Abstract
Objective
To evaluate the effect of a postpartum risk-based low-molecular-weight heparin protocol for venous thromboembolism prevention.
Methods
We conducted a retrospective cohort of postpartum women at a safety-net hospital before (2013), during (2014), and after (2015) implementation of a risk-based enoxaparin thromboembolism prevention protocol. The calculated sample size was based on a primary outcome of enoxaparin administration rate. Secondary outcomes included incidence of postpartum thromboembolism, wound complications, and 30-day readmission rates. The prevalence of thromboembolism risk factors and protocol adherence was evaluated in two groups of women before (May 2013) and after (May 2015) protocol implementation. Exact chi-square or Cochran-Armitage trend tested differences in rates.
Results
Over 3 years, 9,766 deliveries were included. Enoxaparin was administered to 0.28% (95% CI 0.14–0.55) of postpartum women in 2013 (before) compared with 33.46% (95% 31.89–35.07%) after protocol implementation (p<0.001). Although underpowered to detect a difference in these outcomes, no differences were seen in rates of thromboembolism (0.16%, 0.12%, 0.15%, p=.9), wound complication (0.82%, 1.21%, 0.91%, p=0.7), or emergency department visits (8.30%, 7.96%, 8.34%, p=0.9), while readmissions increased (0.79%, 1.27%, 1.42%, p=0.02). Prevalence of thromboembolism risk factors did not differ between women delivered in May 2013 and May 2015. Physician adherence to the protocol was 89.5% in May 2015. Nine women had thromboembolic events after protocol implementation: 5 received appropriate treatment per protocol, but 4 did not. In 2014,3/4 of women with a thromboembolism were inadequately treated compared to 1/5 in 2015 with the addition of a computerized order set.
Conclusion
Implementation of a low-molecular-weight heparin risk-based protocol for postpartum thromboembolism prevention resulted in high physician adherence and over 30% of postpartum women receiving enoxaparin. Prior to implementing such a protocol on a wider scale, a much larger study is needed to evaluate the effect on thromboembolic disease and wound problems.
Introduction
Thromboembolism is one of the leading causes of maternal death in developed countries accounting for approximately 15% of maternal deaths (1). In the United Kingdom, the publication of a Royal College of Obstetricians and Gynaecologists (RCOG) Green-top Guideline (2) with recommendations for risk-based low-molecular-weight heparin prophylaxis coincided with a reduction in maternal deaths from 1.94 deaths per 100,000 live births to 0.79 deaths per 100,000 live births (3). Recently, in the United States, the National Partnership for Maternal Safety (NPMS) released a bundle for thromboembolism prevention advocating for universal risk assessment and development of a prophylaxis protocol that fits each institution’s needs and resources (4).
Protocols and guidelines for thromboembolism prophylaxis are now commonplace for most medical and surgical admissions (5,6). However, there is concern within the obstetric community about application of these risk scoring systems from other specialties to otherwise young and healthy postpartum women given the low incidence of thromboembolism and unproven benefit in this population (7).
At our safety-net teaching hospital, a risk-based thromboembolism prevention protocol adapted from RCOG guidelines was implemented for all postpartum women in January 2014. We report the effect of the implementation of this protocol at a single center in the United States with an annual delivery volume of 3,000–3,500 women. Specifically, we examined the proportion of women who received prophylaxis before and after protocol implementation, provider adherence to prescribing enoxaparin as intended by the protocol, and the effect of the protocol on the rate of thromboembolism and other complications.
Materials and Methods
In 2014, a risk-based enoxaparin thromboembolism prevention protocol was initiated in our safety-net teaching institution in Denver, Colorado. This was a before–after retrospective cohort study of postpartum women from 2013 through 2015. All women who delivered between January 1, 2013 and December 31, 2015 were included. Postpartum women for the 3-year cohort were identified by extracting all deliveries from a Philip’s Healthcare OBTraceVue system (our Labor and Delivery electronic charting system). Women who were incarcerated or received enoxaparin antepartum for another indication (e.g. history of thromboembolism or known thrombophilia) were excluded.
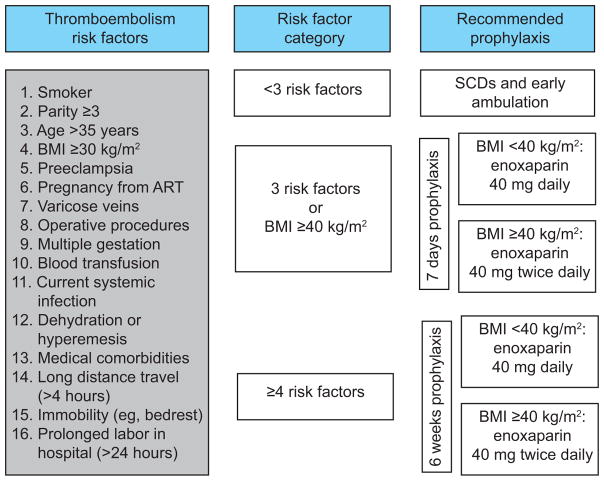
The year of 2013 served as a historical control during which time there was no postpartum thromboembolism protocol, and decisions regarding prophylaxis were made by individual providers. In January 2014, a risk-based protocol modified from RCOG guidelines was developed and initiated through provider education and protocol algorithms that were posted at health care provider work stations (Figure 1). Postpartum women with risk factors for thromboembolism received either 7 days or 6 weeks of enoxaparin prophylaxis, depending on the number of risk factors. Women with a body mass index (BMI) of less than 40 mg/kg2 received 40 mg enoxaparin daily and women with a BMI of 40 mg/kg2 or greater received 40 mg enoxaparin twice daily. Enoxaparin was initiated within 6 hours of a vaginal delivery and within 12 hours of a cesarean delivery. Universal compliance was encouraged.
Figure 1.
Algorithm for implemented risk-based, low-molecular-weight heparin venous thromboembolism prevention protocol in postpartum women. Risk factors selected based on the Royal College of Obstetricians and Gynaecologists Green-top Guideline No. 37a.2 SCD, sequential compression device; BMI, body mass index; ART, assisted reproductive technology.
In January 2015, a hard-stop computerized physician order entry system was added to increase protocol adherence. At the time of protocol implementation, Siemens Soarian LifeLink Clinicals was the order entry system. The implementation consisted of a mandatory section within the post-partum order set that required providers to select all relevant patient risk factors followed by the appropriate thromboembolism prophylaxis regimen based on the protocol algorithm. All women undergoing cesarean deliveries wore sequential compression devices (SCDs) until ambulatory both before and after protocol implementation.
The following information was extracted from the Denver Health Data Warehouse for the entire cohort: date of delivery, maternal age at time of delivery, race, ethnicity, insurance type, enoxaparin administration, incidence of thromboembolism within 12 weeks of delivery, wound complications (separation, seroma, hematoma, infection), 30-day in-patient readmission rates, and unscheduled out-patient visits (emergency department, urgent care, or obstetric triage). The Data Warehouse was queried using the following billing codes: venous thromboembolism 453.X; pulmonary embolism 415.1, 415.11, 415.12, 415.13, 415.19; wound hematoma 090.2, 998.12; wound seroma 998.13; disruption of wound unspecified 998.30, 998.3. Manual chart review confirmed wound complications, thromboembolic events, and readmissions (as well as the associated diagnoses) that were identified using these billing codes. Enoxaparin use was identified from pharmacy administration records.
The Denver Health Data Warehouse is an administrative database maintained by the institution for all in-patient and out-patient encounters within the Denver Health system. Several methods ensure that data from the warehouse are accurate, reliable, and secure. A data quality group comprised of information technology analysts, medical records staff, and super users from core departments (e.g. laboratory, radiology, pharmacy) verify the accuracy, reliability and integrity of these data via random sampling and interface automation. In clinical areas, quality assurance teams comprised of physicians, nurses, and information technology staff continually sample data received for accuracy. Furthermore, for this study, all complication outcome data from the warehouse were confirmed with chart review and no discrepancies were noted.
Chart review was performed to verify the rate of enoxaparin administration in addition to the prevalence of protocol-defined risk factors and protocol adherence by providers. To determine the number of charts that needed to be reviewed, we calculated a sample size based on the anticipated difference in the proportion of women who were receiving postpartum thromboembolism prophylaxis with enoxaparin in 2013 (prior to protocol implementation) and in 2015 (after risk-based protocol implementation). We chose proportions that closely straddled the 50%ile which would require the largest sample size to observe a difference. Therefore, the proportion of women receiving thromboembolism prophylaxis in the 2013 group was assumed to be 43.5% and in the 2015 group, under the alternative hypothesis, was 56.5%. There are at least 250 deliveries per month at Denver Health. A sample size of 500 (250 women in May 2013 and 250 women in May 2015) achieves 80% power to detect a difference of 13% using a two-sided Fisher’s Exact test and α = 0.050. The rate of enoxaparin use among postpartum women was estimated as point prevalence with 95 % confidence intervals.
Risk factors for thromboembolism that were abstracted from the medical record include all of those presented in Figure 1. If BMI was not available at the time of admission, the BMI recorded at the participant’s last prenatal visit was used if this visit was within two weeks of delivery. All other data points were available for all participants. The month of May was chosen in each of these years as this is an academic institution with a change in house staff in mid-June, so our intent was to choose the month with the greatest chance of compliance with the protocol with seasoned providers. In addition, May is several months into the year and full compliance with the protocol following education was anticipated by then.
A detailed chart review was performed for each thromboembolic event, which included identification of protocol-defined risk factors, appropriate use of prophylaxis, and timing of diagnosis. Adherence to protocol was defined as identification of all protocol-defined risk factors and appropriate selection and use of prophylaxis. All chart review performed in this study was completed by one author (ER), a trained physician in obstetrics and gynecology.
Rates are summarized as percent with exact 95% confidence intervals (CI). An exact chi-square was used to test differences in comparisons between 2013 and 2015 for categorical variables. A Cochran-Armitage trend test was used to test for trends in secondary outcomes across years from before protocol implementation through full implementation with an electronic order set.
A p-value less than 0.05 was considered statistically significant. Research Electronic Data Capture (REDCap) was used for data collection and management (8). SAS was used for statistical analysis and GraphPad Prism for creation of figures. This study was approved by the Colorado Multiple Institutional Review Board (COMIRB).
Results
From January 1, 2013 to December 31, 2015 there were 9,786 deliveries identified. Three were excluded due to active incarceration and 17 met exclusion criteria of prior clot (antepartum or prior to pregnancy) or known thrombophilia. Thus, 9,766 deliveries were included over the 3 years with 3157 deliveries in 2013, 3217 in 2014, and 3392 in 2015. During this time, there were 503 women who delivered twice, and 7 women who delivered 3 times. Each delivery from these women is included as each delivery was considered an opportunity to assess risk factors for thromboembolic prophylaxis. Demographics of the cohort are reported in Table 1. There were differences in racial distribution and insurance type over the study period.
Table 1.
Demographics of Study Population
| Characteristic | Value | 2013 n=3157 |
2014 n=3217 |
2015 n=3392 |
p-value |
|---|---|---|---|---|---|
| Maternal age (years) | <18 | 119 (3.8) | 108 (3.4) | 106 (3.1) | 0.668 |
| 18–<35 | 2562 (81.2) | 2590 (80.5) | 2743 (80.9) | ||
| 35–<40 | 373 (11.8) | 423 (13.1) | 436 (12.9) | ||
| 40+ | 103 (3.3) | 96 (3.0) | 107 (3.2) | ||
| Race or ethnicity | Hispanic | 2075 (65.7) | 1951 (60.6) | 1649 (48.6) | <.001 |
| White | 499 (15.8) | 706 (21.9) | 1130 (33.3) | ||
| Black | 381 (12.1) | 394 (12.2) | 434 (12.8) | ||
| Asian | 176 (5.6) | 146 (4.5) | 157 (4.6) | ||
| Native American or Pacific Islander | 22 (0.7) | 16 (0.5) | 17 (0.5) | ||
| Missing | 4 (0.1) | 4 (0.1) | 5 (0.1) | ||
| Insurance type | Medicaid | 2611 (82.7) | 2802 (87.1) | 2893 (85.3) | <.001 |
| Private Insurance | 334 (10.6) | 338 (10.5) | 379 (11.2) | ||
| Self-Pay | 199 (6.3) | 70 (2.2) | 111 (3.3) | ||
| Medicare | 13 (0.4) | 7 (0.2) | 9 (0.3) |
Values presented as: number (percent). P values are from X2.
The rate of enoxaparin administration in 2013 prior to protocol implementation was 0.28% (95% CI, 0.14–0.55%) compared with 33.46% (95% CI, 31.89–35.07%) after protocol implementation with an electronic order set in 2015 (p<0.001).
There were no significant differences in rate of thromboembolism, wound complication, or unscheduled out-patient visits over the 3 years, while the rate of in-patient readmission increased (Table 2). The overall incidence of thromboembolism for the 3 years was 0.14% (95% CI, 0.07–0.22%). The readmission rate increased from 0.8% in 2013 to 1.3% in 2014, and 1.4% in 2015 (p=0.02). However, when readmissions were categorized as resulting from preeclampsia or eclampsia, pyelonephritis, endometritis, wound complication, embolism, or other, the distribution of indication for readmission did not differ over time (p=0.08) (Table 3). Of note, there were no additional thromboembolic events identified upon chart review of all readmissions that were not previously identified with billing code queries.
Table 2.
Venous Thromboembolism Events and Other Complications
| Event or Complication | 2013 n=3157 |
2014 n=3217 |
2015 n=3392 |
p-value |
|---|---|---|---|---|
| Overall venous thromboembolism | 5 (0.16) | 4 (0.12) | 5 (0.15) | 0. 912 |
| Pulmonary embolus | 3 (0.10) | 4 (0.12) | 5 (0.15) | 0. 546 |
| Deep venous thrombosis | 2 (0.06) | 0 (0.0) | 1 (0.03) | 0. 449 |
| Overall wound complications | 23 (0.82) | 35 (1.21) | 29 (0.91) | 0. 736 |
| Hematoma | 2 (0.06) | 6 (0.19) | 3 (0.09) | 0. 786 |
| Infection | 11 (0.35) | 14 (0.44) | 15 (0.44) | 0. 557 |
| Seroma | 9 (0.29) | 4 (0.12) | 2 (0.06) | 0. 020 |
| Wound disruption | 7 (0.22) | 15 (0.47) | 15 (0.44) | 0.153 |
| Readmit within 30 days – Emergency Department, Urgent Care, Obstetric Triage Unit | 262 (8.30) | 256 (7.96) | 283 (8.34) | 0. 938 |
| Readmit within 30 days - Inpatient | 25 (0.79) | 41 (1.27) | 48 (1.42) | 0. 0197 |
Values presented as: Number (percent), p-values from Cochran Armitage test of trend.
Table 3.
Reasons for Inpatient Readmission Over Study Period
| Reason for readmission | 2013 n=25 |
2014 n=41 |
2015 n=48 |
P value |
|---|---|---|---|---|
| Endometritis | 3 (12.0) | 11 (26.8) | 18 (37.5) | 0.08 |
| Preeclampsia or eclampsia | 7 (28.0) | 11 (26.8) | 10 (20.8) | |
| Pyelonephritis | 0 (0.0) | 1 (2.4) | 4 (8.3) | |
| Thromboembolism | 2 (8.0) | 0 (0.0) | 2 (4.2) | |
| Wound complication | 3 (12.0) | 2 (4.9) | 6 (12.5) | |
| Other | 10 (40.0) | 16 (39.0) | 8 (16.7) |
Values presented as number (percent). P value is overall chi square.
Two-hundred-sixty women delivered in May 2013 and 257 in May 2015. Deliveries in these months were reviewed in detail. No women delivered in both time periods. More Hispanic and fewer Medicaid-insured women delivered in May 2013 versus May 2015; other demographics and clinical characteristics did not differ (data not shown). The prevalence of thromboembolism risk factors did not differ between May 2013 and May 2015 (Table 4). BMI ≥ 30 kg/m2, parity ≥ 3, operative procedures (i.e. cesareans and bilateral tubal ligations), ≥24 hours of immobility or prolonged labor, and systemic infection (typically chorioamnionitis) were the most common risk factors identified.
Table 4.
Venous Thromboembolism Risk Factors for Women Who Delivered in May 2013 and May 2015
| Thromboembolism risk factor | May 2013 n=260 |
May 2015 n=257 |
p value | Risk factor missed by provider |
|---|---|---|---|---|
| Age > 35 years | 26 (10.0) | 35 (13.6) | 0.22 | 0 |
| Body mass index ≥ 30 kg/m2 | 144 (55.4) | 137 (53.3) | 0.66 | 2 (0.8) |
| Medical co-morbidity | 7 (2.7) | 6 (2.3) | 1 | 1 (0.4) |
| ≥ 24 hours bedrest or immobility | 54 (20.8) | 41 (16.0)) | 0.17 | 8 (3.1) |
| Operative procedures | 89 (34.2) | 74 (28.8) | 0.19 | 2 (0.8) |
| Parity ≥ 3 | 94 (36.2) | 100 (38.9) | 0.53 | 2 (0.8) |
| Smoker | 19 (7.3) | 16 (6.2) | 0.73 | 4 (1.6) |
| Varicose veins | 3 (1.2) | 1 (0.4) | 0.62 | 0 |
| Blood transfusion | 2 (0.8) | 3 (1.2) | 0.68 | 0 |
| Multiple gestation | 3 (1.2) | 3 (1.2) | 1 | 0 |
| Preeclampsia | 18 (6.9) | 26 (10.1) | 0.21 | 1 (0.4) |
| Current systemic infection | 34 (13.1) | 37 (14.4) | 0.70 | 3 (1.2) |
Values in cells represent N (%). P-values are from X2. There were no instances of assisted reproductive technology, dehydration or hyperemesis, or long distance travel identified.
Consistent with the whole cohort, the rate of thromboembolism prophylaxis with enoxaparin significantly increased before and after implementation of the protocol when comparing the May 2013 and 2015 deliveries; only 0.38% of postpartum women (95%CI, 0.00–1.14) received enoxaparin in May 2013 compared with 31.13% (95%CI, 25.43–36.83) (p<0.001) in May 2015 after full protocol implementation with an electronic order set. Among women receiving enoxaparin, 48.75% had cesarean deliveries, and 51.25% had vaginal deliveries.
Physician adherence to protocol in May 2015 was 89.5% indicating that all appropriate risk factors were selected and enoxaparin was prescribed as delineated by the protocol. Non-adherence to protocol occurred in 27 cases (10.5%). Twenty resulted in under-treatment: 8 received no prophylaxis when indicated for 7 days, 10 received 7 days when indicated for 6 weeks, and 2 received once daily rather than twice daily dosing. Seven cases of non-adherence were due to over-treatment; 2 received 7 days when no prophylaxis was indicated, 4 received 6 weeks rather than 7 days, and one received twice daily instead of daily dosing. The most commonly overlooked risk factors were ≥ 24 hours immobility, prolonged labor, tobacco use, and systemic infection. The most common reasons for over-treatment were miscalculation of BMI and counting age 35 as a risk factor when protocol states age >35.
There were 14 thromboembolic events for the entire cohort (2013–2015). Nine events were after protocol implementation in the years of 2014 and 2015 (Table 5): 5 received appropriate treatment per protocol (2 received no enoxaparin as they only had 2 risk factors for thromboembolism and 3 received 7 days enoxaparin for 3 risk factors), but 4 did not receive appropriate treatment per protocol (one of whom had significant morbidity related to hemorrhage). Three of four women in 2014 with thromboembolism events did not receive adequate prophylaxis per protocol compared to only 1/5 in 2015 with the electronic order set in place. Notably the events in 2014 occurred earlier in the postpartum course (days 1–5), while those in 2015 predominantly occurred after the 7 days of prophylactic enoxaparin (days 6–51). Four out of the 14 women with thromboembolic events had a thrombophilia work up after their diagnoses which were negative. The most common risk factors for women with thromboembolism were BMI ≥30 kg/m2 and operative procedures (cesarean, postpartum bilateral tubal ligation, or dilation and curettage). All women with thromboembolic events had at least one of these risk factors.
Table 5.
Venous Thromboembolism Events After Protocol Implementation
| Case | Clot type | PPD | Delivery type | BMI (kg/m2) | Risk factors | Embolism prophylaxis received | Adequate per protocol |
|---|---|---|---|---|---|---|---|
| 2014 (post-protocol implementation, pre-electronic order set) | |||||||
| 1 | PE | 2 | Scheduled cesarean | 29.4 | Cesarean, parity | SCDs | Yes |
| 2 | PE | 1 | Cesarean in labor | 35.9 | Cesarean, BMI, age, preeclampsia, immobility, infection | SCDs | No |
| 3 | PE | 5 | Scheduled cesarean | 31 | Cesarean-hysterectomy, BMI, transfusion, immobility, infection, parity | SCDs | No |
| 4 | PE | 5 | Vaginal | 33.7 | Tubal ligation, BMI, parity, infection | 7 days enoxaparin | No |
| 2015 (post-protocol implementation with electronic order set) | |||||||
| 5 | PE | 51 | Vaginal | 49 | BMI>40 | 7 days enoxaparin | Yes |
| 6 | PE | 13 | Vaginal | 34 | BMI, immobility | SCDs | Yes |
| 7 | PE | 6 | Cesarean in labor | 30 | Cesarean, BMI, infection | 7 days * enoxaparin | No |
| 8 | DVT andPE | 29 | Vaginal | 32 | Tubal ligation, BMI, parity | 7 days enoxaparin | Yes |
| 9 | PE | 51 | Vaginal | 41 | BMI >40 | 7 days enoxaparin | Yes |
PE is pulmonary embolism. DVT is deep vein thrombosis. PPD is postpartum day. BMI is body mass index. SCDs are sequential compression devices. Adequate per protocol is defined as identifying all correct risk factors and receiving indicated treatment per protocol.
Subject 7 was not started until day four of admission secondary to staff oversight.
Discussion
Implementation of a risk-based thromboembolism prophylaxis protocol increased the use of enoxaparin from less than 1 percent to over 30% of postpartum women for at least 7 days postpartum. Provider adherence to the protocol was excellent at 89.5% demonstrating the feasibility of implementation at our institution. Our sample size was inadequate to evaluate the effect of the protocol on rare outcomes such as thromboembolism and wound complications, and we did not see any differences in the incidence of these events.
Our protocol was based on RCOG guidelines which only require 2 risk factors to meet criteria for 7 days of prophylaxis, and 3 or more risk factors for 6 weeks of prophylaxis (5). Our protocol was less aggressive requiring 3 and 4 or more risk factors for 7 days and 6 weeks of prophylaxis, respectively. Had we followed the RCOG guidelines, over 54% of postpartum women would have met criteria for enoxaparin for at least 7 days postpartum. Notably, 3 women with a thromboembolism received enoxaparin for 7 days and then presented with a symptomatic embolism between 29–51 days postpartum. Per RCOG guidelines, these women would have met criteria for 6 weeks of prophylaxis, which may have prevented these later events.
Consistent with published data from Friedman et al (7), 6 of our 14 thromboembolic events occurred after vaginal deliveries. Thus, limiting the scope of an institutional protocol to cesarean deliveries may be missing up to 50% of those that could potentially benefit from prophylaxis.
The predominant limitation of our study is that the sample size was not large enough to detect a difference in rare events, most importantly thromboembolism. Approximately 10 years of deliveries from both before and after implementation of the protocol (n=32,191 per group) would be required to detect a 50% reduction in thromboembolism from the baseline incidence of 0.1583% to 0.0792% with 80% power and alpha of 0.05 using a two-sided Fisher’s exact test. Our study was also based at a single safety-net hospital with a largely Hispanic, uninsured or publically insured population which limits generalizability. We also may have missed thromboembolic events for women who presented to outside hospitals. However, this is unlikely as the majority of our patients remain in the Denver Health system for urgent care, emergent care and out-patient follow-up after delivery, and the incidence of thromboembolism in our cohort is consistent with population-based data (9). Finally, the lack of effect of the protocol on embolism rates may reflect patient non-compliance with the protocol after discharge from the hospital; however, our findings are indicative of “real world” practice.
Strengths of our study include the high rate (89.5%) of provider adherence to the implemented protocol. The early implementation of a modified RCOG protocol also allows for sharing data about the effect of risk-based protocol implementation at a time when other practices are considering implementation of similar thromboembolism prevention protocols in response to the NPMS bundle release.
Further research is needed to evaluate the utility and cost-effectiveness of implementing such a protocol on a broader scale in the United States. As Sibai and Rouse argue in their editorial addressing the NPMS thromboembolism safety bundle, there could be significant costs associated with nationwide use of a heparin risk-based protocol (7). The wholesale acquisition cost for a single syringe of 40mg enoxaparin at the time of analysis was $4.58. Over a one-month period with full protocol implementation (May 2015), the wholesale cost of enoxaparin was $9425.64. Importantly, this cost estimate does not consider the morbidity and costs associated with embolic events, or other costs associated with implementation of the protocol, such as nursing and physician time.
While a multi-center randomized controlled trial would be ideal to evaluate the effect of a pharmacologic prophylaxis protocol on the incidence of thromboembolism, this is unlikely to be the next step without more observational data. Rodger et al completed a feasibility study for such an RCT; among 1,346 potentially eligible women only 25 were randomized for a randomization rate of 0.7 per center per month (10). A feasible alternative may be to create a multi-center registry of current prophylaxis protocols and incidence of thromboembolic events.
We demonstrate that the implementation of a low-molecular-weight heparin thromboembolism prophylaxis protocol with a computerized order set resulted in high provider adherence to the protocol and a large increase in the rate of use of pharmaceutical prophylaxis for postpartum women at risk for thromboembolism. Whether the implementation of such a protocol results in a reduction of thromboembolic events and ultimately maternal deaths remains unknown.
Acknowledgments
Dr. Metz is supported by the National Institute on Child Health and Human Development under award number 2K12HD001271-16. This project was also supported by Colorado Clinical and Translational Sciences Institute (CTSI) with the Development and Informatics Service Center under award number UL1 RR025780. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors thank Renee Starr, RN, Informatics Specialist, for her assistance with data extraction for this manuscript.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
Each author has indicated that he/she has met the journal’s requirements for authorship.
Presented as a poster at the Society for Maternal-Fetal Medicine 37th Annual Meeting, Las Vegas, Nevada January 23-28 2017.
References
- 1.Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367(9516):1066–74. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 2.Royal College of Obstetritians and Gynaecologists. Reducing the Risk of Thrombosis and Embolism During. Green-top Guidel No 37a [Internet] 2009;(37a):1–35. Available from: http://www.rcog.org.uk/files/rcog-corp/GTG37aReducingRiskThrombosis.pdf.
- 3.Guimicheva B, Czuprynska J, Arya R. The prevention of pregnancy-related venous thromboembolism. Br J Haematol. 2015;168(2):163–74. doi: 10.1111/bjh.13159. [DOI] [PubMed] [Google Scholar]
- 4.D’Alton ME, Friedman AM, Smiley RM, Montgomery DM, Paidas MJ, D’Oria R, et al. National Partnership for Maternal Safety: Consensus Bundle on Venous Thromboembolism. Obstet Gynecol. 2016;128(4):688–98. doi: 10.1097/AOG.0000000000001579. [DOI] [PubMed] [Google Scholar]
- 5.Barbar S, Noventa F, Rossetto V, Ferrari A, Brandolin B, Perlati M, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: The Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450–7. doi: 10.1111/j.1538-7836.2010.04044.x. [DOI] [PubMed] [Google Scholar]
- 6.Bahl V, Hu HM, Henke PK, Wakefield TW, Campbell Da, Caprini Ja. A validation study of a retrospective venous thromboembolism risk scoring method. Ann Surg [Internet] 2010;251(2):344–50. doi: 10.1097/SLA.0b013e3181b7fca6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19779324. [DOI] [PubMed] [Google Scholar]
- 7.Sibai BM, Rouse DJ. Pharmacologic Thromboprophylaxis in Obstetrics: broader use demands better data. Obs Gynecol. 2016;128(4):681–4. doi: 10.1097/AOG.0000000000001656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris Pa, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap) - A metadata driven methodology and workflow process for providing translational research informatict support. J Biomed Inform [Internet] 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2700030/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tepper NK, Boulet SL, Whiteman MK, Monsour M, Marchbanks Pa, Hooper WC, et al. Postpartum Venous Thromboembolism. Obstet Gynecol [Internet] 2014;123(5):987–96. doi: 10.1097/AOG.0000000000000230. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00006250-201405000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Rodger MA, Phillips P, Kahn SR, James AH, Konkle BA. Low-molecular-weight heparin to prevent postpartum venous thromboembolism a pilot randomised placebo-controlled trial. Thromb Haemost. 2015;113(1):212–6. doi: 10.1160/TH14-06-0485. [DOI] [PubMed] [Google Scholar]