An 8-week old male infant presented with failure-to-thrive in the setting of persistent postprandial emesis. After an uneventful pregnancy with routine prenatal care, he was born at full-term. His birth weight was 3kg (14th percentile) and his postnatal course was uncomplicated. Family history was significant for Tourette’s syndrome in his father and paternal uncle, but was otherwise unremarkable. At 4-weeks of age, he developed recurrent non-bloody, non-bilious vomiting after feeding and demonstrated persistent irritability and progressive weight loss. An abdominal ultrasound showed no intra-abdominal pathology, but incidentally revealed a left pleural effusion. A chest radiograph confirmed a large left effusion and demonstrated costovertebral anomalies in the form of butterfly-shaped thoracic vertebral bodies and a partial fusion of several ribs [Figure 1]. Upon admission, vital signs were appropriate for age and weight, length and head circumference were charted at <1st, <1st and the 31th percentile respectively. Upon examination, the infant was found to have subtle dysmorphic features [Figure 2; available at www.jpeds.com]. Breath sounds appeared diminished on the left and a systolic heart murmur was appreciated at the left-upper sternal border. An echocardiogram showed two atrial septal defects with mild to moderate left to right flow, but qualitatively normal right ventricular and valvular function. A thoracostomy tube was placed and 75ml of milk-colored fluid were removed with good lung reexpansion. Laboratory testing indicated a chylous effusion with a pH of 7.57, a WBC of 19,643/mm3 with 94% lymphocytes, and a triglyceride content of 1,062mg/dl. A diagnosis of congenital chylothorax was made and dietary therapy with a medium-chain triglyceride based formula was initiated, leading to temporary improvement only [Figure 1]. Octreotide was introduced and a repeat placement of a thoracostomy tube became necessary. Eventually the pleural effusion subsided and remained controlled on dietary therapy only. Feeding problems persisted, and consequently a gastrojejunostomy tube was placed, leading to improved weight gain. Due to the combination of early-onset chylothorax, costovertebral anomalies, atrial septal defects, hypotonia, feeding difficulties and dysmorphic features, Noonan syndrome was suspected. Genetic testing revealed a known pathogenic variant, c.417G>C (p.Glu139Asp), in exon 4 of the PTPN11 gene. This variant has been previously reported in several families with Noonan syndrome 1–5 and is predicted to be disease causing.
Figure 1.
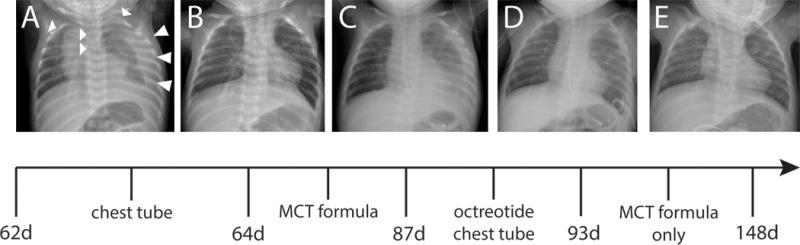
(A) Initial presentation: Large left and subpulmonic effusion. Decreased left lung volume. Streaky opacities in the right lung base and left perihilar region are consistent with atelectasis. Upper thoracic butterfly vertebrae and rib anomalies with a partially fused left cervical and first rib and partial fusion of the posterior second and third ribs are noted. (B) Two days after placement (and accidental removal) of a left chest tube: Near complete resolution of the left pleural effusion. Persistent low lung volumes. (C) Twelve days after drainage and after initiation of a MCT-based formula: Significant reaccumulation of the left pleural effusion. Bilateral hazy opacities representing diffuse atelectasis. (D) After an unsuccessful trial of octreotide, a left pigtail catheter was placed: Significant interval decrease in the left sided pleural effusion. (E) Resolution of the left sided pleural effusion. Lung volumes remain low.
Figure 2.
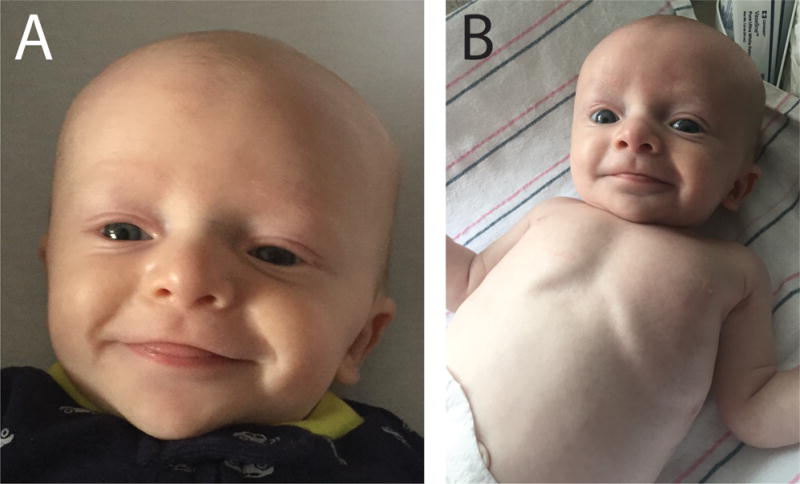
(A) & (B) Pictures taken around the time of the patient’s initial presentation. Dysmorphic facial features include a tall forehead, widely spaced eyes, a flat nasal root, low-set ears, and a cupid’s bow upper lip. A wide inter-nipple distance is also noted.
Congenital chylothorax or chylothorax presenting during infancy is a rare and potentially life-threatening condition with an incidence of about 1:30.000 births.6,7 Acquired causes of early-onset chylothorax include trauma including from thoracic surgery, granulomatous infections, tumors, congenital heart disease, and vascular or lymphatic malformations.8 Associations with genetic syndromes including congenital lymphatic malformation syndromes such as Gorham-Stout, Optiz G/BBB, Hennekam, and Milroy syndromes, and chromosome abnormalities such as Trisomy 21 or Turner syndrome have been reported.8 Chylothorax of variable onset has also been reported in RASopathies 9 such as Noonan syndrome, although detailed descriptions of spontaneous early-onset chylothorax in genetically-confirmed cases of Noonan syndrome have not been reported. Collectively, genetic etiologies may account for up to ~15–30% of cases of early-onset chylothorax.6,7 Noonan syndrome has a broad and variably expressed phenotype that often includes short stature, congenital heart defects, and variable developmental delay.10 Dysmorphic features consist of a broad or webbed neck, pectus excavatum/carinatum and characteristic facial features.11 Less common manifestations involve coagulation defects, ocular abnormalities and lymphatic dysplasia. It is notable that not all individuals develop these manifestations and that many only become apparent after infancy. Features consistent with Noonan syndrome identified in our patient include subtle but characteristic dysmorphic facial features with a typical gestalt [Figure 2], failure to thrive (although with a normal growth trajectory on the Noonan syndrome growth chart), feeding difficulties, costovertebral anomalies and an atrial septum defect. Although individually these findings are non-specific, taken together they strongly suggest a diagnosis of Noonan syndrome. Chylothorax is a rare but important complication of Noonan syndrome. Spontaneous early-onset chylothorax prompting a diagnosis of Noonan syndrome has been reported in a few cases with severe pulmonary lymphangiectasis or significant respiratory distress at birth.13–15 The present case, however, emphasizes the need to consider Noonan syndrome even if the presenting symptom is a more gradual-onset chylothorax. About 50% of Noonan syndrome cases are caused by germline mutations in PTPN11, encoding SHP2, a non-receptor protein tyrosine phosphatase. Most mutations impair SHP2 protein conformation, leading to a gain of function and resulting in increased downstream signaling in the RAS/MAPK pathway 16, thus placing Noonan syndrome in the RASopathy family of diagnoses. Interestingly, this group of disorders is increasingly linked to lymphatic anomalies 17 and inhibitors of the RAS/MAPK pathway are under intense development for cancer, some of which might be applicable as therapies for syndromic lymphatic malformations.
Acknowledgments
Supported by NIH (4 T32 HD 7466-20 [to M.W.]).
We thank the patient and his family for their generous support of this study.
Abbreviations
- MAPK
Mitogen-activated protein kinase
- PTPN11
protein-tyrosine phosphatase, nonreceptor-type, 11
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ko JM, Kim JM, Kim GH, Yoo HW. PTPN11, SOS1, KRAS, and RAF1 gene analysis, and genotype-phenotype correlation in Korean patients with Noonan syndrome. J Hum Genet. 2008;53:999–1006. doi: 10.1007/s10038-008-0343-6. [DOI] [PubMed] [Google Scholar]
- 2.Karow A, Steinemann D, Gohring G, Hasle H, Greiner J, Harila-Saari A, et al. Clonal duplication of a germline PTPN11 mutation due to acquired uniparental disomy in acute lymphoblastic leukemia blasts from a patient with Noonan syndrome. Leukemia. 2007;21:1303–5. doi: 10.1038/sj.leu.2404651. [DOI] [PubMed] [Google Scholar]
- 3.Bertola DR, Pereira AC, Albano LM, De Oliveira PS, Kim CA, Krieger JE. PTPN11 gene analysis in 74 Brazilian patients with Noonan syndrome or Noonan-like phenotype. Genet Test. 2006;10:186–91. doi: 10.1089/gte.2006.10.186. [DOI] [PubMed] [Google Scholar]
- 4.Hung CS, Lin JL, Lee YJ, Lin SP, Chao MC, Lo FS. Mutational analysis of PTPN11 gene in Taiwanese children with Noonan syndrome. J Formos Med Assoc. 2007;106:169–72. doi: 10.1016/S0929-6646(09)60235-7. [DOI] [PubMed] [Google Scholar]
- 5.Tartaglia M, Kalidas K, Shaw A, Song X, Musat DL, van der Burgt I, et al. PTPN11 mutations in Noonan syndrome: molecular spectrum, genotype-phenotype correlation, and phenotypic heterogeneity. Am J Hum Genet. 2002;70:1555–63. doi: 10.1086/340847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bialkowski A, Poets CF, Franz AR, Erhebungseinheit fur seltene padiatrische Erkrankungen in Deutschland Study G Congenital chylothorax: a prospective nationwide epidemiological study in Germany. Arch Dis Child Fetal Neonatal Ed. 2015;100:F169–72. doi: 10.1136/archdischild-2014-307274. [DOI] [PubMed] [Google Scholar]
- 7.Haines C, Walsh B, Fletcher M, Davis PJ. Chylothorax development in infants and children in the UK. Arch Dis Child. 2014;99:724–30. doi: 10.1136/archdischild-2013-304364. [DOI] [PubMed] [Google Scholar]
- 8.Tutor JD. Chylothorax in infants and children. Pediatrics. 2014;133:722–33. doi: 10.1542/peds.2013-2072. [DOI] [PubMed] [Google Scholar]
- 9.Joyce S, Gordon K, Brice G, Ostergaard P, Nagaraja R, Short J, et al. The lymphatic phenotype in Noonan and Cardiofaciocutaneous syndrome. Eur J Hum Genet. 2016;24:690–6. doi: 10.1038/ejhg.2015.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts AE, Allanson JE, Tartaglia M, Gelb BD. Noonan syndrome. Lancet. 2013;381:333–42. doi: 10.1016/S0140-6736(12)61023-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allanson JE. Objective studies of the face of Noonan, Cardio-facio-cutaneous, and Costello syndromes: A comparison of three disorders of the Ras/MAPK signaling pathway. Am J Med Genet A. 2016;170:2570–7. doi: 10.1002/ajmg.a.37736. [DOI] [PubMed] [Google Scholar]
- 12.Allanson JE, Bohring A, Dorr HG, Dufke A, Gillessen-Kaesbach G, Horn D, et al. The face of Noonan syndrome: Does phenotype predict genotype. Am J Med Genet A. 2010;152A:1960–6. doi: 10.1002/ajmg.a.33518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathur D, Somashekar S, Navarrete C, Rodriguez MM. Twin infant with lymphatic dysplasia diagnosed with Noonan syndrome by molecular genetic testing. Fetal Pediatr Pathol. 2014;33:253–7. doi: 10.3109/15513815.2014.904026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prasad R, Singh K, Singh R. Bilateral congenital chylothorax with Noonan syndrome. Indian Pediatr. 2002;39:975–6. [PubMed] [Google Scholar]
- 15.Chan DK, Ho NK. Noonan syndrome with spontaneous chylothorax at birth. Aust Paediatr J. 1989;25:296–8. doi: 10.1111/j.1440-1754.1989.tb01481.x. [DOI] [PubMed] [Google Scholar]
- 16.Niihori T, Aoki Y, Ohashi H, Kurosawa K, Kondoh T, Ishikiriyama S, et al. Functional analysis of PTPN11/SHP-2 mutants identified in Noonan syndrome and childhood leukemia. J Hum Genet. 2005;50:192–202. doi: 10.1007/s10038-005-0239-7. [DOI] [PubMed] [Google Scholar]
- 17.Brouillard P, Boon L, Vikkula M. Genetics of lymphatic anomalies. J Clin Invest. 2014;124:898–904. doi: 10.1172/JCI71614. [DOI] [PMC free article] [PubMed] [Google Scholar]


