Abstract
Objective
To assess the therapeutic efficacy of local injections with Botulinum Toxin Type-A (Btx-A) in improving blood flow to the hands of patients with Raynaud's Phenomenon (RP) secondary to scleroderma.
Methods
In this randomized, double-blind, placebo-controlled clinical trial (ClinicalTrials.gov #NCT02165111), patients with scleroderma-associated RP received Btx-A (50 units in 2.5 mL) in one randomly selected hand and sterile saline (2.5 mL) in the opposite hand. Follow-up at 1 and 4 months post-injection included Laser Doppler Imaging (LDI) of hands, patient-reported outcomes, and physical exam. We compared outcomes using paired t-test and population average generalized models with generalized estimating equations.
Results
Of 40 patients enrolled, 25 had limited and 15 diffuse scleroderma. From baseline to 1-month follow-up, there was a greater reduction in average blood flow in Btx-A hands compared to placebo. The model estimated that this difference was statistically significant (average difference: -30.08 flux units, 95% CI: -56.19 to -3.98, p-value=0.024). This difference was mainly influenced by patients with longstanding RP and diffuse scleroderma. Change in blood flow at 4-month follow-up was not significantly different between groups. Clinical measures improved slightly for Btx-A hands in QuickDASH, McCabe score, pain VAS, and Raynaud's Condition Score.
Conclusion
Our laboratory-based LDI flow data do not support using Btx-A to treat RP in all scleroderma patients. The secondary clinical outcomes suggest some positive, but questionably clinically meaningful effect. The role of Btx-A in treating RP should be further studied with more homogeneous patient populations and in unique clinical situations like acute digital ischemia.
Introduction
Raynaud's phenomenon (RP) is present in most patients with scleroderma and is often the presenting symptom of the disease process.1 This painful and debilitating condition results from increased vascular reactivity combined with structural vascular disease in the cutaneous arterial supply to the digits.1-3 RP strongly impacts patient quality of life4 and can result in recurrent digital ulcers and critical ischemic events in scleroderma,5 which may require partial amputations of the affected digits.
Despite the heavy burden of RP on patients with scleroderma, treatment options are not ideal. Symptom control and prevention of disease progression rely on non-drug measures coupled with pharmacological treatment with vasodilators such as calcium channel blockers, phosphodiesterase-5 inhibitors, endothelin receptor antagonists and prostacyclins.1 If this treatment fails or the patient cannot tolerate its side effects, patients are left with few options. Surgical treatment, requiring time- and skill-intensive microsurgery, consists in peri-arterial sympathectomy and is generally limited to patients with RP refractory to medical therapy.6 Repair of larger vessel occlusive disease is a very uncommon surgical option.
Botulinum Toxin Type-A (Btx-A) injected locally into the hand is considered a possible alternative therapy for RP. Several mechanisms are proposed for the potential effect of Btx-A on RP, involving the inhibition of sympathetic adrenergic or cholinergic vasoconstriction, sensory nerves and/or endothelial exocytosis of endothelin-1.3, 7 Studies in animal models find a positive effect of Btx-A increasing vascular blood flow by inhibiting sympathetic vasoconstriction.8 Observational studies involving patients with RP report that Btx-A injected into the perivascular space of the affected digits provides significant symptomatic relief.9-15 Most of these studies are retrospective case series;10, 11, 13, 15 the two prospective cohort studies published have low internal validity due to lack of randomization, placebo controls, and/or blinding.12, 14 Most studies have had heterogeneous patient populations, including patients with primary RP, secondary RP associated with other connective tissue disorders, and/or acute digital ischemia.10, 11, 13, 15 Only two studies have focused specifically on RP secondary to scleroderma,12, 14 limiting the interpretation of the evidence for this patient population. Anecdotal evidence from one case series suggests that patients with scleroderma may be less responsive to Btx-A injections compared to other patients with RP.11
We conducted a randomized, double-blind, placebo-controlled clinical trial to determine if local injections with Btx-A would be an efficacious intervention to control RP by improving blood flow and symptoms in patients with RP secondary to scleroderma.
Patients and Methods
Study design and setting
This was a randomized, double-blind, parallel-group, placebo-controlled, clinical trial conducted at the Johns Hopkins Scleroderma Center in Baltimore, MD, USA (ClinicalTrials.gov # NCT02165111). Participants were enrolled between January and May 2015; follow-up was completed by September 2015.
Participants
Eligible participants included adults aged 18 or over, with bilateral RP16 and scleroderma. Patients enrolled had a diagnosis of scleroderma defined by 1980 American College of Rheumatology (ACR) criteria,17 2013 ACR/EULAR criteria, having at least 3/5 CREST (calcinosis, Raynaud's phenomenon, esophageal dysmotility, sclerodactyly, telangiectasia) syndrome criteria, or having definite Raynaud's phenomenon, abnormal nailfold capillaries and a scleroderma-specific autoantibody. Patients were excluded if they had active infections in either hand, acute digital ischemia, a history of myasthenia gravis, any known allergies or hypersensitivity to Botulinum toxin preparations, prior administration of Botulinum toxin vaccine, current use of aminoglycoside antibiotics, prior upper extremity vascular surgery, including surgical sympathectomy, and those who were pregnant or lactating.
Interventions
Participants received Btx-A (OnabotulinumtoxinA, Allergan plc, Dublin, Ireland) in one randomly-selected hand and sterile saline in the opposite hand. Injections were performed by two hand surgeons, using a 3 mL syringe and 30-gauge needle through the dorsal surface in 7 locations per hand: adjacent to the 2nd, 3rd, and 4th common digital arteries through the web spaces (10 units each=30 units or 1.5mL), and the radial side of the index finger proximal phalanx base, the ulnar side of the small finger proximal phalanx base, and each side of the thumb proximal phalanx base (5 units each=20 units or 1mL). Figure 1 illustrates our injection protocol. Hands in the experimental group received 50 units of Btx-A in total, reconstituted in 2.5 mL of sterile saline. Hands in the control group received 2.5 mL of sterile saline in total as placebo.
Figure 1.
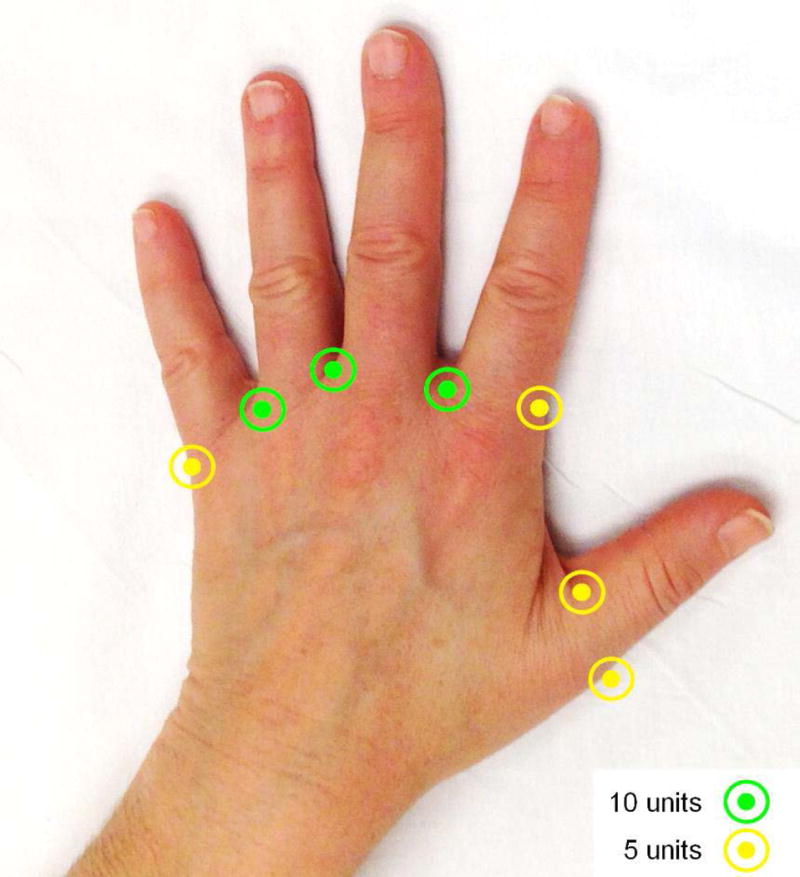
Sites of injection and dose.
Randomization, blinding, and allocation concealment
Our study pharmacist generated a random sequence with blocks of 4 patients for study arm allocation in a 1:1 ratio using Microsoft Excel 2007 (Microsoft Corp., Redmond, WA). Study participants and all study team members (including hand surgeons and the study statistician), except for the study pharmacist, were blinded to treatment allocation and the size of randomization blocks. Both injections were clear and visually not distinguishable. The study coordinator enrolled all study participants. The study pharmacist assigned treatment allocation and maintained allocation concealment throughout the study period.
Data collection and outcomes
Follow-up consisted of a safety phone call (or optional in-person study visit) at 7 days (±3 days) post-injection and in-person study visits at 1 month (±8 days) and 4 months (±15 days) post-injection. In-person visits included non-invasive Laser Doppler Imaging (LDI) of hands, pulse oximetry, and physical exam for digital ulceration. Patient-reported outcomes were collected specifically for each hand, using instruments validated in other upper extremity and hand conditions (QuickDASH,18 McCabe Cold Sensitivity Score,19 and VAS pain scale)20 and specifically for RP (Raynaud's Condition Score (RCS)).21 Patients were asked at each visit their opinion on which hand they thought had been treated with Btx-A. This helped assess the success of blinding.22
Our primary outcome was the change in blood flow from baseline to 1-month follow-up, measured using the Moor LDI2-IR scanner (Moor Instruments Ltd., UK). By scanning tissues with a low-energy laser beam, LDI quantifies the Doppler effect between red blood cells and the scanner to determine blood flow speed, allowing non-invasive, objective measurement of superficial cutaneous blood flow.23 LDI has been proposed as an outcomes measure for microvascular research,24 and has been extensively used for scleroderma-associated secondary RP.23, 25-28 The study room temperature was maintained at 77° Fahrenheit (25° Celsius) and the study staff waited 30 minutes after patient arrival in the room to allow temperature equilibration before performing scans. LDI readings were analyzed using Moor LDI Review v6.0 software (Moor Instruments Ltd., UK), plotting regions of interest including the 2nd, 3rd, and 4th digits, limited by a line from the 4th web space to the lateral aspect of 2nd digit over the flare of the metacarpophalangeal joint. Mean flux units within this region were calculated and three consecutive scans were averaged for each visit. Intra-class correlation of consecutive scans in each arm within a patient was 95.6%, calculated using a three-level linear mixed effect model with participant and arm as random intercepts.
Secondary outcomes were the change in blood flow to 4-months post-injection, patient-reported outcomes (QuickDASH, McCabe Cold Sensitivity Score, and VAS pain scale) at 1-month and 4-months post-injection, and weekly RCS completed until 17 weeks post-injection. Tertiary outcomes were the number of active digital ulcers per physical exam (defined clinically by the investigators) and standardized photography of the digits.
Weekly ambient temperature was collected at study participant's zip codes from a publicly available website (www.weather.com). Clinical characteristics relating to patient disease severity were tabulated from a prospective database maintained by the Johns Hopkins Scleroderma Center, including history of renal crisis or pulmonary hypertension, scleroderma subtype, diagnosis year, RP onset year, and Medsger RP severity score. Medsger severity scale is a set of validated severity grading scales for each organ system involved in scleroderma. The scale for peripheral vascular involvement classifies patients into grade 0: no RP, grade 1: RP, grade 2: digital pitting scars, grade 3: digital tip ulcerations, or grade 4: digital gangrene.29
Sample size
Using data from the literature,30 a sample of 40 study participants, or 80 paired blood flow measurements, was estimated to provide at least 80% power to detect a paired difference of 1.3 kHz, equivalent to 87% difference from baseline in blood flow measurements, with a type I error of 5% and conservatively assuming a within-person correlation of blood flow measurements between 0.3 and 0.5 and standard deviation of less than 2.9 kHz for the paired difference.
Statistical methods
Continuous variables were summarized using means and standard deviations (SD) or medians and interquartile ranges (IQR), as appropriate, and categorical variables using proportions. An interim analysis was performed for the primary outcome once the first 20 patients had completed 1-month post-injection study visits, using paired t-test. A stopping rule established that if either group was found to be significantly better over the other, study enrollment and interventions would stop.
The final analysis assessed changes in blood flow and secondary outcomes by fitting generalized linear population-average models with robust variance using generalized estimating equations and exchangeable working correlation structure. Models included treatment arm (Btx-A vs. placebo), time point (1 month vs. baseline and 4 months vs. baseline) and their interaction. Interaction terms tested whether blood flow (or secondary outcome) trajectories differed by treatment arm. Unlike other secondary variables, RCS data included weekly measurements for the study duration (17 weeks). A separate model estimated changes in RCS over time and, to adjust for ambient temperature, temperature data were included as a fixed effect in the model. Significance levels were set at alpha≤0.001 for the interim analysis, alpha≤0.049 for final analysis of the primary outcome (to account for the interim analysis),31 and alpha≤0.050 for all other analyses.
Before conducting data analysis or unblinding, 4 variables were defined for sub-group analysis: 1) cutaneous subtype (diffuse skin disease vs limited scleroderma), classified according to Leroy et al.32 2) RP severity, quantified as the maximum ever recorded peripheral vascular disease/RP score from first scleroderma center visit to study enrollment from the Medsger severity scale;29 3) years since RP onset; and 4) calcium channel blockers use at baseline. This analysis was intended for hypothesis generation, rather than hypothesis testing, and to potentially guide future studies.
Ethical considerations
This study conforms to the Declaration of Helsinki ethical principles for medical research. Institutional Review Board (IRB) approval was obtained from the Johns Hopkins Medicine IRB (protocol #NA_00087346). Three independent faculty members at our institution served as Adverse Events Committee, monitoring and overseeing serious and/or unexpected adverse events. This study is registered on ClinicalTrials.gov (NCT02165111). No major changes to the protocol were made during the study in regards to eligibility criteria, interventions, examinations, data collection, analytical methods, or outcomes.
Results
Patient characteristics
Forty patients were enrolled, all of whom completed 1-week post-injection phone calls and 1- and 4-months post-injection study follow-up visits. One patient chose the optional 1-week post-injection study visit; all others chose the safety phone call.
Of the study participants, 25 had limited and 15 diffuse scleroderma. Thirty-one patients (78%) were female, 9 (22%) were male. Mean age was 51.9 years (SD: 12.3, range: 21-75), median time since scleroderma diagnosis was 14 years (IQR: 6-19.5), median time since RP onset was 15.6 years (IQR: 10.7-23.2). Maximum ever recorded Medsger score of severity of vascular disease was 1 for 11 patients (28.21%), 2 for 15 patients (38.46%), and 3 for 13 patients (33%). One patient did not have the severity score recorded.
The most common comorbidities were hypertension (43%) and dyslipidemia (43%), followed by COPD (15%), atrial fibrillation (10%), arthritis (8%), diabetes (3%), and coronary artery disease (3%). At baseline, two (5%) patients had a history of scleroderma renal crisis and one (3%) had pulmonary hypertension. Two (5%) patients were current smokers; 10 (25%) were former smokers. The median number of pack-years for these 12 patients was 20 (IQR: 15.63-28.75).
Most participants were being treated for RP at baseline with calcium channel blockers (72.5%). Other drug therapy with vasoactive potential, not necessarily being used for the treatment of RP, included phosphodiesterase-5 inhibitors (17.5%), fluoxetine (12.5%), and losartan (12.5%). One patient was treated with oral bosentan for pulmonary hypertension throughout study duration.
Baseline Outcome Measurements
Hands allocated to Btx-A had similar baseline measures as hands allocated to placebo for blood flow, QuickDASH score, McCabe Cold Sensitivity Score, VAS pain scale score, RCS, and number of active ulcers (table 1).
Table 1.
Primary and secondary outcomes comparing changes from baseline to 1 and 4 months post-injection in hands injected with Botulinum toxin A vs. placebo.
| Baseline | 1 month | 4 months | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Test | Btx-A | Placebo | Btx-A | Placebo | Interaction p-value* | Btx-A | Placebo | Interaction p-value* | |
| LDI Blood flow (flux units)* | 398.24 | 395.65 | 362.06 | 389.55 | 0.024 | 359.75 | 361.33 | 0.796 | |
| QuickDASH score | 27.34 | 30.76 | 26.96 | 29.11 | 0.504 | 24.15 | 26.10 | 0.388 | |
| McCabe score | 221.79 | 221.15 | 185.31 | 187.19 | 0.938 | 182.19 | 182.5 | 0.906 | |
| VAS Pain score | 3.43 | 3.67 | 2.68 | 3.05 | 0.683 | 1.99 | 1.84 | 0.327 | |
| Oxygensaturation (%) | 90.95 | 92.2 | 94.1 | 95.21 | 0.917 | 92.49 | 95.31 | 0.489 | |
| Number of ulcers | 0.53 | 0.55 | 0.45 | 0.53 | 0.697 | 0.28 | 0.36 | 0.572 | |
LDI: Laser Doppler Imaging. VAS: Visual Assessment Scale.
Interaction p-values from Generalized Estimating Equations (GEE) compared change in outcome from baseline to follow-up visit between treatment arms.
Interim Analysis
Our interim analysis comparing the blood flow change to 1-month post-injection between groups did not show a significant difference (p=0.741). Therefore, study recruitment continued until the target sample size was achieved and follow-up was completed.
LDI blood Flow
The mean baseline blood flow in Btx-A hands was 398.24 (SD: 190.27) flux units, which was not significantly different from 395.65 (SD: 189.17) flux units in the placebo group (p=0.842). Our primary outcome, the change in blood flow from baseline to 1-month follow-up, showed a decrease of 36.19 flux units in Btx-A hands (95% Confidence Interval [CI]: -78.49 to 6.12 flux units). In contrast, blood flow decreased by 6.10 flux units in placebo hands (95% CI: -46.28 to 34.07 flux units; figure 2A). The statistical model showed significant evidence for this between-arm difference (average difference: -30.08 flux units, 95% CI: -56.19 to -3.98, interaction p-value=0.024). The absolute blood flow at 1-month follow-up was 362.06 (SD: 190.94) flux units in Btx-A hands, which was significantly lower than the mean 389.55 (SD: 182.81) flux units for placebo (p=0.018).
Figure 2.
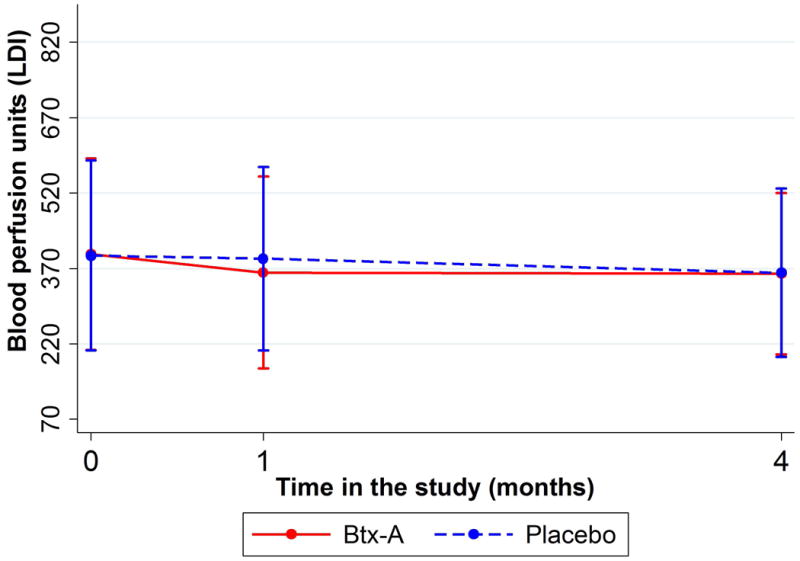
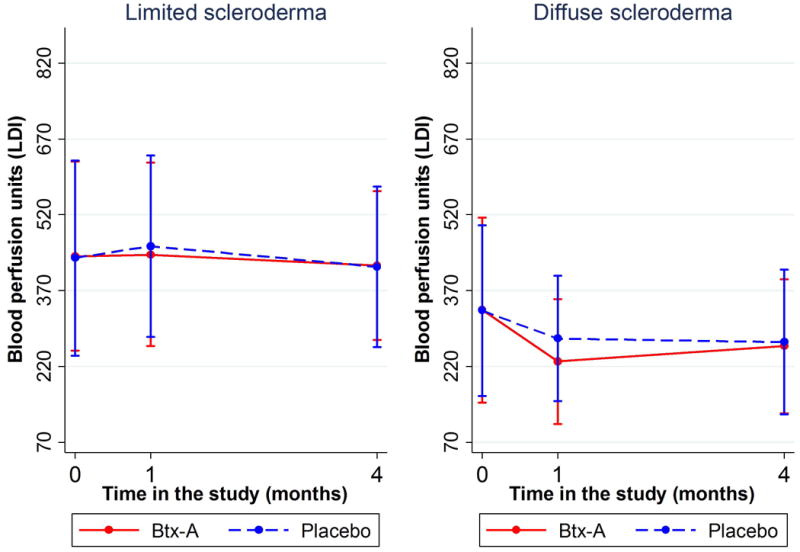
Figure 2A (top): Change in blood perfusion (measured with LDI scanner) by treatment arm over time. Presented as means and standard deviation bars. Figure 2B (bottom): Change in blood perfusion (measured with LDI scanner) over time by type of scleroderma. Presented as means and standard deviation bars.
The change in blood flow from baseline to 4-month follow-up was not significantly different between arms (p=0.796). Mean absolute blood flow at 4-month follow-up was 359.75 (SD: 160.36) flux units for Btx-A and 361.33 (SD: 167.63) flux units for placebo; this comparison was not significantly different (p=0.898). Blood flow data are shown in table 1.
Secondary Outcome measures
Changes in QuickDASH scores were not significantly different between arms at 1-month (p=0.504) or 4-month follow-up (p=0.388). Absolute scores were slightly lower in the Btx-A arm compared to placebo at baseline (3.42 points lower), 1-month follow-up (2.15 points lower), and 4-month follow-up (1.95 points lower); these between-arm differences however were not statistically significant.
McCabe Cold Sensitivity scores in Btx-A hands were slightly better than in the placebo arm (1.88 points lower at 1-month follow-up and 0.31 points lower at 4-month follow-up). However, these between-arm differences were not significant (p=0.834 and p=0.963, respectively). Rates of change in McCabe scores from baseline were not significant at 1-month (p=0.938) or 4-months post-injection (p=0.906).
Change in VAS pain from baseline was not significantly different between study groups (p=0.683 at 1-month, p=0.327 at 4-month follow-up). VAS pain scores were slightly lower in Btx-A, compared to placebo, at 1-month (0.38 cm lower) but not at 4-month follow-up (0.15 cm higher). These between-arm differences were not statistically significant (p=0.121 and p=0.585, respectively).
Oxygen saturation from pulse oximetry was slightly higher in the placebo arm at 1-month follow-up, compared to the Btx-A arm (mean: 95.21% vs. 94.10%, respectively). However, the between-group difference was not statistically significant (p=0.318) and remained nonsignificant at 4-month follow-up (95.31% vs 92.49%, p=0.074).
Patient-reported RCS (scored 1 to 10, with 10 being severe) decreased by 0.18 points weekly in Btx-A hands (95% CI: 0.13-0.22, p<0.001) and 0.14 points weekly in placebo hands (95% CI: 0.11-0.18, p<0.001). The interaction term comparing these slopes between groups showed weak statistical evidence (p=0.063). After adjusting for weekly ambient temperature at the patient's zip code, there was statistically significant evidence for a difference between these two rates of decline (p=0.045), suggesting that a faster decline in Btx-A hands (figure 3).
Figure 3.
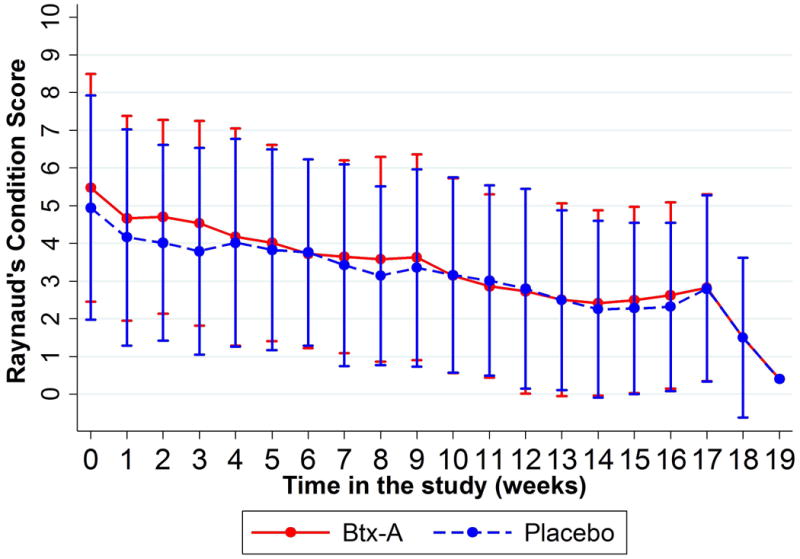
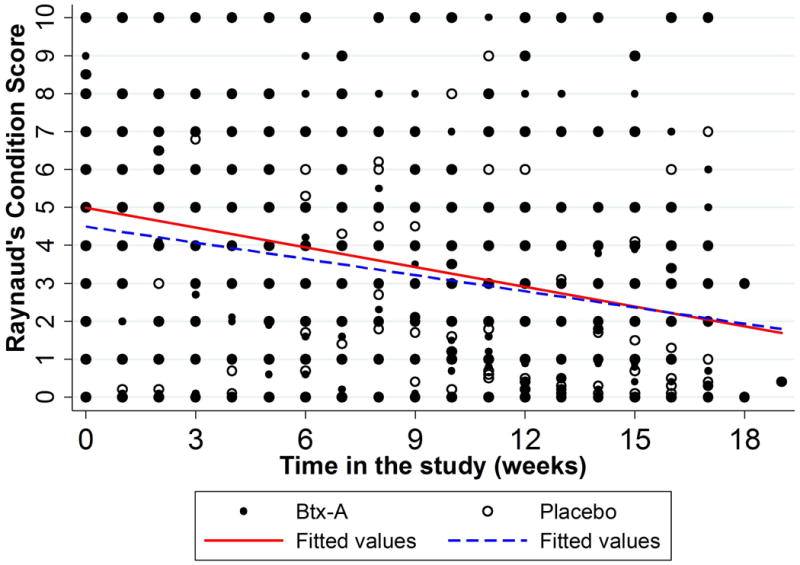
Figure 3A (left): Observed Raynaud's Condition Score throughout study period. Presented as means and standard deviation bars. Figure 3B (right): Fitted Raynaud's Condition Score measurements throughout study period (unadjusted). Presented as mean rates of decline by study group.
At baseline, 8 (20%) Btx-A and 10 (25%) placebo hands had active ulcers. The Relative Risk (RR) of developing new ulcers was 16.67% higher in placebo hands at 1-month follow-up, compared to Btx-A hands (RR: 1.17, 95% 0.65-2.10), and 27% higher at 4-month follow-up (RR: 1.27, 95% CI: 0.68-2.37). These differences between groups were not significantly different (p=0.608 and p=0.447, respectively). Changes over time in the number of new ulcers were also not statistically significant at 1-month (p=0.697) or 4-month follow-up (p=0.572). Secondary outcomes are shown in table 1.
All patients were asked at each visit which hand they thought received Btx-A. At 1-week post-injection, 11 (27.5%) patients guessed correctly; 8 of these and 3 additional patients (27.5%), also guessed correctly at 1-month follow-up. At 4-month follow-up, 19 (47.50%) patients guessed correctly, 11 of which had guessed correctly at previous visits. None of these guesses happened more often than what would be expected by chance (p>0.999, p=0.706, p=0.275, respectively).
Subgroup analysis
A subgroup analysis according to cutaneous subtype, RP disease duration, RP severity, and baseline treatment with calcium channel blockers (CCBs) was done for hypothesis generation and to potentially guide future research. This analysis showed that the negative trend observed in the full analysis was mainly driven by patients with longer time since RP onset (>15.56 years) and those with diffuse scleroderma (figure 2B). Table 2 summarizes these results (expanded in supplementary material).
Table 2.
Subgroup analysis results for Laser Doppler Imaging (LDI) blood flow at 1-month follow-up.
| Absolute LDI Blood Flow at 1-month | Change in LDI Blood Flow at 1-month | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Group | n | Between-arm difference | 95%CI | p-value* | Between-arm difference | 95%CI | Interaction p-value** |
| All | 40 | -27.49 | -50.20 to -4.79 | 0.018 | -30.08 | -56.19 to -3.98 | 0.024 |
|
| |||||||
| Limited | 25 | -16.78 | -48.07 to 14.51 | 0.293 | -20.31 | -50.78 to 10.16 | 0.191 |
| Diffuse | 15 | -45.34 | -75.24 to -15.44 | 0.003 | -46.37 | -94.25 to 1.52 | 0.058 |
|
| |||||||
| Short duration of RP | 20 | -3.52 | -28.15 to 21.17 | 0.780 | -37.16 | -74.74 to 0.42 | 0.053 |
| Long duration of RP | 20 | -51.46 | -87.21 to -15.71 | 0.005 | -23.01 | -59.96 to 13.95 | 0.222 |
|
| |||||||
| On CCBs at baseline | 29 | -22.73 | -51.82 to 6.36 | 0.126 | -37.52 | -71.61 to -3.42 | 0.031 |
| Not on CCBs at baseline | 11 | -40.03 | -71.40 to -8.66 | 0.012 | -10.47 | -40.15 to 19.20 | 0.489 |
|
| |||||||
| Medsger Severity 1 | 11 | -28.44 | -64.73 to -7.84 | 0.124 | -42.63 | -95.53 to 10.26 | 0.114 |
| Medsger Severity 2 | 15 | -33.81 | -67.90 to 0.29 | 0.052 | -30.41 | -67.43 to 6.62 | 0.107 |
| Medsger Severity 3 | 13 | -15.29 | -65.60 to -35.02 | 0.551 | -10.77 | -61.21 to 39.7 | 0.676 |
Btx-A: Botulinum toxin A. CI: Confidence Interval. RP: Raynaud's Phenomenon. CCB: Calcium Channel Blocker.
P-values from the model compared absolute outcomes at 1-month follow-up visit between treatment arms (Btx-A - placebo).
Interaction p-values from the model compared changes in outcomes from baseline to 1-month follow-up visit between treatment arms (Btx-A - placebo).
Adverse reactions
Two participants (5%) experienced weakness of the intrinsic muscles of the hand after receiving study injections. Both cases were in Btx-A-treated hands and no cases were observed in placebo-treated hands. The two patients reported weakness 7 days post-injection and fully recovered by 3 weeks in one case and 9 weeks in the other case. Both patients were able to guess correctly which hand had received Btx-A.
Discussion
Btx-A, as administered in our study, did not significantly improve blood flow to the hands of patients with scleroderma-associated RP. In fact, the change in blood flow from baseline to 1-month follow-up declined more with Btx-A than placebo (p=0.024). On average, blood perfusion decreased 30.08 flux units (7.7% from baseline) among Btx-A hands. Absolute blood flow at 1-month follow-up was also lower for Btx-A hands compared to placebo (p=0.018).
Interestingly, patients reported slightly better outcomes for hand function (QuickDASH scores), cold sensitivity (McCabe scores), and pain (VAS pain scale) in Btx-A hands. However, these differences were not statistically significant. Patients reported a greater reduction in RP severity, measured with the Raynaud's Condition Score (RCS), a validated patient-reported scale of the impact of RP. Although smaller than the reported minimum clinically important difference for changes in RCS,21 this difference was statistically significant after adjusting for local ambient temperature (p=0.045).
We enrolled patients during winter and spring of a particularly cold winter. This made interpreting our findings more complex, as most patients reported subjective relief in both hands over time. Fortunately, randomized within-patient placebo controls allowed meaningful comparisons between study arms.
Interestingly, a planned subgroup analysis showed that the overall negative results observed in the full analysis were mainly influenced by patients with longer time since RP onset (>15.56 years) and those with diffuse scleroderma. This suggests that differences in response to Btx-A may exist in subgroups of patients with scleroderma. We observed significantly worse blood flow among patients on CCBs at baseline. However, this finding is likely not clinically important due to a significantly lower absolute blood flow observed among patients not on CCBs and McCabe Cold Sensitivity scores that appeared to favor the CCBs group (data not shown). Accounting for differences in responses in this subgroup may be important for future studies.
To our knowledge, this is the first randomized, placebo-controlled, double-blind, clinical trial evaluating Btx-A injections in patients with RP secondary to scleroderma. In contrast to other studies, our study demonstrated a significant decrease in blood flow with Btx-A.
A first case series, published by Sycha et al. in 2004, showed positive results from Btx-A in two patients with primary RP and RP secondary to mixed connective tissue disease.13 Their promising results included subjective (VAS pain scale) and objective RP outcomes (laser Doppler interferometry). This was followed by a case series by Van Beek et al in 2009,15 where 11 patients with RP associated with various connective tissue disorders received up to 100 units of Btx-A per hand treated. Almost all patients (82%), including those with limited and diffuse scleroderma, reported improvement in the severity and frequency of their vasospastic episodes. Unlike our study, Van Beek et al included patients with acute digital ischemia and did not objectively measure perfusion.
A larger, uncontrolled, retrospective case series reported similar results injecting 50-100 units of Btx-A into each hand, using LDI as an outcomes measure.11 A heterogeneous patient population was included with both primary and secondary RP; some with critical ischemia. Blood flow increased by up to 425% in hands treated with Btx-A. Eighty-four percent of patients reported improvement in pain. Three patients with scleroderma participated in this study: 2 with diffuse disease had improved blood flow but no symptom relief and 1 with limited disease had improved blood flow and 13 months of pain relief.
Observational studies reviewed by Iorio et al, reported positive results.9 Two additional prospective studies, which did not incorporate ideal controls, focused specifically on scleroderma patients and also showed benefits from Btx-A treatment.12, 14 To our knowledge, robust studies using randomization, blinding, or placebo controls are not published.
It is important to recognize that the effect of an intervention measured in a controlled laboratory setting may have very different biological implications compared to patient-reported clinical outcomes. Interestingly, our subjective, patient-reported measures suggested slight symptom relief in Btx-A hands, as measured using patient-reported weekly RCS. Although the size of this effect was small, this finding may be important given that this subjective outcome was reported while patients were effectively blinded to treatment allocation. We also found small differences that favored Btx-A in other secondary outcomes (hand function, cold sensitivity, pain, and number of ulcers), but were not statistically significant.
This apparent disconnect between patient-reported outcomes and objective vascular laboratory measures, such as LDI-determined blood flow, is reported by others. Pauling et al did not find significant correlations between subjective results from a RCS diary and objective results from either laser speckle contrast imaging or infrared thermography.33 Neumeister et al found that scleroderma patients showed improvement in LDI blood flow despite not reporting subjective pain relief after Btx-A injections.11
Our study has several limitations. We chose instruments that were validated for hand and upper extremity conditions, but not specifically for scleroderma in the case of the McCabe and QuickDASH scales. We studied a heterogeneous group of scleroderma patients, showing that results were generally more favorable for patients with limited scleroderma and those with earlier RP who may have more modifiable disease. Stratification into more homogeneous patient populations may benefit future studies. The role for Btx-A alone or in combination with another vasoactive drug was not studied. It also remains to be established if Btx-A would be helpful in treating critical digital ischemia.
It is possible that our negative results are in part due to our screening methods. We may have included patients with larger vessel disease such as radial or ulnar artery occlusion, as we did not perform a pre-study Allen's test and did not perform digital arteriograms or magnetic resonance arteriograms before the study injections. This type of screening was implemented by some of the observational studies. 9, 12, 15 However, Uppal et al did not report screening patients with arteriograms and nevertheless reported positive results.14
Our study did not include an option for dose escalation in that we intended to evaluate a standardized injection protocol and dosing that would enhance patient safety and minimize risk of loss of hand function. We observed a lower incidence of intrinsic muscle weakness in our study (5%) compared to previous studies (9%-27%).9 Previous studies used Btx-A doses from 10 to 100 units per treated hand. A dose as low as 10 units of Btx-A has been reported to show favorable results.9 We used a more distal site of injection than reported in some studies (figure 1). We also did this to prevent hand weakness. The lower incidence of intrinsic muscle weakness observed in this study also limited unblinding from drug effects to a small proportion of our study population.
We may have been underpowered to detect clinically meaningful changes in perfusion. Available data from the literature have not examined perfusion in flux units, which meant that our power calculation was based on different units of perfusion (kHz) that are not convertible into flux units. We believe, however, that measurements in flux units capture more blood flow information than simple frequency.
Our study design was novel and a major strength of this study as we had patients serve as their own controls. This provided methodological advantages but also limited our ability to discern whether a high within-patient correlation may have biased our results towards the null or whether there could be any contralateral improvement after unilateral Btx-A treatment, which has been observed with digital sympathectomy.34
We considered multiple study designs, including using in-house cold challenge tests. We decided not to use a cold challenge test for the following reasons: first, due to the mechanism of action of Btx-A and previous reports,9-15 it was important for us to capture longer-term changes in blood flow in a real-world setting, rather than a laboratory contrived setting, as these do not necessarily correlate with each other.35 Second, cold challenges would have made our study logistics difficult due to patients serving as their own controls. Finally, the resulting measurements would have lacked comparability with previous Btx-A studies using LDI.10, 11, 13
Additionally, this study was conducted at a large tertiary center which may have decreased the generalizability of our findings. Finally, we made our study follow-ups at 1-month and 4-months post-treatment. It is possible that benefit occurred earlier and then disappeared. However, previous reports suggested long-term benefit and we felt that prolonged benefit would be clinically important. It is possible that acute effects may help acute digital ischemia.
Our primary outcome, laboratory-based data do not support the use of Btx-A in the treatment of RP in all scleroderma patients without acute digital ischemia. However, there were secondary clinical outcomes suggesting some benefit. Raynaud's severity, as reported by the patient showed a small, but statistically significant improvement in hands treated with Btx-A. Further research is required to fully understand the role of Btx-A in the treatment of RP.
Supplementary Material
Acknowledgments
This study was supported by Allergan via an independent and unrestricted research grant. Allergan had the opportunity to review the final version of the manuscript to address any factual inaccuracies or request the redaction of information deemed to be proprietary or confidential and ensure that study support was disclosed.
We would like to thank Dr. Lisa Ishii, Dr. Barbara De Lateur, Dr. Chad Gordon for their contribution as members of the Adverse Events Committee for this study and Dr. Aleksandra Beselman, Dr. Jim Monolakis and Chris Reynolds for their contribution as study pharmacists. We would also like to thank the Johns Hopkins Scleroderma Center staff, particularly Dr. Zsuzsanna McMahan, Margaret Sampedro, Adrianne Woods, Madeline Myers, Felicia Davis, Regina Greco, and Consuelo Laws. We acknowledge support from the Martha McCrory Professorship, the Scleroderma Research Foundation, and the National Center for Research Resources and the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health through Grant Number 1UL1TR001079.
Financial support: This study was supported by Allergan via an independent and unrestricted research grant. Allergan had the opportunity to review the final version of the manuscript to address any factual inaccuracies or request the redaction of information deemed to be proprietary or confidential and ensure that study support was disclosed. We acknowledge support from the Martha McCrory Professorship, the Scleroderma Research Foundation, and the National Center for Research Resources and the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health through Grant Number 1UL1TR001079.
References
- 1.Cappelli L, Wigley FM. Management of Raynaud Phenomenon and Digital Ulcers in Scleroderma. Rheumatic diseases clinics of North America. 2015;41(3):419–38. doi: 10.1016/j.rdc.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Block JA, Sequeira W. Raynaud's phenomenon. Lancet (London, England) 2001;357(9273):2042–8. doi: 10.1016/S0140-6736(00)05118-7. [DOI] [PubMed] [Google Scholar]
- 3.Wigley FM, Flavahan NA. Raynaud's Phenomenon. New England Journal of Medicine. 2016;375(6):556–65. doi: 10.1056/NEJMra1507638. [DOI] [PubMed] [Google Scholar]
- 4.Frantz C, Avouac J, Distler O, Amrouche F, Godard D, Kennedy AT, et al. Impaired quality of life in systemic sclerosis and patient perception of the disease: A large international survey. Seminars in arthritis and rheumatism. 2016 doi: 10.1016/j.semarthrit.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Hummers LK, Wigley FM. Management of Raynaud's phenomenon and digital ischemic lesions in scleroderma. Rheumatic diseases clinics of North America. 2003;29(2):293–313. doi: 10.1016/s0889-857x(03)00019-x. [DOI] [PubMed] [Google Scholar]
- 6.Chiou G, Crowe C, Suarez P, Chung L, Curtin C, Chang J. Digital Sympathectomy in Patients With Scleroderma: An Overview of the Practice and Referral Patterns and Perceptions of Rheumatologists. Annals of plastic surgery. 2015;75(6):637–43. doi: 10.1097/SAP.0000000000000614. [DOI] [PubMed] [Google Scholar]
- 7.Flavahan NA. A vascular mechanistic approach to understanding Raynaud phenomenon. Nature reviews Rheumatology. 2015;11(3):146–58. doi: 10.1038/nrrheum.2014.195. [DOI] [PubMed] [Google Scholar]
- 8.Stone AV, Koman LA, Callahan MF, Eckman DM, Smith BP, Plate JF, et al. The effect of botulinum neurotoxin-A on blood flow in rats: a potential mechanism for treatment of Raynaud phenomenon. The Journal of hand surgery. 2012;37(4):795–802. doi: 10.1016/j.jhsa.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 9.Iorio ML, Masden DL, Higgins JP. Botulinum toxin A treatment of Raynaud's phenomenon: a review. Seminars in arthritis and rheumatism. 2012;41(4):599–603. doi: 10.1016/j.semarthrit.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Neumeister MW. Botulinum toxin type A in the treatment of Raynaud's phenomenon. The Journal of hand surgery. 2010;35(12):2085–92. doi: 10.1016/j.jhsa.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Neumeister MW, Chambers CB, Herron MS, Webb K, Wietfeldt J, Gillespie JN, et al. Botox therapy for ischemic digits. Plast Reconstr Surg. 2009;124(1):191–201. doi: 10.1097/PRS.0b013e3181a80576. [DOI] [PubMed] [Google Scholar]
- 12.Serri J, Legre R, Veit V, Guardia C, Gay AM. [Botulinum toxin type A contribution in the treatment of Raynaud's phenomenon due to systemic sclerosis] Annales de chirurgie plastique et esthetique. 2013;58(6):658–62. doi: 10.1016/j.anplas.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Sycha T, Graninger M, Auff E, Schnider P. Botulinum toxin in the treatment of Raynaud's phenomenon: a pilot study. European journal of clinical investigation. 2004;34(4):312–3. doi: 10.1111/j.1365-2362.2004.01324.x. [DOI] [PubMed] [Google Scholar]
- 14.Uppal L, Dhaliwal K, Butler PE. A prospective study of the use of botulinum toxin injections in the treatment of Raynaud's syndrome associated with scleroderma. The Journal of hand surgery, European volume. 2014;39(8):876–80. doi: 10.1177/1753193413516242. [DOI] [PubMed] [Google Scholar]
- 15.Van Beek AL, Lim PK, Gear AJ, Pritzker MR. Management of vasospastic disorders with botulinum toxin A. Plast Reconstr Surg. 2007;119(1):217–26. doi: 10.1097/01.prs.0000244860.00674.57. [DOI] [PubMed] [Google Scholar]
- 16.Wigley FM. Clinical practice. Raynaud's Phenomenon. The New England journal of medicine. 2002;347(13):1001–8. doi: 10.1056/NEJMcp013013. [DOI] [PubMed] [Google Scholar]
- 17.Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis and rheumatism. 1980;23(5):581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 18.Beaton DE, Wright JG, Katz JN. Development of the QuickDASH: comparison of three item-reduction approaches. The Journal of bone and joint surgery American volume. 2005;87(5):1038–46. doi: 10.2106/JBJS.D.02060. [DOI] [PubMed] [Google Scholar]
- 19.McCabe SJ, Mizgala C, Glickman L. The measurement of cold sensitivity of the hand. The Journal of hand surgery. 1991;16(6):1037–40. doi: 10.1016/s0363-5023(10)80065-6. [DOI] [PubMed] [Google Scholar]
- 20.Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27(1):117–26. doi: 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 21.Khanna PP, Maranian P, Gregory J, Khanna D. The minimally important difference and patient acceptable symptom state for the Raynaud's condition score in patients with Raynaud's phenomenon in a large randomised controlled clinical trial. Annals of the rheumatic diseases. 2010;69(3):588–91. doi: 10.1136/ard.2009.107706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulz KF, Grimes DA. Blinding in randomised trials: hiding who got what. Lancet (London, England) 2002;359(9307):696–700. doi: 10.1016/S0140-6736(02)07816-9. [DOI] [PubMed] [Google Scholar]
- 23.Correa MJ, Andrade LE, Kayser C. Comparison of laser Doppler imaging, fingertip lacticemy test, and nailfold capillaroscopy for assessment of digital microcirculation in systemic sclerosis. Arthritis research & therapy. 2010;12(4):R157. doi: 10.1186/ar3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fullerton A, Stucker M, Wilhelm KP, Wardell K, Anderson C, Fischer T, et al. Guidelines for visualization of cutaneous blood flow by laser Doppler perfusion imaging. A report from the Standardization Group of the European Society of Contact Dermatitis based upon the HIRELADO European community project. Contact dermatitis. 2002;46(3):129–40. doi: 10.1034/j.1600-0536.2002.460301.x. [DOI] [PubMed] [Google Scholar]
- 25.Herrick AL, Hutchinson C. Vascular imaging. Best practice & research Clinical rheumatology. 2004;18(6):957–79. doi: 10.1016/j.berh.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Anderson ME, Hollis S, Moore T, Jayson MI, Herrick AL. Non-invasive assessment of vascular reactivity in forearm skin of patients with primary Raynaud's phenomenon and systemic sclerosis. British journal of rheumatology. 1996;35(12):1281–8. doi: 10.1093/rheumatology/35.12.1281. [DOI] [PubMed] [Google Scholar]
- 27.Aghassi D, Monoson T, Braverman I. Reproducible measurements to quantify cutaneous involvement in scleroderma. Archives of dermatology. 1995;131(10):1160–6. [PubMed] [Google Scholar]
- 28.Figueiras E, Roustit M, Semedo S, Ferreira LF, Crascowski JL, Humeau A. Sample entropy of laser Doppler flowmetry signals increases in patients with systemic sclerosis. Microvascular research. 2011;82(2):152–5. doi: 10.1016/j.mvr.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Medsger T, Jr, Silman A, Steen V, Black C, Akesson A, Bacon P, et al. A disease severity scale for systemic sclerosis: development and testing. The Journal of rheumatology. 1999;26(10):2159–67. [PubMed] [Google Scholar]
- 30.Gush RJ, Taylor LJ, Jayson MI. Acute effects of sublingual nifedipine in patients with Raynaud's phenomenon. Journal of cardiovascular pharmacology. 1987;9(5):628–31. doi: 10.1097/00005344-198705000-00018. [DOI] [PubMed] [Google Scholar]
- 31.McPherson K. Statistics: the problem of examining accumulating data more than once. The New England journal of medicine. 1974;290(9):501–2. doi: 10.1056/NEJM197402282900907. [DOI] [PubMed] [Google Scholar]
- 32.LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA, Jr, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. The Journal of rheumatology. 1988;15(2):202–5. [PubMed] [Google Scholar]
- 33.Pauling JD, Shipley JA, Hart DJ, McGrogan A, McHugh NJ. Use of Laser Speckle Contrast Imaging to Assess Digital Microvascular Function in Primary Raynaud Phenomenon and Systemic Sclerosis: A Comparison Using the Raynaud Condition Score Diary. The Journal of rheumatology. 2015;42(7):1163–8. doi: 10.3899/jrheum.141437. [DOI] [PubMed] [Google Scholar]
- 34.Wasserman A, Brahn E. Systemic sclerosis: bilateral improvement of Raynaud's phenomenon with unilateral digital sympathectomy. Seminars in arthritis and rheumatism. 2010;40(2):137–46. doi: 10.1016/j.semarthrit.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Wise RA, Malamet R, Wigley FM. Acute effects of nifedipine on digital blood flow in human subjects with Raynaud's phenomenon: a double blind placebo controlled trial. The Journal of rheumatology. 1987;14(2):278–83. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


