Abstract
Heterotopic ossification (HO, also termed myositis ossificans) is the formation of extra-skeletal bone in muscle and soft tissues. HO is a tissue repair process gone awry, and is a common complication of surgery and traumatic injury. Medical strategies to prevent and treat HO fall well short of addressing the clinical need. Better characterization of the tissues supporting HO is critical to identifying therapies directed against this common and sometimes devastating condition. The physiologic processes of osteogenesis and angiogenesis are highly coupled and interdependent. However, little efforts have been made to document the vascular patterning within heterotopic ossification. Here, surgical pathology case files of 29 human HO specimens were examined by vascular histomorphometric analysis. Results demonstrate a temporospatial patterning of HO vascularity that depends on the ‘maturity’ of the bony lesion. In sum, human HO demonstrates a time and space dependent pattern of vascularization suggesting a coupled pathophysiologic process involving the coordinate processes of osteogenesis and angiogenesis. Further imaging studies may be used to further characterize vasculogenesis within HO and whether anti-angiogenic therapies are a conceivable future therapy for this common condition.
Keywords: angiogenesis, vasculogenesis, osteogenesis, ectopic bone, heterotopic bone
1. Introduction
Heterotopic ossification (HO, also termed myositis ossificans) is the pathologic development of extra-skeletal bone which develops after musculoskeletal trauma, spinal cord injury, or severe burns. In a distinct but related genetic disease, patients with an activating mutation in the type I bone morphogenetic protein (BMP) receptor ACVR1 develop ectopic bone after minimal soft tissue trauma(1). HO is surprisingly common, and has been reported to occur at a frequency of 90% after certain types of hip arthroplasty or acetabular fractures(2-7). HO represents a major health burden to the patient, associated with pain, mobility impairments, deep vein thrombosis, nerve entrapment, and poor wound healing. Reports of disability associated with HO are variable, but may exceed a frequency of 20%, owing to soft-tissue loss, joint contracture, and chronic pain(8).
The histopathologic appearance of heterotopic ossification evolves over time. Early lesions demonstrate a hypercellular proliferation of spindle cells often with little bone matrix. Spindled areas often have features of nodular fasciitis (NF), including high numbers of normal mitotic figures, scattered multinucleated giant cells, and extravasated erythrocytes. As ossification ensues, woven bone with prominent osteoblast lining is characteristic. Cartilage and endochondral ossification may be seen, but are usually focal and not seen in most cases. More mature lesions show thickened trabeculae of lamellar bone with less prominent osteoblastic rimming. Late in the evolution of HO, lesions develop an appearance similar to compact bone. On intact surgical pathology specimens, a zonation phenomenon is often appreciated, with the most mature bone tissue observed at the periphery. On resection, HO is most often bounded by a thick fibrous capsule. Despite the well characterized phasic appearance of HO that forms a temporospatial continuum, a detailed vascular histomorphometric analysis is lacking. Here, we report on the vascular patterning of human HO with a particular emphasis on vascular modeling across the histologic spectrum of HO.
2. Materials and Methods
2.1. Identification of HO samples
35 cases of heterotopic ossification (HO) were identified in our surgical pathology archives (Johns Hopkins University and University of California, Los Angeles). Cases were obtained under IRB approval with waiver of informed consent. All material was coded so as to protect the confidentiality of personal health information. Six cases were excluded due to insufficient material to analyze. A total of 29 cases were included in the study, with two independent pathologists verifying the diagnostic accuracy (E.M., A.W.J.).
2.2. Image acquisition and categorization of HO samples
Random histologic images were taken of each sample with the goal of encompassing distinct areas of each lesion. Depending on the size of the specimen, between 3 and 17 images of each case were taken. A total of 177 images were analyzed. Next, images were examined (by M.C. and A.W.J) and categorized base on their predominant histologic appearance. The following five subcategories were employed:
nodular fasciitis-like – sheets and fascicles of plump spindled myofibroblastic cells with minimal bone formation;
HO with woven bone – composed predominantly of woven bone with prominent osteoblastic rimming;
HO with lamellar bone – composed predominantly of thickened trabeculae of lamellar bone;
fibrous capsule – the thickened fibrous capsule immediately peripheral to lesional tissue;
cartilaginous areas – areas of prominent cartilaginous differentiation or endochondral ossification.
2.3. Vascular histomorphometric analysis of HO samples
Each image was analyzed in a blinded fashion to determine the following parameters: blood vessel number (as defined by the total vessel number per 10× field), blood vessel area (as defined by the total vascular area per 10× field), blood vessel density (as defined as blood vessel area / tissue area per 10× field), blood vessel size (as defined by blood vessel area/number per 10× field), and blood vessel wall thickness (as defined by mean vessel wall thickness per 10× field). Vessel number was counted manually, while all other calculations were performed using Adobe Photoshop CS6 (San Jose, CA). Vessel area was determined using the magic wand tool in Adobe Photoshop in conjunction with the measurement log function to measure area in pixel units. Density was calculated by dividing vessel area by total photograph tissue area, as determined using Adobe Photoshop. To account for the difference between a large number of small vessels and a small number of large vessels, vessel area was divided by vessel number to determine average vessel size in each slide. Vessel thickness was measured using the “ruler tool” in Adobe Photoshop and thickness was measured in pixel units. Use of Adobe Photoshop based quantifications are illustrated in Supplemental Figure 1. Graphical representations were constructed using GraphPad Prism (La Jolla, CA).
3. Results
3.1. Patient demographics and histologic appearance
A total of 29 cases of heterotopic ossification (HO) were included in our study. Patients were evenly distributed by age (14 male, 15 female). Mean patient age was 37.9 years (range: 6-76) at the time of resection. HO affected the lower extremity in most cases (n=17), followed by the upper extremity (n=7), or back/paraspinal area (n=3). Many cases were juxta-articular, including the hip (n=4), knee (n=5), elbow (n=1), wrist or ankle (n=3). Patient demographics and specific location of each lesion are further detailed in Supplemental Table 1.
Typical histologic features of each HO were next examined, using our previously described categories to describe the histologic spectrum of HO (Fig. 1). Categories included:
nodular fasciitis (NF)-like areas, in which sheets and fascicles of spindled fibroblastic / myofibroblastic cells were observed resembling nodular fasciitis or granulation tissue (Fig. 1A,B). Only scattered / minimal bone formation was observed in these areas, which tended to involve the central aspects of the lesion. Blood vessels in NF-like areas consisted of numerous, slender, elongated, and thin-walled vascular channels with a similar orientation to the spindled stroma. Samples with NF-like areas were less common (n=11 cases).
HO with woven bone, in which the lesional bone is predominantly composed of woven bone with prominent osteoblastic rimming (Fig. 1C,D). Blood vessels within the interstices between woven bone trabeculae were thin walled, slightly dilated, and numerous. All cases showed at least some areas with woven bone.
HO with lamellar bone, in which the lesional bone is predominantly composed of thickened trabeculae of lamellar bone (Fig. 1E,F). These more mature zones were generally seen farther toward the periphery of the lesion. Blood vessels within the marrow spaces were thin-walled, variably sized and often ectactic or dilated, resembling sinusoids of bone marrow. All cases showed at least some areas with lamellar bone.
fibrous capsule, in which compressed fibrous tissue was obtained immediately peripheral to lesional tissue (Fig. 1G,H), which occasionally demonstrated a more edematous appearance. A mixture of thick and thin-walled vessels resembling arterial and venous channels were observed within the fibrous capsule areas. Samples with a fibrous capsule were identified in n=21 cases.
cartilaginous areas, in which areas of prominent cartilaginous differentiation or endochondral ossification were present (Fig. 1I,J). Rare blood vessels were observed within cartilaginous areas, mostly circumscribing these areas. Cartilaginous areas were found in n=8 cases.
Figure 1.
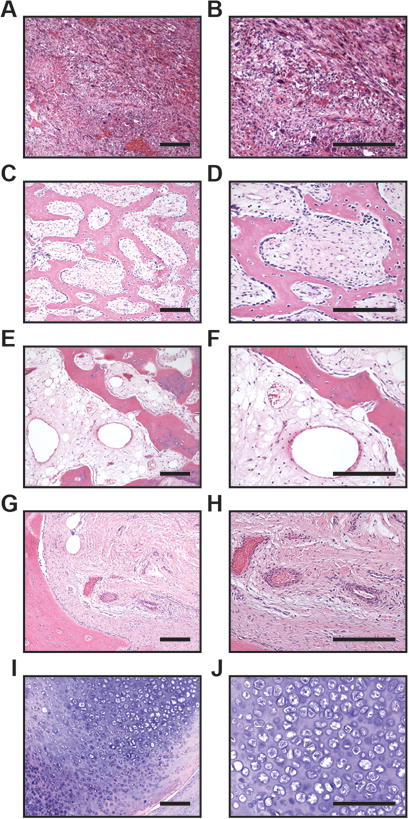
Representative histologic images of heterotopic ossification. (A,B) Typical histologic images of areas with nodular fasciitis-like appearance. Lesion is highly cellular and composed of sheets and fascicles of spindled cells, with scattered multinucleated giant cells, rare foci of bone matrix formation, and numerous slender, elongated capillary-type vessels. (C,D) Typical areas of HO with woven bone. Decalcified section demonstrates anastomosing trabeculae of woven bone with prominent osteoblast lining. The intervening fibrous stroma houses thin-walled capillaries and veins. (E,F) Typical areas of HO with lamellar bone. Decalcified sections composed of thickened trabeculae of lamellar bone. The intervening stroma is composed of fibrous and adipose tissue, with increasing numbers of dilated vessels resembling sinusoids of the bone marrow. (G,H) Typical appearance of outer aspect of HO with fibrous capsule immediately peripheral to lesional tissue. Dense collagenous tissue houses a mixture of thick-walled arteries as well as veins. (I,J) Typical foci of cartilage and endochondral ossification, with rare vessels predominantly at the periphery of cartilage lobules. Scale bar: 100 um.
By definition, all cases had at least some reactive bone formation. 4 of 29 cases showed areas with well-developed bone tissue resembling compact bone. Although this was a distinguishing feature in a minority of cases, the abundance of bone matrix and low apparent vascularization in these areas prompted exclusion of these areas from further study. No cases included features of malignancy, such as a disorderly growth pattern of hyperchromatic or pleomorphic cells, significant cellular atypia, or atypical mitotic figures.
3.2. Vascular histomorphometry
Next, vascular indices were examined across the histologic spectrum of HO. Blood Vessel Number (BV.N) was high across most histologic areas of HO, with mean BV.N of ~8-10 per 10× field (Fig. 2A). Cartilaginous areas were the exception, which generally showed few blood vessels that often skirted the outside of the chondrocytic areas (mean BV.N of ~2 per 10× field). Between histologic areas of HO, a trend towards increased BV.N was observed among NF-like and woven bone areas.
Figure 2.
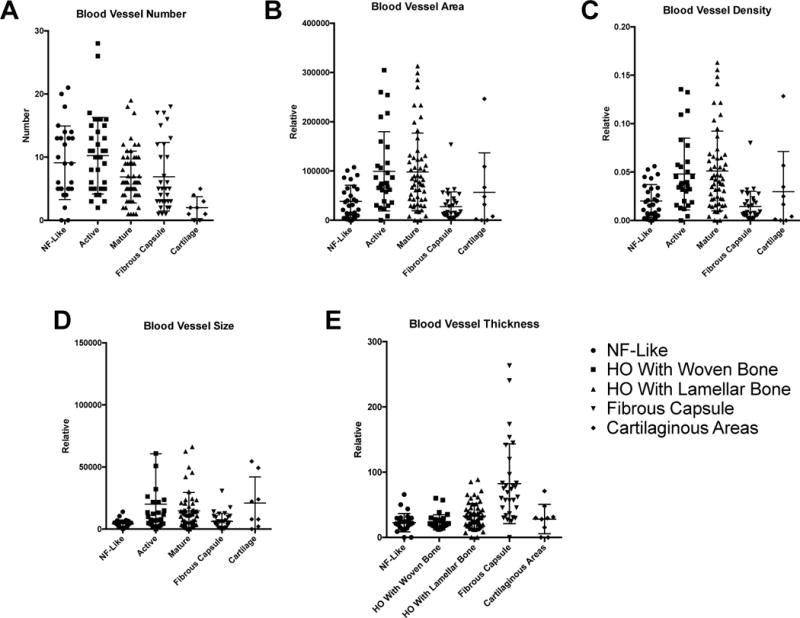
Histomorphometric analysis of vascularity among samples of heterotopic ossification. Vascular parameters were assessed among random histologic fields of HO, and categorized by their predominant histologic appearance into five categories: ‘NF-like, woven bone, lamellar bone, fibrous capsule, cartilage.’ Each dot represents a single photographic image. Means and SEM are depicted. (A) Blood vessel number per 10× field, (B) relative blood vessel area per 10× field, (C) relative blood vessel density per 10× field, (D) relative blood vessel size per 10× field, and (E) relative blood vessel thickness per 10× field.
Blood vessel area (BV.Ar) and (BV.D) were next examined across the histologic spectrum of HO (Fig. 2B,C), with similar trends observed with both indices. NF-like areas showed relative low BV.Ar and BV.D, predominantly owing to the small size of individual capillary-type vessels. Areas of woven bone and lamellar bone showed high BV.Ar and BV.D, although large variability was observed between images. Vessels within the fibrous capsule showed relative reduced BV.Ar and BV.D in comparison to the lesional HO tissue. This represented a predominance of small- and medium-sized arteries and veins within the capsule tissue. Within cartilaginous areas, BV.Ar and BV.D was variable but overall low.
Blood vessel size next examined across the histologic spectrum of HO (Fig. 2D). Vessels within NF-like areas and within fibrous capsule were typically small in size, representing slender, elongated capillary-type vessels. Areas of woven bone showed more variable distribution in blood vessel size, characterized by numerous capillaries interspersed with a fraction of larger caliber veins. Areas of lamellar bone showed the greatest number of larger vessels, which often resembled the sinusoids of normal bone marrow. Within cartilaginous areas, blood vessel size was variable.
Blood vessel wall thickness was finally examined across the histologic spectrum of HO (Fig. 2E). Most histologic areas were composed of predominately thin-walled capillaries and veins, including NF-like areas, woven bone, lamellar bone, and cartilaginous areas. Notably, the fibrous capsule was distinguished from other areas of HO by a larger number of thick-walled arteries.
4. Discussion
HO is a benign ossifying process of the soft tissues whose pathogenesis still remains poorly understood. As HO generally follows local trauma and/or inflammatory insults, it is generally conceived of as a tissue repair process gone awry. The histologic spectrum of HO is wide and is separated spatially and temporally. To some extent this likely reflects the evolving ossification of a lesion, which represents a combination of endochondral and intramembranous bone formation. Here, our work suggests that temporospatial spectrum of HO also includes significant vascular modeling and remodeling.
Briefly, each characteristic ‘zone’ of HO depicts a distinct vascular appearance. The earliest stage of the lesion – the NF-like stage – displays the highest number of thin-walled capillaries, which are of the smallest caliber. HO with woven bone is marked by a high number of vessels, with variably enlarged size. As the lesion matures, HO areas with lamellar bone have fewer vessels but these thin-walled, dilated vessels are of the largest size. Cartilaginous areas are typically avascular, with small numbers of vessels in the nearby stroma.
To our knowledge, this is the first study in which detailed vascular histomorphometric analysis was been performed on the pathologic process of heterotopic ossification. Our findings suggest that a distinctive vascular patterning occurs within HO that coincides with the lesion’s ossification and maturation, and that angiogenesis and osteogenesis are coupled processes in HO. Of course, there is significant evidence supporting how angiogenesis and osteogenesis are related. While the exact triggers of blood vessel formation in osteogenesis are not known, it has been shown that osteoblasts play a regulatory role in angiogenesis as sensors of hypoxia. In response to low oxygen levels, osteoblasts activate the hypoxia-inducible factor alpha (HIF-α) pathway(9). In turn, the HIF-α pathway up-regulates vascular endothelial growth factor (VEGF), a cytokine that is essential in vessel formation, as well as other angiogenic factors (10). Various normal and pathologic conditions require osteoblasts to respond to low oxygen and nutrient levels. During fracture healing, for example, increased VEGF expression has been shown to support angiogenesis during skeletal repair(11). Similarly, in mice models, inducing the HIF-α pathway through low oxygen levels promotes long bone modelling and acquisition(9). Conversely, mice with mutant HIF-α develop narrow bones with thinner cortices thought to be secondary to impaired vasculature(12). It has also been shown that the HIF-α pathway can be genetically and pharmacologically manipulated to promote bone healing(13). These studies, along with our observations, lend support to the view of HO as a coordinate pathologic process of osteogenesis and angiogenesis.
If HO represents an interdependent process of osteogenesis and angiogenesis, it is intriguing to make conjectures about the outcome of anti-angiogenic therapies with HO. Experimental studies have clearly shown a dynamic temporospatial pattern of VEGF expression that coincides with vascular proliferation in HO. However, differences in model systems, timepoints, and methods of analysis have resulted in some heterogeneous observations. For example, some experimental HO studies have observed VEGF expression and vascular proliferation as early as 48 hours after incitement of the HO process(14), occurring even before frank cartilage was observed. In other experimental models, VEGF expression and vascular invasion was observed predominantly after chondrogenesis - as cartilage was invaded by vessels and replaced by new-formed bone(15, 16). With these observations in mind, it is unclear what the exact phenotypic consequences would be of anti-angiogenic therapies on the histopathology of HO. Undoubtedly, this would depend on the timing of therapy, as well as the extent to which cartilaginous differentiation was present within a given lesion.
Importantly, the histopathologic appearance of HO is ultimately a spectrum that differs both spatially across a lesion as well as temporally across periods in time. With this in mind, we recognize that our categorization of HO images is artefactual and does not represent a rigid framework for future studies. Instead, we used simple categorical assignments so as to provide a frame of reference for vascular histomorphometric analysis. We anticipate that future studies that use radiographic detection of bone and vasculature could provide far more detailed, correlative assessments of vascularity within HO.
For the practicing pathologist, the most important diagnostic distinction is between HO and extraskeletal osteosarcoma (OS). Presence of clinical risk factors for HO may suggest the diagnosis, including trauma, orthopaedic surgery, or traumatic brain injury. A typical radiographic appearance suggestive of HO is a well-defined lesion with a zonal mineralization pattern. This includes peripheral radiodensity and central lucency, imparting an appearance of so called ‘eggshell calcification.’ Comparisons to prior radiographic imaging may be helpful to assure a benign diagnosis. Helpful histologic findings of HO include presence of bone ‘maturation’ and spatial zonation with more peripherally mature bony elements. As well, the absence of frankly sarcomatous features is an important diagnostic distinction, including absence of anaplasia and atypical mitotic figures. The most challenging distinction is between early HO with nodular fasciitis-like features and extraskeletal OS. Any challenging biopsy that presents a diagnostic dilemma should be reviewed in the context of clinical and radiographic findings, with a low threshold for consulting an orthopaedic pathologist. Like HO, extraskeletal OS is a highly vascular lesion with high microvascular density(17). Angiogenic markers, such as tumor-derived VEGF expression, as well as indices of vascularity correlate with outcome in OS(18, 19). Elevated tumor and circulating VEGF levels are associated with pulmonary metastasis(20), although this is not a universal finding across all studies(17). To date, it is unclear if the histologic appearance or histomorphometric analysis of blood vessels has any helpful role in distinguishing HO from extraskeletal OS.
In sum, human HO demonstrates a consistent pattern of vascularization suggesting a highly coupled pathophysiologic process involving the coordinate processes of osteogenesis and angiogenesis. Studies such as these beg the question of whether anti-angiogenic therapies are a conceivable future therapy for this common condition.
Supplementary Material
Schematic of vascular histomorphometric analysis. High magnification image of fibrous capsule shown. (A) Original picture, demonstrating a high density of vessels. (B) Identification of arteries (A), veins (V), and capillaries (C) by light microscopy. Blood vessel number determined by a manual count per 10× field. (C) Manual tracing for blood vessels performed in Adobe Photoshop using the magic wand tool. Used to determine blood vessel area, blood vessel density, and blood vessel size. (D) Blood vessel wall thickness, determined by using the ruler tool in Adobe Photoshop.
Acknowledgments
We thank Drs. Scott Nelson and Evelina Kodrat for their sharing of case files.
The present work was supported by the NIH/NIAMS (K08 AR068316), Orthopaedic Research and Education Foundation with funding provided by the Musculoskeletal Transplant Foundation, and Catherine and Constantinos J. Limas Research Award.
References
- 1.Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nature genetics. 2006;38(5):525–7. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 2.Maender C, Sahajpal D, Wright TW. Treatment of heterotopic ossification of the elbow following burn injury: recommendations for surgical excision and perioperative prophylaxis using radiation therapy. J Shoulder Elbow Surg. 2010;19(8):1269–75. doi: 10.1016/j.jse.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 3.Bedi A, Zbeda RM, Bueno VF, Downie B, Dolan M, Kelly BT. The incidence of heterotopic ossification after hip arthroscopy. Am J Sports Med. 2012;40(4):854–63. doi: 10.1177/0363546511434285. [DOI] [PubMed] [Google Scholar]
- 4.Cipriano CA, Pill SG, Keenan MA. Heterotopic ossification following traumatic brain injury and spinal cord injury. J Am Acad Orthop Surg. 2009;17(11):689–97. doi: 10.5435/00124635-200911000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Rath E, Sherman H, Sampson TG, Ben Tov T, Maman E, Amar E. The incidence of heterotopic ossification in hip arthroscopy. Arthroscopy. 2013;29(3):427–33. doi: 10.1016/j.arthro.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Medina A, Shankowsky H, Savaryn B, Shukalak B, Tredget EE. Characterization of heterotopic ossification in burn patients. J Burn Care Res. 2014;35(3):251–6. doi: 10.1097/BCR.0b013e3182957768. [DOI] [PubMed] [Google Scholar]
- 7.Chen HC, Yang JY, Chuang SS, Huang CY, Yang SY. Heterotopic ossification in burns: our experience and literature reviews. Burns. 2009;35(6):857–62. doi: 10.1016/j.burns.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Ranganathan K, Loder S, Agarwal S, Wong VW, Wong VC, Forsberg J, et al. Heterotopic Ossification: Basic-Science Principles and Clinical Correlates. J Bone Joint Surg Am. 2015;97(13):1101–11. doi: 10.2106/JBJS.N.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Wan C, Deng L, Liu X, Cao X, Gilbert SR, et al. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest. 2007;117(6):1616–26. doi: 10.1172/JCI31581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akeno N, Robins J, Zhang M, Czyzyk-Krzeska MF, Clemens TL. Induction of vascular endothelial growth factor by IGF-I in osteoblast-like cells is mediated by the PI3K signaling pathway through the hypoxia-inducible factor-2alpha. Endocrinology. 2002;143(2):420–5. doi: 10.1210/endo.143.2.8639. [DOI] [PubMed] [Google Scholar]
- 11.Street J, Winter D, Wang JH, Wakai A, McGuinness A, Redmond HP. Is human fracture hematoma inherently angiogenic? Clinical orthopaedics and related research. 2000;(378):224–37. doi: 10.1097/00003086-200009000-00033. [DOI] [PubMed] [Google Scholar]
- 12.Riddle RC, Leslie JM, Gross TS, Clemens TL. Hypoxia-inducible factor-1alpha protein negatively regulates load-induced bone formation. The Journal of biological chemistry. 2011;286(52):44449–56. doi: 10.1074/jbc.M111.276683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan C, Gilbert SR, Wang Y, Cao X, Shen X, Ramaswamy G, et al. Activation of the hypoxia-inducible factor-1alpha pathway accelerates bone regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(2):686–91. doi: 10.1073/pnas.0708474105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dilling CF, Wada AM, Lazard ZW, Salisbury EA, Gannon FH, Vadakkan TJ, et al. Vessel formation is induced prior to the appearance of cartilage in BMP-2-mediated heterotopic ossification. J Bone Miner Res. 2010;25(5):1147–56. doi: 10.1359/jbmr.091031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ueno T, Kagawa T, Kanou M, Fujii T, Fukunaga J, Mizukawa N, et al. Immunolocalization of vascular endothelial growth factor during heterotopic bone formation induced from grafted periosteum. Ann Plast Surg. 2004;53(2):150–4. doi: 10.1097/01.sap.0000110752.55981.41. [DOI] [PubMed] [Google Scholar]
- 16.Kakudo N, Kusumoto K, Wang YB, Iguchi Y, Ogawa Y. Immunolocalization of vascular endothelial growth factor on intramuscular ectopic osteoinduction by bone morphogenetic protein-2. Life Sci. 2006;79(19):1847–55. doi: 10.1016/j.lfs.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 17.Ek ET, Ojaimi J, Kitagawa Y, Choong PF. Does the degree of intratumoural microvessel density and VEGF expression have prognostic significance in osteosarcoma? Oncol Rep. 2006;16(1):17–23. [PubMed] [Google Scholar]
- 18.Lammli J, Fan M, Rosenthal HG, Patni M, Rinehart E, Vergara G, et al. Expression of Vascular Endothelial Growth Factor correlates with the advance of clinical osteosarcoma. Int Orthop. 2012;36(11):2307–13. doi: 10.1007/s00264-012-1629-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coomber BL, Denton J, Sylvestre A, Kruth S. Blood vessel density in canine osteosarcoma. Can J Vet Res. 1998;62(3):199–204. [PMC free article] [PubMed] [Google Scholar]
- 20.DuBois S, Demetri G. Markers of angiogenesis and clinical features in patients with sarcoma. Cancer. 2007;109(5):813–9. doi: 10.1002/cncr.22455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schematic of vascular histomorphometric analysis. High magnification image of fibrous capsule shown. (A) Original picture, demonstrating a high density of vessels. (B) Identification of arteries (A), veins (V), and capillaries (C) by light microscopy. Blood vessel number determined by a manual count per 10× field. (C) Manual tracing for blood vessels performed in Adobe Photoshop using the magic wand tool. Used to determine blood vessel area, blood vessel density, and blood vessel size. (D) Blood vessel wall thickness, determined by using the ruler tool in Adobe Photoshop.


