Abstract
Vitamin D and its’ metabolites are a crucial part of the endocrine system that controls whole body calcium homeostasis. The goal of this hormonal control is to regulate serum calcium levels so that they are maintained within a very narrow range. To achieve this goal, regulatory events occur in coordination at multiple tissues, e.g. the intestine, kidney, bone, and parathyroid gland. Production of the vitamin D endocrine hormone, 1,25 dihydroxyvitamin D (1,25(OH)2 D) is regulated by habitual dietary calcium intake and physiologic states like growth, aging, and the menopause. The molecular actions of 1,25(OH)2 D on calcium regulating target tissues are mediated predominantly by transcription controlled by the vitamin D receptor. The primary role for 1,25(OH)2 D during growth is to increase intestinal calcium absorption so that sufficient calcium is available for bone mineralization. However, vitamin D also has specific actions on kidney and bone.
Keywords: absorption, excretion, homeostasis, parathyroid hormone, endocrinology
I. An Overview of Whole Body Calcium Homeostasis
The term calcium homeostasis refers to the regulation of extracellular fluid (ECF) calcium ion concentration within a very narrow range (i.e. ECF = 88 mg/dL; total serum calcium = 9.5 mg/dL; free ionized calcium 5.0 mg/dL (Houillier et al., 2006)). Cell biologists focus on how this concentration is used to form an inward calcium gradient across cell membranes (7,000:1 extracellular:intracellular) and how this gradient can be released and used for cell signaling (Clapham, 2007). In contrast, the physiologic perspective views calcium homeostasis as a system whereby serum calcium levels are controlled by the movement of calcium through/among several tissues (intestine, kidney, bone) and regulated by endocrine hormones produced by multiple tissues (kidney, parathyroid gland, thyroid gland). In this light, intestinal calcium absorption from the diet is a disturbing signal that adds calcium to the ECF after a meal and the bone (through formation and resorption), as well as the kidney (by reabsorptive processes that control urinary excretion), must then respond to the disturbance and control it.
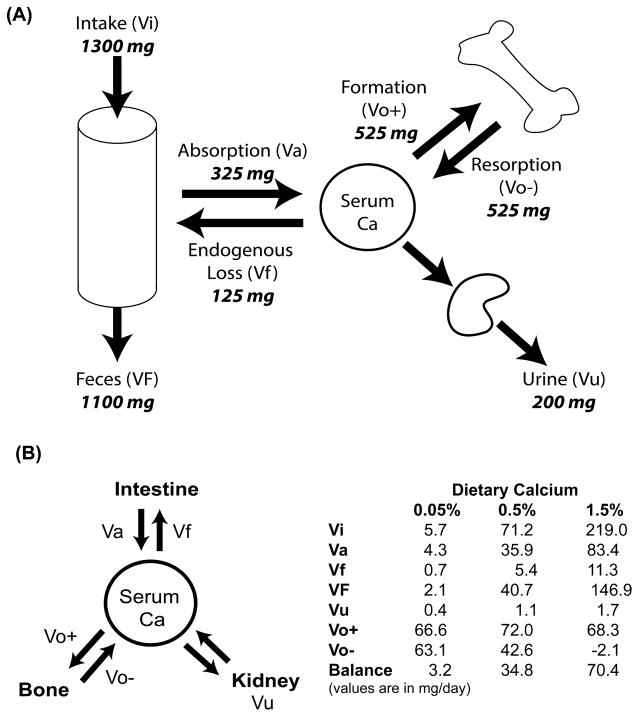
The critical role of intestine, kidney, and bone in calcium homeostasis can be seen from calcium kinetic studies using oral delivery and intravenous infusion of stable calcium isotopes. In adult women, calcium absorption is moderately efficient (Figure 1A, Va/Vi*100 = 25% of a daily intake of 1300 mg). The absorbed calcium joins the serum pool and is distributed to several pools depending upon need. In growth-stable adult women, the amount of calcium that is deposited in bone due to formation (Vo+) is equal to the amount released due to bone resorption (Vo−), i.e. bone turnover is in balance. Losses of calcium from the central pool through urine and fecal endogenous excretion and these are equal to the amount of calcium that was absorbed (i.e. net calcium absorption (Va – Vf) is equal to urinary calcium losses (Vu)). Calcium homeostasis can change dramatically under various physiologic or dietary conditions. For example, Wastney et al. (Wastney et al., 1996) found that while 22 year-old women had moderate calcium absorption efficiency and were in bone balance, growing 13 year-old girls absorbed calcium more efficiently (38% vs 22% per day when intake was 1330 mg/d), excreted less calcium in the urine (100 vs 203 mg/d), and had a positive bone balance (282 mg/d) that reflected more bone formation than resorption. Similarly, Bronner and Aubert (Bronner and Aubert, 1965) used calcium kinetics to show how reducing dietary calcium intake from excess (1.5% calcium in the diet) to deficient (0.05% calcium) could enhance bone resorption, improve intestinal calcium absorption efficiency, and reduce renal calcium loss in growing rats (Figure 1B).
Figure 1. Whole body calcium balance and its control by habitual dietary calcium intake.
(A) Calcium kinetic studies show that calcium homeostasis is controlled by the balance between calcium absorption (Va) and excretion (urine, Vu; feces, VF, endogenous fecal losses, Vf) as well as the rate of bone formation (Vo+) and resorption (Vo−). Calcium balance = calcium intake (Vi) – (VF+Vu), Net calcium absorption = Va-Vf; bone balance = bone formation (Vo+) – bone resorption (Vo−). (B) Habitual dietary calcium intake alters calcium homeostasis (using data reported in Bronner and Aubert (Bronner and Aubert, 1965).
II. Vitamin D Metabolites are a Part of the Hormonal Network that Controls Whole Body Calcium Homeostasis
The change in whole body calcium homeostasis induced by altering dietary calcium levels is preceded by changes in circulating hormone levels, i.e. calcium loading and deprivation regulate plasma levels of the vitamin D hormone 1,25 dihydroxyvitamin D (1,25(OH)2 D) (Adams et al., 1979, Song et al., 2003). 1,25(OH)2 D is derived from vitamin D3, which can be produced from 7-dehydrocholesterol in ultraviolet B exposed skin (Webb et al., 1988) or obtained from the diet or as supplements. In the liver, vitamin D is hydroxylated by the 25-hydroxylase to form 25 hydroxyvitamin D (25OH D), a stable metabolite that is used as a measure of vitamin D status. Under normal physiologic conditions the circulating endocrine hormone 1,25(OH)2 D is produced mainly within the kidney where the actions of two enzymes, the 25 hydroxyvitamin D, 1α hydroxylase (CYP27B1) that produces 1,25(OH)2 D from 25OH D, and the 25 hydroxyvitamin D, 24 hydroxylase (CYP24A1) that is the first step in 1,25(OH)2 D degradation, are balanced to influence the amount of 1,25(OH)2 D available for release into the circulation (Hewison et al., 2000).
Vitamin D and vitamin D metabolites are bound in the circulation by a number of factors including albumin, lipoproteins, and the Vitamin D Binding Protein (DBP) (White and Cooke, 2000). The primary role of DBP is to prevent renal loss of vitamin D. During renal filtration, vitamin D metabolite-DBP complexes are actively reabsorbed by the membrane receptor megalin and its partner cubulin (Leheste et al., 2003). As a result, DBP knockout mice have accelerated clearance of 25OHD from blood, reduced serum levels of 25OH D and 1,25(OH)2 D, and are more sensitive to dietary vitamin D deficiency that wild-type mice (Safadi et al., 1999). Studies on the metabolic clearance of various vitamin D analogs suggests that the affinity of a vitamin D metabolite or analog for DBP is correlated with the half-life in circulation (Dusso et al., 1991). However, DBP is not required for cellular uptake of vitamin D metabolites; uptake of 25OHD into the liver is faster (Safadi et al., 1999) and tissue accumulation of 1,25(OH)2 D is not altered (Zella et al., 2008) in DBP knockout mice. This suggests that cells either accumulate free vitamin D metabolites or that other serum proteins like albumin are involved in cellular vitamin D uptake (Chun et al., 2014).
A. Regulation of Vitamin D Metabolism or Action by Other Hormones
The renal production of 1,25(OH)2 D is strongly regulated by elevations in serum PTH that are initiated by transient changes in serum calcium levels such as those that occur with variations in dietary calcium intake (Boyle et al., 1971, Grubenmann et al., 1978, DeLuca, Seshadri et al., 1985). The changes in serum calcium levels are sensed by the calcium sensing receptor (CaSR), a glycoprotein that is a G-protein coupled receptor located on the cell surface of chief cells in the parathyroid gland (Hebert et al., 1997). When calcium binds to the CaR it activates several intracellular signaling enzymes including: phospholipase C which then activates protein kinase C, phospholipase D and A2 that mediate generation of arachadonic acid for regulation of cyclooxygenase and lipoxygenase pathways, and ERK, JNK, and p38 MAP kinases. Changes in ionized serum calcium can alter the PTH production at multiple levels (Juppner et al., 2000). PTH secretion increases within seconds of sensing hypocalcemia while intracellular PTH degradation is rapidly suppressed by hypocalcemia and increases in the amount of intact PTH available for secretion are present within 30 minutes. Finally, transcriptional regulation of the PTH gene and stabilization of the PTH mRNA occurs within hours of sensing low serum calcium levels.
PTH has several critical functions related to calcium homeostasis (i.e. promoting bone resorption (Silva and Bilezikian, 2015) and renal calcium reabsorption (Lau and Bourdeau, 1995, Moor and Bonny, 2016)). In addition, dietary calcium deprivation increases serum PTH levels and so high PTH is associated with increased calcium absorption during periods of low dietary calcium intake. However, the effect of PTH on calcium absorption is indirect and mediated through the ability of PTH to regulate renal vitamin D metabolism and control serum levels of the vitamin D hormone, 1,25 dihydroxyvitamin D (1,25(OH)2 D) (Zierold et al., 2003, Armbrecht et al., 2003). PTH stimulates CYP27B1 gene expression by binding to a cell surface receptor, increasing cAMP production, and activation of the cyclic AMP response element binding protein (CREB) transcription factor (Armbrecht et al., 2003). PTH can also reduce renal 1,25(OH)2 D degradation by reducing the half-life of the CYP24A1 mRNA (Zierold et al., 2003, Armbrecht et al., 2003). 1,25(OH)2 D is a potent suppressor of PTH gene expression (Nishishita et al., 1998) as well as expression of CYP27B1 (Turunen et al., 2007) leading to the feed-back inhibition of its own production. The vitamin D-mediated suppression of gene expression requires vitamin D response elements in the promoter of the PTH gene (Russell et al., 1999, Mackey et al., 1996) or CYP27B1 gene (Turunen et al., 2007) and, presumabley, recruitment co-repressors with histone deaceylase activity to keep these genes in a transcriptionally repressed state.
While many hormones have independent effects on calcium homeostasis, several hormones in addition to PTH indirectly influence calcium homeostasis through their effects on the regulation of vitamin D metabolism or action. During pregnancy, maternal calcium requirements increase significantly because of the skeletal development that occurs during the third trimester of pregnancy. As such, serum 1,25(OH)2 D levels are elevated during late pregnancy (Ritchie et al., 1998) due to PTH-independent, placenta 1,25(OH)2 D production (Breslau and Zerwekh, 1986). Increased calcium loss during lactation also has a large impact on maternal calcium homeostasis. During lactation, prolactin has direct effects on calcium homeostasis but can also influence it by increasing renal 1,25(OH)2 D production (Robinson et al., 1982). Growth hormone and its physiologic mediator insulin-like growth factor I control linear bone growth, bone mass accrual, and intestinal calcium absorption (Zhang et al., 2011, Fleet et al., 1994, Mora et al., 1999, Boot et al., 1997, Rudman et al., 1990). This is due to in part to activation of renal CYP27B1 and the elevation of serum 1,25(OH)2 D levels (Zoidis et al., 2002). Growth hormone can also prevent the loss of intestinal VDR that occurs in ovariectomized rats (Chen et al., 1997) suggesting that growth hormone increases the cell sensitivity to 1,25(OH)2 D by regulating tissue VDR levels. There are well-established negative effects of glucocorticoids treatment on bone (Weinstein, 2012) and calcium absorption (Hahn et al., 1981). For example, the association between serum 1,25(OH)2D and calcium absorption was blunted after corticosteroid treatment (Morris et al., 1990), suggesting the existence of glucocorticoid-induced intestinal resistance to vitamin D action. Several early reports showed that hypothyroidism increased (Lekkerkerker et al., 1971) and hyperthyroidism reduced (Haldimann et al., 1980) intestinal calcium absorption. This is an indirect effect of thyroid hormones mediated by transcriptional repression of CYP27B1 gene expression (Kozai et al., 2013) that alters serum 1,25(OH)2 D levels (Bouillon et al., 1980). Fibroblast growth factor 23 (FGF23) is a phosphatonin that controls Pi metabolism (see below) in part by suppressing renal CYP27B1 mRNA levels, CYP27B1 enzymatic activity, and 1,25(OH)2 D production (Shimada et al., 2001, Perwad et al., 2007). The FGF23-mediated suppression of serum 1,25(OH)2 D should strongly suppress intestinal calcium absorption, however no studies have been done to formally test this hypothesis.
Estrogen loss has many effects on calcium balance in post-menopausal women, e.g. increasing bone resorption, reducing calcium absorption and increasing in urinary calcium loss (Heaney et al., 1978, Riggs et al., 2002). Low estrogen levels seen in post-menopausal women are associated with reduced serum 1,25(OH)2 D level (Gallagher et al., 1980) but ovariectomy does not reduce serum 1,25(OH)2 D levels in rats (Pavlovitch et al., 1980). In contrast, oophorectomy reduced 1,25(OH)2 D-induced intestinal calcium absorption in young women and that this was reversed by estrogen repletion (Gennari et al., 1990). Other studies suggest that the impact of estrogen loss on the intestinal responsiveness to 1,25(OH)2 D is due to reduced VDR levels (Chen et al., 1997, Arjmandi et al., 1994, Liel et al., 1999), although the loss of tissue VDR levels following estrogen loss is not seen in all studies (Colin et al., 1999).
III. Critical Role of VDR in Control of Calcium Homeostasis
It is well established that the physiologic actions of 1,25(OH)2 D are mediated by transcriptional events resulting from activation of the nuclear vitamin D receptor, VDR (Christakos et al., 2016). The importance of VDR in whole body calcium homeostasis is clear from the disruption in calcium metabolism seen in people with type II genetic rickets resulting from inactivating mutations in the VDR gene (Tiosano et al., 2011) as well as in multiple VDR knockout mouse lines (Li et al., 1997, Yoshizawa et al., 1997, Van Cromphaut et al., 2001). Collectively, mouse models show that global VDR deletion causes a massive disruption in calcium metabolism in growing mice that includes severe hypocalcemia, elevated serum PTH and 1,25(OH)2 D levels, disruption of the growth plate causing growth arrest, and severe osteomalacia.
Several lines of evidence show that regulating intestinal calcium absorption is the single most important role for VDR during growth. First, VDR deletion causes a greater than 70% reduction in calcium absorption efficiency in growing mice (Van Cromphaut et al., 2001, Song et al., 2003). However, the abnormal calcium homeostasis seen in VDR knockout mice can be prevented by feeding a rescue diet with high lactose, high calcium, and high phosphorus levels that promotes vitamin D-independent calcium absorption (Amling et al., 1999). Second, intestine-specific VDR deletion results in a phenotype that is consistent with dietary calcium deficiency and the phenotype of the global VDR knockout mouse (i.e. osteomalacia, reduced serum calcium, elevated serum 1,25(OH)2 D and PTH levels) (Lieben et al., 2012). FInally, my group showed that intestine epithelium-specific, transgenic expression of VDR could restore normal calcium absorption efficiency to VDR knockout mice and this was sufficient to normalize serum PTH, serum calcium, and bone mineral density (Xue and Fleet, 2009).
While the presence of the VDR is an absolute requirement for efficient calcium absorption (Van Cromphaut et al., 2001, Song et al., 2003), intestinal VDR level or function may also be an important regulator of intestinal calcium absorption efficiency. Low intestinal VDR levels have been associated with the loss of basal and vitamin D-responsive calcium absorption during aging (Bullamore et al., 1970, Nordin et al., 2004, Ebeling et al., 1992) and after estrogen depletion (Chen et al., 1997, Gennari et al., 1990, Liel et al., 1999). In addition, my group and others have provided direct experimental evidence demonstrating the importance of VDR level in the control of vitamin D-regulated intestinal calcium absorption from studies using inducible over-expression of VDR in Caco-2 cells (Shao et al., 2001) and from mouse studies that show intestinal resistance to 1,25(OH)2 D action when intestinal VDR levels are genetically manipulated to be low (i.e. 10% normal (Lieben et al., 2015), 50% normal (Song and Fleet, 2007)). Altered VDR function can also impair intestinal calcium absorption. People with the longer, less transcriptionally active VDR encoded by the “f” allele of the Fok I gene polymorphism have reduced calcium absorption efficiency compared to individuals with longer VDR encoded by the “F” allele (Ames et al., 1999, Jurutka et al., 2000, Huang et al., 2006). These data show that alterations in VDR level and/or function can impact intestinal calcium absorption and the intestinal responses to the increased serum 1,25(OH)2 D levels that result from habitual low dietary calcium intake.
In addition to the critical role of VDR signaling for the control of intestinal calcium absorption, there is evidence that VDR is also an important regulator of calcium homeostasis at the level of the kidney and bone. In the kidney, the VDR is crucial for the control of 1,25(OH)2 D production (Wang et al., 2015) where it is involved in the transcriptional upregulation of CYP24A1 (Iida et al., 1993), the suppression of CYP27B1 expression in the proximal convoluted tubule (PCT) (Turunen et al., 2007), and the suppression of PTH production (Nishishita et al., 1998). The loss of these functions explains why serum 1,25(OH)2 D levels are so high in VDR knockout mice (Li et al., 1997, Yoshizawa et al., 1997, Van Cromphaut et al., 2001). In addition to the role of VDR in renal vitamin D metabolism, Li et al. (Li et al., 2001) showed that urinary excretion of calcium was not different between 3-month old wild-type and VDR knockout mice fed a chow diet (1% calcium, 0.85% phosphorus) even though the knockout mice had severe hypocalcemia, indicating poor renal calcium reabsorption in VDR knockout mice despite signals that should promote urinary calcium retention. Consistent with this hypothesis, feeding VDR knockout mice the high calcium rescue diet (2% calcium, 1.2% phosphorus) for one week normalized serum calcium but urinary calcium was increased 2-fold relative to wild-type mice. Similar to this, we found that urinary calcium was lower in hypocalcemic, 2-month old VDR knockout mice but it was elevated 3-fold in VDR knockout mice where intestine-specific transgenic expression of human VDR restored intestinal calcium absorption and normalized serum calcium levels (Xue and Fleet, 2009). This is consistent with the hypothesis that vitamin D stimulates renal calcium reabsorption and that the loss of VDR eliminates this regulatory pathway.
Our study in VDR knockout mice with intestine-specific, transgenic VDR expression also revealed an interesting bone phenotype. Rather than simply normalizing bone phenotypes to levels seen in control mice, a number of femoral bone phenotypes were increased by 13–18% (e.g. bone mineral density, cortical or trabecular bone area) (Xue and Fleet, 2009). This could be explained by several studies on of the effects of VDR deletion in osteoblasts. First, VDR could release a constraint on osteoblast differentiation. This was shown by Sooy et al. (Sooy et al., 2005) who reported that calvarial osteoblast progenitors from VDR knockout mice could form more bone in vitro, a effect they proposed was due to releasing vitamin D-mediated suppression of the transcription factor RUNX2 (Drissi et al., 2002). Second, osteoblasts from VDR knockout mice also have defective signaling to osteoclasts. Normally, osteoblasts induce osteoclast differentiation when RANK (receptor for the activation of the NFκβ) on the cell surface of osteoclast progenitors is activated by RANKL (RANK ligand), a cell surface ligand expressed on the surface of osteoblasts (Boyle et al., 2003). RANKL action can be antagonized by osteoprotegerin (OPG), a secreted protein produced by osteoblasts that binds to RANK, blocks RANKL binding to RANK, and prevents RANKL-mediated activation of osteoclast differentiation. 1,25(OH)2 D activates this system by inducing RANKL expression (Kitazawa et al., 2008) and suppressing OPG expression (Notoya et al., 2004, Lee et al., 2002). Thus, VDR deletion in osteoblasts could increase bone mass by reducing osteoclast production. Consistent with this, Yamamoto et al. (Yamamoto et al., 2013) found that osteoblast-specific deletion of VDR in mice cause a 20% increase in bone mass and this was associated with reduced bone resorption (e.g. 30% lower osteoclast surface) and a 50% reduction in bone RANKL expression in VDR knockout mice.
The studies from VDR knockout mice that I discussed above clearly link vitamin D signaling through the VDR to all three tissues critical to the control of calcium homeostasis. In the next sections I will discuss specific mechanisms of vitamin D action at the intestine and in the kidney. Another review in this special issue will discuss the role of vitamin D signaling in bone.
IV. Vitamin D-Mediated Regulation of Intestinal Calcium Absorption
In 1937 Nicolaysen first reported the dependence of intestinal calcium absorption on vitamin D in rats (Nicolaysen). Others later showed that intestinal calcium absorption efficiency is reduced by more than 75% during vitamin D deficiency (Pansu et al., 1983, Sheikh et al., 1988). Studies in humans show that calcium malabsorption occurs in the later stages of vitamin D deficiency (serum 25OH D levels < 10 nmol/L) (Need et al., 2008) when secondary hyperparathyroidism can’t maintain serum 1,25(OH)2 D (and calcium absorption) presumably because there is not enough 25OH D for conversion to 1,25(OH)2 D. Intestinal calcium absorption efficiency also falls dramatically with aging and this decline is due to the age-related fall in serum 1,25(OH)2 D levels as well as intestinal resistance to the actions of 1,25(OH)2 D (Wood et al., 1998, Pattanaungkul et al., 2000, Scopacasa et al., 2004)
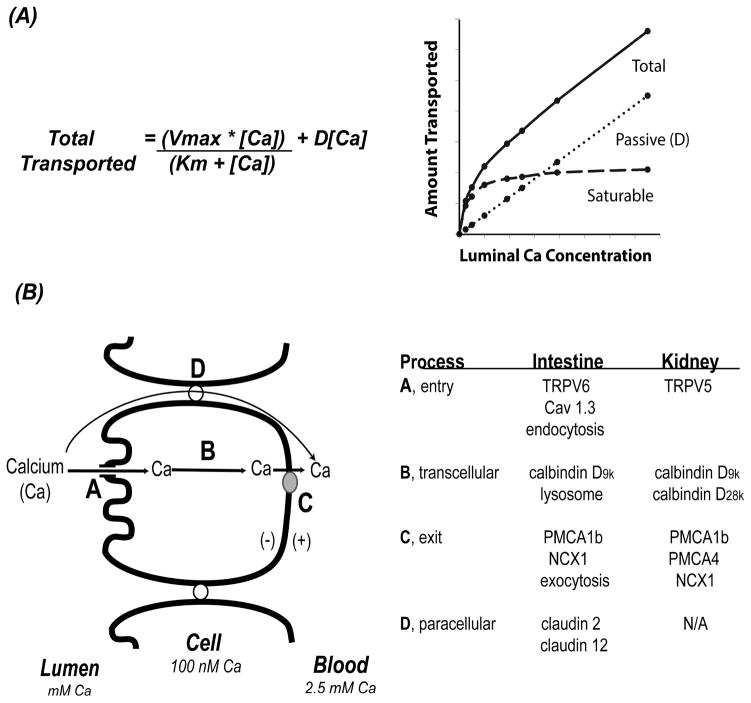
By examining the efficiency of absorption across a wide range of luminal calcium concentrations, it’s clear that the transfer of calcium across the intestinal barrier occurs through both saturable and non-saturable pathways that can be modeled mathematically using a modified Michaelis-Menten equation (Wasserman and Taylor, 1969, Pansu et al., 1981, Heaney et al., 1975, Sheikh et al., 1990) (see Figure 2A). Data from studies using ion microscopy on chick intestine (Chandra et al., 1990, Fullmer et al., 1996), in situ ligated loops of rat duodenum (Pansu et al., 1983), and differentiated monolayers of the human intestinal cell line Caco-2 (Giuliano and Wood, 1991) show that the saturable component of duodenal calcium absorption is transcellular and 1,25(OH)2 D-regulated. 1,25(OH)2 D increases Vmax (the maximal capacity of transport) consistent with an increase in the production of intestinal calcium transporters. Saturable calcium absorption is an energy dependent pathway (Favus et al., 1983) that is most prevalent in the duodenum and jejunum. The saturable pathway is absent in the ileum (Pansu et al., 1983) but studies show that vitamin D regulated calcium absorption also occurs in the in the large intestine (Favus et al., 1981, Favus and Langman, 1984, Karbach and Rummel, 1987, Karbach and Feldmeier, 1993, Barger-Lux et al., 1989) and that VDR expression in these segments are necessary for normal calcium homeostasis (Christakos et al., 2016, Reyes-Fernandez and Fleet, 2016).
Figure 2. Models for intestinal calcium absorption and renal calcium reabsorption.
(A) Kinetic modeling of intestinal mineral absorption shows that both saturable and non-saturable pathways exist. Total transport is the sum of a linear, concentration-dependent, non-saturable transport process (defined by a straight line) and a saturable component that can be defined by the Michaelis-Menton equation. [Ca] = luminal concentration of calcium; D = the slope of the non-saturable linear component assuming the intercept equals zero; Vmax = the maximum transport rate seen for the saturable transport component; Km = the luminal concentration of calcium at ½ the Vmax. (B) A summary of models for calcium absorption across the intestinal barrier. Potential mechanisms for entry, A, intracellular movement, B, and exit, C, from the cells are provided in the table for the intestine and kidney. D = the paracellular movement; N/A = not applicable.
The non-saturable component of calcium absorption is a linear function of luminal calcium concentration and it occurs throughout the length of the intestine (at 13% of luminal load per hour in humans (Sheikh et al., 1990)). There is some evidence that the non-saturable portion of calcium absorption in the human ileum is also vitamin D sensitive (Sheikh et al., 1990). Under adequate-to-high calcium intakes, the proportion of calcium transported in any given segment is determined by the presence of the saturable and non-saturable pathways, the residence time in the segment, and the solubility of calcium within the intestinal segment. As a result, even though calcium solubility is low and the saturable pathway is absent or very low, the total amount of calcium absorption is greatest in the ileum since the residence time in this segment is 10 times longer than in the more proximal intestinal segments (Marcus and Lengemann, 1962, Duflos et al., 1995).
A. Molecular Models of Vitamin D Regulated calcium absorption
Several models have been proposed to describe the mechanism for vitamin D mediated intestinal calcium absorption.
1. Facilitated Diffusion
In 1986 Bronner et al. (Bronner et al., 1986) summarized the data supporting the facilitated diffusion model (Figure 2B). They argued that the kinetic analysis of transcellular transport, brush border membrane uptake, and basolateral membrane extrusion identified the intracellular diffusion of calcium across the cytoplasm as the rate limiting step in calcium absorption. Subsequent research has both supported and refuted key aspects of this model.
Vitamin D-dependent brush border membrane calcium uptake is thought to be mediated by the transient receptor potential cation channel vanilloid family member 6 (TRPV6 aka CaT1 or ECAC2) (Peng et al., 1999). The gene for this apical membrane calcium channel is regulated by 1,25(OH)2 D (Meyer et al., 2007, Fleet et al., 2002) and its induction precedes the increase in duodenal calcium absorption that occurs following 1,25(OH)2 D injection (Song et al., 2003). In addition, intestine-specific transgenic expression of TRPV6 alone can increase calcium absorption efficiency and prevent abnormal calcium homeostasis in VDR knockout mice (Cui et al., 2012). However, 1,25(OH)2 D-induced intestinal calcium absorption was not reduced in TRPV6 knockout mice (Kutuzova et al., 2008, Benn et al., 2008) even though the induction of calcium absorption caused by low calcium intake was reduced by 40% in TRPV6 knockout mice (Pickert et al., 2009) and in mice with a non-functional D541A variant TRPV6 (Woudenberg-Vrenken et al., 2012). Because of the inconsistent data in TRPV6 knockout mice, the L-type calcium channel Cav1.3 was proposed as an alternative channel for apical membrane calcium uptake (Kellett). However, several studies do not support an essential role for Cav1.3 in either basal or vitamin D-stimulated intestinal calcium absorption. (Xue and Fleet, 2009, Reyes-Fernandez and Fleet, 2015).
Bronner et al. (Bronner et al., 1986) proposed that the key mediator of intracellular diffusion of calcium during absorption is the cytoplasmic calcium binding protein calbindin D (calbindin D9k in mammals, calbindin D28k in chicks) (Christakos et al., 1992). Intestinal calbindin levels positively correlate to calcium absorption over a wide range of biological conditions (Bronner et al., 1986) and their levels change in response to vitamin D deficiency, 1,25(OH)2 D injection, and VDR deletion (Van Cromphaut et al., 2001, Song et al., 2003, Wasserman and Taylor, 1966), suggesting the calbindin D9k and D28k genes might be vitamin D target genes mediating the effect of the hormone on calcium absorption (Bronner and Buckley, 1982). However several lines of evidence suggest that calbindins are more likely intracellular calcium buffers than intracellular calcium ferries. First, calbindin D protein remained high even after 1,25(OH)2 D-induced calcium absorption had returned to normal in chicks (Spencer et al., 1978) and mice (Song et al., 2003) and neither basal nor 1,25(OH)2 D-induced calcium absorption are reduced in calbindin D9k null mice (Benn et al., 2008, Akhter et al., 2007). This suggests that calbindin levels alone are neither sufficient nor necessary to drive intestinal calcium absorption. However, high calcium absorption resulting from intestine-specific, transgenic TRPV6 expression increases intestinal calbindin D9k levels even in VDR knockout mice (Cui et al., 2012). This suggests that calbindin D9k is an intracellular calcium buffer that increases in response to elevated transcellular calcium absorption and that it is not a facilitator of transcellular calcium movement.
The final step in the facilitated diffusion model is the extrusion of calcium from the cell. This is an energy dependent process (Favus et al., 1983) that is mediated by the plasma membrane calcium ATPase 1b (PMCA1b). PMCA1b expression is reduced by vitamin D deficiency and increased by vitamin D repletion and low dietary calcium intake (Wasserman et al., 1992, Cai et al., 1993). Deletion of PMCA1b (Atp2b1) or 4.1R, a protein that stabilizes PMCA1b in the basolateral membrane, reduces both basal and 1,25(OH)2 D-induced intestinal calcium absorption (Liu et al., 2013, Ryan et al., 2015). Some have proposed that basolateral calcium extrusion may also occur through a sodium-calcium exchanger (van Corven et al., 1985) but sodium-potassium pump inhibitors that disrupt the sodium gradient necessary for sodium-calcium exchange did not reduce duodenal calcium transport in rats (Favus et al., 1983).
2. Vesicular Transport
Sequestering calcium into vesicles within the cell is an alternative to the ferry/buffer role proposed for calbindin D during transcellular intestinal calcium absorption (Figure 2B). In support of this model, several groups have reported that 1,25(OH)2 D treatment increased the number of lysosomes in chick intestine (Davis and Jones, 1982), the release of lysosomal enzymes from isolated rat enterocytes (Nemere and Szego, 1981), the activity and cycling of lysosomes (Warner and Coleman, 1975), and the level of lysosomal calcium (Nemere et al., 1986). Also, calcium has been associated with endosomes in the brush border region of intestinal epithelial cells prior to its appearance in lysosomes during calcium absorption, (Nemere and Norman, 1988). These observations may explain why preventing lysosomal calcium accumulation with lysosomal pH disrupting agents blocks vitamin D-mediated calcium absorption (Nemere et al., 1986) through a mechanism that is independent of ATP-mediated calcium extrusion (Favus et al., 1989). Calbindin D28k was reported in chick intestinal endosomes and lysosomes after 1,25(OH)2 D treatment (Nemere et al., 1986) but calbindin D9k hasn’t been reported in endosomes from mammalian intestinal epithelial cells. As a result, although these data suggest that vesicular movement is a legitimate pathway for uptake and movement of calcium through intestinal epithelial cells, it isn’t clear what makes vesicular transport specific for calcium.
3. Transcaltachia
The facilitated diffusion model for vitamin D-mediated calcium absorption requires transcriptional events mediated through the VDR. In contrast, transcaltachia has been described as the rapid absorption of calcium that occurs within minutes of exposing chick enterocytes to 1,25(OH)2 D (Nemere et al., 1984). Transcaltachia occurs only in response to serosal 1,25(OH)2 D exposure which suggests that there is a membrane receptor on the basolateral surface in absorptive epithelial cells. Some research suggests transcaltachia represents a novel, non-nuclear role for the vitamin D receptor (Huhtakangas et al., 2004), a hypothesis supported by the observation that mouse intestinal VDR was found associated with caveolae (Huhtakangas et al., 2004), from structural modeling showing an alternative ligand binding pocket (Mizwicki et al., 2004), and from studies showing that specific vitamin D analogs designed for the alternative binding pocket can stimulate transcaltachia in chick intestine (Norman et al., 2002). Alternatively, transcaltachia may be regulated by the membrane associated rapid response steroid binding protein (MARRS) (Nemere et al., 2004), a multi-functional protein that regulates glucose sensing and is also known as ERp57, PLCα, or PDIA3. Because MARRS has multiple functions, it is difficult to distinguish whether its role in glucose sensing has an indirect impact on cellular calcium movement. With that in mind, intestine-specific deletion of MARRS in mice reduced cellular 1,25(OH)2 D binding, disrupted 1,25(OH)2 D regulated calcium and phosphate uptake into enterocytes (Nemere et al., 2010, Nemere et al., 2012), and reduced basal calcium absorption in by 30% (Nemere et al., 2012). Unfortunately, two essential pieces of data are missing and this has limited the acceptance of transcaltachia as physiologically important pathway for calcium homeostasis. First, no one has reported the existence of rapid fluxes in serum 1,25(OH)2 D needed for transcaltachia, particularly during the consumption of calcium-rich meals when transcaltachia would have to occur for the physiologic benefit of the process to be realized. Second, there have been no reported adverse effects of MARRS deletion on bone, despite the critical importance of intestinal calcium absorption for normal bone growth (Xue and Fleet, 2009).
4. Regulated Paracellular Movement through Tight Junctions
In addition to the models for transcellular calcium absorption, some studies have shown that vitamin D signaling increases diffusional, presumably paracellular fluxes across the intestine, particularly in the jejunum and ileum (Sheikh et al., 1990, Karbach). By using Ussing chambers on rat duodenum, Tudpor et al. (Tudpor et al., 2008) found that 1,25(OH)2 D induced ion movement and transepithelial electrical resistance (TEER) without affecting manitol flux. This suggests that the effect of vitamin D was due to a change in the charge selectivity of the tight junction. Fujita et al. (Fujita et al., 2008) proposed that this effect was due 1,25(OH)2 D-mediated induction of the tight junction proteins claudin 2 and claudin 12 (Figure 2B). The mRNA levels for these proteins fall dramatically in the jejunum of VDR knockout mice, and siRNA against claudin 2 or 12 can reduce calcium permeability in Caco-2 cell monolayers. However, claudin 2 and 12 expression is highest in the ileum (Fujita et al., 2006) and in the undifferentiated crypt cells of the small intestine (Rahner et al., 2001). Thus, these proteins are not present in the intestinal segments (duodenum) or differentiated epithelial cells where 1,25(OH)2 D regulates the saturable component of calcium absorption. This suggests that their impact may be on the linear diffusional component of calcium transport, which may explain why the non-saturable component of ileal calcium absorption is reduced in chronic renal disease patients with low serum 1,25(OH)2 D levels (Sheikh et al., 1990).
V. Vitamin D-Mediated Regulation of Calcium Transport Across Renal Epithelial Cells
The ability of the kidney to reabsorb calcium from the renal filtrate is highly efficient; approximately 1400 liters of blood containing 135 grams of calcium is filtered through the kidney each day yet only a liter or two of urine containing 175 mg of calcium is made each day. Renal calcium reabsorption occurs through a number of mechanisms that are unique to each segment of the kidney tubule – the full complexity of this process was recently reviewed by Moor and Bonny (Moor and Bonny, 2016). Approximately 90% of renal calcium reabsorption occurs in the proximal tubule and thick ascending limb of the loop of Henle. However, the primary location for vitamin D mediated renal calcium reabsorption is the distal convoluted tubule (DCT), a segment that account for about 8% of total renal calcium reabsorption.
The movement of calcium across the DCT epithelium has many similarities to what has already been described for the intestinal epithelium except that paracellular transport is minimal due to the expression of claudins that do not have cation pore properties (Hou et al., 2013) (Figure 2B). Like intestinal calcium absorption, DCT calcium reabsorption is thought be mediated by three steps: apical membrane uptake, facilitated intracellular diffusion, and ATP-dependent calcium extrusion. The entry of free calcium into the cell is primarily through transient receptor potential cation channel subfamily V member 5 (TRPV5, aka CaT2 or ECAC1) (Hoenderop et al., 1999, Hoenderop et al., 2000, Peng et al., 2000). Deletion of TRPV5 in mice has physiologic effects that are less extreme than the VDR knockout mouse (Hoenderop et al., 2003). While TRPV5 deletion increased urinary calcium excretion by 4-fold, the mice grew normally and had normal serum calcium levels. This is because calcium homeostasis adapted to increase serum 1,25(OH)2 D by 2-fold leading to a 50% increase in intestinal calcium absorption and increases in osteoclast surface that reduced bone mass significantly. This is similar to what happens to calcium metabolism in klotho knockout mice (Alexander et al., 2009). Klotho is normally a transmembrane protein that serves as a co-receptor for the FGF receptor (FGFR1c in proximal tubular epithelial cells) that mediates the cellular actions of FGF23 to control phosphate metabolism (Erben). Some of the effects of klotho deficiency are due to the loss of FGF23-mediated suppression of renal 1,25(OH)2 D production, accounting for elevated serum 1,25(OH)2 D levels in klotho knockout mice (Alexander et al., 2009). However, a soluble form of α klotho can be generated when α and β secretases cleave the membrane form of klotho. Cell based studies show that soluble klotho can act as a β glucuronidase to hydrolyze TRPV5-associated carbohydrates thereby increasing TRPV5 mediated calcium uptake by 178% in HEK293 cells (Chang et al., 2005). Additional studies showed that glycosylation of TRPV5 prevents accumulation of TRVP5 in the plasma membrane but doesn’t change its ability to transport calcium. While this suggests an essential, vitamin D-independent role of soluble klotho in renal calcium reabsorption (i.e. consistent with data showing elevated urinary calcium in mice fed low vitamin D diets that prevent klotho-deletion associated elevations in serum 1,25 (OH)2 D (Alexander et al., 2009)), the physiologic significance of soluble klotho isn’t yet secure since it is not clear how production of soluble klotho is regulated (Xu and Sun, 2015).
Despite the critical importance of TRPV5 in DCT calcium transport and the fact that a pharmacologic dose of 1,25(OH)2 D increased TRPV5 mRNA by 6-fold (Song et al., 2003), the effect of altering dietary calcium levels on TRPV5 is small and VDR deletion actually increased renal TRPV5 mRNA levels (Song et al., 2003). This suggests that the primary action of vitamin D on renal calcium reabsorption is not through transcriptional regulation of TRPV5.
Intracellular calcium fluxes during renal calcium reabsorption are thought to be controlled by calbindins but their role has been more difficult to determine because, unlike humans that express only calbindin D28k in the kidney, mice expression both calbindin D9k and calbindin D28k (Christakos et al., 1992). Of the two forms found in mouse kidney, calbindin D9k is more sensitive to VDR deletion (completely lost vs reduced 60%), dietary calcium restriction (increased 100% vs no change), and 1,25(OH)2 D injection (increased 6-fold vs 2.5 fold) compared to calbindin D28k (Song et al., 2003, Song et al., 2003). Although calbindin D9k (Akhter et al., 2007) and calbindin D28k knockout mice grow normally have no disruption of calcium homeostasis, calbindin D28k/calbindin D9k double knockout mice are more sensitive to the effects of a low calcium diet (Ko et al., 2009), suggesting an essential role for the calcium binding function of these proteins in physiology. Because TRPV5 activity is rapidly suppressed when intracellular calcium levels increase (Hoenderop et al., 2001), renal calbindins may buffer increases in intracellular calcium that would down-regulate TRPV5.
The final step in the transcellular calcium transport is the active extrusion of calcium. This may be mediated by the action of plasma membrane calcium ATPase (PMCA) family members or by a sodium-calcium exchanger (NCX1). Although both the activity and amount of the PMCAs are regulated by 1,25(OH)2 D (Pannabecker et al., 1995), this step appears to be due to effects on PMCA1b mRNA stability (Glendenning et al., 2000). Although all of the work on vitamin D and PMCA has been done on PMCA1b, some have suggested that PMCA4 is important (van der Hagen et al., 2014). However, a recent study shows that PMCA4 knockout mice don’t experience renal calcium wasting, demonstrating that it is not essential for renal calcium reabsorption (van Loon et al., 2016). While NCX1 is important for renal calcium reabsorption in the DCT, its expression is not regulated by the loss of vitamin D signaling in CYP27B1 knockout mice (Hoenderop et al., 2002), vitamin D deficiency in wild-type mice (Ko et al., 2009) or VDR deletion in normal rat kidney cells (Xi et al., 2011).
VI. Summary and Conclusions
Since the initial discovery of vitamin D as an essential nutrient for normal growth and bone development by McCollum in 1922 (McCollum et al., 1922), we have learned a remarkable amount about how vitamin D is produced in skin, how it is metabolized to the active hormone 1,25(OH)2 D, and how that hormone regulates genes that control calcium homeostasis. This this review, I have reviewed the data that shows the importance for vitamin D and signaling through the VDR in the control of intestinal calcium absorption, renal calcium metabolism, bone metabolism, and even vitamin D metabolism. The evidence shows that the most important role that vitamin D plays during growth is the control of calcium absorption.
Highlights.
Calcium homeostasis controls serum calcium levels within a narrow range
The vitamin D endocrine system controls whole body calcium homeostasis
Habitual dietary calcium intake and physiologic states control vitamin D metabolism
The primary role of vitamin D is to regulate intestinal calcium absorption.
Vitamin D also regulates urinary calcium excretion and bone metabolism
Acknowledgments
Funding: This review did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors
Abbreviations
- 1,25(OH)2 D
1,25 dihydroxyvitamin D
- 25OH D
25 hydroxyvitamin D
- CaSR
calcium sensing receptor
- CYP24A1
25 hydroxyvitamin D, 24 hydroxylase
- CYP27B1
25 hydroxyvitamin D, 1α hydroxylase
- DBP
vitamin D binding protein
- DCT
distal convoluted tubule
- ECF
extracellular fluid
- FGF23
fibroblast growth factor 23
- MARRS
membrane associated rapid response steroid binding protein
- NCX1
sodium-calcium exchanger 1
- OPG
osteoprotegerin
- PTH
parathyroid hormone
- PCT
proximal convoluted tubule
- PMCA
plasma membrane calcium ATPase
- RANK
receptor for the activation of NFκβ
- RANKL
ligand for RANK
- TRPV5
transient receptor potential cation channel subfamily V member 5
- TRPV6
transient receptor potential cation channel subfamily V member 6
- VDR
vitamin D receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Houillier P, et al. What serum calcium can tell us and what it can’t. Nephrol Dial Transplant. 2006;21:29–32. doi: 10.1093/ndt/gfi268. [DOI] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 2007;131:1047–58. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Wastney ME, et al. Differences in calcium kinetics between adolescent girls and young women. Am J Physiol. 1996;271:R208–R216. doi: 10.1152/ajpregu.1996.271.1.R208. [DOI] [PubMed] [Google Scholar]
- Bronner F, Aubert JP. Bone metabolism and regulation of the blood calcium level in rats. Am J Physiol. 1965;209:887–90. doi: 10.1152/ajplegacy.1965.209.5.887. [DOI] [PubMed] [Google Scholar]
- Adams ND, et al. The effects of oral CaCO3 loading and dietary calcium deprivation on plasma 1,25-dihydroxyvitamin D concentrations in healthy adults. J Clin Endocrinol Metab. 1979;48:1008–16. doi: 10.1210/jcem-48-6-1008. [DOI] [PubMed] [Google Scholar]
- Song Y, et al. Calcium transporter 1 and epithelial calcium channel messenger ribonucleic acid are differentially regulated by 1,25 dihydroxyvitamin D3 in the intestine and kidney of mice. Endocrinology. 2003;144:3885–94. doi: 10.1210/en.2003-0314. [DOI] [PubMed] [Google Scholar]
- Webb AR, et al. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67:373–378. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- Hewison M, et al. 1alpha-Hydroxylase and the action of vitamin D. J Mol Endocrinol. 2000;25:141–148. doi: 10.1677/jme.0.0250141. [DOI] [PubMed] [Google Scholar]
- White P, Cooke N. The multifunctional properties and characteristics of vitamin D-binding protein. Trends Endocrinol Metab. 2000;11:320–327. doi: 10.1016/s1043-2760(00)00317-9. [DOI] [PubMed] [Google Scholar]
- Leheste JR, et al. Hypocalcemia and osteopathy in mice with kidney-specific megalin gene defect. FASEB J. 2003;17:247–249. doi: 10.1096/fj.02-0578fje. [DOI] [PubMed] [Google Scholar]
- Safadi FF, et al. Ostopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. J Clin Invest. 1999;103:239–251. doi: 10.1172/JCI5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusso AS, et al. On the mechanisms for the selective action of vitamin D analogs. Endocrinology. 1991;128:1687–92. doi: 10.1210/endo-128-4-1687. [DOI] [PubMed] [Google Scholar]
- Zella LA, et al. Vitamin D-binding protein influences total circulating levels of 1,25-dihydroxyvitamin D3 but does not directly modulate the bioactive levels of the hormone in vivo. Endocrinology. 2008;149:3656–3667. doi: 10.1210/en.2008-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun RF, et al. Vitamin D and DBP: the free hormone hypothesis revisited. J Steroid Biochem Mol Biol. 2014;144(Pt A):132–7. doi: 10.1016/j.jsbmb.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle IT, et al. Regulation by calcium of in vivo synthesis of 1,25-dihydroxycholecalciferol and 21,25-dihydroxycholecalciferol. Proc Natl Acad Sci U S A. 1971;68:2131–4. doi: 10.1073/pnas.68.9.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubenmann W, et al. Effects of calcium intake and renal function on plasma immunoreactive parathyroid hormone levels in rats. Horm Metab Res. 1978;10:438–43. doi: 10.1055/s-0028-1093409. [DOI] [PubMed] [Google Scholar]
- DeLuca HF. The vitamin D system in the regulation of calcium and phosphorus metabolism. Nutr Rev. 1979;37:161–93. doi: 10.1111/j.1753-4887.1979.tb06660.x. [DOI] [PubMed] [Google Scholar]
- Seshadri MS, et al. Bioactive parathyroid hormone in the rat: effects of calcium and calcitriol. Endocrinology. 1985;117:2417–23. doi: 10.1210/endo-117-6-2417. [DOI] [PubMed] [Google Scholar]
- Hebert SC, et al. Role of the Ca(2+)-sensing receptor in divalent mineral ion homeostasis. J Exp Biol. 1997;200(Pt 2):295–302. doi: 10.1242/jeb.200.2.295. [DOI] [PubMed] [Google Scholar]
- Juppner H, et al. Parathyroid hormone and parathyroid hormone-related peptide in the regulation of calcium homeostasis and bone development. In: DeGroot L, Jameson J, editors. Endocrinology. W.B. Saunders Company; Philadelphia, PA: 2000. pp. 969–998. [Google Scholar]
- Silva BC, Bilezikian JP. Parathyroid hormone: anabolic and catabolic actions on the skeleton. Curr Opin Pharmacol. 2015;22:41–50. doi: 10.1016/j.coph.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau K, Bourdeau JE. Parathyroid hormone action in calcium transport in the distal nephron. Curr Opin Nephrol Hypertens. 1995;4:55–63. doi: 10.1097/00041552-199501000-00008. [DOI] [PubMed] [Google Scholar]
- Moor MB, Bonny O. Ways of calcium reabsorption in the kidney. Am J Physiol Renal Physiol. 2016;310:F1337–50. doi: 10.1152/ajprenal.00273.2015. [DOI] [PubMed] [Google Scholar]
- Zierold C, et al. Regulation of 25-hydroxyvitamin D3-24-hydroxylase mRNA by 1,25-dihydroxyvitamin D3 and parathyroid hormone. J Cell Biochem. 2003;88:234–237. doi: 10.1002/jcb.10341. [DOI] [PubMed] [Google Scholar]
- Armbrecht HJ, et al. Hormonal regulation of 25-hydroxyvitamin D3-1alpha-hydroxylase and 24- hydroxylase gene transcription in opossum kidney cells. Arch Biochem Biophys. 2003;409:298–304. doi: 10.1016/s0003-9861(02)00636-7. [DOI] [PubMed] [Google Scholar]
- Armbrecht HJ, et al. PTH increases renal 25(OH)D3-1alpha -hydroxylase (CYP1alpha) mRNA but not renal 1,25(OH)2D3 production in adult rats. Am J Physiol Renal Physiol. 2003;284:F1032–F1036. doi: 10.1152/ajprenal.00306.2002. [DOI] [PubMed] [Google Scholar]
- Nishishita T, et al. A negative vitamin D response DNA element in the human parathyroid hormone-related peptide gene binds to vitamin D receptor along with Ku antigen to mediate negative gene regulation by vitamin D. J Biol Chem. 1998;273:10901–10907. doi: 10.1074/jbc.273.18.10901. [DOI] [PubMed] [Google Scholar]
- Turunen MM, et al. Selective use of multiple vitamin D response elements underlies the 1 alpha,25-dihydroxyvitamin D-3-mediated negative regulation of the human CYP27B1 gene. Nucleic Acids Res. 2007;35:2734–2747. doi: 10.1093/nar/gkm179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J, et al. Vitamin D receptor interactions with the rat parathyroid hormone gene: synergistic effects between two negative vitamin D response elements. J Bone Miner Res. 1999;14:1828–1837. doi: 10.1359/jbmr.1999.14.11.1828. [DOI] [PubMed] [Google Scholar]
- Mackey SL, et al. Vitamin D receptor binding to the negative human parathyroid hormone vitamin D response element does not require the retinoid X receptor. Mol Endocrinol. 1996;10:298–305. doi: 10.1210/mend.10.3.8833658. [DOI] [PubMed] [Google Scholar]
- Ritchie LD, et al. A longitudinal study of calcium homeostasis during human pregnancy and lactation and after resumption of menses. Am J Clin Nutr. 1998;67:693–701. doi: 10.1093/ajcn/67.4.693. [DOI] [PubMed] [Google Scholar]
- Breslau NA, Zerwekh JE. Relationship of estrogen and pregnancy to calcium homeostasis in pseudohypoparathyroidism. J Clin Endocrinol Metab. 1986;62:45–51. doi: 10.1210/jcem-62-1-45. [DOI] [PubMed] [Google Scholar]
- Robinson CJ, et al. Role of Prolactin in Vitamin-D Metabolism and Calcium-Absorption During Lactation in the Rat. J Endocrinol. 1982;94:443–453. doi: 10.1677/joe.0.0940443. [DOI] [PubMed] [Google Scholar]
- Zhang Q, et al. Insulin-like growth factor-1 increases bone calcium accumulation only during rapid growth in female rats. J Nutr. 2011;141:2010–6. doi: 10.3945/jn.111.142679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleet JC, et al. Growth hormone and parathyroid hormone stimulate intestinal calcium absorption in aged female rats. Endocrinology. 1994;134:1755–60. doi: 10.1210/endo.134.4.8137740. [DOI] [PubMed] [Google Scholar]
- Mora S, et al. Serum levels of insulin-like growth factor I and the density, volume, and cross-sectional area of cortical bone in children. J Clin Endocrinol Metab. 1999;84:2780–2783. doi: 10.1210/jcem.84.8.5874. [DOI] [PubMed] [Google Scholar]
- Boot AM, et al. Changes in bone mineral density, body composition, and lipid metabolism during growth hormone (GH) treatment in children with GH deficiency. J Clin Endocrinol Metab. 1997;82:2423–2428. doi: 10.1210/jcem.82.8.4149. [DOI] [PubMed] [Google Scholar]
- Rudman D, et al. Effects of human growth hormone in men over 60 years old. N Engl J Med. 1990;323:1–6. doi: 10.1056/NEJM199007053230101. [DOI] [PubMed] [Google Scholar]
- Zoidis E, et al. IGF-I and GH stimulate Phex mRNA expression in lungs and bones and 1,25-dihydroxyvitamin D(3) production in hypophysectomized rats. Eur J Endocrinol. 2002;146:97–105. doi: 10.1530/eje.0.1460097. [DOI] [PubMed] [Google Scholar]
- Chen C, et al. Modulation of intestinal vitamin D receptor by ovariectomy, estrogen and growth hormone. Mech Ageing Dev. 1997;99:109–122. doi: 10.1016/s0047-6374(97)00094-8. [DOI] [PubMed] [Google Scholar]
- Weinstein RS. Glucocorticoid-induced osteoporosis and osteonecrosis. Endocrinol Metab Clin North Am. 2012;41:595–611. doi: 10.1016/j.ecl.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn TJ, et al. Effects off short term glucocorticoid administration on intestinal calcium absorption and circulating vitamin D metabolite concentrations in man. Journal of Clinical Endocrinology & Metabolism. 1981;52:111–5. doi: 10.1210/jcem-52-1-111. [DOI] [PubMed] [Google Scholar]
- Morris HA, et al. Malabsorption of calcium in corticosteroid-induced osteoporosis. Calcif Tissue Int. 1990;46:305–308. doi: 10.1007/BF02563820. [DOI] [PubMed] [Google Scholar]
- Lekkerkerker JF, et al. Enhancement of calcium absorption in hypothyroidism. Observations with a new method measuring calcium absorption. Isr J Med Sci. 1971;7:399–400. [PubMed] [Google Scholar]
- Haldimann B, et al. Intestinal calcium absorption in patients with hyperthyroidism. Journal of Clinical Endocrinology & Metabolism. 1980;51:995–7. doi: 10.1210/jcem-51-5-995. [DOI] [PubMed] [Google Scholar]
- Kozai M, et al. Thyroid hormones decrease plasma 1alpha,25-dihydroxyvitamin D levels through transcriptional repression of the renal 25-hydroxyvitamin D3 1alpha-hydroxylase gene (CYP27B1) Endocrinology. 2013;154:609–22. doi: 10.1210/en.2012-1647. [DOI] [PubMed] [Google Scholar]
- Bouillon R, et al. Influence of thyroid function on the serum concentration of 1,25-dihydroxyvitamin D3. Journal of Clinical Endocrinology & Metabolism. 1980;51:793–7. doi: 10.1210/jcem-51-4-793. [DOI] [PubMed] [Google Scholar]
- Shimada T, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perwad F, et al. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro. Am J Physiol Renal Physiol. 2007;293:F1577–F1583. doi: 10.1152/ajprenal.00463.2006. [DOI] [PubMed] [Google Scholar]
- Heaney RP, et al. Menopausal changes in calcium balance performance. J Lab Clin Med. 1978;92:953–963. [PubMed] [Google Scholar]
- Riggs BL, et al. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- Gallagher JC, et al. Effect of estrogen on calcium absorption and serum vitamin D metabolites in postmenopausal osteoporosis. J Clin Endocrinol Metab. 1980;51:1359–1364. doi: 10.1210/jcem-51-6-1359. [DOI] [PubMed] [Google Scholar]
- Pavlovitch H, et al. Lack of effect on ovariectomy on the metabolism of vitamin D and intestinal calcium-binding protein in female rats. Journal of Endocrinology. 1980;86:419–24. doi: 10.1677/joe.0.0860419. [DOI] [PubMed] [Google Scholar]
- Gennari C, et al. Estrogen preserves a normal intestinal responsiveness to 1,25- dihydroxyvitamin D3 in oophorectomized women. J Clin Endocrinol Metab. 1990;71:1288–1293. doi: 10.1210/jcem-71-5-1288. [DOI] [PubMed] [Google Scholar]
- Arjmandi BH, et al. In vivo effect of 17 β-estradiol on intestinal calcium absorption in rats. Bone and Mineral. 1994;26:181–189. doi: 10.1016/s0169-6009(08)80062-1. [DOI] [PubMed] [Google Scholar]
- Liel Y, et al. Estrogen increases 1,25-dihydroxyvitamin D receptors expression and bioresponse in the rat duodenal mucosa. Endocrinology. 1999;140:280–285. doi: 10.1210/endo.140.1.6408. [DOI] [PubMed] [Google Scholar]
- Colin EM, et al. Evidence for involvement of 17 β-estradiol in intestinal calcium absorption independent of 1,25-dihydroxyvitamin D3 level in the Rat. J Bone Miner Res. 1999;14:57–64. doi: 10.1359/jbmr.1999.14.1.57. [DOI] [PubMed] [Google Scholar]
- Christakos S, et al. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol Rev. 2016;96:365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiosano D, et al. Calcium absorption, kinetics, bone density, and bone structure in patients with hereditary vitamin D-resistant rickets. J Clin Endocrinol Metab. 2011;96:3701–9. doi: 10.1210/jc.2011-1432. [DOI] [PubMed] [Google Scholar]
- Li YC, et al. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci U S A. 1997;94:9831–5. doi: 10.1073/pnas.94.18.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa T, et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet. 1997;16:391–396. doi: 10.1038/ng0897-391. [DOI] [PubMed] [Google Scholar]
- Van Cromphaut SJ, et al. Duodenal calcium absorption in vitamin D receptor-knockout mice: functional and molecular aspects. Proc Natl Acad Sci USA. 2001;98:13324–13329. doi: 10.1073/pnas.231474698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, et al. Vitamin D receptor (VDR) knockout mice reveal VDR-independent regulation of intestinal calcium absorption and ECaC2 and calbindin D9k mRNA. J Nutr. 2003;133:374–80. doi: 10.1093/jn/133.2.374. [DOI] [PubMed] [Google Scholar]
- Amling M, et al. Rescue of the skeletal phenotype of vitamin D receptor ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analysis. Endocrinology. 1999;140:4982–4987. doi: 10.1210/endo.140.11.7110. [DOI] [PubMed] [Google Scholar]
- Lieben L, et al. Normocalcemia is maintained in mice under conditions of calcium malabsorption by vitamin D-induced inhibition of bone mineralization. J Clin Invest. 2012;122:1803–15. doi: 10.1172/JCI45890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue YB, Fleet JC. Intestinal Vitamin D Receptor Is Required for Normal Calcium and Bone Metabolism in Mice. Gastroenterology. 2009;136:1317–1327. doi: 10.1053/j.gastro.2008.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullamore JR, et al. Effect of age on calcium absorption. Lancet. 1970;2:535–537. doi: 10.1016/s0140-6736(70)91344-9. [DOI] [PubMed] [Google Scholar]
- Nordin BE, et al. Effect of age on calcium absorption in postmenopausal women. Am J Clin Nutr. 2004;80:998–1002. doi: 10.1093/ajcn/80.4.998. [DOI] [PubMed] [Google Scholar]
- Ebeling PR, et al. Evidence of an age-related decrease in intestinal responsiveness to vitamin-D - relationship between serum 1,25-dihydroxyvitamin-D3 and intestinal vitamin-D receptor concentrations in normal women. J Clin Endocrinol Metab. 1992;75:176–182. doi: 10.1210/jcem.75.1.1320048. [DOI] [PubMed] [Google Scholar]
- Shao A, et al. Increased vitamin D receptor level enhances 1,25-dihydroxyvitamin D3- mediated gene expression and calcium transport in Caco-2 cells. J Bone Miner Res. 2001;16:615–624. doi: 10.1359/jbmr.2001.16.4.615. [DOI] [PubMed] [Google Scholar]
- Lieben L, et al. Extra-intestinal calcium handling contributes to normal serum calcium levels when intestinal calcium absorption is suboptimal. Bone. 2015;81:502–12. doi: 10.1016/j.bone.2015.08.023. [DOI] [PubMed] [Google Scholar]
- Song Y, Fleet JC. Intestinal resistance to 1,25 dihydroxyvitamin D in mice heterozygous for the vitamin D receptor knockout allele. Endocrinology. 2007;148:1396–402. doi: 10.1210/en.2006-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames SK, et al. Vitamin D receptor gene Fok1 polymorphisms predicts calcium absorption and bone mineral density in children. J Bone Miner Res. 1999;14:740–746. doi: 10.1359/jbmr.1999.14.5.740. [DOI] [PubMed] [Google Scholar]
- Jurutka PW, et al. The polymorphic N terminus in human vitamin D receptor isoforms influences trascriptional activity by modulating interaction with transcription factor IIB. Mol Endocrinol. 2000;14:401–420. doi: 10.1210/mend.14.3.0435. [DOI] [PubMed] [Google Scholar]
- Huang ZW, et al. Relationship between the absorption of dietary calcium and the Fok I polymorphism of VDR gene in young women. Zhonghua Yu Fang Yi Xue Za Zhi. 2006;40:75–78. [PubMed] [Google Scholar]
- Wang Y, et al. The vitamin D receptor in the proximal renal tubule is a key regulator of serum 1alpha,25-dihydroxyvitamin D3. Am J Physiol Endocrinol Metab. 2015;308:E201–5. doi: 10.1152/ajpendo.00422.2014. [DOI] [PubMed] [Google Scholar]
- Iida K, et al. Distribution of 1,25-dihydroxyvitamin D3 receptor and 25-hydroxyvitamin D3-24-hydroxylase mRNA expression along rat nephron segments. Biochem Biophys Res Commun. 1993;194:659–64. doi: 10.1006/bbrc.1993.1872. [DOI] [PubMed] [Google Scholar]
- Li YC, et al. Effects of vitamin D receptor inactivation on the expression of calbindins and calcium metabolism. Am J Physiol Endocrinol Metab. 2001;281:E558–E564. doi: 10.1152/ajpendo.2001.281.3.E558. [DOI] [PubMed] [Google Scholar]
- Sooy K, et al. Osteoblasts lacking the vitamin D receptor display enhanced osteogenic potential in vitro. J Cell Biochem. 2005;94:81–87. doi: 10.1002/jcb.20313. [DOI] [PubMed] [Google Scholar]
- Drissi H, et al. 1,25-(OH)2-vitamin D3 suppresses the bone-related Runx2/Cbfa1 gene promoter. Exp Cell Res. 2002;274:323–333. doi: 10.1006/excr.2002.5474. [DOI] [PubMed] [Google Scholar]
- Boyle WJ, et al. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- Kitazawa R, et al. Modulation of mouse RANKL gene expression by Runx2 and vitamin D3. J Cell Biochem. 2008;105:1289–97. doi: 10.1002/jcb.21929. [DOI] [PubMed] [Google Scholar]
- Notoya M, et al. Runx-2 is not essential for the vitamin D-regulated expression of RANKL and osteoprotegerin in osteoblastic cells. Biochem Biophys Res Commun. 2004;324:655–60. doi: 10.1016/j.bbrc.2004.09.101. [DOI] [PubMed] [Google Scholar]
- Lee SK, et al. 1,25(OH)2 vitamin D3-stimulated osteoclast formation in spleen-osteoblast cocultures is mediated in part by enhanced IL-1 alpha and receptor activator of NF-kappa B ligand production in osteoblasts. J Immunol. 2002;169:2374–2380. doi: 10.4049/jimmunol.169.5.2374. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, et al. Vitamin D receptor in osteoblasts is a negative regulator of bone mass control. Endocrinology. 2013;154:1008–20. doi: 10.1210/en.2012-1542. [DOI] [PubMed] [Google Scholar]
- Nicolaysen R. XV. Studies upon the mode of action of vitamin D III the influence of vitamin D on the absorption of calcium and phosphorus in the rat. Biochem J. 1937;37:122–129. doi: 10.1042/bj0310122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pansu D, et al. Duodenal and ileal calcium absorption in the rat and effects of vitamin D. Am J Physiol. 1983;244:G695–G700. doi: 10.1152/ajpgi.1983.244.6.G695. [DOI] [PubMed] [Google Scholar]
- Sheikh MS, et al. Role of vitamin D-dependent and vitamin D-independent mechanisms in absorption of food calcium. J Clin Invest. 1988;81:126–132. doi: 10.1172/JCI113283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Need AG, et al. Vitamin D Metabolites and Calcium Absorption in Severe Vitamin D Deficiency. J Bone Miner Res. 2008 doi: 10.1359/jbmr.080607. [DOI] [PubMed] [Google Scholar]
- Wood RJ, et al. Intestinal calcium absorption in the aged rat: evidence of intestinal resistance to 1,25(OH)2 vitamin D. Endocrinology. 1998;139:3843–3848. doi: 10.1210/endo.139.9.6176. [DOI] [PubMed] [Google Scholar]
- Pattanaungkul S, et al. Relationship of intestinal calcium absorption to 1,25-dihydroxyvitamin D [1,25(OH)2D] levels in young versus elderly women: evidence for age- related intestinal resistance to 1,25(OH)2D action. J Clin Endocrinol Metab. 2000;85:4023–4027. doi: 10.1210/jcem.85.11.6938. [DOI] [PubMed] [Google Scholar]
- Scopacasa F, et al. Relation between calcium absorption and serum calcitriol in normal men: evidence for age-related intestinal resistance to calcitriol. Eur J Clin Nutr. 2004;58:264–269. doi: 10.1038/sj.ejcn.1601777. [DOI] [PubMed] [Google Scholar]
- Wasserman RH, Taylor AN. Some aspects of the intestinal absorption of calcium, with special reference to vitamin D. In: Comar CL, Bronner F, editors. Mineral Metabolism, An Advanced Treatise. Academic Press; New York: 1969. pp. 321–403. [Google Scholar]
- Pansu D, et al. Effect of Ca intake on saturable and nonsaturable components of duodenal Ca transport. Am J Physiol. 1981;240:32–7. doi: 10.1152/ajpgi.1981.240.1.G32. [DOI] [PubMed] [Google Scholar]
- Heaney RP, et al. Calcium absorption as a function of calcium intake. Journal of Laboratory & Clinical Medicine. 1975;85:881–90. [PubMed] [Google Scholar]
- Sheikh MS, et al. In vivo intestinal absorption of calcium in humans. Miner Electrolyte Metab. 1990;16:130–146. [PubMed] [Google Scholar]
- Chandra S, et al. Ion microscopic imaging of calcium transport in the intestinal tissue of vitamin D-deficient and vitamin D-replete chickens: a 44Ca stable isotope study. Proc Natl Acad Sci USA. 1990;87:5715–5719. doi: 10.1073/pnas.87.15.5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullmer CS, et al. Ion microscopic imaging of calcium during 1,25-dihydroxyvitamin D-mediated intestinal absorption. Histochem Cell Biol. 1996;106:215–222. doi: 10.1007/BF02484403. [DOI] [PubMed] [Google Scholar]
- Giuliano AR, Wood RJ. Vitamin D-regulated calcium transport in Caco-2 cells: unique in vitro model. Am J Physiol. 1991;260:G207–G212. doi: 10.1152/ajpgi.1991.260.2.G207. [DOI] [PubMed] [Google Scholar]
- Favus MJ, et al. Effects of trifluoperazine,ouabain, and ethacrynic acid on intestinal calcium. Am J Physiol. 1983;244:G111–G115. doi: 10.1152/ajpgi.1983.244.2.G111. [DOI] [PubMed] [Google Scholar]
- Favus MJ, et al. Kinetic characteristics of calcium absorption and secretion by rat colon. Am J Physiol. 1981;240:G350–G354. doi: 10.1152/ajpgi.1981.240.5.G350. [DOI] [PubMed] [Google Scholar]
- Favus MJ, Langman CB. Effects of 1,25 dihydroxyvitamin D3 on colonic calcium transport in vitamin D-deficient and normal rats. Am J Physiol. 1984;246:G268–G273. doi: 10.1152/ajpgi.1984.246.3.G268. [DOI] [PubMed] [Google Scholar]
- Karbach U, Rummel W. Calcium transport across the colon ascendens and the influence of 1,25-dihydroxyvitamin D3 and dexamethasone. Eur J Clin Invest. 1987;17:368–374. doi: 10.1111/j.1365-2362.1987.tb02202.x. [DOI] [PubMed] [Google Scholar]
- Karbach U, Feldmeier H. The cecum is the site with the highest calcium absorption in rat intestine. Dig Dis Sci. 1993;38:1815–1824. doi: 10.1007/BF01296104. [DOI] [PubMed] [Google Scholar]
- Barger-Lux MJ, et al. Time course of calcium absorption in humans: evidence for a colonic component. Calcif Tissue Int. 1989;44:308–11. doi: 10.1007/BF02556309. [DOI] [PubMed] [Google Scholar]
- Reyes-Fernandez PC, Fleet JC. Compensatory Changes in Calcium Metabolism Accompany the Loss of Vitamin D Receptor (VDR) From the Distal Intestine and Kidney of Mice. J Bone Miner Res. 2016;31:143–51. doi: 10.1002/jbmr.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus CS, Lengemann FW. Absorption of Ca 45 and Sr 85 from solid and liquid food at various levels of the alimentary tract of the rat. J Nut. 1962;77:155–160. doi: 10.1093/jn/77.2.155. [DOI] [PubMed] [Google Scholar]
- Duflos C, et al. Calcium solubility, intestinal sojourn time and paracellular permeability codetermine passive calcium absorption in rats. J Nutr. 1995;125:2348–2355. doi: 10.1093/jn/125.9.2348. [DOI] [PubMed] [Google Scholar]
- Bronner F, et al. An analysis of intestinal calcium transport across the rat intestine. Am J Physiol. 1986;250:G561–G569. doi: 10.1152/ajpgi.1986.250.5.G561. [DOI] [PubMed] [Google Scholar]
- Peng JB, et al. Molecular cloning and characterization of a channel-like transporter mediated intestinal calcium absorption. J Biol Chem. 1999;274:22739–22746. doi: 10.1074/jbc.274.32.22739. [DOI] [PubMed] [Google Scholar]
- Meyer MB, et al. Characterizing early events associated with the activation of target genes by 1,25-dihydroxyvitamin D3 in mouse kidney and intestine in vivo. J Biol Chem. 2007;282:22344–22352. doi: 10.1074/jbc.M703475200. [DOI] [PubMed] [Google Scholar]
- Fleet JC, et al. Vitamin D-inducible calcium transport and gene expression in three Caco-2 cell lines. Am J Physiol. 2002;283:G618–G625. doi: 10.1152/ajpgi.00269.2001. [DOI] [PubMed] [Google Scholar]
- Cui M, et al. Villin promoter-mediated transgenic expression of transient receptor potential cation channel, subfamily V, member 6 (TRPV6) increases intestinal calcium absorption in wild-type and vitamin D receptor knockout mice. J Bone Miner Res. 2012;27:2097–107. doi: 10.1002/jbmr.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutuzova GD, et al. TRPV6 is not required for 1alpha,25-dihydroxyvitamin D3-induced intestinal calcium absorption in vivo. Proc Natl Acad Sci U S A. 2008;105:19655–9. doi: 10.1073/pnas.0810761105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benn BS, et al. Active intestinal calcium transport in the absence of transient receptor potential vanilloid type 6 and calbindin-D9k. Endocrinology. 2008;149:3196–205. doi: 10.1210/en.2007-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickert G, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206:1465–72. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woudenberg-Vrenken TE, et al. Functional TRPV6 channels are crucial for transepithelial Ca2+ absorption. Am J Physiol Gastrointest Liver Physiol. 2012;303:G879–85. doi: 10.1152/ajpgi.00089.2012. [DOI] [PubMed] [Google Scholar]
- Kellett GL. Alternative perspective on intestinal calcium absorption: proposed complementary actions of Ca(v)1.3 and TRPV6. Nutr Rev. 2011;69:347–370. doi: 10.1111/j.1753-4887.2011.00395.x. [DOI] [PubMed] [Google Scholar]
- Reyes-Fernandez PC, Fleet JC. Luminal glucose does not enhance active intestinal calcium absorption in mice: evidence against a role for Ca(v)1.3 as a mediator of calcium uptake during absorption. Nutr Res. 2015;35:1009–15. doi: 10.1016/j.nutres.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakos S, et al. Molecular aspects of the calbindins. J Nutr. 1992;122:678–82. doi: 10.1093/jn/122.suppl_3.678. [DOI] [PubMed] [Google Scholar]
- Wasserman RH, Taylor AN. Vitamin D3-induced calcium-binding proteins in chick intestinal mucosa. Science. 1966;252:791–793. doi: 10.1126/science.152.3723.791. [DOI] [PubMed] [Google Scholar]
- Bronner F, Buckley M. The molecular nature of 1,25-(OH)2-D3-induced calcium-binding protein biosynthesis in the rat. Adv Exp Med Biol. 1982;151:355–360. doi: 10.1007/978-1-4684-4259-5_41. [DOI] [PubMed] [Google Scholar]
- Spencer R, et al. The relationship between vitamin D-stimulated calcium transport and intestinal calcium-binding protein in the chicken. Biochem J. 1978;170:93–101. doi: 10.1042/bj1700093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhter S, et al. Calbindin D9k is not required for 1,25-dihydroxyvitamin D3-mediated Ca2+ absorption in small intestine. Arch Biochem Biophys. 2007;460:227–32. doi: 10.1016/j.abb.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Wasserman RH, et al. Vitamin-D and mineral deficiencies increase the plasma membrane calcium pump of chicken intestine. Gastroenterology. 1992;102:886–894. doi: 10.1016/0016-5085(92)90174-w. [DOI] [PubMed] [Google Scholar]
- Cai Q, et al. Vitamin D and adaptation to dietary calcium and phosphate deficiencies increase intestinal plasma membrane calcium pump gene expression. Proc Natl Acad Sci U S A. 1993;90:1345–1349. doi: 10.1073/pnas.90.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, et al. Impaired intestinal calcium absorption in protein 4.1R-deficient mice due to altered expression of plasma membrane calcium ATPase 1b (PMCA1b) J Biol Chem. 2013;288:11407–15. doi: 10.1074/jbc.M112.436659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan ZC, et al. Deletion of the intestinal plasma membrane calcium pump, isoform 1, Atp2b1, in mice is associated with decreased bone mineral density and impaired responsiveness to 1, 25-dihydroxyvitamin D3. Biochem Biophys Res Commun. 2015;467:152–6. doi: 10.1016/j.bbrc.2015.09.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Corven EJ, et al. Distribution of Ca2+-ATPase, ATP-dependent Ca2+-transport, calmodulin and vitamin D-dependent Ca2+-binding protein along the villus-crypt axis in rat duodenum. Biochim Biophys Acta. 1985;820:274–282. doi: 10.1016/0005-2736(85)90121-x. [DOI] [PubMed] [Google Scholar]
- Davis WL, Jones RG. Lysosomal proliferation in rachitic avian intestinal absorptive cells following 1,25-dihydroxycholecalciferol. Tissue and Cell. 1982;14:585–95. doi: 10.1016/0040-8166(82)90049-0. [DOI] [PubMed] [Google Scholar]
- Nemere I, Szego CM. Early actions of parathyroid hormone and 1,25-dihydroxycholecalciferol on isolated epithelial cells from rat intestine: 1. Limited lysosomal enzyme release and calcium uptake. Endocrinology. 1981;108:1450–1462. doi: 10.1210/endo-108-4-1450. [DOI] [PubMed] [Google Scholar]
- Warner RR, Coleman JR. Electron probe analysis of calcium transport by small intestine. J Cell Biol. 1975;64:54–74. doi: 10.1083/jcb.64.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemere I, et al. 1, 25 dihydroxyvitamin D3-mediated intestinal calcium transport. Biochemical identification of lysozomes containing calcium and calcium-binding protein (calbindin-D 28k) J Biol Chem. 1986;261:16106–16114. [PubMed] [Google Scholar]
- Nemere I, Norman AW. 1,25-Dihydroxyvitamin D3-mediated vesicular transport of calcium in intestine: time-course studies. Endocrinology. 1988;122:2962–9. doi: 10.1210/endo-122-6-2962. [DOI] [PubMed] [Google Scholar]
- Favus MJ, et al. Effects of quinacrine on calcium active transport by rat intestinal epithelium. Am J Physiol. 1989;257:G818–G822. doi: 10.1152/ajpgi.1989.257.5.G818. [DOI] [PubMed] [Google Scholar]
- Nemere I, et al. Calcium transport in perfused duodena from normal chicks: Enhancement within fourteen minutes of exposure to 1,25 dihydroxyvitamin D3. Endocrinology. 1984;115:1476–1483. doi: 10.1210/endo-115-4-1476. [DOI] [PubMed] [Google Scholar]
- Huhtakangas JA, et al. The vitamin D receptor is present in caveolae-enriched plasma membranes and binds 1 alpha,25(OH)(2)-vitamin D-3 in vivo and in vitro. Mol Endocrinol. 2004;18:2660–2671. doi: 10.1210/me.2004-0116. [DOI] [PubMed] [Google Scholar]
- Mizwicki MT, et al. Identification of an alternative ligand-binding pocket in the nuclear vitamin D receptor and its functional importance in 1alpha,25(OH)2-vitamin D3 signaling. Proc Natl Acad Sci USA. 2004;101:12876–12881. doi: 10.1073/pnas.0403606101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AW, et al. Molecular tools for study of genomic and rapid signal transduction responses initiated by 1 alpha,25(OH)2-vitamin D3. Steroids. 2002;67:457–466. doi: 10.1016/s0039-128x(01)00167-2. [DOI] [PubMed] [Google Scholar]
- Nemere I, et al. Identification and characterization of 1,25D3-membrane-associated rapid response, steroid (1,25D3-MARRS) binding protein. J Steroid Biochem Mol Biol. 2004;89–90:281–285. doi: 10.1016/j.jsbmb.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Nemere I, et al. Intestinal cell calcium uptake and the targeted knockout of the 1,25D3-MARRS (membrane-associated, rapid response steroid-binding) receptor/PDIA3/Erp57. J Biol Chem. 2010;285:31859–66. doi: 10.1074/jbc.M110.116954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemere I, et al. Intestinal cell phosphate uptake and the targeted knockout of the 1,25D3-MARRS receptor/PDIA3/ERp57. Endocrinology. 2012;153:1609–15. doi: 10.1210/en.2011-1850. [DOI] [PubMed] [Google Scholar]
- Nemere I, et al. Role of the 1,25D3-MARRS receptor in the 1,25(OH)(2)D(3)-stimulated uptake of calcium and phosphate in intestinal cells. Steroids. 2012 doi: 10.1016/j.steroids.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Karbach U. Paracellular Calcium Transport Across the Small Intestine. J Nutr. 1992;122:672–677. doi: 10.1093/jn/122.suppl_3.672. [DOI] [PubMed] [Google Scholar]
- Tudpor K, et al. 1,25-dihydroxyvitamin D3 rapidly stimulates the solvent drag-induced paracellular calcium transport in the duodenum of female rats. J Physiol Sci. 2008;58:297–307. doi: 10.2170/physiolsci.RP002308. [DOI] [PubMed] [Google Scholar]
- Fujita H, et al. Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol Biol Cell. 2008;19:1912–1921. doi: 10.1091/mbc.E07-09-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H, et al. Differential expression and subcellular localization of claudin-7, -8, -12, -13, and -15 along the mouse intestine. J Histochem Cytochem. 2006;54:933–944. doi: 10.1369/jhc.6A6944.2006. [DOI] [PubMed] [Google Scholar]
- Rahner C, et al. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology. 2001;120:411–422. doi: 10.1053/gast.2001.21736. [DOI] [PubMed] [Google Scholar]
- Hou J, et al. Claudins and the kidney. Annu Rev Physiol. 2013;75:479–501. doi: 10.1146/annurev-physiol-030212-183705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenderop JGJ, et al. Molecular identification of the apical Ca2+ channel in 1,25-dihydroxyvitamin D3-responsive epithelia. J Biol Chem. 1999;274:8375–8378. doi: 10.1074/jbc.274.13.8375. [DOI] [PubMed] [Google Scholar]
- Hoenderop JG, et al. Localization of the epithelial Ca(2+) channel in rabbit kidney and intestine. J Am Soc Nephrol. 2000;11:1171–1178. doi: 10.1681/ASN.V1171171. [DOI] [PubMed] [Google Scholar]
- Peng JB, et al. A rat kidney-specific calcium transporter in the distal nephron. J Biol Chem. 2000;275:28186–28194. doi: 10.1074/jbc.M909686199. [DOI] [PubMed] [Google Scholar]
- Hoenderop JG, et al. Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J Clin Invest. 2003;112:1906–14. doi: 10.1172/JCI19826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander RT, et al. Klotho prevents renal calcium loss. J Am Soc Nephrol. 2009;20:2371–9. doi: 10.1681/ASN.2008121273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erben RG. Update on FGF23 and Klotho signaling. Mol Cell Endocrinol. 2016;432:56–65. doi: 10.1016/j.mce.2016.05.008. [DOI] [PubMed] [Google Scholar]
- Chang Q, et al. The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–493. doi: 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- Xu Y, Sun Z. Molecular basis of Klotho: from gene to function in aging. Endocr Rev. 2015;36:174–93. doi: 10.1210/er.2013-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko SH, et al. Effect of dietary calcium and 1,25-(OH)2D3 on the expression of calcium transport genes in calbindin-D9k and -D28k double knockout mice. Biochem Biophys Res Commun. 2009;379:227–32. doi: 10.1016/j.bbrc.2008.12.029. [DOI] [PubMed] [Google Scholar]
- Hoenderop JG, et al. Function and expression of the epithelial Ca(2+) channel family: comparison of mammalian ECaC1 and 2. J Physiol. 2001;537:747–761. doi: 10.1111/j.1469-7793.2001.00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannabecker TL, et al. Vitamin D-dependent transcriptional regulation of the intestinal plasma membrane calcium pump. Biochem Biohphys Res Commun. 1995;213:499–505. doi: 10.1006/bbrc.1995.2159. [DOI] [PubMed] [Google Scholar]
- Glendenning P, et al. Calcitriol upregulates expression and activity of the 1b isoform of the plasma membrane calcium pump in immortalized distal kidney tubular cells. Arch Biochem Biophys. 2000;380:126–132. doi: 10.1006/abbi.2000.1908. [DOI] [PubMed] [Google Scholar]
- van der Hagen EA, et al. Coordinated regulation of TRPV5-mediated Ca(2)(+) transport in primary distal convolution cultures. Pflugers Arch. 2014;466:2077–87. doi: 10.1007/s00424-014-1470-x. [DOI] [PubMed] [Google Scholar]
- van Loon EP, et al. Calcium Extrusion Pump PMCA4: A New Player in Renal Calcium Handling? PLoS One. 2016;11:e0153483. doi: 10.1371/journal.pone.0153483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenderop JG, et al. Modulation of renal Ca2+ transport protein genes by dietary Ca2+ and 1,25-dihydroxyvitamin D3 in 25-hydroxyvitamin D3-1alpha-hydroxylase knockout mice. FASEB J. 2002;16:1398–1406. doi: 10.1096/fj.02-0225com. [DOI] [PubMed] [Google Scholar]
- Ko SH, et al. Dietary calcium and 1,25-dihydroxyvitamin D3 regulate transcription of calcium transporter genes in calbindin-D9k knockout mice. J Reprod Dev. 2009;55:137–42. doi: 10.1262/jrd.20139. [DOI] [PubMed] [Google Scholar]
- Xi Q, et al. Adenovirus-delivered microRNA targeting the vitamin D receptor reduces intracellular Ca(2)(+) concentrations by regulating the expression of Ca(2)(+)-transport proteins in renal epithelial cells. BJU Int. 2011;107:1314–9. doi: 10.1111/j.1464-410X.2010.09444.x. [DOI] [PubMed] [Google Scholar]
- McCollum EV, et al. An experimental demonstration of the existance of a vitamin which promotes calcium deposition. 1922:293–312. [Google Scholar]