Abstract
There is growing evidence suggesting greater severity and worse outcomes in children with mixed as compared to single respiratory virus infections. However, studies that assess the risk factors that may predispose a child to a mixture of respiratory syncytial virus (RSV) and adenoviral infections, are scarce. In a retrospective cohort study, the study investigated the epidemiology of RSV and adenovirus infections and predictors of mixed RSV-adenoviral infections in young children hospitalized with acute lower respiratory infection in Bogota, Colombia, South America, over a 2-year period 2009–2011. Of a total of 5,539 children admitted with a diagnosis of acute lower respiratory infection, 2,267 (40.9%) who were positive for RSV and/or adenovirus were selected. Out the total number of cases, 1,416 (62.5%) infections occurred during the 3-month period from March to May, the first rainy season of Bogota, Colombia. After controlling for gender, month when the nasopharyngeal sample was taken, and other pre-existing conditions, it was found that an age greater than 6 months (OR:1.74; CI 95%:1.05–2.89; P = 0.030) and malnutrition as a comorbidity (OR:9.92; CI 95%:1.01–100.9; P = 0.049) were independent predictors of mixed RSV-adenoviral infections in the sample of patients. In conclusion, RSV and adenovirus are significant causes of acute lower respiratory infection in infants and young children in Bogota, Colombia, especially during the first rainy season. The identified predictors of mixed RSV-adenoviral infections should be taken into account when planning intervention, in order to reduce the burden of acute lower respiratory infection in young children living in the country.
Keywords: acute respiratory infection, adenovirus infections, co-infection, pediatrics, respiratory syncytial virus
INTRODUCTION
Acute lower respiratory infection is one of the most important causes of morbidity and mortality among children under 5 years of age throughout the world [Costa et al., 2006]. Although acute lower respiratory infection is the most common cause of morbidity in high-income countries, it is an even greater health problem in low- and middle-income countries [Berman, 1991; Savitha et al., 2007; Nair et al., 2010]. Viral pathogens are the most common causes of acute lower respiratory infection in infants and young children [Jennings et al., 2004]. Respiratory syncytial virus (RSV) is the leading viral cause of acute lower respiratory infection worldwide [Weber et al., 1998] and the most important viral pathogen in infancy, especially among young infants less than 6 months of age [Langley and Anderson, 2011]. However, despite the undoubted importance of RSV as a respiratory pathogen in young children, other viruses have also been reported to be important causes of acute lower respiratory infection in young children, such as adenoviruses, influenza, parainfluenza, rhinoviruses, and human metapneumovirus [Calvo et al., 2008]. Specifically, adenoviruses play a significant role in pediatric acute lower respiratory infection, since it has been reported that they are the second most frequent respiratory viral pathogens after RSV [Palomino et al., 2004; De Paulis et al., 2011]. In some investigations, it had been reported that adenoviruses are responsible for 4–10% of cases of pneumonia and up to 10% of cases of bronchiolitis [Hong et al., 2001]. In contrast to RSV, adenoviruses can be isolated throughout the entire year, following a nonseasonal (perennial) pattern [Videla et al., 1998; Larranaga et al., 2000], and in some cases these infections produce a more severe disease than does RSV, causing severe pneumonia, a prolonged hospital stay, admission to pediatric intensive care units, the need for respiratory support, and death [Palominio et al., 2000; Kim et al., 2003; Lewis et al., 2009]. However, despite the undoubted importance of investigating RSV and adenoviruses due to the associated high morbidity and mortality rates in infants and young children in low- and middle-income countries, only a limited amount of information is available about the pediatric epidemiology of these viral respiratory infections in these countries. Accordingly, there is a critical need for studies that may lead to a better understanding of the epidemiology, clinical characteristics, seasonality, and risk factors that may predispose a child to viral coinfections. Although still controversial, there is growing evidence suggesting greater severity and worse outcomes in children with mixed compared to single respiratory virus infections [Papadopoulos et al., 2002; Semple et al., 2005; Rodriguez et al., 2013]. These kinds of studies are essential for discovering the occurrence and the seasonal pattern of the viruses, and therefore for predicting epidemics and planning preventive measures, especially for high-risk pediatric groups, in an effort to try to reduce the extensive burden of the disease.
The aim of the present study was to investigate the epidemiology, clinical characteristics, and seasonality of RSV and adenoviral infections in a large cohort of Colombian children less than 3 years of age hospitalized due to acute lower respiratory infection and to determine the risk factors that may predispose a child to mixed RSV-adenoviral infections.
MATERIAL AND METHODS
Study Population and Procedures
Details of the study design and methods have been reported elsewhere [Rodriguez et al., 2013]. Briefly, in a retrospective cohort study, a consecutive sample of pediatric patients hospitalized at the Fundacion Hospital La Misericordia in Bogota, Colombia aged less than 36 months with a diagnosis of acute lower respiratory infection who were RSV and/or adenoviruses positive, determined through nasopharyngeal aspirates, was examined during a 2-year period (between May 1st, 2009 and May 31st, 2011). After reviewing the electronic medical records, the following demographic and clinical data was collected: date of admission, age, primary diagnosis at admission, presence of comorbidities, number of days with respiratory symptoms, and month and year of nasopharyngeal aspirates sampling. Likewise, variables related to disease-severity markers were collected, such as need for oxygen administration, need for home oxygen therapy, pediatric intensive care unit admission, length of stay in the pediatric intensive care unit, need for endotracheal intubation, use of antibiotics, length of hospital stay, and mortality.
Nasopharyngeal aspirates for virus detection was taken immediately upon admission to the emergency department or within 48 hr of admission using a standardized technique, and was processed immediately or stored at 4 °C until the next working day (in a phosphate-buffered saline transport medium at 2–8°C for 24 hr or at 70°C for more than 24 hr). Those children screened were tested for RSV antigen using a rapid immunoassay method (Abbott Test Pack RSV Rapid Diagnostic Kit, Abbott, IL) and for adenovirus using a rapid immunochromatographic test method (Adeno Respi-Strip; Coris BioConcept, Gembloux, Belgium). If more than one sample of a patient who was tested for RSV or adenoviruses turned out to be positive, it was considered that there were separate episodes when the dates of the tests were separated by more than 4 weeks or if at least one negative test was recorded in the interim.
The study protocol was approved by the local ethics board.
Statistical Analysis
Continuous variables are presented as mean ± standard deviation (SD) or median (interquartile range), whichever is appropriate. Categorical variables are presented as numbers (percentage). Differences in continuous variables between patients with single RSV infections, single adenovirus infections, and mixed RSV-adenovirus infections were analyzed using one-way analysis of variance (ANOVA) with the post-hoc Tukey test or the non-parametric ANOVA (Kruskall-Wallis) test, as appropriate. Differences in continuous variables between patients with a single respiratory infection and those with dual respiratory infections were analyzed using the unpaired t-test or the Mann-Whitney U test, whichever was appropriate. Associations between categorical variables were analyzed using the Chi-square test or Fisher’s exact test, whichever was appropriate. To identify factors independently associated with mixed RSV-adenoviral infections, logistic regression models were adjusted by including the following predictor variables in the models: age, presence of comorbidities, and month of nasopharyngeal aspirates sampling. All statistical tests were two-tailed, and the significance level used was 0.05. The data were analyzed with the Statistical Package Stata 12.0 (Stata Corporation, College Station, Texas).
RESULTS
General Characteristics
From May 1st, 2009 to May 31st, 2011, a total of 5,539 patients were admitted with a diagnosis of acute lower respiratory infection, of whom 2,267 (40.9%) were selected for study because they were positive for VSR and/or adenovirus. Of the 2,267 participants, 1,265 (55.8%) were males, and the median (interquartilic range [IQR]) age was 7.0 (3.0–14.0) months. The age group distribution was: 1,087 (47.9%) less than 6 months, 524 (23.1%) between 7 and 12 months, 464 (20.5%) between 13 and 24 months, and 192 (8.5%) between 25 and 36 months. Of the total of 2,267 cases included in the study, 1,991 (87.8%) were single RSV infections, 205 (9.0%) were single adenovirus infections, and the remaining 71 (3.1%) were mixed RSV-adenovirus infections. The median (IQR) of the number of days with respiratory symptoms before admission was 3.0 (2.0–5.0) days. Regarding the clinical diagnosis at admission, 1,641 (72.4%) patients were diagnosed as having bronchiolitis, 313 (13.8%) as having pneumonia, and the remaining 313 (13.8%) as having other diagnoses such as viral laryngotracheitis (croup), apparent life threatening event (ALTE), and acute viral nasopharyngitis. The RSV and/or adenoviral infections were acquired in the community in 2,172 (95.8%) cases and were transmitted nosocomially in the remaining 95 (4.2%) cases.
Comorbidities
On analyzing for the presence of comorbidities, it was found that 956 (42.2%) children showed comorbid conditions: 744 (32.8%) patients had some preexisting respiratory condition, 96 (4.2%) patients had a history of prematurity, 24 (1.1%) patients had a history of a congenital heart disease, 17 (0.7%) patients had some preexisting renal disease, 13 (0.6%) patients had a previous diagnosis of cancer, 4 (0.2%) patients suffered from malnutrition, and the remaining 58 (2.6%) patients had other comorbidity conditions.
Seasonal Distribution of RSV and Adenoviral Infections
During the periods 2009–2010 and 2010–2011, there were 1,283 (56.6%) and 984 (43.4%) cases of RSV and/or adenovirus bronchiolitis, respectively. On analyzing the data about seasonal distribution of the RSV and adenoviral infections, it was found that 1,416 (62.5%) infections occurred during the 3-month period from March to May, and 851 (37.5%) infections occurred in the remaining 9 months of the year. Upon analyzing the seasonal distribution of cases, differentiating by type of viral infection, it was found that out of the total of 1,191 single RSV infections, 1,266 (63.6%) infections occurred during the 3-month period from March to May, and the remaining 725 (36.4%) in the remaining 9 months of the year. Adenovirus was detected throughout the two years (excepting January and February 2010), but a peak occurred during the months of March through May in both years. Out of the total of 205 single adenoviral infections, 98 (47.8%) occurred during the 3-month period from March to May, and 107 (52.2%) in the remaining 9 months of the year. Finally, out of the total of 71 mixed RSV-adenovirus infections, 52 (73.2%) occurred during the 3-month period from March to May and the remaining 19 (26.8%) in the other 9 months of the year. Figure 1 shows the monthly number of RSV and adenoviral acute lower respiratory infection covering the period from May 2009 through May 2011, along with the monthly variation of the average precipitation.
Fig. 1.
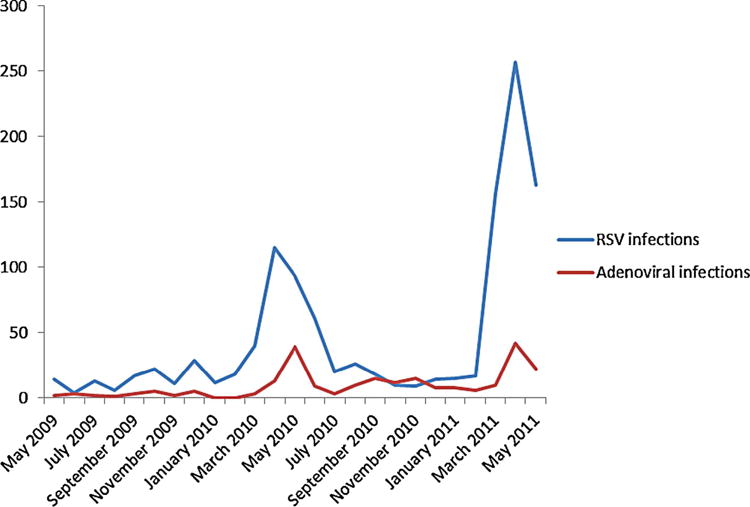
Monthly number of RSV and adenoviral acute lower respiratory infections. The X-axis shows the date (covering the period from May 2009 through May 2011). The Y-axis shows the monthly number of RSV and adenoviral acute lower respiratory infections
Differences Between Single RSV, Single Adenoviral, and Mixed RSV-Adenoviral Infections
Patients with single adenoviral infections were significantly older than patients with single RSV and mixed RSV-adenoviral infections (12.0 [7.0–22.0] vs. 6.0 [2.0–13.0] vs. 8.0 [4.0–15.0] months, respectively, P < 0.001). Significant differences were seen in disease-severity parameters, such as length of hospital stay, need for oxygen administration, and need for home oxygen administration between patients with mixed RSV-adenoviral infections and those in the other two viral groups. Although it was found that patients with mixed RSV-adenoviral infections were at a higher risk of needing endotracheal intubation than those in the other two groups, statistical significance was not achieved. Table I provides descriptive statistics for these and other variables of interest in the study, differentiated by the type of viral infection. Table II shows the underlying conditions in children included in the study, differentiated by single (RSV or adenovirus) versus dual (RSV and adenovirus) respiratory infections.
TABLE I.
Main Differences Between RSV, Adenoviral and Mixed RSV-Adenoviral Acute Lower Respiratory Infectionsa
| Variable | Single RSV infections (n = 1991) | Single adenoviral infections (n = 205) | Mixed RSV-adenoviral infections (n = 71) | P-value |
|---|---|---|---|---|
| Age (months), median (IQR) | 6,0 (2,0–13,0) | 12,0 (7,0–22,0)c,d | 8,0 (4,0–15,0) | < 0,001 |
| Days with respiratory symptoms | 3,0 (2,0–5,0) | 4,0 (2,0–8,0)c | 4,0 (2,0–5,0) | 0,020 |
| Presence of any comorbidity | 835 (41,9%) | 86 (42,0%) | 35 (49,3%) | 0,466 |
| Length of hospital stay (days) | 8,0 (5,0–11,0) | 7,0 (4,0–13,5)c,d | 10,0 (6,0–17,0)c | 0,029 |
| Need for oxygen administration | 1886 (94,7%) | 155 (75,6%)c,d | 69 (97,2%) | < 0,001 |
| Need for home oxygen therapy | 908 (45,6%) | 68 (33,2%)c,d | 32 (45,1%) | 0,003 |
| PICU admission b | 156 (7,8%) | 15 (7,3%) | 11 (15,5%) | 0,061 |
| Length of stay in the PICU (days) | 5,0 (3,0–10,0) | 13,0 (5,0–29,0)c | 6,0 (3,0–11,0) | 0,017 |
| Need for endotracheal intubation | 87 (4,4%) | 12 (5,9%) | 7 (9,9%) | 0,070 |
| Use of antibiotics | 494 (24,8%) | 77 (37,6%)c | 25 (35,2%)c | < 0,001 |
| Mortality | 23 (1,2%) | 5 (2,4%) | 2 (2,8%) | 0,165 |
RSV, respiratory syncytial virus.
PICU, pediatric intensive care unit.
Significantly different from single RSV infections (post hoc Tukey test, P < 0.05).
Significantly different from mixed RSV-adenoviral infections (post hoc Tukey test, P < 0.05).
TABLE II.
Underlying Conditions in Children Included in the Study, Differentiated by Single (RSV or Adenovirus) Versus Dual (RSV and Adenovirus) Respiratory Infections
| Type of underlying condition | Single respiratory infections n = 2196 (%) | Dual respiratory infections n = 71 (%) | P-value |
|---|---|---|---|
| Respiratory disease | 716 (32,6) | 28 (39,4) | 0,228 |
| Prematurity | 94 (4,3) | 2 (2,8) | 0,767 |
| Congenital heart disease | 23 (1,0) | 1 (1,4) | 0,536 |
| Renal disease | 17 (0,8) | 0 (0,0) | 0,464 |
| Cancer | 12 (0,5) | 1 (1,4) | 0,340 |
| Malnutrition | 3 (0,1) | 1 (1,4) | 0,120 |
| Other comorbidities | 56 (2,6) | 2 (2,8) | 0,703 |
Disease Severity and Mortality Data
Regarding the severity of the respiratory disease, 488 (21.5%) children were admitted to the pediatric intensive care unit. Out of the total of 2,267 patients included in the study, 2,110 (93.1%) required supplemental oxygen administration, 106 (4.7%) required endotracheal intubation, 596 (26.3%) were given antibiotics, and 1,008 (44.5%) required home oxygen therapy. Children who were admitted to the pediatric intensive care unit, compared to children who did not require pediatric intensive care unit admission, were significantly younger (1.0 [1.0–2.0] vs. 2.0 [1.0–2.0] months old, P < 0.001), underwent a significantly higher number of days with supplemental oxygen use (12.5 [9.0–18.0] vs. 7.0 [4.0–9.0] days, P < 0.001), were more likely to be given antibiotics (255 [52.3%) vs. 341 [19.2%] patients, respectively, P < 0.001), were more likely to have a comorbidity (252 [51.6%] vs. 704 [39.6%] patients, respectively, P < 0.001), were more likely to have a nosocomial-acquired viral infection (41 [8.4%] vs. 52 [2.9%] patients, respectively, P < 0.001), were more likely to require home oxygen administration (308 [63.1%] vs. 700 [39.3%] patients, respectively, P < 0.001), and were more likely to have a mixed RSV-adenoviral infection (29 [5.9%] vs. 42 [2.4%] patients, respectively, P < 0.001).
The mortality rate among patients included in the study during the 2-year period was 1.3%. Children who died, compared to children who survived, were more likely to have a comorbidity (19 [63.3%] vs. 937 [41.9%] patients, respectively, P = 0.018), and were more likely to have a nosocomial-acquired viral infection (6 [20.0%] vs. 87 [3.9%] patients, respectively, P < 0.001).
Effect of Risk Factors on Mixed RSV-Adenoviral Infections
The predictive variables included in the multivariate models were: age, gender, presence of comorbidities, and month of nasopharyngeal aspirates sampling. After controlling for gender, month of nasopharyngeal aspirates sampling, a previous history of prematurity, having some preexisting respiratory condition, a previous history of a congenital heart disease, having some preexisting renal disease, and the presence of cancer as a comorbidity, it was found that an age greater than 6 months and malnutrition as a comorbidity were independent predictors of mixed RSV-adenoviral infections in the sample of patients. Nasopharyngeal aspirates sampling during the 3-month period from March to May was nearly significant (P = 0.068) (Table III).
TABLE III.
Predictors of Dual (RSV and Adenovirus) Respiratory Infections in Multivariate Analysis
| Variable | Odds ratio (OR) (CI 95%) | P-value |
|---|---|---|
| Male gender | 1,21 (0,74–1,97) | 0,444 |
| Age greater than 6 months | 1,74 (1,05–2,89) | 0,030 |
| Prematurity | 0,83 (0,19–3,55) | 0,812 |
| Preexisting respiratory disease | 1,35 (0,82–2,24) | 0,237 |
| Congenital heart disease | 1,36 (0,18–10,47) | 0,763 |
| Malnutrition | 9,92 (1,01–100,9) | 0,049 |
| Cancer | 2,21 (0,27–17,79) | 0,453 |
| Other comorbidities | 1,37 (0,32–5,87) | 0,670 |
| NPA sampling during March to May* | 1,64 (0,96–2,80) | 0,068 |
Nasopharyngeal aspirates (NPA) in a month other than during the 3-month period from March to May, the period of the first rainy season in Colombia.
DISCUSSION
The present study shows that RSV and adenoviruses are significant causes of acute lower respiratory infection in infants and young children in low- and middle-income countries, especially during the first rainy season, which is defined as the 3-month period from March to May in Colombia. Additionally, the findings of the present study suggest that an age greater than 6 months and malnutrition as a comorbidity were independent predictors of mixed RSV-adenoviral infections in the study population.
The present findings could have important consequences as a consideration for future decision-making, such as the decision to test for adenovirus in patients already infected with RSV, especially if the patient’s condition is getting worse, due to the growing evidence suggesting greater severity and worse outcomes in children with dual compared to single respiratory virus infections. Detection of mixed RSV-adenoviral infections may have implications for patient management, such as lowering the threshold for admission to the hospital for children with acute lower respiratory infection or more closely monitoring the care of patients, in an effort to reduce the impact of the burden of acute lower respiratory infection in pediatric patients.
In agreement with other reports of studies done in countries located in tropical and sub-tropical regions with seasonal rainfall, the present study shows that the seasonality of RSV is associated with the rainy period of the city [Weber et al., 1998]. Likewise, although during the two analyzed periods adenoviruses were isolated throughout the year, the frequency of adenovirus infections was also higher during the 3-month period from March to May. However, the increase of cases during this 3-month period was less marked than in patients with single RSV infections. This pattern of isolation of adenoviruses differs from reports in Brazil and other tropical countries, in which adenovirus infections have been observed year round, without a clear seasonality [Vieira et al., 2001], but agrees with reports from other countries in which adenovirus are usually more frequent in winter and spring [Zou et al., 2012], with the findings of a previous report from Colombia in which adenovirus was detected throughout the year (excepting January) but peaks occurred in March, July, October, and December [Herrera-Rodriguez et al., 2007], and with local epidemiological surveillance reports (Secretary of Health of Bogota).
The reported rate of dual respiratory viral infections varies widely, ranging from zero in population-based cases and case-control studies [Lazar et al., 2004; van Woensel et al., 2006] to 100% in a single case report. The rate of dual respiratory infection in the study was only 3.1%, which is lower than in the majority of the previous reports. This could be caused by differences in study design, the performance ability and availability of diagnostic methods for viral detection, the number and type of pathogens assessed in each of the different studies, patient populations (e.g., differences in age, socioeconomic status, genetic immune response, previous immune condition, and comorbid conditions), and in the time of year of the study period (e.g., winter vs. summer or during epidemics of respiratory virus infections). In the present study, the only test for RSV and adenovirus was that using rapid immunochromatographic test methods, which may explain the lower rate of dual respiratory infection in comparison with other studies that simultaneously tested for multiple respiratory viruses and that used new and highly sensitive molecular amplification methods for viral detection. Therefore, it is highly probable that the actual rate of dual respiratory infections in the present study is greater than the one that was reported.
The findings with respect to predictors of mixed viral respiratory infections are consistent with other studies in which age greater than 6 months and a history of immunosupression were shown to be predictors of viral coinfection [Chorazy et al., 2013]. Cilla et al., in a study performed on children aged less than 3 years old with community-acquired pneumonia, noted that the percentage of coinfection was low for adenovirus (0.3%), and children aged less than 12 months were more likely to have a viral coinfection [Cilla et al., 2008]. With respect to the seasonal distribution of the respiratory infections, while in the present study the majority (73.2%) of the mixed RSV-adenovirus infections occurred during the 3-month period from March to May and nasopharyngeal aspirates sampling during the 3-month period from March to May was somewhat associated with mixed RSV-adenoviruses infections in the multivariate analysis, in the aforementioned study by Cilla et al., viral coinfections were more frequent in the cold months, when the number of circulating respiratory viruses is higher than in the remaining months. Taken together, these findings suggest that age, time of year in which viral circulation occurs, and conditions that are associated with immunodeficiency such as malnutrition could enhance the susceptibility of children to viral coinfections. There are a number of possible explanations for these associations. The immaturity of the immune system of young children, the lower levels of cellular immunity and poor antibody response during the acute phase of RSV infections in young infants, the lack of previous exposure to respiratory viruses, facilitation of infection with a second virus in children already infected by RSV, the protection provided by maternal antibodies against adenoviruses, which normally occurs in infants younger than 6 months, a more prolonged shedding of respiratory viruses in younger children compared to older children, and a greater incidence of viral respiratory infection as a whole in young children could explain the association between age and mixed-RSV infections that was found in the present study [Drews et al., 1997; Hong et al., 2001; Queiroz et al., 2002]. The greater probability of viral coinfections during the 3-month period from March to May could be due to the fact that the percentage of coinfection of each virus is determined by the greater or lesser possibility of its seasonal circulation’s coinciding with that of the other virus [Cilla et al., 2008], because both RSV and adenovirus detection peaked during the months of March through May for both years. With respect to the interaction between nutrition, immunodeficiency, and susceptibility to viral infections, there is convincing evidence showing that malnutrition is the primary cause of immunodeficiency worldwide, with infants, children, adolescents, and the elderly being the most-affected groups [Katona and Katona-Apte, 2008], and that an impaired T-cell response secondary to effects relating to thymic architecture and function is the most common effect of malnutrition on the immune system, which could enhance the susceptibility of children to viral coinfections. There are several mechanisms that lead to an altered immune response in the malnourished host during infectious exposure, increasing the severity and morbidity of clinical infections. These mechanisms include alterations in the production of cytokines by the adaptive immune system, an impaired cytokine response to antigens [Rodriguez et al., 2005], increased levels of circulating proinflammatory cytokines [Dulger et al., 2002], blunting of the acute phase response to infection [Manary et al., 2004], a weaker ability to produce a compensatory anti-inflammatory response [Schultz et al., 2004], and impairment of chemotaxis, phagocytosis, and microbial killing mechanisms due to a reduction of the production of key mediators such as complement C3, leukotrienes, cathelicidin antimicrobial peptide, and leptin. However, given that the finding of malnutrition as an independent predictor of mixed RSV-adenoviral infections is based on a limited number of patients, the results from such analyses need to be interpreted with caution.
The main limitations of the present study are the small number of predictor variables included in the multivariate analysis and the fact that it was conducted in a specialized, tertiary referral hospital. Although a small number of predictor variables in the multivariate analysis was included, it can be seen that the majority of the variables that have been reported in the literature as potential confounders was included. However, as is the case for other observational epidemiologic studies, residual confounding cannot be excluded, so interpretation of the results needs to be cautious. The fact that the study was conducted in a specialized, tertiary referral hospital makes it likely that the patients included represent the extreme of the spectrum of severity of all patients with RSV and adenovirus infection, which could limit the generalization of the results to other contexts. The main strength of this study is a sample size greater than that of similar epidemiological reports, thus reducing random error and increasing the accuracy of the estimates.
In conclusion, the present study shows that RSV and adenoviruses are significant causes of acute lower respiratory infection in infants and young children in low- and middle-income countries, especially during the first rainy season. The identified predictors of mixed RSV-adenoviral infections should be taken into account when planning intervention, in order to reduce the burden of acute lower respiratory infection in young children living in these regions.
Acknowledgments
We thank Mr. Charlie Barrett for his editorial assistance.
Grant sponsor: National Institutes of Health (NIH) Career Development Award; Grant numbers: K12HL090020; K12HD001399–13.
Institution at which the work was performed: Fundacion Hospital La Misericordia Bogota Colombia
References
- Berman S. Epidemiology of acute respiratory infections in children of developing countries. Rev Infect Dis. 1991;13:S454–S462. doi: 10.1093/clinids/13.supplement_6.s454. [DOI] [PubMed] [Google Scholar]
- Calvo C, Garcia-Garcia ML, Blanco C, Vazquez MC, Frias ME, Perez-Brena P, Casas I. Multiple simultaneous viral infections in infants with acute respiratory tract infections in Spain. J Clin Virol. 2008;42:268–272. doi: 10.1016/j.jcv.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorazy ML, Lebeck MG, McCarthy TA, Richter SS, Torner JC, Gray GC. Polymicrobial Acute Respiratory Infections in a Hospital-Based Pediatric Population. Pediatr Infect Dis J. 2013;33:460–466. doi: 10.1097/INF.0b013e31828683ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilla G, Oñate E, Perez-Yarza EG, Montes M, Vicente D, Perez-Trallero E. Viruses in community-acquired pneumonia in children aged less than 3 years old: High rate of viral coinfection. J Med Virol. 2008;80:1843–1849. doi: 10.1002/jmv.21271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LF, Yokosawa J, Mantese OC, Oliveira TF, Silveira HL, Nepomuceno LL, Moreira LS, Dyonisio G, Rossi LM, Oliveira RC, Ribeiro LZ, Queiroz DA. Respiratory viruses in children younger than five years old with acute respiratory disease from 2001 to in Uberlandia, MG, Brazil. Mem Inst Oswaldo Cruz. 2006;101:301–306. doi: 10.1590/s0074-02762006000300014. [DOI] [PubMed] [Google Scholar]
- De PM, Gilio AE, Ferraro AA, Ferronato AE, do Sacramento PR, Botosso VF, Oliveira DB, Marinheiro JC, Harsi CM, Durigon EL, Vieira SE. Severity of viral coinfection in hospitalized infants with respiratory syncytial virus infection. J Pediatr (Rio J) 2011;87:307–313. doi: 10.2223/JPED.2100. [DOI] [PubMed] [Google Scholar]
- Drews AL, Atmar RL, Glezen WP, Baxter BD, Piedra PA, Green-berg SB. Dual respiratory virus infections. Clin Infect Dis. 1997;25:1421–1429. doi: 10.1086/516137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulger H, Arik M, Sekeroglu MR, Tarakcioglu M, Noyan T, Cesur Y, Balahoroglu R. Pro-inflammatory cytokines in Turkish children with protein-energy malnutrition. Mediators Inflamm. 2002;11:363–365. doi: 10.1080/0962935021000051566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Rodriguez DH, de la Hoz F, Marino C, Ramirez E, Lopez JD, Velez C. Adenovirus in children under five years of age. Circulation patterns and clinical and epidemiological characteristics in Colombia, 1997–2003. Rev Salud Publica (Bogota) 2007;9:420–429. doi: 10.1590/s0124-00642007000300010. [DOI] [PubMed] [Google Scholar]
- Hong JY, Lee HJ, Piedra PA, Choi EH, Park KH, Koh YY, Kim WS. Lower respiratory tract infections due to adenovirus in hospitalized Korean children: Epidemiology, clinical features, and prognosis. Clin Infect Dis. 2001;32:1423–1429. doi: 10.1086/320146. [DOI] [PubMed] [Google Scholar]
- Jennings LC, Anderson TP, Werno AM, Beynon KA, Murdoch DR. Viral etiology of acute respiratory tract infections in children presenting to hospital: Role of polymerase chain reaction and demonstration of multiple infections. Pediatr Infect Dis J. 2004;23:1003–1007. doi: 10.1097/01.inf.0000143648.04673.6c. [DOI] [PubMed] [Google Scholar]
- Katona P, Katona-Apte J. The interaction between nutrition and infection. Clin Infect Dis. 2008;46:1582–1588. doi: 10.1086/587658. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Hong JY, Lee HJ, Shin SH, Kim YK, Inada T, Hashido M, Piedra PA. Genome type analysis of adenovirus types 3 and 7 isolated during successive outbreaks of lower respiratory tract infections in children. J Clin Microbiol. 2003;41:4594–4599. doi: 10.1128/JCM.41.10.4594-4599.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley GF, Anderson LJ. Epidemiology and prevention of respiratory syncytial virus infections among infants and young children. Pediatr Infect Dis J. 2011;30:510–517. doi: 10.1097/INF.0b013e3182184ae7. [DOI] [PubMed] [Google Scholar]
- Larranaga C, Kajon A, Villagra E, Avendano LF. Adenovirus surveillance on children hospitalized for acute lower respiratory infections in Chile (1988–1996) J Med Virol. 2000;60:342–346. [PubMed] [Google Scholar]
- Lazar I, Weibel C, Dziura J, Ferguson D, Landry ML, Kahn JS. Human metapneumovirus and severity of respiratory syncytial virus disease. Emerg Infect Dis. 2004;10:1318–1320. doi: 10.3201/eid1007.030983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PF, Schmidt MA, Lu X, Erdman DD, Campbell M, Thomas A, Cieslak PR, Grenz LD, Tsaknardis L, Gleaves C, Kendall B, Gilbert D. A community-based outbreak of severe respiratory illness caused by human adenovirus serotype 14. J Infect Dis. 2009;199:1427–1434. doi: 10.1086/598521. [DOI] [PubMed] [Google Scholar]
- Manary MJ, Yarasheski KE, Berger R, Abrams ET, Hart CA, Broadhead RL. Whole-body leucine kinetics and the acute phase response during acute infection in marasmic Malawian children. Pediatr Res. 2004;55:940–946. doi: 10.1203/01.pdr.0000127017.44938.6d. [DOI] [PubMed] [Google Scholar]
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O’Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sedyaningsih Sutanto A, Ngama M, Munywoki PK, Kartasasmita C, Simoes EA, Rudan I, Weber MW, Campbell H. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomino MA, Larranaga C, Avendano LF. Hospital-acquired adenovirus 7h infantile respiratory infection in Chile. Pediatr Infect Dis J. 2000;19:527–531. doi: 10.1097/00006454-200006000-00007. [DOI] [PubMed] [Google Scholar]
- Palomino MA, Larranaga C, Villagra E, Camacho J, Avendano LF. Adenovirus and respiratory syncytial virus-adenovirus mixed acute lower respiratory infections in Chilean infants. Pediatr Infect Dis J. 2004;23:337–341. doi: 10.1097/00006454-200404000-00012. [DOI] [PubMed] [Google Scholar]
- Papadopoulos NG, Moustaki M, Tsolia M, Bossios A, Astra E, Prezerakou A, Gourgiotis D, Kafetzis D. Association of rhinovirus infection with increased disease severity in acute bronchiolitis. Am J Respir Crit Care Med. 2002;165:1285–1289. doi: 10.1164/rccm.200112-118BC. [DOI] [PubMed] [Google Scholar]
- Queiroz DA, Durigon EL, Botosso VF, Ejzemberg B, Vieira SE, Mineo JR, Yamashita C, Hein N, Lopes CL, Cacharo AL, Stewien KE. Immune response to respiratory syncytial virus in young Brazilian children. Braz J Med Biol Res. 2002;35:1183–1193. doi: 10.1590/s0100-879x2002001000011. [DOI] [PubMed] [Google Scholar]
- Rodriguez L, Gonzalez C, Flores L, Jimenez- Zamudio L, Graniel J, Ortiz R. Assessment by flow cytometry of cytokine production in malnourished children. Clin Diagn Lab Immunol. 2005;12:502–507. doi: 10.1128/CDLI.12.4.502-507.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez DA, Rodriguez-Martinez CE, Cardenas AC, Quilaguy IE, Mayorga LY, Falla LM, Nino G. Predictors of severity and mortality in children hospitalized with respiratory syncytial virus infection in a tropical region. Pediatr Pulmonol. 2014;49:269–276. doi: 10.1002/ppul.22781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitha MR, Nandeeshwara SB, Pradeep Kumar MJ, ul-Haque K, Raju CK. Modifiable risk factors for acute lower respiratory tract infections. Indian J Pediatr. 2007;74:477–482. doi: 10.1007/s12098-007-0081-3. [DOI] [PubMed] [Google Scholar]
- Schultz C, Temming P, Bucsky P, Gopel W, Strunk T, Hartel C. Immature anti-inflammatory response in neonates. Clin Exp Immunol. 2004;135:130–136. doi: 10.1111/j.1365-2249.2004.02313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple MG, Cowell A, Dove W, Greensill J, McNamara PS, Halfhide C, Shears P, Smyth RL, Hart CA. Dual infection of infants by human metapneumovirus and human respiratory syncytial virus is strongly associated with severe bronchiolitis. J Infect Dis. 2005;191:382–386. doi: 10.1086/426457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Woensel JB, Bos AP, Lutter R, Rossen JW, Schuurman R. Absence of human metapneumovirus co-infection in cases of severe respiratory syncytial virus infection. Pediatr Pulmonol. 2006;41:872–874. doi: 10.1002/ppul.20459. [DOI] [PubMed] [Google Scholar]
- Videla C, Carballal G, Misirlian A, Aguilar M. Acute lower respiratory infections due to respiratory syncytial virus and adenovirus among hospitalized children from Argentina. Clin Diagn Virol. 1998;10:17–23. doi: 10.1016/s0928-0197(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Vieira SE, Stewien KE, Queiroz DA, Durigon EL, Torok TJ, Anderson LJ, Miyao CR, Hein N, Botosso VF, Pahl MM, Gilio AE, Ejzenberg B, Okay Y. Clinical patterns and seasonal trends in respiratory syncytial virus hospitalizations in Sao Paulo. Brazil Rev Inst Med Trop Sao Paulo. 2001;43:125–131. doi: 10.1590/s0036-46652001000300002. [DOI] [PubMed] [Google Scholar]
- Weber MW, Mulholland EK, Greenwood BM. Respiratory syncytial virus infection in tropical and developing countries. Trop Med Int Health. 1998;3:268–280. doi: 10.1046/j.1365-3156.1998.00213.x. [DOI] [PubMed] [Google Scholar]
- Zou L, Zhou J, Li H, Wu J, Mo Y, Chen Q, Fang L, Wu D, Wu J, Ke C. Human adenovirus infection in children with acute respiratory tract disease in Guangzhou, China. APMIS. 2012;120:683–688. doi: 10.1111/j.1600-0463.2012.02890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


