Abstract
The purpose of the current study was to examine the independent and interactive effects of social adversity (SA) and HIV infection on subcortical shape alterations and cognitive functions. Participants included HIV+ (n = 70) and HIV− (n = 23) individuals who underwent MRI, neurocognitive and clinical assessment, in addition to completing questionnaires from which responses were used to create an SA score. Bilateral amygdalae and hippocampi were extracted from T1-weighted images. Parametric statistical analyses were used to compare the radial distance of the structure surface to a median curve to determine the presence of localized shape differences as a function of HIV, SA and their interaction. Next, multiple regression was used to examine the interactive association between HIV and SA with cognitive performance data. An HIV*SA interactive effect was found on the shape of the right amygdala and left hippocampus. Specifically, HIV-infected participants (but not HIV-uninfected controls) who evidenced higher levels of SA displayed an inward deformation of the surface consistent with reduced volume of these structures. We found interactive effects of HIV and SA on learning/memory performance. These results suggest that HIV+ individuals may be more vulnerable to neurological and cognitive changes in the hippocampus and amygdala as a function of SA than HIV-individuals, and that SA indicators of childhood SES and perceived racial discrimination are important components of adversity that are associated with cognitive performance.
Keywords: Human immunodeficiency virus, Hippocampus, Amygdala, Adversity, Structural neuroimaging, Cognition
Introduction
The range of adversities that have been found to result in long-lasting changes in brain structure and function are diverse, with several studies demonstrating how socioeconomic position at the individual, familial and community levels influence well-being and disease risk (Adler et al. 1994; Lawson et al. 2013; Lorant et al. 2003; McEwen and Gianaros 2010). A number of prospective studies have also documented the negative effects of adversity on cognitive functioning, such that prolonged exposure to adversity is associated with impairments in emotional regulation (Tottenham et al. 2010) and learning and memory (Richards and Wadsworth 2004; Shonkoff et al. 2012). Notably, disruption of the hypothalamic-pituitary-adrenal (HPA) axis, the body’s central stress response system, has been a focus of investigation and considered an underlying mechanism by which adverse events, and its resultant stress, engender such changes (Lupien et al. 2009; Sapolsky 1996), particularly in brain regions such as the hippocampus and amygdala, which are abundant in glucocorticoid receptors (Herman and Cullinan 1997). Disruption of the HPA-axis has been associated with loss of neuronal connections and smaller hippocampal volume (Rao et al. 2010; Sapolsky 1996; Woon and Hedges 2008) and altered amygdala connectivity (Thomason et al. 2015).
However, there is very little known about the interaction between social adversity (SA) and other cognitive risk factors such as HIV-serostatus. Approximately 50% of people living with HIV (PLWH) demonstrate cognitive impairment at some point during the course of HIV disease (Heaton et al. 2010, 2011). Cognitive compromise has been observed in domains such as attention, reduced psychomotor speed, executive function, learning/memory, and motor function (Becker et al. 2011; Heaton et al. 2011; Heaton et al. 2004; Maki et al. 2009; Tozzi et al. 2007). Studies suggest that functional and structural abnormalities of subcortical regions underlie these cognitive deficits (Castelo et al. 2006; Moore et al. 2006; Maki et al. 2009).
Socioeconomic disadvantage
SES disadvantage is high among PLWH, with up to 45% unemployed (Rabkin et al. 2004; Ibrahim et al. 2008). Lower SES among HIV-infected individuals has been linked to a host of adverse outcomes such as increased morbidity and mortality (Cunningham et al. 2005), and neurocognitive outcomes (Arentoft et al. 2015). A number of recent studies have indicated that SES factors are associated with hippocampal size across the lifespan (Noble et al. 2012; Hanson et al. 2011; Jednoróg et al. 2012; Staff et al. 2012). However, most of the research in this area has focused on family-level or individual-level SES. Given that community-level SES has been shown to predict health outcomes over and above the effects of individual SES (e.g., Winkleby and Cubbin 2003), it is also important to assess the role of perceived community SES in predicting neural and cognitive outcomes.
Racial/ethnic discrimination
Social disadvantage linked to ethnic/racial identity can contribute to poor outcomes even when controlling for SES (e.g., Williams and Mohammed 2009). Perceived racial/ethnic discrimination has been linked to a number of health outcomes including lower cognitive performance (Barnes et al. 2012; Taylor and Turner 2002; Thames et al. 2013; Williams et al. 2016), which may be caused by stress that emerges from perceptions of threat. Considering that African Americans account over 50% of the HIV-infected population (CDC 2015), perceived racial discrimination is an important factor to consider when investigating the effects of social adversity within this population. Prior work has shown that higher levels of perceived discrimination related to poorer cognitive test performance, particularly in the domain of episodic memory and perceptual speed in a large cohort of community-dwelling African American older adults (Barnes et al. 2012). In a sample of young-to-middle aged Black/African American and White/Caucasian adults, higher scores on perceived racial discrimination resulted in underperformance on measures of learning and memory among Black participants when tested by a White examiner (Thames et al. 2013). Results from these studies suggest that perceived discrimination operates in the same fashion as other chronic stressors (i.e., disruption in neural mechanisms that underlie emotional regulation and memory function).
Studies have documented dysregulation of the HPA axis and blunted adrenocorticotropic hormone (ACTH) responses among HIV-infected individuals (Kumar et al. 2003, 1993; Patterson et al. 2013), thus it is plausible to expect that HIV status may increase vulnerability to SA effects on brain structure and function, particularly in limbic structures. To our knowledge, few studies have directly addressed the interactive association between HIV and SA. Clark et al. (2012) studied HIV-infected and HIV-uninfected individuals who were classified into high- vs. low-early life stress (ELS) groups (quantified through a self-report questionnaire assessing the occurrence of 17 adverse life events). An interaction between HIV status and ELS severity indicated that HIV-infected individuals in the high ELS group evidenced larger right amygdala volume, and these abnormalities were associated with reduced processing/psychomotor speed (Clark et al. 2012). Spies and Seedat (2014) examined an all-female HIV-infected and HIV-uninfected cohort and narrowed their focus of ELS to histories of maltreatment including emotional, physical and sexual abuse and emotional and physical neglect. In this study, HIV-infected women with ELS had the lowest mean regional volume in several subcortical regions (e.g., hippocampus). These volume reductions were associated with neurocognitive performance, such that HIV-infected females with ELS evidenced lower scores, as compared to all other comparison groups. The Women’s Interagency HIV Study (WIHS), a large and comprehensive study of HIV-infected and HIV-uninfected women, reported a significant HIV by stress interaction (using the Perceived Stress Scale) in the neurocognitive domain of verbal memory (Rubin et al. 2015). Only among the HIV-infected group were associations found between high stress and lower verbal memory performance, indicating that high levels of perceived stress contribute to the deficits in verbal memory observed in WIHS women. While these studies provide valuable insights into the potential vulnerability to stress experienced by HIV-infected individuals, several questions remain with regard to the effects of multiple adversities that are experienced in both childhood and adulthood, including stressors that are specific to one’s personal identity (e.g., racial discrimination).
Although the independent effects of HIVand SA have been well established, the interactive effects of SA and HIV on neurological integrity require further study, particularly as it pertains to adversities that may be common to this population (e.g., socioeconomic disadvantage, racial discrimination). We have chosen to employ the method of 3D shape analyses due to its sensitivity to regional (e.g. specific nuclei) changes not detectable by standard volumetric methods (Fernández-Espejo et al. 2011; Hughes et al. 2012; Kim et al. 2013). Studies have used shape analysis in various populations including Bipolar Disorder (Ong et al. 2012), Huntington’s disease (Van den Bogaard et al. 2011), Mild Cognitive Impairment and Alzheimer’s Disease (Costafreda et al. 2011), and more recently, HIV (Wade et al. 2015). Therefore, the current study examined the effects of SA and HIV on subcortical morphometry and neurocognition in a group of HIV-infected and HIV-uninfected Caucasian and African-American individuals. We expected to observe significant main and interactive effects of HIV and SA on subcortical shape abnormalities and neurocognitive functioning, such that HIV-infected individuals with higher adversity scores would demonstrate a greater magnitude of hippocampal and amygdala shape abnormalities and lower cognitive performance (particularly in the domain of memory) as compared to HIV-uninfected individuals.
Method
A total of 93 participants (HIV-infected, n = 70) and (HIV-uninfected, n = 23) were enrolled as part of a larger study examining the effects of HIV on neurocognitive functioning among African American and Caucasian individuals (NIMH K23 MH095661). There were no differences between the study sample and the larger sample on a number of demographic and clinical characteristics. Participants were recruited from various local community clinics and HIV service agencies in the Greater Los Angeles area. All procedures received approval by the University of California, Los Angeles (UCLA) Institutional Review Board (IRB; #12–000406). Participants were included in the study if they were over the age of 18 years (range 23–75), reported English as their primary language, scored ≥26 on the Mini-Mental Status Exam (MMSE; Folstein et al. 1975), self-identified as African-American or Caucasian, and were able to provide informed consent (assessed by the participant communicating their understanding of the consent form). Participants were excluded if they reported either current abuse/dependence of cocaine or amphetamines, past stimulant abuse/dependence, or current/past diagnosis of a psychotic-spectrum disorder (assessed by a modified version of the SCID; Spitzer et al. 1995). Recent illicit drug use was assayed via urine toxicology as well as a self-report drug screen (i.e., Brief Drug History Questionnaire). Participants were excluded if they screened positive for stimulants or hallucinogens. Participants with CNS confounds (e.g., HIV-associated opportunistic infections or neurosyphilis), Hepatitis C coinfection, (confirmed by serology), major head injury (loss of consciousness >30 min), or contraindication to the safe use of MRI, were also excluded. Our HIV+ participants were a chronically infected population (average years with HIV = 12), stable on ARVs (as demonstrated by a threshold below virologic failure (< 5000 copies/mL) (Alvarez-Uria et al. 2012; WHO 2010) or undetectable (< 20 copies/mL) plasma viral loads and self-reported continuous ART for at least 3 months), and clinically stable (as indicated by CD4+ cells/mm3) (see Table 1).
Table 1.
Demographic and characteristics (N = 93)
| HIV+ (n = 70) | HIV−(n = 23) | statistic, p-value | effect size | |
|---|---|---|---|---|
| Age | 52.04(11.02) | 49.35 (12.74) | t = .974, p = 0.33 | d = 0.22 |
| Sex (% male) | 85% | 47% | χ2 =. 2.91, p = 0.08 | d = 0.35 |
| Ethnicity (%) | χ2 = .93, p = 0.63 | d = 0.20 | ||
| African American | 60% | 60% | – | |
| Caucasian | 40% | 40% | – | |
| Education (years) | 13.44 (2.14) | 14.22 (2.08) | t = −1.53, p = 0.13 d | = 0.36 |
| WRAT-4 reading raw score | 59.57 (6.67) | 61.57 (7.59) | t = −1.19, p = 0.24 | d = 0.27 |
| Social adversity score (total) | 1.03 (1.00) | 1.45 (1.01) | t = −1.74, p = 0.09 | d = 0.41 |
| “0” | 36% | 22% | – | |
| “1” | 33% | 36% | – | |
| “2” | 20% | 27% | – | |
| “3” | 11% | 14% | – | |
| Individual adversity indicators | ||||
| Financial Strain | NS | |||
| “0” (no) | 85% | 85% | ||
| “1” (yes) | 15% | 15% | ||
| Current SES (Hollingshead index) | 40.91 (10.76) | 42.36 (11.31) | F = .297, p = .58 | d = 0.13 |
| Childhood neighborhood SES | ||||
| “1” (low income/poor) | 24% | 22% | NS | |
| “2” (middle class) | 60% | 50% | ||
| “3” (upper middle class) | 12% | 18% | ||
| “4” (wealthy) | 3% | 9% | ||
| Current community SES | ||||
| “1” (low income/poor) | 33% | 27% | NS | |
| “2” (middle class) | 48% | 46% | ||
| “3” (upper middle class) | 13% | 22% | ||
| “4” (wealthy) | 4% | 4% | ||
| Perceived racial discrimination (PED-Q) | 36.77 (16.11) | 29.41 (11.06) | F = 5.77, p = 0.02 | d = 0.58 |
| Global z-score | −.002 (.65) | .27 (.43) | F = 3.0, p = .09 | d = 0.49 |
| Processing Speed z-score | −.05 (.80) | .16 (.78) | F = 1.18, p = 0.28 | d = 0.26 |
| Attention/WM z-score | −.07 (.91) | .22 (.71) | F = 1.86, p = 0.17 | d = 0.35 |
| Fluency z-score | −.01 (.85) | .04 (.85) | F = .08, p = 0.77 | d = 0.05 |
| Learning/Mem z-score | −.10 (.80) | .32 (.98) | F = 3.59, p = 0.05 | d = 0.46 |
| Executive z-score | −.11 (.89) | .34 (.59) | F = 4.96, p = 0.03 | d = 0.59 |
| Motor T-score | .96 (.98) | 1.11 (.74) | F = .462, p = 0.49 | d = 0.17 |
| BDI-II score | 9.75 (8.77) | 4.61 (5.34) | t = 2.65, p = 0.01 | d = 0.71 |
| Recent plasma CD4 count | 604.79 (293.25) | – | – | |
| Nadir CD4 count | 206.61 (150.38) | – | – | |
| *% undetectable plasma viral load | 54% | – | – | |
| % on cART | 95% | – | – | |
| Current psychiatric history | ||||
| Major Depressive Disorder (MDD) | 6% | 6% | NS | |
| Bipolar Disorder | 0% | 0% | NS | |
| Past psychiatric history | ||||
| Major depressive disorder | 16% | 18% | NS | |
| Age of onset of MDD | 23.5 (6.62) | 22.02 (5.65) | NS | |
| Bipolar disorder | 0% | 0% | NS | |
| Current drug use | ||||
| Alcohol | 2% | 4% | NS | |
| Marijuana | 3% | 0 | NS | |
| Past Drug Use | ||||
| Alcohol | 9% | 12% | χ2 = .39, p = 0.53 | d = 0.13 |
| Marijuana | 22% | 25% | χ2 = .11, p = .85 | d = 0.03 |
| Cocaine | 6% | 12% | χ2 = .35, p = .48 | d = 0.06 |
| Stimulants | 6% | 0 | χ2 = 1.37,p = .29 | d = 0.24 |
| Past drug abuse/dependence | ||||
| Alcohol | 23% | 16% | χ2 = .39, p = .53 | d = 0.13 |
| Marijuana | 25% | 23% | NS | |
| Urine toxicology results (% positive) | ||||
| Amphetamine | 0% | 0% | NS | |
| Barbiturates | 0% | 0% | NS | |
| Cocaine | 0% | 0% | NS | |
| Benzodiazepines | 34% | 9% | χ2 = 5.62, p = 0.01 | d = 0.50 |
| Marijuana | 23% | 22% | NS | |
| Methadone | 1% | 0% | NS | |
| Opiates | 10% | 0% | χ2 = 2.48, p = 0.11 | d = 0.33 |
| PCP | 0% | 0% | NS | |
| MDMA | 0% | 0% | NS |
Undetectable viral load ≤20 copies/mL
NS = Not significant p > .90
Measures
Social adversity
A composite SA score was derived to capture the following domains: (1) perceived racial/ethnic discrimination (Perceived Ethnic Discrimination Questionnaire (PED-Q; Contrada et al. 2001); (2) financial strain (i.e., “have you ever not received medical care because of financial problems?”); (3) perceived childhood neighborhood SES; (4) perceived current neighborhood SES, and (5) current personal SES (Hollingshead index of Social Status; Hollingshead 1975). These domains have each been described as indicators of adversity (Montez et al. 2016; Taylor and Turner 2002; Wicks et al. 2005; Williams et al. 2016). Aggregating multiple sources of stress into a composite index has become an increasingly used method among researchers interested in examining the influence of cumulative adversity on physical health and mental health (Steptoe and Marmot 2003; Troxel et al. 2003; Toussaint et al. 2016). Using procedures based upon methods described by Troxel et al. (2003), each indicator was dichotomized into “0” or “1” using the top 20–30% of the sample distribution, except for financial strain, which was dichotomized at the top 13% (based on its distribution). Next, we summed the dichotomous values into one score to represent an index of social adversity. Total possible scores range from 0 to 5. Of the full sample, 33%, 34%, 22%, 7%, 4%, 0% had scores of 0, 1, 2, 3, 4, & 5, respectively. Given the low sample representation for scores 3 and 4, they were combined, resulting in a range from 0 to 3.
Additional details regarding this SA index can be found in Williamson et al. (2016) and Table 1.
Neurocognitive performance
Participants underwent a comprehensive neurocognitive battery assessing the following domains: attention/concentration and information processing speed (Wechsler Adult Intelligence Scale-4th Edition [WAIS-IV]-Coding and Symbol Search; Trail Making Test-Part A; and Stroop Interference Test-Color and Word Naming), verbal fluency (Controlled Oral Word Association Test-FAS and Animal Fluency), learning and memory (Hopkins Verbal Learning Test-Revised [HVLT-R], Total Learning; Brief Visuospatial Memory Test-Revised [BVMT], Total Learning; Delayed Recall from HVLT and BVMT), and executive functioning (Trail Making Test-Part B; Stroop Interference Test-Interference score; and WAIS-IV-Letter-Number Sequencing). We converted raw scores from each test into standardized z-scores using published normative data (e.g., Heaton et al. 2004) and then averaged them to create global neurocognitive performance and cognitive domain composites.
MRI acquisition
T1-weighted data was collected using a 12-channel head coil on Siemens Tim Trio 3 T scanner (Siemens Medical Solution, Erlangen, Germany) at the Center for Cognitive Neuroscience (Los Angeles, California). Structural MP-RAGE T1-weighted scans were acquired with 120–1.0 mm sagittal slices, FOV = 256 mm (A-P) × 192 mm (FH), matrix =256–192, TR = 450.0 ms, TE = 10.0 ms, Flip Angle =8, voxel size =1.0 mm × 0.94 mm × 0.94 mm. All data was visually inspected for artifacts and data prior to analyses. No scans demonstrated significant artifact. Therefore, all scans (N = 96) were included in the preprocessing pipeline.
During the shape analysis pipeline, registration/alignment and intensity inhomogeneity were assessed for artifacts and outliers. Scans that did not pass quality assurance criteria were either discarded or successfully rerun through the previous pipeline step (e.g., successfully registered on a second run through FSL FIRST). Three scans were not included because they did not pass registration or were found to have outliers during the pipeline process.
Analytic plan
Prior to conducting primary analyses, HIV status groups were compared on variables of age, ethnicity/race, sex, education, current depression (as assessed by Beck Depression Inventory-2 [BDI-II; Beck et al. 1996]), current or past diagnosis of Major Depressive Disorder, and SA score. Bivariate correlations and Chi-square analyses investigated relationships between demographic and clinical variables and SA score. Any self-reported mood or demographic variables that showed group differences at p < .05 were added as covariates when analyzing shape differences between the groups. We included sex, depression, current drug use (i.e., benzodiazepines and opiates) and past cocaine use as covariates in the models.
Shape and processing analysis
The following preprocessing steps were completed using FSL version 5.0. T-1 anatomical scans subsequently underwent intensity inhomogeneity normalization using the MNI “nu_correct” tool (www.bic.mni.mcgill.ca/software/). Next, we used an automated, model-based subcortical segmentation protocol (FSL FIRST) to extract the bilateral amygdala and hippocampus. Analyses compared the radial distance of structure surface to a median curve to determine the presence of localized shape differences. For HIV group comparisons, parametric statistical analyses were conducted at each voxel comprising the surface (i.e., vertex) of each structure relative to the mean surface of the control group. Statistically significant results could either show atrophy (vectors significantly below the median surface) or hypertrophy (significantly above the median surface). In the analyses, the design matrix was demeaned so that the HIV− group had a value of −0.5 and the HIV+ group had a valued of +0.5. We report corrected F-statistics using family-wise error at p < .05. These analyses determined (1) Shape differences between HIV-infected and HIV-uninfected groups, (2) Relationships between SA score and shape alterations, (3) HIV*SA interactive effects on shape alterations.
Neurocognitive outcomes
Two stepwise linear regressions were conducted using SPSS version 23.0 to determine whether there was a significant interaction between HIV-status and SA (interaction term added in step 2) in predicting learning/memory and executive functioning performance over and above HIV-status and SA alone (added in step 1). We restricted our analyses to these cognitive domains for the following reasons (1) these domains are conceptually linked to functions of our brain regions of interest, and (2) to reduce the number of statistical comparisons. Prior to analyses, model assumptions were checked, including screening for outliers and evaluating for multicollinearity, with tolerance values of <0.40 and variance inflation factor values of >2.5 suggestive of multicollinearity (Allison 2012). Covariates included age, sex, current drug use, past cocaine use, WRAT, and depression scores given their influence on cognitive performance and trends for HIV group differences in our sample. Covariates were entered into the first step. HIV status (dummy coded) and SA score (centered) were entered as predictors in the second step. In the final step, the interaction term (i.e., HIV*SA) was entered.
Results
HIV-status group comparisons on demographics, social adversity, and neurocognition
Table 1 provides summary statistics for HIV group comparisons. Independent samples t-tests, Analysis of Variance, and Chi-square tests were conducted to compare HIV status group differences in demographic characteristics (e.g., age, education, sex), SA, depression symptoms, drug use variables, and neurocognitive functioning. There were no statistically significant HIV group differences in age, WRAT-4 performance, education, adversity scores, ethnicity, or past and current Major Depressive Disorder (all p’s > .10). There was a trend for differences in the distributions of sex between groups (p = .08), with a greater proportion of men in the HIV-infected group. There were significant HIV group differences in BDI-II scores and in the cognitive domains of learning/memory and executive functioning with HIV+ individuals demonstrating higher BDI-II scores, and performing significantly lower in the learning/memory and executive functioning domains. There were no significant HIV group differences in other cognitive domains.
Relationships between social adversity, demographic, cognitive and clinical variables
There were no statistically significant associations between age and SA score, rs (93) = −.09, p = .36. However, there was a significant association between education and SA score, rs (93) = −.38, p < .0001. There were no sex differences in SA score (t = −.895, p = .35). Among HIV+ individuals, there was no association between SA and nadir CD4, current CD4 or viral load (all p’s > .10). SA score was negatively correlated with cognitive performance in learning/memory rs (93) = −.21, p = .04 and executive functioning domains, rs (93) = −.24, p = .02. There were no associations between SA score and cognitive domains of attention/processing speed rs (93) = −.12, p = .24, motor functioning, rs (93) = −.03, p = .75, or verbal fluency, rs (93) = −.16, p = .12. Table 2 shows the correlations between the five indicators of adversity.
Table 2.
Correlations between five indicators of social adversity
| Measure | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1. Financial strain | – | ||||
| 2. Child neighborhood SES | −.17 | – | |||
| 3. Current neighborhood SES | −.19 | −.09 | – | ||
| 4. Current SES | −.12 | .13 | .24* | – | |
| 5. Perceived discrimination | −.16 | −.08 | −.32* | −.21* | – |
p < .05
Interactive effect of HIV and social adversity on subcortical shape
We observed a statistically significant (i.e., p < .05 adjusted for multiple comparisons) HIV*SA interaction that was associated with reductions on the surface of the left hippocampus and right amygdala. Specifically, HIV-infected participants who reported higher levels of SA displayed the greatest deformation/atrophy in vertices on the surface of these two subcortical structures (Fig. 1). The interactive association was found for 18% of the vertices comprising the left hippocampal surface and 46% of the vertices comprising the right amygdala. There was no significant HIV*SA interaction on reductions in vertices in the left amygdala. Notably, however, increased SA scores (independent of HIV status) were associated with significantly reduced vertices in the left amygdala (Fig. 2), accounting for 41% of the voxels comprising the surface of the left amygdala.
Fig. 1.
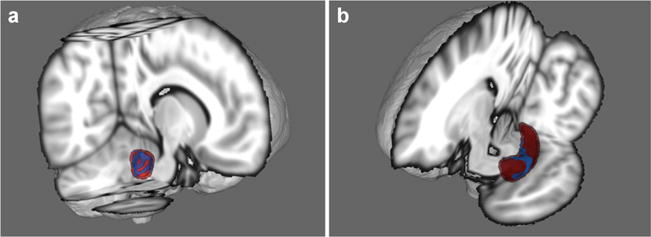
Area in blue represents the surface region where a significant HIV*Adversity interaction was observed. Fig. A shows the right amygdala and Fig. B shows the left hippocampus (seen from sagittal and coronal views, respectively). HIV+ participants with higher adversity scores evidenced the greatest atrophy of these regions
Fig. 2.
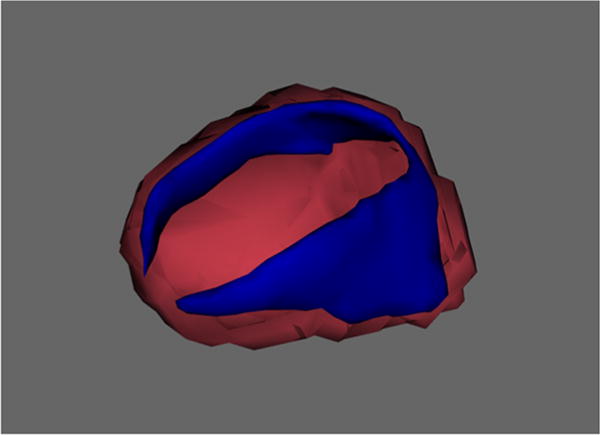
Effect of Social Adversity Score on Amygdala Vertex Area in blue represents the surface region of the amygdala that was associated with social adversity
Subcortical shape and cognitive function
We explored the association between subcortical shape and performance across all cognitive domains. Given that these analyses were exploratory, we used a threshold of p < .05, uncorrected for multiple comparisons. Please see Table 3 and Fig. 3 for details. Left hippocampal shape was positively associated with domains of learning/memory, verbal fluency, executive functioning, and attention/processing speed. Right hippocampal shape was positively associated with domains of learning and memory, executive functioning, verbal fluency, and motor functioning. Right amygdala shape was only associated with verbal fluency, whereas left amygdala shape was positively associated with domains of attention/processing speed, verbal fluency, and motor functioning.
Table 3.
Percent of hippocampal and amygdala vertices associated with cognitive domains
| Variable | Attention/processing speed | Motor functioning | Verbal fluency | Learning/memory | Executive function |
|---|---|---|---|---|---|
| Left Amygdala | 37% | 38% | 80% | NS | NS |
| Right Amygdala | NS | NS | 12% | NS | NS |
| Left Hippocampus | 7% | NS | 1% | 18% | 6% |
| Right Hippocampus | NS | 38% | 33% | 34% | 28% |
Fig. 3.
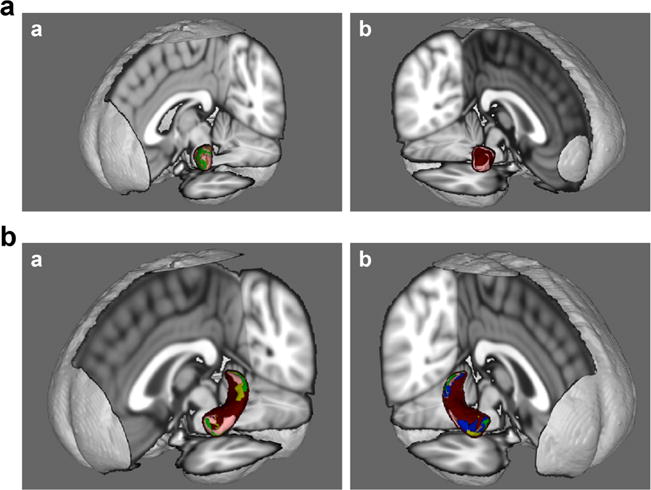
a Amygdala shape correlations with cognitive domains (Figure a = left amygdala; Figure b = right amygdala). Green = attention/information processing speed; Yellow = learning/memory; Pink = verbal fluency; Blue = executive functioning Brown = motor functioning b Hippocampal shape correlations with cognitive domains (Figure a = left hippocampus; Figure b = right hippocampus). Green = attention/information processing speed; Yellow = learning/memory; Pink = verbal fluency; Blue = executive functioning; Brown = motor functioning.
Interactive effects of HIV and SA on learning/memory and executive function
As previously mentioned, multiple regression analyses were conducted to assess possible interactions between HIV-status and SA on learning/memory functioning. The final step of the regression was statistically significant (R2 = .27, F (3, 89) = 4.29, p < .04) indicating an HIV*SA interaction (b = .39, p = .04). Inspection of beta weights associated with SA and learning/memory relationships demonstrated strong negative relationships for HIV− (b = .−.59, p = .003) and HIV+ (b = −.80, p = .001) individuals.
We observed main effects for HIV (b = −.28, p = .005) and SA (b = −.34, p = .001) on executive function, but there was no statistically significant interactive association as indicated by the final step of the model (ΔR2 = .002, F (3, 89) = .273, p = .603).
Individual effects of components of SA on subcortical shape and cognitive domains of learning/memory and executive function
Finally, we explored the individual contribution from each of the five indices (i.e., financial strain, racial/ethnic discrimination, neighborhood SES during childhood, current neighborhood SES, current personal SES that were used to derive the SA score in a series of post-hoc analyses for the entire sample. Again, considering these results are exploratory, we did not correct for multiple comparisons. Instead, we report correlations that were statistically significant at p < .05. Table 4 reports the percent of vertices from the surface of the amygdala and hippocampus that were statistically significant. Left amygdala shape was negatively associated with financial strain and current SES. Right amygdala was positively associated with childhood neighborhood SES and negatively associated with financial strain. Left hippocampal shape was positively correlated with current SES and childhood neighborhood SES. Left hippocampal shape was negatively correlated with financial strain and racial discrimination. Right hippocampal shape was positively correlated with current SES and childhood neighborhood SES, and negatively correlated with financial strain (see Fig. 4 for details).
Table 4.
Percent of hippocampal and amygdala vertices associated with social adversity indicators
| Variable | Financial strain | Current SES | Childhood neighborhood SES | Racial discrimination | Current neighborhood SES |
|---|---|---|---|---|---|
| Left amygdala | 15% | 4% | NS | NS | NS |
| Right amygdala | 10% | NS | 48% | NS | NS |
| Left hippocampus | 24% | 3% | 6% | 5% | NS |
| Right hippocampus | 29% | 3% | 50% | NS | NS |
Fig. 4.
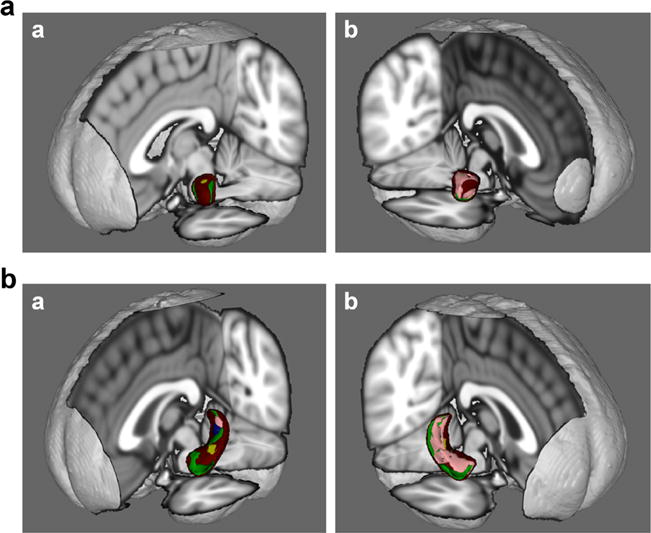
a Amygdala shape correlations with social adversity indicators (Figure a = left amygdala; Figure b = right amygdala). Green = financial strain; Yellow = current SES; Pink = childhood neighborhood SES; Blue = racial discrimination; Brown = current neighborhood SES b. Hippocampal shape correlations with social adversity indicators (Figure a = left hippocampus; b = right hippocampus). Green = financial strain; Yellow = current SES; Pink = childhood neighborhood SES; Blue = racial discrimination; Brown = current neighborhood SES.
Results from stepwise regressions indicated that childhood neighborhood SES (ΔR2 =. .21, b = −.48, p = .03) and perceived discrimination (ΔR2 = .18, b = −.44, p = .02) were each associated of lower performance in learning/memory, whereas there was no significant contribution of the other indicators (i.e., financial strain, current neighborhood SES, current SES).
We performed the same analyses using executive functioning as our outcome variable of interests for the entire sample. Results from a separate hierarchical regression indicated that the model with childhood neighborhood SES (ΔR2 = .45, b = −.70, p = .002) was statistically significant in association with performance in executive functioning, whereas none of the other indicators individually contributed to performance in executive function.
Discussion
The purpose of the current study was to examine levels of exposure to social adversity in our HIV-infected and HIV-uninfected groups, and to determine the effects of cumulative SA, HIV, and the interaction between these two factors on subcortical shape abnormalities in regions of the hippocampus and amygdala—structures that are particularly vulnerable to the effects of HIV and social stress/adversity and play an important role in cognitive and psychological functioning. We also investigated these associations among neurocognitive domains (i.e., learning/memory and executive functioning) that are conceptually linked to these regions of interest.
We observed interactive effects of HIV and SA on the surface of the left hippocampus and right amygdala. A significant main effect of SA was found in the surface of the left amygdala, such that greater SA scores were associated with reductions in the surface area of this region. These findings suggest that reductions in the surface area of the left hippocampus and right amygdala were specific to the HIV-infected group, whereas the association between SA and left amygdala shape were found in both HIV status groups. Notably, there were no significant differences in the SA score between HIV status groups, suggesting that both groups experienced comparable levels of adversity. We were not surprised to observe similar levels of adversity between our HIV-infected and HIV-uninfected comparison groups, as we make efforts to recruit an HIV-uninfected comparison group that is demographically similar to our HIV-infected participants. Together, our results demonstrated that the association of SA on left hippocampal and right amygdala structures were found among HIV-infected (but not HIV-uninfected) individuals, which suggests that exposure to adversity may be deleterious to these regions among PLWH. This is consistent with other studies examining HIV and social stress among men and women (Clark et al. 2012; Spies and Seedat 2014; Rubin et al. 2015). Additionally, our findings build upon previous studies that have found that HIV-infected individuals show dysregulation of the HPA axis and a blunted ACTH response (Kumar et al. 1993; Patterson et al. 2013), which are posited mechanisms for the observed effects in the current study. After controlling for potential demographic confounds (e.g., depressive symptoms), we found a significant interaction between HIVand SA in the right amygdala, indicating that the right amygdala demonstrated greater abnormalities in the surface shape for those in the HIV-infected group, relative to our HIV-uninfected group. These findings are similar to those reported by Clark et al. (2012), which also demonstrated abnormalities in the right amygdala as a function of exposure to adversity and HIV-status.
While the HIV*SA interaction was found in association with the right amygdala, we do not interpret this as a lateralized effect considering that an association between SA and deformations was found in the left amygdala. Instead, the regions that were found to be associated with the interaction were those that survived multiple comparisons. Inspection of images without correction for multiple comparisons demonstrated interactive associations with bilateral hippocampi and amygdalae. Nevertheless, our findings are consistent with those reported by Clark et al., in which a main effect of ELS was found in the left amygdala, whereas interactive effects were found in the right amygdala. Examination of individual factors that composed our SA composite was also generally consistent with findings by Hanson et al. (2015), which observed smaller left amygdala volume among children from low SES backgrounds compared to children from high SES backgrounds. Notably, we found strong positive correlations between childhood neighborhood SES and right amygdala and right hippocampus, suggesting that larger surface of the right amygdala and hippocampus surface (which is related to volume) was observed among individuals who reported higher SES neighborhoods in childhood than those who reported lower SES neighborhoods. However, indicators of other types of adversity (i.e., current SES, perceived racial discrimination, and financial strain) were either associated with bilateral surface regions of the amygdala and hippocampus (i.e., financial strain) or exclusively associated with the left amygdala (i.e., current SES) and left hippocampus (i.e., perceived racial discrimination). Therefore, it is possible that we observed a main effect of SA in the left amygdala, and not the right, because a majority of the adversity indicators correlated with left-hemispheric regions. More studies are needed with larger samples to directly test lateralized effects in relation to different types of adversity.
We also found HIV*SA interactive associations with deformations of the left hippocampus, which was not found by Clark et al., but is consistent with findings from Spies et al. (2016). One plausible explanation for the differences between our findings and those reported by Clark et al. may be due to characteristics of the sample or due to the different measures of SA used between the studies. Clark et al. (2012) used a measure specific to early life stress, which retrospectively captured a range of events that occurred only in childhood. Focusing solely on early life stress may explain why the findings from Clark et al. (2012) were specific to enlargement of the amygdala. Thus, we posit that the timing of stress exposure may have differential effects on amygdala development, which has been suggested by others (Lupien et al. 2009). Hyperactivity of the HPA-axis has been associated with pre-and postnatal adversities (Bosch et al. 2012; Entringer et al. 2009; Hellemans et al. 2010). The timing of adversity is associated with differential cortisol stress response in adulthood, with increases occurring around ages 6 and 7 (Bosch et al. 2012; Pesonen et al. 2010) and decreases associated with ages 12–16 (Bosch et al. 2012). Thus, in adulthood, observations of reduced volumes of these brain regions could be a strong marker of the time of exposure to adversity or the duration rather than of the effects of specific traumas on various brain regions.
Our findings are similar to those reported by Rubin et al. (2015), whereby the association between stress and memory function was found among HIV-infected women. In the current study, although the associations of SA and learning/memory were strong for both groups, the association was stronger for HIV+ individuals. This is important given that the current study used a mixed cohort of men and women and did not assess recent perceived stress. It would be interesting to examine whether the time of assessment may have a greater impact on memory functions for HIV-infected individuals, as they may present with existing memory problems due to the disease.
While studies of brain volume have provided valuable insights into the effects of chronic stress and HIV on the CNS, the current study extends the literature by providing information about the location and pattern of structural change through shape analysis methods and its relationship to memory. Disease processes often affect structure in a non-uniform fashion, or subregions may be differentially affected (i.e., reduction in some areas and thinning in others). Volumetric analysis may obscure these non-uniform changes (Tang et al. 2014), whereas shape analytic methods allow for more fine-grained analysis, capable of detecting both subtle and nonuniform changes (Csernansky et al. 2004; Wade et al. 2015). Our findings demonstrate distinct structural deformations associated with HIV and SA that coincide with performance on our neurocognitive findings.
There are limitations to this study worth noting. First, as mentioned previously, the SA index was a combination of adversity that targets the individual (e.g., perceived racial discrimination) as well as their community (e.g., community SES). There is no “gold standard” with how social adversity is operationally defined in the literature, which may partly contribute to inconsistencies in study findings across studies of adversity. Another limitation worth noting is that our HIV-infected group was three times larger than the HIV-uninfected group, resulting in broader estimates of the outcome in the HIV+ group. While smaller sample sizes result in less reliable estimates, this would be particularly problematic if HIV status was confounded with the SA measure, or if we failed to observe a statistically significant interaction. Further, our measure of SA was largely retrospective (with the exception of current SES), whereas prospective studies measuring adversity may provide a more comprehensive picture of “exposure” to adversity. Finally, we were limited in our scope of brain regions investigated in association with SA. 3D shape analyses allows for the automated segmentation of subcortical/limbic regions, but does not provide information about cortical regions such as the prefrontal cortex, which are known to be associated with adversity (Shonkoff et al. 2012). Despite these limitations, we were able to demonstrate that our measure of SA shows strong associations with brain abnormalities, which is consistent with our knowledge of the stress response (Eiland and McEwen 2012; Frodl and O’Keane 2013; Herman and Cullinan 1997; Jacobson and Sapolsky 1991;Hayano et al. 2009; Massana et al. 2003; Weniger et al. 2008).
Second, there was a slight male/female imbalance between the HIV status groups, with more males in HIV+ group. Nevertheless, our findings are consistent with studies that have used primarily female cohorts (Maki et al. 2009; Maki et al. 2015; Rubin et al. 2015). Nevertheless, replication in a larger sample stratified by sex is ideal for future studies.
These limitations aside, the current study provides important information for how adversities, particularly those that are associated with childhood SES and perceived racial/ethnic discrimination, impact brain structure and function as well as potentially increase vulnerability to more severe neurological changes for HIV-infected individuals who already experience compromises due to the disease. Exposures to racial/ethnic discrimination has been demonstrated to have powerful effects on a number of cognitive and health related outcomes in general populations as well as those with HIV (Barnes et al. 2012; Schuster et al. 2005; Taylor and Turner 2002; Thames et al. 2013; Williams et al. 2016). Our findings suggest that perceived racial/ethnic discrimination is an important type of adversity that warrants consideration in relationship to cognitive outcomes. Further, the results herein have implications for other neurological populations (e.g., Mild Cognitive Impairment) where existing brain abnormalities may be exacerbated in the context of chronic adversity. We also found that learning/memory and executive functioning were critical domains implicated in both HIV and SA, with childhood SES and perceived racial discrimination most strongly correlated with these functions. Our results open up questions about whether HIV-infected individuals are more susceptible to neural changes as a function of SA, and if these neural changes increase vulnerability to poor cognitive outcomes. More longitudinal studies are needed to explore this complex relationship. Future studies should also investigate if abnormalities in subcortical regions affected by SA are associated with HPA-axis dysregulation (e.g., hypercortisolism, reduced glucocorticoid receptor sensitivity) among PLWH.
These findings have important public health implications as they suggest that CNS effects of exposure to adversity may be more burdensome in the context of HIV-infection. Considering that our findings were driven by SES-related factors and perceived racial/ethnic discrimination, this suggests that clinicians and other professionals working with HIV-infected individuals should be aware of the importance of assisting with resources (e.g., psychotherapy to target feelings around discrimination, financial assistance programs in the community) for HIV-infected individuals.
Acknowledgments
We would like to thank William Cunningham, M.D., M.P.H. and Ronald Hays, Ph.D. for their helpful comments and suggestions on the manuscript.
We would like to acknowledge the following funding sources:
National Institute of Mental Health (NIMH) K23 MH095661 (PI: A. Thames)
Dr. Kuhn is supported through a National Institute of Mental Health T32 Postdoctoral Fellowship (Kuhn, MH 19535).
Mr. Williamson is supported through a National Institute of Mental Health Predoctoral Fellowship (Williamson, MH 15750)
Footnotes
Compliance with ethical standards This study was funded by National Institute of Mental Health (NIMH) K23 MH095661 (PI: A. Thames)
Conflict of interest Dr. April Thames declares that she has no conflict of interest.
Dr. Taylor Kuhn declares that he has no conflict of interest.
Ms. Zanjbeel Mahmood declares that she has no conflict of interest.
Dr. Robert Bilder declares that he has no conflict of interest.
Mr. Timothy Williamson declares that he has no conflict of interest.
Dr. Elyse Singer declares that she has no conflict of interest.
Dr. Alyssa Arentoft declares no conflict of interest.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study. All procedures were approved by UCLA Institutional Review Board.
References
- Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, et al. Socioeconomic status and health: the challenge of the gradient. American Psychologist. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- Allison P. When can you safely ignore multicollinearity? Statistical Horizons. Last modified September. 2012;10:2012. [Google Scholar]
- Alvarez-Uria G, Naik PK, Pakam R, Midde M. Early HIV viral load determination after initiating first-line antiretroviral therapy for indentifying patients with high risk of developing virological failure: data from a cohort study in a resource-limited setting. Tropical Medicine & International Health. 2012;17:1152–1155. doi: 10.1111/j.1365-3156.2012.02982.x. [DOI] [PubMed] [Google Scholar]
- Arentoft A, Byrd D, Monzones J, Coulehan K, Fuentes A, Rosario A, et al. Socioeconomic status and neuropsychological functioning: associations in an ethnically diverse HIV+ cohort. The Clinical Neuropsychologist. 2015;29(2):232–254. doi: 10.1080/13854046.2015.1029974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes LL, Lewis TT, Begeny CT, Yu L, Bennett DA, Wilson RS. Perceived discrimination and cognition in older African Americans. Journal of the International Neuropsychological Society. 2012;18(5):856. doi: 10.1017/S1355617712000628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the beck depression inventory-II. San Antonio: Psychological Corporation. 1996;1:82. [Google Scholar]
- Becker BW, Thames AD, Woo E, Castellon SA, Hinkin CH. Longitudinal change in cognitive function and medication adherence in HIV-infected adults. AIDS and Behavior. 2011;15(8):1888– 1894. doi: 10.1007/s10461-011-9924-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch NM, Riese H, Reijneveld SA, Bakker MP, Verhulst FC, Ormel J, Oldehinkel AJ. Timing matters: long term effects of adversities from prenatal period up to adolescence on adolescents’ cortisol stress response. The TRAILS study. Psychoneuroendocrinology. 2012;37(9):1439–1447. doi: 10.1016/j.psyneuen.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Castelo JMB, Sherman SJ, Courtney MG, Melrose RJ, Stern CE. Altered hippocampal-prefrontal activation in HIV patients during episodic memory encoding. Neurology. 2006;66(11):1688–1695. doi: 10.1212/01.wnl.0000218305.09183.70. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control. HIV in African Americans. Fast Facts. 2015 Retrived online from: www.cdc.gov/hif/pdf/HIV-AA-english-508.pdf.
- Clark US, Cohen RA, Sweet LH, Gongvatana A, Devlin KN, Hana GN, et al. Effects of HIV and early life stress on amygdala morphometry and neurocognitive function. Journal of the International Neuropsychological Society. 2012;18(04):657–668. doi: 10.1017/S1355617712000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contrada RJ, Ashmore RD, Gary ML, Coups E, Egeth JD, Sewell A, et al. Measures of ethnicity-related stress: psychometric properties, ethnic group differences, and associations with well-being. Journal of Applied Social Psychology. 2001;31(9):1775–1820. [Google Scholar]
- Costafreda SG, Dinov ID, Zhuowen T, Shi Y, Liu CY, Kloszewska I, Mecocci P, Soininen H, Tsolaki M, Vellas B, Wahlund LO, Spenger C, Toga AW, Lovestone S, Simmons A. Automated hippocampal shape analysis predicts the onset of dementia in mild cognitive impairment. NeuroImage. 2011;56(1):212–219. doi: 10.1016/j.neuroimage.2011.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernansky JG, Schindler MK, Splinter NR, Wang L, Gado M, Selemon LD, et al. Abnormalities of thalamic volume and shape in schizophrenia. American Journal of Psychiatry. 2004;161(5):896–902. doi: 10.1176/appi.ajp.161.5.896. [DOI] [PubMed] [Google Scholar]
- Cunningham WE, Hays RD, Duan N, Andersen R, Nakazono TT, Bozzette SA, Shapiro MF. The effect of socioeconomic status on the survival of people receiving care for HIV infection in the United States. Journal of Health Care for the Poor and Underserved. 2005;16(4):655–676. doi: 10.1353/hpu.2005.0093. [DOI] [PubMed] [Google Scholar]
- Eiland L, McEwen BS. Early life stress followed by subsequent adult chronic stress potentiates anxiety and blunts hippocampal structural remodeling. Hippocampus. 2012;22(1):82–91. doi: 10.1002/hipo.20862. [DOI] [PubMed] [Google Scholar]
- Entringer S, Buss C, Kumsta R, Hellhammer DH, Wadhwa PD, Wust S. Prenatal psychosocial stress exposure is associated with subsequent working memory performance in young women. Behavioral Neuroscience. 2009;123:886–893. doi: 10.1037/a0016265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Espejo D, Bekinschtein T, Monti MM, Pickard JD, Junque C, Coleman MR, Owen AM. Diffusion weighted imaging distinguishes the vegetative state from the minimally conscious state. NeuroImage. 2011;54(1):103–112. doi: 10.1016/j.neuroimage.2010.08.035. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frodl T, O’Keane V. How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiology of Disease. 2013;52:24–37. doi: 10.1016/j.nbd.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Chandra A, Wolfe BL, Pollak SD. Association between income and the hippocampus. PloS One. 2011;6(5):e18712. doi: 10.1371/journal.pone.0018712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, et al. Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biological Psychiatry. 2015;77(4):314–323. doi: 10.1016/j.biopsych.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayano F, Nakamura M, Asami T, Uehara K, Yoshida T, Roppongi T, et al. Smaller amygdala is associated with anxiety in patients with panic disorder. Psychiatry and Clinical Neurosciences. 2009;63(3):266–276. doi: 10.1111/j.1440-1819.2009.01960.x. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. Journal of the International Neuropsychological Society. 2004;10(03):317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy CHARTER study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, LeBlanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. Journal of Neurovirology. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans KG, Sliwowska JH, Verma P, Weinberg J. Prenatal alcohol exposure: fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neuroscience & Biobehavioral Reviews. 2010;34(6):791–807. doi: 10.1016/j.neubiorev.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo–pituitary–adrenocortical axis. Trends in Neurosciences. 1997;20(2):78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB, Redlich FC. Social class and mental illness: a community study. 1958. American Journal of Public Health. 2007;97(10):1756–1757. doi: 10.2105/ajph.97.10.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes EJ, Bond J, Svrckova P, Makropoulos A, Ball G, Sharp DJ, David Edwards A, Hajnal JV, Counsell SJ. Regional changes in thalamic shape and volume with increasing age. NeuroImage. 2012;63(3):1134–1142. doi: 10.1016/j.neuroimage.2012.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim F, Anderson J, Bukutu C, Elford J. Social and economic hardship among people living with HIV in London. HIV Medicine. 2008;9(8):616–624. doi: 10.1111/j.1468-1293.2008.00605.x. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical Axis. Endocrine Reviews. 1991;12(2):118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- Jednoróg K, Altarelli I, Monzalvo K, Fluss J, Dubois J, et al. Correction: the influence of socioeconomic status on children’s brain structure. PloS One. 2012;7(10) doi: 10.1371/journal.pone.0042486. 10.1371/annotation/47661de2-2c53-4396-9f88-06b5ad233566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Evans GW, Angstadt M, Ho SS, Sripada CS, Swain JE, et al. Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proceedings of the National Academy of Sciences. 2013;110(46):18442–18447. doi: 10.1073/pnas.1308240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Kumar AM, Morgan R, Szapocznik J, Eisdorfer C. Abnormal pituitary-adrenocortical response in early HIV-1 infection. Journal of Aquired Immune Deficiency Syndrome. 1993;6:61–65. [PubMed] [Google Scholar]
- Kumar M, Kumar AM, Waldrop D, Antoni MH, Eisdorfer C. HIV-1 infection and its impact on the HPA Axis, cytokines, and cognition. Stress. 2003;6(3):167–172. doi: 10.1080/10253890310001605376. [DOI] [PubMed] [Google Scholar]
- Lawson GM, Duda JT, Avants BB, Wu J, Farah MJ. Associations between children’s socioeconomic status and prefrontal cortical thickness. Developmental Science. 2013;16(5):641–652. doi: 10.1111/desc.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorant V, Deliege D, Eaton W, Robert A, Philippot P, Ansseau M. Socioeconomic inequalities in depression: a meta-analysis. American journal of epidemiology. 2003;157(2):98–112. doi: 10.1093/aje/kwf182. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Maki PM, Cohen MH, Weber K, Little DM, Fornelli D, Rubin LH, et al. Impairments in memory and hippocampal function in HIV-positive vs HIV-negative women: a preliminary study. Neurology. 2009;72(19):1661–1668. doi: 10.1212/WNL.0b013e3181a55f65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Rubin LH, Valcour V, Martin E, Crystal H, Young M, et al. Cognitive function in women with HIV findings from the Women’s interagency HIV study. Neurology. 2015;84(3):231–240. doi: 10.1212/WNL.0000000000001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massana G, Serra-Grabulosa JM, Salgado-Pineda P, Gastó C, Junqué C, Massana J, et al. Amygdalar atrophy in panic disorder patients detected by volumetric magnetic resonance imaging. NeuroImage. 2003;19(1):80–90. doi: 10.1016/s1053-8119(03)00036-3. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Annals of the New York Academy of Sciences. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montez JK, Bromberger JT, Harlow SD, Kravitz HM, Matthews KA. Life-course socioeconomic status and metabolic syndrome among midlife women. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2016 doi: 10.1093/geronb/gbw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DJ, Masliah E, Rippeth JD, Gonzalez R, Carey CL, Cherner M, et al. Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS. 2006;20(6):879–887. doi: 10.1097/01.aids.0000218552.69834.00. [DOI] [PubMed] [Google Scholar]
- Noble KG, Grieve SM, Korgaonkar MS, Engelhardt LE, Griffith EY, Williams LM, Brickman AM. Hippocampal volume varies with educational attainment across the life-span. Frontiers in Human Neuroscience. 2012;6:307. doi: 10.3389/fnhum.2012.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong D, Walterfang M, Malhi GS, Styner M, Velakoulis D, Pantelis C. Size and shape of the caudate nucleus in individuals with bipolar affective disorder. The Australian and New Zealand Journal of Psychiatry. 2012;46(4):340–351. doi: 10.1177/0004867412440191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson S, Moran P, Epel E, Sinclair E, Kemeny M, Deeks SG, Bacchetti P, Acree M, Epling L, Kirschbaum C, Hecht FM. Cortisol patterns are associated with T cell activation in HIV. PLOS One. 2013 doi: 10.1371/journal.pone.0063429. Retrieved online from http://www.plosone.org. [DOI] [PMC free article] [PubMed]
- Pesonen AK, Räikkönen K, Feldt K, Heinonen K, Osmond C, Phillips DI, et al. Childhood separation experience predicts HPA axis hormonal responses in late adulthood: a natural experiment of World War II. Psychoneuroendocrinology. 2010;35(5):758–767. doi: 10.1016/j.psyneuen.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Rabkin JG, McElhiney M, Ferrando SJ, Van Gorp W, Lin SH. Predictors of employment of men with HIV/AIDS: a longitudinal study. Psychosomatic Medicine. 2004;66(1):72–78. doi: 10.1097/01.psy.0000108083.43147.6d. [DOI] [PubMed] [Google Scholar]
- Rao U, Chen LA, Bidesi AS, Shad MU, Thomas MA, Hammen CL. Hippocampal changes associated with early-life adversity and vulnerability to depression. Biological Psychiatry. 2010;67(4):357–364. doi: 10.1016/j.biopsych.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards M, Wadsworth MEJ. Long term effects of early adversity on cognitive function. Archives of Disease in Childhood. 2004;89(10):922–927. doi: 10.1136/adc.2003.032490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Cook JA, Weber KM, Cohen MH, Martin E, Valcour V, Milam J, Anastos K, Young MA, Alden C, Gustafson DR, Maki PM. The association of perceived stress and verbal memory is greater in HIV-infected versus HIV-uninfected women. Journal of Neurovirology. 2015;21(4):422–432. doi: 10.1007/s13365-015-0331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Why stress is bad for your brain. Science. 1996;273(5276):749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- Schuster MA, Collins R, Cunningham WE, Morton SC, Zierler S, Wong M, Kanouse DE. Perceived discrimination in clinical care in a nationally representative sample of HIV-infected adults receiving health care. Journal of General Internal Medicine. 2005;20(9):807–813. doi: 10.1111/j.1525-1497.2005.05049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, Garner AS, Siegel BS, Dobbins MI, Earls MF, McGuinn L, et al. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129(1):e232–e246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- Spies G, Seedat S. Depression and resilience in women with HIV and early life stress: does trauma play a mediating role? A cross-sectional study. 2014;4(2) doi: 10.1136/bmjopen-2013-004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies G, Fennema-Notestine C, Cherner M, Seedat S. Changes in cognitive function in women with HIV infection and early life stress. AIDS Care. 2016;29(1):14–23. doi: 10.1080/09540121.2016.1204417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. Structured clinical interview for DSM-IV (SCID) New York: Biometrics Research; 1995. [DOI] [PubMed] [Google Scholar]
- Staff RT, Murray AD, Ahearn TS, Mustafa N, Fox HC, Whalley LJ. Childhood socioeconomic status and adult brain size: childhood socioeconomic status influences adult hippocampal size. Annals of Neurology. 2012;71(5):653–660. doi: 10.1002/ana.22631. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Marmot M. Burden of psychosocial adversity and vulnerability in middle age: associations with biobehavioral risk factors and quality of life. Psychosomatic Medicine. 2003;65(6):1029–1037. doi: 10.1097/01.psy.0000097347.57237.2d. [DOI] [PubMed] [Google Scholar]
- Tang X, Holland D, Dale AM, Younes L, Miller MI. Shape abnormalities of subcortical and ventricular structures in mild cognitive impairment and Alzheimer’s disease: detecting, quantifying, and predicting. Human Brain Mapping. 2014;35(8):3701–3725. doi: 10.1002/hbm.22431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J, Turner J. Perceived discrimination, social stress, and depression in the transition to adulthood: racial contrasts. Social Psychology Quarterly. 2002;65(3):213–225. [Google Scholar]
- Thames AD, Hinkin CH, Byrd DA, Bilder RM, Duff KJ, Mindt MR, et al. Effects of stereotype threat, perceived discrimination, and examiner race on neuropsychological performance: simple as black and white? Journal of the International Neuropsychological Society. 2013;19(05):583–593. doi: 10.1017/S1355617713000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Marusak HA, Tocco MA, Vila AM, McGarragle O, Rosenberg DR. Altered amygdala connectivity in urban youth exposed to trauma. Social Cognitive and Affective Neuroscience. 2015;10(11):1460–1468. doi: 10.1093/scan/nsv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental Science. 2010;13(1):46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussaint L, Shields GS, Dorn G, Slavich GM. Effects of lifetime stress exposure on mental and physical health in young adulthood: how stress degrades and forgiveness protects health. Journal of Health Psychology. 2016;21(6):1004–1014. doi: 10.1177/1359105314544132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Bellagamba R, Corpolongo A, Salvatori MF, Visco-Comandini U, et al. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. Journal of Acquired Immune Deficiency Syndromes. 2007;45(2):174–182. doi: 10.1097/QAI.0b013e318042e1ee. [DOI] [PubMed] [Google Scholar]
- Troxel WM, Matthews KA, Bromberger JT, Sutton-Tyrrell K. Chronic stress burden, discrimination, and subclinical carotid artery disease in African American and Caucasian women. Health Psychology. 2003;22(3):300. doi: 10.1037/0278-6133.22.3.300. [DOI] [PubMed] [Google Scholar]
- Van den Bogaard SJ, Dumas EM, Ferrarini L, Milles J, van Buchem MA, van der Grond J, Roos RA. Shape analysis of subcortical nuclei in Huntington’s disease, global versus local atrophy–A results from the TRACK-HD study. Journal of Neurological Sciences. 2011;307(1–2):60–68. doi: 10.1016/j.jns.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Wade BS, Valcour VG, Wendelken-Riegelhaupt L, Esmaeili-Firidouni P, Joshi SH, Gutman BA, Thompson PM. Mapping abnormal subcortical brain morphometry in an elderly HIV+ cohort. NeuroImage: Clinical. 2015;9:564–573. doi: 10.1016/j.nicl.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weniger G, Lange C, Sachsse U, Irle E. Amygdala and hippocampal volumes and cognition in adult survivors of childhood abuse with dissociative disorders. Acta Psychiatrica Scandinavica. 2008;118(4):281–290. doi: 10.1111/j.1600-0447.2008.01246.x. [DOI] [PubMed] [Google Scholar]
- WHO. Antiretroviral therapy for HIV infection in adults and adolescents 2010. 2010 http://www.who.int/hiv/pub/arv/adult2010/en/index.html. Accessed 29 Sept 2016.
- Wicks S, Hjern A, Gunnell D, Lewis G, Dalman C. Social adversity in childhood and the risk of developing psychosis: a national cohort study. American Journal of Psychiatry. 2005;162(9):1652–1657. doi: 10.1176/appi.ajp.162.9.1652. [DOI] [PubMed] [Google Scholar]
- Williams DR, Mohammed SA. Discrimination and racial disparities in health: evidence and needed research. Journal of Behavioral Medicine. 2009;32(1):20–47. doi: 10.1007/s10865-008-9185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: patterns and prospects. Health Psychology. 2016;35(4):407. doi: 10.1037/hea0000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson TJ, Mahmood Z, Kuhn TK, Thames AD. Differential relationships between social adversity and depressive symptoms by HIV status and racial/ethnic identity. Health Psychology. 2016 doi: 10.1037/hea0000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkleby MA, Cubbin C. Influence of individual and neighbourhood socioeconomic status on mortality among black, Mexican-American, and white women and men in the United States. Journal of Epidemiology and Community Health. 2003;57(6):444–452. doi: 10.1136/jech.57.6.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woon FL, Hedges DW. Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: a meta-analysis. Hippocampus. 2008;18(8):729–736. doi: 10.1002/hipo.20437. [DOI] [PubMed] [Google Scholar]


