Abstract
Background
Vaccine-derived polioviruses (VDPVs), strains of poliovirus mutated from the oral polio vaccine, pose a challenge to global polio eradication. Immunodeficiency-related vaccine-derived polioviruses (iVDPVs) are a type of VDPV which may serve as sources of poliovirus reintroduction after the eradication of wild-type poliovirus. This review is a comprehensive update of confirmed iVDPV cases published in the scientific literature from 1962 to 2012, and describes clinically relevant trends in reported iVDPV cases worldwide.
Methods
We conducted a systematic review of published iVDPV case reports from January 1960 to November 2012 from four databases. We included cases in which the patient had a primary immunodeficiency, and the vaccine virus isolated from the patient either met the sequencing definition of VDPV (>1% divergence for serotypes 1 and 3 and >0.6% for serotype 2) and/or was previously reported as an iVDPV by the World Health Organization.
Results
We identified 68 iVDPV cases in 49 manuscripts reported from 25 countries and the Palestinian territories. 62% of case patients were male, 78% presented clinically with acute flaccid paralysis, and 65% were iVDPV2. 57% of cases occurred in patients with predominantly antibody immunodeficiencies, and the overall all-cause mortality rate was greater than 60%. The median age at case detection was 1.4 years [IQR: 0.8, 4.5] and the median duration of shedding was 1.3 years [IQR: 0.7, 2.2]. We identified a poliovirus genome VP1 region mutation rate of 0.72% per year and a higher median percent divergence for iVDPV1 cases. More cases were reported from high income countries, which also had a larger age variation and different distribution of immunodeficiencies compared to upper and lower middle-income countries.
Conclusion
Our study describes the incidence and characteristics of global iVDPV cases reported in the literature in the past five decades. It also highlights the regional and economic disparities of reported iVDPV cases.
Keywords: Oral polio vaccine, Immunodeficiency, iVDPV, Polio eradication, Poliomyelitis
1. Introduction
The prevalence of poliomyelitis has decreased dramatically since the 1960s with the development and widespread use of the live attenuated Sabin oral poliovirus vaccine (OPV). OPV became the backbone of worldwide vaccine strategies launched by the Global Polio Eradication Initiative (GPEI) due to its low cost, ability to induce mucosal immunity, and ease of administration [1,2]. However, the potential for OPV to mutate into neurovirulent strains through prolonged intestinal replication—which can result in vaccine-derived poliovirus (VDPV) outbreaks—poses a challenge to global polio eradication [3–5]. Given their relation to key polio endgame issues such as OPV cessation and vaccine stockpile design, VDPVs thus have implications for vaccination policies worldwide [6,7]. Numerous studies have been conducted to assess the risks, costs, and benefits of different eradication options, including the evaluation of scenarios with VDPVs [8–11].
VDPVs are identified based on their degree of genetic divergence from the parent OPV strain and can be comparable to wild-type poliovirus in their capacity to cause paralysis [12]. By definition, VDPVs of serotypes 1 and 3 have >1% genetic divergence, whereas VDPVs of serotype 2 have >0.6% divergence from parent OPV strains [12]. VDPVs are classified into 3 categories: cVDPVs, aVDPVs, and iVDPVs. Circulating vaccine-derived polioviruses (cVDPVs) occur when VDPVs establish and sustain circulation in under-immunized communities for an extended period of time [9,13–16]. Ambiguous vaccine-derived polioviruses (aVDPVs) are either isolated from people with no known immunodeficiency or from sewage whose ultimate source is unknown [16–18]. Immunodeficiency-related vaccine-derived polioviruses (iVDPVs) are a special case of VDPVs in which patients have a primary immunodeficiency (PID) [16, 19]. Unlike immunocompetent persons, who excrete the vaccine virus for a limited period of time, some immunodeficient persons are unable to clear intestinal replication of the vaccine virus after exposure to OPV [19,20]. In this regard, iVDPVs pose a significant threat to the eradication campaign, as individuals that harbor the vaccine virus for prolonged periods of time could serve as sources of poliovirus reintroduction after polio eradication [16,21, 22].
Several key efforts have been made to achieve better assessment of the risks posed by iVDPVs in recent years. The GPEI maintains a registry of iVDPV cases. Although this registry is not publicly available, a list of these cases is periodically published in the World Epidemiological Record (WER) and Morbidity and Mortality Weekly Report (MMWR) [12,23–31]. Additional studies have been launched to search for potential iVDPV carriers. Although these studies either identified no or few cases, they are an important step toward understanding the true risk and prevalence of iVDPVs, as well as in confirming the feasibility and value of conducting polio surveillance programs [32–36].
A review by Kew et al. [19] providing a comprehensive overview of VDPVs included a brief chapter summarizing the main features of iVDPVs and a list of documented iVDPV excretors from 1962 to 2003. A review by Tebbens et al. [21] investigating the risks of continued vaccination with OPV modeled the occurrence of paralysis due to infection with the vaccine virus by type of VDPV. A list of iVDPV cases reported in 1962–2005 was presented, which the authors drew upon for inputs to their model. They also stratified their analysis by income level of the country in which a case was reported to assess regional differences. A review by Van de Ven et al. [37] explored the role of prolonged gastrointestinal infections in causing inflammatory enteropathy in patients with a PID. Poliovirus was among the enteroviruses examined, and the authors identified a select number of cases that were iVDPVs, with the most recent case reported in 2005.
In light of the initiatives launched by the GPEI and other researchers, as well as prior reviews regarding iVDPVs, we present the results of a systematic review of reported iVDPV cases in the literature from 1962 to 2012. The aims of this study are twofold. The first is to present general characteristics and significant trends observed amongst iVDPV cases over five decades, elucidating key findings that have implications for polio eradication such as patterns in poliovirus genome evolution, regional differences in case prevalence, and information regarding case detection and transmission. Over 30 iVDPV cases have been reported since 2006, and given the increasing attention paid to iVDPVs as the world approaches polio eradication, our review provides an update on confirmed iVDPV cases published in the scientific literature.
Second, we aim to foster greater interest and further research regarding iVDPVs that will be useful in the development of appropriate vaccination strategies and policies to achieve and maintain polio eradication. To date, there are no licensed antiviral compounds for poliovirus. There are several antiviral agents such as pirodavir and pleconaril that are known to inhibit poliovirus replication and hence chronic excretion, but data have shown that pleconaril lacks efficacy against certain serotypes of poliovirus [38]. Thus far, the Polio Antivirals Initiative (PAI) has reported pocapavir as a promising agent [39–41].
2. Methods
2.1. Search strategy
Published citations of iVDPV cases from January 1960 to November 2012 were obtained using search strings developed for PubMed, Science Direct, Scopus, and Web of Science, respectively. Key words used to identify citations containing iVDPV cases included immunodeficiency, immunocompromised, immunosup-pressed, polio, poliomyelitis, “vaccine derived polioviruses”, VDPV, iVDPV, “oral poliovirus vaccine”, OPV, “acute flaccid paralysis”, AFP, “vaccine associated paralytic poliomyelitis”, recombinant, chronic, long-term, persistent, prolonged, and excretion. Key words were arranged according to syntax and format restrictions within each database. All four search strings (listed in Supplementary Table 1) were inputted in their respective databases on November 22, 2012. No citations published after this date were included in our study. Only citations published in English, Chinese, Spanish, and French were considered.
2.2. Screening process: inclusion and exclusion criteria
After the removal of duplicate citations, two teams—each consisting of two researchers (J.G. and N.S., M.H. and S.B.W.)—identified potential cases by reviewing the titles and abstracts of the search string results. Citations focused on non-human studies, non-polio infections, non-primary immunodeficiencies, vaccine safety guidelines and contraindications, and general immunization policies were excluded at this stage.
Two researchers (J.G. and S.B.W.) then reviewed all potential cases in full to determine if they met our inclusion criteria. We included cases in which (1) the patient had a PID reported by the authors, and (2) the vaccine virus isolated from the patient met the sequencing definition of VDPV (>1% divergence for serotypes 1 and 3 and >0.6% for serotype 2) and/or the case was identified by the World Health Organization (WHO) or Centers for Disease Control (CDC) in the WER or MMWR as an iVDPV case. We excluded manuscripts focused upon cVDPVs, aVDPVs and reports of immunodeficient persons shedding a poliovirus but which had no sequencing data and had never been reported in a WER/MMWR. Fig. 1 depicts the screening process.
Fig. 1.
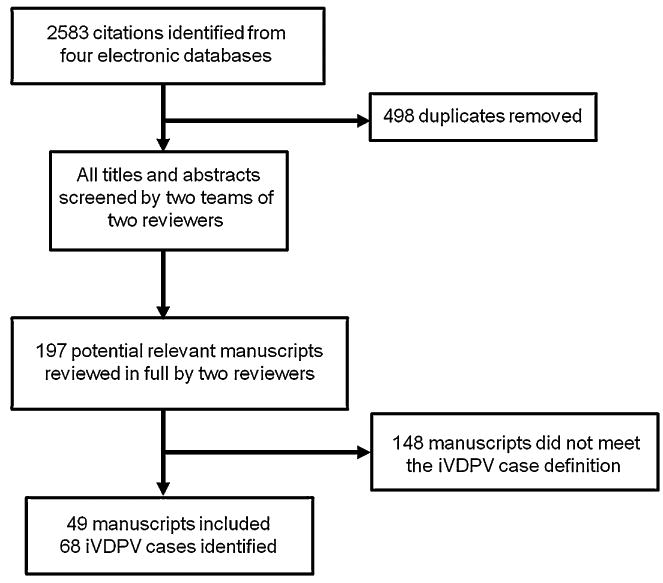
Screening process for included manuscripts. This figure demonstrates the screening process for included and excluded citations from PubMed, Science Direct, Scopus, and Web of Science. Cases were included if (1) the patient had a PID reported by the authors, and (2) the vaccine virus isolated from the patient met the sequencing definition of VDPV (>1% divergence for serotypes 1 and 3 and >0.6% for serotype 2) and/or the case had been previously reported as an iVDPV by the World Health Organization.
2.3. Data extraction
Cases were extracted in duplicate by two researchers (J.G. and S.B.W.) and recorded according to the following parameters: year of case detection, gender and age of the patient, case country of origin, type of PID, serotype of shed virus, percent divergence of shed virus, duration of shedding, vaccination history, treatment status, presence of acute flaccid paralysis (AFP), and survival status. The recorded age was the age of onset of paralysis for the patient or the age of detection in the case of asymptomatic patients. Country of case origin was the country where the iVDPV case was detected. Data on the types of treatment received, the cause of death, and the reason for cessation of shedding were recorded for a smaller subset of cases that had this information available.
When discrepancies arose between data extracted by the two researchers (J.G., S.B.W.), a third researcher (M.H. or N.S.) was consulted. Many cases were described in more than one citation, and when discrepancies arose amongst the sources themselves, we extracted data from the most detailed citation or data that was reported in the majority of the related citations.
The classification of the income level of the country was designated according to criteria proposed by the World Bank [42]. Economies are divided according to 2012 gross national income (GNI) per capita, calculated using the World Bank Atlas method. The groups are low income, $1035 or less; lower middle income, $1036–$4085; upper middle income, $4086–$12,615; and high income, $12,616 or more.
Immune disorders were broadly classified per the Expert Committee of the International Union of Immunological Societies [43] as follows: predominantly antibody deficiency — including agammaglobulinemia, hypogammaglobulinemia, common variable immunodeficiency, x-linked agammaglobulinemia, or other antibody deficiency; combined immunodeficiency — including severe combined immunodeficiency (SCID), major histocompatibility complex class II deficiency, human leukocyte antigen deficiency; PID-not specified—cases whose immune disorders were not specified by the authors; or PID-other—which was comprised of immune disorders that did not fall into the previous categories.
Duration of shedding was measured from the last OPV dose administered to the last stool sample in which OPV was detected (calculated definition); this is a more conservative way to estimate duration of shedding [18,44,45]. When it was not possible to calculate the duration of shedding from the data in the case report, we used the duration of shedding reported by the author (reported definition).
2.4. Statistical analysis
Statistical analyses were performed in R (version 3.0.1) [46]. Single and multivariable linear regressions were used to examine the relationship between duration of shedding and iVDPV genetic divergence. The Wilcoxon signed-rank test was performed to assess the different genetic divergence of iVDPV serotypes. Categorical data were compared using a 2-tailed Fisher exact test. A p-value of ≤0.05 was considered statistically significant.
3. Results
3.1. Prevalence and location of iVDPV cases
68 iVDPV cases from 49 manuscripts published between January 1960 and November 2012 met our inclusion criteria. We excluded eight manuscripts that described immunodeficient persons shedding poliovirus but did not report sequencing data to confirm that this shed virus was a VDPV [47–54]. None of the cases reported in these manuscripts was ever reported in a WER/MMWR. Most of these manuscripts were published before the onset of routine OPV sequencing.
Fig. 2 shows the iVDPV case incidence over time. A noticeable increase in the number of cases occurred in the early 2000s. The highest number of reported cases occurred in 2011 (n = 12). Fig. 3 illustrates iVDPV case incidence over time by income level of the country where the case was detected. From 1960 to the early 1990s, iVDPV cases were reported only in high income countries. However, a decade later, the rapid increase in the number of cases reported in lower middle and upper middle income countries occurred simultaneously with the decline in case incidence in high income countries. 64% (9/14) and 41% (11/27) of the cases in lower and upper middle income countries, respectively, were reported in 2010–2012, whereas high income countries did not have a clear peak in case incidence.
Fig. 2.
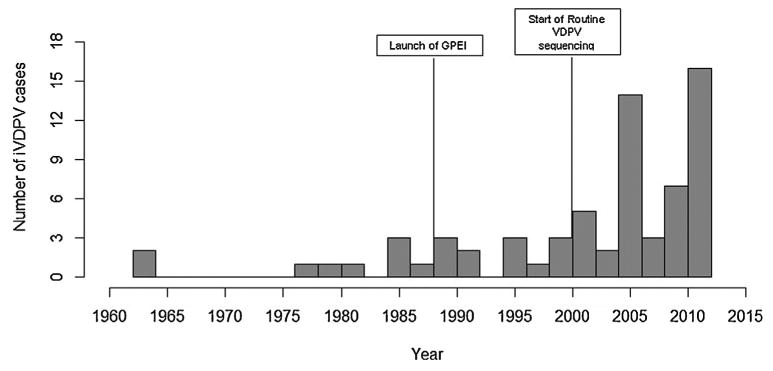
iVDPV incidence over time. This figure demonstrates the incidence of reported iVDPV cases included in our analysis.
Fig. 3.
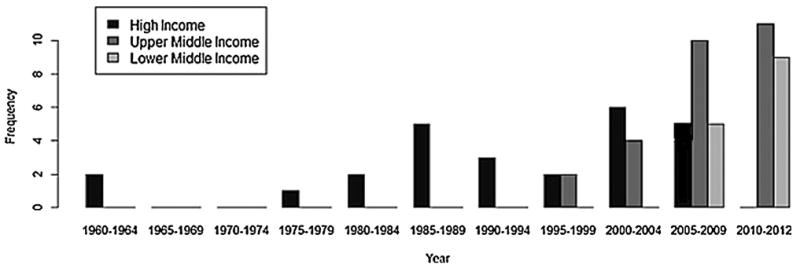
iVDPV incidence by income level of country of case origin overtime. This figure demonstrates iVDPV incidence by income status of the country of origin. Income level of the country is designated according to 2012 GNI per capita, calculated using the World Bank Atlas method. The groups are stratified as follows: low income: $1035 or less; lower middle income, $1036–$4085; upper middle income, $4086–$12,615; and high income, $12,616 or more.
Fig. 4 shows the geographical distribution of reported iVDPV cases, and the corresponding number of cases for each respective country. Twenty-five countries and the Palestinian territories (West Bank and Gaza Strip) reported iVDPV cases, although most countries reported only one case. Half (n = 34) of the cases included in our analysis were from four countries: Iran, the United States, U.K., and China. Nine cases were reported in four African countries. More countries in Asia and the Middle East appear to have reported ≥1 case, but iVDPV case incidence was not isolated to a particular region.
Fig. 4.
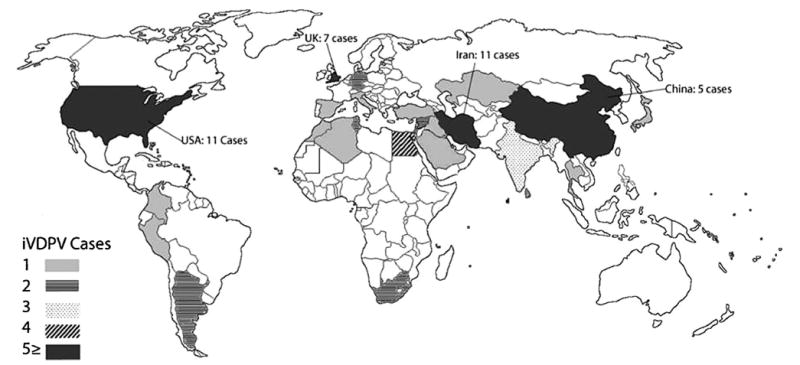
Reported iVDPV cases from 1960 to November 2012. This figure demonstrates the geographical representation of the iVPDV cases identified in our review, and the corresponding number of cases for each respective country. Twenty-five countries and the Palestinian territories (West Bank and Gaza Strip) reported iVDPV cases, although most countries reported only one case. Half (n = 34) of cases included in our analysis were from four countries: Iran, the United States, U.K., and China. Nine cases were reported in four African countries. More countries in Asia and the Middle East appear to have reported ≥1 case, but iVDPV case incidence was not isolated to a particular region.
3.2. Characteristics of iVDPV cases
Table 1 summarizes characteristics for the included iVDPV cases. Over 60% of case patients were male, 78% of the cases presented clinically with AFP, and 65% of the cases were iVDPV2. There were six cases of concurrent multi-serotype infections: three were iVDPV1 and iVDPV2 co-infections, and the other three were iVDPV2 and iVDPV3 co-infections. Over half of all cases occurred in patients with immunodeficiencies classified as predominantly antibody deficiencies, and the overall all-cause mortality rate was greater than 60%.
Table 1.
Characteristics of iVDPV cases.
| Characteristic | Number of cases* (n = 68) | Results |
|---|---|---|
| Gender, % male (n) | 55 | 62% (34) |
| Median age at time of case detection (IQR) | 63 | 1.4 years (0.82, 4.5) |
| Died, % (n) | 58 | 62% (36) |
| Median time between paralysis and death (IQR) | 15 | 0.5 years (0.25, 2.0) |
| Median calculated duration of shedding1 (IQR) | 20 | 1.1 years (0.64, 2.9) |
| Median reported duration of shedding2 (IQR) | 47 | 1.3 years (0.67, 2.0) |
| Median combined duration of shedding3 (IQR) | 67 | 1.3 years (0.67, 2.2) |
| Presented with acute flaccid paralysis, % (n) | 67 | 78% (53) |
| Serotype identified and divergence | 74† | |
| Serotype 1 | ||
| Median percent divergence (IQR) | 15 | 3.2% (2.4, 4.0) |
| Serotype 2 | ||
| Median percent divergence (IQR) | 48 | 2.0% (1.4, 2.3) |
| Serotype 3 | ||
| Median percent divergence (IQR) | 11 | 2.0% (1.3, 2.2) |
| Immune disorder | 68 | |
| Predominantly antibody immunodeficiency, % | 39 | 57% |
| Combined Immunodeficiency, % | 15 | 22% |
| Primary immunodeficiency (other), % | 1 | 1.5% |
| Primary immunodeficiency (unspecified), % | 13 | 19% |
This table describes characteristics of iVDPV cases included in our analysis.
Only cases with available information were analyzed for each parameter. Duration of shedding was recorded according to the following definitions:
Calculated as the time from last OPV administration to last positive stool sample.
As reported by author of original case report.
Combined calculated and reported values.
There were more serotypes detected than cases because 6 iVDPVs had multi-serotype infections.
Immune disorders were categorized as follows: predominantly antibody deficiency = agammaglobulinemia, hypogammaglobulinemia, common variable immunodeficiency, x-linked agammaglobulinemia, or other antibody deficiency; combined immunodeficiency = severe combined immunodeficiency, major histocompatibility complex class II deficiency, human leukocyte antigen deficiency; primary immunodeficiency-other = immune disorders that either did not fall into the previous categories; or primary immunodeficiency-not specified = cases whose immune disorders were not specified by the authors.
The median age at the time of case detection was 1.4 years [interquartile range (IQR): 0.8, 4.5]. In the cohort of case patients that were reported to be alive at the time of last follow up, the median age at case detection was 4.5 years [IQR: 1.2, 9.1] compared with 1.3 years [IQR: 0.7, 2.0] in case patients that had died. Duration of shedding was also examined separately according to survival status. While the overall median duration of shedding was 1.3 years [IQR: 0.7, 2.2], the duration for the surviving cohort was 1.5 years [IQR: 0.4, 3.0].
Of 68 cases, two cases that were iVDPV2 had divergence less than 1% (at 0.88% and 0.9% respectively) [44,55]. iVDPV1 cases had a higher median percent divergence than both iVDPV2 and iVDPV3 cases (3.2% for iVDPV1 compared to 2% for both iVDPV2 and iVDPV3; p = 0.004, Wilcoxon signed-rank test). The IQR for median percent divergence of iVDPV1 [2.4%, 4.0%] did not overlap with the IQRs of iVDPV2 [1.4%, 2.3%] and iVDPV3 [1.3%, 2.2%] cases.
The relationship between the percent divergence of the iVDPV isolate and the duration of shedding (both calculated and reported definitions included) for a given case is presented in Fig. 5 (n = 57). The overall trend presents a positive linear association between percent divergence at the VP1 (viral protein 1) region of the poliovirus genome and duration of shedding. The majority of cases shed for less than three years. Single and multivariable regressions were performed to further assess the effect of shedding duration and serotype on divergence. Shedding duration and age were statistically associated with divergence — each additional year of shedding and age corresponded to an increase in the percent divergence by 0.72% and 0.08%, respectively, even after controlling for gender, presence of AFP, serotype, and survival status (p<3e−15; p<0.00013).
Fig. 5.
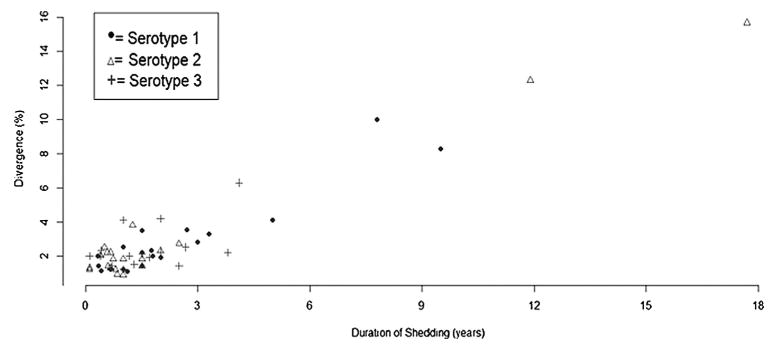
Correlation of duration of shedding and the genetic divergence of iVDPVs. This figure demonstrates the relationship of percent genetic divergence of OPV strains at the VP1 region with the duration of shedding (for both calculated and reported definitions) of the iVDPV cases included in our analysis. Single and multivariable regressions were performed. Shedding duration and age were statistically associated with divergence and age — each additional year of shedding and age corresponded to an increase in the percent divergence by 0.72% and 0.08%, even after controlling for gender, presence of AFP, serotype, and survival status (p<3e−15; p< 0.00013). However, it is important to note that there are limitations in this analysis given the high mortality rate among case patients if the patient was still shedding at the time of death and the inherent bias in that shedding is only reported starting at case detection. Both these factors may have artificially shortened the measure for duration of shedding.
Analyses were conducted to further examine the relationship between immunodeficiency and survival status of the cases. 89% (8/9) of patients with SCID died, whereas the mortality rate for patients with an immunodeficiency classified as a predominantly antibody deficiency was 50% (17/34). There was a lower survival rate for females compared to males, however, this was not statistically significant (24% versus 44%, Fisher exact test, p = 0.22). Data on the interval from the onset of AFP to patient death were available for only 15 cases. The median time to death was six months [IQR: 4.0, 24.0]. iVDPV classified as predominantly antibody deficiency constituted 69% (18/26) and 57% (16/28) of cases in high income and upper middle income countries, respectively, but only 36% (5/14) in lower middle income countries (Fisher exact test, p = 0.051). In fact, PID-not specified cases comprised the largest group for lower middle income countries (46%, 6/13).
Patients from lower middle income countries had a mortality rate of 80% (8/10), compared to 58% (14/24) for both high income and upper middle income countries. However, this difference was not statistically significant (Fisher exact test, p = 0.49). Additionally, there is a much larger variation in age at presentation for cases from high income countries compared to upper and lower middle income countries (IQRs for median age at presentation are [0.9, 13.8], [0.8, 2.0], and [0.5, 1.7] for high, upper middle, and lower middle income countries, respectively).
The majority of cases (93%, 63/68) occurred in countries that exclusively used OPV at the time of case presentation. Germany and the United States reported one and two iVDPV cases, respectively, after their switch to an IPV-only regimen [56–58]. Italy and the West Bank and Gaza Strip reported an iVDPV case when using a mixed IPV/OPV vaccination schedule [12,55].
87% (20/23) of cases stopped excretion either randomly or through death. Only three cases stopped excretion after treatment for their immunodeficiency [33,45,59]. The majority (11/13) of deaths were reported to be due to the patient's PID, and only two cases were specified to be a result of paralytic poliomyelitis [44,45].
Five iVDPV cases were identified as contacts exposed to a recent OPV recipient [45,57,58,60,61]. Two of these cases were household contacts of a healthy infant vaccinated with OPV [45,57]. Other cases occurred through exposure to OPV in communities with low vaccine coverage [58], and in the case of one patient, the pediatric ward where he had worked as a nurse [60]. Two of the contact cases had received at least one IPV dose [45,60]. 14 cases (21%) were identified through methods that did not include detection of AFP.
4. Discussion
This study is a comprehensive update of confirmed iVDPV cases published in the scientific literature from 1962 to 2012. We found that the majority of reported iVDPV cases had underlying antibody deficiencies, presented with AFP, and died with an overall mortality rate of 62%. We identified a poliovirus genome VP1 mutation rate of 0.72% per year through regression analysis, as well as a higher median percent divergence for iVDPV1 cases. This mutation rate, which approaches the estimated poliovirus genome mutation rate of 1% per year [31], was comparable across serotypes as it held true when serotype was added as a control variable in the multivariable regression analysis. Accordingly, it follows that given the same mutation rate, the longer average shedding duration of iVDPV1—with a median length of 2.67 years—resulted in more mutations and hence a higher median reported divergence than the other serotypes.
Our study highlights important aspects of iVDPVs that could influence the post eradication strategy. The current vaccination strategy recommended by the WHO—the replacement of the triva-lent OPV (tOPV) with the combined regimen of bivalent type 1/type 3 OPV (bOPV) and 1 IPV dose—was made based on the elimination of wild poliovirus type 2 [56,62,63]. However, a large proportion of our identified cases shed iVDPV2. In fact, only 30% of patients shed exclusively iVDPV1 or iVDPV3. Furthermore, the majority of cVDPV outbreaks worldwide are caused by serotype 2 [9,64,65], and uncertainty remains regarding IPVs ability to provide enough mucosal protection against serotype 2 to prevent outbreaks caused by re-introduction of VDPV-2 in the post-tOPV era [66].
Second, more than 20% of the iVDPV cases included in our analysis were asymptomatically shedding divergent virus. This alludes to a likely much larger pool of infected poliovirus patients that still remain undetected and highlights the underestimation of iVDPV prevalence already noted in the literature [67]. Together, these results demonstrate the well-known risks that immunodeficient individuals pose to polio eradication: they may unknowingly serve as point sources for iVDPV—particularly iVDPV2—reintroduction of polio [22]. Hence, strengthening polio surveillance programs to identify iVDPVs is critical in the post-eradication era, particularly given the suspected iVDPV cases identified in some studies [68–71]. Previous studies launched to identify iVDPVs have demonstrated the practicality of these surveillance programs [34,35,72], and a multinational surveillance study led by the Jeffrey Modell Foundation is currently underway [73].
Third, five iVDPV cases identified in our analysis did not receive OPV directly and instead acquired OPV from exposure to vaccinated family members or contacts. This emphasizes the dangers that continued use of OPV poses even if guidelines are followed and immunodeficient persons are not vaccinated directly.
There were limitations to our study. Since the primary source of data was case reports, there was variation in the details reported for each case resulting in missing information for certain parameters. However, we applied a conservative and consistent approach to the data extraction, omitted equivocal information for data quality purposes, and also sought a third party reviewer to resolve any discrepancies. In order to accurately assess the relationship between the percent divergence of iVDPV strains and the duration of shedding, the same measure for duration of shedding (last OPV to last positive sample) was calculated for each case when available. When this information was missing, we relied upon the duration of shedding measure provided by the authors of the case report; the method by which the authors calculated this measure is unclear. Thus there may be heterogeneity in our duration of shedding calculations. However, median duration of shedding between these two definitions was similar: 1.1 years [IQR: 0.64, 2.9] and 1.3 years [IQR: .67, 2.0] for calculated and reported definitions, respectively.
In addition, the duration of shedding in our analysis may have been artificially shortened by the high mortality rate among cases if the patient was still shedding at the time of death and the inherent bias in that shedding is only reported starting at case detection. Lastly, we only included iVDPV cases reported in manuscripts cataloged in PubMed, Scopus, Science Direct, and Web of Science. These databases do not routinely catalog regional polio meeting reports, which may have reported additional iVDPV cases. We also did not include cases that lacked sequencing data and were not reported in a WER/MMWR to confirm that the shed virus was an iVDPV. Thus the true incidence of reported or detected iVDPV cases is likely higher than we report here.
The majority of our cases presented with AFP. However, this reflects the source of our data (published literature) as many iVDPV shed OPV asymptomatically [32,33,35]. The majority of our cases occurred in patients with immunodeficiencies classified as predominantly antibody deficiencies, which is likely due in part to the differential prevalence of combined immunodeficiencies and predominantly antibody deficiencies. SCID, a combined immunodeficiency and the most severe form of PID, is associated with a high mortality rate; this may contribute to the lower prevalence of combined immunodeficiencies compared to predominantly antibody deficiencies identified in the general population as well as our study [74]. Given the higher mortality rate of SCID patients, it is likely that some SCID patients died before they were diagnosed with iVDPV in our sample [43].
Finally, a main implication of our study is the regional and economic disparities seen in the iVDPV cases. The trends in increasing incidence over time (Fig. 2) can be accounted for by the launch of the GPEI and the start of routine surveillance programs and VPDV sequencing by the WHO in 2001. The shift in the incidence of reported iVDPV cases from high income to upper middle and lower middle income countries (Fig. 3) is reflective of a few factors at play. First, the decline of iVDPV cases in high-income countries of iVDPV cases in the past decade is due to the change in vaccination schedules in the early 2000s for many of these countries. Specifically, countries including the U.S., U.K., and others in Europe switched from OPV and mixed OPV/IPV regimens to the exclusive use of IPV [75–77].
Second, the regional shift in iVDPV incidence is also reflective of a change in the level of polio surveillance in recent years. Implementation of intensified surveillance for VDPVs and special studies of iVDPV excretion among patients with PIDs in developing and middle-income countries began in the mid-2000s, and the results of these efforts as presented in this study are apparent [12,32,34,35]. In other words, countries that have the means to perform VDPV sequencing—either through their own resources or from support provided by others—and the healthcare services to properly diagnosis immune disorders are overrepresented. The fact that no iVDPV cases were reported in the lowest income countries provides a nuance to this phenomenon, in that polio surveillance may be available in these countries but they still lack the resources and healthcare infrastructure to diagnosis surviving patients with PID. In this regard, these economic and regional differences demonstrate the underreporting of cases and hence the overall underestimation of iVDPV incidence in the literature. In fact, the other differences identified—the larger variation in age for high income countries and the higher mortality rate and proportion of PID-not specified cases from lower middle income countries—are also likely a consequence of economic and regional disparities.
5. Conclusion
This study is a comprehensive update of confirmed iVDPV cases published in the scientific literature spanning five decades. It describes clinically relevant trends in reported iVDPV cases worldwide and also highlights the regional and economic disparities of reported iVDPV cases. These results may be informative in designing policy in the post-eradication era, given the relevance of iVDPV cases to polio endgame issues such as OPV cessation and ecologic poliovirus surveillance.
Supplementary Material
Acknowledgments
We would like to give thanks to Meira F. Halpern and Stacy Huang for their feedback on the manuscript, and the librarians at Stanford University and University of Central Florida for their statistical and technical support.
Abbreviations
- AFP
acute flaccid paralysis
- aVDPV
ambiguous vaccine-derived poliovirus
- cVDPV
circulating vaccine-derived poliovirus
- GNI
gross national income
- GPEI
Global Polio Eradication Initiative
- IPV
inactivated polio vaccine
- iVDPV
immunodeficiency-related vaccine-derived poliovirus
- MMWR
Morbidity and Mortality Weekly Report
- OPV
oral polio vaccine
- PAI
Polio Antivirals Initiative
- PID
primary immunodeficiency
- SCID
severe combined immunodeficiency
- VDPV
vaccine-derived poliovirus
- VP1
viral protein 1
- WER
World Epidemiological Record
Footnotes
Conflict of interest: Authors do not have any conflict of interest.
Author contributions: J.G. and S.B.W. equally contributed to the literature search, study extraction, data analysis, drafts, and revisions of the manuscript. N.S., M.H. and Y.M. participated in the study design. M.H. and N.S. participated in the initial screening process, supervised the methodologies of the project and extensively reviewed drafts of the manuscript. Y.M. critically reviewed versions of the manuscript.
Appendix A. Supplementary data: Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/ j.vaccine.2015.01.018.
References
- 1.Liu X, Levin A, Makinen M, Day J. OPV vs IPV: past and future choice of vaccine in the global polio eradication program. Partn Health Reform Proj. 2003 [Google Scholar]
- 2.Heymann DL, Sutter RW, Aylward RB. A vision of a world without polio: the OPV cessation strategy. Biologicals. 2006;34(2):75–9. doi: 10.1016/j.biologicals.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Aylward B, Yamada T. The polio endgame. N Engl J Med. 2011;364(24):2273–5. doi: 10.1056/NEJMp1104329. [DOI] [PubMed] [Google Scholar]
- 4.Nathanson N. Eradication of poliovirus: fighting fire with fire. J Infect Dis. 2011;203(7):889–90. doi: 10.1093/infdis/jiq148. [DOI] [PubMed] [Google Scholar]
- 5.Bhaumik S. Polio eradication: current status and challenges. J Family Med Prim Care. 2012;1(2):84–5. doi: 10.4103/2249-4863.104936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson KM, Duintjer Tebbens RJ. National choices related to inactivated poliovirus vaccine, innovation and the endgame of global polio eradication. Expert Rev Vaccines. 2014;13(2):221–34. doi: 10.1586/14760584.2014.864563. [DOI] [PubMed] [Google Scholar]
- 7.Duintjer Tebbens RJ, Pallansch MA, Alexander JP, Thompson KM. Optimal vaccine stockpile design for an eradicated disease: application to polio. Vaccine. 2010;28(26):4312–27. doi: 10.1016/j.vaccine.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Duintjer Tebbens RJ, Pallansch MA, Kalkowska DA, Wassilak SGF, Cochi SL, Thompson KM. Characterizing poliovirus transmission and evolution: insights from modeling experiences with wild and vaccine-related polioviruses. Risk Anal. 2013;33(4):703–49. doi: 10.1111/risa.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duintjer Tebbens RJ, Pallansch MA, Kim JH, Burns CC, Kew OM, Oberste MS, et al. Oral poliovirus vaccine evolution and insights relevant to modeling the risks of circulating vaccine-derived polioviruses (cVDPVs) Risk Anal. 2013;33(4):680–702. doi: 10.1111/risa.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson KM, Duintjer Tebbens RJ. Current polio global eradication and control policy options: perspectives from modeling and prerequisites for oral poliovirus vaccine cessation. Expert Rev Vaccines. 2012;11(4):449–59. doi: 10.1586/erv.11.195. [DOI] [PubMed] [Google Scholar]
- 11.Thompson KM, Duintjer Tebbens RJ, Pallansch MA, Kew OM, Sutter RW, Aylward RB, et al. The risks, costs, and benefits of possible future global policies for managing polioviruses. Am J Public Health. 2008;98(7):1322–30. doi: 10.2105/AJPH.2007.122192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Update on vaccine-derived polioviruses detected worldwide, April 2011-June 2012. Wkly Epidemiol Rec. 2012;87(38):358–68. [PubMed] [Google Scholar]
- 13.Kew O, Morris-Glasgow V, Landaverde M, Burns C, Shaw J, Garib Z, et al. Outbreak of poliomyelitis in Hispaniola associated with circulating type 1 vaccine-derived poliovirus. Science. 2002;296(5566):356–9. doi: 10.1126/science.1068284. [DOI] [PubMed] [Google Scholar]
- 14.Center for Disease Control and Prevention. Circulation of a type 2 vaccine-derived poliovirus – Egypt, 1982–1993. MMWR Morb Mortal Wkly Rep. 2001;50(3):41–2. 51. [PubMed] [Google Scholar]
- 15.Center for Disease Control and Prevention. Public health dispatch: poliomyelitis—Madagascar, 2002. MMWR Morb Mortal Wkly Rep. 2002;51(28):622. [Google Scholar]
- 16.Vaccine-derived Polioviruses (VDPV) [accessed 12.01.13];Global Polio Eradication Initiative. 2013 http://www.polioeradication.org/Polioandprevention/Thevirus/Vaccinederivedpolioviruses.aspx.
- 17.Martín J. Vaccine-derived poliovirus from long term excretors and the end game of polio eradication. Biologicals. 2006;34(2):117–22. doi: 10.1016/j.biologicals.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Minor P. Vaccine-derived poliovirus (VDPV): impact on poliomyelitis eradication. Vaccine. 2009;27(20):2649–52. doi: 10.1016/j.vaccine.2009.02.071. [DOI] [PubMed] [Google Scholar]
- 19.Kew OM, Sutter RW, de Gourville EM, Dowdle WR, Pallansch MA. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annu Rev Microbiol. 2005;59:587–635. doi: 10.1146/annurev.micro.58.030603.123625. [DOI] [PubMed] [Google Scholar]
- 20.Minor P. Emerging/disappearing viruses future issues concerning polio eradication. Virus Res. 2002;82(1–2):33–7. doi: 10.1016/s0168-1702(01)00384-7. [DOI] [PubMed] [Google Scholar]
- 21.Duintjer Tebbens RJ, Pallansch MA, Kew OM, Cáceres VM, Jafari H, Cochi SL, et al. Risks of paralytic disease due to wild or vaccine-derived poliovirus after eradication. Risk Anal. 2006;26(6):1471–505. doi: 10.1111/j.1539-6924.2006.00827.x. [DOI] [PubMed] [Google Scholar]
- 22.Pliaka V, Kyriakopoulou Z, Markoulatos P. Risks associated with the use of live-attenuated vaccine poliovirus strains and the strategies for control and eradication of paralytic poliomyelitis. Expert Rev Vaccines. 2012;11(5):609–28. doi: 10.1586/erv.12.28. [DOI] [PubMed] [Google Scholar]
- 23.Center for Disease Control and Prevention. Prolonged poliovirus excretion in an immunodeficient person with vaccine-associated paralytic poliomyelitis. MMWR Morb Mortal Wkly Rep. 1997;46(28):641–3. [PubMed] [Google Scholar]
- 24.Center for Disease Control and Prevention. Poliovirus infections in fourunvacci-nated children – Minnesota, August–October 2005. MMWR Morb Mortal Wkly Rep. 2005;54(41):1053–5. [PubMed] [Google Scholar]
- 25.Center for Disease Control and Prevention. Update on vaccine-derived polioviruses. MMWR Morb Mortal Wkly Rep. 2006;55(40):1093–7. [PubMed] [Google Scholar]
- 26.Center for Disease Control and Prevention. Update on vaccine-derived polioviruses – worldwide, January 2006–August 2007. MMWR Morb Mortal Wkly Rep. 2007;56(38):996–1001. [PubMed] [Google Scholar]
- 27.Center for Disease Control and Prevention. Update on vaccine-derived polioviruses – worldwide, January 2008–June 2009. MMWR Morb Mortal Wkly Rep. 2009;58(36):1002–6. [PubMed] [Google Scholar]
- 28.Center for Disease Control and Prevention. Update on vaccine-derived polioviruses – worldwide, July 2009–March 2011. MMWR Morb Mortal Wkly Rep. 2011;60(25):846–50. [PubMed] [Google Scholar]
- 29.Vaccine-derived polioviruses – update. Wkly Epidemiol Rec. 2006;81(42):398–404. [PubMed] [Google Scholar]
- 30.Vaccine-derived polioviruses detected worldwide January 2008–June 2009. Wkly Epidemiol Rec. 2009;84(38):390–6. [PubMed] [Google Scholar]
- 31.Vaccine-derived polioviruses detected worldwide, July 2009–March 2011. Wkly Epidemiol Rec. 2011;86(27):277–88. [PubMed] [Google Scholar]
- 32.Halsey NA, Pinto J, Espinosa-Rosales F, Faure-Fontenla MA, da Silva E, Khan AJ, et al. Search for poliovirus carriers among people with primary immune deficiency diseases in the United States, Mexico, Brazil, and the United Kingdom. Bull World Health Organ. 2004;82(1):3–8. [PMC free article] [PubMed] [Google Scholar]
- 33.Fiore L, Plebani A, Buttinelli G, Fiore S, Donati V, Marturano J, et al. Search for poliovirus long-term excretors among patients affected by agammaglobulinemia. Clin Immunol. 2004;111(1):98–102. doi: 10.1016/j.clim.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 34.Sazzad HM, Rainey JJ, Mach O, Sutter R, Diordista S, Kawser CA, et al. The feasibility of identifying children with primary immunodeficiency disorders: preparation for the polio post-eradication era in Bangladesh. Vaccine. 2012;30(36):5396–400. doi: 10.1016/j.vaccine.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 35.de Silva R, Gunasena S, Ratnayake D, Wickremesinghe GD, Kumarasiri CD, Pushpakumara Ba, et al. Prevalence of prolonged and chronic poliovirus excretion among persons with primary immune deficiency disorders in Sri Lanka. Vaccine. 2012;30(52):7561–5. doi: 10.1016/j.vaccine.2012.10.035. [DOI] [PubMed] [Google Scholar]
- 36.Diamanti E, Ibrahimi B, Tafaj F, Mezini E, Dodbiba A, Dobi V, et al. Surveillance of suspected poliomyelitis in Albania, 1980–1995: suggestion of increased risk of vaccine associated poliomyelitis. Vaccine. 1998;16(9–10):940–8. doi: 10.1016/s0264-410x(98)80025-x. [DOI] [PubMed] [Google Scholar]
- 37.van de Ven AA, Hoytema van Konijnenburg DP, Wensing AM, van Montfrans JM. The role of prolonged viral gastrointestinal infections in the development of immunodeficiency-related enteropathy. Clin Rev Allergy Immunol. 2012;42(1):79–91. doi: 10.1007/s12016-011-8292-9. [DOI] [PubMed] [Google Scholar]
- 38.De Palma AM, Pürstinger G, Wimmer E, Patick AK, Andries K, Rombaut B, et al. Potential use of antiviral agents in polio eradication. Emerg Infect Dis. 2008;14(4):545–51. doi: 10.3201/eid1404.070439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The task force for global health. [accessed 15.07.14];Polio Antivirals Initiative. 2014 http://www.taskforce.org/our-work/programs/polio-antivirals-initiative.
- 40.Collett MS, Hincks JR, Oberste S. Anti-poliovirus activity of pocapavir in a human mopv1 challenge model. Presentation at Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); 2013. [abstract nr G-996b] [Google Scholar]
- 41.Modlin JF. Antiviral drugs for poliomyelitis. Presented at the Polio Eradication Symposium, Infectious Diseases (ID) Week; 2013. [Google Scholar]
- 42.Income levels. [accessed 15.01.13];World Bank's Annu World Dev Rep. 2013 http://wdronline.worldbank.org/worldbank/a/incomelevel.
- 43.Buckley RH. Chapter 116: Evaluation of suspected immunodeficiency. In: Behrman RE, Jenson HB, Stanton BF, editors. Nelson textbook of pediatrics. Elsevier/Saunders; 2012. pp. 715–22. [Google Scholar]
- 44.Khetsuriani N, Prevots DR, Quick L, Elder ME, Pallansch M, Kew O, et al. Persistence of vaccine-derived polioviruses among immunodeficient persons with vaccine-associated paralytic poliomyelitis. J Infect Dis. 2003;188(12):1845–52. doi: 10.1086/379791. [DOI] [PubMed] [Google Scholar]
- 45.Shahmahmoodi S, Mamishi S, Aghamohammadi A, Aghazadeh N, Tabatabaie H, Gooya MM, et al. Vaccine-associated paralytic poliomyelitis in immunodeficient children, Iran, 1995–2008. Emerg Infect Dis. 2010;16(7):1133–6. doi: 10.3201/eid1607.091606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.R Core Team. 2013. R: a language and environment for statistical computing. http://www.r-project.org/
- 47.Feigin RD, Guggenheim MA, Johnsen SD. Vaccine-related paralytic poliomyelitis in an immunodeficient child. J Pediatr. 1971;79(4):642–7. doi: 10.1016/s0022-3476(71)80313-x. [DOI] [PubMed] [Google Scholar]
- 48.Lopez C, Biggar WD, Park BH, Good RA. Nonparalytic poliovirus infections in patients with severe combined immunodeficiency disease. J Pediatr. 1974;84(4):497–502. doi: 10.1016/s0022-3476(74)80667-0. [DOI] [PubMed] [Google Scholar]
- 49.Saulsbury FT, Winkelstein JA, Davis LE, Hsu SH, D'Souza BJ, Gutcher GR, et al. Combined immunodeficiency and vaccine-related poliomyelitis in a child with cartilage-hair hypoplasia. J Pediatr. 1975;86(6):868–72. doi: 10.1016/s0022-3476(75)80216-2. [DOI] [PubMed] [Google Scholar]
- 50.Davis LE, Bodian D, Price D, Butler IJ, Vickers JH. Chronic progressive poliomyelitis secondary to vaccination of an immunodeficient child. N Engl J Med. 1977;297(5):241–5. doi: 10.1056/NEJM197708042970503. [DOI] [PubMed] [Google Scholar]
- 51.Wright PF, Hatch MH, Kasselberg AG, Lowry SP, Wadlington WB, Karzon DT. Vaccine-associated poliomyelitis in a child with sex-linked agammaglobulinemia. J Pediatr. 1977;91(3):408–12. doi: 10.1016/s0022-3476(77)81309-7. [DOI] [PubMed] [Google Scholar]
- 52.Gaebler JW, Kleiman MB, French ML, Chastain G, Barrett C, Griffin C. Neurologic complications in oral polio vaccine recipients. J Pediatr. 1986;108(6):878–81. doi: 10.1016/s0022-3476(86)80920-9. [DOI] [PubMed] [Google Scholar]
- 53.Benabdellah A, Nimour M. Congenital immunodeficiency revealed by an acute anterior poliomyelitis. A case report Med Trop (Mars) 2000;60(4):363–4. [PubMed] [Google Scholar]
- 54.Cherkasova EA, Yakovenko ML, Rezapkin GV, Korotkova EA, Ivanova OE, Eremeeva TP, et al. Spread of vaccine-derived poliovirus from a paralytic case in an immunodeficient child: an insight into the natural evolution of oral polio vaccine. J Virol. 2005;79(2):1062–70. doi: 10.1128/JVI.79.2.1062-1070.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buttinelli G, Donati V, Fiore S, Marturano J, Plebani A, Balestri P, et al. Nucleotide variation in Sabin type 2 poliovirus from an immunodeficient patient with poliomyelitis. J Gen Virol. 2003;84(pt (5)):1215–21. doi: 10.1099/vir.0.18974-0. [DOI] [PubMed] [Google Scholar]
- 56.Sutter RW, Kew OM, Cochi SL, Aylward B. Poliovirus vaccine-live. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 6th. Philadelphia: Saunders Elsevier; 2012. pp. 598–645. [Google Scholar]
- 57.DeVries AS, Harper J, Murray A, Lexau C, Bahta L, Christensen J, et al. Vaccine-derived poliomyelitis 12 years after infection in Minnesota. N Engl J Med. 2011;364(24):2316–23. doi: 10.1056/NEJMoa1008677. [DOI] [PubMed] [Google Scholar]
- 58.Alexander JP, Ehresmann K, Seward J, Wax G, Harriman K, Fuller S, et al. Transmission of imported vaccine-derived poliovirus in an undervaccinated community in Minnesota. J Infect Dis. 2009;199(3):391–7. doi: 10.1086/596052. [DOI] [PubMed] [Google Scholar]
- 59.Gumede N, Muthambi V, Schoub BD. Immunodeficiency-associated vaccine-derived poliovirus type 3 in infant, South Africa, 2011. Emerg Infect Dis. 2012;18(6):992–4. doi: 10.3201/eid1806.120037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Misbah SA, Lawrence PA, Kurtz JB, Chapel HM. Prolonged faecal excretion of poliovirus in a nurse with common variable hypogammaglobulinaemia. Postgrad Med J. 1991;67(785):301–3. doi: 10.1136/pgmj.67.785.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hidalgo S, García Erro M, Cisterna D, Freire MC. Paralytic poliomyelitis caused by a vaccine-derived polio virus in an antibody-deficient Argentinean child. Pediatr Infect Dis J. 2003;22(6):570–2. [PubMed] [Google Scholar]
- 62.Polio Eradication & Endgame Strategic Plan 2013–2018. [accessed 25.04.13];Global Polio Eradication Initiative. 2013 http://www.polioeradication.org/Portals/0/Document/Resources/StrategyWork/EndgameStratPlan_20130409_ENG.pdf.
- 63.World Health Organization. Polio vaccines: WHO position paper, January 2014 – recommendations. Vaccine. 2014;32(33):4117–8. doi: 10.1016/j.vaccine.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 64.Moturi EK, Porter KA, Wassilak SG, Tangermann RH, Diop OM, Burns CC, et al. Progress toward polio eradication – Worldwide, 2013–2014. MMWR Morb Mortal Wkly Rep. 2014;63(21):468–72. [PMC free article] [PubMed] [Google Scholar]
- 65.Center for Disease Control and Prevention. Acute flaccid paralysis associated with circulating vaccine-derived poliovirus – Philippines, 2001. MMWR Morb Mortal Wkly Rep. 2001;50(40):874–5. [PubMed] [Google Scholar]
- 66.Hird TR, Grassly NC. Systematic review of mucosal immunity induced by oral and inactivated poliovirus vaccines against virus shedding following oral poliovirus challenge. PLoS Pathog. 2012;8(4) doi: 10.1371/journal.ppat.1002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kew O. Reaching the last one percent: progress and challenges in global polio eradication. CurrOpin Virol. 2012;2(2):188–98. doi: 10.1016/j.coviro.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 68.Hovi T, Shulman LM, van der Avoort H, Deshpande J, Roivainen M, De Gourville EM. Role of environmental poliovirus surveillance in global polio eradication and beyond. Epidemiol Infect. 2012;140(1):1–13. doi: 10.1017/S095026881000316X. [DOI] [PubMed] [Google Scholar]
- 69.Černáková B, Sobotová Z, Rovný I, Bláhova Š, Roivainen M, Hovi T. Isolation of vaccine-derived polioviruses in the Slovak Republic. Eur J Clin Microbiol Infect Dis. 2005;24(6):438–9. doi: 10.1007/s10096-005-1331-9. [DOI] [PubMed] [Google Scholar]
- 70.Blomqvist S, Savolainen C, Laine P, Hirttiö P, Lamminsalo E, Penttilä E, et al. Characterization of a highly evolved vaccine-derived poliovirus type 3 isolated from sewage in Estonia. J Virol. 2004;78(9):4876–83. doi: 10.1128/JVI.78.9.4876-4883.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shulman LM, Manor Y, Handsher R, et al. Molecular and antigenic characterization of a highly evolved derivative of the type 2 oral poliovaccine strain isolated from sewage in Israel. J Clin Microbiol. 2000;38(10):3729–34. doi: 10.1128/jcm.38.10.3729-3734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li L, Ivanova O, Triki H. Poliovirus excretion among persons with primary immune deficiency disorders: summary of a seven-country study series. J Infect Dis. 2014;210(suppl. 1):S368–72. doi: 10.1093/infdis/jiu065. http://jid.oxfordjournals.org/content/210/suppl1/S368.long. [DOI] [PubMed] [Google Scholar]
- 73.Tinder P. Jeffrey Modell Foundation Announces Global Polio Surveillance Study. [accessed 15.07.14];Vaccine News. 2014 http://vaccinenewsdaily.com/medical_countermeasures/328498-jeffrey-modell-foundation-announces-global-polio-surveillance-study/3.
- 74.Buckley RH, Schiff RI, Schiff SE, Markert ML, Williams LW, Harville TO, et al. Human severe combined immunodeficiency: genetic, phenotypic, and functional diversity in one hundred eight infants. J Pediatr. 1997;130(3):378–87. doi: 10.1016/s0022-3476(97)70199-9. [DOI] [PubMed] [Google Scholar]
- 75.Sutter RW, Prevots DR, Cochi SL. Poliovirus vaccines. Progress toward global poliomyelitis eradication and changing routine immunization recommendations in the United States. Pediatr Clin North Am. 2000;47(2):287–308. doi: 10.1016/s0031-3955(05)70208-x. [DOI] [PubMed] [Google Scholar]
- 76.National Travel Health Network and Centre. [accessed 20.01.13];Polio vaccine information. 2004 http://www.unidocs.co.uk/docs/travel/polio_vaccine.pdf.
- 77.Vidor E, Plotkin SA. Poliovirus vaccine—inactivated. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 6th. Philadelphia: Saunders Elsevier; 2012. pp. 573–97. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


