Abstract
Objective
Adiponectin is found in human serum in three groups of multimers (high, medium, and low molecular weight). Previously, we reported two ethnic-specific variants in ADIPOQ, G45R (Hispanic Americans) and R55C (African Americans). Although carriers of both variants had mean adiponectin levels ≤20% of those of non-carriers, they were not clinically different from non-carriers. To compare carriers of both variants and non-carriers, relative quantification of adiponectin isoforms to total adiponectin was performed on serum samples.
Methods
The multimeric patterns of serum adiponectin in G45R carriers (n=23), R55C carriers (n=3), and Hispanic and African American non-carriers (n=84 and 44, respectively) from the IRAS Family Study were explored using native western blotting and densitometry.
Results
Serum samples from carriers showed an absence of the high molecular weight (HMW) isoform and a marked reduction in the medium molecular weight isoform but an approximate two-fold increase in the amount of the low molecular weight isoform (LMW). Thus, individuals making only LMW adiponectin are metabolically normal.
Conclusions
The results contrast with the proposed biological importance of the HMW multimer. This suggests that the LMW isoform may functionally compensate for some of the loss/reduction of the higher-order multimers in carriers of the G45R and R55C mutations.
Key terms: Adipokines, African American, Ethnic Minorities, Family Studies, Hispanics
Introduction
Adiponectin, encoded by ADIPOQ, is an adipokine exclusively synthesized and secreted by adipocytes into serum (1). Levels of circulating adiponectin have been shown to be inversely correlated with body weight and cardiometabolic disease (2, 3, 4, 5) and have been the subject of extensive investigation in metabolic disease in humans. The adiponectin monomer contains a collagen-like domain which facilitates assembly of multimers containing carboxyl terminal globular domains believed to be the active component of the molecule. In normal human serum, adiponectin is distributed in three distinct isoforms: low molecular weight (LMW) trimers, medium molecular weight (MMW) hexamers, and high molecular weight (HMW) oligomers (1, 6). The HMW oligomer, comprised of 12–18 adiponectin monomers, has previously been reported to be the most biologically active of the three isoforms in both humans and mice (7, 8), although there is not a firm consensus on this position in the literature. In addition, there is evidence that the ratio of HMW to total adiponectin is a better predictor of insulin resistance and metabolic syndrome compared with total adiponectin levels alone (6, 9).
Previously, we have reported identification of two ethnic-specific novel coding variants in the ADIPOQ gene: G45R in Hispanic Americans (10) and R55C in African Americans (11). These coding variants are located in the collagen-like domain of ADIPOQ. Total serum adiponectin levels had previously been quantified by radioimmunoassay (RIA) (12) in individuals harboring the variants and were found to be ≤ 20% of the mean adiponectin levels observed in non-carriers (10). Despite the documented inverse correlation between cardiometabolic disease and total serum adiponectin levels (2), these G45R and R55C mutation carriers were statistically no different in measures of metabolic health (e.g. insulin sensitivity, body mass index) and were not clinically different from related individuals who do not harbor the G45R or R55C variants (10, 11). Based on the seemingly contradictory results observed in individuals with mutations, we have carried out a focused analysis of the adiponectin isoforms from these subjects in an effort to explain the surprising lack of association with clinical characteristics commonly associated with a reduction in serum adiponectin levels.
Methods
Materials
Primary rabbit polyclonal antibodies against mouse and human adiponectin were obtained from Biovendor R&D Products (Asheville, NC). Fluorescently labeled 800CW goat anti-rabbit IgG secondary antibody, nitrocellulose membranes, and Odyssey tris-buffered saline blocking buffer were purchased from LI-COR Biotechnology (Lincoln, NE). Reference HMW adiponectin isoforms were purchased from R&D Systems (Minneapolis, MN). Control pooled mouse serum and the Life Technologies total adiponectin enzyme-linked immunosorbent assay (ELISA) were acquired from Thermo-Fisher Scientific (Waltham, MA).
Samples
Human Subjects
The primary source of human samples was the Insulin Resistance Atherosclerosis Family Study (IRASFS), a multi-center, family-based study designed to identify the genetic determinants of insulin resistance and visceral adiposity in Hispanic American and African American populations (13). Serum samples from Hispanic American (N = 107) and African American (N = 47) IRASFS participants were analyzed for adiponectin multimers (see below). An additional 10 African American samples from other cohorts were included for comparison (14, 15). Archived serum samples, stored at −80°C, were used for all analyses. Written informed consent was obtained from all participants prior to sample collection, and Institutional Review Board approval was secured at all clinical and analysis sites.
Mice
Blood collection procedures were approved by the Institutional Animal Care and Use Committee at Wake Forest School of Medicine, Winston-Salem, North Carolina. Whole blood was collected from two fourteen-month-old C57BL/6J female mice. Serum was separated from whole blood using standard procedure. Briefly, blood samples were allowed to clot at 23°C for 60 min, followed by centrifugation at 1,500 × g for 15 min at 4°C to separate the serum from the clot. Serum was removed from each sample and stored in a clean microcentrifuge tube at −80°C.
ELISA
A subset of 26 IRASFS Hispanic American serum samples was re-quantified in this study using the Life Technologies total adiponectin ELISA according to the manufacturer’s protocol. In brief, antigen in the samples was bound to antibody-coated wells. A detection antibody was then added to each well, followed by horse radish peroxidase (HRP) solution. Finally, tetramethylbenzidine substrate was used to detect HRP activity. Addition of stop solution changed the reaction color for detection at 450 nm by a microplate reader.
Western Blots
Native (non-denaturing, non-reducing) western blots were performed to separate and visualize the adiponectin isoforms within each serum sample (human and mouse). Each 20 μL sample consisted of 0.5 μL serum, 10 μL standard native loading buffer, and 9.5 μL 50 mM tris buffer, pH 6.8. Samples were loaded onto hand-cast 6% polyacrylamide gels in tris-glycine buffer without sodium dodecyl sulfate (SDS) and run for 3 h 30 min at 125 V on ice. Following electrophoresis, gels were soaked for 15 min in 0.1% SDS to confer a net negative charge to the separated proteins and facilitate the unidirectional transfer to a nitrocellulose membrane. Protein transfer was conducted in tris-glycine buffer with 20% methanol at 25 V for 1 h. After transfer, the membranes were fixed in 5% acetic acid for 15 min, rinsed with deionized water, and allowed to air-dry for several minutes. Adiponectin was probed using a 1:2500 dilution of a species-specific polyclonal rabbit anti-adiponectin antibody. A fluorescently labeled secondary antibody (IRDye 800CW Goat anti-Rabbit IgG) was used to detect the adiponectin-bound primary antibody. Following washing to remove excess unbound antibody, the membranes were dried for 24 h and scanned using an Odyssey Classic Infrared Imaging System (LI-COR Biosciences). Scans were saved in a high-resolution image format for subsequent analysis.
Densitometry Analysis and Quantification
Fiji Image J software (16, 17) was used to quantify the adiponectin isoforms for each sample. Each image was separated out by color channel (red, blue, and green). Subsequent steps were performed using the green channel image. Three regions corresponding to the high, medium, and low molecular weight bands of adiponectin were delineated. Rectangles were drawn around each lane of the gel, and the total intensity of the signal within each designated isoform region was measured. The signal score was calculated by subtracting the background signal from the total intensity. The average intensity of the marker lanes from the red channel image was used as the reference signal, and each signal score was divided by the reference signal to obtain the final adjusted signal score, or relative intensity value. Densitometry traces were graphed based on the relative intensity values with Microsoft Excel, and total adiponectin was estimated for each sample by summing the relative intensities for that sample. Total adiponectin levels and isoform-to-total adiponectin percentages were determined based on these values.
Results
Table 1 lists relevant demographic information for the IRASFS participants whose sera were tested in this study. Among the non-carrier samples were Hispanic American and African American individuals that previously had low (bottom 20%) total plasma adiponectin levels by RIA (12). These samples were chosen for analysis to investigate the possibility that they also showed isoform distribution differences such as those observed with the carrier samples. In addition, in Hispanic American only, non-carriers with normal/average plasma adiponectin levels were included. Thus, there were three Hispanic American test groups and two African American test groups. Across most measures, the sample subgroups were not noticeably different (e.g. body mass index). For some traits, these small samples may differ (e.g. acute insulin response [AIR]; Table 1). The ranges of AIR measurements were large, however, the median values show more modest differences (e.g. Hispanic American normal adiponectin: 810.80 pmol/L; Hispanic American, low adiponectin: 625.50 pmol/L; Hispanic American, G45R carriers: 497.80 pmol/L). Moreover, in the original analyses (10, 11), there were no significant differences based on genotype found in the overall samples (Hispanic American, n=1240; African American, n=566).
Table 1.
Phenotypic summaries of IRASFS Hispanic and African American participants
| Hispanic Americans | African Americans1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Non-carriers, standard2 | Non-carriers, low2 | G45R Carriers | Non-carriers, low2 | R55C Carriers | ||||||
| 26 | 58 | 23 | 44 | 3 | |||||||
|
| |||||||||||
| Traits | Mean | Range | Mean | Range | Mean | Range | Mean | Range | Mean | Range | |
| Age (years) | 43.37 | 20–73 | 39.06 | 19–76 | 41.28 | 21–70 | 41.32 | 20–68 | 45.51 | 33–56 | |
| % Female | 61.5 | 21.4 | 56.5 | 45.5 | 66.7 | ||||||
| % T2D | 7.7 | 20.7 | 13 | 18.2 | 66.7 | ||||||
| Adiponectin (μg/mL)3 | 13.84 | 7.24–20.22 | 4.46 | 2.34–5.81 | 2.20 | 1.20–3.00 | 2.87 | 1.20–3.67 | 1.34 | 1.20–1.54 | |
|
| |||||||||||
| Glucose Homeostasis | Insulin Sensitivity Index (SI; ×10−5 min−1/[pmol/L]) | 1.64 | 0.13–5.01 | 1.51 | 0.21–4.68 | 2.41 | 0.31–7.83 | 1.07 | 0.09–3.40 | 0.59 | 0.53–0.64 |
| Acute Insulin Response (AIR; pmol/L) | 1188.78 | 338.45– 3900.30 | 907.73 | 51.20– 3668.90 | 776.60 | 33.80– 3531.20 | 1105.60 | 57.80– 4078.20 | 2322.65 | 136.6– 4508.7 | |
| Disposition Index (DI; SI × AIR; ×10−5 min−1) | 1561.08 | 101.01– 5988.99 | 1033.81 | 46.01– 4616.52 | 1411.52 | 170.75– 6365.01 | 988.11 | 48.92– 2903.89 | 1478.98 | 72.40– 2885.57 | |
| Fasting Glucose (mg/dL) | 95.86 | 80.5–125 | 96.76 | 77–119.5 | 83.39 | 81–123 | 101.48 | 82.5–122.5 | 71.50 | 92–122.5 | |
| Fasting Insulin (μU/mL) | 16.20 | 4–36 | 20.98 | 3–65 | 18.95 | 4–40 | 21.13 | 3–108 | 23.501 | 6–31 | |
| Metabolic Clearance of Insulin (min−1) | 4.75 | 1.79–8.98 | 4.14 | 0.96–9.17 | 5.77 | 2.48–11.48 | 3.56 | 1.18–7.24 | 2.95 | 2.68–3.22 | |
|
| |||||||||||
| Adiposity | Body Mass Index; BMI (kg/m2) | 30.2 | 17.8–37.5 | 30.2 | 21.8–40.0 | 29.9 | 20.6–45.4 | 31.9 | 22.1–47.7 | 30.4 | 26.5–34.9 |
| Visceral Adipose Tissue (cm2) | 124.36 | 37.16– 220.93 | 123.21 | 53.08– 255.06 | 106.43 | 17.43– 216.52 | 105.35 | 38.30– 246.75 | 105.96 | 76.53– 144.65 | |
| Subcutaneous Adipose Tissue (cm2) | 400.34 | 67.21– 668.19 | 327.37 | 127.53– 655.98 | 294.86 | 40.42– 519.66 | 360.22 | 81.54– 708.89 | 418.71 | 256.69– 590.70 | |
| Waist Circumference (cm) | 93.25 | 71.75– 112.90 | 96.70 | 73.50– 127.90 | 92.23 | 67.30– 120.30 | 96.18 | 69.95– 127.20 | 97.23 | 92.35– 105.65 | |
| Liver Density (HU) | 52.94 | 25.71– 66.53 | 43.38 | 13.95– 69.25 | 49.38 | 22.07– 61.72 | 49.36 | 10.42–62.04 | 61.41 | 61.00–61.66 | |
|
| |||||||||||
| Lipids | Triglycerides (mg/dL) | 116.12 | 24–234 | 158.14 | 32–506 | 124.04 | 43–264 | 91.18 | 36–19367.00 | 32–99 | |
| High Density Lipoproteins (mg/dL) | 39.92 | 21–63 | 33.47 | 22–50 | 36.87 | 24–51 | 38.86 | 27–63 | 35.00 | 27–43 | |
| Low Density Lipoproteins (mg/dL) | 108.08 | 34–177 | 110.89 | 65–215 | 112.87 | 59–162 | 111.75 | 51–167 | 139.33 | 109–170 | |
| Total Cholesterol (mg/dL) | 171.23 | 102–246 | 174.86 | 117–337 | 174.57 | 115–228 | 168.82 | 99–238 | 187.67 | 150–233 | |
|
| |||||||||||
| Hypertension | Systolic Blood Pressure (mm) | 119.27 | 98–197 | 120.21 | 93–169 | 110.78 | 90–132 | 121.00 | 89–164 | 122.33 | 112–133 |
| Diastolic Blood Pressure (mm) | 79.46 | 64–99 | 77.78 | 57–101 | 71.78 | 56–88 | 78.27 | 59–101 | 74.67 | 71–80 | |
| Albumin Creatinine Ratio (American; mg/g) | 16.25 | 3.65– 109.44 | 20.90 | 2.22– 412.24 | 18.33 | 2.77–91.65 | 39.41 | 1.95– 1282.76 | 383.02 | 2.27– 1142.22 | |
|
| |||||||||||
| Inflammation | IL-6 (pg/mL) | 2.63 | 0.71–8.43 | 2.21 | 0.25–9.96 | 2.71 | 0.40–7.95 | 4.03 | 0.26–20.77 | 8.63 | 0.63–17.12 |
| C-Reactive Protein (mg/mL) | 6.26 | 0.13–32.81 | 3.00 | 0.18–14.28 | 4.03 | 0.13–13.63 | 3.19 | 0.64–13.10 | 2.55 | 0.88–3.41 | |
Due to sample availability, all IRASFS African American samples included in this study were either non-carriers measured to have low adiponectin levels by RIA or R55C carriers
“Standard” refers to those samples with total adiponectin levels measured to fall within the mean +/− 1 standard deviation of all samples measured; those samples in the bottom 10% of all samples measured were classified as “low”12.
Adiponectin levels as measured by RIA12.
ELISA
Serum adiponectin levels of the 26 Hispanic American samples from the IRASFS measured by the ELISA ranged from 1.51–29.37 ng/mL (mean = 9.34 ng/mL; SD = 6.54). Plasma adiponectin levels had previously been measured in these IRASFS participants using RIA (12). This assay was followed by measurement on a subset of samples (N=55) using a total adiponectin ELISA (EMD Millipore; Billerica, MA) (10). Among the three quantification assays, the highest correlation was between the results of the two ELISAs (r = 0.91). There was relatively poor correlation (r = 0.56) between the measurements obtained by the prior RIA and ELISA performed in this study, although this could be due in part to the use of serum compared to plasma as the source of adiponectin.
Western Blots
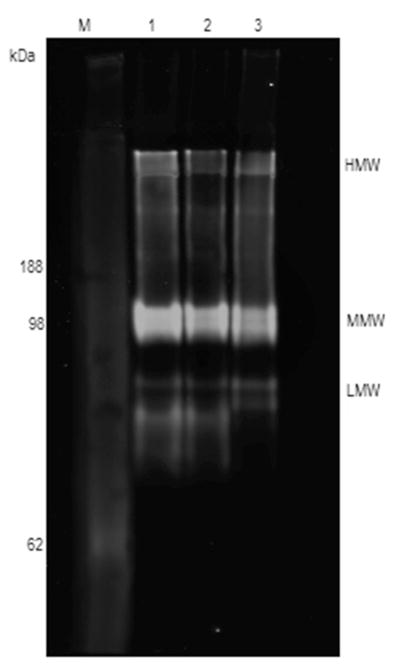
Fluorescent imaging of western blots showed that HMW isoforms were undetectable or greatly reduced and the MMW isoform was markedly reduced in G45R and R55C carrier samples (Figure 1A and 1B, respectively). This was accompanied by a noticeable increase in the amount of LMW isoform in samples from both G45R and R55C carriers compared to non-carrier samples. In contrast to the human samples, fluorescent images of mouse serum immunoblots showed only the HMW and MMW isoforms of adiponectin (Figure 2), consistent with previously published results (7). Mouse samples incubated with human antibody and human samples incubated with mouse antibody do show cross-reactivity, although the LMW isoform was only faintly detected in human samples when probed with mouse antibody (data not shown).
Figure 1.
(A) IRASFS Hispanic American and (B) IRASFS African American serum samples blotted under native conditions. Carriers of the G45R mutation (A; Lanes 1–5) and the R55C mutation (B; Lanes 8, 10, and 11) show a distinctively different isoform pattern from Hispanic (A; Lanes 6–13) and African American (B; Lanes 1–7, 9, 12, 13) non-carriers. The HMW isoform is drastically reduced or undetectable in all mutation carriers, and the MMW isoform is also markedly decreased. However, there is a corresponding increase in the LMW isoform. It is worth noting that samples in panel A, lanes 6–8 are from Hispanic American non-carriers, low (RIA) adiponectin levels. These samples display the same isoform pattern as the Hispanic American normal (RIA) adiponectin level non-carriers (A; Lanes 9–13).
Figure 2.
Samples from female C57BL/6J mice (Lanes 1–2) and pooled mouse sera (Lane 3). The predominant isoform detected in the mouse is the MMW hexamer.
Densitometry and Quantification
Representative densitometry traces illustrating the distribution of the three adiponectin isoforms are shown for a Hispanic American G45R carrier (Figure 3A), a Hispanic American non-carrier (Figure 3B), an African American R55C carrier (Figure 3C), and an African American non-carrier (Figure 3D). The same patterns were consistently observed among individual members of each respective group. The average percentage of HMW to total adiponectin was determined from the quantification of blots for each group as well (Figure 4). Interestingly, the Hispanic American non-carriers, low (RIA) adiponectin level test group appeared to have a lower HMW percentage than the Hispanic American non-carriers, normal adiponectin levels test group. Mean percentages of HMW for each group were: G45R carriers, 1.0% (0.3–1.6%); G45 non-carriers, low (RIA) adiponectin levels, 8.7% (1.8–15.4%); G45 non-carriers, normal adiponectin levels, 14.1% (5.6–24.9%); R55C carriers, 2.6% (1.2–6.1%); and (low RIA) R55 non-carriers, 6.1% (1.3–20.5%). The mean percentages of MMW for each group were: G45R carriers, 4.6% (0.96–33.3%); G45 non-carriers, low (RIA) adiponectin levels, 17.9% (10.4–30.2%); G45 non-carriers, normal adiponectin levels, 23.5% (2.8–38.2%); R55C carriers, 3.4% (1.8–6.1%); and (low RIA) R55 non-carriers, 18.3% (8.7–32.5%). In addition, with densitometry, we estimate that the LMW isoform synthesis is 2.2-fold higher in the G45R and 2.5-fold higher in the R55C carriers than in non-carriers.
Figure 3.
Representative densitometry traces from a Hispanic American G45R carrier (A), a Hispanic American non-carrier (B), an African American R55C carrier (C), and an African American non-carrier (D). Both the G45R carrier (A) and the R55C carrier (C) show minimal detection of the HMW multimers, although the R55C carrier has a small MMW peak. Characteristic of all carriers, both show a tall broad peak corresponding to the LMW isoform. The non-carriers (B, D) display the more typical sharp thin HMW peak followed by two small peaks of lower magnitude, then a narrow MMW peak and a moderately broad LMW peak.
Figure 4.
The mean ratio of the high molecular weight isoform to total adiponectin levels. Carriers of the G45R and R55C variants have markedly smaller HMW to total adiponectin ratios than non-carriers. Interestingly, the difference between carriers and non-carriers is more pronounced in the Hispanic Americans than in the African Americans included in this study.
Discussion
In this study, we performed native western blotting experiments with densitometry analysis of adiponectin in serum samples from Hispanic American and African American IRASFS participants. The motivation was to use western blots to assess serum adiponectin from individuals harboring the G45R or R55C ADIPOQ variants. It is important to note that before either variant had been identified, carriers were determined to have low total adiponectin levels by RIA, yet were not clinically different from their family members with higher adiponectin levels (10, 11, 12). Herein, we show that the difference is not the total adiponectin concentration, but the pattern of adiponectin isoform distribution between carriers of the G45R and R55C variants and non-carriers from the same populations. Working back to the original reports, we conclude that variable isoform distributions were not significantly different in their associations with cardiometabolic traits. With this, secretion of the LMW isoform of adiponectin is substantially increased in G45R and R55C mutation carriers.
It is striking that the low total adiponectin levels in G45R and R55C carriers determined previously by RIA (12) were so different from the current western blot analysis. However, a marked increase in the LMW isoform and considerably decreased HMW and MMW was observed in Western blot analysis of the mutant carriers. This observation substantially changes the context of the discussion of why individuals with the G45R or R55C variants do not differ clinically from non-carriers. The apparent increase in LMW adiponectin in carriers of these mutations, leading to higher levels of total adiponectin, may functionally compensate for the lack of the HMW isoform. This advantage is not observed in carriers of other ADIPOQ variants, e.g. G84R and G90S, who, despite producing normal levels of LMW and MMW isoforms, are unable to form the HMW multimers and overall have lower total adiponectin levels and higher incidence of metabolic disease (18, 19). This scenario, however, results in further questions. First, it has been reported that alterations to the HMW multimer are in some cases correlated to increases in insulin sensitivity (7, 8), but carriers of ADIPOQ G45R or R55C variants lacking the HMW isoform do not differ phenotypically from Hispanic American or African American non-carriers. Thus, characterization of the isoform distribution of the G45R and R55C carriers provides interesting insight into the metabolic action of adiponectin isoforms suggesting that LMW multimers may be comparably active and suggest a need for investigation into the role of different isoforms. It is notable that endogenous LMW adiponectin makes up a lower proportion of the total adiponectin in mice (18), which was replicated using this experimental approach (data not shown). These dramatic differences suggest that the metabolic influences of adiponectin isoforms may be different in this model system compared to humans.
Based on the discrepancy between the total adiponectin levels measured by RIA versus those measured by other antibody-dependent assays (ELISA and western blot), it is likely that some antibodies may be subject to preferential detection of one isoform over others. In this case, it appears the RIA may have preferentially detected the HMW isoform of adiponectin, resulting in an inaccurate quantification of total adiponectin levels of those participants carrying the G45R mutation. It is therefore worth noting that caution must be taken when selecting an antibody-based quantification system to measure serum adiponectin, or any protein with multiple isoforms, to ensure the desired isoform(s) is detected. Here, rabbit polyclonal anti-human adiponectin antibody was used to successfully detect adiponectin isoforms, but other polyclonal antibodies have been used with similar results. In addition, we have used a monoclonal anti-human adiponectin antibody and observed nearly identical results (data not shown). This study underscores the importance of antibody validation for the intended purpose of an experiment or assay.
Conclusion
In summary, analysis of naturally occurring mutations in ADIPOQ reveal insights into adiponectin biology including a possible compensatory mechanism to explain the clinically normal phenotype in G45R and R55C carriers who lack the HMW isoform.
Study Importance Questions.
What is already known about this subject?
The structure of adiponectin and its formation into multimers is well-known.
The current literature of adiponectin multimers suggests that the high molecular weight isoform may be the most biologically active and that changes to this isoform have been shown to be related to increases in insulin sensitivity. However, it is unclear whether this is a direct or indirect effect.
What does your study add?
The results of our research suggest that the high molecular weight isoforms may not be critical for metabolic health, as carriers of two unique ADIPOQ mutations lack the high molecular weight multimers but are not clinically different from non-carriers.
It is possible there is a compensatory effect in these carriers, however, given the corresponding twofold increase in the low molecular weight adiponectin isoform we observed.
Acknowledgments
Funding: National Institutes of Health under Award Numbers R01 HG007112 and R01 DK071891; Wake Forest School of Medicine Center on Diabetes, Obesity, and Metabolism – pilot funding
The authors would like to thank the participants of the IRAS Family Study, without whom this research would not have been possible, and Swapan K. Das, Ph.D. for his generous contribution of an R55C serum sample from the AAGMEx study.
Footnotes
Disclosure: The authors declare no conflict of interest.
References
- 1.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 2.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 3.Frühbeck G, Gómez-Ambrosi J, Muruzábal FJ, Burrell MA. The adipocyte: a model for integration of endocrine and metabolic signaling in energy metabolism regulation. Am J Physiol Endocrinol Metab. 2001;280:E827–847. doi: 10.1152/ajpendo.2001.280.6.E827. [DOI] [PubMed] [Google Scholar]
- 4.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 5.Rutkowski JM, Scherer PE. Isolation and quantitation of adiponectin higher order complexes. Methods Enzymol. 2014;537:243–259. doi: 10.1016/B978-0-12-411619-1.00013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang ZV, Scherer PE. Adiponectin, the past two decades. J Mol Cell Biol. 2016;8:93–100. doi: 10.1093/jmcb/mjw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 8.Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 9.Hara K, Horikoshi M, Yamauchi T, Yago H, Miyazaki O, Ebinuma H, et al. Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care. 2006;29:1357–1362. doi: 10.2337/dc05-1801. [DOI] [PubMed] [Google Scholar]
- 10.Bowden DW, An SS, Palmer ND, Brown WM, Norris JM, Haffner SM, et al. Molecular basis of a linkage peak: exome sequencing and family-based analysis identify a rare genetic variant in the ADIPOQ gene in the IRAS Family Study. Hum Mol Genet. 2010;19:4112–4120. doi: 10.1093/hmg/ddq327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.An SS, Palmer ND, Hanley AJ, Ziegler JT, Brown WM, Freedman BI, et al. Genetic analysis of adiponectin variation and its association with type 2 diabetes in african americans. Obesity (Silver Spring) 2013 doi: 10.1002/oby.20419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo X, Saad MF, Langefeld CD, Williams AH, Cui J, Taylor KD, et al. Genome-wide linkage of plasma adiponectin reveals a major locus on chromosome 3q distinct from the adiponectin structural gene: the IRAS family study. Diabetes. 2006;55:1723–1730. doi: 10.2337/db05-0428. [DOI] [PubMed] [Google Scholar]
- 13.Henkin L, Bergman RN, Bowden DW, Ellsworth DL, Haffner SM, Langefeld CD, et al. Genetic epidemiology of insulin resistance and visceral adiposity. The IRAS Family Study design and methods. Ann Epidemiol. 2003;13:211–217. doi: 10.1016/s1047-2797(02)00412-x. [DOI] [PubMed] [Google Scholar]
- 14.Divers J, Wagenknecht LE, Bowden DW, Carr JJ, Hightower RC, Xu J, et al. Ethnic differences in the relationship between albuminuria and calcified atherosclerotic plaque: the African American-diabetes heart study. Diabetes Care. 2010;33:131–138. doi: 10.2337/dc09-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma NK, Sajuthi SP, Chou JW, Calles-Escandon J, Demons J, Rogers S, et al. Tissue-Specific and Genetic Regulation of Insulin Sensitivity-Associated Transcripts in African Americans. J Clin Endocrinol Metab. 2016;101:1455–1468. doi: 10.1210/jc.2015-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, et al. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278:40352–40363. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 19.Vasseur F, Helbecque N, Dina C, Lobbens S, Delannoy V, Gaget S, et al. Single-nucleotide polymorphism haplotypes in the both proximal promoter and exon 3 of the APM1 gene modulate adipocyte-secreted adiponectin hormone levels and contribute to the genetic risk for type 2 diabetes in French Caucasians. Hum Mol Genet. 2002;11:2607–2614. doi: 10.1093/hmg/11.21.2607. [DOI] [PubMed] [Google Scholar]