Abstract
Background
Telehealth offers opportunities to extend clinical and research interventions for pediatric obesity.
Objectives
To assess utility of a telephone intervention, implemented through a national primary care pediatric research network, for promoting differentiation in dietary intake, consistent with either a low-glycemic load (Low GL) or Low Fat prescription, among overweight/obese school-age children.
Methods
Five-week telephone dietary counseling intervention for parents of overweight/obese school-age children recruited through the Slone Center Office-based Research Network. Parent-child dyads were randomized to Low GL or Low Fat diet. Primary outcomes were dietary GL and dietary fat, adjusted for energy intake and assessed by 24-hour dietary recall.
Results
Subjects were randomized to Low GL (n=11, 8.1±1.7 years, 45.5% male) or Low Fat (n=11, 8.2±2.0 years, 36.4% male), with no baseline differences. Overall, 86% of subjects attended at least 4 of 5 counseling sessions, and study completion rate was 91% (based on completion of the final dietary recalls). Reported satisfaction was high. In adjusted analyses limited to “recall completers,” reduction in dietary GL (g/1000 Kcal) achieved within the Low GL group was significant (p=0.01) and greater than the change in dietary GL in the Low Fat group (mean ± SE; −12.9 ± 4.4 vs. 5.1 ± 4.9, p=0.03). Similarly, reduction in dietary fat (% of total energy) within the Low Fat group was significant (−5.6 ± 2.5, p=0.046) but with no difference between groups (p=0.25).
Conclusion
A telephone-based dietary intervention for overweight/obese children, implemented through a national pediatric research network, fostered prescribed dietary changes. ClinicalTrials.gov registration: NCT00620152
Keywords: pediatric, treatment, obesity, telehealth, practice-based research, diet
Introduction
Obesity is a prevalent chronic disease of childhood (1), yet pediatric weight management programs struggle to retain patients (2), limiting effectiveness of interventions. Telehealth modalities for convenient implementation of dietary interventions in primary care, ranging from telephone to videoconferencing (3-7), are critical given the widespread problem of pediatric obesity and limited resources in academic medical centers. Telephone interventions are increasingly used for health promotion with potential advantages over face-to-face and written interventions including lower cost, convenience, and privacy (8). For younger children, interventions targeting parents have been advocated as a means to involve families and garner parental support in providing healthful foods and modeling healthful behaviors (9). Multifaceted interventions for pediatric obesity have shown success (10), yet few studies directly compare dietary treatments independent of other facets (11).
Our aim was to assess utility of a telephone intervention, implemented through a national primary care pediatric research network, for promoting differentiation in dietary intake, consistent with either a low-glycemic load (Low GL) or Low Fat prescription, among overweight/obese school-age children. We chose the telephone as the most basic form of telehealth to avoid potential challenges of more sophisticated telehealth platforms that could impede development of relationships with primary care practices. We focused on dietary intake to inform planning of future trials aimed at comparing dietary interventions for pediatric obesity over the long term (12). We evaluated the telephone intervention through a national network, recognizing that collaboration between an obesity clinic in an academic medical center and a primary care network in future trials would offer opportunities to conduct well-powered dietary intervention studies with high external validity. We hypothesized that the telephone intervention would elicit dietary changes consistent with randomly-assigned prescriptions.
Methods
Study Overview
A 5-week telephone intervention was designed to counsel parents/guardians (hereafter referred to as “parents”) on dietary prescriptions. Parent-child dyads were randomized to either a Low GL or Low Fat diet, representing diets along the spectrum of possible approaches for treating obesity in school-age children (13). Primary outcomes were dietary GL (g/1000 Kcal) and dietary fat (% of total energy) assessed by 24-hour dietary recall interviews. The study was conducted at Boston Children’s Hospital (BCH) with subjects recruited through the Slone Center Office-Based Research (SCOR) Network coordinated by the Slone Epidemiology Center at Boston University School of Medicine (14). The study was approved by the BCH IRB.
Subjects
Subjects were aged 5-10 years, with body mass index (BMI) for sex and age ≥85th percentile within the prior 6 months. Parent and child had to be living in the same household with a working telephone, and conversant in English. Parent had to be literate in English. Children were excluded for major chronic medical illness (e.g., diabetes, inflammatory bowel disease), psychiatric disorders, obesity-associated genetic syndromes, current participation in another obesity-related research study or formal weight-loss program, being related to or living with another study participant, or following a specialized diet.
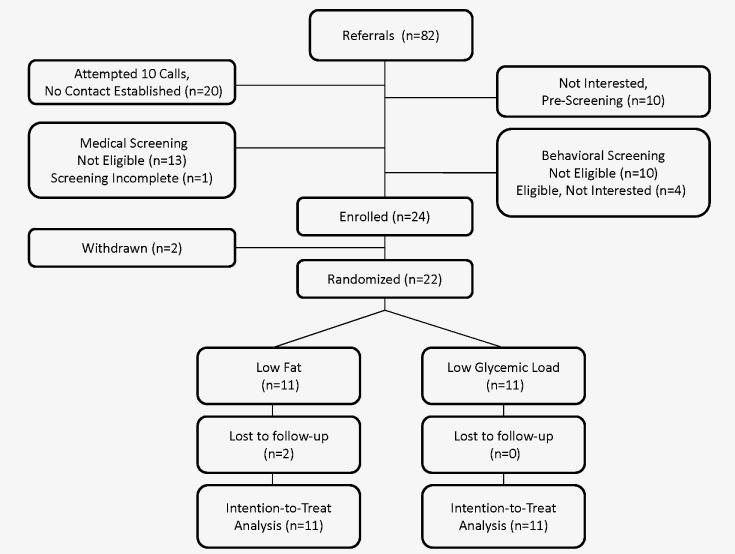
At the time of the study, the SCOR Network included approximately 500 pediatricians/family physicians (~75%/25%) with ~60% group-private practice/30% solo-private practice/5% clinic/5% other and ~40% suburban/30% rural/25% urban/5% other. Subjects were recruited from 8 sites located in Concord, NH; Lexington, KY; Lockport, NY; State College, PA; Boston, MA; Austin, TX; San Marcos, TX; and Indianapolis, IN. A preliminary phase from April-July 2008, with 21 referrals and 10 randomized subjects, informed modifications to screening and intervention protocols presented herein. Subjects, who participated in the preliminary phase, were not included in the main analysis. Based on this phase, readiness to make dietary changes was added as an inclusion criterion, as described below. The main study recruited from August 2008-February 2009 with 82 referrals and 22 randomized subjects (Figure 1).
Figure 1.
Screening and Enrollment
Enrollment and Randomization
Recruitment and enrollment was a multistep process. First, during face-to-face visits, referring physicians reviewed introductory brochures with patients who met age and BMI criteria. There was limited uptake of a site stipend ($400), offered for chart review to identify and contact additional patients. After verbal permission from the parent, the physician faxed a referral to BCH, including the patient’s most recent height and weight. Second, a research assistant (RA) performed telephone screening with each parent to confirm eligibility (Figure 1, “medical screening”). Third, families passing initial screening spoke with the dietitian, who assessed readiness to make dietary change (15), motivation for study participation, support for making dietary changes, and current dietary practices (Figure 1, “behavioral screening”). Final eligibility was confirmed by the study directors (ETR and CBE) and principal investigator (DSL).
A parent completed the informed consent process by telephone, and assent was obtained from children aged 7 years and older. Subjects were randomized by an RA to either a Low GL or Low Fat diet after two baseline dietary recall interviews. Families were not formally masked to group assignment but were only told that both groups would follow healthful nutritional programs differing in emphasized foods. Randomization was stratified by site. Separate randomization envelopes for each site were prepared in advance by the BCH Clinical Research Center in randomly permuted blocks of 2 and 4, preventing anticipation of assignments.
Intervention
The telephone intervention (Table S1) involved 5 weekly contacts with a dietitian, with each call ~30 minutes in duration. The Low GL and Low Fat interventions were comparable with the exception of the dietary prescription, which varied in carbohydrate-to-fat ratio. All appointments were scheduled by an RA at the time of consent, and standardized procedures were used to handle missed sessions consistently.
The patient-centered counseling model (16) was used to foster dietary adherence (17, 18). Targeted constructs included readiness to change, self-efficacy, health beliefs, behavioral clues, and self-control. Counseling sessions were audiorecorded, and a subset was reviewed by the senior author (CBE) for consistency with targeted constructs and differentiation in dietary prescriptions. Printed materials tailored to the assigned dietary prescription were mailed to the family before sessions 1, 3, and 5. These included nutritionally-themed “games” for children such as word jumbles and word searches linked to the assigned diet. Sessions 2 and 4 were focused less on nutrition education and more on dietary review, counseling, and support.
The Low GL diet had a target macronutrient composition of 40-45% of energy from carbohydrate, 30-35% from fat, and 20-25% from protein and was prescribed ad libitum, based on data suggesting less hunger and energy intake among children in response to low- vs. high-glycemic index (GI) meals (19). Nutrition education focused on replacing high- or moderate-GI sources of carbohydrate with low-GI sources or healthful fat (e.g., unsaturated). Parents were instructed to offer their children ample amounts of non-starchy vegetables, fruits, and legumes and limit provision of starchy vegetables, refined grains, and sweets. These guidelines fostered adequate intake of dietary fiber and consumption of healthful fat. “Food Choice Lists” delineated low-, moderate-, and high-GL foods, taking GI into account.
The Low Fat diet had a target macronutrient composition of 50-55% of energy from carbohydrate, 25-30% from fat, and 20-25% from protein and was also prescribed ad libitum, based on the contention that individuals eating less fat consume fewer calories because fat has a higher energy density and is more satiating than carbohydrate (20). Nutrition education focused on choosing foods containing ≤3 grams of fat/serving, limiting added fats, and using low-fat meal preparation strategies. Parents were instructed to offer their children ample amounts of grains, vegetables, and fruits and limit high-fat foods. Some attention was given to whole grains to foster adequate dietary fiber intake. “Food Choice Lists” delineated low-, moderate-, and high-fat foods.
Data Collection
Subjects’ most recent height and weight were provided by their referring physician to calculate BMI. These data were collected using standard clinical measurement practices. Demographic data were collected by telephone from the parent.
A diet technician, masked to group assignment and with no responsibilities for implementing the intervention, conducted unannounced 24-hour dietary recall interviews by telephone with the child, assisted by the parent. Two interviews were conducted the week prior to randomization and another two during the second week following completion of the intervention. Dietary data were collected by a multiple-pass method using the Nutrient Data System for Research software versions 2006-2008 (Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN) (21-23). To facilitate portion size estimation, the parent used a picture guide, provided via email or mail.
After the final recall interview, the diet technician surveyed the parent about his/her satisfaction with the study, on a scale of 1 (not at all satisfied) to 10 (extremely satisfied) and the child’s apparent level of interest in the study during participation, on a scale of 0 (not at all interested) to 4 (extremely interested).
The parent and child each received a gift card at the completion of the study (up to $50 for parent and $20 for child based on number of completed sessions and dietary recall interviews).
Sample Size
Power calculations were based on a Student’s t-test with 2-sided type I error rate of 0.05, using cross-sectional standard deviation of the outcome and pre-post correlation within subjects estimated from a previous study (17). With 15 subjects/arm, the study would have 80% power with α=0.05, to detect a differential change of ~9 g/1000 kcal in glycemic load and 5% of energy from fat, deemed achievable based on prior studies (17, 22). Due to the extended recruitment period and funding limits, the actual sample comprising 11 subjects/arm provided 80% power to detect a differential change of ~21 g/1000 kcal in glycemic load and ~12% of energy from fat.
Statistical Analysis
The primary a priori analysis employed the intention-to-treat principle, classifying each subject according to his/her randomly assigned diet regardless of duration or compliance. Imputation for missing data utilized the last observation carried forward. Secondary analyses were conducted to evaluate outcomes for the subgroup of subjects who completed the final dietary recall interviews (hereafter referred to as “recall completers”). All tests were two-tailed with significance level of p<0.05. SAS software (version 9.2, Cary, NC) was used for all computations.
Baseline characteristics were compared between groups by Student’s t-test (continuous variables) or Fisher’s exact test (categorical variables). Pre-specified primary outcomes were change in dietary glycemic load (g/1000 Kcal) and dietary fat (% of total energy). Change in total calories was a secondary outcome. Data from the two dietary recall interviews at each time point were averaged. Within-group changes were assessed by paired t-test. Between-group changes were compared by Student’s t-test. We evaluated baseline characteristics deemed potentially relevant to the efficacy of a dietary change intervention (race/ethnicity, practice site, and BMI category) by analysis of covariance.
Results
Study Sample
Baseline characteristics of the 22 randomized subjects (11 Low GL, 11 Low Fat) are summarized in Table 1. The two groups were balanced with no significant differences. Subjects were recruited from 7 of 8 participating SCOR Network sites. Participating parents were predominantly mothers (100% Low GL vs. 72.7% Low Fat, p=0.21), with approximately 50% self-reporting very good/excellent health (54.5% Low GL vs. 46.1% Low Fat, p=0.75).
Table 1.
Characteristics of subjects, compared by study arm.*
| Low GL (n=11) | Low Fat (n=11) | P | |
|---|---|---|---|
| Child | |||
| Age, yr | 8.1 ± 1.7 | 8.2 ± 2.0 | 0.90 |
| Male | 5 (45.5) | 4 (36.4) | 1.0 |
| BMI, kg/m2 | 24.4±3.6 | 24.4±3.0 | 0.99 |
| BMI ≥ 99th percentile | 4 (36.4) | 5 (45.5) | 1.0 |
| Race/ethnicity | 1.0 | ||
| Non-Hispanic white | 8 (72.7) | 8 (72.7) | |
| Non-Hispanic black | 1 (9.1) | 0 (0) | |
| Hispanic | 2 (18.2) | 3 (27.3) | |
| Practice Recruitment Site† | 0.94 | ||
| New Hampshire | 4 (36.4) | 2 (18.2) | |
| New York | 1 (9.1) | 1 (9.1) | |
| Massachusetts | 1 (9.1) | 0 (0) | |
| Kentucky | 0 (0) | 1 (9.1) | |
| Pennsylvania | 2 (18.2) | 2 (18.2) | |
| Texas | 2 (18.2) | 3 (27.3) | |
| Indiana | 1 (9.1) | 2 (18.2) | |
| Parent/guardian | |||
| Education | 0.62 | ||
| High school/Some College | 5 (45.5) | 7 (64.6) | |
| College/Graduate School | 6 (54.5) | 4 (36.4) | |
| Annual Income | 0.64 | ||
| $50,000 or less | 4 (36.4) | 3 (27.3) | |
| More than $50,000 | 7 (63.6) | 8 (72.7) | |
| Insured§ | 11 (100) | 10 (90.9) | 1.0 |
Mean ± standard deviation. For categorical variables, N (%).
No subjects recruited from practice site in Austin, TX.
Both parent/guardian and child have health insurance.
Dietary Outcomes
Dietary outcomes are presented in Table 2. Dietary GL, fat, and total energy were comparable in the two groups at baseline. In the intention-to-treat analyses (all subjects), there were no significant between- or within-group differences for changes in dietary fat. There was a significantly lower GL in the Low GL compared to the Low Fat group post intervention (61.2 ± 3.7 vs. 77.2 ± 2.8, p=0.003). However, change in GL did not differ between groups (p=0.06). The Low GL group also had a significant within-group decrease in total energy intake (−376 ± 89, p<0.005) and significantly lower total energy intake compared to the Low Fat group at the end of the intervention (p=0.001). Change in total energy intake, however, did not differ between groups (p=0.06).
Table 2.
Dietary characteristics compared by study arm.
| Unadjusted* | Adjusted§ | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Low GL (n=11) |
Low Fat (n=11) |
P | Low GL (n=11) |
Low Fat (n=9) |
P | |
| Percentage of energy from fat, % | ||||||
| Baseline | 32.0 ± 1.0 | 30.8 ± 2.3 | 0.63 | |||
| Post-intervention | 29.8 ± 1.8 | 27.2 ± 1.5 | 0.28 | |||
| Change | −2.2 ± 2.0 | −3.5 ± 2.5 | 0.68 | −1.2 ± 2.2 | −5.6 ± 2.5† | 0.25 |
| Glycemic load, g/1000 kcal | ||||||
| Baseline | 71.3 ± 2.2 | 75.7 ± 3.7 | 0.32 | |||
| Post-intervention | 61.2 ± 3.7 | 77.2 ± 2.8 | 0.003 | |||
| Change | −10.1 ± 5.3 | 1.5 ± 2.7 | 0.06 | −12.9 ± 4.4†† | 5.1 ± 4.9 | 0.03 |
| Total energy, kcal | ||||||
| Baseline | 1373 ± 89 | 1481 ± 101 | 0.43 | |||
| Post-intervention | 997 ± 75 | 1384 ± 72 | 0.001 | |||
| Change | −376 ± 89††† | −97 ± 107 | 0.06 | −350 ± 128† | −150 ± 143 | 0.35 |
Mean ± standard error. GL, glycemic load
Intention to treat analysis, P-value for comparison between groups.
Limited to “recall completers” and adjusted for race/ethnicity, practice site, and BMI
Within-group change, p<0.05
Within-group change, p=0.01
Within-group change, p<0.005
Two subjects in the Low Fat group were lost to follow-up and therefore did not complete the final dietary recall interviews. Unadjusted analyses limited to “recall completers” (data not shown) were qualitatively unchanged from intention-to-treat analyses. However, when analyses limited to “recall completers” were adjusted for race/ethnicity, practice site, and BMI percentile (Table 2), reduction in dietary GL achieved within the Low GL group was significant (p=0.01) and greater than the change in dietary GL in the Low Fat group (mean ± SE; −12.9 ± 4.4 vs. 5.1 ± 4.9, p=0.03). Similarly, adjusted for the same covariates, the reduction in dietary fat achieved within the Low Fat group was significant (−5.6 ± 2.5, p=0.046) but with no difference between groups. There were no independent differences for change in dietary GL or fat by race/ethnicity, practice site, or BMI percentile.
Process Measures and Experience
Overall, 59% of subjects completed all 5 counseling sessions, and 86% completed at least 4. There were no differences in number (4.6 ± 0.5 vs. 4.2 ± 1.1, p=0.23) or length of completed counseling sessions (42.1 ± 5.0 vs. 39.3 ± 6.1 minutes, p=0.26) between the Low GL and Low Fat groups. Among “recall completers” (91% of total sample), there was an overall positive experience with the intervention and there were no significant differences between groups for satisfaction with scheduling, counseling, printed materials for parents, printed materials for children, or child’s interest (Table S2). However, compared to the Low GL group, the Low Fat group reported higher overall satisfaction (9.4 ± 0.7 vs. 10.0 ± 0, p=0.01). No serious harm or unintended effects were reported in either group.
Discussion
We implemented a telephone-based dietary research protocol involving screening, consent, and intervention delivery (nutrition education and dietary counseling) through collaboration with a pediatric practice-based research network. Overall, we found that a telephone-based intervention targeting parents of overweight or obese school-age children fostered prescribed dietary changes in the short term, with high rates of adherence in completing scheduled telephone calls, and intervention satisfaction. Our findings have important implications for 1) partnerships between obesity clinics in academic medical centers and primary care providers in community settings and 2) design and implementation of future trials to compare distinct dietary interventions for treating pediatric obesity.
Results are consistent with those of previous studies describing successful impact of telehealth interventions targeting parents as the agent of change on the dietary intake of preschool and school-age children (3, 4, 7). For example, Wyse et al. conducted the Healthy Habits trial, a cluster randomized controlled trial of preschool-age children in New South Wales, Australia (3). Parents either received 4 weekly telephone calls facilitated by a trained interviewer using a computer-assisted telephone protocol and supplemented by mailed written materials or a booklet on the Australian dietary guidelines and how to meet them. The investigators found significant improvement (p<0.001) in fruit and vegetable intake at 2 months compared to controls, and significant reduction in “non-core foods” which included those high in fat, salt, or sugar (3). The effect on fruits and vegetable intake was maintained at 6 months (4) and 12 months (24). Davis et al. evaluated a family-based behavioral group for school-age children offered through telehealth and demonstrated increased fruit and vegetable intake and decreased BMI z-score, not significantly different from the face-to-face physician intervention (7). A similar study comparing outcomes of a telephone vs. telehealth via videoconference demonstrated comparable satisfaction and outcomes (25). Therefore, opportunities offered by telehealth to extend the reach of obesity treatment have received increasing attention (7, 25, 26), with focus on new methods to scale successful intervention strategies that have been implemented in primary care, such as motivational interviewing (27).
Dietary intake data have important implications for using telehealth to enhance external validity of broader scale nutrition research aiming to evaluate effects of diets varying in composition for treating pediatric obesity. We detected a group difference for change in dietary GL in adjusted analyses, and changes for GL and dietary fat within respective groups, suggesting that telehealth holds promise for delivering distinct dietary prescriptions to achieve differentiation in self-reported intake among children in their normal environments. Moreover, by reducing geographic barriers to recruiting and counseling patients in randomized trials of dietary interventions, telehealth is a viable option for evaluating generalizability of dietary treatments across demographic groups.
Strengths of the study include the geographic heterogeneity of the sample, good subject adherence with the protocol, and high retention rate. Nevertheless, despite sociodemographic heterogeneity in education and income, the sample was predominantly non-Hispanic White and represented families who were ready to change, as a criterion for eligibility, thereby limiting generalizability. Next steps to evaluate broader generalizability of findings must include collaboration with primary care providers who treat ethnically and racially diverse patients and those at all stages of change. Sample size may have limited power to assess changes in primary outcomes. Although we demonstrated a mechanism to conduct dietary trials within a network, our challenges with recruitment, though common (28, 29), underscore another area for continued planning. In addition, the primary outcome measure was based on dietary assessment and therefore social desirability bias could have influenced our results (30). Further, while families were not formally masked to group assignment, the interventions were not labeled as “low-glycemic load” or “low fat,” nor were families aware of the dietary outcomes of interest. Finally, we did not have a control group and thus could not estimate the extent of the effects achieved beyond usual care. Therefore, we cannot compare either intervention effect to changes resulting from external influences. However, given the short duration of the intervention and challenges of obesity treatment, a significant external contribution to observed effects is unlikely.
In conclusion, our telephone-based dietary intervention for overweight/obese children fostered prescribed dietary changes and highlights opportunities for further developing partnerships between academic medical centers and primary care providers. Next steps include incorporating other modalities of telehealth (6); extending intervention delivery over a longer period to assess durability and into a larger, more heterogeneous patient population to enhance generalizability; incorporating physical activity and tools recognized to be successful in primary care (27); conducting cost analyses (with implications for reimbursement models); and assessing clinical outcomes, such as BMI, and patient-reported outcomes, such as quality of life.
Supplementary Material
What is already known about this subject.
Management of pediatric obesity remains a challenge with finite resources available from academic medical centers.
Exploring strategies for intervention in primary care settings may offer increased opportunities for treatment and research, as through pediatric primary care research networks.
Telehealth and telephone interventions have been explored to extend the reach of pediatric weight management services.
What this study adds
A telephone-based dietary intervention targeted at parents of overweight/obese school-age children fostered dietary changes consistent with well differentiated prescriptions.
Research involving long-distance screening, consent, and dietary intervention can be delivered by telephone with fidelity and satisfaction through a primary care research network.
Acknowledgements
ETR, CBE, LV, AAM, CR and DSL conceptualized the study design; CR, ETR, CBE, and DSL developed the intervention; CR and PG recruited subjects, acquired data and implemented the intervention; ETR, CBE, and LV supervised data collection; ETR performed the data analysis, and all authors were involved in interpretation of data. ETR and CBE prepared the first draft of the manuscript. All other authors critically reviewed and revised the manuscript. All authors reviewed and approved the final version. The study was funded by the New Balance Foundation. Additional support included the Centers for Disease Control and Prevention grant K01DP000089 (ETR) and National Institute of Diabetes, Digestive and Kidney Diseases grant K24DK082730 (DSL). We would like to thank the participating patients/families, project coordinator at the Slone Epidemiology Center, Richard Vezina MPH, the physicians at the participating SCOR Network sites, the Clinical Research Center at Boston Children’s Hospital for statistical support (Henry Feldman PhD) and database development, Roula Zoghbi Smith MPH (now at Texas Children’s Hospital, Houston, TX) for project administration, Margaret Lovesky Apura RD, MPH for guidance on intervention materials, and Linda Seger-Shippee for conducting the 24-hour dietary recall interviews.
Abbreviations
- BCH
Boston Children’s Hospital
- SCOR
Slone Center Office-based Research
- RA
research assistant
- GL
glycemic load
- GI
glycemic index
Footnotes
Disclosure/Conflict of interest: Dr. Rhodes receives research funding from Merck. Dr. Ludwig received royalties for books on obesity and nutrition. The remaining authors have no disclosures. The study sponsors had no role in study design; collection, analysis, or interpretation of data; writing the report; or decision to submit the paper for publication.
References
- 1.Rhodes ET, Ludwig DS. Childhood obesity as a chronic disease: keeping the weight off. Jama. 2007;298(14):1695–6. doi: 10.1001/jama.298.14.1695. [DOI] [PubMed] [Google Scholar]
- 2.Skelton JA, Beech BM. Attrition in paediatric weight management: a review of the literature and new directions. Obes Rev. 2011;12(5):e273–81. doi: 10.1111/j.1467-789X.2010.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wyse R, Wolfenden L, Campbell E, Campbell KJ, Wiggers J, Brennan L, et al. A cluster randomized controlled trial of a telephone-based parent intervention to increase preschoolers' fruit and vegetable consumption. Am J Clin Nutr. 2012;96(1):102–10. doi: 10.3945/ajcn.111.030585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fletcher A, Wolfenden L, Wyse R, Bowman J, McElduff P, Duncan S. A randomised controlled trial and mediation analysis of the 'Healthy Habits', telephone-based dietary intervention for preschool children. Int J Behav Nutr Phys Act. 2013;10:43. doi: 10.1186/1479-5868-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen GM, Irby MB, Boles K, Jordan C, Skelton JA. Telemedicine and pediatric obesity treatment: review of the literature and lessons learned. Clinical obesity. 2012;2(3-4):103–11. doi: 10.1111/j.1758-8111.2012.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipana LS, Bindal D, Nettiksimmons J, Shaikh U. Telemedicine and face-to-face care for pediatric obesity. Telemedicine journal and e-health : the official journal of the American Telemedicine Association. 2013;19(10):806–8. doi: 10.1089/tmj.2012.0292. [DOI] [PubMed] [Google Scholar]
- 7.Davis AM, Sampilo M, Gallagher KS, Landrum Y, Malone B. Treating rural pediatric obesity through telemedicine: outcomes from a small randomized controlled trial. Journal of pediatric psychology. 2013;38(9):932–43. doi: 10.1093/jpepsy/jst005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McBride CM, Rimer BK. Using the telephone to improve health behavior and health service delivery. Patient Educ Couns. 1999;37(1):3–18. doi: 10.1016/s0738-3991(98)00098-6. [DOI] [PubMed] [Google Scholar]
- 9.Golan M, Crow S. Targeting parents exclusively in the treatment of childhood obesity: long-term results. Obes Res. 2004;12(2):357–61. doi: 10.1038/oby.2004.45. [DOI] [PubMed] [Google Scholar]
- 10.Oude Luttikhuis H, Baur L, Jansen H, Shrewsbury VA, O'Malley C, Stolk RP, et al. Interventions for treating obesity in children. Cochrane Database Syst Rev. 2009;(1):CD001872. doi: 10.1002/14651858.CD001872.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Collins CE, Warren J, Neve M, McCoy P, Stokes BJ. Measuring effectiveness of dietetic interventions in child obesity: a systematic review of randomized trials. Arch Pediatr Adolesc Med. 2006;160(9):906–22. doi: 10.1001/archpedi.160.9.906. [DOI] [PubMed] [Google Scholar]
- 12.Ebbeling CB, Leidig MM, Sinclair KB, Hangen JP, Ludwig DS. A reduced-glycemic load diet in the treatment of adolescent obesity. Archives of pediatrics & adolescent medicine. 2003;157(8):773–9. doi: 10.1001/archpedi.157.8.773. [DOI] [PubMed] [Google Scholar]
- 13.Moreno LA, Ochoa MC, Warnberg J, Marti A, Martinez JA, Marcos A. Treatment of obesity in children and adolescents. How nutrition can work? Int J Pediatr Obes. 2008;3(Suppl 1):72–7. doi: 10.1080/17477160801897158. [DOI] [PubMed] [Google Scholar]
- 14.Vernacchio L, Corwin MJ, Vezina RM, Pelton SI, Feldman HA, Coyne-Beasley T, et al. Xylitol syrup for the prevention of acute otitis media. Pediatrics. 2014;133(2):289–95. doi: 10.1542/peds.2013-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller WR, Rollnick S. Motivational Interviewing: Preparing People to Change Addictive Behavior. Guilford Press; New York: 1991. [Google Scholar]
- 16.Rosal MC, Ebbeling CB, Lofgren I, Ockene JK, Ockene IS, Hebert JR. Facilitating dietary change: the patient-centered counseling model. Journal of the American Dietetic Association. 2001;101(3):332–41. doi: 10.1016/S0002-8223(01)00086-4. [DOI] [PubMed] [Google Scholar]
- 17.Ebbeling CB, Leidig MM, Feldman HA, Lovesky MM, Ludwig DS. Effects of a low-glycemic load vs low-fat diet in obese young adults: a randomized trial. Jama. 2007;297(19):2092–102. doi: 10.1001/jama.297.19.2092. [DOI] [PubMed] [Google Scholar]
- 18.Ebbeling CB, Feldman HA, Chomitz VR, Antonelli TA, Gortmaker SL, Osganian SK, et al. A randomized trial of sugar-sweetened beverages and adolescent body weight. N Engl J Med. 2012;367(15):1407–16. doi: 10.1056/NEJMoa1203388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ludwig DS, Majzoub JA, Al-Zahrani A, Dallal GE, Blanco I, Roberts SB. High glycemic index foods, overeating, and obesity. Pediatrics. 1999;103(3):e26. doi: 10.1542/peds.103.3.e26. [DOI] [PubMed] [Google Scholar]
- 20.Astrup A. The role of dietary fat in the prevention and treatment of obesity. Efficacy and safety of low-fat diets. Int J Obes Relat Metab Disord. 2001;25(Suppl 1):S46–50. doi: 10.1038/sj.ijo.0801698. [DOI] [PubMed] [Google Scholar]
- 21.Lytle LA, Nichaman MZ, Obarzanek E, Glovsky E, Montgomery D, Nicklas T, et al. Validation of 24-hour recalls assisted by food records in third-grade children. The CATCH Collaborative Group. J Am Diet Assoc. 1993;93(12):1431–6. doi: 10.1016/0002-8223(93)92247-u. [DOI] [PubMed] [Google Scholar]
- 22.Ramon-Krauel M, Salsberg SL, Ebbeling CB, Voss SD, Mulkern RV, Apura MM, et al. A low-glycemic-load versus low-fat diet in the treatment of fatty liver in obese children. Child Obes. 2013;9(3):252–60. doi: 10.1089/chi.2013.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.French SA, Sherwood NE, JaKa MM, Haapala JL, Ebbeling CB, Ludwig DS. Physical changes in the home environment to reduce television viewing and sugar-sweetened beverage consumption among 5- to 12-year-old children: a randomized pilot study. Pediatr Obes. 2015 doi: 10.1111/ijpo.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolfenden L, Nathan N, Williams CM, Delaney T, Reilly KL, Freund M, et al. A randomised controlled trial of an intervention to increase the implementation of a healthy canteen policy in Australian primary schools: study protocol. Implement Sci. 2014;9:147. doi: 10.1186/s13012-014-0147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis AM, Sampilo M, Gallagher KS, Dean K, Saroja MB, Yu Q, et al. Treating rural paediatric obesity through telemedicine vs. telephone: Outcomes from a cluster randomized controlled trial. J Telemed Telecare. 2015 doi: 10.1177/1357633X15586642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irby MB, Boles KA, Jordan C, Skelton JA. TeleFIT: adapting a multidisciplinary, tertiary-care pediatric obesity clinic to rural populations. Telemedicine journal and e-health : the official journal of the American Telemedicine Association. 2012;18(3):247–9. doi: 10.1089/tmj.2011.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Resnicow K, McMaster F, Bocian A, Harris D, Zhou Y, Snetselaar L, et al. Motivational interviewing and dietary counseling for obesity in primary care: an RCT. Pediatrics. 2015;135(4):649–57. doi: 10.1542/peds.2014-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong JM, Gallagher M, Gooding H, Feldman HA, Gordon CM, Ludwig DS, et al. A randomized pilot study of dietary treatments for polycystic ovary syndrome in adolescents. Pediatr Obes. 2016;11(3):210–20. doi: 10.1111/ijpo.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warren JM, Golley RK, Collins CE, Okely AD, Jones RA, Morgan PJ, et al. Randomised controlled trials in overweight children: practicalities and realities. Int J Pediatr Obes. 2007;2(2):73–85. doi: 10.1080/17477160601133671. [DOI] [PubMed] [Google Scholar]
- 30.Guinn CH, Baxter SD, Royer JA, Hardin JW, Mackelprang AJ, Smith AF. Fourth-grade children's dietary recall accuracy for energy intake at school meals differs by social desirability and body mass index percentile in a study concerning retention interval. J Health Psychol. 2010;15(4):505–14. doi: 10.1177/1359105309353814. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.