Abstract
Research on balance and mobility in older adults has been conducted primarily in lab-based settings in individuals who live in the community. Although they are at greater risk of falls, residents of long-term care facilities, specifically residential care communities (RCCs), have been investigated much less frequently. We sought to determine the feasibility of using portable technology-based measures of balance and muscle strength (i.e. an accelerometer and a load cell) that can be used in any RCC facility. Twenty-nine subjects (age 87±6 years) living in RCCs participated. An accelerometer placed on the back of the subjects measured body sway during different standing conditions. Sway in antero-posterior and medio-lateral directions were calculated. Lower extremity strength was measured with a portable load cell and the within-visit reliability was determined. Assessments of grip strength, gait speed, frailty, and comorbidity were also examined. A significant increase in postural sway in both the AP and ML directions occurred as the balance conditions became more difficult due to alteration of sensory feedback (p < 0.001) or reducing the base of support (p < 0.001). There was an association between increased sway and increased frailty, more comorbidities and slower gait speed. All strength measurements were highly reliable (ICC = 0.93 to 0.99).An increase in lower extremity strength was associated with increased grip strength and gait speed. The portable instruments provide inexpensive ways for measuring balance and strength in the understudied RCC population, but additional studies are needed to examine their relationship with functional outcomes.
Keywords: long-term care, residential care communities, accelerometer, standing balance, and muscle strength
Introduction
In 2012, approximately 2.1 million people lived in long-term care facilities in the US. Two-thirds lived in nursing homes and one-third resided in residential care communities (RCCs).1An RCC is defined as a facility that provides room, board with at least two meals a day, help with personal care such as bathing and dressing, and health-related services, such as medication management.1 Older adults residing in long-term care facilities are at greater risk of falling and sustaining an injury compared to community dwellers2. Another report estimates 21% of residents of RCCs fell in the previous 3 months, and that 29% of residents require assistance with walking or locomotion.3 Because individuals living in RCCs represent a group that is transitioning to needing greater assistance and having greater fall risk, it is important to investigate the fall risk factors in this population.
Lower-extremity muscle weakness and balance impairment are two of the many risk factors that have been associated with mobility limitations and falls in older adults.2,4–7 Research related to balance and mobility in older adults has been conducted primarily in lab-based settings with individuals who reside in the community. The laboratory-based tests are usually expensive and not portable, so the application of these tests has been limited to larger cohorts. Recent technological advancements have provided inexpensive and portable quantitative tools to assess balance, such as accelerometers and the Wii balance board™ (Nintendo, Redmond, WA), in older adults,8–10 and those with Parkinson disease,11 multiple sclerosis,12 stroke.13 Little data are available on use of these technologies to assess sway and strength in RCCs.
The current gold standard method to measure lower extremity muscle strength is using computerized isokinetic dynamometry.14 The high cost, low portability, and time-consumption are drawbacks that have limited the application of computerized isokinetic dynamometry in residential care communities. Another method to assess strength in a clinical setting is manual muscle testing. Although it is the most frequently used technique to quantify muscle strength and easier to use, it lacks sensitivity and responsiveness and is subject to a ceiling effect.15,16 Handheld dynamometers have been commonly used in different settings to objectively quantify muscle strength. Even though handheld dynamometers have good reliability in different populations, they have some important limitations such as difficulty in stabilizing the subject, and they are influenced by the strength of the examiner especially for large muscle groups.17,18 The concept of using a simple strain-gauge uniaxial load cell device has been proposed before but it has not been tested in who live in residential care communities.19 A uniaxial load cell device provides an easy and reliable way to overcome the aforementioned drawbacks and quantify muscle strength in different settings outside the clinic.
The purpose of the present study is the validation of upright balance and examining the within-day reliability of lower extremity muscle strength measurements in older adults living in RCCs, by using an inexpensive and portable device that can be used in any long-term care facility.
Methods
Subjects and Setting
Twenty-nine participants (8 male, meanage 87±6 years) enrolled in this study. All subjects were recruited using a convenience sample of older adults living in three RCC facilities, who were already enrolled in a program to examine functional assessments of residents in RCC settings. Inclusion criteria were: (a) age 65 years or older, (b) living independently in RCC facilities (i.e. requiring little or no assistance with the activities of daily living), and (c) cognitively able to provide informed consent. Exclusion criteria included (a) subjects who were not able to ambulate for 1 minute (assistive devices were allowed), and (b) any medical or neurological condition that prevented subjects from performing maximal muscle action. The study was approved by the Institutional Review Board (IRB) of the University of Pittsburgh.
Instrumentation
Balance Accelerometry
Balance accelerometry was adapted from part of the National Institutes of Health (NIH) toolbox project.20 The system consists of a dual axis accelerometer (ADXL213AE, Analog Devices, Inc., Norwood, MA) oriented to record mediolateral (M-L) and anteroposterior (A-P) acceleration of the body. The acceleration is transmitted via a Bluetooth transmitter to a laptop computer at 50 Hz and with 16-bit accuracy. The system was affixed to the subject's back at the level of the iliac crest using Velcro and a gait belt. A custom written Labview program was used to acquire the data (Figure 1).
Figure 1.
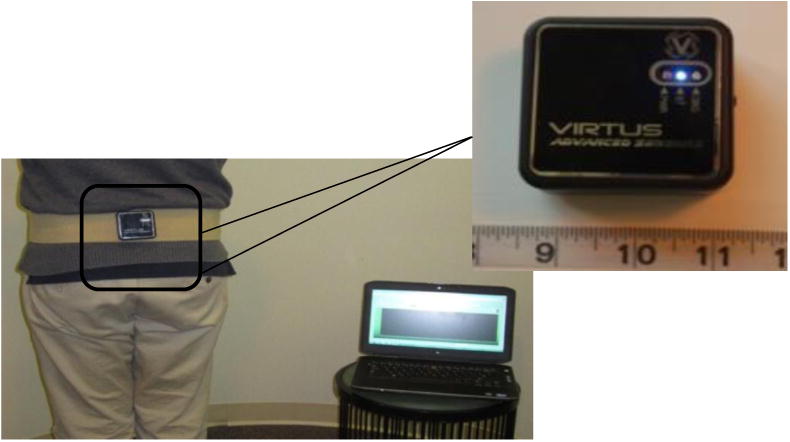
An accelerometer placed on back of subject, and a Bluetooth transmitter is used to record the data on a laptop computer.
Uni-axial load cell for assessment of lower extremity strength
A uni-axial load cell (Measurement Specialties, ELPF-T3E-500L, Redmond, WA) was used to measure the lower extremity strength. The load cell has a maximum capacity of 2225 N. The load cell was connected to an amplifier that displayed the instantaneous and maximum force exerted on the load cell. The load cell was arranged in series with straps (two cuffs) that fit around the limb on one end and a stable object on the other end. Muscle groups included hip flexors and abductors, knee extensors and flexors, and ankle dorsiflexors and plantarflexors (Figure 2).
Figure 2.
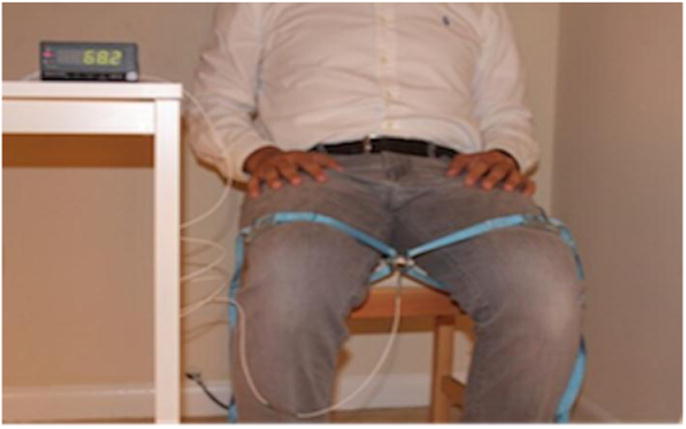
A uni-axial load cell placed between the subject's legs and secured with straps to measure hip abduction muscle strength, which is displayed by the indicator on the table.
Procedure
Standing Balance Test
The protocol consisted of multiple tests. The first test was based on the modified Clinical Test of Sensory Interaction in Balance (mCTSIB,),21 and had 4 different standing conditions designed to alter the sensory feedback: (1) standing with feet together on firm surface with eyes open (FIRM-EO); (2) standing with feet together on firm surface with eyes closed (FIRM-EC); (3) standing with feet together on foam surface with eyes open (FOAM-EO); (4) standing with feet together on foam surface with eyes closed (FOAM-EC). The foam surface that was used in the testing consisted of an Airex Balance Pad (Advanced Medical Technology Inc., Watertown, MA), with a thickness was 6 cm. Under the 4 conditions, subjects stood for 30s, as still as possible, facing a wall with their arms crossed in front of their chest. A research coordinator guarded the subject from behind in case a loss of balance occurred. Between condition, subjects had a seated rest break for 30-60 s. If subjects were not able to complete a trial, they were given one other opportunity to perform it.
For the second balance test, subjects performed Short Physical Performance Battery (SPPB).22 First, subjects stood for 10 sec with three different bases of support: feet together, semi-tandem (one foot halfway in front of the other), and tandem (heel of one foot directly in front of the toes of the other foot). Subjects could choose which foot to put in front. If subjects were unable to complete, they continued to the next condition and the investigator documented that subject wasn't able to complete the task. Next, subjects performed repeated sit-to-stand movements, and finally gait speed was timed for 4 meters. The sway during these final two components was not considered for further analysis.
Lower Extremity Strength Testing
Strength measurements included three maximum voluntary isometric contractions (MVIC) for six different muscle groups. All of the testing was done in sitting position. Testing positions have been adopted from studies that have used isokinetic14, and isometric dynamometers39; details about the device positions for each muscle group are summarized in Table 1. The test was done with the subject's dominant leg in all tests except for the hip abductor strength, which was necessarily tested bilaterally. The dominant leg was identified by asking the subject the side of hand dominance. All the trials were done with the participant sitting in a chair. The tone and words of encouragement used by the examiner were standardized. During each trial, the subject increased force up to a maximum over the course of five seconds. Thirty seconds of rest was provided between trials. The peak value was recorded from the amplifier. The average of the three trials was used in the data analysis. All of the measurements were taken by a physical therapist.
Table 1. Testing positions for strength measurements. Subject was seated in a standard height 4-legged chair in all positions.
| Isometric Action | Testing Device Position |
|---|---|
| Knee extension | The subject was seated with hips and knees flexed 90 deg. One cuff of the device was placed around the chair leg and the other cuff around the ankle of the subject's dominant leg. The subject extended the knee gradually until the maximum force was attained over the course of five seconds |
| Knee flexion | The subject was seated with hips and knees flexed 90 deg. One cuff was placed around subject's ankle and the other cuff was attached to fixed object in front of the subject. The subject flexed the knee gradually until the maximum force was attained |
| Hip abduction | The subject was seated with hips and knees flexed 90 deg, with knees touching at midline. One cuff was placed around the right thigh and the other cuff around the left thigh, just proximal to the knee joint. The subject moved both knees laterally until the maximum force was attained |
| Hip flexion | The subject was seated with hips and knees flexed 90 deg. One cuff was placed around the thigh just proximal to the knee joint and the second cuff was attached to the ground by having the examiner step on it. The subject flexed the hip in a superior direction until the maximum force was attained |
| Ankle plantarflexion and dorsiflexion | The subject was seated with hips flexed 90 deg. The dominant leg was extended at the knee. One cuff was placed around the distal part of the foot approximately at the first metatarso-phalangeal joint, and the other cuff was attached to a fixed object (e.g. arm of the chair). For ankle plantarflexion, the examiner held a bar with the other cuff attached to it. Subject plantarflexed or dorsiflexed the ankle until the maximum force was attained |
Secondary outcome measures
Duke Comorbidity Index
The Duke Comorbidity Index is a simple patient self-report measure that contains 18-item list of chronic conditions. The index has been validated for various subgroups such as community-older adults and stroke population.23,24
Grip strength
A JAMAR PLUS+® digital hand dynamometer (Sammons Preston, Bolingbrook, IL) was used to measure grip strength. Four trials were performed after one practice trial, with the dominant hand. The average of the peak force was calculated.
Gait speed
Gait speed was measured over 4 meters at self-selected pace. This measure assesses usual walking speed over short distance. It represents a quick, inexpensive and highly reliable tool to use with older adults.25 Participants stood with both feet touching the start line and asked to walk at his/her usual speed until he/she passed a cone that was placed at the end of the 4 meters. The average of two trials was used in the data analysis.
Fried's Frailty Index
Fried's phenotype of frailty26 was used to assess frailty; scoring is based on presence of five frailty indicators: weight loss (self-report of unintentional weight loss of 10 pounds or more in the past year), exhaustion (identified by subject responses to the questions from the Center for Epidemiological Studies depression (CES-D) scale: “I felt that everything I did was an effort” and “I could not get going”), slow gait speed (time to walk 15 feet, adjusted for gender and height), weakness (grip strength, using an established cutoff stratified by gender and BMI), and low physical activity (measured using subject responses to a leisure time questionnaire, the Minnesota Leisure Time Activities Questionnaire).26 Each indicator is assigned a value of 0 or 1. Subjects were divided into three groups based on total score: Nonfrail = 0 indicators, Prefrail = 1-2 indicators, Frail = 3 or more indicators.26
Data Processing
The balance accelerometry data were visually inspected and sudden, extraneous movements were removed. The first and last five seconds for the mCTSIB conditions were not included in the analysis in order to avoid transition effects. Using a custom written Matlab program, the accelerometry data were lowpass-filtered using a 4th order Butterworth filter with a cutoff frequency of 2 Hz. The Root Mean Square (RMS), and the Normalized Path Length (NPL) of the acceleration were calculated for both the anteroposterior (AP) and mediolateral (ML) directions (Figure 3). The RMS and NPL were calculated as follows:
Figure 3.
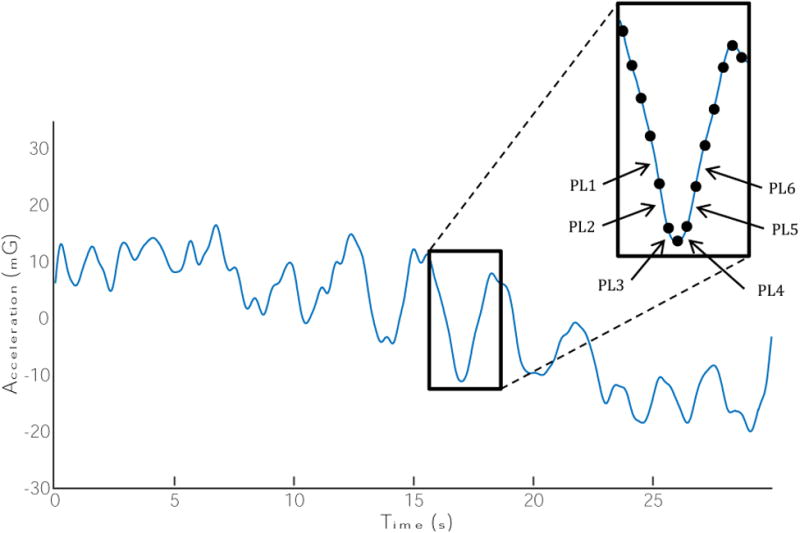
Representative sample of postural acceleration in the antero-posterior direction during standing on level surface with eyes open. The root-mean-square (RMS) is the standard deviation of the points, referenced to the mean value of the entire acceleration curve. The normalized path length (NPL) is the sum of the absolute value of the individual path length distances (as shown in the inset figure, PL1, PL2, …, PL6), divided by the length of time that it takes to travel this distance. This is performed for the entire length of time for the trial.
Where N is the number of samples, and t equals to the time duration, and a is the acceleration data at time sample j. The mG stands for milli-Gravitational acceleration, where 1 mG = 0.0098 m/s2 and mG/s is the milli-Gravitional acceleration divided by the time duration in s.
Statistical Analysis
SPSS version 21.0 was used for all data analyses. The Friedman test was used to examine if significant differences existed in the postural sway among the mCTSIB and SPPB balance tasks. Post hoc pairwise comparisons were made with Wilcoxon signed ranks tests. In order to assess the test-retest reliability for the lower extremity muscle strength testing across all three contractions, the intra-class correlation coefficient (ICC) was calculated.27 The standard error of measurement (SEM) for each of the muscle strength conditions was calculated.
Where SD is the standard deviation and r is the intraclass correlation coefficient. A Spearman rank correlation was used to examine the relationship between postural sway and muscle strength measurements with gait speed, number of comorbidities, grip strength and frailty index. A Mann-Whitney U test was used to determine if there is a significant difference between fallers and non-fallers in terms of body sway and lower extremity strength.
Results
Participant Characteristics
A total of 29 subjects living in RCCs were assessed using balance accelerometry and muscle strength measurements. The average age of the participants was 87 ± 6 years (range: 72-98 years). Seventy-two percent (21/29) of the participants were female. About 66% (19/29) had fallen at least once in the previous year (Table 2). Reduced grip strength, after adjusting for gender and BMI, was the most common frailty indicator (72% of subjects), followed by reduced walking speed (45%), low physical activity (41%), exhaustion (21%), and unintentional weight loss (3%) (Table 3).
Table 2. Demographic and clinical characteristics of subjects.
| Variable | Mean (SD) | Range |
|---|---|---|
| Age (y) | 87 (6) | 72 - 98 |
| Body mass index (kg/m2) | 28.1 (5.6) | 21 - 47 |
| Number of comorbidities | 7 (3) | 2 - 13 |
| Falls in the last year | Number of subjects | % |
| 0 | 10 | 34 |
| 1 | 8 | 28 |
| 2 or more | 11 | 38 |
| Fried's Frailty Index | ||
| Nonfrail | 4 | 14 |
| Prefrail | 18 | 62 |
| Frail | 7 | 24 |
Table 3. Percentage of subjects who had the presence of an individual frailty indicator.26.
| Frailty Markers | Total % (n=29) |
|---|---|
| Weight loss: lost >10 lbs unintentionally | 3 |
| Low physical activity (kcals/week) | 41 |
| Reduced walking time/15 feet | 45 |
| Reduced grip strength (kg) | 72 |
| Exhaustion (self-report) | 21 |
Balance Accelerometry
A significant increase in RMS and NPLs way in both the AP and ML directions occurred as the balance conditions became more difficult due to alteration of sensory feedback in the mCTSIB(p < 0.001), or reducing the base of support in the SPPB(p < 0.001). RMS sway in the AP direction was increased by at least 50% when subjects closed their eyes compared with eyes open, or stood on foam compared with standing on level surface (Figure 4, top). In the ML direction, RMS sway increased by at least 60% with eyes closed, and by at least 100% when standing on foam compared to level surface (Figure 4, top). Similarly, the NPL sway in both directions was increased by at least 50% during eyes closed compared with eyes open, and when standing on compliant surface as compared to standing on the firm surface (Figure 4b, bottom). During the SPPB, RMS and NPL sway in both AP and ML directions were increased by at least 60% when subjects stood in the tandem position compared with standing with feet together and in semi-tandem, and RMS sway in the ML direction was increased by about 25% during semi-tandem stance compared with feet together (Figure 5).
Figure 4.
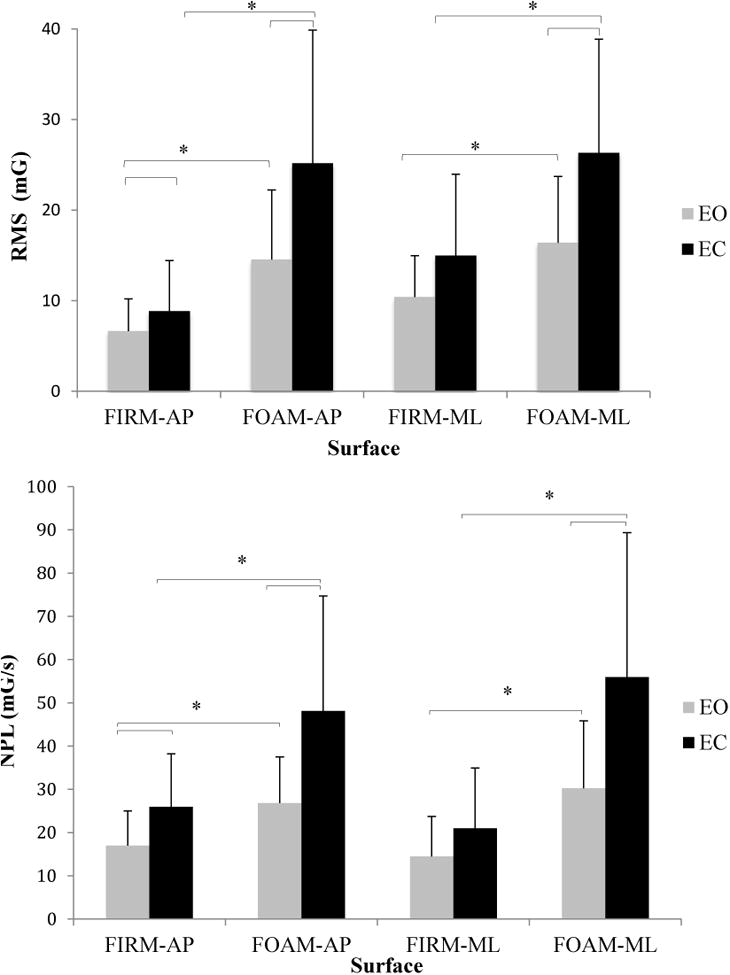
A) Effect of vision (Eyes Open: EO, and Eyes Closed, EC) and surface conditions (Firm, Foam) on root-mean-square (RMS, Top) and normalized path length (NPL, Bottom) sway acceleration for antero-posterior (AP) and mediolateral (ML) directions. (Error bars represent ± 1 standard deviation); mG: milli-Gravitational acceleration, mG/s:milli-Gravitational acceleration divided by time duration.
*: indicates significant difference with p < 0.05.
Figure 5.
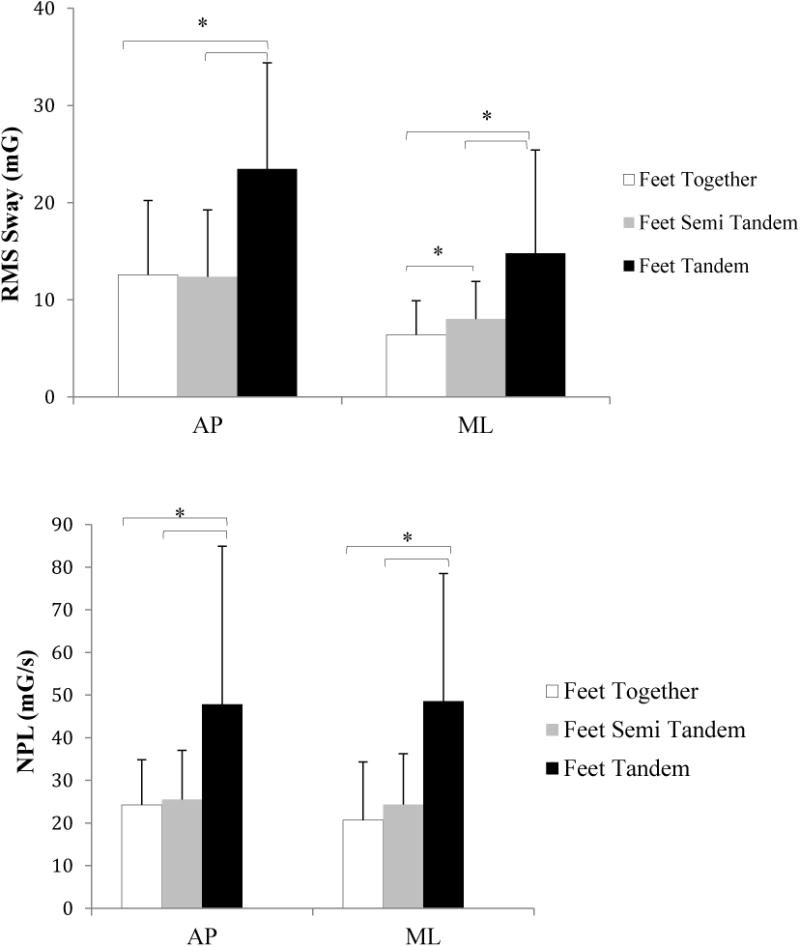
Effect of base of support on root-mean-square (RMS, Top) and normalized path length (NPL, Bottom) sway acceleration for antero-posterior (AP) and mediolateral (ML) directions.(error bars represent ± 1 standard deviation); mG: milli-Gravitational acceleration, mG/s:milli-Gravitational acceleration divided by time duration.
*: indicates a significant difference with p < 0.05.
There was a statistically significant correlation between the total amount of RMS sway in the AP and ML directions and number of frailty indicators during standing with eyes open on compliant surface, (AP: Spearman rho = 0.40, p = 0.04, ML: Spearman rho = 0.53, p = 0.006). In addition, when subjects stood on a firm surface with eyes open, AP RMS sway was significantly correlated with the number of frailty indicators (Spearman rho = 0.42, p = 0.03).RMS sway in the ML direction during semi-tandem stance was significantly different between fallers and non-fallers (U = 28, p = 0.004), but no other sway measures were associated with fall history (Figure 6).
Figure 6.
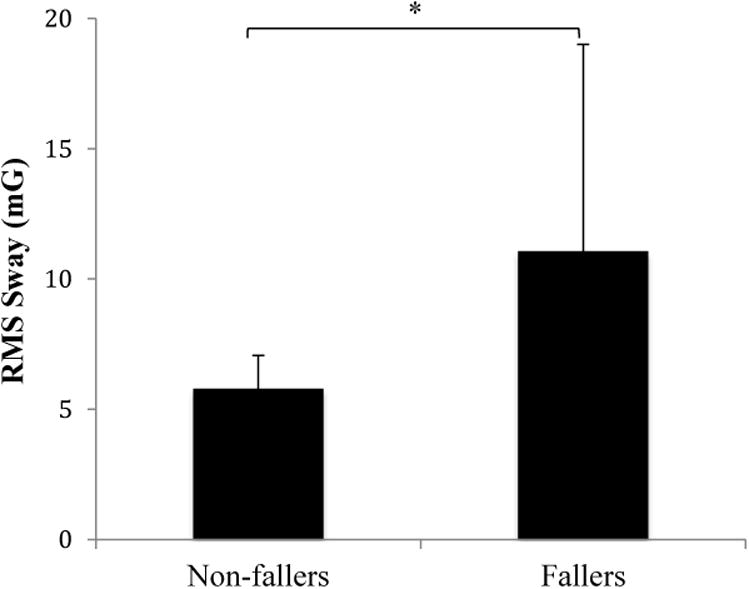
Root-mean-square (RMS, mG) sway acceleration for ML direction during semi-tandem stance between fallers and non-fallers; mG: milli-Gravitational acceleration.
*: significant differences with p < 0.05.
A number of the balance accelerometry measurements were correlated with clinically important measures, specifically the Duke Comorbidity Index and gait speed (Table 4). The number of comorbidities was significantly associated with increases in both AP and ML sway while subjects stood on foam with EC (Spearman rho = 0.39 AP, 0.41 ML, p < 0.04), semi-tandem (Spearman rho = 0.57 AP, 0.46 ML, p < 0.015) and tandem conditions (Spearman rho = 0.47 AP,0.51 ML, p<0.020). Elevated RMS and NPL sway in the ML direction during tandem standing was associated slower gait speed (Spearman rho =-0.65for RMS, -0.42 for NPL, p < 0.04).
Table 4. Spearman rank correlation coefficients between balance accelerometry conditions, and Duke Comorbidity Index and gait speed.
| Duke Comorbidity Index | Gait speed | |
|---|---|---|
| Foam EC (AP-RMS) | 0.39* | -0.06 |
| Foam EC (ML-RMS) | 0.41* | 0.10 |
| Feet tandem (AP-RMS) | 0.47* | -0.39 |
| Feet tandem (AP-NPL) | 0.57* | -0.38 |
| Feet tandem (ML-RMS) | 0.52* | -0.65* |
| Feet tandem (ML-NPL) | 0.42* | -0.42* |
| Semi-tandem (AP-NPL) | 0.57* | -0.13 |
| Semi-tandem (ML-RMS) | 0.46* | -0.07 |
| Semi-tandem (ML-NPL) | 0.50* | 0.08 |
indicates significant correlation coefficient p < 0.05.
Strength measurements
Descriptive statistics and test-retest reliability data for strength measurements are presented in Table 5. Test-retest reliability was calculated from 3 repeated tests and all measurements were highly reliable (ICC = 0.93 to 0.99). The standard error of the measurement was less than 24 N. There was a significant correlation between reduced knee flexion, ankle dorsiflexion, and hip abduction strength and an increased number of frailty indicators (Spearman rho = -0.42– -0.54, p < 0.04). In order to validate the utility of these strength measurements, we correlated the strength with the clinical measures (Table 6). An increase in lower extremity strength performance was associated with increased grip strength except for hip flexion strength (Spearman rho = 0.56 – 0.76, p <0.007) and increased gait speed except for ankle plantarflexion and hip flexion (Spearman rho = 0.49 – 0.52, p < 0.02). In addition, a decrease in the number of comorbidities was associated with greater knee flexion and ankle dorsiflexion strength (Spearman rho = -0.47, Spearman rho =-0.48, p <0.03).
Table 5. Mean (SD) lower extremity strength performance and test-retest reliability, indicated by the intraclass correlation coefficient (ICC), and standard error of measurement (SEM).
| Isometric Action (N) | Mean (SD) | ICC (CI 95%) | SEM |
|---|---|---|---|
| Knee Extension | 163.7 (74.5) | 0.95 (0.91-0.97) | 16.6 |
| Knee Flexion | 122.5 (45.1) | 0.98 (0.97-0.99) | 6.4 |
| Hip Abduction | 177.4 (74.5) | 0.99 (0.98-0.99) | 23.5 |
| Hip Flexion | 141.1 (48.0) | 0.97 (0.95-0.98) | 8.2 |
| Ankle PF | 267.5 (114.7) | 0.97 (0.95-0.98) | 19.8 |
| Ankle DF | 115.6 (47.0) | 0.93 (0.87-0.96) | 12.3 |
Table 6. Spearman rank correlation coefficients between lower extremity strength, and Duke Comorbidity Index, gait speed, and grip strength.
| Isometric Action | Duke Comorbidity Index | Gait speed | Grip strength |
|---|---|---|---|
| Knee Extension | -0.13 | 0.49* | 0.56* |
| Knee Flexion | -0.47* | 0.50* | 0.76* |
| Hip Abduction | -0.25 | 0.52* | 0.68* |
| Hip Flexion | -0.11 | 0.23 | 0.34 |
| Ankle PF | -0.07 | 0.19 | 0.66* |
| Ankle DF | -0.48* | 0.52* | 0.65* |
indicates significant correlation coefficient p < 0.05
Discussion
The purpose of the present study was to demonstrate the feasibility of measuring balance performance using low-cost accelerometers and lower extremity muscle strength using a uni-axial load cell, in older adults living in residential care communities. Body sway measured with the accelerometer increased as the balance task became progressively more difficult by reducing the degree of sensory input or by narrowing the base of support. These findings demonstrate the ability of the accelerometer measurements to measure body sway in community settings. In addition, the test-retest reliability for the lower extremity measurements was high.
When somatosensory cues relative to a stable surface were reduced by standing on a foam pad, the older adults produced higher body sway than standing on firm surface.28 The current findings are in accordance with other research using similar technology.8,9,29,30 Our research extends the previous work by demonstrating feasibility in a residential environment in older adults who had mean age that was greater than many of the other studies investigating older adults. Moreover, our results showed that the NPL for AP sway during the eyes closed on foam condition was greater than the sway of healthy older adults aged 66-85 in a previous study that used the same accelerometer, which further validates the measurements.31 In order to further establish face validity, the magnitude of postural sway in both AP and ML directions during standing on foam EC, semi-tandem and tandem conditions was correlated with the total number of comorbid medical conditions, indicating that greater sway was related to a greater number of comorbidities.
The current findings also suggest that the sway measurements reflect the frailty status of older adults. The results should be interpreted cautiously given the small sample size in this study. Also, an increase in body sway is not specifically an indication for frailty. For instance, people with vestibular dysfunction or peripheral neuropathy may have increased sway, without being classified as frail.32 Nonetheless, our results are in general agreement with previous studies in terms of differences in postural balance in older adults with varying degrees of frailty.33–35 In addition, postural sway in the ML direction during semi-tandem stance was significantly increased in subjects with a history of falling compared with those who did not fall in the previous year. Semi-tandem stance places more emphasis on the control of stance in the ML direction, which has been shown to be related to fall history in previous studies.36–38
Our results indicate excellent relative reliability across the test trials for all the included muscle groups. In addition, the mean force production was consistent with previous reported values from adults 81-89 years old that were obtained using a stationary dynamometer.39 Four out of the six strength values (except hip flexion and ankle plantarflexion) were correlated with grip strength and gait speed. It is possible that hip flexion and ankle plantarflexion were not as highly correlated with grip strength because the examiner provided some of the resistance, rather than the cuff being attached to an immovable object. As a result, the examiner may have influenced these recordings.
The common method to objectively quantify postural stability is the use of force platforms, which measure the ground reaction forces and moments (as represented by the center of pressure, or COP) that control the movements of the center of mass. Although force platforms are a reliable and sensitive tool,40 the cost, required space, and lack of portability result in limited clinical usage, as well as limit the ability to use it across different populations such as residential care communities. In addition, there are alternatives such as TekScan MatScan41, and Wii Balance Board 10 which have shown good reliability in estimating COP. Although these tools can provide information about the control of the movements, it is recognized that these measurements correlate highly with the movement of the center of mass that can be estimated from body-worn accelerometers, which overcome the limitations of cost and portability.9 Therefore, implementing such technology will help investigators access understudied populations such as residents of RCC, and will allow clinicians to examine measurements in real-life environments. This should ultimately help clinicians and therapists develop interventions based on the subject's balance assessment. Finally, the uniaxial load cell provides a reliable and inexpensive method to quantify muscle strength in different environments outside the clinic.
One issue regarding clinical research in long term care facilities (including RCC) is the range of function of the residents. Long term care centers can include skilled, assisted living, independent, dementia units and short term care. Furthermore, residents often transition from one level to another depending on acute illness, fractures, family requests or physician input/supervision. Therefore, rather than classify or characterize residents by level of care, functional status or frailty measures are often used to characterize a cohort. Given the important relationships between falls, balance and lower extremity strength, developing low-cost and portable assessments of balance and strength are essential for monitoring the health status of the residents.
There were several limitations to this study: Given that this sample was a pilot study to examine the feasibility of obtaining these measurements in an RCC environment, future studies with larger sample sizes are needed to comprehensively examine the relationships between balance and mobility measurements. Second, other psychometric properties such as responsiveness, and test-retest reliability for the sway measurements were not examined in this study.
Conclusion
Sway increased as the testing conditions became more difficult, and correlated with grip strength, gait speed, and number of comorbidities in the RCC population. In addition, sway increased with the number of frailty indicators. Furthermore, a portable load cell provides a feasible, reliable, and inexpensive method for testing lower extremity muscle strength, in a frail cohort in the long-term care setting.
Acknowledgments
This study was supported by funds from the National Institutes of Health, National Institute on Aging (R01AG028068). The authors gratefully acknowledge the support from the University of Pittsburgh Pepper Center, and the UPMC Aging institute. We also thank staff and participants of the following sites from which data has been collected: Asbury Heights site, Seneca Hills Village, and Cumberland Woods Village.
Footnotes
Conflicts of interest: On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- 1.Hyattsville MD. National Center for Health Statistics. Retrieved from http://www.cdc.gov/nchs/data/nsltcp/long_term_care_services_2013.pdf.
- 2.Rubenstein LZ. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing. 2006;35(Suppl 2):ii37–ii41. doi: 10.1093/ageing/afl084. [DOI] [PubMed] [Google Scholar]
- 3.Harris-Kojetin L, Sengupta M, Park-Lee E, et al. Long-Term Care Providers and services users in the United States: data from the National Study of Long-Term Care Providers, 2013-2014. Vital Health Stat 3. 2016;(38):x–xii. 1-105. [PubMed] [Google Scholar]
- 4.Koster A, Penninx BWJH, Newman AB, et al. Lifestyle factors and incident mobility limitation in obese and non-obese older adults. Obesity (Silver Spring) 2007;15(12):3122–3132. doi: 10.1038/oby.2007.372. [DOI] [PubMed] [Google Scholar]
- 5.Gill TM, Gahbauer EA, Murphy TE, Han L, Allore HG. Risk factors and precipitants of long-term disability in community mobility: A cohort study of older persons. Ann Intern Med. 2012;156(2):131–140. doi: 10.7326/0003-4819-156-2-201201170-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rivera JA, Fried LP, Weiss CO, Simonsick EM. At the tipping point: predicting severe mobility difficulty in vulnerable older women. J Am Geriatr Soc. 2008;56(8):1417–1423. doi: 10.1111/j.1532-5415.2008.01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Summary of the Updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc. 2011;59(1):148–157. doi: 10.1111/j.1532-5415.2010.03234.x. [DOI] [PubMed] [Google Scholar]
- 8.O'Sullivan M, Blake C, Cunningham C, Boyle G, Finucane C. Correlation of accelerometry with clinical balance tests in older fallers and non-fallers. Age Ageing. 2009;38(3):308–313. doi: 10.1093/ageing/afp009. [DOI] [PubMed] [Google Scholar]
- 9.Whitney SL, Roche JL, Marchetti GF, et al. A comparison of accelerometry and center of pressure measures during computerized dynamic posturography: a measure of balance. Gait Posture. 2011;33(4):594–599. doi: 10.1016/j.gaitpost.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang WD, Chang WY, Lee CL, Feng CY. Validity and reliability of wii fit balance board for the assessment of balance of healthy young adults and the elderly. J Phys Ther Sci. 2013;25(10):1251–1253. doi: 10.1589/jpts.25.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horak FB, Mancini M. Objective biomarkers of balance and gait for Parkinson's disease using body-worn sensors. Mov Disord. 2013;28(11):1544–1551. doi: 10.1002/mds.25684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spain RI, Mancini M, Horak FB, Bourdette D. Body-worn sensors capture variability, but not decline, of gait and balance measures in multiple sclerosis over 18 months. Gait Posture. 2014;39(3):958–964. doi: 10.1016/j.gaitpost.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Culhane KM, O'Connor M, Lyons D, Lyons GM. Accelerometers in rehabilitation medicine for older adults. Age Ageing. 2005;34(6):556–560. doi: 10.1093/ageing/afi192. [DOI] [PubMed] [Google Scholar]
- 14.Stark T, Walker B, Phillips JK, Fejer R, Beck R. Hand-held dynamometry correlation with the gold standard isokinetic dynamometry: a systematic review. PM R. 2011;3(5):472–479. doi: 10.1016/j.pmrj.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 15.Frese E, Brown M, Norton BJ. Clinical reliability of manual muscle testing. Middle trapezius and gluteus medius muscles. Phys Ther. 1987;67(7):1072–1076. doi: 10.1093/ptj/67.7.1072. [DOI] [PubMed] [Google Scholar]
- 16.Conable KM, Rosner AL. A narrative review of manual muscle testing and implications for muscle testing research. J Chiropr Med. 2011;10(3):157–165. doi: 10.1016/j.jcm.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stone CA, Nolan B, Lawlor PG, Kenny RA. Hand-held dynamometry: tester strength is paramount, even in frail populations. J Rehabil Med. 2011;43(9):808–811. doi: 10.2340/16501977-0860. [DOI] [PubMed] [Google Scholar]
- 18.Wikholm JB, Bohannon RW. Hand-held Dynamometer Measurements: Tester Strength Makes a Difference. J Orthop Sport Phys Ther. 1991;13(4):191–198. doi: 10.2519/jospt.1991.13.4.191. [DOI] [PubMed] [Google Scholar]
- 19.Dura JV, Gianikellis K, Forner AA. A STRAIN-GAUGE UNIAXIAL LOAD CELL TO EVALUATE MUSCULAR STRENGTH LEVEL IN ISOMETRIC EXERCISE. ISBS - Conf Proc Arch. 1996;1(1) [Google Scholar]
- 20.Rine RM, Schubert MC, Whitney SL, et al. Vestibular function assessment using the NIH Toolbox. Neurology. 2013;80(11 Suppl 3):S25–S31. doi: 10.1212/WNL.0b013e3182872c6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shumway-Cook a, Horak FB. Assessing the influence of sensory interaction of balance. Suggestion from the field. Phys Ther. 1986;66:1548–1550. doi: 10.2522/ptj.20080227. [DOI] [PubMed] [Google Scholar]
- 22.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 23.Rigler SK, Studenski S, Wallace D, Reker DM, Duncan PW. Co-morbidity adjustment for functional outcomes in community-dwelling older adults. Clin Rehabil. 2002;16(4):420–428. doi: 10.1191/0269215502cr515oa. [DOI] [PubMed] [Google Scholar]
- 24.Studenski SA, Lai SM, Duncan PW, Rigler SK. The impact of self-reported cumulative comorbidity on stroke recovery. Age Ageing. 2004;33(2):195–198. doi: 10.1093/ageing/afh056. [DOI] [PubMed] [Google Scholar]
- 25.Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13(10):881–889. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- 26.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 27.Shrout PE, Fleiss JL. Intraclass Correlations : Uses in Assessing Rater Reliability. 1979;86(2):420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 28.Wolfson L, Whipple R, Derby CA, et al. A dynamic posturography study of balance in healthy elderly. Neurology. 1992;42(11):2069–2075. doi: 10.1212/wnl.42.11.2069. [DOI] [PubMed] [Google Scholar]
- 29.Kamen G, Patten C, Du CD, Sison S. An accelerometry-based system for the assessment of balance and postural sway. Gerontology. 1998;44(1):40–45. doi: 10.1159/000021981. [DOI] [PubMed] [Google Scholar]
- 30.Moe-Nilssen R, Helbostad JL. Trunk accelerometry as a measure of balance control during quiet standing. Gait Posture. 2002;16(1):60–68. doi: 10.1016/s0966-6362(01)00200-4. [DOI] [PubMed] [Google Scholar]
- 31.Marchetti GF, Bellanca J, Whitney SL, et al. The development of an accelerometer-based measure of human upright static anterior- posterior postural sway under various sensory conditions: test-retest reliability, scoring and preliminary validity of the Balance Accelerometry Measure (BAM) J Vestib Res. 2013;23(4-5):227–235. doi: 10.3233/VES-130490. [DOI] [PubMed] [Google Scholar]
- 32.Allum JH, Adkin AL, Carpenter MG, Held-Ziolkowska M, Honegger F, Pierchala K. Trunk sway measures of postural stability during clinical balance tests: effects of a unilateral vestibular deficit. Gait Posture. 2001;14(3):227–237. doi: 10.1016/s0966-6362(01)00132-1. [DOI] [PubMed] [Google Scholar]
- 33.Dayhoff NE, Suhrheinrich J, Wigglesworth J, Topp R, Moore S. Balance and muscle strength as predictors of frailty among older adults. J Gerontol Nurs. 1998;24(7):18–27. doi: 10.3928/0098-9134-19980701-06. quiz 54-55. [DOI] [PubMed] [Google Scholar]
- 34.Schwenk M, Mohler J, Wendel C, et al. Wearable sensor-based in-home assessment of gait, balance, and physical activity for discrimination of frailty status: baseline results of the Arizona frailty cohort study. Gerontology. 2015;61(3):258–267. doi: 10.1159/000369095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martínez-Ramírez A, Lecumberri P, Gómez M, Rodriguez-Mañas L, García FJ, Izquierdo M. Frailty assessment based on wavelet analysis during quiet standing balance test. J Biomech. 2011;44(12):2213–2220. doi: 10.1016/j.jbiomech.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 36.Doheny EP, McGrath D, Greene BR, et al. Displacement of centre of mass during quiet standing assessed using accelerometry in older fallers and non-fallers. Conf Proc Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Conf. 2012;2012:3300–3303. doi: 10.1109/EMBC.2012.6346670. [DOI] [PubMed] [Google Scholar]
- 37.McGrath D, Doheny EP, Walsh L, et al. Taking balance measurement out of the laboratory and into the home: discriminatory capability of novel centre of pressure measurement in fallers and non-fallers. Conf Proc Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Conf. 2012;2012:3296–3299. doi: 10.1109/EMBC.2012.6346669. [DOI] [PubMed] [Google Scholar]
- 38.Maki BE, Holliday PJ, Topper AK. A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population. J Gerontol. 1994;49(2):M72–M84. doi: 10.1093/geronj/49.2.m72. [DOI] [PubMed] [Google Scholar]
- 39.Hasegawa R, Lee C, Koizumi D, Rogers ME. Threshold of lower body muscular strength necessary to perform ADL independently in community-dwelling older adults. Clin Rehabil. 2008;22:902–910. doi: 10.1177/0269215508094713. [DOI] [PubMed] [Google Scholar]
- 40.Monsell EM, Furman JM, Herdman SJ, Konrad HR, Shepard NT. Computerized dynamic platform posturography. Otolaryngol Head Neck Surg. 1997;117(4):394–398. doi: 10.1016/S0194-5998(97)70132-3. [DOI] [PubMed] [Google Scholar]
- 41.Brenton-Rule A, Mattock J, Carroll M, et al. Reliability of the TekScan MatScan® system for the measurement of postural stability in older people with rheumatoid arthritis. J Foot Ankle Res. 2012;5:21. doi: 10.1186/1757-1146-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]


