Abstract
Objectives
To characterize the clinical presentation and outcome in infants <6 months of age with Kawasaki disease (KD) and to describe the use of newer anti-inflammatory therapies in this young population.
Study design
We evaluated 88 infants < 6 months old and 632 ≥ 6 months old treated for KD. We compared differences in laboratory data, response to treatment, and coronary artery outcomes between the two cohorts. Fisher exact test was used to analyze categorical variables, while the Wilcoxon Rank Sum test was used for continuous variables.
Results
The majority of children in both cohorts were diagnosed and treated within the first 10 days of illness (median illness day 6 in both cohorts). For patients treated within the first 10 days after fever onset, a larger proportion of infants < 6 months old had a dilated or aneurysmal coronary artery on the initial echocardiogram compared with those ≥ 6 months old (43.4% vs. 19.5%). 18.6% of infants < 6 months old who had a normal echocardiogram at diagnosis, developed a dilated or aneurysmal coronary artery on a subsequent echocardiogram within 8 weeks of diagnosis. Twenty-eight infants < 6 months old received a single dose of infliximab without any untoward effects.
Conclusions
Despite treatment in the first 10 days, infants < 6 months old with acute KD are more likely to develop coronary artery abnormalities. Thus, the development of adjunctive therapies to reduce coronary artery damage should target this population.
Keywords: Kawasaki Disease, infants, coronary artery aneurysm, delayed diagnosis
Infants < 6 months of age are reported to have a higher prevalence of incomplete Kawasaki disease (KD), delayed diagnosis and treatment, coronary artery abnormalities (CAA), and intravenous immunoglobulin (IVIG) resistance. 1-7 These adverse outcomes may be intertwined as incomplete clinical signs can lead to delayed diagnosis, late treatment, and increased risk of aneurysms that develop in up to 25% of untreated or late treated patients with KD. However, limited information is available for this population. The aims of this study were to characterize the clinical presentation and outcome in infants with KD < 6 months of age and to describe the use of newer anti-inflammatory therapies in this young population.
Methods
We evaluated 88 infants < 6 months old and 632 ≥ 6 months old treated for KD between January 1, 2004 and December 31, 2013. Of the 88 infants, 53 were treated at Rady Children's Hospital San Diego (RCHSD) and had data prospectively collected, while 35 had data collected retrospectively at Children's Hospital Orange County (CHOC). All of the patients ≥ 6 months were treated at RCHSD and had data prospectively collected. We collected demographic and clinical data, including age, ethnicity, illness day at diagnosis (illness day 1 = first day of fever), response to intravenous immunoglobulin (IVIG) therapy, coronary artery status, complete blood count, erythrocyte sedimentation rate, and plasma concentrations of C-reactive protein (CRP), alanine aminotransferase (ALT), and γ-glutamyl transferase (GGT) prior to IVIG treatment. We age-adjusted hemoglobin concentrations and expressed the values as standard deviations from the mean (zHgb) according to the following formula: ([observed hemoglobin] – [Mean hemoglobin for age])/standard deviation for age.8 The standard deviations were estimated as one-quarter of the reported range of normal hemoglobin concentrations for each age interval.
Complete KD presentation was defined as fever ≥3 days and ≥4 of the following clinical signs: bilateral conjunctival injection, polymorphous skin rash, changes in the lips or oral mucosa, changes (edema/redness/peeling) of the extremities, and unilateral cervical lymphadenopathy. Patients with fever ≥ 3 days plus ≤4 criteria with or without echocardiogram abnormalities were classified as incomplete KD. The response to IVIG therapy was classified as resistant, responsive, late treatment (≥11 days after fever onset), not treated, or unevaluable (received infliximab in addition to IVIG as initial therapy either for coronary artery aneurysms or because of clinical trial participation). IVIG-resistance was defined as persistent or recrudescent fever (T ≥38.0°C rectally or orally) at least 36 hours but not longer than 7 days after completion of the first IVIG infusion.
Echocardiograms were performed according to a standard protocol applied to all patients with KD. The standard of care at both centers is to perform an echocardiogram at the time of initial diagnosis and at 2 weeks after diagnosis in all patients with KD. In addition, infants and children with coronary artery damage have echocardiograms performed more frequently and at similar intervals (i.e. every one to two weeks depending on severity of illness) as part of standard of care at both institutions. Patients were classified at RCHSD as having normal (<2.5 standard deviation units [Z-score] from the mean, normalized for body surface area), dilated (Z-score ≥2.5 to <4), or aneurysmal (Z ≥ 4; Z>10 for giant aneurysm) coronary arteries on the basis of the maximal internal diameters of the right coronary artery (RCA) and left anterior descending artery (LAD) measured by echocardiography at the time of diagnosis and up to 8 weeks after onset of fever. The worst-ever Z-score (Z-worst) for either the RCA or LAD at any time point during the first 6 weeks after fever onset or the Z-score of the largest aneurysm in any coronary artery segment was used for the continuous variable analysis. Body surface area and Z-scores were calculated using the Haycock and the Dallaire equations, respectively.9 At CHOC, coronary artery abnormalities were classified as normal, dilated or aneurysmal (focal dilation of an arterial segment at least 1.5 times the diameter of the adjacent segment). The study received Institutional Review Board approval at the University of California, San Diego and CHOC.
Statistical Analyses
The data for infants < 6 months old were combined for the two sites and compared with infants and children ≥ 6 months old. Worst Z-score on any echocardiogram among patients was compared for infants < 6 months old vs. those ≥ 6 months old for RCHSD only as Z-score data were not available for CHOC. Fisher exact test was used to analyze categorical variables, while the Wilcoxon Rank Sum test was used for continuous variables.
For laboratory values that were outside the dynamic range of the test (i.e., ESR of ≥140 mm/h, ALT ≤3 mg/dL and CRP ≤0.3 mg/dL), the maximal or minimal value detected by the assay was used, as appropriate. All statistical analyses were performed in the statistical software R version 3.1.0 (available at: http://www.R-project.org). No adjustments were made for multiple testing. P-values less than 0.05 were considered statistically significant.
Results
Of the patients in this study, 88 (12.2%) were < 6 months old at the time of diagnosis with KD and the remaining 632 patients (87.8%) were between 6 months and 17 years old (Table I). As is seen typically in KD, a predominance of the patients were male in both cohorts. There were more patients with KD of Asian ethnicity in the < 6 month old cohort and a larger number of patients that were of mixed racial background in those ≥ 6 months. Both cohorts had a median illness day of 6 days at the time of diagnosis and the majority in both cohorts (86.4% in < 6 months old and 86.2% in ≥ 6 months old) were diagnosed and treated within the first 10 days of illness.
Table 1. Demographic and clinical characteristics, diagnostic criteria, treatment and treatment responses of the study populations.
| < 6 months old (CHOC & RCHSD) N=88 | ≥ 6 months old (RCHSD only) N=632 | P value | |
|---|---|---|---|
|
| |||
| Age at diagnosisa | 4.1 months (3.1 – 5.0) | 2.9 years (1.7 to 4.8 years) | -- |
|
| |||
| Male sex, N (%) | 62 (70.5) | 385 (60.1) | NS |
|
| |||
| Race/Ethnicity, N (%) | |||
| White | 18 (20.5) | 148 (23.4) | 0.001b |
| Hispanic | 29 (33) | 215 (34) | |
| Asian | 27 (30.7) | 90 (14.2) | |
| African-American | 3 (3.4) | 26 (4.1) | |
| Native American | 0 (0) | 3 (0.5) | |
| Native Hawaiian or Pacific Islander | 1 (1.1) | 2 (0.3) | |
| More than one race | 8 (9.1) | 135 (21.4) | |
| Other | 2 (2.2) | 6 (1) | |
| Unknown | 0 (0) | 7 (1.1) | |
|
| |||
| Days of illness at diagnosisa | 6 (4-8) | 6 (5-8) | NS |
|
| |||
| Number of subjects (%) diagnosed at: | |||
| ≤ 10 days | 76 (86.4) | 545 (86.2) | NS |
| >10 days | 12 (13.6) | 87 (13.8) | |
|
| |||
| White blood cell count (×103) a | 14.7 (12.0 – 19.8) | 13.1 (10.3 – 17.1) | 0.003 |
|
| |||
| zHgba,c | -1.7 (-2.5 to -0.8) | -1.2 (-2.2 to -0.3) | 0.002 |
|
| |||
| Platelets (×103) a | 451 (372 – 577) | 366 (285 - 469) | <0.001 |
|
| |||
| Erythrocyte sedimentation rate (mm/hr) a | 55 (33 - 67) | 58 (39 - 74) | NS |
|
| |||
| C-reactive protein (mg/dl) a | 11.8 (6 - 18.1) | 6.5 (3.4 - 14.5) | <0.001 |
|
| |||
| Alanine aminotransferase (U/L) a | 26 (17 - 39) | 39 (20 - 103) | <0.001 |
|
| |||
| Gamma glutamyl transferase (U/L) a | 52 (26 - 93) | 37 (17 - 117) | NS |
|
| |||
| Albumin a | 3.2 (2.8-3.8) | 3.9 (3.5-4.2) | <0.001 |
|
| |||
| Clinical Presentation, N (%) d | |||
| Rash | 81 (92.1) | 577 (91.3) | NS |
| Conjunctival injection | 81 (92.1) | 581 (91.9) | NS |
| Oral changes | 75 (85.2) | 583 (92.3) | 0.04 |
| Unilateral cervical | 5 (5.7) | 201 (31.9) | <0.001 |
| lymphadenopathy | 58 (65.9) | 533 (84.3) | <0.001 |
| Extremity changes | |||
|
| |||
| Complete KDe, N (%) | 45 (51.1) | 505 (79.9) | <0.001 |
|
| |||
| Incomplete KD, N (%) | 43 (48.9) | 127 (20.1) | |
| By laboratory criteriaf | 24/88 (27.3) | 111/632 (17.6) | <0.001 |
| By echo criteriag | 19/88 (21.6) | 16/632 (2.5) | <0.001 |
|
| |||
| IVIG, N (%)h | 86 (97.7) | 615 (97.8) | NS |
|
| |||
| Treated after 10th day of illness, N (%) | 10 (11.6) | 70 (11.3) | NS |
|
| |||
| IVIG resistancei | 7 (13.0) | 75 (16.6) | NS |
| 2nd IVIG | 4 (57.1) | 28 (37.3) | |
| Infliximab | 3 (42.9) | 47 (62.7) | |
|
| |||
| Indications for infliximab N (%)j | 22 (28.9) | 94 (17.2) | 0.02 |
| Clinical trial k | 5 (22.7) | 67 (71.3) | |
| Coronary artery abnormality k | 17 (77.3) | 24 (25.5) | |
| Otherk,l | 0 (0) | 3 (3.2) | |
NS = Not significant
All values expressed as median (25% to 75% IQR)
For ethnicity, there was a significant difference in the groups labeled ‘Asian’ and ‘More than one race’
zHgb is the SD units from the mean for age-adjusted hemoglobin values 1
These clinical features are based on the American Heart Association criteria for KD. Oral changes include erythematous oropharynx or lips or strawberry tongue. Cervical lymphadenopathy is unilateral and is a lymph node at least 1.5 cm.
Complete KD as defined by the AHA with at least 4 of the 5 clinical criteria
Incomplete KD by laboratory evaluation is defined by the AHA with <4 clinical criteria and laboratory inflammation (ESR ≥ 40 mm/hr or CRP ≥ 3 mg/dl with ≥ 3 supplementary labs elevated: albumin levels of <3.0 g/dL, anemia for age, elevation of ALT level, >450 000 platelets per mm3 after the seventh day, white blood cell count of >15 000 cells per mm3, and >10 white blood cells per high-power field in the urine)2
Incomplete KD by echocardiographic criteria is defined by the AHA with <4 clinical criteria and z score of LAD or RCA ≥2.52
All subjects who received IVIG were given 2 g/kg
Only subjects treated with IVIG within the first 10 days of illness who did not receive infliximab as part of a clinical trial, or for the indications of echo abnormality or severity of illness within 36 hours of IVIG infusion (N=54 for < 6 months; N= 451 for ≥ 6 months), were included in the denominator
Using denominator of all subjects who received IVIG in the first 10 days of illness (N=76 for <6 months; N=545 for ≥ 6 months old)
Using denominator of subjects who received infliximab for an indication other than treatment resistance and were treated in the first 10 days of illness (N=22 for <6 months; N=94 for subjects ≥ 6 months old)
Indications for infliximab included severe reactive arthritis, severe systemic inflammation, and KD shock syndrome
[1] Gunn L, Nechyba C. The Harriet Lane Handbook:16th edition. Philadelphia: Mosby; 2002.
[2] Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004; 110:2747-71.
Overall, oral changes, unilateral cervical lymphadenopathy, and extremity changes were more common in the older group (p = 0.04, <0.001, and <0.001, respectively) (Table I). Although there was a larger proportion of patients with complete KD at RCHSD (33/53, 62.3%) than at CHOC (12/35, 34.3%), this proportion was higher for patients older than 6 months old (505/632, 79.9%). Patients <6 month old were more likely to be diagnosed based on an abnormal baseline echocardiogram than by laboratory evaluation as per the 2004 AHA KD guidelines (Table I). With respect to laboratory data, the median WBC, platelet count and CRP were higher and alanine aminotransferase and albumin were lower in the < 6 month group (Table I). We also compared the demographic, clinical and laboratory data between infants < 6 months old with and without coronary artery abnormalities and there were no significant differences in sex, ethnicity, clinical presentation and baseline laboratory data between these two groups.
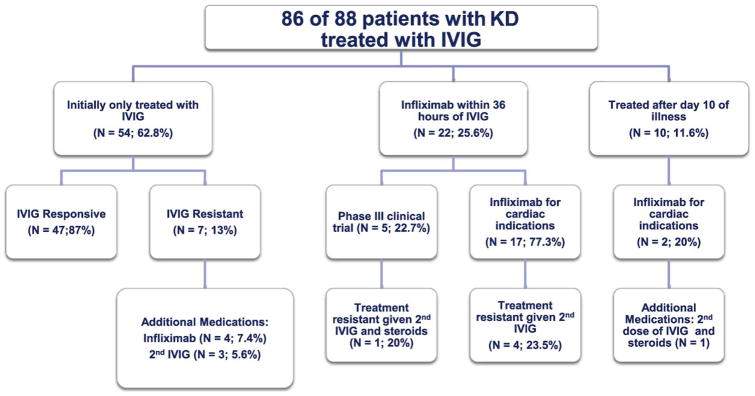
All patients were treated with IVIG (2 g/kg) and aspirin except for patients who presented late in the course of illness and no longer had evidence of systemic inflammation (2 of 88 <6 months old (2.3%); 17 of 632 ≥ 6 months old (2.7%)) (Table I). At RCHSD, the clinical practice was to administer infliximab (5 mg/kg) to all patients with an initial RCA or LAD Z score ≥ 2.5 and as the first re-treatment for IVIG-resistance. At CHOC, the clinical practice was to administer infliximab to all patients with a coronary artery dilatation or aneurysm and to administer a second dose of IVIG for IVIG-resistance. Infants <6 months old were more likely to receive infliximab for coronary artery dilation by echocardiogram as compared with those ≥ 6 months old who were more likely to have received infliximab as part of a Phase III clinical trial. Of the 86 infants treated with IVIG, 22 (25.6%) received infliximab within 36 hours of IVIG either as part of a Phase III clinical trial (N=5, 22.7%) or for a dilated or aneurysmal coronary artery on the first echocardiogram (N=17, 77.3%) (Figure 1; available at www.jpeds.com). At the two sites combined, a total of 28 infants < 6 months old received a single dose of infliximab without any untoward effects at either the time of administration or over a follow up period of at least one year. Because of theoretical concerns about the safety of infliximab administration to patients who had recently received live virus vaccines, we analyzed antecedent rotavirus vaccine administration in our < 6 month old cohort. Of the 21 infants < 6 months old who received infliximab at RCHSD, 8 (38.1%) had received one dose of rotavirus vaccine within 3 months of receiving infliximab (4 of these (19%) within 1 month) without any side effects. Contrary to published experience, the prevalence of IVIG resistance for patients with KD treated with IVIG alone within the first 10 days of illness was similar between those <6 months old and those ≥ 6 months old (13% and 16.6%, respectively).
Figure 1. Treatments administered to infants < 6 months old.
For patients treated within the first 10 days after fever onset at both sites, a larger proportion of infants < 6 months old had a dilated or aneurysmal coronary artery on the initial echocardiogram as compared with those ≥ 6 months old (35.5% dilated, 6.6% aneurysm, 1.3% giant aneurysm vs. 16.7% dilated, 2.6% aneurysm, 0.2% giant aneurysm, P<0.001) (Table II). Aneurysms and giant aneurysms were significantly more common over the first 8 weeks since diagnosis at both sites in infants < 6 months old compared with those ≥ 6 months old (13.2% aneurysm and 5.3% giant aneurysm vs. 4.4% aneurysm and 0.6% giant aneurysm, P<0.001). Eight of the 43 patients (18.6%) < 6 months old who had a normal echocardiogram at diagnosis developed a dilated or aneurysmal coronary artery on a subsequent echocardiogram within 8 weeks of diagnosis. Of these, 7 infants, including the two who developed aneurysms, had an abnormal echocardiogram within 2 weeks of the initial normal echocardiogram (median 7 days, range 2-13 days). One infant had a borderline dilated coronary artery (Z 2.1) which steadily increased in size over the course of a month and then qualified as a dilated coronary artery by AHA criteria (Z 2.6).
Table 2. Coronary Artery Abnormalities in KD Subjects Treated in the First 10 Days of Illness*.
| < 6 months old (CHOC & RCHSD) N=76 | ≥ 6 months old (RCHSD only) N=545 | P value | |
|---|---|---|---|
|
| |||
| Baseline coronary artery status, N (%) | <0.001 | ||
| Normal | 43 (56.6) | 439 (80.6) | |
| Dilated | 27 (35.5) | 91 (16.7) | |
| Aneurysm | 5 (6.6) | 14 (2.6) | |
| Giant aneurysm | 1 (1.3) | 1 (0.2) | |
|
| |||
| Worst coronary artery status | <0.001 | ||
| Normal | 35 (46.1) | 414 (76.0) | |
| Dilated | 27 (35.5) | 104 (19.1) | |
| Aneurysm | 10 (13.2) | 24 (4.4) | |
| Giant aneurysm | 4 (5.3) | 3 (0.6) | |
Subjects were classified at RCHSD as having normal (<2.5 standard deviation units [Z-score] from the mean, normalized for body surface area), dilated (Z-score ≥2.5 to <4), or aneurysmal (Z ≥ 4; Z>10 for giant aneurysm) coronary arteries on the basis of the maximal internal diameters of the right coronary artery (RCA) and left anterior descending artery (LAD) measured by echocardiography at the time of diagnosis and up to 8 weeks after onset of fever. At CHOC, coronary artery abnormalities were classified as normal, dilated or aneurysmal (focal dilation of an arterial segment at least 1.5 times the diameter of the adjacent segment). The worst coronary artery status was based on the echocardiograms conducted in the first 8 weeks of illness.
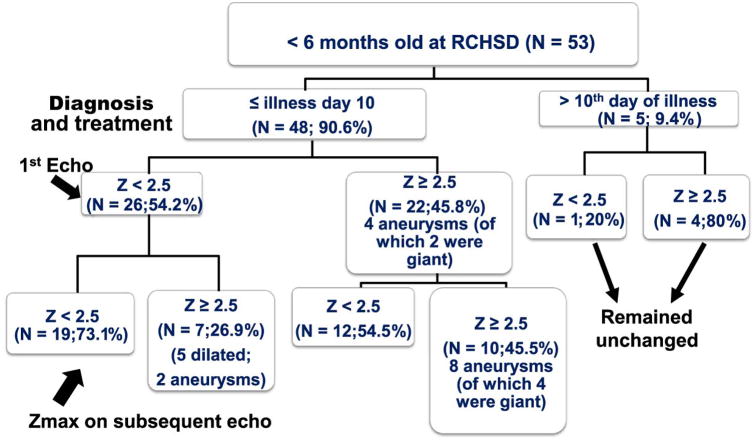
Coronary artery Z-score data were available for all patients with KD treated at RCHSD. In infants < 6 months old diagnosed and treated for KD at RCHSD within the first 10 days of illness (N=48), only 26 (54.2%) had normal Z scores on their initial echocardiogram (Figure 2; available at www.jpeds.com). Seven of the 26 (26.9%) with initially normal echocardiograms went on to have new abnormalities by echo including five who developed new dilation (Z-score 2.6-3.8) and two who developed aneurysms with maximal Z scores of 5.7 and 7.2. In contrast, only 30 of 439 patients (6.8%) older than 6 months treated within the first 10 days of illness had an initial normal echo with subsequent abnormalities on follow-up echoes. Of the 22 infants < 6 months (45.8%) who had an initial abnormal echocardiogram, 10 remained abnormal with Z-scores ranging from 2.8-12.2. Four patients had giant aneurysms with Z-scores from 11.1-12.2. Thus, overall, 17 (35.4%) infants < 6 months old had persistent coronary artery dilation or aneurysm despite timely treatment within the first 10 days after onset of fever.
Figure 2. Progression of coronary artery Z-scores of patients with KD < 6 months old by illness day at diagnosis.
Discussion
Our study emphasizes the poor outcomes in a young patient population despite timely diagnosis and treatment. The first report of the clinical picture of infants younger than 6 months of age was published in 1986 by Burns et al.3 Of the eight infants with KD in that study, six developed coronary artery aneurysms and two of these died from the coronary artery vasculitis.
In this current study with data combined from both sites, even with timely diagnosis and treatment with IVIG in the first 10 days of illness, nearly 20% of infants < 6 months had an aneurysm or giant aneurysm as compared with 5% of infants ≥ 6 months old. Furthermore, over 50% of the infants had coronary artery abnormalities on their initial echocardiogram. One limitation of our study is that Z-scores of the coronary arteries were only available at RCHSD. However, as with our study, other recent studies of this young population have also had a high prevalence of CAA including 35% in a study from Chandigarh, India and 30.8% in a study in Anyang, Korea.1, 7 Also, in our study, infants < 6 months of age were more likely to be of Asian descent. This is consistent with data from Japan where the highest incidence of KD is seen in infants less than a year old.10
As compared with the published literature where age < 6 months is considered a risk factor for late diagnosis, the median illness day for diagnosis in our study was day 6, which afforded the opportunity to understand how the coronary artery damage can evolve in these infants despite timely diagnosis and treatment 11. It is alarming that more than 25% of infants < 6 months old with a normal echocardiogram initially went on to develop a dilated or aneurysmal coronary artery, most within the first two weeks of treatment, emphasizing the importance of careful and frequent echocardiographic monitoring of these infants.
Age < 6 months old has been reported to be an important variable in predicting IVIG resistance. 12, 13 In our study the prevalence of IVIG-resistance was similar between those < 6 months old and those as least 6 months of age. This may in part be because so many infants received alternative therapies, most notably infliximab, early on in the course of illness either as part of a clinical trial or for a cardiac indication, thus making it impossible to determine if those patients were resistant to IVIG alone. Infliximab was well tolerated by these infants, even with recent administration of rotavirus vaccine in a subset of these patients.
The percentage of infants presenting with incomplete KD has also varied widely in the published literature, ranging from 19 to 88% depending on the definition used and the method of data collection.1, 7, 14, 15 In our study, the proportion of patients with KD < 6 months old presenting with incomplete KD varied by site. At RCHSD, the prevalence of incomplete KD in infants < 6 months old (37.8%) was similar to those older than 6 months old. It should be noted that there were methodological differences in reporting as at RCHSD the data were prospectively collected on standardized case report forms, whereas at CHOC data were retrospectively collected by chart review. This may have influenced the quality of the data. In addition, patients at RCHSD were evaluated prospectively by one of two experienced clinicians specializing in KD, whereas at CHOC one of five experienced infectious disease clinicians evaluated and treated the patients. Of the infants with incomplete KD in our study, 21.6% were diagnosed based on an abnormal first echocardiogram as compared with only 2.5% in those older than 6 months of age. This further supports the recommendation in the AHA KD Guidelines “Infants ≤ 6 months old on day ≥ 7 of fever without other explanation should undergo laboratory testing and, if evidence of systemic inflammation is found, an echocardiogram, even if the infants have no clinical criteria”, stressing the importance of an echocardiogram in diagnosing KD in a febrile infant.16
Given the high prevalence of coronary artery abnormalities in this young population even with timely diagnosis and treatment in the first 10 days of illness, the development of adjunctive therapies to prevent coronary artery abnormalities in young infants should be encouraged.
Acknowledgments
Supported by the National Heart, Lung, and Blood Institute (HL69413 and K24 HL074864 [J.B.]), Rady Children's Hospital San Diego Physician Development Fund (to A.T), the National Center for Research Resources and the National Center for Advancing Translational Sciences (UL1 TR001414 [to A.T.]) and The Macklin Foundation.
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Singh S, Agarwal S, Bhattad S, Gupta A, Suri D, Rawat A, et al. Kawasaki disease in infants below 6 months: a clinical conundrum? International journal of rheumatic diseases. 2016 doi: 10.1111/1756-185X.12854. [DOI] [PubMed] [Google Scholar]
- 2.Rosenfeld EA, Corydon KE, Shulman ST. Kawasaki disease in infants less than one year of age. J Pediatr. 1995;126:524–9. doi: 10.1016/s0022-3476(95)70344-6. [DOI] [PubMed] [Google Scholar]
- 3.Burns JC, Wiggins JW, Jr, Toews WH, Newburger JW, Leung DY, Wilson H, et al. Clinical spectrum of Kawasaki disease in infants younger than 6 months of age. J Pediatr. 1986;109:759–63. doi: 10.1016/s0022-3476(86)80689-8. [DOI] [PubMed] [Google Scholar]
- 4.Manlhiot C, Yeung RS, Clarizia NA, Chahal N, McCrindle BW. Kawasaki disease at the extremes of the age spectrum. Pediatrics. 2009;124:e410–5. doi: 10.1542/peds.2009-0099. [DOI] [PubMed] [Google Scholar]
- 5.Pannaraj PS, Turner CL, Bastian JF, Burns JC. Failure to diagnose Kawasaki disease at the extremes of the pediatric age range. Pediatr Infect Dis J. 2004;23:789–91. doi: 10.1097/01.inf.0000134312.39744.a4. [DOI] [PubMed] [Google Scholar]
- 6.Song D, Yeo Y, Ha K, Jang G, Lee J, Lee K, et al. Risk factors for Kawasaki disease-associated coronary abnormalities differ depending on age. Eur J Pediatr. 2009;168:1315–21. doi: 10.1007/s00431-009-0925-0. [DOI] [PubMed] [Google Scholar]
- 7.Yoon YM, Yun HW, Kim SH. Clinical Characteristics of Kawasaki Disease in Infants Younger than Six Months: A Single-Center Study. Korean circulation journal. 2016;46:550–5. doi: 10.4070/kcj.2016.46.4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunn L, Nechyba C. The Harriet Lane Handbook. 16th. Philadelphia: Mosby; 2002. [Google Scholar]
- 9.Dallaire F, Dahdah N. New equations and a critical appraisal of coronary artery Z scores in healthy children. J Am Soc Echocardiogr. 2011;24:60–74. doi: 10.1016/j.echo.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Makino N, Nakamura Y, Yashiro M, Ae R, Tsuboi S, Aoyama Y, et al. Descriptive epidemiology of Kawasaki disease in Japan, 2011-2012: from the results of the 22nd nationwide survey. J Epidemiol. 2015;25:239–45. doi: 10.2188/jea.JE20140089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minich LL, Sleeper LA, Atz AM, McCrindle BW, Lu M, Colan SD, et al. Delayed diagnosis of Kawasaki disease: what are the risk factors? Pediatrics. 2007;120:e1434–40. doi: 10.1542/peds.2007-0815. [DOI] [PubMed] [Google Scholar]
- 12.Egami K, Muta H, Ishii M, Suda K, Sugahara Y, Iemura M, et al. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. The Journal of pediatrics. 2006;149:237–40. doi: 10.1016/j.jpeds.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi T, Inoue Y, Takeuchi K, Okada Y, Tamura K, Tomomasa T, et al. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. 2006;113:2606–12. doi: 10.1161/CIRCULATIONAHA.105.592865. [DOI] [PubMed] [Google Scholar]
- 14.Joffe A, Kabani A, Jadavji T. Atypical and complicated Kawasaki disease in infants. Do we need criteria? West J Med. 1995;162:322–7. [PMC free article] [PubMed] [Google Scholar]
- 15.No SJ, Kim DO, Choi KM, Eun LY. Do predictors of incomplete Kawasaki disease exist for infants? Pediatr Cardiol. 2013;34:286–90. doi: 10.1007/s00246-012-0440-3. [DOI] [PubMed] [Google Scholar]
- 16.Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110:2747–71. doi: 10.1161/01.CIR.0000145143.19711.78. [DOI] [PubMed] [Google Scholar]