Abstract
Time perception is a fundamental component of everyday life. Although time can be measured using standard units, the relationship between an individual’s experience of perceived time and a standard unit is highly sensitive to context. Stressful and threatening stimuli have been previously shown to produce time distortion effects, such that individuals perceive the stimuli as lasting for different amounts of time as compared to a standard unit. As a highly social species, humans are acutely sensitive to social stressors; however, time distortion effects have not been studied in the context of social stress. We collected psychophysiological (electrocardiogram and impedance cardiography) and time perception data before, during, and after a modified version of the Trier Social Stress Test for 42 participants. Based on prior theories and evidence from the time perception literature, we hypothesized that experiencing a stressful event would result in time distortion. This hypothesis was supported by the data, with individuals on average reproducing short and long duration negative and positive stimuli as lasting longer after experiencing social stress, t(41) = −3.55, p = .001, and t(41) = −4.12, p <.001 for negative stimuli, and t(41)5 −2.43, p = .02, and t(41) = −3.07, p = .004 for positive stimuli. However, changes in time perception were largely unrelated to psychophysiological reactivity to social stress. These findings are in line with some other studies of time distortion, and provide evidence for the interoceptive salience model of time perception. Implications for mechanisms of time distortion are discussed.
Descriptors: Social stress, Psychophysiology, Time perception, Heart rate variability, Impedance cardiography
Time perception is central to nearly all aspects of everyday life, from deciding when to fulfill basic needs like sleeping and eating to coordinating complex social interactions with others. However, there is a dynamic relationship between an individual’s perception of time and a standard example of the same time unit (e.g., 5 s), such that time may be perceived as lasting shorter or longer than the standard unit depending on the context.
Perceived time is highly sensitive to the effects of context (e.g., Angrilli, Cherubini, Pavese, & Manfredini, 1997). Research indicates that people tend to experience perceived time distortion in response to negative or aversive stimuli across a number of sensory modalities (van Wassenhove, Buonomano, Shimojo, & Shams, 2008). Threatening, negative, or fearful stimuli tend to give rise to a feeling that time is slowing down or expanding (Bar-Haim, Kerem, Lamy, & Zakay, 2010; Droit-Volet & Gil, 2009, 2015), and this effect is commonly referred to as time dilation. Time dilation has been demonstrated for angry faces (Gil, Niedenthal, & Droit-Volet, 2007), fear cuing pictures (Grommet et al., 2011), and 95 dB white noise (Droit-Volet, Mermillod, Cocenas-Silva, & Gil, 2010). In addition, several previous studies have provided evidence that time dilation is a robust effect at a variety of stimulus durations (Droit-Volet & Meck, 2007; Sucala & David, 2012). While these studies provide evidence that time dilation can occur during the presentation of a threatening or negative stimulus, it is not presently known whether stressful experiences can alter subsequent time perception, or whether time dilation effects are specific to negative stimuli as compared to positive or neutral stimuli.
One previously proposed mechanism for time dilation is through changes in psychophysiological response (Craig, 2009; Droit-Volet & Meck, 2007; Zakay & Block, 1997), though this idea has yet to be clearly operationalized or robustly tested. According to Craig (2009), the interoceptive salience model proposes the idea that time perception is influenced by psychophysiological processes associated with emotion, and, therefore, based on this model we would expect that the stressful experiences that produce psychophysiological responses may also influence time estimation. One recent study provided some support for the interoceptive salience model by demonstrating that increased skin conductance (i.e., a physiological change) in response to negative stimuli is associated with time dilation (Mella, Conty, & Pouthas, 2011). However, many other studies have utilized stimuli designed to induce arousal via emotional response when measuring time dilation (e.g., Angrilli et al., 1997; Droit-Volet et al., 2010) without measuring psychophysiological responses. The present paper aims to further examine the relationship between psychophysiological response to a stressful situation, specifically cardiac autonomic reactivity, and the subsequent experience of time distortion for not only negative, but also positive and neutral stimuli.
Researchers have conceptualized stress as a coordinated response of multiple bodily systems to a potential threat in the environment (Lupien, McEwen, Gunnar, & Heim, 2009; McEwen, 2005). For social mammals, social stressors can result in distinct patterns of psychophysiological and behavioral responses (Norman, Hawkley, Cole, Berntson, & Cacioppo, 2012; Roelofs, Hagenaars, & Stins, 2010). Social stressors are deemed such because the proximate stimulus generating a response is based on an actual or potential social experience. Furthermore, the underlying psychological mechanism of social stressors is based on the negative emotions, like fear and anxiety, that they can evoke (Lazarus, 1999). Prior research indicates that cardiac autonomic responses to social stressors that involve social evaluative threat are both robust and reliable (Bosch et al., 2009; Yim, Quas, Rush, Granger, & Skoluda, 2015). The cardiac autonomic responses produced by social stressors also have implications for time perception under the interoceptive salience model, which suggests that perceived time is sensitive to changes in cardiorespiratory functioning (Craig, 2009). Thus, social stressors are not only a particularly potent way to evoke psychophysiological responses in the lab, but also an ideal method for studying the effects of stress on time perception.
The purpose of the present study was to examine individual differences in perceived time distortion as a function of experiencing social stress by comparing prestressor and poststressor time reproductions of positive, neutral, and negative pictures. Essentially, this research asks whether experiencing social stress has differential time distortion effects on stimuli of different valences, and whether time distortion, particularly for negative images, is affected by individual differences in psychophysiological responses to social stress as would be expected based on prior research (e.g., Mella et al., 2011). Unlike prior studies (e.g., Bar-Haim et al., 2010; Buetti & Lleras, 2012) in which participants engaged in a timing task with stressful stimuli, here the stress manipulation is separate from the experimental stimuli, which are positive, negative, or neutral but are not intended to be threatening or stressful. It is generally hypothesized that this type of time distortion occurs as a result of changes in psychophysiological responses produced by these negative or aversive stimuli (Droit-Volet et al., 2010; Mella et al., 2011). However, it is unknown whether engaging in a stressful task designed to elicit a psychophysiological response will affect subsequent time perception. Although less is known about time distortion effects for positive and neutral stimuli, we anticipate that experiencing a stressor might increase time dilation for these stimuli as well, but to a lesser extent than negative stimuli given that positive and neutral stimuli are not expected to compound the effects of a stressor the way that negative stimuli might.
This study addresses several specific research questions. First, does experiencing a stressor affect time estimation? We hypothesize that participants will show evidence of time dilation for negative stimuli after experiencing a stressor. Less is known about time distortion effects for positive stimuli, and thus we did not advance specific hypotheses as to the effects of stress on time distortion for these stimuli. Second, we address the question of whether sympathetic and parasympathetic aspects of psychophysiological response to a stressful task are predictive of time distortion. In this context, it is important to measure both systems because they both innervate the heart, and the extent to which heart rate is affected by a stressful situation could also affect time perception under the interoceptive salience model (Craig, 2009). We hypothesize that participants who exhibit greater sympathetic nervous system activation and greater parasympathetic withdrawal in response to a stressor will also experience more time distortion (i.e., larger differences in prestressor and poststressor time estimations), and we would expect this effect to be strongest for negative stimuli.
Method
Participants
Participants for this study (N = 53; 21 females) were recruited as part of a larger study conducted at the University of Chicago, and were compensated with $10 cash or course credit. Ages ranged from 18 to 23 with an average of 19.95 (SD = 1.3). Ninety-three percent of the sample reported being current undergraduate students. Data were excluded from this subsample for a variety of reasons, including taking antidepressant, antiseizure, or pain medication within 12 h of participating, which can affect recording of autonomic nervous system (ANS) measures (2 participants), not following timing task instructions (3 participants), not following experimenter instructions (1 participant), or equipment failure (5 participants); leaving analyzable data from 42 participants (16 females). Demographic information for the current study sample is reported in Table 1.
Table 1.
Demographic Information and Self-Reported State Anxiety
| Mean ± SEM or % | |
|---|---|
| Age | 19.95 ± 0.2 |
| BMI | 22.84 ± 0.4 |
| Percent female | 38% |
| Race/ethnicity | |
| White | 33% |
| Asian | 33% |
| Black | 0% |
| Hispanic | 12% |
| Other or multiracial | 22% |
| STAI-state (pre-TSST) | 37.02 ± 1.5 |
| STAI-state (post-TSST) | 41.02 ± 1.5 |
Note. There was a significant difference between STAI-state (pre-TSST) and STAI-state (post-TSST), t(41) = −2.94, p < .01. BMI = body mass index; STAI = State-Trait Anxiety Inventory.
Procedure
Participants began the experiment with a 5-min passive baseline measure of autonomic cardiac activity. After the passive baseline, participants completed a series of questionnaires assessing loneliness, depression, perceived stress, fear of negative evaluation, and feelings of anxiety (state and trait). With the exception of state anxiety, the questionnaire measures were not significantly related to our main research questions and therefore will not be discussed further. Participants then completed a practice time reproduction task, which involved passively viewing colored shapes and then reproducing their duration. Following the passive viewing, a question mark appeared on the screen to indicate the transition to the reproduction phase. During the reproduction phase of each trial, participants were instructed to press the space bar once to begin the reproduction and once again to end their estimation of the duration of time that the stimulus had appeared on the screen. This reproduction method has shown higher accuracy than other methods of time reproduction (Mioni, Stablum, McClintock, & Grondin, 2014). The stimuli were presented in E-Prime 2.0 (E-Prime, Pittsburgh, PA) and were displayed for 400, 1,650, 2,900, or 4,150 ms. These durations were chosen to provide four evenly spaced time points spanning a range from fewer than 500 ms to more than 4,000 ms; different subsets of this range have been examined in other studies of time distortion (e.g., Bar-Haim et al., 2010; Mereu & Lleras, 2013; Tipples, 2011), though previous studies tended to focus on either the shorter or longer end of the range and beyond (for exceptions, see Lewis & Miall, 2003; Pollatos, Yeldesbay, Pikovsky, & Rosenblum, 2014). Following the practice, participants completed a prestress measure of time distortion. This measure involved the same interval reproduction task that participants completed as practice of time estimation except that it contained novel pictures from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2008). A further description of the pictures and presentation duration is discussed below.
Following this prestress timing task, participants completed a modified version of the Trier Social Stress Test1 (TSST; Kirschbaum, Pirke, & Hellhammer, 1993) in which participants were shown a scenario and then given 2 min to mentally prepare a speech before being asked to deliver their speech to a video camera. Participants were told that their speeches would be evaluated by experts on poise, articulation, and content. Participants were not told ahead of time how long their speeches must be, but the speeches were cut off after 3 min for all participants. Immediately following the TSST, participants completed a posttest time reproduction task that was identical to the prestress timing task except that it contained novel IAPS pictures. Pre- and poststress picture sets were approximately equal on normed values for valence and intensity. Finally, participants completed a second series of questionnaires to gather demographic information as well as information about feelings of anxiety (state), and medical information (height, weight, and contraceptive use/menstrual cycle information from female participants). Afterward, participants were debriefed, thanked, and dismissed.
Materials
IAPS picture stimuli
Before and after the TSST, participants viewed positive, negative, and neutral IAPS pictures that were presented in randomized order. Each time, participants viewed 48 pictures (16 of each valence) that were presented for one of four durations (400, 1,650, 2,900, or 4,150 ms). Prior to data collection, a total of 96 pictures were separated into two groups of 48 pictures, and the order of these two sets of pictures (i.e., before or after TSST) was counterbalanced across participants. Likewise, we made an effort to counterbalance picture durations such that some participants viewed certain pictures for short durations, while other participants viewed those same pictures for longer durations. This was done to avoid timing effects based on the duration of specific pictures. Pictures were always presented in a random order, irrespective of picture valence. A full list of the IAPS pictures used can be found in the Appendix.
Anxiety questionnaire measures
The State-Trait Anxiety Inventory (STAI; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983) contains two 20-item scales, one for measuring current state anxiety and the other for measuring trait level anxiety. For this study, we focused on the state scale, which was administered at the beginning and the end of the study. Example items include “I feel anxious” and “I feel at ease” (reverse scored); participants responded using a 4-point scale ranging from 1 = not at all to 4 = very much so. Responses for the state scale assessed at the beginning of the study ranged from 21 to 56. Similarly, responses on the state scale when it was presented at the end of the study ranged from 22 to 59.
Autonomic nervous system measures
Cardiovascular measures of sympathetic and parasympathetic cardiac control were derived from preejection period (PEP) and high (respiratory) frequency (0.12–0.40 Hz) heart rate variability (HF HRV), respectively. PEP, calculated from impedance cardiography (ICG), is the period between the electrical invasion of the ventricular myocardium (Q wave of the electrocardiogram [ECG]) and the opening of the aortic valve (B notch). PEP depends on the time development of intra-ventricular pressure, and it is widely used as an index of cardiac contractility. Because variations in contractility are largely under sympathetic control, PEP is commonly used as a noninvasive measure of sympathetic cardiac control (Berntson, Norman, Hawkley, & Cacioppo, 2008; Sherwood et al., 1990). PEP is measured in milliseconds, and lower PEP values represent higher levels of sympathetic cardiac control.
HF HRV is a rhythmic fluctuation of heart rate in the respiratory frequency band and has been shown to be a relatively pure index of parasympathetic control (Berntson et al., 1997). The ECG was obtained using the standard Lead II configuration, and the impedance cardiogram was obtained using the standard tetrapolar electrode system and procedures described elsewhere (Sherwood et al., 1990).
ECG and basal thoracic impedance (Zo) was measured using a Bionex system with a sampling rate of 1000 Hz (Mindware, Gahanna, OH). All ECG and ICG data were visually inspected, preprocessed, and analyzed using Mindware Software version 3.1.1. (Mindware). For each subject, impedance data were ensemble-averaged to produce estimates of PEP. For this study, we used the previously specified B-point calculation method of 55% of dZ/dt peak plus a constant of four (Lozano et al., 2007), and the Q-point calculation was based on the onset of the R wave (Berntson, Lozano, Chen, & Cacioppo, 2004). Respiration was also derived from ICG, and fluctuations in respiration did not influence our results. Following visual inspection and correction of the interbeat interval series derived from the ECG, HF HRV was derived by spectral analysis following procedures that have been previously specified (Berntson et al., 1997). The interbeat interval series was time-sampled at 4 Hz (with interpolation) to yield an equal interval time series. This time series was detrended (linear), end tapered, and submitted to a fast Fourier transform. HF HRV spectral power was then integrated over the respiratory frequency band (0.12–0.40 Hz). HF HRV is represented as the natural log of the heart period variance in the respiratory band (in ms2).
For every participant, both HF HRV and PEP were collected over 5 min at three different points throughout the study. Data were preprocessed in 1-min segments that were then averaged to derive values for baseline, during the stressor2, and poststressor for use in analyses. The poststressor measures were taken during the second iteration of the time reproduction task.
Deriving the Data Analytic Sample
All timing data were examined for outliers, and values exceeding ± 2.5 SD from the sample mean for that timing interval were excluded from further analyses. This resulted in the exclusion of 46 individual values across pretest and posttest for all participants, which represents a 1.1% rate of outliers in this data set. Averaged raw values for each valence and duration are presented in Table 2. Before conducting statistical analyses, timing data were converted to proportions by dividing raw values by stimulus durations (e.g., reproduction durations for stimuli lasting 400 ms were all divided by 400); this was done in order to compare across stimulus duration. Additionally, participants were excluded from the data set if the correlation between the actual stimulus durations and their reproductions was less than 0.80 (three participants, referenced above as the majority of the participants that did not follow task instructions). Three participants were missing data for one of the three times PEP was measured (baseline, during TSST, or after TSST); data from these participants were excluded from repeated measures analyses involving PEP.
Table 2.
Average Timing Values by Duration and Type of Stimulus
| Duration | Prestressor | Poststressor | Δ Post—Pre |
|---|---|---|---|
| Negative | |||
| 400 | 490.41 (26.8) | 565.67 (34.3) | 75.26 (21.2) |
| 1,650 | 1,546.55 (55.6) | 1,635.23 (62.0) | 88.67 (40.7) |
| 2,900 | 2,513.45 (68.6) | 2,657.87 (69.8) | 144.42 (62.3) |
| 4,150 | 3,381.37 (71.2) | 3,651.14 (70.3) | 269.77 (65.5) |
| Neutral | |||
| 400 | 492.39 (24.6) | 504.51 (25.2) | 19.43 (27.7) |
| 1,650 | 1,505.16 (49.2) | 1,579.79 (58.4) | 74.63 (42.4) |
| 2,900 | 2,457.38 (61.6) | 2,611.29 (58.5) | 153.92 (47.7) |
| 4,150 | 3,553.01 (69.2) | 3,548.88 (67.0) | −4.14 (66.2) |
| Positive | |||
| 400 | 475.52 (21.3) | 534.98 (31.1) | 59.46 (24.5) |
| 1,650 | 1,539.79 (51.5) | 1,598.27 (57.8) | 58.48 (45.4) |
| 2,900 | 2,522.63 (68.9) | 2,590.18 (63.0) | 67.55 (46.7) |
| 4,150 | 3,430.46 (66.6) | 3,589.24 (70.9) | 158.78 (51.8) |
Note. All values are in milliseconds. Values presented are raw mean scores with standard errors in parentheses.
Results
We examined heart rate (HR), HF HRV, and PEP at baseline, during the TSST, and after the TSST using three separate repeated measures analyses of variance (ANOVAs) with time as a within-subject factor. For HR, there was a significant within-subject effect of time, F(1.5,63.3) = 61.22, p <.001, Cohen’s d = 2.44, with degrees of freedom adjusted using a Greenhouse-Geisser correction for violation of sphericity. Planned comparisons indicated that HR increased from baseline during the TSST, t(41) = −7.77, p <.001, and decreased after the TSST ended, t(41)58.92, p <.001. For HF HRV, there was a marginally significant within-subject effect, F(2,82) = 2.91, p = .060, Cohen’s d = 0.50. Follow up t tests indicated that HF HRV did not significantly differ from baseline during the TSST, t(41)5 0.991, p = .328; however, there was a significant increase in HF HRV after the TSST, compared to during the TSST, t(41) = −2.50, p = .016. There was also a significant within-subject effect for PEP, F(1.4,53.5) = 6.99, p = .005, Cohen’s d = 0.86, with degrees of freedom adjusted using a Greenhouse-Geisser correction for violation of sphericity. Compared to baseline, PEP decreased during the TSST, t(40) = 2.24, p =.031, and this was followed by an increase in PEP after the TSST compared to during the TSST, t(38) = −3.60, p = .001. These results are displayed visually in Figure 1.
Figure 1.
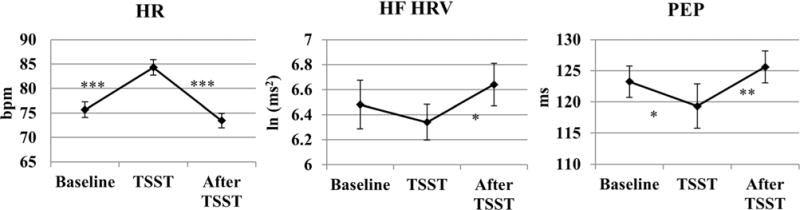
Changes from baseline during and after the TSST for heart rate (HR), high frequency heart rate variability (HF HRV), and preejection period (PEP). ***p<.001; **p< .01; *p<.05. Error bars represent standard error values.
In addition to changes in HR, HF HRV, and PEP, we also found changes in feelings of state anxiety from pre-TSST to post-TSST. There was a significant increase in self-reported state anxiety following the TSST, t(41) = −2.94, p =.005; means and standard errors for these measures can be found in Table 1. Changes in self-reported state anxiety were not correlated with post-TSST time reproductions (rs range from .02 to .20, all ps > .21); however, there was a significant positive correlation between change in self-reported state anxiety and pre-TSST time reproductions for negative pictures at the 4,150-ms duration, such that participants who reported more increases in state anxiety after the TSST also exhibited longer reproductions for negative pictures before the TSST, r = .36, p < .05 (all other rs ranged from −.05 to .24, all ps > .12). Changes in self-reported anxiety were not significantly correlated with changes from baseline during the TSST for HR, HF HRV, or PEP (rs range from −.01 to .08, all ps > .61).
Initial comparisons of time estimations found that changes from pre-TSST to post-TSST were not significantly different when comparing proportion values for the three supra second durations, 1,650 ms, 2,900 ms, and 4,150 ms, F(2,82) = 0.23, p =.796. Thus, for all analyses we focused on only the shortest and longest durations, 400 ms and 4,150 ms, respectively. Differences were calculated by subtracting pre-TSST time reproduction values from post-TSST values. Using these difference scores, we examined changes in time reproductions with a 3 (Valence: negative, neutral, positive) × 2 (Stimulus Duration: 400 ms, 4,150 ms) within-subject repeated measures ANOVA. There was a significant main effect for valence, F(1.5,60.7) = 3.66, p =.043, Cohen’s d = 0.59, with degrees of freedom adjusted using a Greenhouse-Geisser correction for violation of sphericity. Follow-up t tests indicated that time reproductions were significantly longer at post-TSST for negative pictures at both the 400-ms and 4,150-ms durations, t(41) = −3.55, p =.001, and t(41) = −4.12, p <.001, respectively. Reproductions of positive pictures were also found to be longer at post-TSST for both durations, t(41) = −2.43, p = .020, and t(41) = −3.07, p = .004, respectively. There were no significant differences for time reproductions of neutral pictures at either duration. Additionally, there was a nonsignificant main effect for duration, F(1,40) = 2.92, p = .095. Visual depictions of these results are displayed in Figure 2.
Figure 2.
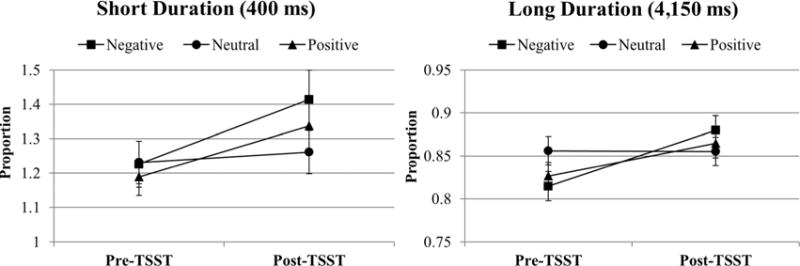
Time reproductions by valence for short and long durations.
There were no significant relationships between baseline measures of HR, HF HRV, and PEP and pre-TSST time duration values. To test the hypothesis that changes in heart rate, HF HRV, or PEP relate to time distortion, we correlated changes in these measures from baseline to during the TSST with changes in time reproduction intervals from pre-TSST to post-TSST for stimuli for which there was a significant effect of the TSST on time distortion (i.e., negative and positive pictures). There were no significant relationships between changes in HR (rs range from −.11 to .19, all ps>.24) or HF HRV (rs range from −.02 to .04, all ps> .81) for any of the time reproduction variables. There was, however, a significant correlation between change in PEP and change in time reproduction for negative pictures at the 400-ms duration, r = −.33, p = .034. This relationship is depicted in Figure 3. Change in PEP was not significantly correlated with time reproduction for negative pictures at 4,150 ms or for positive pictures at either duration (rs range from −.15 to .12, all ps > .35). There were also significant positive correlations between post-TSST HR and changes in time reproductions for short-duration negative and positive pictures (r = .39, p = .011, and r = .31, p = .044, respectively). No significant relationships emerged between post-TSST HF HRV or PEP values and any measures of time distortion.
Figure 3.
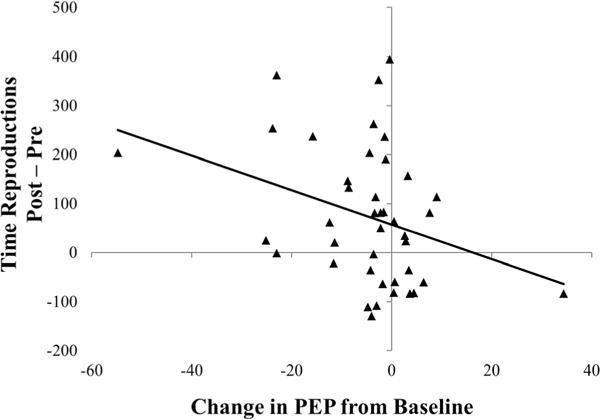
Relationship between change in time reproductions for short duration (400 ms) negative pictures and change in PEP during modified TSST.
Discussion
The aim of the present study was to examine whether individuals would show differences in time perception as a function of experiencing a social stressor known to produce reliable psychophysiological effects. We found that, compared to neutral pictures, participants showed evidence of time dilation for negative and positive pictures after experiencing a social stressor. Participants in this study also showed expected patterns of ANS activity (e.g., increases in heart rate and decreases in PEP compared to baseline) during the TSST, and self-reported state anxiety also increased as a function of participating in the TSST. These changes in psychophysiological response were largely unrelated to time dilation, with the exception of time reproductions for negative stimuli presented for very short (400-ms) durations. We did, however, find that higher post-TSST HR was significantly related to increased time dilation for short-duration negative and positive pictures, which offers some support for the interoceptive salience model of time perception (Craig, 2009). Unlike some previous time distortion studies (e.g., Mella et al., 2011), changes in psychophysiological response were measured during a separate stressful task, and we were interested in carryover effects to a subsequent timing task. Similar to past studies of time distortion (Angrilli et al., 1997; Grommet et al., 2011), IAPS pictures were chosen as stimuli in order to examine whether stimuli valence (i.e., positive, negative, or neutral) would have an effect on reproduction intervals in a systematic way as a result of the TSST. Following the social stress manipulation, participants showed evidence of time dilation for negative pictures, which is consistent with prior research (e.g., Angrilli et al., 1997; Grommet et al., 2011; Mereu & Lleras, 2013). However, we also found evidence for time dilation effects for positive pictures that were not present for neutral pictures, which suggests that time dilation effects might be more likely to occur for valenced stimuli. Future studies should consider including both positive and negative stimuli when examining time distortion effects.
This study also sought to further test the interoceptive salience model of time perception (Craig, 2009) by investigating the relationship between psychophysiological responses to social stress and the effects that these responses might have on time perception. Although the results did not indicate that changes in heart rate or HF HRV during the TSST had any effect on subsequent time reproductions, decreases in PEP from baseline were associated with increased time reproductions for short-duration negative pictures. This finding is consistent with prior studies that examined skin conductance responses and time perception for longer intervals (Droit-Volet et al., 2010; Mella et al., 2011). Participants did show changes in time reproductions from pre-TSST to post-TSST for both negative and positive stimuli, as well as significant changes in heart rate and PEP during the manipulation; however, in most instances (e.g., for positive pictures and for all pictures presented for the longer duration), it appears that ANS reactivity alone may not provide a complete explanation as to why changes in time reproductions occurred. Additionally, it is important to note that for our data baseline ANS measures were not systematically related to pre-TSST time reproductions. This contrasts with prior studies that have found effects of resting state ANS variables on time perception (e.g., Cellini et al., 2015; Pollatos et al., 2014).
Different paradigms have been used to assess time distortion throughout the time perception literature. Researchers have uncovered effects of time distortion for different types of stimuli using the oddball paradigm, where a target is presented in the middle of a set of standards and then is judged to be shorter or longer than the standards (e.g., van Wassenhove et al., 2008). Similar to the oddball paradigm, the temporal bisection task involves training participants on short and long durations and then asking them to decide which of the two durations a series of intermediate durations are more similar to (e.g., Droit-Volet & Gil, 2015; Grommet et al., 2011). In time reproduction tasks, the comparison of the reproduced interval to the target interval is the primary variable of interest, whereas the oddball paradigm and temporal bisection task focus on the point at which a stimulus is equally likely to be categorized as short or long. The use of a time reproduction paradigm in this study affords a more nuanced view of the effects of social stress on time perception that might not have been captured with a different timing paradigm. Additionally, future studies that use time reproduction paradigms should continue to include both subsecond and suprasecond intervals, given (a) the relationship between psychophysiological responses to social stress and time dilation for negative pictures at specifically subsecond intervals presented here, and (b) both are used throughout the time perception literature.
The present study has several limitations to consider. First, participants did not receive any training that might have helped improve their timing accuracy for the different durations they were presented. If participants had been trained on the reproduction intervals, we would expect less between-subject variability in time reproductions and may have been able to detect more subtle changes in time distortion in response to the TSST. Additionally, this study used a within-subject design to compare pre-TSST and post-TSST time reproductions; however, future studies could consider utilizing a between-subjects design to compare effects of the TSST to a passive control condition, or a within-subject design that involves both TSST and control sessions. Another limitation of this study is the fact that the method of time reproduction, though overall quite accurate, might not be ideal for use with very short durations where it can be less accurate than other methods (Mioni et al., 2014). Future studies should consider stimulus length when determining which reproduction method to use. It is also possible that the post-TSST ANS data may have been influenced by the presence of valenced stimuli during the post-TSST time reproduction task and/or by the effort participants exerted during that task. Additionally, we did not measure participants ratings of the emotional intensity of the IAPS stimuli. There is some evidence that ratings of emotional intensity might affect the relationship between psychophysiological response and experiences of time distortion (Mella et al., 2011), and future studies should consider adding these ratings to further examine these relationships.
Our results indicate that participants show increased time dilation for negative and positive pictures after completing the social stress task, demonstrating that time perception can be disrupted by a stressful event that is unrelated to the stimuli being timed. Likewise, participants clearly displayed psychophysiological reactivity to the TSST, and changes from baseline in PEP during the TSST were associated with increased time dilation for negative pictures presented for very short durations following the TSST. The extent to which psychophysiological responses influence time perception and the effects that this relationship could have on subsequent behavior in stressful situations is a potentially fruitful avenue for future research.
Acknowledgments
The authors would like to thank the members of the Social Psychophysiology and Neuroendocrinology Lab for their feedback on this project, and Mario Palmisano for his help with data collection. Kathryne Van Hedger is now at Department of Psychiatry and Behavioral Neuroscience, University of Chicago. Elizabeth A. Necka is now at National Institutes of Health.
Appendix
IAPS Pictures Used as Stimuli
| Picture type | Picture label |
|---|---|
| Negative | 1275, 5973, 7360, 9101, 9280, 9301, 9630, 9830, 1274, 7359, 9090, 9290, 9300, 9373, 9390, 9470, 2700, 2717, 3300, 6311, 7361, 9342, 9424, 9530, 2055, 2278, 3216, 3220, 9265, 9041, 9419, 9592 |
| Neutral | 5471, 7006, 7030, 7038, 7050, 7186, 7242, 7500, 5520, 7009, 7037, 7041, 7056, 7170, 7235, 7249, 2191, 2210, 2397, 2441, 2499, 2595, 2850, 2890, 2038, 2200, 2215, 2385, 2445, 2512, 2840, 9070 |
| Positive | 1722, 5270, 5660, 7250, 7280, 7289, 7430, 7470, 1590, 5450, 5849, 7260, 7390, 7400, 7480, 7508, 2339, 2346, 4606, 4623, 4625, 8120, 8461, 8496, 2310, 2345, 4610, 4617, 4624, 4641, 8371, 8540 |
Footnotes
This version did not include the mental arithmetic portion of the task.
Data collected during the stressor were recorded over a 2-min preparation and during the 3-min speech. There were no significant differences between preparation and speech for HF HRV or PEP. HR increased slightly during speech compared to preparation, but both values were significantly higher than baseline and poststressor, and thus were combined for parsimony.
References
- Angrilli A, Cherubini P, Pavese A, Manfredini S. The influence of affective factors on time perception. Perception & Psychophysics. 1997;59(6):972–982. doi: 10.3758/BF03205512. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Kerem A, Lamy D, Zakay D. When time slows down: The influence of threat on time perception in anxiety. Cognition & Emotion. 2010;24(2):255–263. doi: 10.1080/02699930903387603. [DOI] [Google Scholar]
- Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, VanderMolen MW. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Lozano DL, Chen YJ, Cacioppo JT. Where to Q in PEP. Psychophysiology. 2004;41(2):333–337. doi: 10.1111/j.1469-8986.2004.00156.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Norman GJ, Hawkley LC, Cacioppo JT. Cardiac autonomic balance versus cardiac regulatory capacity. Psychophysiology. 2008;45(4):643–652. doi: 10.1111/j.1469-8986.2008.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch JA, de Geus EJC, Carroll D, Goedhart AD, Anane LA, Veldhuizen van Zanten JJ, Edwards KM. A general enhancement of autonomic and cortisol responses during social evaluative threat. Psychosomatic Medicine. 2009;71(8):877–885. doi: 10.1097/PSY.0b013e3181baef05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buetti S, Lleras A. Perceiving control over aversive and fearful events can alter how we experience those events: An investigation of time perception in spider-fearful individuals. Frontiers in Psychology. 2012;3 doi: 10.3389/fpsyg.2012.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellini N, Mioni G, Levorato I, Grondin S, Stablum F, Sarlo M. Heart rate variability helps tracking time more accurately. Brain and Cognition. 2015;101:57–63. doi: 10.1016/j.bandc.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Craig AD. Emotional moments across time: A possible neural basis for time perception in the anterior insula. Philosophical Transactions of the Royal Society B–Biological Sciences. 2009;364(1525):1933–1942. doi: 10.1098/rstb.2009.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droit-Volet S, Gil S. The time-emotion paradox. Philosophical Transactions of the Royal Society B–Biological Sciences. 2009;364(1525):1943–1953. doi: 10.1098/rstb.2009.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droit-Volet S, Gil S. The emotional body and time perception. Cognition & Emotion. 2015;29:1–13. doi: 10.1080/02699931.2015.1023180. [DOI] [PubMed] [Google Scholar]
- Droit-Volet S, Meck WH. How emotions colour our perception of time. Trends in Cognitive Sciences. 2007;11(12):504–513. doi: 10.1016/j.tics.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Droit-Volet S, Mermillod M, Cocenas-Silva R, Gil S. The effect of expectancy of a threatening event on time perception in human adults. Emotion. 2010;10(6):908–914. doi: 10.1037/a0020258. [DOI] [PubMed] [Google Scholar]
- Gil S, Niedenthal PM, Droit-Volet S. Anger and time perception in children. Emotion. 2007;7(1):219–225. doi: 10.1037/1528-3542.7.1.219. [DOI] [PubMed] [Google Scholar]
- Grommet EK, Droit-Volet S, Gil S, Hemmes NS, Baker AH, Brown BL. Time estimation of fear cues in human observers. Behavioural Processes. 2011;86(1):88–93. doi: 10.1016/j.beproc.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The Trier Social Stress Test—A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Affective ratings of pictures and instruction manual Technical Report A-8. Gainesville, FL: University of Florida; 2008. [Google Scholar]
- Lazarus RS. Stress and emotion: A new synthesis. New York, NY: Springer Publishing Company; 1999. [Google Scholar]
- Lewis PA, Miall RC. Distinct systems for automatic and cognitively controlled time measurement: Evidence from neuroimaging. Current Opinion in Neurobiology. 2003;13(2):250–255. doi: 10.1016/s0959-4388(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Lozano DL, Norman G, Knox D, Wood BL, Miller BD, Emery CF, Berntson GG. Where to B in dZ/dt. Psychophysiology. 2007;44(1):113–119. doi: 10.1111/j.1469-8986.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stressed or stressed out: What is the difference? Journal of Psychiatry & Neuroscience. 2005;30(5):315–318. [PMC free article] [PubMed] [Google Scholar]
- Mella N, Conty L, Pouthas V. The role of physiological arousal in time perception: Psychophysiological evidence from an emotion regulation paradigm. Brain and Cognition. 2011;75(2):182–187. doi: 10.1016/j.bandc.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Mereu S, Lleras A. Feelings of control restore distorted time perception of emotionally charged events. Consciousness and Cognition. 2013;22(1):306–314. doi: 10.1016/j.concog.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Mioni G, Stablum F, McClintock SM, Grondin S. Different methods for reproducing time, different results. Attention Perception & Psychophysics. 2014;76:675–681. doi: 10.3758/s13414-014-0625-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman GJ, Hawkley LC, Cole SW, Berntson GG, Cacioppo JT. Social neuroscience: The social brain, oxytocin, and health. Social Neuroscience. 2012;7(1):18–29. doi: 10.1080/17470919.2011.568702. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Yeldesbay A, Pikovsky A, Rosenblum M. How much time has passed? Ask your heart. Frontiers in Neurorobotics. 2014;8:1–9. doi: 10.3389/fnbot.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs K, Hagenaars MA, Stins J. Facing freeze: Social threat induces bodily freeze in humans. Psychological Science. 2010;21(11):1575–1581. doi: 10.1177/0956797610384746. [DOI] [PubMed] [Google Scholar]
- Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, Vandoornen LJP. Methodological guidelines for impedance cardiography. Psychophysiology. 1990;27(1):1–23. doi: 10.1111/j.1469-8986.1990.tb02171.x. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Sucala M, David D. Slowing down the clock: A review of experimental studies investigating psychological time dilation. Journal of General Psychology. 2012;139:230–243. doi: 10.1080/00221309.2012.695410. [DOI] [PubMed] [Google Scholar]
- Tipples J. When time stands still: Fear-specific modulation of temporal bias due to threat. Emotion. 2011;11(1):74–80. doi: 10.1037/a0022015. [DOI] [PubMed] [Google Scholar]
- van Wassenhove V, Buonomano DV, Shimojo S, Shams L. Distortions of subjective time perception within and across senses. PLOS ONE. 2008;3(1):e1437. doi: 10.1371/journal.pone.0001437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim IS, Quas JA, Rush EB, Granger DA, Skoluda N. Experimental manipulation of the Trier Social Stress Test-Modified (TSST-M) to vary arousal across development. Psychoneuroendocrinology. 2015;57:61–71. doi: 10.1016/j.psyneuen.2015.03.021. [DOI] [PubMed] [Google Scholar]
- Zakay D, Block RA. Temporal cognition. Current Directions in Psychological Science. 1997;6(1):12–16. doi: 10.1111/1467-8721.ep11512604. [DOI] [Google Scholar]


