumour size, cm | 2.5 [1.5–3.2] |
| Resection type | |
| Lobectomy | 141 (84.4) |
| Segmental resection | 13 (7.8) |
| Nonanatomic (wedge) | 8 (4.8) |
| Bilobectomy | 5 (3.0) |
| T stage | |
| T1 | 86 (51.5) |
| T2 | 54 (32.3) |
| T3 | 7 (4.2) |
| T4 | 2 (1.2) |
| Benign | 10 (6.0) |
| Metastases to lung | 8 (4.8) |
| Cumulative stage | |
| Occult cancer | 2 (1) |
| 1A | 71 (43) |
| 1B | 40 (24) |
| 2A | 15 (9) |
| 2B | 6 (4) |
| 3A | 14 (8) |
| 4 | 7 (4) |
| Benign tumour | 10 (6) |
| FEV1, % predicted | 85 [71–94] |
| DLCO, % predicted | 80 [69–93] |
DLCO = diffusing capacity of the lungs for carbon monoxide; FEV1 = forced expiratory volume in 1 second; IQR = interquartile range; RATS = robotic-assisted thoracic surgery.
The median LOS was 4 (IQR 3–6) days, and the median duration of chest tube drainage was 3 (IQR 2–5) days (Table 2). The median total duration of surgery was 270 (IQR 233–326) minutes, whereas the median time on the robotic console was 142 (IQR 111–192) minutes. There were 12 conversions to thoracotomy: 1 owing to hemorrhage, 8 owing to difficult dissections (adhesions, tumour location) and 3 owing to intraoperative injury to adjacent organs. Fourteen patients (8.4%) experienced intraoperative complications; 70 (41.9%) patients experienced postoperative complications, but only 14 (8.4%) of them were classified as grade III or higher according to the Ottawa Thoracic Morbidity and Mortality Classification System.7 The median number of lymph nodes harvested was 8 (IQR 5–10; Table 3). There were no deaths at 90-day follow-up (Table 2).
Table 2.
Perioperative outcomes of patients undergoing RATS (n = 167)
| Outcome | No. (%) or median [IQR] |
|---|---|
| Length of stay, d | 4 [3–6] |
| Chest tube duration, d | 3 [2–5] |
| Total duration of surgery, min | 270 [233–326 |
| Total console time, min | 142 [111–192] |
| Conversion | 12 (7.2) |
| Intraoperative complications | 14 (8.4) |
| Prolonged air leak* | 16 (9.6) |
| Postoperative complications† | 14 (8.4) |
| In-hospital mortality | 0 (0.0) |
| 90-day mortality | 0 (0.0) |
IQR = interquartile range; RATS = robotic-assisted thoracic surgery.
Longer than 5 d.
Ottawa Thoracic Morbidity and Mortality Classification System.
Table 3.
Upstaging and nodal counts (n = 18)*
| Upstaging | Tertile; no. (%) | ||
|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | |
| N0/NX to N1 | 6 (55) | 4 (36) | 1 (9) |
| N0/NX to N2 | 1 (17) | 5 (83) | — |
| N1 to N2 | 1 (100) | — | — |
IQR = interquartile range.
Total no. of lymph nodes harvested: median 8 (IQR 5–10).
A total of 157 cases performed by 3 surgeons were included in the temporal analysis. Cases for the fourth surgeon were not included because that surgeon had not performed the 20 cases required for proficiency.4 There were no significant differences in patient baseline characteristics between tertiles, although differences in patient sex approached significance (p = 0.06; Table 4). The majority of patients in each tertile (t) underwent a robotic lobectomy (n = 49, 82% in t1; n = 45, 96% in t2; n = 38, 76% in t3), whereas the rest underwent either segmental or sublobar nonanatomic resection. There appeared to be differences in resection type by tertile (p = 0.023). Total duration of surgery decreased significantly (p < 0.001) over the learning curve (309.0 min in t1, 258.5 min in t2, 236.0 min in t3), as did time on the robotic console (172.0 min in t1, 136.0 min in t2, 116.0 min in t3, p < 0.001). There were no differences in median LOS (4 d), chest tube duration (3 d), or number of lymph nodes harvested (n = 8) among tertiles (Table 5). The overall intraoperative complication rate was 9%, with 7 of 60 patients in t1, 2 of 47 patients in t2 and 5 of 50 patients in t3 experiencing a total of 18 intraoperative complications. The overall postoperative complication rate was 8%, with 4 of 60 patients in t1, 3 of 47 patients in t2 and 5 of 50 patients in t3 experiencing a total of 16 postoperative complications. The differences in complications between tertiles were not statistically significant (p = 0.28 and p = 0.80, respectively; Table 5). Rates of conversion to thoracotomy increased from 3% and 4% in t1 and t2, respectively, to 14% in t3 (p = 0.07). Reasons for conversion in t1 included airway injury (n = 1 of 2) and vascular injury (n = 1 of 2). Patients in t2 were converted for adhesions (n = 1 of 2) and central tumour location (n = 1 of 2). In t3, 4 of 7 patients were converted owing to adhesions, 2 of 7 owing to difficult dissection and tumour size and 1 of 7 owing to vascular injury.
Table 4.
Demographic and clinical characteristics of patients undergoing RATS (n = 167), by tertile
| Characteristic | Tertile; median [range] or no. (%) | p value | ||
|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | ||
| Age, yr | 66.5 [44–86] | 67.0 [29–88] | 67.5 [27–85] | 0.92 |
| Male sex | 34 of 60 (57) | 16 of 47 (34) | 21 of 50 (42) | 0.06 |
| T stage | 0.030 | |||
| T0/TX | 2 (3.4) | 1 (2.1) | 0 (0) | |
| T1 | 41 (68.3) | 23 (48.9) | 27 (54.0) | |
| T2 | 16 (26.7) | 20 (42.6) | 18 (36.0) | |
| T3 | 0 (0) | 2 (4.3) | 5 (10.0) | |
| T4 | 1 (1.7) | 1 (2.1) | 0 (0) | |
| N stage | 0.42 | |||
| NX | 3 (5.0) | 0 (0) | 2 (4.0) | |
| N0 | 48 (80.0) | 38 (80.8) | 43 (86.0) | |
| N1 | 4 (6.7) | 6 (12.8) | 3 (6.0) | |
| N2 | 5 (8.3) | 3 (6.4) | 2 (4.0) | |
| Tumour size, cm | 2.15 [0.9–5.8] | 2.7 [0.9–6.0] | 2.5 [1.0–11.0] | 0.13 |
| FEV1 | 82.00 [52–120] | 84.00 [43–109] | 88.00 [65–105] | 0.48 |
| DLCO | 79.00 [29–112] | 79.5 [46–108] | 84.5 [57–111] | 0.30 |
DLCO = diffusing capacity of the lungs for carbon monoxide; FEV1 = forced expiratory volume in 1 s; IQR = interquartile range; RATS = robotic-assisted thoracic surgery.
Table 5.
Comparison of outcomes by tertile
| Outcome | Tertile; median or no. (%)* | p value | ||
|---|---|---|---|---|
| Tertile 1 (n = 60) | Tertile 2 (n = 47) | Tertile 3 (n = 50) | ||
| Total duration of surgery, min | 309.0 | 258.5 | 236.0 | < 0.001 |
| Total console time, min | 172.0 | 136.0 | 116.0 | < 0.001 |
| Conversion | 2 (3.0) | 2 (4.3) | 7 (14.0) | 0.10 |
| LOS (d, median) | 4.0 | 4.0 | 4.0 | 0.16 |
| Chest tube duration, d | 3.0 | 3.0 | 3.0 | 0.91 |
| No. of lymph nodes harvested | 8 | 8 | 8 | 0.39 |
| No. of patients with intraoperative complications† | 8 | 2 | 8 | 0.28 |
| No. of patients with postoperative complications† | 4 | 3 | 5 | 0.80 |
LOS = length of stay in hospital.
Unless indicated otherwise.
Ottawa Thoracic Morbidity and Mortality Classification System.
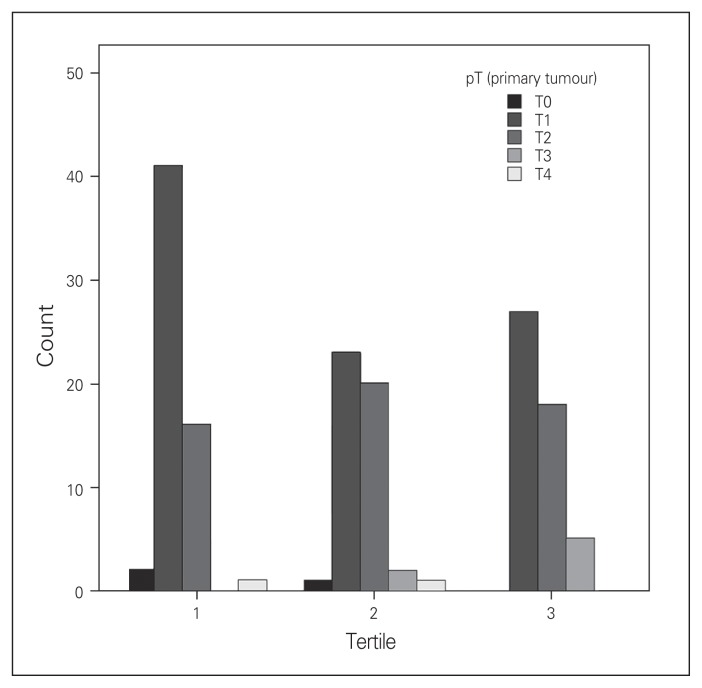
There was a significant difference in T stage across tertiles, with patients in later tertiles having a higher prevalence of T2 and T3 tumours (p = 0.030; Fig. 1 and Table 4). There were no significant differences in N stage (p = 0.42) or disease type (benign v. malignant, p = 0.37).
Fig. 1.
Pathologic T-stage (pT), by tertile.
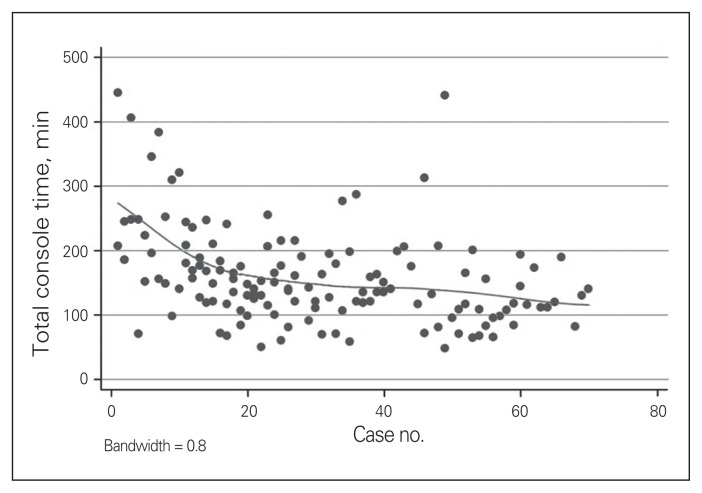
To determine the effects of a learning curve over time, we created a scatterplot showing the linear association between case number and console time (Fig. 2). The plot shows a steady decline in duration of surgery until approximately case 20, followed by a plateau in duration for the next 21–40 cases. The overall downward slope indicates that console time decreased as surgeons become more proficient with it. A plot comparing case number to duration of surgery showed similar results. Linear regression analysis revealed a strong association between console time and case number, when case number is less than 20. Specifically, console time was shown to decrease by 6.64 ± 1.84 minutes/case until case 20 (p = 0.001). These findings were not significant for cases 21–40 (p = 0.54) or after the 41st case (p = 0.42). Similarly, total duration of surgery decreased by 8.04 ± 1.78 minutes/case until case 20 (p < 0.001). Total duration of surgery for cases 21–40 and cases 41 onward did not decrease significantly (p = 0.73 and p = 0.20, respectively).
Fig. 2.
Total console time, by case number.
Discussion
This is the first Canadian series on RATS resection for lung cancer. There were no deaths within 90 days of the operation. Postoperative hospital LOS and duration of chest tube drainage in this series were comparable to reported rates in the literature.8–10 Although the median overall duration of surgery (270 min) was slightly greater than the average reported time of 190 minutes found in the literature,10,11 it fell within reported ranges of 104–399 minutes.2,3,12 Total duration of surgery became comparable to reported literature rates as the learning curve plateaued. The conversion rate of 12% is comparable to larger series by Park and colleagues9 and Cerfolio and colleagues, 2 but higher than the 1.5% conversion in a 200-patient series by Dylewski and colleagues.8 An increase in conversion rate across the tertiles was noted (14% in t3 v. 3% in t1, p = 0.10). Although this difference was not statistically significant, likely owing to small numbers, it does have important clinical implications. The higher conversion rate in t3 likely reflects increasing surgeon comfort with RATS and willingness to perform more difficult resections, as evidenced by the higher T stage in this tertile. Moreover, the data demonstrate that although conversions in t1 occurred because of intraoperative injury, those in t3 were largely attributed to difficult dissection.
The significant decrease of total duration of surgery and time on the robotic console across the tertiles confirms the presence of a learning curve at approximately 20 cases, as reported by Gharagozloo and colleagues4 and Veronesi and colleagues.5,13 Other sources suggest that surgeons proficient in VATS can expect similar durations of surgery and LOS after completing just 6 robotic resections,14 although our analysis did not confirm this finding. Further use of univariate regression did not reveal any significant evidence of a second learning curve beyond the 20th case. However, it is possible that a second learning curve exists, but is masked owing to confounding factors, such as increased T stage, adhesions and a willingness of surgeons to attempt more complex dissections while maintaining quality indicators (e.g., nodal counts).
The ease of lymph node dissection with the robotic platform is thought to be one of the advantages of RATS over VATS. The rate of upward N stage migration in this series was 10.8%, which is slightly lower than published rates of RATS nodal upstaging of 13.2%.15 Our rates were comparable to reported ranges of VATS nodal upstaging, reported to be 8.1%–15.2%,15–17 and thoracotomy, reported to be 11.5%–13.1%.17
This series provides comparable results to the robotic literature and confirms the safety of robotic resection for pulmonary lobectomy. However, only a few direct comparisons of the impact of RATS versus VATS on patient outcomes have been completed to date. We recently conducted a meta-analysis that demonstrated no differences in patient outcomes through the use of RATS versus VATS, with the exception of cost.11 To further explore these differences, we completed a cost analysis comparing RATS and VATS for lung resection. Preliminary data demonstrate that robotic lobectomy is associated with increased costs in a Canadian context owing to operating room time and costs of disposables (RATS: $15 247, 95% CI $15 643–$18 945; VATS: $12 131, 95% CI $13 218–$15 879) with a statistically significant difference of $3116 (p < 0.001), creating a significant barrier for uptake.9,18,19 This issue is even more pronounced in the publicly funded Canadian health care system, where robotic surgery is supported only by philanthropic initiatives. Perhaps the most comparable comparison to the Canadian system is the United Kingdom’s publicly funded National Health Service (NHS). A recent white paper published by the NHS presents evidence to inform whether robotic thoracic surgery is sustainable within a publicly funded system.20 Evidence regarding safety, perioperative outcomes, quality of life outcomes, learning curves and cost-effectiveness was used to inform the recommendation. The findings demonstrated that the expensive console, coupled with the additional disposable costs were not sustainable in the publicly funded system. The NHS concluded that “there is not sufficient evidence to support a proposal for the routine commissioning of robotic-assisted lung resection for primary lung cancer.” Rather, the real potential value of RATS may lie in the development of novel localization and resection techniques (ClinicalTrials.gov: NCT02570958).
Limitations
Although this study is one of the larger series reported in the literature, it is limited by its retrospective nature. Outcomes by tertile should be interpreted with caution owing to limited sample size. It is also highly likely that this sample is subject to selection bias, as surgeons initially selected less complex cases for RATS until proficiency with the robotic platform was reached. Additionally, prospective data and cost–utility research will confirm whether the technology provides a sustainable modality for Canadian lung cancer care; research is currently underway in the form of a multicentre randomized controlled trial being conducted in Ontario with participation from St. Joseph’s Healthcare Hamilton, McMaster University, and the University Health Network, University of Toronto (ClinicalTrials.gov: NCT02617186).
Conclusion
We present the first series of RATS lobectomy for lung cancer in Canada and demonstrate favourable outcomes with this new technology. As experience with robotic lung resection grew, surgeons appeared willing to complete more complex resections. A prospective randomized clinical trial is underway to further elucidate differences in outcomes and costs between the 2 platforms in the Canadian public health care system.
Footnotes
This work has been presented, in part, at the Canadian Association of Thoracic Surgeons annual meeting in Québec, Que., Sept. 19, 2015, and at the World Conference on Lung Cancer 16th annual meeting in Denver, Colo., Sept. 6, 2015.
Competing interests: None declared.
Contributors: C. Fahim, W. Hanna, T. Waddell and K. Yasufuku designed the study. All authors acquired the data, which C. Fahim and W. Hanna analyzed. C. Fahim and W. Hanna wrote the article, which all authors reviewed and approved for publication.
References
- 1.Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg. 2008;86:2008–16. doi: 10.1016/j.athoracsur.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic resection with 4 arms. J Thorac Cardiovasc Surg. 2011;142:740–6. doi: 10.1016/j.jtcvs.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 3.Melfi FM, Mussi A. Robotically assisted lobectomy: learning curve and complications. Thorac Surg Clin. 2008;18:289–95. doi: 10.1016/j.thorsurg.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Gharagozloo F, Margolis M, Tempesta B, et al. Robot-assisted lobectomy for early-stage lung cancer: report of 100 consecutive cases. Ann Thorac Surg. 2009;88:380–4. doi: 10.1016/j.athoracsur.2009.04.039. [DOI] [PubMed] [Google Scholar]
- 5.Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg. 2010;140:19–25. doi: 10.1016/j.jtcvs.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 6.Noyez L. Cumulative sum analysis: a simple and practical tool for monitoring and auditing clinical performance. Health Care Current Reviews. 2013;2:1. [Google Scholar]
- 7.Seely AJ, Ivanovic J, Threader J, et al. Systematic classification of morbidity and mortality after thoracic surgery. Ann Thorac Surg. 2010;90:936–42. doi: 10.1016/j.athoracsur.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Dylewski MR, Ohaeto AC, Pereira JF. Pulmonary resection using a total endoscopic robotic video-assisted approach. Semin Thorac Cardiovasc Surg. 2011;23:36–42. doi: 10.1053/j.semtcvs.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Park BJ, Melfi F, Mussi A, et al. Robotic lobectomy for non-small cell lung cancer (NSCLC): long-term oncologic results. J Thorac Cardiovasc Surg. 2012;143:383–9. doi: 10.1016/j.jtcvs.2011.10.055. [DOI] [PubMed] [Google Scholar]
- 10.Cao C, Manganas C, Ang SC, et al. A systematic review and meta-analysis on pulmonary resections by robotic video-assisted thoracic surgery. Ann Cardiothorac Surg. 2012;1:3–10. doi: 10.3978/j.issn.2225-319X.2012.04.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agzarian J, Fahim C, Shargall Y, et al. The use of robotic-assisted thoracic surgery for lung resection: a comprehensive systematic review. Semin Thorac Cardiovasc Surg. 2016;28:182–92. doi: 10.1053/j.semtcvs.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Augustin F, Bodner J, Maier H, et al. Robotic-assisted minimally invasive vs. thoracoscopic lung lobectomy: comparison of perioperative results in a learning curve setting. Langenbecks Arch Surg. 2013;398:895–901. doi: 10.1007/s00423-013-1090-5. [DOI] [PubMed] [Google Scholar]
- 13.Veronesi G. Robotic surgery for the treatment of early-stage lung cancer. Curr Opin Oncol. 2013;25:107–14. doi: 10.1097/CCO.0b013e32835daf4f. [DOI] [PubMed] [Google Scholar]
- 14.Louie BE, Farivar AS, Aye RW, et al. Early experience with robotic lung resection results in similar operative outcomes and morbidity when compared with matched video-assisted thoracoscopic surgery cases. Ann Thorac Surg. 2012;93:1598–1604. doi: 10.1016/j.athoracsur.2012.01.067. [DOI] [PubMed] [Google Scholar]
- 15.Lee BE, Shapiro M, Rutledge JR, et al. Nodal upstaging in robotic and video assisted thoracic surgery lobectomy for clinical N0 lung cancer. Ann Thorac Surg. 2015;100:229–33. doi: 10.1016/j.athoracsur.2015.03.109. [DOI] [PubMed] [Google Scholar]
- 16.Zhou H, Tapias LF, Gaissert HA, et al. Lymph node assessment and impact on survival in video-asisted thoracoscopic lobectomy or segmentectomy. Ann Thorac Surg. 2015;100:910–6. doi: 10.1016/j.athoracsur.2015.04.034. [DOI] [PubMed] [Google Scholar]
- 17.Licht PB, Jørgensen OD, Ladegaard L, et al. A national study of nodal upstaging after thoracoscopic versus open lobectomy for clinical stage I lung cancer. Ann Thorac Surg. 2013;96:943–49. doi: 10.1016/j.athoracsur.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Kaur MN, Shiwcharan A, Patterson L, et al. Robotic-assisted thoracoscopic lobectomy versus video-assisted thoracoscopic lobectomy for early stage non-small cell lung cancer: a healthcare resource utilization analysis. Abstract presented at the 16th Annual Canadian Surgery Forum; Toronto, ON. September 8–11. [Google Scholar]
- 19.Swanson SJ, Miller DL, McKenna RJ, et al. Comparing robot-assisted thoracic surgical lobectomy with conventional video-assisted thoracic surgical lobectomy and wedge resection: results from a multihospital database (Premier) J Thorac Cardiovasc Surg. 2014;147:929–37. doi: 10.1016/j.jtcvs.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 20.Clinical Commissioning Policy Proposition. Robotic assisted lung resection for primary lung cancer. NHS England B10X03/01. [accessed 2016 Apr 11]. Available: www.engage.england.nhs.uk/consultation/clinical-commissioning-wave2/user_uploads/b10-x03-robtc-lung-resctn-policy-prop.pdf.