Abstract
HIV-infected individuals (HIV+) has 2–3 times the rate of tobacco smoking than the general population, and whether smoking may lead to greater psychiatric symptoms or cognitive deficits remains unclear. We evaluated the independent and combined effects of being HIV+ and chronic tobacco-smoking on impulsivity, psychopathological symptoms and cognition. 104 participants [27 seronegative (SN)-non-Smokers, 26 SN-Smokers, 29 HIV+non-Smokers, 22 HIV+Smokers] were assessed for psychopathology symptoms (Symptom Checklist-90, SCL-90), depressive symptoms (Center for Epidemiologic Studies-Depression Scale, CES-D), impulsivity (Barratt Impulsiveness Scale, BIS), decision-making (The Iowa Gambling Task, IGT, and Wisconsin Card Sorting Test, WCST), and cognition (seven neurocognitive domains). Both HIV+ and Smoker groups had higher SCL-90 and CES-D scores, with highest scores in HIV+Smokers. On BIS, both HIV+ and Smokers had higher Total Impulsiveness scores, with higher behavioral impulsivity in Smokers, highest in HIV+Smokers. Furthermore, across the four groups, HIV+Smokers lost most money and made fewest advantageous choices on the IGT, and had highest percent errors on WCST. Lastly, HIV+ had lower z-scores on all cognitive domains, with the lowest scores in HIV+Smokers.
These findings suggest that HIV-infection and chronic tobacco smoking may lead to additive deleterious effects on impulsivity, psychopathological (especially depressive) symptoms and cognitive dysfunction. Although greater impulsivity may be premorbid in HIV+ and Smokers, the lack of benefits of nicotine in chronic Smokers on attention and psychopathology, especially those with HIV-infection, may be due to the negative effects of chronic smoking on dopaminergic and cardio-neurovascular systems. Tobacco smoking may contribute to psychopathology and neurocognitive disorders in HIV+ individuals.
Keywords: HIV, Tobacco use, Decision making, Psychopathology
Introduction
Since the introduction of combined antiretroviral therapy (cART) in the mid 1990s, HIV-infected (HIV+) individuals are living longer (National Center for Health Statistics, 2015). These aging HIV+ individuals often have more chronic co-morbid health problems, including those associated with chronic tobacco use disorder. Furthermore, the tobacco smoking rate is almost three times (42%) (Tesoriero et al., 2010; Mascolini, 2013) more prevalent in HIV+ compared to the averaged rate of smokers in the U.S. (15.1%) (Centers for Disease Control and Prevention, 2015). The higher smoking prevalence is partly due to the greater risk factors related to tobacco use disorder, such as lower socioeconomic status, greater substance abuse, and homosexual/bisexual status (Centers for Disease Control and Prevention, 2015). Whether tobacco use in HIV+ individuals might lead to greater impulsivity and more psychopathological symptoms, or might improve cognition as observed in seronegative (SN) individuals (Rezvani and Levin, 2001), remain unclear.
Both HIV+ individuals and tobacco Smokers typically have greater impulsivity (Mitchell, 1999; Martin et al., 2004). HIV+ patients also showed abnormal prefrontal and subcortical brain networks, which may lead to dysfunction in controlled processing, response inhibition and working memory (Farinpour et al., 2000; Martin et al., 2001). For example, HIV+ individuals performed worse than SN substance-dependent individuals on a gambling task, by exhibiting higher levels of cognitive impulsivity and making more disadvantageous choices (Martin et al., 2004), and HIV+ persons engaged in more risk-taking behaviors (Owe-Larsson et al., 2009). These risk taking behaviors may lead to negative consequences since HIV+ men with greater impulsivity had lower educational attainment, lower income, were more likely to be unemployed, had more psychiatric diagnoses, higher depressive symptom scores and more unprotected sex (Semple et al., 2006). Similarly, tobacco smokers were found to be more impulsive than non-smokers on 26 out of 28 personality questionnaires and tasks, and preferred smaller immediate rewards rather than larger more delayed rewards on a behavioral choice task (Mitchell, 1999). Together, these findings indicate greater impulsivity in both HIV+ individuals and in tobacco smokers on various dimensions.
Higher rates of psychopathology were also observed in both HIV+ and tobacco-smoking populations. For instance, more symptoms of depression and anxiety were observed in HIV+ participants compared to SN controls, often as a consequence of living and coping with their HIV+ status (Owe-Larsson et al., 2009; Jin et al., 2010; Rezaei et al., 2013). Tobacco smokers were also found to have higher rates of anxiety and depression than non-smokers (Breslau, 1995), as well as more neurotic traits (Gilbert and Gilbert, 1995).
Relative to impulsivity and psychopathology, the cognitive effects associated with HIV-infection and tobacco-smoking were studied more extensively. HIV infection may cause brain injury via direct neurotoxic effects from the viral proteins and indirect effects from neuroinflammation (Levy, 2007), which in turn may lead to HIV-associated neurocognitive disorders (HAND) (Antinori et al., 2007b). Even with effective cART and aviremia, many HIV+ patients continued to have cognitive impairments (Robertson et al., 2007; Heaton et al., 2010; Simioni et al., 2010), including diminished processing and psychomotor speed, as well as deficits in executive function and attention/working memory (Martin et al., 2003; Heaton et al., 2010; Heaton et al., 2011a). In contrast, the highly addictive psychoactive compound nicotine in tobacco (Dani and De Biasi, 2001) can improve both attention and memory (Rezvani and Levin, 2001; Heishman et al., 2010). Therefore, HIV+ individuals may self-medicate with tobacco for their cognitive deficits or psychopathological symptoms (Pomerleau and Pomerleau, 1985; Carmody, 1989). However, nicotine withdrawal may also lead to anxiety, depression and concentration problems (Breslau, 1995; Gilbert and Gilbert, 1995).
Overall, HIV infection and tobacco-smoking each appears to have negative effects on impulsivity and psychopathology, but opposite influences on cognition. However, it remains unclear how the combination of both factors will influence such behavioral outcomes. While HIV+ women who smoked tobacco cigarettes had better frontal/executive performance than the non-smokers (Wojna et al., 2007), HIV+ mixed gender participants who were current smokers had worse learning, memory, and global cognitive functioning scores than the non-smokers (Bryant et al., 2013). However, in the latter study, the smokers with worse cognitive function also had lower education and higher rates of hepatitis C virus infection than the non-smokers (Bryant et al., 2013). Furthermore, HIV+ women who used other substances (cocaine or heroin) showed additional negative effects on verbal memory, processing speed and executive function, compared to those who did not use substances, even after adjustment for group difference in past tobacco smoking (Meyer et al., 2013).
The current study aims to evaluate the independent and combined effects of being HIV+ and chronic tobacco-smoking on impulsivity, psychopathological symptoms and cognitive functions. Since both HIV infection and tobacco smoking may lead to greater impulsivity and psychopathology, we expected HIV+Smokers to show even more of these symptoms. However, since HIV-infection and tobacco-smoking might lead to opposite effects on cognition, we expected HIV+Smokers to show relatively normal levels of cognition compared to age and education-matched SN-non-Smokers.
Methods
Research participants
104 participants were enrolled across four groups (27 SN non-Smoker, 29 SN-Smokers, 26 HIV+non-Smokers, and 22 HIV+Smokers) for a 2×2 design, with HIV-serostatus and tobacco smoking status as factors. These participants were recruited from the local community, via flyers, web-based advertisements, word-of-mouth, or local health care providers, and were screened by telephone (n=164). Potentially qualified participants were further examined by a physician and their medical records were reviewed to ensure they fulfilled the study criteria. The inclusion criteria for HIV+ participants were: 1) men or women of any ethnicity, age 18–65 years; 2) HIV+ (verified by medical records); 3) stable on a cART regimen for ≥6 months; 4) nadir CD4 count<500 cells/mm3; 5) negative urine toxicology screen (including methamphetamine, amphetamine, cocaine, marijuana, benzodiazepine, barbiturates and opiates) on the day of evaluation. All SN participants were confirmed to be HIV-1 negative with the Clearview® COMPLETE HIV 1/2 test kits (Alere, Waltham, MA) in addition to fulfilling Inclusion criteria 1 & 5 above. Tobacco Smokers were current daily users, at least 1/2 pack cigarettes per day, for at least past 2 years, while non-Smokers had less than 2 lifetime pack-years and were not currently smoking. The exclusion criteria for all groups were: 1) History of co-morbid psychiatric illnesses including major unipolar or bipolar affective disorders (except for reactive depression), schizophrenia, obsessive compulsive disorder, and attention deficit hyperactive disorder; 2) confounding neurological disorders (e.g. dementia syndromes other than HAND, multiple sclerosis, Parkinson disease, brain infections including Hepatitis C, neoplasm, cerebral palsy or significant head trauma with coma and prolonged hospitalization); 3) significant abnormal screening blood tests indicating a chronic medical condition that might influence brain function; 4) current or history of moderate or severe other substance use disorders, including methamphetamines, cocaine, alcohol, opiates and marijuana, according to DSM-5 criteria; therefore, individuals who had only “mild substance use disorders” or “recreational substance use” were allowed in the study. 5) pregnancy (if female of child bearing potential); 6) Inability to read at 8th grade level and could not provide consent. Prior to the studies, all participants were verbally informed of the study procedures and signed a written informed consent form, which along with the protocol were approved by the University of Hawaii Cooperative Committee on Human Studies. Each participant completed the following assessments for clinical and medical evaluations, psychopathological symptoms, impulsivity, decision -making and cognition.
Clinical and Medical Evaluations
Each participant was evaluated by a study physician with a structured neuropsychiatric examination, including detail substance use histories and neurological examination, and medical record review. For HIV+ participants, they were additionally assessed with HIV Dementia Scale, Karnofsky score, and their nadir and most recent CD4 cell counts and plasma viral loads were obtained from their medical records. Tobacco usage and Fagerstrom scores were obtained in the Tobacco smokers. All participants also had screening blood tests if none were performed within the past 6 months, an electrocardiogram and urine toxicology screen.
Assessments for Psychopathological symptoms
Symptom Checklist-90 (SCL-90) is a 90-item test that assessed how distressed the participant felt for a range of symptoms within the past week. Responses range from 0 to 4 (0=Not at all, 1=A little bit, 2=Moderately, 3=Quite a bit, 4=Extremely). The questions were categorized into nine subscales with t-score conversion for the following dimensions: Depression (DEP), Anxiety (ANX), Somatization (SOM), Obsessive Compulsiveness (O-C), Hostility (HOS), Interpersonal Sensitivity (I-S), Phobic Anxiety (PHB), Paranoid Ideation (PAR), and Psychoticism (PSY). These nine subscales were further grouped into global indices: General Symptom Index (GSI) is a measure of overall psychological distress; Positive Symptom Distress Index (PSDI) is a measure of symptom intensity; and Positive Symptom Total (PST) provides the number of self reported symptoms (Pearson Education).
Center for Epidemiologic Studies-Depression Scale (CES-D) is a 20 item self-reported questionnaire regarding how often the participant might have experienced specific symptoms associated with depression over the past week. Item responses range from 0 (Rarely or None of the Time) to 3 (Most or Almost all the Time). A score of >16 identifies those at risk for clinical depression (Radloff, 1977; Lewinsohn et al., 1997).
Assessments for Impulsiveness and Decision Making
The Barratt Impulsiveness Scale (BIS) (Patton et al., 1995) is a self-administered 30-item questionnaire that assesses different personality constructs for the degree of impulsiveness. Each item was rated from 1 (rarely/never) to 4 (almost always/always). Three type of scores were calculated from the questionnaire and used for subsequent analyses, including a total score, and two empirical based categories of “cognitive” and “behavioral” impulsivity (see Reise et al., 2013).
The Iowa Gambling Task (IGT)(Bechara and Martin, 2004) evaluated risk-taking behaviors, using computerized display of four decks of cards to be selected by the participants. The amount of reward varied, with two smaller reward decks ($50) and two larger reward decks ($100). The losses in the decks also varied, with smaller losses in the lower reward decks, and larger penalties in the large reward decks. These penalties also occurred in random intervals. Participants were instructed to choose the cards to maximize their “winnings,” and the computer screen continuously displayed the total amount of money won and the amount won and lost each time a card was drawn.
The Wisconsin Card Sorting Test (WCST Computerized version 4: CV4) involves multiple cognitive processes (problem solving, set shifting, working memory and attention) that assessed executive function, learning and cognitive flexibility. The test involves matching stimulus cards with one of four category cards, in which the stimuli are multidimensional according to color (C), shape (S) and number (N); each dimension defined a sorting rule. By trial and error, the participant had to determine a preordained sorting rule given just the feedback (“Right” or “Wrong”) on the screen after each sort. After 10 consecutive correct sorts, the rule would change without the participant being informed. Up to six attempts were allotted to derive a new rule, providing five rule shifts in the following sequence (C-S-N-C-S-N), with each rule attainment referred to as ‘completing a category’. The testing continued until all 128 cards were sorted and irrespective of whether the participant completed all the rule shifts. Two types of errors were possible: perseverative errors, in which the participant made the same response with a wrong sorting rule, and non-perseverative errors. The correct response rate and response error rate were calculated from each participant’s performance.
Neurocognitive Performance
Each participant also completed a battery of neuropsychological tests that evaluated seven cognitive domains, along with assessments for their activities of daily living, that are required to determine the HAND or HAND-equivalent status of the participants.(Antinori et al., 2007a) The seven domains included processing speed, learning, memory, verbal fluency, executive function, attention, and fine motor function (see Table 2 for tests used for each domain). Z-scores were generated for these domains, adjusted for age and education, using our normative database containing data from 507 healthy participants administered the same tests in our laboratory.
Table 2.
Cognitive Domain Z-Scores1 of the Study Participants (Mean±S.E.)
| Cognitive Domain | SN Controls | SN Tobacco Smokers | HIV+ Participants | HIV+ Tobacco Smokers | Two-way ANOVA p-value
|
||
|---|---|---|---|---|---|---|---|
| n=25 | n=25 | n=25 | n=22 | IV effect | Tobacco effect | HIV x Tobacco interaction | |
| Fluency | 0.37±0.12 | 0.05±0.10 | −0.04±0.10 | −0.18±0.13 | 0.04 | 0.20 | 0.37 |
| Executive function | 0.18±0.12 | −0.26±0.11 | −0.36±0.14 | −0.61±0.14 | 0.02 | 0.19 | 0.40 |
| Speed | −0.05±0.09 | −0.23±0.09 | −0.36±0.08 | −0.55±0.13 | 0.03 | 0.23 | 0.91 |
| Attention/WM | −0.03±0.13 | −0.39±0.10 | −0.54±0.13 | −0.58±0.13 | 0.02 | 0.46 | 0.19 |
| Learning | 0.16±0.11 | −0.27±0.10 | −0.31±0.14 | −0.53±0.16 | 0.03 | 0.14 | 0.37 |
| Memory | 0.16±0.11 | −0.12±0.10 | −0.25±0.13 | −0.47±0.13 | 0.01 | 0.30 | 0.55 |
| Motor | 0.03±0.12 | −0.17±0.09 | −0.30±0.11 | −0.68±0.18 | 0.02 | 0.29 | 0.74 |
| Global | 0.13±0.08 | −0.20±0.06 | −0.27±0.09 | −0.55±0.12 | 0.003 | 0.07 | 0.59 |
| # (%) with HAND or HAND-equivalent | 6 (24%) | 12 (48%) | 8 (32%) | 14 (63.6%) | |||
| Subtypes of HAND or HAND-equivalent | 6 ANI | 10 ANI 2 MND |
5 ANI 2 MND 1 HAD |
4 ANI 5 MND 5 HAD |
|||
- Fluency: DKEFS or Ruff Figural Design Fluency and Verbal Fluency (with letters FAS)
- Executive Functions: DKEFS CWI or Stroop Interference and Trail Making Test B
- Speed of Information Processing: Symbol Digit, DKEFS Trail-making Number Sequencing or Trail Making Test A, DKEFS Color naming or Stroop Color Naming, and California Computerized Assessment Package (CalCAP) Simple Reaction Time
- Attention/Working Memory: Arithmetic from Wechsler Adult Intelligence Scale-VI, Digit Span Backward, Letter-Number Sequencing, Arithmetic and Paced Auditory Serial Addition Test 1
- Learning: Rey Auditory Verbal Learning Test Trial 5; Rey-Osterreith Complex Figure Test-Immediate Recall
- Memory: Rey Auditory Verbal Learning Test Delayed Recall (Trial 7); Rey Complex Figure-Delayed Recall
- Motor Skills: Grooved Pegboard Dominant and Non-dominant hands.
HAND: HIV-associated Neurological Disorder(s)
ANI: Asymptomatic neurocognitive impairment
MND: Minor neurocognitive disorder
HAD: HIV-associated dementia or HAD-equivalent
Statistical analyses
The demographic variables across the four groups were compared using one-way ANOVA or Chi-square. Comparison of the scores from all instruments and questionnaires assessed in the four participant groups were performed using a two-way ANCOVA, with HIV and smoking status as between-participant factors, with education and age as covariates. Two-way ANOVA was performed on the cognitive domain z-scores, without additional covariates, since these scores were adjusted for age and education. Possible relationships between cognitive performance on these tasks and other tasks that showed group differences were explored using Pearson correlations.
Results
Participant Characteristics (Table 1)
Table 1.
Clinical Characteristics of the Study Participants (Mean±S.E.)
| SN Controls | SN Tobacco Smokers | HIV + Participants | HIV+ Tobacco Smokers | p-value | |
|---|---|---|---|---|---|
| n=25 | n=25 | n=25 | n=22 | ||
| Age (years) | 43.8±11.4 | 44.0±9.3 | 47.3±11.8 | 45.9±11.2 | 0.62 |
| Education (years) | 15.6±3.7 | 13.4±1.9 | 15.4±2.3 | 14.1±2.4 | 0.02* |
| Males (%)/Females (%) | 23(92)/2(8) | 23(92)/2(8) | 24(96)/1(4) | 20(91)/2(9) | 0.62 |
| Race | 0.89 | ||||
| White/Asian/Black/Native-Hawaiian | 12/8/0 | 9/6/1 | 12/6/1 | 12/3/1 | |
| Pacific Islander/Mixed Race | 2/3 | 3/6 | 3/3 | 1/5 | |
| HIV Disease Severity | |||||
| CD4 count (#cells/mm3) | 549.9±356.3 | 551.9±233.5 | 0.98 | ||
| Nadir CD4 (#cells/mm3) | 206.4±133.3 | 219.2±137.1 | 0.77 | ||
| HIV duration (months) | 185.0±254.9 | 126.3±89.4 | 0.33 | ||
| # (%) with undetectable virus | 19 (76%) | 20 (91%) | .17 | ||
| # (%) taking antiretroviral medications | 22 (88%) | 20 (91%) | .75 | ||
| Plasma HIV RNA (copies/mL)** | 9,260±39,493 | 159±471 | 0.31 | ||
| Log plasma HIV RNA | 2.1±1.0 | 1.7±0.5 | 0.10 | ||
| Karnofsky score (max. 100) | 93.15±1.28 | 93.18±1.91 | 0.99 | ||
| HIV Dementia Scale | 15.05±0.34 | 15.13±0.52 | 14.46±0.40 | 12.82±0.86 | 0.10 |
| # HAND or HAND-Equivalent (%) | 6 (24%) | 9 (36%) | 7 (28%) | 11 (50%) | 0.25 |
| # On antidepressants/anxiolytics (%)*** | 2 (8%) | 4 (16%) | 8 (32%) | 9 (40%) | 0.03* |
| Tobacco usage | |||||
| Fagerstrom score | 4.8±2.4 | 3.4±2.2 | 0.11 | ||
| Daily average tobacco use (#cigarettes) | 17.9±8.7 | 17.9±12.4 | 0.995 | ||
| Total lifetime tobacco used (pack years) | 22.3±15.1 | 22.6±18.4 | 0.96 | ||
| Duration of tobacco use (years) | 24.4±11.9 | 25.9±12.1 | 0.68 | ||
| Marijuana (MJ) usage | |||||
| # Marijuana users past month (%) | 3 (12%) | 5 (20%) | 6 (24 %) | 6 (27.3%) | 0.59 |
| Daily average MJ used (g) | 0.34±1.41 | 0.13±0.20 | 0.22±0.40 | 0.54±1.00 | 0.41 |
| Total lifetime MJ used (kg) | 2.6±10.4 | 0.62±1.20 | 1.73±4.15 | 3.98±9.72 | 0.46 |
| Duration of MJ use (years) | 4.9±9.3 | 8.5±11.9 | 13.4±14.6 | 14.3±13.2 | 0.03* |
| Median years since last use (range) | 12.2 (0–39.8) | 1.7 (0–29.9) | 3.4 (0–30.4) | 1.1 (0–39.7) | 0.21 |
| Alcohol usage | |||||
| # Alcohol users past month (%) | 14 (56%) | 8 (32%) | 10 (40%) | 8 (36.4%) | 0.33 |
| Daily average alcohol used (mL) | 9.9±14.4 | 13.1±32.6 | 13.1±32.6 | 33.5±56.4 | 0.33 |
| Total lifetime alcohol used (Liters) | 96.5±150.8 | 140.2±352.9 | 362.5±1311.4 | 276.8±540.1 | 0.57 |
| Median lifetime alcohol used (Liters, range) | 65.8 (0.004–596) | 68.4 (2.2–1533) | 88.9 (9.5–6748) | 53.1 (0.33–2491) | 0.64 |
| Duration of alcohol use (years) | 20.1±14.6 | 15.3±15.9 | 18.3±15.0 | 22.2±12.3 | 0.54 |
| Median days since last use (range) | 0 (0–12,926) | 20 (0–2,002) | 30 (0–4,848) | 91 (0–3,666) | 0.29 |
P-value <0.05 (from 1-way ANOVA, Kruskal-Wallis or chi-square test)
Plasma HIV RNA was calculated from 6 (HIV+) and 2 (HIV+Smoker) participants with detectable viruses.
- SN: venlafaxine (n=1); trazadone (n=1)
- SN-Smokers: bupropion (n=1); fluoxetine (n=2); Clordiazepoxide (n=1)
- HIV+: venlafaxine (n=2); paroxetine (n=4); trazodone (n=3); clonazepam (n=1); fluoxetine (n=1); aprazolam (n=1); buspirone (n=1); bupropion (n=1); aripiprazole (n=1); diazepam (n=1); tempazepam (n=1)
- HIV+Smokers: paroxetine (n=2); quetiapine (n=1); fluoxetine (n=2); mirtazapine (n=1); diazepam (n=1); aripiprazole (n=2); olanzapine (n=2); citalopram (n=2); bupropion (n=3); venlafaxine (n=1); aprazolam (n=1); clonazepam (n=1)
Age, gender and racial distributions were similar among the four groups. The SN-Smoker group tended to have lower education than either the SN or HIV+ groups. The two HIV+ groups had similar clinical measures of HIV disease severity, and the two tobacco-Smoker groups had similar tobacco usage. In addition, the four groups had similar marijuana (MJ) usage variables, which included the number of participants who used MJ in the past 30 days, their daily averaged MJ used and the total lifetime MJ used, as well as median years since last MJ use. However, both HIV+ groups used MJ for longer duration (HIV+ vs. SN: p=0.03; HIV+Smokers vs. SN; p=0.02). By design, none of the participants had severe alcohol use disorder, and all alcohol usage variables were also not different across the four groups.
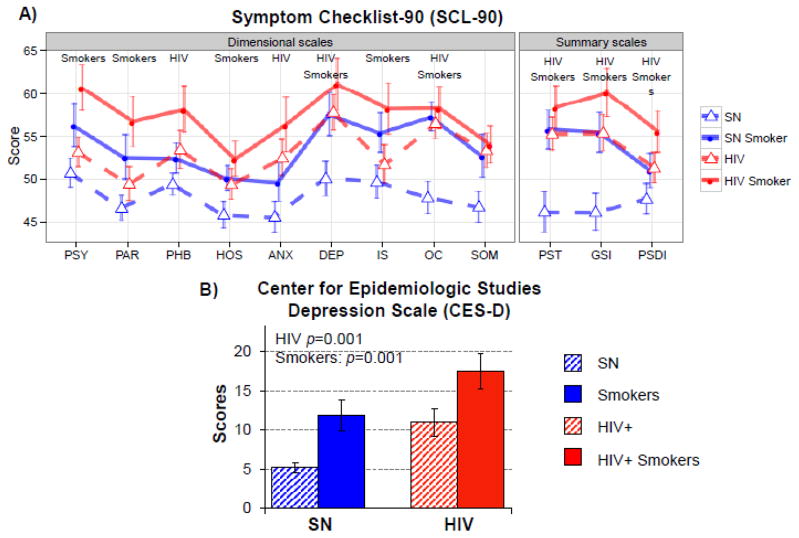
Symptom Checklist-90 (SCL90, Figure 1A)
Figure 1. Psychopathological Symptoms and Depression Scale.
A) On Symptom Checklist-90, both tobacco-Smokers and HIV+ participants had higher scores and more psychopathological symptoms than SN. HIV+Smokers had the highest scores, with more symptoms, on depression and obsessive compulsiveness, as well as the global scores. B) On the Center for Epidemiological Studies-Depression (CES-D) scale, HIV+ participants (regardless of smoking status) and tobacco-Smokers (regardless of HIV serostatus) had greater depressive symptoms, with the HIV+Smoker group reporting the greatest depressive symptoms.
Regardless of smoking status, HIV+ participants reported more symptoms, which led to higher summary scores of PST (Positive Symptom Total), GSI (General Symptom Index) and PSDI (Positive Symptom Distress Index) (all F>4, p<0.05). Similarly, regardless of HIV serostatus, Smokers had higher summary scores on all three summary measures (all F>4, p<0.05). Hence, the HIV+Smokers consistently had the highest scores for all subscales. The HIV+ participants showed greater anxiety, phobic anxiety, depression, and obsessive compulsiveness (all F>5, p<0.05), while both Smoker groups showed greater psychoticism, paranoid ideation, hostility, depression, interpersonal sensitivity, and obsessive compulsiveness (all F>5, p<0.05).
Center for Epidemiologic Studies – Depression Scale (CES-D, Figure 1B)
Similar to the finding of higher depressive symptoms on SCL-90, HIV participants (F(1, 96)=10.9, p=0.001) and Smoker participants (F(1, 96)=11.3, p=0.001) had higher CES-D scores than SN controls. Hence, HIV+Smokers had the highest CES-D scores (17.4±11.2), indicating possible clinical depression in some of the participants. Since more HIV+ participants were using antidepressant or anxiolytic medications (see Table 1), the use of these medications was also included as a covariate, but the group differences on CES-D score remain significant.
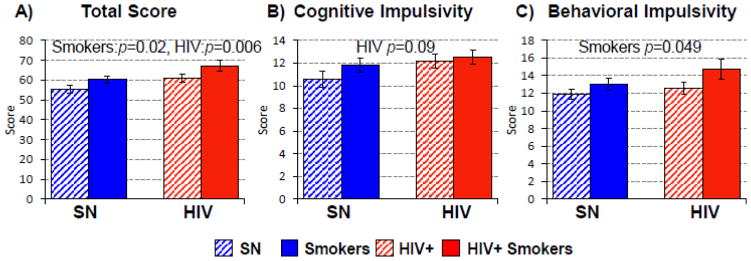
Barratt Impulsiveness Scale (BIS, Figure 2)
Figure 2. Barratt Impulsivity Scale.
(A) The Barratt Impulsivity Scale shows higher Total Scores in both HIV+ individuals (regardless of smoking status) and in Tobacco Smokers (regardless of HIV status), and the highest Total score in the HIV+Smoker group. (B & C) On the two-factor model, HIV+ participants tended to have higher scores than SN on Cognitive Impulsivity, while chronic Smokers had higher Behavioral Impulsivity. Therefore, HIV+Smokers had the highest scores on both cognitive and behavioral impulsivity scores.
Both HIV (F(1, 85)=8.0, p=0.006) and Smoker (F(1, 85)=5.5, p=0.02) participants were more impulsive with higher Total Impulsiveness scores. Hence, HIV+Smokers showed additively higher Total Impulsive scores. The scores were also calculated according to the two factor model (Reise et al., 2013) using cognitive and behavioral impulsivity indices. Smoker participants had higher behavioral impulsiveness scores: F(1, 85)=4.0, p=0.049), while HIV+ participants tended to have higher cognitive impulsiveness scores (F(1, 85)=3.0, p=0.09).
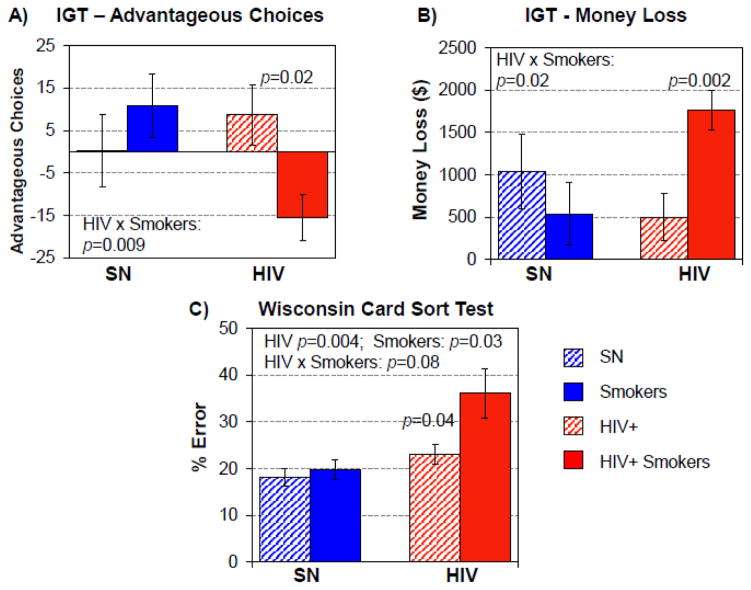
The Iowa Gambling Task (IGT, Figure 3)
Figure 3. Iowa Gambling Task (IGT) and Wisconsin Card Sort Test.
(A & B) The Iowa Gambling Task (IGT) shows that the effect of HIV+ depends on smoking status for advantageous choices made and for the money lost. While SN Smokers had better performance than SN-nonSmokers, with more advantageous choices and loss less money, HIV+Smokers made more disadvantageous choices and lost more money than HIV+ non-Smokers. C) On the Wisconsin Card Sort Task, HIV+ participants, significantly had higher % errors, significantly higher in those who were Smokers, and Smokers (regardless of HIV serostatus) also tended to have more errors; therefore, HIV+Smokers had the highest error percentages.
All four groups lost money on the IGT, thus the analyses were conducted on total amount of money lost in addition to the number of advantageous choices made in the task. Although HIV+ subjects and Smokers did not perform differently, an interaction effect was found on total money lost (p=0.009) and the number of advantageous choices made (p=0.02, Figure 3). Post-hoc t-tests show that HIV+Smokers lost more money) than HIV+non-Smokers [$1,764±237 versus $497±282, t(41)= 3.1, p= 0.004]. Furthermore, HIV+Smokers made more disadvantageous choices (−15.5±5.4, negative values for net disadvantageous choices) than HIV+non-Smokers (8.7±7.0, positive values for net advantageous choices), t(39)=2.2, p=0.004.
Wisconsin Card Sorting Test (WCST)
More errors on the WCST were made by both HIV+ participants (F(1, 65)=9.0, p=0.004) and Smoker participants (F(1, 65)=4.9, p = 0.03) compared to the SN controls. Although HIV+ and Smoking status showed only a trend for an interaction effect, HIV+ Smokers showed an additive effect with the highest error percentage (36.1±5.3%, Figure 3). Correct response rates did not differ between groups.
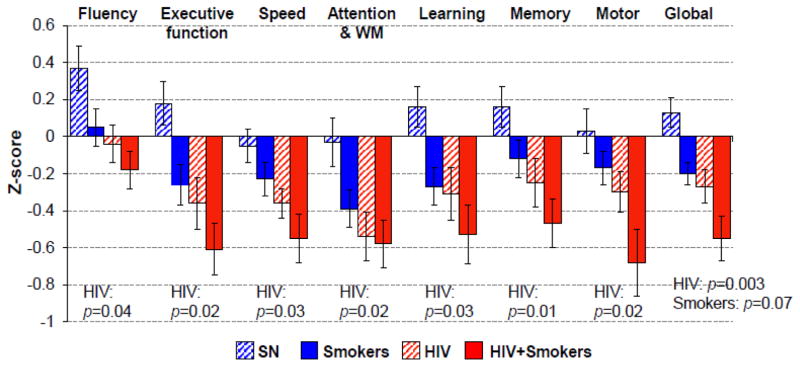
Neurocognitive Performance and Cognitive Domain Z-Scores (Table 2, Figure 4)
Figure 4. Performance on Cognitive Domain (Z-scores).
Performance (Z-scores) in the seven cognitive domains assessed across all four groups. HIV+ participants (regardless of Smoker status) performed worse on all seven domains, which led to worse performance on the Global score. SN-Smokers also tended to perform worse than SN non-smokers which led to a trend for the lower Global score. Hence HIV+ and Smoker status showed an additive effect, leading to lowest scores in all domain scores and the Global scores in HIV+Smokers.
Of the seven cognitive domains assessed, regardless of tobacco-Smoking status, HIV+ participants had significantly lower scores on all domains, including fluency (F(1, 98)=4.2, p=0.04), executive function (F(1, 99) =5.5, p=0.02), speed (F(1, 99)=5.1, p=0.03), attention/WM (F(1, 99)=5.4, p=0.02), learning (F(1, 94)=4.3, p=0.03), memory (F(1, 95)=6.9, p=0.01), motor (F(1, 96)=5.3, p=0.02), as well as global (F(1, 103)=9.6, p=0.003). Although Smokers showed only non-significantly lower scores than non-Smokers, HIV+Smokers again showed additive effects of HIV+ and Smoker status and had the lowest global z-scores, which was significantly lower compared to SN controls (p=0.007). Co-varying for group difference in marijuana use duration did not change the significance of the results. The four participant groups also performed similarly on the HIV dementia scale, although the HIV+Smoker group showed poorer performance compared to SN controls (posthoc t-test: HIV+Smoker vs. SN: p=0.02). Regardless of HIV serostatus, the Smokers had a non-significantly greater proportion of subjects with deficits that were consistent with HAND or HAND-equivalent.
Correlations between Cognitive Domain Z-scores, Decision-making or Psychopathological Symptoms
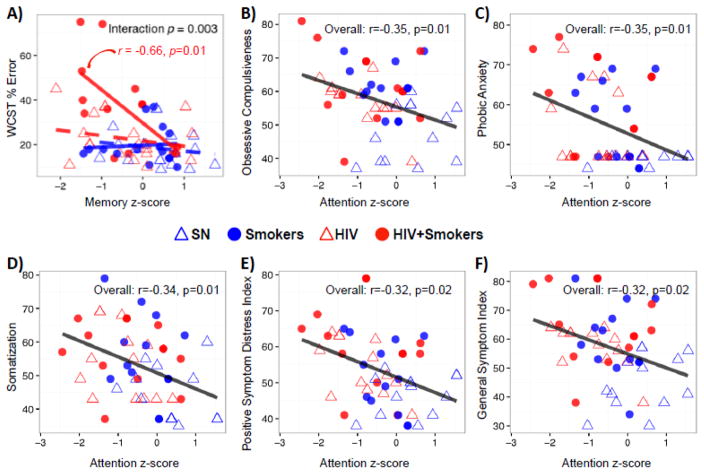
Only HIV+Smokers with lower memory z-scores had higher error rates on the WCST (r=−0.66, p=0.01, Figure 5A), but no significant correlations were seen for the other participant groups. In addition, no correlations were found between cognitive domain z-scores or tasks and tobacco-usage (cumulative lifetime number of cigarettes smoked or the average number of cigarettes smoked per day). Cognitive domain z-scores also did not correlate with the scores on BIS or the Iowa Gambling tasks that showed significant HIV or tobacco-Smoker effects. However, regardless of HIV or tobacco use status, individuals with lower attention z-scores had greater psychopathological symptoms (Figures 5B–F).
Figure 5. Correlations Between Cognitive Function and Psychopathological Symptoms.
A) Correlations between memory z-score and WCST % error: only HIV+ smokers with poorer memory had more errors on the WCST (r=−0.66, p=0.01); the other three groups showed no significant correlations (r= 0.09 to −0.27; p=0.24 to 0.74). B–F): Lower attention z-scores were associated with greater psychopathological symptoms, including obsessive compulsiveness, phobic anxiety, somatization, positive symptom distress index as well as general symptom index.
Discussion
This cross-sectional study used a 2×2 design to explore the independent and combined effects of HIV-infection and chronic current tobacco-use, in participants with mild or no other substance use disorders, on several dimensions related to psychopathological symptoms, cognition and behavior. We found that regardless of tobacco use, HIV+ participants had more psychopathological symptoms, greater cognitive impulsivity and poorer cognitive performance. However, regardless of HIV status, tobacco smokers also had more psychopathological symptoms, greater behavioral impulsivity but non-significantly worse cognitive performance. Therefore, HIV+Smokers consistently demonstrated additive adverse effects of HIV-infection and tobacco smoking, with the worst performance or scores on these psychological, behavioral and cognitive measures. Lastly, those with the lower memory scores made more errors on decision making task while those with poorest attention also had more psychopathological symptoms.
Psychopathological symptoms
The more prevalent psychopathological symptoms in our HIV+ participants are consistent with prior studies (Owe-Larsson et al., 2009; Jin et al., 2010; Rezaei et al., 2013), with anxiety disorders and depression being the most common psychiatric conditions (Elliott, 1998; Morrison et al., 2014). The Smokers in our study also had more psychological distress than non-Smokers overall, which is consistent with tobacco smokers having the tendency for more neurotic traits (Gilbert and Gilbert, 1995), as well as more anxiety and depression (Breslau, 1995) than non-smokers. Whether smoking leads to or worsens the depression via neurochemical alterations, or is used as self-medication by smokers for their depressive and psychological symptoms (Kassel et al., 2003) remains unclear. Most tobacco smokers claim that smoking alleviates their anxiety and leads to calming effects, demonstrating beneficial effects of nicotine (Heishman et al., 2010); however, negative correlations between smoking and affect were observed (Dierker et al., 2014), as well as subtle attention/impulsivity deficits (Wagner et al., 2013). Therefore, the highest summary scores on all psychopathological symptoms in our HIV+Smokers amongst the four groups suggest additive negative effects from both being HIV-seropositive and chronic tobacco smoking. Lastly, regardless of HIV or tobacco use status, the correlations between greater psychopathological symptoms and lower attention scores suggest that these symptoms may be distracting to these individuals and may ultimately impact their daily lives and cognitive function.
Cognitive Flexibility, Impulsivity and Decision-Making
Both HIV+ participants and the Smokers in our study made more errors on WCST, indicating poorer learning, executive function and cognitive flexibility, which again led to an additive effect on poorer performance in HIV+Smokers. The poorer performance on learning and executive function is consistent with findings in HIV+ patients maintained on cART (Heaton et al., 2011b). However, given the high prevalence of tobacco use amongst HIV+ patients,(Tesoriero et al., 2010) those in the earlier studies might have been tobacco smokers also. Earlier studies of HIV+ participants found poorer performance on WCST only in those with AIDS compared to the SN controls (Villa et al., 1993), or in those with smaller striatal structures, especially caudate and putamen (Correa et al., 2016), although information regarding the smoking status of these participants were not reported. Since the caudate and putamen have the highest densities of dopaminergic synapses and reciprocal projections to the orbitofrontal brain regions, deficits in dopaminergic system would lead to impairments in the frontostriatal or prefrontal networks, which may lead to poorer inhibitory control, attention/working memory and decision-making (Braver and Cohen, 2000).
Furthermore, since nicotinic receptors modulate dopamine release in the striatum, blockade of nicotinic receptors with antagonists, deletion of endogenous acetylcholine, or disruption of nicotinic receptor genes, all may lead to markedly decreased dopamine release (Zhou et al., 2001). Therefore, in chronic tobacco smokers, dopaminergic function or release may be down-regulated by nicotinic blockade. A recent 6-[18F]fluoro-L-dihydroxyphenylalanine (18F-DOPA)-PET study showed that nicotine-dependent smokers had lower dopamine synthesis capacity that appears to normalize with abstinence (Rademacher et al., 2015). Since HIV+ participants also showed decreased dopamine transporters (Wang et al., 2004b), and lower D2 receptors in those who were also tobacco smokers (Chang et al., 2008a), HIV+Smokers would likely have even lower dopaminergic function. The lower DAT would lead to poorer performance on WCST, with greater preservative and total errors, as shown in healthy individuals (Yen et al., 2015). Our HIV+Smokers indeed had the highest percent errors on WCST.
On the IGT, HIV+ participants and SN controls behaved differently depending on their tobacco use status. While SN Smokers made better choices and loss less money, HIV+Smokers made more disadvantageous choices compared to the other three groups, and are consistent with the more disadvantageous choices made on the IGT by HIV+ participants, including those with substance-dependence (Martin et al., 2004), compared to those without HIV-infection (Martin et al., 2004; Hardy et al., 2006).
Compared to SN controls, HIV+ participants showed non-significantly higher cognitive impulsivity scores on BIS, which might be related to their impaired attention (Sorenson et al., 1994; Chang et al., 2001). However, greater cognitive impulsivity on IGT were not related to the working memory deficits, which require significant attention, in HIV+ participants (Martin et al., 2004; Hardy et al., 2006);. The greater risk-taking behaviors in HIV+ individuals (Owe-Larsson et al., 2009) might have contributed to a premorbid predisposition for smoking, consistent with findings linking impulsivity to nicotine sensitivity (Perkins et al., 2008). Our chronic Smokers were also more impulsive, but their impulsivity symptoms involved greater behavioral impulsivity, which is consistent with prior studies using personality and behavioral measures (Mitchell, 1999). The HIV+Smokers in our study had the greatest cognitive and behavioral impulsivity scores, which might have resulted from the additive effects of HIV-associated brain injury and chronic tobacco smoking on the underlying neural circuitry involved in decision-making processes (Janes et al., 2012).
Cognitive Performance
HIV+ participants in the current study had greater deficits in all domains, including memory and executive function, which are more commonly found in cART-treated patients (Heaton et al., 2011b), but they also had poorer performance on speed, and motor function, similar to the deficits reported in HIV+ patients prior to cART (Heaton et al., 2011b). Poorer cognitive performance in HIV+ participants may result from multiple HIV-mediated mechanisms that lead to their persistent neuroinflammation (Chang et al., 2003; Chang et al., 2008c; Chang et al., 2014) and declined neuronal function (Chang et al., 2004; Ernst et al., 2009). Specifically, persistent neuroinflammation may result from ongoing neuroimmune response to reservoirs of CNS infection within perivascular macrophages, microglia and astrocytes (Dahl et al., 2014; Brown, 2015). In addition, declined neuronal function may be related to decreased brain glutamate (Ernst et al., 2010) that resulted from increased release (Musante et al., 2010) and decreased reuptake of glutamate by infected or activated astrocytes (Vesce et al., 1997; Zou and Crews, 2005), disruption of cellular energetic with accumulation of glycolytic intermediates (Dickens et al., 2015), proteins and lipids (Bandaru et al., 2013), or dysfunction of dopaminergic terminals, as shown by the lower levels of [C-11-cocaine] reuptake, indicating decreased presynaptic dopamine transporters (Wang et al., 2004a; Chang et al., 2008b).
Similar to prior studies that found poorer working memory in chronic tobacco smokers (Ernst et al., 2001), both Smoker groups in the current study also performed more poorly on executive function and memory domains (including working memory and learning tasks) than SN controls. The additive effects of HIV and chronic smoking led to the poorest cognitive performance in our HIV+Smoker group, across the four groups, which is also consistent with an earlier study that found worse memory and global functioning scores in HIV+Smokers compared to HIV+ non-Smokers (Bryant et al., 2013). However, the HIV+Smokers in this earlier study also had a higher prevalence of hepatitis C virus co-infection, which might have contributed to the poorer cognitive performance (Fialho et al., 2016; Ibrahim et al., 2016), but we excluded such participants in the current study. Since nicotine is neuroprotective (Vieira-Brock et al., 2015), the additive adverse effects of tobacco smoking on cognitive performance in HIV+ participants may be due to chronic tobacco use’s negative influence on cardiovascular risk factors, which in turn may contribute to poorer cognition in HIV+ patients (Becker et al., 2009; Fabbiani et al., 2013), Since smoking cessation may decrease the cardiovascular risks in HIV+ patients (Petoumenos et al., 2011), future studies should evaluate whether smoking cessation may also lead to improved cognition in HIV+Smokers.
Limitation and Conclusion
A major limitation of this study is the cross-sectional design, which could not establish a causal relationship between HIV infection and/or chronic tobacco smoking on these psychological symptoms, cognitive and behavioral measures. However, we carefully matched the subject characteristics across the four participants groups, including similar marijuana and alcohol usage variables, and excluded or controlled for many potential confounding variables, in order to minimize the variability beyond the main factors assessed (HIV-serostatus and Tobacco-Smoking) across the subject groups. Nevertheless, longitudinal studies are needed to evaluate whether smoking cessation may lead to decreased impulsivity and improve psychological symptoms and cognitive function in HIV patients or those with HAND.
HIV-related behavior and cognitive impairments can have adverse effects on daily function, antiretroviral adherence, and quality of life (Heaton et al., 2004; Scott et al., 2011; Thames et al., 2011), while HIV-infection and substance use may have additive adverse effects on neurocognitive functions involving the prefrontal-subcortical systems (Rippeth et al., 2004). The additive negative effects of HIV and tobacco smoking on the various cognitive domains indicate that assessments of the risk factor for HAND should include tobacco smoking. Tobacco cessation programs also should be recommended to HIV+Smokers to minimize the deleterious effects of smoking on HAND. Although acute administration of nicotine can improve affect as well as attention and memory in healthy controls (Rezvani and Levin, 2001; Perkins et al., 2008), such beneficial effect may not be present in those with HIV-associated brain injury. Future studies with administration of nicotine or other dopamine agonist to HIV+ participants with and without tobacco smoking may provide additional insights regarding how the nicotinic or dopaminergic system might be impacted in these individuals.
Acknowledgments
This work was supported by the National Institutes of Health grants (2K24-DA16170; U54-NS56883; G12 MD007601). We are grateful to our research participants and the referral physicians from our community providers, including Dr. Drew Kovach, Dr. Dominic Chow, Dr. Jennifer Frank, Dr. Cyril Goshima, and the personnel at the Life Foundation, the Gregory House and at Save the Food Basket. We also appreciate the meticulous and hard work from the multiple clinical and technical research staff members (especially Mark Lum, B.S.) who assisted in the data collection of this study.
Footnotes
Conflict of Interest Statement: The authors have declared that no conflict of interest exists.
References
- Antinori A, Arendt G, Becker J, Brew B, Byrd D, Cherner M, Clifford D, Cinque P, Epstein L, Goodkin K. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007a;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007b;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandaru VV, Mielke MM, Sacktor N, McArthur JC, Grant I, Letendre S, Chang L, Wojna V, Pardo C, Calabresi P, Munsaka S, Haughey NJ. A lipid storage-like disorder contributes to cognitive decline in HIV-infected subjects. Neurology. 2013;81:1492–1499. doi: 10.1212/WNL.0b013e3182a9565e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Martin E. Impaired decision making related to working memory deficits in individuals with substance addictions. Neuropsychology. 2004;18:152–162. doi: 10.1037/0894-4105.18.1.152. [DOI] [PubMed] [Google Scholar]
- Becker JT, Kingsley L, Mullen J, Cohen B, Martin E, Miller EN, Ragin A, Sacktor N, Selnes OA, Visscher BR. Vascular risk factors, HIV serostatus, and cognitive dysfunction in gay and bisexual men. Neurology. 2009;73:1292–1299. doi: 10.1212/WNL.0b013e3181bd10e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Cohen JD. On the control of control: The role of dopamine in regulating prefrontal function and working memory. Control of cognitive processes: Attention and performance. 2000;XVIII:713–737. [Google Scholar]
- Breslau N. Psychiatric comorbidity of smoking and nicotine dependence. Behavior genetics. 1995;25:95–101. doi: 10.1007/BF02196920. [DOI] [PubMed] [Google Scholar]
- Brown A. Understanding the MIND phenotype: macrophage/microglia inflammation in neurocognitive disorders related to human immunodeficiency virus infection. Clinical and translational medicine. 2015;4:7. doi: 10.1186/s40169-015-0049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant VE, Kahler CW, Devlin KN, Monti PM, Cohen RA. The effects of cigarette smoking on learning and memory performance among people living with HIV/AIDS. AIDS care. 2013;25:1308–1316. doi: 10.1080/09540121.2013.764965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody TP. Affect Regulation, Tobacco Addiction, and Smoking Cessation. Journal of psychoactive drugs. 1989;21:331–342. doi: 10.1080/02791072.1989.10472175. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. HIV and Substance Use in the United States. 2015 http://www.cdc.gov/hiv/riskbehaviors/substanceuese.html.
- Chang L, Tomasi D, Yakupov R, Lozar C, Arnold S, Caparelli E, Ernst T. Adaptation of the attention network in human immunodeficiency virus brain injury. Annals of neurology. 2004;56:259–272. doi: 10.1002/ana.20190. [DOI] [PubMed] [Google Scholar]
- Chang L, Wang GJ, Volkow ND, Ernst T, Telang F, Logan J, Fowler JS. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. Neuroimage. 2008a;42:869–878. doi: 10.1016/j.neuroimage.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Wang G-J, Volkow ND, Ernst T, Telang F, Logan J, Fowler JS. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. Neuroimage. 2008b;42:869–878. doi: 10.1016/j.neuroimage.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Jiang C, Cunningham E, Buchthal S, Douet V, Andres M, Ernst T. Effects of APOE ε4, age, and HIV on glial metabolites and cognitive deficits. Neurology. 2014;82:2213–2222. doi: 10.1212/WNL.0000000000000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Speck O, Miller EN, Braun J, Jovicich J, Koch C, Itti L, Ernst T. Neural correlates of attention and working memory deficits in HIV patients. Neurology. 2001;57:1001–1007. doi: 10.1212/wnl.57.6.1001. [DOI] [PubMed] [Google Scholar]
- Chang L, Wong V, Nakama H, Watters M, Ramones D, Miller EN, Cloak C, Ernst T. Greater than age-related changes in brain diffusion of HIV patients after 1 year. J Neuroimmune Pharmacol. 2008c;3:265–274. doi: 10.1007/s11481-008-9120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Ernst T, Witt MD, Ames N, Walot I, Jovicich J, DeSilva M, Trivedi N, Speck O, Miller EN. Persistent brain abnormalities in antiretroviral-naive HIV patients 3 months after HAART. Antivir Ther. 2003;8:17–26. [PubMed] [Google Scholar]
- Correa DG, Zimmermann N, Netto TM, Tukamoto G, Ventura N, de Castro Bellini Leite S, Cabral RF, Fonseca RP, Bahia PR, Gasparetto EL. Regional Cerebral Gray Matter Volume in HIV-Positive Patients with Executive Function Deficits. Journal of neuroimaging: official journal of the American Society of Neuroimaging. 2016 doi: 10.1111/jon.12327. [DOI] [PubMed] [Google Scholar]
- Dahl V, Peterson J, Fuchs D, Gisslen M, Palmer S, Price RW. Low levels of HIV-1 RNA detected in the cerebrospinal fluid after up to 10 years of suppressive therapy are associated with local immune activation. AIDS. 2014;28:2251–2258. doi: 10.1097/QAD.0000000000000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, De Biasi M. Cellular mechanisms of nicotine addiction. Pharmacology Biochemistry and Behavior. 2001;70:439–446. doi: 10.1016/s0091-3057(01)00652-9. [DOI] [PubMed] [Google Scholar]
- Dickens AM, Anthony DC, Deutsch R, Mielke MM, Claridge TD, Grant I, Franklin D, Rosario D, Marcotte T, Letendre S, McArthur JC, Haughey NJ. Cerebrospinal fluid metabolomics implicate bioenergetic adaptation as a neural mechanism regulating shifts in cognitive states of HIV-infected patients. AIDS. 2015;29:559–569. doi: 10.1097/QAD.0000000000000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierker LC, Avenevoli S, Stolar M, Merikangas K. Smoking and depression: an examination of mechanisms of comorbidity. American Journal of Psychiatry. 2014 doi: 10.1176/appi.ajp.159.6.947. [DOI] [PubMed]
- Elliott A. Anxiety and HIV infection. STEP perspective. 1998;98:11. [PubMed] [Google Scholar]
- Ernst M, Heishman SJ, Spurgeon L, London ED. Smoking history and nicotine effects on cognitive performance. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2001;25:313–319. doi: 10.1016/S0893-133X(01)00257-3. [DOI] [PubMed] [Google Scholar]
- Ernst T, Jiang CS, Nakama H, Buchthal S, Chang L. Lower brain glutamate is associated with cognitive deficits in HIV patients: a new mechanism for HIV-associated neurocognitive disorder. Journal of magnetic resonance imaging: JMRI. 2010;32:1045–1053. doi: 10.1002/jmri.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Yakupov R, Nakama H, Crocket G, Cole M, Watters M, Ricardo-Dukelow ML, Chang L. Declined neural efficiency in cognitively stable human immunodeficiency virus patients. Annals of neurology. 2009;65:316–325. doi: 10.1002/ana.21594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbiani M, Ciccarelli N, Tana M, Farina S, Baldonero E, Di Cristo V, Colafigli M, Tamburrini E, Cauda R, Silveri MC, Grima P, Di Giambenedetto S. Cardiovascular risk factors and carotid intima-media thickness are associated with lower cognitive performance in HIV-infected patients. HIV medicine. 2013;14:136–144. doi: 10.1111/j.1468-1293.2012.01044.x. [DOI] [PubMed] [Google Scholar]
- Farinpour R, Martin EM, Seidenberg M, Pitrak DL, Pursell KJ, Mullane KM, Novak RM, Harrow M. Verbal working memory in HIV-seropositive drug users. Journal of the International Neuropsychological Society. 2000;6:548–555. doi: 10.1017/s1355617700655042. [DOI] [PubMed] [Google Scholar]
- Fialho R, Pereira M, Bucur M, Fisher M, Whale R, Rusted J. Cognitive impairment in HIV and HCV co-infected patients: a systematic review and meta-analysis. AIDS care. 2016:1–14. doi: 10.1080/09540121.2015.1108385. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Gilbert BO. Personality, psychopathology, and nicotine response as mediators of the genetics of smoking. Behavior genetics. 1995;25:133–147. doi: 10.1007/BF02196923. [DOI] [PubMed] [Google Scholar]
- Hardy DJ, Hinkin CH, Levine AJ, Castellon SA, Lam MN. Risky decision making assessed with the gambling task in adults with HIV. Neuropsychology. 2006;20:355. doi: 10.1037/0894-4105.20.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, McCutchan JA, Reicks C, Grant I. The impact of HIV-associated neuropsychological impairment on everyday functioning. Journal of the International Neuropsychological Society. 2004;10:317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte T, Atkinson J. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, LeBlanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. Journal of neurovirology. 2011a;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011b;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology. 2010;210:453–469. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim I, Salah H, El Sayed H, Mansour H, Eissa A, Wood J, Fathi W, Tobar S, Gur RC, Gur RE, Dickerson F, Yolken RH, El Bahaey W, Nimgaonkar V. Hepatitis C virus antibody titers associated with cognitive dysfunction in an asymptomatic community-based sample. J Clin Exp Neuropsychol. 2016;38:861–868. doi: 10.1080/13803395.2016.1168780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Nickerson LD, Kaufman MJ. Prefrontal and limbic resting state brain network functional connectivity differs between nicotine-dependent smokers and non-smoking controls. Drug and alcohol dependence. 2012;125:252–259. doi: 10.1016/j.drugalcdep.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Zhao G, Zhang F, Feng L, Wu N. The psychological status of HIV-positive people and their psychosocial experiences in eastern China. HIV medicine. 2010;11:253–259. doi: 10.1111/j.1468-1293.2009.00770.x. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychological bulletin. 2003;129:270. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Levy J. HIV and the pathogenesis of AIDS. Washington DC: ASM Press; 2007. [Google Scholar]
- Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging. 1997;12:277–287. doi: 10.1037//0882-7974.12.2.277. [DOI] [PubMed] [Google Scholar]
- Martin EM, Sullivan TS, Reed RA, Fletcher TA, Pitrak DL, Weddington W, Harrow M. Auditory working memory in HIV-1 infection. Journal of the International Neuropsychological Society. 2001;7:20–26. doi: 10.1017/s1355617701711022. [DOI] [PubMed] [Google Scholar]
- Martin EM, Pitrak DL, Rains N, Grbesic S, Pursell K, Nunnally G, Bechara A. Delayed nonmatch-to-sample performance in HIV-seropositive and HIV-seronegative polydrug abusers. Neuropsychology. 2003;17:283. doi: 10.1037/0894-4105.17.2.283. [DOI] [PubMed] [Google Scholar]
- Martin EM, Pitrak DL, Weddington W, Rains NA, Nunnally G, Nixon H, Grbesic S, Vassileva J, Bechara A. Cognitive impulsivity and HIV serostatus in substance dependent males. Journal of the International Neuropsychological Society. 2004;10:931–938. doi: 10.1017/s1355617704107054. [DOI] [PubMed] [Google Scholar]
- Mascolini M. Smoking Rate Twice Higher With Than Without HIV in First National US Study. 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, GA. 2013. [Google Scholar]
- Meyer VJ, Rubin LH, Martin E, Weber KM, Cohen MH, Golub ET, Valcour V, Young MA, Crystal H, Anastos K. HIV and recent illicit drug use interact to affect verbal memory in women. Journal of acquired immune deficiency syndromes. 2013;63:67. doi: 10.1097/QAI.0b013e318289565c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology. 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Morrison MF, Petitto JM, Ten Have T, Gettes DR, Chiappini MS, Weber AL, Brinker-Spence P, Bauer RM, Douglas SD, Evans DL. Depressive and anxiety disorders in women with HIV infection. American Journal of Psychiatry. 2014 doi: 10.1176/appi.ajp.159.5.789. [DOI] [PubMed] [Google Scholar]
- Musante V, Summa M, Neri E, Puliti A, Godowicz TT, Severi P, Battaglia G, Raiteri M, Pittaluga A. The HIV-1 viral protein Tat increases glutamate and decreases GABA exocytosis from human and mouse neocortical nerve endings. Cerebral cortex. 2010;20:1974–1984. doi: 10.1093/cercor/bhp274. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics. Health, United States, 2014: With Special Feature on Adults Aged 55–64. Hyattsville, Maryland: 2015. [PubMed] [Google Scholar]
- Owe-Larsson M, Säll L, Salamon E, Allgulander C. HIV infection and psychiatric illness. African journal of psychiatry. 2009:12. doi: 10.4314/ajpsy.v12i2.43729. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. Journal of clinical psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Pearson Education I Symptom Checklist-90-Revised (SCL-90-R®) In.
- Perkins KA, Lerman C, Coddington SB, Jetton C, Karelitz JL, Scott JA, Wilson AS. Initial nicotine sensitivity in humans as a function of impulsivity. Psychopharmacology. 2008;200:529–544. doi: 10.1007/s00213-008-1231-7. [DOI] [PubMed] [Google Scholar]
- Petoumenos K, Worm S, Reiss P, de Wit S, d’Arminio Monforte A, Sabin C, Friis-Moller N, Weber R, Mercie P, Pradier C, El-Sadr W, Kirk O, Lundgren J, Law M. Rates of cardiovascular disease following smoking cessation in patients with HIV infection: results from the D:A:D study(*) HIV medicine. 2011;12:412–421. doi: 10.1111/j.1468-1293.2010.00901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS. Neuroregulators and the reinforcement of smoking: towards a biobehavioral explanation. Neuroscience & Biobehavioral Reviews. 1985;8:503–513. doi: 10.1016/0149-7634(84)90007-1. [DOI] [PubMed] [Google Scholar]
- Rademacher L, Prinz S, Winz O, Henkel K, Dietrich CA, Schmaljohann J, Mohammadkhani Shali S, Schabram I, Stoppe C, Cumming P, Hilgers RD, Kumakura Y, Coburn M, Mottaghy FM, Grunder G, Vernaleken I. Effects of Smoking Cessation on Presynaptic Dopamine Function of Addicted Male Smokers. Biological psychiatry. 2015 doi: 10.1016/j.biopsych.2015.11.009. [DOI] [PubMed] [Google Scholar]
- Radloff LS. Center for Epidemiologic Studies Depression Scale. 1977. [DOI] [PubMed] [Google Scholar]
- Reise SP, Moore TM, Sabb FW, Brown AK, London ED. The Barratt Impulsiveness Scale–11: Reassessment of its structure in a community sample. Psychological assessment. 2013;25:631. doi: 10.1037/a0032161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaei S, Taramian S, Kafie SM. Psychopathological Dimensions in Substance Abusers with and without HIV/AIDS and Healthy Matched Group. Addiction & health. 2013;5:115. [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Cognitive effects of nicotine. Biological psychiatry. 2001;49:258–267. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, Wolfson T, Grant I. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. Journal of the International Neuropsychological Society. 2004;10:1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, McArthur JC, Collier AC, Evans SR, Ellis RJ. The prevalence and incidence of neurocognitive impairment in the HAART era. Aids. 2007;21:1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Vigil O, Heaton RK, Schweinsburg BC, Ellis RJ, Grant I, Marcotte TD. A neuropsychological investigation of multitasking in HIV infection: Implications for everyday functioning. Neuropsychology. 2011;25:511. doi: 10.1037/a0022491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple SJ, Zians J, Grant I, Patterson TL. Methamphetamine use, impulsivity, and sexual risk behavior among HIV-positive men who have sex with men. Journal of Addictive Diseases. 2006;25:105–114. doi: 10.1300/J069v25n04_10. [DOI] [PubMed] [Google Scholar]
- Simioni S, Cavassini M, Annoni J-M, Abraham AR, Bourquin I, Schiffer V, Calmy A, Chave J-P, Giacobini E, Hirschel B. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. Aids. 2010;24:1243–1250. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- Sorenson DJ, Martin EM, Robertson LC. Visual attention in HIV-1 infection. Neuropsychology. 1994;8:424. [Google Scholar]
- Tesoriero JM, Gieryic SM, Carrascal A, Lavigne HE. Smoking among HIV positive New Yorkers: prevalence, frequency, and opportunities for cessation. AIDS Behav. 2010;14:824–835. doi: 10.1007/s10461-008-9449-2. [DOI] [PubMed] [Google Scholar]
- Thames AD, Kim MS, Becker BW, Foley JM, Hines LJ, Singer EJ, Heaton RK, Castellon SA, Hinkin CH. Medication and finance management among HIV-infected adults: The impact of age and cognition. Journal of Clinical and Experimental Neuropsychology. 2011;33:200–209. doi: 10.1080/13803395.2010.499357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesce S, Bezzi P, Rossi D, Meldolesi J, Volterra A. HIV-1 gp120 glycoprotein affects the astrocyte control of extracellular glutamate by both inhibiting the uptake and stimulating the release of the amino acid. FEBS letters. 1997;411:107–109. doi: 10.1016/s0014-5793(97)00674-1. [DOI] [PubMed] [Google Scholar]
- Vieira-Brock PL, McFadden LM, Nielsen SM, Ellis JD, Walters ET, Stout KA, McIntosh JM, Wilkins DG, Hanson GR, Fleckenstein AE. Chronic Nicotine Exposure Attenuates Methamphetamine-Induced Dopaminergic Deficits. J Pharmacol Exp Ther. 2015;355:463–472. doi: 10.1124/jpet.114.221945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa G, Monteleone D, Marra C, Bartoli A, Antinori A, Pallavicini F, Tamburrini E, Izzi I. Neuropsychological abnormalities in AIDS and asymptomatic HIV seropositive patients. Journal of Neurology, Neurosurgery & Psychiatry. 1993;56:878–884. doi: 10.1136/jnnp.56.8.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M, Schulze-Rauschenbach S, Petrovsky N, Brinkmeyer J, von der Goltz C, Gründer G, Spreckelmeyer KN, Wienker T, Diaz-Lacava A, Mobascher A. Neurocognitive impairments in non-deprived smokers—results from a population-based multi-center study on smoking-related behavior. Addiction biology. 2013;18:752–761. doi: 10.1111/j.1369-1600.2011.00429.x. [DOI] [PubMed] [Google Scholar]
- Wang G-J, Chang L, Volkow ND, Telang F, Logan J, Ernst T, Fowler JS. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain. 2004a;127:2452–2458. doi: 10.1093/brain/awh269. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Chang L, Volkow ND, Telang F, Logan J, Ernst T, Fowler JS. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain. 2004b;127:2452–2458. doi: 10.1093/brain/awh269. [DOI] [PubMed] [Google Scholar]
- Wojna V, Robles L, Skolasky RL, Mayo R, Selnes O, de la Torre T, Maldonado E, Nath A, Meléndez LM, Lasalde-Dominicci J. Associations of cigarette smoking with viral immune and cognitive function in human immunodeficiency virus-seropositive women. Journal of neurovirology. 2007;13:561–568. doi: 10.1080/13550280701620747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen CH, Yeh YW, Liang CS, Ho PS, Kuo SC, Huang CC, Chen CY, Shih MC, Ma KH, Peng GS, Lu RB, Huang SY. Reduced Dopamine Transporter Availability and Neurocognitive Deficits in Male Patients with Alcohol Dependence. PLoS One. 2015;10:e0131017. doi: 10.1371/journal.pone.0131017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nature neuroscience. 2001;4:1224–1229. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]
- Zou JY, Crews FT. TNF alpha potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: neuroprotection by NF kappa B inhibition. Brain Res. 2005;1034:11–24. doi: 10.1016/j.brainres.2004.11.014. [DOI] [PubMed] [Google Scholar]