Abstract
Objective
To directly compare effort of breathing between high flow nasal cannula (HFNC), nasal intermittent mechanical ventilation (NIMV), and nasal continuous positive airway pressure (NCPAP).
Study design
This was a single center prospective cross-over study for patients <6 months in the cardiothoracic or pediatric intensive care unit receiving nasal noninvasive respiratory support after extubation. We measured effort of breathing using esophageal manometry with pressure-rate product (PRP) on all 3 modes. NIMV synchrony was determined by comparing patient efforts (esophageal manometry) with mechanically delivered breaths (spirometry in ventilator circuit). On NIMV, PRP and synchrony was also measured after adding a nasal clip on 26 patients.
Results
Forty-two children were included. Median (IQR) age was 2 (0.5, 4) months. There was no difference in median PRP between HFNC 6 liters per minute, 355 (270,550), NIMV 12/5 cm H2O, 341 (235, 472), and NCPAP 5 cm H2O, 340 (245,506) (P = .33). Results were similar regardless of HFNC flow rate or NIMV inspiratory pressure. Median PRP on CPAP of 5 cm H2O prior to extubation 255 (176, 375) was significantly lower than all postextubation values (P < .002). On NIMV, less than 50% of patient efforts resulted in a ventilator breath, which was not improved with a nasal clip (P > .07)). However, as NIMV synchrony improved (>60%), PRP on NIMV was lower than on HFNC.
Conclusions
For infants, effort of breathing is similar on HFNC, NIMV, and NCPAP after extubation, regardless of flow rate or inspiratory pressure. We speculate that bi-level NIMV may be superior if high levels of synchrony can be achieved.
Pediatric practitioners use nasal modes of respiratory support such as humidified high flow nasal cannula (HFNC), nasal intermittent mechanical ventilation (NIMV), and nasal continuous positive airway pressure (NCPAP) to improve gas exchange, and work of breathing.1 Pediatric observational data support lower intubation rates and reduced costs with NCPAP2 and NIMV.3-5 These modes are increasingly used after extubation to prevent extubation failure,6 although pediatric data are sparse.7-9
HFNC is sometimes used interchangeably with NCPAP or NIMV. There are few pediatric data comparing clinical outcomes between these 3 nasal modes of respiratory support. Although there are some data comparing physiologic response of each mode,10,11 existing studies assessing work or effort of breathing are mostly based on subjective clinical scoring systems.12 We sought to determine if there is a significant difference in objective measures of patient effort of breathing between these modes for infants (<6 months of age) when used after extubation. We hypothesized that NIMV would produce the greatest reduction of effort of breathing, dependent on patient synchrony.
Methods
We conducted a prospective cross-over cohort study in the medical-surgical pediatric intensive care unit (ICU) and cardiothoracic ICU at Children's Hospital Los Angeles from July 2013 until October 2014. The Institutional Review Board at Children's Hospital Los Angeles gave full approval for this study. This was ancillary to a previously published (parent) study, which contains further details about study methodology.13 Patients were eligible if they were between 37 weeks corrected gestational age and 6 months, intubated >12 hours, had no contraindication to a nasoesophageal catheter or respiratory inductance plethysmoghy bands, and were placed on a nasal mode of respiratory support (HFNC, NIMV, or NCPAP) by the primary team within 1 hour of extubation. Patients on home continuous positive airway pressure (CPAP) or bi-level positive airway pressure were excluded. Informed consent was obtained from the parent/guardian.
We placed an esophageal balloon catheter prior to extubation (Avea SmartCath 6F or 7F, CareFusion, Houten, The Netherlands), respiratory inductance plethysmography bands (Respiband Plus; Viasys Healthcare, Hoechberg, Germany) around the chest and abdomen, and connected a calibrated pneumotachometer (Viasys Variflex 51000-40094; Viasys Healthcare) to the endotracheal tube. Pressure-rate product (PRP), the product of the respiratory rate and peak to trough change in esophageal pressure was measured using previously described methods.11
After extubation, HFNC was delivered with an O2/air blender and a heated humidifier (MR850, Fisher and Paykel Healthcare Limited, Auckland, New Zealand). NIMV and NCPAP were provided either through the Servo-I ventilator system (Servo-I; Maquet, Solna, Sweden) or through the Avea Ventilator System (CareFusion, Yorba Linda, California) using an ICU ventilator equipped with software for air-leak compensation during noninvasive ventilation. To provide NIMV, the Avea was placed in NIMV mode and the Servo-I was placed in NIV pressure control mode, both of which provide time-triggered breaths that are pressure controlled and time cycled. Because both ventilator modes are time triggered this delivers a mandatory breath at a set rate per minute. In addition to time triggering, only the Servo-I in NIV PC mode allows additional flow triggering at the ventilator. However, the additional flow triggering only occurs if the patient is able to generate a sufficiently high peak inspiratory flow rate and the leak at the nasal interface is minimal. Both systems interfaced with the Ram Cannula (NeoTech Products, Valencia, California). For a subset of patients (see below) a nasal clip (Neoseal; NeoTech Products) was added while on NIMV. No proximal trigger device was used, and ventilator settings were not specifically adjusted to improve synchrony.
Prior to extubation, we recorded 5 minutes of steady state spontaneous breathing on CPAP of 5 cm H2O (CPAP 5), as part of the parent study. After extubation, the choice of using noninvasive respiratory support (NRS) and initial NRS mode was left to the primary team. All patients received HFNC of 4, 6, and 8 liters per minute; NCPAP 5 cm H2O (NCPAP 5); and NIMV with an expiratory positive airway pressure of 5 cm H2O, respiratory rate of 20 and a driving pressure (delta P) of 8, 12, and 16 cm H2O. These settings were chosen based on settings used in previous work, in conjunction with standard ventilator settings used in our ICUs.14,15 The initial mode of nasal noninvasive ventilation was determined by the clinical team.
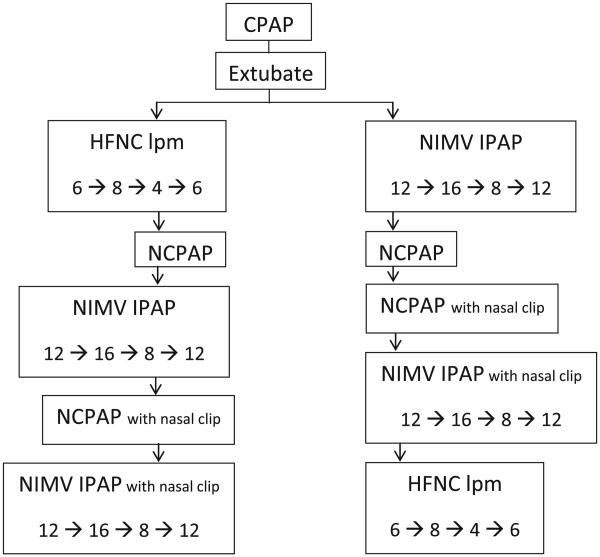
Once the patient stabilized on the initial mode, we began the protocol. The sequence of the flow titrations is displayed in Figure 1 (available at www.jpeds.com). Patients were maintained on each setting for 5-10 minutes prior to study recordings or when switching modes. Measurements were recorded for a minimum of 2 minutes after the patient stabilized on each setting. Patients with upper airway obstruction (UAO) following extubation (as gauged by the UAO tool in the parent study) had recordings postponed until resolution of symptoms, often after administration of racemic epinephrine. We ensured that inspiratory flow limitation was no longer present, and PRP had reached steady state conditions (was no longer changing in response to UAO treatments) before starting measurements. No other respiratory treatments were permitted until the study protocol was complete.
Figure 1.
Sequence of interventions. Patients received the following order of flow titrations. HFNC flow and inspiratory positive airway pressure (IPAP) changes were made after steady state was achieved and at least 2 minutes of recordings were obtained.
After enrolling 14 patients, we observed low levels of NIMV synchrony and amended the study protocol to test whether addition of a nasal clip device (Neoseal) could achieve better synchrony while patients were on NIMV and reduce effort of breathing. No other changes to the protocol were made to improve synchrony other than the addition of the nasal clip.
Synchrony was quantified by comparing ventilator delivered breaths (by connecting the pneumotachometer through the noninvasive ventilator circuit), with patient efforts (negative deflections in esophageal pressure) with measured recordings taken during 1-minute of steady state breathing. Specifically, we required that ventilator delivered airflow (as measured by spirometry) occurred during the inspiratory phase as defined by continued negative deflections of esophageal pressure. Percent synchrony equals the percentage of ventilator breaths synchronous with patient effort.
Statistical Analyses
Our primary objective was to determine if effort of breathing as measured by PRP was different between HFNC (HFNC 6 liters per minute [HFNC 6]) vs NIMV (NIMV with a driving pressure [delta P] of 12 cm H2O [NIMV12]) vs NCPAP 5). HFNC 6 and NIMV 12 were used as primary settings for comparisons, as they are commonly used initial settings. Median PRP measured over 2 minutes on HFNC 6 was compared with median PRP on NIMV 12 and median PRP on NCPAP 5 using Friedman ANOVA.
Secondary objectives were to determine how flow rate of HFNC or inspiratory pressure on NIMV changed PRP, and whether these values approximated pre-extubation values on CPAP 5. To do so, we compared median PRP under all study conditions and CPAP 5 with Friedman ANOVA.
Our final objective was to analyze the effects of NIMV synchrony. We compared the percentage of synchronous breaths, stratified by NIMV setting, before and after introduction of the nasal clip using χ2 tests. We compared PRP, stratified by NIMV setting, before and after introduction of the nasal clip using a Wilcoxon signed rank test. To explore whether NIMV synchrony contributed to a potential improvement in effort of breathing of NIMV over HFNC, we calculated a ratio of PRP on potentially equivalent NIMV and HFNC settings (HFNC 4 was considered equivalent to NIMV 8, HFNC 6 to NIMV 12, and HFNC 8 to NIMV 16). We graphed the ratio of PRP against the percentage of breaths on that NIMV setting which were synchronous to determine if there was a dose response to reductions on effort of breathing and synchrony. Sensitivity analysis regarding synchrony was performed by excluding patients on the Avea ventilator because it does not allow additional flow triggering during NIMV in the mode we used.
Results
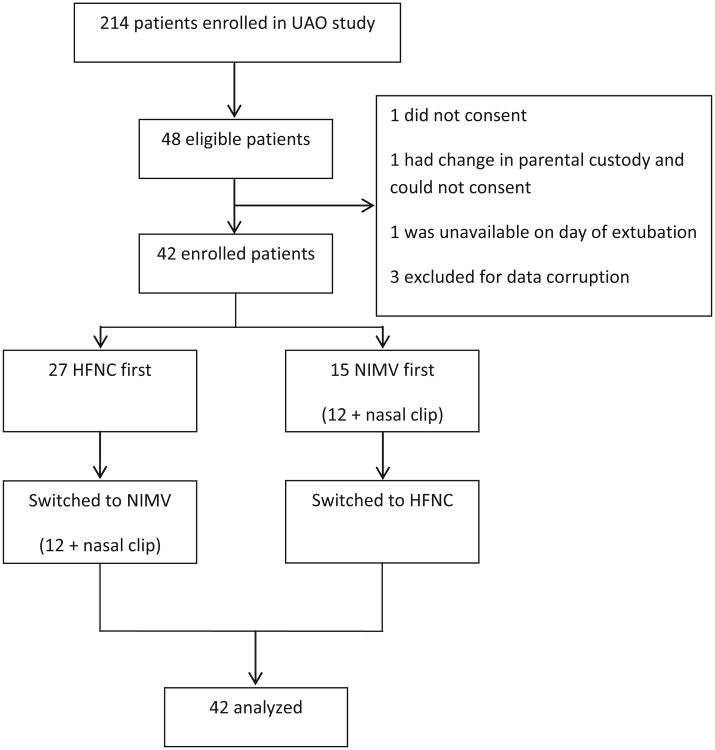
Of 48 eligible infants from the parent study, 45 were enrolled. Three infants had corrupted data, with problems with data recordings on 1 or all of the study conditions, precluding calculation of effort of breathing. This left 42 infants for analysis (Figure 2). The median (range) age was 2 (0.25- 6) months, 52% male, and 71% had congenital heart disease. Median weight was 3.6 kg (2.2-9.6), with median length of invasive ventilation of 7 days (1-40). Clinicians chose HFNC as the initial mode of NRS for 27 (64%) children. Median (range) duration of NRS post extubation was 1 (0.1-29) day (Table). Three patients were on the Avea and 39 were on the Servo-I.
Figure 2.
CONSORT diagrammatic representation of screening, enrollment, and cross-over.
Table. Patient characteristics described as median and ranges or number and percent of total studied population.
| Age (range) | 2 mo (0.25-6) |
| Male (%) | 22 (52%) |
| Ethnicity | |
| Hispanic | 26 (61%) |
| White | 14 (33%) |
| Other | 3 (6%) |
| Weight (range) | 3.6 kg (2.2-9.6) |
| Reason for intubation | |
| Congenital heart disease surgery (%) | 30 (71%) |
| Respiratory failure (%) | 10 (23%) |
| Length of mechanical ventilation (range) | 7 d (2-30) |
| Length of noninvasive ventilation (range) | 1 (0.1-29 d) |
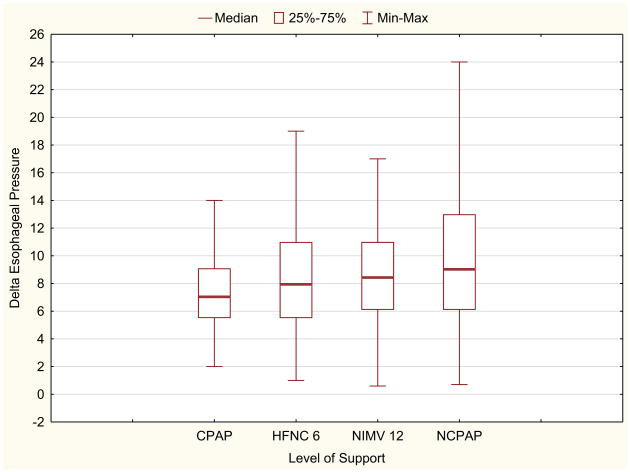
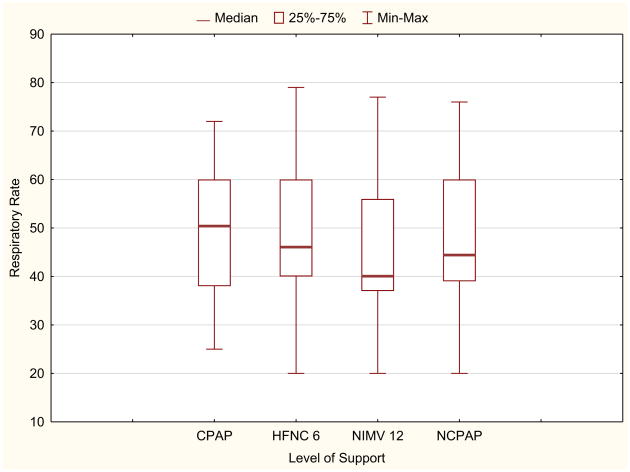
Effort of breathing, as defined by pressure-rate product as a function of mode of ventilation and level of support is presented in Figure 3. For the primary objective, there was no difference in median PRP between HFNC 6 (355; IQR 270, 550), NIMV 12 (341; IQR 235, 472), and NCPAP 5 (340; IQR 245,506) (P = .33). When comparing all 8 study conditions, there was a significant difference in median PRP (Freidman ANOVA P = .002). Post hoc analysis indicated that PRP on CPAP before extubation (255; IQR 176, 375) was lower than all postextubation values (all P < .002). There was no difference in PRP between the different flow rates of HFNC or inspiratory pressures on NIMV (Figure 3). When examining the individual components of PRP (delta esophageal pressure and respiratory rate), median delta esophageal pressure was similar between all modes and settings (P = .11, Figure 4; available at www.jpeds.com). Respiratory rate did not differ between conditions (P = .14), with a general trend that respiratory rate on NIMV was lower than on HFNC (Figure 5; available at www.jpeds.com). When restricting the analysis to the subgroup with the highest effort of breathing (PRP >300), PRP was not different between HFNC of 6 and NIMV of 12, although this analysis was limited by small sample size (N = 9; P = .57).
Figure 3.
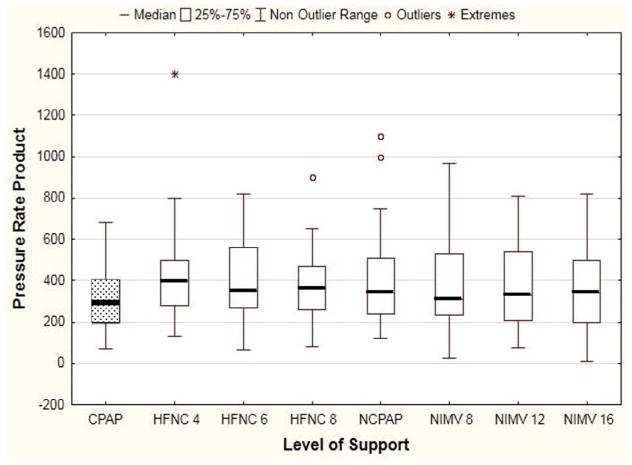
Effort of breathing by support. The primary analysis demonstrated effort of breathing before extubation on CPAP was lower than all other conditions (P = .002). However, there is no difference in effort of breathing between HFNC and NIMV, regardless of flow rate or inspiratory pressure (Kruskal-Wallis ANOVA P = .15). CPAP values highlighted with dotted box.
Figure 4.
There is no statistically significant difference in change in esophageal pressure, regardless of the mode of noninvasive support (P = .11). Max, maximum; Min, minimum.
Figure 5.
Respiratory rate as a function of NRS. Data presented as median (bar), IQR (box), and range (whiskers). There is a trend for difference in median respiratory rate for all conditions (Kruskal-Wallis ANOVA P = .14), although this was not statistically significant, with the lowest respiratory rate seen on NIMV 12.
When looking at all NIMV settings, 50% of patient breath attempts resulted in a ventilator-delivered breath. The addition of a nasal clip did not significantly improve synchrony Figure 6 (available at www.jpeds.com) (n = 26, NIMV 12, P = .07; NIMV 8, P = .35; NIMV 16, P = .40), nor did it significantly change PRP (Figure 7; available at www.jpeds.com) (NIMV 12, P = .49; NIMV 8, P = .9; NIMV 16, P = .71).
Figure 6.
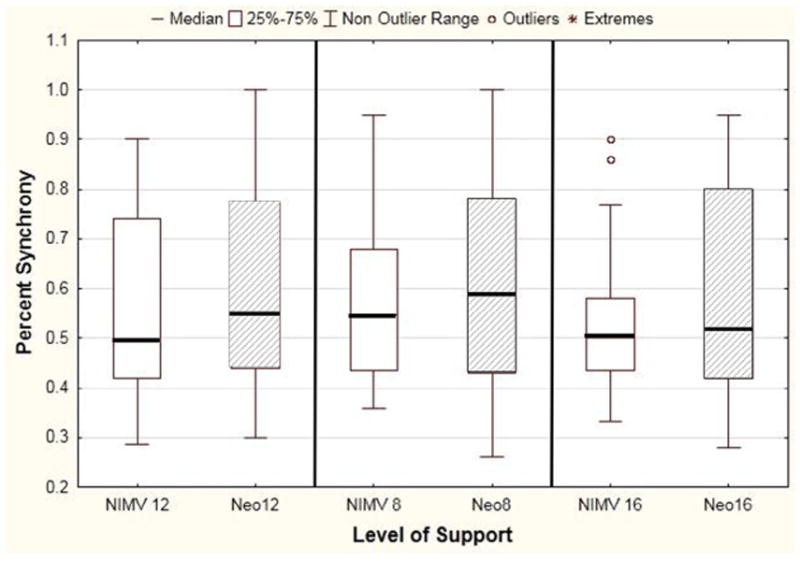
Effect of Neoseal nasal clip on synchrony, stratified by level of NIMV support. Data presented as median (bar), IQR (box), and range (whiskers), with Neoseal indicated by the shaded box. The Neoseal nasal clip altered synchrony from a median of 50%-55% (NIMV 12, P 07; NIMV 8, P = .35; NIMV 16, P = .40).
Figure 7.
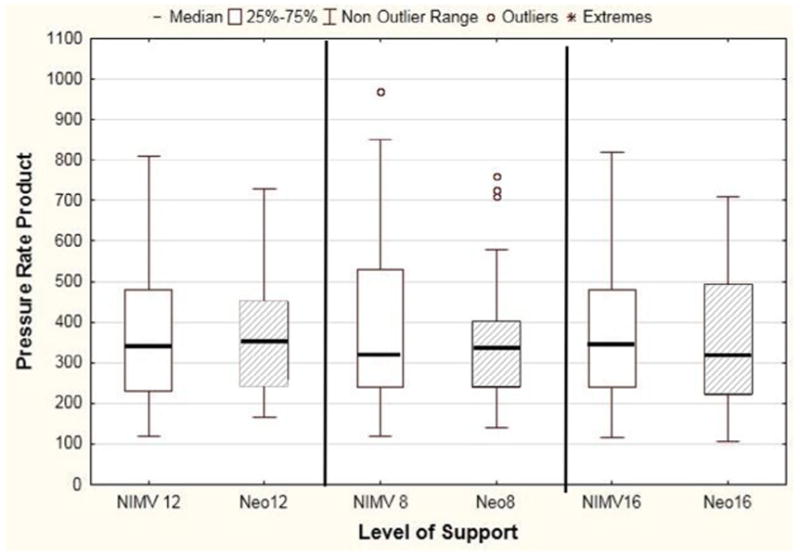
Effect of Neoseal on effort of breathing. There was no difference in median effort of breathing regardless of level of NIMV support with the addition of the nasal clip (NIMV 12, P = .49; NIMV 8, P = .9; NIMV 16, P = .71). Shaded area corresponds with NIMV with Neoseal.
For all patients (regardless of nasal clip), we graphed the ratio of PRP on NIMV/PRP on HFNC vs percent of synchronous breaths on NIMV (Figure 8). NIMV appears to result in lower PRP than HFNC as NIMV synchrony improves (R = −0.28, P = .022). It appears as if NIMV may become superior to HFNC when patient synchrony exceeds 60%. When excluding patients on the Avea ventilator (n = 3), there was no difference in synchrony (analysis not shown).
Figure 8.
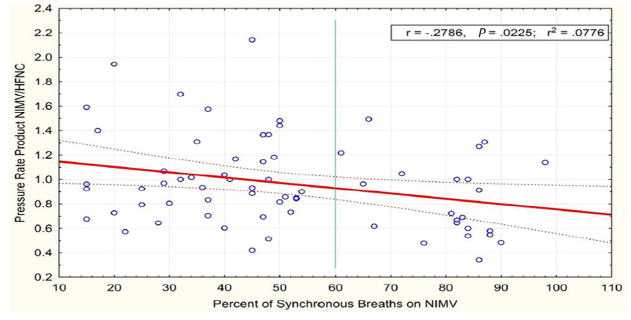
Scatterplot and linear regression line assessing the relationship between the percentage of synchronous breaths on NIMV (x-axis) and the ratio of PRP on NIMV/HFNC. Patients who achieved more synchrony had a larger reduction in effort of breathing on NIMV compared with HFNC (NIMV 12 vs HFNC 6 and NIMV 12 with NeoSeal vs HFNC 6). (R = −0.28, P = .022, r2 = 0.0778). PRP ratios less than 1 demonstrate decreased effort of breathing on NIMV than HFNC. The regression line crosses 1 when the percentage synchrony exceeds 60%, suggesting NIMV results in lower effort of breathing than HFNC when synchrony exceeds 60% (vertical line).
Discussion
We demonstrated through a physiologic cross-over study in infants after extubation that HFNC, NCPAP, and NIMV result in similar patient effort of breathing, regardless of flow rate for HFNC or inspiratory pressure for NIMV. However, if a high level (at least 60%) of NIMV synchrony can be achieved, NIMV may be associated with a lower effort of breathing compared with HFNC. Effort of breathing while intubated on CPAP 5 is significantly lower than effort of breathing on any noninvasive nasal therapy after extubation, confirming previous studies and re-enforcing the point that pressure support is not needed to overcome “the resistance of the endotracheal tube” even for small infants.16,17
We chose to study a relatively objective and direct measure of effort of breathing (PRP) because reducing patient work of breathing through the effective use of mechanical ventilation (both invasive and noninvasive) can prevent respiratory failure. As many have highlighted, patients who develop respiratory failure after extubation (with or without noninvasive ventilation) have high morbidity and mortality.18 Our results are congruent with published neonatal studies. Saslow et al19 measured work of breathing in 18 preterm infants and demonstrated no significant difference between NCPAP compared with the 3 levels of HFNC. Aghai et al20 compared NCPAP with NIMV in 15 preterm neonates and found no significant difference in lung compliance, tidal volume, respiratory rate, minute ventilation, or phase angle.
The neonatal literature suggests synchronized modes of noninvasive ventilation may be superior to nonsynchronized modes in reducing oxygen dependence,21 desaturations,22 and incidence of bronchopulmonary dysplasia.23 Other pediatric investigators found that NCPAP improved effort of breathing more than bi-level positive pressure in a cohort of children with UAO, largely because of asynchrony with the bi-level support.24 Moreover, a recently published pediatric study showed improvements in electrical activity of the diaphragm as a surrogate for effort of breathing when better synchrony is achieved on NRS.25 Similarly, our study highlights the importance of synchrony with NIMV in reducing effort of breathing. We found that NIMV may only offer an advantage in reducing effort of breathing over HFNC when good synchrony can be achieved.
Inadequate synchrony can be a consequence of a multitude of factors and may lead to NRS failure. Adult studies support NRS asynchrony as a risk factor for reintubation, and neonatal studies support an association of asynchrony with inadequate gas exchange and persistent tachypnea. Although there are many types of asynchrony, we primarily concentrated on ineffective efforts.15 Ineffective effort and late cycling are often coupled together and are most likely secondary to the magnitude of leak between the device interface and patient, a major consideration for nasal modes of NRS in infants.26,27
Even though we found no significant difference in effort of breathing between HFNC, NIMV, or NCPAP for infants <6 months of age after extubation, the likely mechanisms of action of these modes differ.28,29 The most commonly accepted hypothesis is that HFNC results in washout of nasopharyngeal dead space.30 Other studies have suggested an alteration in upper airway resistance results in improved lung recruitment.31,32 Most of the NIMV data has been generated in preterm infants, and it is generally considered to work by depositing bi-level positive pressure via nasal prongs, which theoretically improves the patency of the upper airway and may provide some lung recruitment.20 In premature infants with underdeveloped respiratory drive, it can initiate inspiratory reflexes and activate respiratory drive.33 NCPAP is thought to provide the same alteration in upper airway resistance and create deposition of continuous positive pressure into the airways.20 As such, it may be that certain subgroups (based on underlying need for lung recruitment or disease state) will be better served with NIMV vs HFNC or vice versa. That should be an area of future research.
There are several limitations to our study. Our enrollment included a majority of patients who were electively extubated to NRS. Most patients had minimal residual respiratory disease, with relatively low PRP values. Although the results were similar when we restricted analysis to patients with higher baseline PRP values, limited sample size precludes generalizing these findings to children who may have more significant lung disease. There is published literature showing the efficacy of NRS to reduce intubation rates in children with lung disease, such as bronchiolitis or pneumonia.34 In addition, we did not randomize which mode of ventilation was studied first, largely because some patients were not consented until after extubation when the decision was made to initiate noninvasive ventilation. This decision was often made urgently, and we could not delay clinical care for randomization as the clinical team had decided on a starting mode of NRS. Although alternative approaches such as randomization prior to extubation in the event of NRS use could have been considered, this would have involved obtaining study consent on many patients who would not ultimately receive a study intervention, and was not feasible due to limited study resources. We chose not to randomize the order after the patient had already been exposed to the initial mode (either NIMV or HFNC) because we felt it was unlikely to have a meaningful impact on the results of the study as the exposure already happened. Although the effects of these interventions on effort of breathing are nearly immediate, it is possible that the lack of randomization may have affected our results. The Ram Cannula was used as the nasal interface for all nasal support (HFNC, NCPAP, and NIMV). We chose the same nasal interface to minimize patient discomfort and agitation (thus, increased effort of breathing) when switching between the modes. However, the shape and caliber of the nasal prongs in the Ram Cannula may not be optimal for HFNC. This could underestimate the effects of HFNC and makes it even less likely that NIMV or NCPAP is superior to HFNC. Moreover, we did not examine other interfaces such as oro-nasal masks.
We were unable to achieve good synchrony on the majority of patients, even with a nasal clip. We did not attempt to improve synchrony further by altering mode (ie, pressure support instead of assist control), or using different triggering devices (ie, neurally adjusted ventilation). Further innovation is needed to improve patient noninvasive ventilator synchrony, particularly investigating the effect of the inspiratory time, ventilator triggers, ventilator modes, minimizing leak, and other methods of triggering. In addition, the analysis comparing PRP on HFNC vs potentially equivalent pressures on NIMV as a function of synchrony is exploratory. We do not have data to confirm equivalent flow rates of HFNC and NIMV pressure. Moreover, we did not study other levels of NCPAP, and some investigators have found reduced effort of breathing with higher levels of CPAP.24 Our sample size was limited for all of the multiple comparisons, although we were adequately powered to detect differences for our primary comparison of interest (CPAP 5 vs HFNC 6 vs NIMV 12/6). However, the lack of any clear trends that one of these modes is superior to another makes it unlikely that we would have found an effect, even with a larger sample size. Finally, we examined short-term effects on effort of breathing not long-term outcomes such as an ICU length of stay or ventilator-free days.
For infants less than 6 months of age, there is no difference in effort of breathing between HFNC, NIMV, or NCPAP after extubation, regardless of flow rate or inspiratory pressure. However, patient-NIMV synchrony is an important factor in determining effort of breathing, and if high levels of synchrony can be achieved, NIMV may reduce patient effort of breathing over HFNC. Nonetheless, effort of breathing while intubated on CPAP 5 is still lower than effort of breathing on all nasal modes of respiratory support after extubation. This should be factored into clinical decision making for assessing extubation readiness for infants.
Acknowledgments
Funded by the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NIH/NICHD) (1K23HL103785 [to R.K.]). The authors declare no conflicts of interest.
Glossary
- CPAP
Continuous positive airway pressure
- CPAP 5
CPAP of 5 cm H2O
- HFNC
Humidified high flow nasal cannula
- HFNC 6
HFNC 6 liters per minute
- ICU
Intensive care unit
- NCPAP
Nasal continuous positive airway pressure
- NCPAP 5
NCPAP of 5 cm H2O
- NIMV
Nasal intermittent mechanical ventilation
- NIMV 12
NIMV with a driving pressure of 12 cm H2O
- NRS
Noninvasive respiratory support
- PRP
Pressure-rate product
- UAO
Upper airway obstruction
References
- 1.Nava S. Behind a mask: tricks, pitfalls, and prejudices for noninvasive ventilation. Respir Care. 2013;58:1367–76. doi: 10.4187/respcare.02457. [DOI] [PubMed] [Google Scholar]
- 2.Essouri S, Laurent M, Chevret L, Durand P, Ecochard E, Gajdos V, et al. Response to Jones et al.: revisiting the benefits of nCPAP versus intubation for severe bronchiolitis. Intensive Care Med. 2014;40:764. doi: 10.1007/s00134-014-3255-2. [DOI] [PubMed] [Google Scholar]
- 3.Essouri S, Chevret L, Durand P, Haas V, Fauriux B, Devictor D. Noninvasive positive pressure ventilation: five years of experience in a pediatric intensive care unit. Pediatr Crit Care Med. 2006;7:329–34. doi: 10.1097/01.PCC.0000225089.21176.0B. [DOI] [PubMed] [Google Scholar]
- 4.Essouri S, Durand P, Chevret L, Balu L, Devictor D, Fauroux B, et al. Optimal level of nasal continuous positive airway pressure in severe viral bronchiolitis. Intensive Care Med. 2011;37:2002–7. doi: 10.1007/s00134-011-2372-4. [DOI] [PubMed] [Google Scholar]
- 5.Ganu S, Gautam A, Wilkins B, Egan J. Increase in use of noninvasive ventilation for infants with severe bronchiolitis is associated with decline in intubation rates over a decade. Intensive Care Med. 2012;38:1177–83. doi: 10.1007/s00134-012-2566-4. [DOI] [PubMed] [Google Scholar]
- 6.Bajaj A, Rathor P, Sehgal V, Shetty A. Efficacy of noninvasive ventilation after planned extubation: a systematic review and meta-analysis of randomized controlled trials. Heart Lung. 2015;44:150–7. doi: 10.1016/j.hrtlng.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Mayordomo-Colunga J, Medina A, Rey C, Concha A, Menendez S, Los Arcos M, et al. Noninvasive ventilation after extubation in paediatric patients: a preliminary study. BMC Pediatr. 2010;10:29. doi: 10.1186/1471-2431-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabrini L, Landoni G, Oriani A, Plumari VP, Nobile L, Greco M, et al. Noninvasive ventilation and survival in acute care settings: a comprehensive systematic review and meta-analysis of randomized controlled trials. Crit Care Med. 2015;43:880–8. doi: 10.1097/CCM.0000000000000819. [DOI] [PubMed] [Google Scholar]
- 9.Sağiroğlu G, Baysal A, Copuroğlu E, Gül Y, Karamustafaoğlu Y, Dogukan M. Does early use of bi-level positive airway pressure (BIPAP) in cardiothoracic intensive care unit prevent reintubation? Int J Clin Exp. 2014;7:3439–46. [PMC free article] [PubMed] [Google Scholar]
- 10.Rubin S, Ghuman A, Deakers T, Khemani R, Ross P, Newth CJ. Effort of breathing in children receiving high-flow nasal cannula. Pediatr Crit Care Med. 2014;15:1–6. doi: 10.1097/PCC.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 11.Esteban A, Frutos-Vivar F, Ferguson ND, Arabi Y, Apezequia C, Gonzalez M, et al. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med. 2004;350:2452–60. doi: 10.1056/NEJMoa032736. [DOI] [PubMed] [Google Scholar]
- 12.Cambonie G, Milési C, Jaber S, Amsallem F, Barbotte E, Picaud JC, et al. Nasal continuous positive airway pressure decreases respiratory muscles overload in young infants with severe acute viral bronchiolitis. Intensive Care Med. 2008;34:1865–72. doi: 10.1007/s00134-008-1201-x. [DOI] [PubMed] [Google Scholar]
- 13.Khemani RG, Hotz J, Morzov R, Flink R, Kamerkar A, Ross PA, et al. Evaluating risk factors for pediatric post-extubation upper airway obstruction using a physiology- based tool. Am J Respir Crit Care Med. 2016;193:198–209. doi: 10.1164/rccm.201506-1064OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urbano Villaescusa J, Mencia Bartolome S, Cidoncha Escobar E, Lopez-Herce Cid J, Sanitago Lozano MJ, Carrillo Alvarez A. Experience with high-flow nasal cannula oxygen therapy in children. An Pediatr (Barc) 2008;68:4–8. doi: 10.1157/13114463. [DOI] [PubMed] [Google Scholar]
- 15.Vignaux L, Vargas F, Roeseler J, Tassaux D, Thille AW, Kossowsky MP, et al. Patient- ventilator asynchrony during noninvasive ventilation for acute respiratory failure: a multicenter study. Intensive Care Med. 2009;35:840–6. doi: 10.1007/s00134-009-1416-5. [DOI] [PubMed] [Google Scholar]
- 16.Willis BC, Graham AS, Yoon E, Wetzel RC, Newth CJ. Pressure-rate products and phase angles in children on minimal support ventilation and after extubation. Intensive Care Med. 2005;31:1700–5. doi: 10.1007/s00134-005-2821-z. [DOI] [PubMed] [Google Scholar]
- 17.Khemani RG, Hotz J, Morzov R, Flink RC, Kamerkar A, LaFortune M, et al. Pediatric extubation readiness tests should not use pressure support. Intensive Care Med. 2016;42:1214–22. doi: 10.1007/s00134-016-4387-3. [DOI] [PubMed] [Google Scholar]
- 18.Glossop AJ, Shephard N, Bryden DC, Mills GH. Noninvasive ventilation for weaning, avoiding reintubation after extubation and in the postoperative period: a meta-analysis. Br J Anaesth. 2012;109:305–14. doi: 10.1093/bja/aes270. [DOI] [PubMed] [Google Scholar]
- 19.Saslow JG, Aghai ZH, Nakhla TA, Hart JJ, Lawrysh R, Stahl GE, et al. Work of breathing using high-flow nasal cannula in preterm infants. J Perinatol. 2006;26:476–80. doi: 10.1038/sj.jp.7211530. [DOI] [PubMed] [Google Scholar]
- 20.Aghai ZH, Saslow JG, Nakhla T, Milcarek B, Hart J, Lawrysh-Plunkett R, et al. Synchronized nasal intermittent positive pressure ventilation (SNIPPV) decreases work of breathing (WOB) in premature infants with respiratory distress syndrome (RDS) compared to nasal continuous positive airway pressure (NCPAP) Pediatr Pulmonol. 2006;41:875–81. doi: 10.1002/ppul.20461. [DOI] [PubMed] [Google Scholar]
- 21.Gizzi C, Papoff P, Giordano I, Massenzi L, Barbàra CS, Campelli M, et al. Flow- synchronized nasal intermittent positive pressure ventilation for infants <32 weeks' gestation with respiratory distress syndrome. Crit Care Res Pract. 2012;2012:301818. doi: 10.1155/2012/301818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gizzi C, Montecchia F, Panetta V, Castellano C, Mariani C, Campelli M, et al. Is synchronised NIPPV more effective than NIPPV and NCPAP in treating apnoea of prematurity (AOP)? A randomised cross-over trial. Arch Dis Child Fetal Neonatal Ed. 2015;100:F17–23. doi: 10.1136/archdischild-2013-305892. [DOI] [PubMed] [Google Scholar]
- 23.Ramanathan R. Nasal respiratory support through the nares: its time has come. J Perinatol. 2010;30(Suppl):S67–72. doi: 10.1038/jp.2010.99. [DOI] [PubMed] [Google Scholar]
- 24.Essouri S, Nicot F, Clément A, Garabedian EN, Roger G, Lofaso F, et al. Noninvasive positive pressure ventilation in infants with upper airway obstruction: comparison of continuous and bilevel positive pressure. Intensive Care Med. 2005;31:574–80. doi: 10.1007/s00134-005-2568-6. [DOI] [PubMed] [Google Scholar]
- 25.Ducharme-Crevier L, Beck J, Essouri S, Jouvet P, Emeriaud G. Neurally adjusted ventilatory assist (NAVA) allows patient-ventilator synchrony during pediatric noninvasive ventilation: a crossover physiological study. Crit Care. 2015;19:44. doi: 10.1186/s13054-015-0770-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozyilmaz E, Ugurlu AO, Nava S. Timing of noninvasive ventilation failure: causes, risk factors, and potential remedies. BMC Pulm Med. 2014;14:19. doi: 10.1186/1471-2466-14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang L, Mendler MR, Waitz M, Schmid M, Hassan MA, Hummler HD. Effects of synchronization during noninvasive intermittent mandatory ventilation in preterm infants with respiratory distress syndrome immediately after extubation. Neonatology. 2015;108:108–14. doi: 10.1159/000431074. [DOI] [PubMed] [Google Scholar]
- 28.Spentzas T, Minarik M, Patters AB, Vinson B, Stidham G. Children with respiratory distress treated with high-flow nasal cannula. J Intensive Care Med. 2009;24:323–8. doi: 10.1177/0885066609340622. [DOI] [PubMed] [Google Scholar]
- 29.Sztrymf B, Messika J, Bertrand F, Hurel D, Leon R, Dreyfuss D, et al. Beneficial effects of humidified high flow nasal oxygen in critical care patients: a prospective pilot study. Intensive Care Med. 2011;37:1780–6. doi: 10.1007/s00134-011-2354-6. [DOI] [PubMed] [Google Scholar]
- 30.Frizzola M, Miller TL, Rodriguez ME, Zhu Y, Rojas J, Hesek A, et al. Highflow nasal cannula: impact on oxygenation and ventilation in an acute lung injury model. Pediatr Pulmonol. 2011;46:67–74. doi: 10.1002/ppul.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sivieri EM, Gerdes JS, Abbasi S. Effect of HFNC flow rate, cannula size, and nares diameter on generated airway pressures: an in vitro study. Pediatr Pulmonol. 2013;48:506–14. doi: 10.1002/ppul.22636. [DOI] [PubMed] [Google Scholar]
- 32.Arora B, Mahajan P, Zidan MA, Sethuraman U. Nasopharyngeal airway pressures in bronchiolitis patients treated with high-flow nasal cannula oxygen therapy. Pediatr Emerg Care. 2012;28:1179–84. doi: 10.1097/PEC.0b013e318271a671. [DOI] [PubMed] [Google Scholar]
- 33.Hutchison AA, Bignall S. Noninvasive positive pressure ventilation in the preterm neonate: reducing endotrauma and the incidence of bronchopulmonary dysplasia. Arch Dis Child Fetal Neonatal Ed. 2008;93:F64–8. doi: 10.1136/adc.2006.103770. [DOI] [PubMed] [Google Scholar]
- 34.Cavari Y, Sofer S, Rozovski U, Lazar I. Noninvasive positive pressure ventilation in infants with respiratory failure. Pediatr Pulmonol. 2012;47:1019–25. doi: 10.1002/ppul.22561. [DOI] [PubMed] [Google Scholar]