Abstract
Positive, negative, and cognitive symptoms of schizophrenia may affect functional outcomes. However, these factors alone do not account for a large percentage of variance in outcomes. We investigated demographic, cognitive, symptom, and functional capacity predictors of current functional status in 280 outpatients with schizophrenia or schizoaffective disorder. Functional decline over the lifespan was also examined in a subset of participants. Stepwise regressions modeled predictors of current functional status and functional decline as measured by the Assessment of Lifespan Functioning Attainment (ALFA). ALFA functional domains included paid employment, independence in living situation, romantic relationships, close friendships, and recreational engagement. More severe depressive symptoms were consistently associated with worse current community integration (lower levels of close friendships and recreational engagement). Better working memory performance was associated with higher rates of current paid employment. There were no consistent modifiable predictors of decline in functioning, but women reported less functional decline in the domains of employment and close friendships than men. Better cognitive performance was associated with less decline in living independence and romantic relationships, but more decline in paid employment and recreational engagement. Increased assessment and treatment of comorbid depressive symptoms may improve functional outcomes in people with schizophrenia.
Keywords: psychosis, depression, cognition, lifespan functioning
1. Introduction
Schizophrenia is now largely considered to be a group of syndromes, rather than a single illness, due to significant genetic (Sebat et al., 2009; Stefansson et al., 2008; Walsh et al., 2008), symptom (Carpenter and Buchanan, 1994; Liddle and Morris, 1991; Wagman, 1988), and social risk factor (Cantor-Graae and Selten, 2005; Janssen et al., 2004; Zammit et al., 2004) heterogeneity. While different factors are associated with the various syndromes (Liddle, 1987; Liddle et al., 1992; Silverstein et al., 2000; Williams et al., 2000), schizophrenia spectrum disorders consistently lead to poor functional outcomes across multiple domains, including employment, living independence, and social functioning (Green et al., 2000; Green et al., 2004; Harvey et al., 1998).
Currently validated measures of real world functioning (Leifker et al., 2011) only consider a snapshot in time and do not provide a comprehensive lifespan perspective for outcomes relevant to schizophrenia. In addition, the factors predicting current functional status may be different from predictors of functional decline. Moreover, the factors associated with functional outcomes vary greatly (Barnes et al., 2008; Kolakowska et al., 1985; Marwaha and Johnson, 2004; Milev et al., 2005; Sabbag et al., 2012; Wölwer et al., 2014). Therefore, improved characterization of the set of factors that moderate functional outcomes in schizophrenia may be useful in developing and targeting treatments.
Studies of lifespan functioning in schizophrenia are very few and have been limited primarily to investigations of neurocognitive impairment (Friedman et al., 2001; Kalache et al., 2014; Kurtz, 2005) or qualitative assessments (Shepherd et al., 2012). A new scale, the Assessment of Lifespan Functioning Attainment (ALFA;(Joseph et al., 2015), enables the quantitative assessment of various stages in lifespan functioning including current status and post-psychosis decline for five different functional domains: paid employment, living independence, romantic relationships, close friendships, and recreational engagement.
The aim of this study was to model predictors of current functional status and post-psychosis functional decline using the ALFA scale in a large sample of individuals with schizophrenia-spectrum disorders. We predicted that demographic, illness burden, cognitive, and functional capacity factors would account for a significant amount of variance in functional outcomes.
2. Materials and Methods
2.1 Study Participants and Procedures
Outpatients with schizophrenia or schizoaffective disorder (n=280; 59% with schizophrenia, 41% with schizoaffective disorder) were recruited from the University of California, San Diego (UCSD) Outpatient Psychiatric Services clinic and the broader San Diego community and were enrolled in a study examining genetic predictors of cognitive and functional outcome in schizophrenia. The same sample was used in our initial descriptive and factor analytic study of the ALFA (Joseph et al., 2015). The study was approved by the Institutional Review Board and all participants provided written informed consent. Each participant completed the study assessments within a two-week period.
Participants were excluded if they: 1) had a DSM-IV TR (APA, 2000) diagnosis of substance abuse or dependence within six months of study entry; 2) had a diagnosis of intellectual disability or neurological disorders affecting cognitive functioning (including brain injury with loss of consciousness >10 minutes); 3) were not fluent English speakers. The demographic and symptom characteristics, as well as current functional status for the five ALFA domains, are shown in Table 1. Information regarding current functional status was available for all 280 participants, whereas information on decline in functioning was available for a subset of the sample with psychosis onset after age 18 (n=93; see Table 1).
Table 1.
Demographic and clinical characteristics of the study sample (n=280) and subsample (n=93) with decline in functioning data available.
| Sample (n=280) | Subsample (n=93) | |||
|---|---|---|---|---|
| %/M | SD | %/M | SD | |
| Sex (% Male) | 66.8 | - | 63.4 | - |
| Race (% White) | 46.8 | - | 38.7 | - |
| Ethnicity (% Hispanic) | 20.0 | - | 15.1 | - |
| Suicide attempt (% reporting at least one lifetime attempt) |
46.8 | - | 40.9 | - |
| Current marital status (% Single, never married) |
58.2 | - | 50.5 | - |
| Current smoker (%) | 61.8 | - | 59.1 | - |
| Current paid employment (%) | 7.9 | - | 5.4 | - |
| Current living independence (%) | 76.4 | - | 75.3 | - |
| Current romantic relationships (%) | 40.7 | - | 44.1 | - |
| Current close friendships (%) | 75.0 | - | 72.0 | - |
| Current recreational engagement (%) | 65.0 | - | 57.0 | - |
| Age, years | 48.1 | 10.2 | 49.0 | 8.9 |
| Education, years | 12.3 | 2.4 | 12.5 | 2.5 |
| Age of psychosis onset, years | 22.2 | 9.7 | 28.4 | 8.3 |
| Duration of psychosis, years | 25.9 | 12.2 | 20.7 | 9.4 |
| Premorbid IQ estimate | 92.8 | 15.4 | 92.6 | 13.4 |
| HAMD depressive symptom severity | 6.2 | 5.8 | 6.3 | 6.4 |
| SANS negative symptom severity | 29.2 | 17.3 | 21.5 | 16.7 |
| SAPS positive symptom severity | 27.6 | 16.9 | 19.4 | 13.0 |
| Antisocial characteristics | 1.7 | 2.4 | 1.3 | 2.0 |
| Total chlorpromazine equivalent (mg) | 386.8 | 249.9 | 380.9 | 206.7 |
Note. HAMD = Hamilton Depression Rating Scale, SANS = Scale for Assessment of Negative Symptoms, SAPS = Scale for the Assessment of Positive Symptoms.
2.2 Psychiatric and Substance History Measures
Psychiatric history indices were obtained from the Diagnostic Interview for Genetics Studies (Nurnberger et al., 1994). These indices included history of suicide attempts, history of heavy alcohol and substance use, history of smoking, and history of antisocial personality characteristics prior to age 15. Heavy alcohol use was defined as ≥8 drinks per week for women and ≥15 drinks per week for men (Dawson et al., 2005). Heavy substance use for cannabis, cocaine, and other stimulants was defined as 30 or more days of continuous substance use.
2.3 Current Symptom Assessments
Scale for Assessment of Positive Symptoms (SAPS)
(Andreasen, 1984). The SAPS was used to assess four positive symptom domains of psychopathology in schizophrenia: 1) hallucinations; 2) delusions; 3) bizarre behavior; and 4) formal thought disorder.
Scale for Assessment of Negative Symptoms (SANS)
(Andreasen, 1983). The SANS was used to assess negative symptoms of psychopathology in schizophrenia in five domains: 1) affective flattening or blunting; 2) alogia; 3) avolition-apathy; 4) attention; and 5) anhedonia-asociality.
Hamilton Depression Scale (HAMD)
(Hamilton, 1960). The HAMD was used to assess current depression symptoms.
2.4 Functional Capacity
UCSD Performance-based Skills Assessment 2 (UPSA-2)
(Patterson and Goldman, 2005). The UPSA-2 is a measure of functional capacity that assesses ability to perform tasks related to independent living skills in six domains: finance (e.g., write a check to pay a utilities bill), communication (e.g., call a doctor to reschedule an appointment), transportation, recreation planning, household chores (e.g., grocery shopping) and medication management. Prior studies suggest good test-retest reliability for the original UPSA (Leifker et al., 2010), which does not include the medication management ability assessment (MMAA). For the UPSA-2, raw subscale scores for all domains except the MMAA were converted to a composite score out of 100 (Patterson and Goldman, 2005). For the MMAA, a total raw score was computed.
2.5 Self-Reported Functioning
Assessment of Lifespan Functioning Attainment (ALFA)
(Joseph et al., 2015). The ALFA is a quantitative self-report measure of past and current functioning comprising five domains: 1) paid employment (including full-time post-secondary education); 2) living independence; 3) participation in romantic relationships; 4) maintenance of close friendships; and 5) engagement in recreational activities with non-family members. In part 1, current status for each domain was coded 0 for “not participating” and 1 for “currently participating.” In part 2, to determine variation in functioning for specific epochs of adulthood (i.e., age 18–20, 21–30, 31–40, 41–50, etc., up to the individual’s current age) participants were queried as to the number of years that they were engaged in activities corresponding to each ALFA domain. The percentage of years of engagement in each domain from age of 18 to age of psychosis onset was defined as “Pre-Psychosis Functioning,” and percentage of years of engagement in each domain from age of psychosis onset to current age was defined as “Post-Psychosis Functioning”; higher values represent better outcomes. The difference in percentages between Post-Psychosis and Pre-Psychosis Functioning was defined as “Post-Psychosis Decline”; higher values represent less decline, whereas lower values represent greater decline.
2.6 Cognitive Measures
Premorbid intellectual functioning was estimated with the Wide Range Achievement Test III (WRAT-III) reading subtest (Wilkinson, 1993). Current cognitive functioning was measured with the MATRICS Consensus Cognitive Battery (MCCB; Nuechterlein et al., 2008),which includes the following domains: 1) speed of processing (Symbol Coding, Animal Naming, Trail Making Test, Part A); 2) attention/vigilance (Continuous Performance Test-Identical Pairs); 3) working memory (Spatial Span, Letter-Number Span); 4) verbal learning (Hopkins Verbal Learning Test-Revised); 5) visual learning (Brief Visuospatial Memory Test-Revised); 6) reasoning and problem solving (Mazes); and 7) social cognition (Mayer-Salovey-Caruso Emotional Intelligence Test (Nuechterlein et al., 2008). The age- and sex-corrected T-scores for each domain were used as cognitive indices.
Additional cognitive tasks were administered to supplement the MCCB domains of attention and working memory and to measure speeded switching and planning, which are not measured by the MCCB. The Digit Span subtest from the Wechsler Adult Intelligence Scale, Third Edition (Wechsler, 1997) was administered as an additional measure of attention. The longest digit sequence participants repeated correctly was designated as the maximum span score for the forward condition. Reading Span (Conklin et al., 2000; Kremen et al., 2007) was included as a supplemental measure of working memory ability. In this test, participants read groups of sentences of about 14 words aloud (beginning with two sentences, then increasing), and were then asked to recall the last word of each sentence in the set. The Delis-Kaplan Executive Function System (Delis et al., 2001) Trail Making and Tower Tests were also administered to measure speeded switching and planning abilities.
2.7 Statistical Analyses
To reduce the total number of predictor variables in our regression models, point biserial correlations were computed between the demographic, clinical, functional capacity, and cognitive measures and current functioning and post-psychosis decline for the five ALFA domains. All measures correlated with a p value of ≤.05 were included in the regression models.
Stepwise regressions were performed for current functional status and post-psychosis decline in the five ALFA domains. For the stepwise logistic regressions predicting current functional status, we included demographic, psychiatric history, current symptom severity, and current cognitive functioning in the model. For the post-psychosis decline regressions, demographic, psychiatric history, and cognitive scores were entered, given that demographic and history variables cannot change and cognitive performance is thought to be stable in schizophrenia patient populations (Palmer et al., 2009). We excluded current symptom severity factors from the post-psychosis decline regressions as symptom severity may change considerably over time.
3. Results
3.1 Correlates of Current Functioning and Post-Psychosis Decline in ALFA Domains
Significant bivariate correlates of current functioning are shown in Table 2. Cognitive measures were correlated with current functioning for all ALFA domains except romantic relationships. Higher levels of depressive symptoms were associated with worse current functioning in close friendships and recreational engagement. Other variables were less consistently predictive of current functioning and there were no significant correlates of current romantic relationships. The significant correlates of current functioning were modest, ranging from r = .12−.18.
Table 2.
Point biserial correlates of current functioning and bivariate correlates of post-psychosis decline in functioning (p <.05).
| ALFA Domain – Current Functioning |
Measures | r | P |
|---|---|---|---|
| Paid employment | Antisocial characteristics | .178 | .003 |
| Reading Span | .154 | .013 | |
| Premorbid IQ | .130 | .036 | |
| History of Heavy Marijuana Use | .123 | .044 | |
| Living independence | D-KEFS Trails switching | −.127 | .047 |
| Current smoker | −.120 | .046 | |
| History of Heavy Drinking | −.145 | .026 | |
| History of Heavy Stimulant Use | −.141 | .020 | |
| Close friendships | D-KEFS Trails number sequencing | −.160 | .014 |
| HAMD depressive symptom severity | −.125 | .041 | |
| History of Heavy Stimulant Use | .160 | .010 | |
| Recreational engagement | MCCB visual learning | −.128 | .042 |
| HAMD depressive symptom severity | −.156 | .011 | |
| History of Heavy Stimulant Use | .146 | .018 | |
|
ALFA Domain – Decline in Functioning |
Measures | r | P |
| Paid employment | Participant Sex | .394 | <.001 |
| Premorbid IQ | −.301 | .005 | |
| D-KEFS Tower test | −.375 | <.001 | |
| Living independence | D-KEFS Trails motor speed | .238 | .032 |
| Close friendships | Participant Sex | .374 | <.001 |
| Antisocial characteristics | −.221 | .036 | |
| Recreational engagement | MCCB verbal learning | −.249 | .021 |
| Participant Sex | .223 | .034 | |
| D-KEFS Trails number sequencing | −.226 | .045 | |
| Romantic relationships | MCCB attention/vigilance | .306 | .004 |
| Suicide attempt | .231 | .027 |
Note. ALFA = Assessment of Lifespan Functioning Attainment; D-KEFS = Delis-Kaplan Executive Function System; HAMD = Hamilton Depression Rating Scale; MCCB = MATRICS Consensus Cognitive Battery
Correlates of post-psychosis decline in functioning are shown in Table 2 and were generally stronger (r =.22- .39) compared to the correlates of current functioning. Female sex was associated with less decline in years of paid employment, close friendships and recreational engagement. Declines in all ALFA domains except close friendships were associated with several cognitive measures. Current cognitive performance was associated with greater decline in some domains and less decline in others. The remaining variables were less consistently predictive of functional decline.
3.2 Multivariate Predictors of Current Functioning
The stepwise logistic regressions modeling current ALFA domain functioning are shown in Table 3. Better working memory, as measured by the reading span task, predicted current paid employment. Together, the predictor variables explained 5.5% of the variance in current employment. Having a history of heavy stimulant use was significantly associated with living independently, in a model explaining 3.3% of the variance in current living independence. Slower number sequencing, less severe depressive symptoms, and a lack of prior heavy stimulant use predicted current participation in close friendships; together, the predictor variables explained 11.1% of the variance in close friendships. Less severe depressive symptoms, poorer visual learning scores, and a lack of prior heavy stimulant use predicted current recreational engagement; together, the predictor variables explained 8.8% of the variance in recreational engagement. None of the modeled variables predicted current participation in romantic relationships.
Table 3.
Stepwise logistic regression models predicting current functioning.
| ALFA Domain |
Predictors | B | SE B | Wald | P |
Odds Ratio |
95% CI |
|---|---|---|---|---|---|---|---|
| Paid employment (N = 254) |
Reading Span | .165 | .069 | 5.757 | .016 | 1.180 | 1.031 – 1.350 |
| Antisocial characteristics |
.103 | ||||||
| Premorbid IQ | .192 | ||||||
| History of Heavy Marijuana Use |
.159 | ||||||
| Nagelkerke R2 | .055 | ||||||
| Model χ2 | 5.918 | .015 | |||||
| Living independence (N = 246) |
History of Heavy Stimulant Use |
.851 | .398 | 4.572 | .033 | 2.341 | 1.073 – 5.107 |
| History of Heavy Alcohol Use |
.139 | ||||||
| D-KEFS Trails switching |
.064 | ||||||
| Current smoker | .327 | ||||||
| NagelkerkeR2 | .033 | ||||||
| Model χ2 | 4.345 | .037 | |||||
| Close friendships (N = 235) |
D-KEFS Trails number sequencing |
−.120 | .057 | 4.383 | .036 | .887 | .792 – .992 |
| HAMD depressive symptoms |
−.055 | .028 | 3.994 | .046 | .946 | .897 – .999 | |
| History of Heavy Stimulant Use |
−1.588 | .634 | 6.268 | .012 | .204 | .059 – .708 | |
| NagelkerkeR2 | .111 | ||||||
| Model χ2 | 17.112 | .001 | |||||
| Recreational engagement (N = 252) |
MCCB visual learning |
−.026 | .011 | 5.393 | .020 | .974 | .953 – .996 |
| HAMD depressive symptoms |
−.055 | .025 | 4.937 | .026 | .947 | .902 – .994 | |
| History of Heavy Stimulant Use |
−1.025 | .433 | 5.618 | .018 | .359 | .154 – .837 | |
| NagelkerkeR2 | .088 | ||||||
| Model χ2 | 16.042 | .001 |
Note. ALFA = Assessment of Lifespan Functioning Attainment; D-KEFS = Delis-Kaplan Executive Function System; HAMD = Hamilton Depression Rating Scale; MCCB = MATRICS Consensus Cognitive Battery
3.3 Multivariate Predictors of Post-Psychosis Decline in Functioning
Stepwise linear regressions used to model post-psychosis decline in ALFA domain functioning are shown in Table 4. In a multivariate context, lower premorbid IQ, worse planning (Tower test scores), and female sex predicted less decline in paid employment; together, the predictor variables explained 27.2% of the variance in employment decline. Female sex predicted less decline in close friendships in a multivariate model explaining 13.5% of the variance in friendship decline. Better performance on the motor speed condition of D-KEFS Trails predicted less decline in living independence in a multivariate model explaining 5.7% of the variance, and better performance on attention/vigilance tasks on the MCCB predicted less decline in romantic relationships in a model explaining 9.4% of the variance. Poorer verbal learning scores predicted less decline in recreational engagement; together, the predictor variables explained 8.1% of the variance in recreational decline.
Table 4.
Stepwise linear regression models predicting post-psychosis decline in functioning.
| ALFA Domain |
Predictor | B | SE B | p | t | p |
|---|---|---|---|---|---|---|
| Paid employment (N = 87) |
Participant Sex | 17.544 | 5.768 | .300 | 3.041 | .003 |
| Premorbid IQ | −.465 | .203 | −.224 | −2.292 | .024 | |
| D-KEFS Tower test | −2. 353 | 1.048 | −.231 | −2.246 | .027 | |
| R2 | .272 | |||||
| F for R2 | 5.251 | .024 | ||||
| Living independence (N = 81) |
D-KEFS Trails motor speed (5) | 2.406 | 1.103 | .238 | 2.182 | .032 |
| R2 | .057 | |||||
| F for R2 | 4.759 | .032 | ||||
| Close friendships (N = 91) |
Participant Sex | 23.697 | 6.367 | .367 | 3.722 | <.001 |
| Antisocial characteristics | −.178 | −1.820 | .072 | |||
| R2 | .135 | |||||
| F for R2 | 13.851 | <.001 | ||||
| Recreational engagement (N = 79) |
MCCB verbal learning | −1.010 | .388 | −.285 | −2.605 | .011 |
| Participant Sex | .181 | 1.591 | .116 | |||
| D-KEFS Trails number sequencing (2) | −.170 | −1.528 | .131 | |||
| R2 | .081 | |||||
| F for R2 | 6.787 | .011 | ||||
| Romantic relationships (N = 87) |
MCCB attention/vigilance | 1.003 | .338 | .306 | 2.968 | .004 |
| Suicide attempt | .089 | .806 | .422 | |||
| R2 | .094 | |||||
| F for R2 | 8.808 | .004 |
Note. ALFA = Assessment of Lifespan Functioning Attainment; D-KEFS = Delis-Kaplan Executive Function System; MCCB = MATRICS Consensus Cognitive Battery
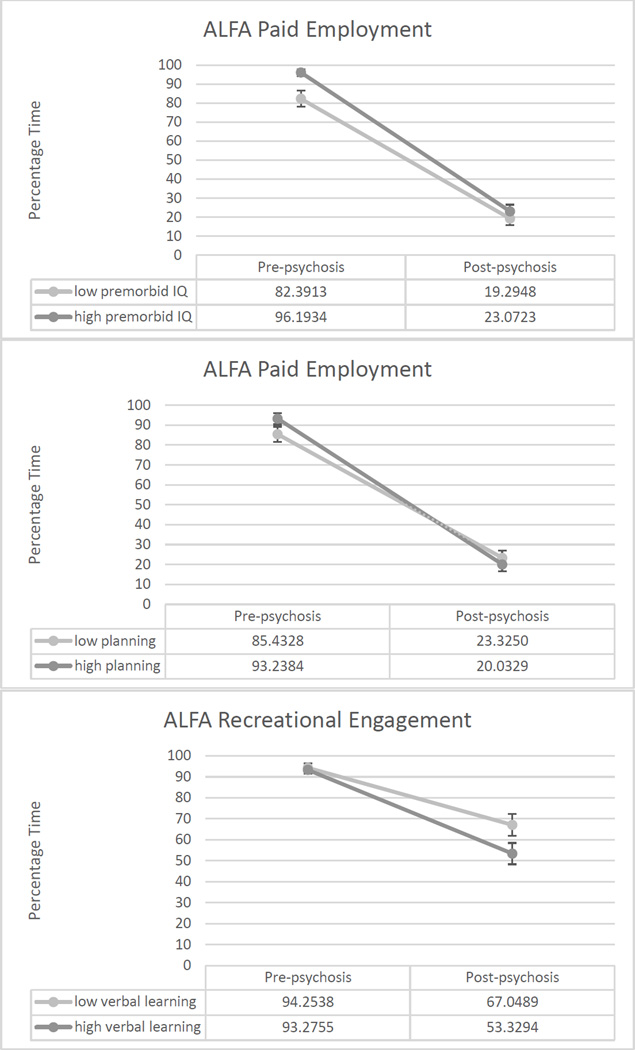
Worse cognitive performance was associated with less decline in functioning in three cases, as described above; Figure 1 shows graphs of these unexpected results. In two of the three cases, it appeared that worse cognitive performance was associated with lower levels of pre-psychosis functioning. Thus, it may be the case that those with worse cognitive performance had lower levels of functioning early in life and had less room to decline; conversely, those with higher levels of cognitive performance may have functioned better early in life and had more room to decline.
Figure 1.
Graphs of three results showing worse cognitive performance associated with less functional decline.
4. Discussion
This study used demographic, clinical, functional capacity, and cognitive measures to model predictors of current functioning and post-psychosis decline in multiple domains relevant to schizophrenia-spectrum disorders. Regarding correlates of current functioning, in a multivariate context, severity of depression symptoms was associated with worse current functioning in the domains of recreational engagement and close friendships. Reducing depressive symptoms may deserve increased focus as a way to improve functional outcomes (Bowie et al., 2010; Sabbag et al., 2012). In addition, our study sample included a substantial proportion of individuals with schizoaffective disorder, which may have influenced outcomes. Future studies including symptom assessment scales such as the Calgary Depression Scale (Addington et al., 1996; Addington et al., 1990) and Clinical Assessment Interview for Negative Symptoms (CAINS; Kring et al., 2013) may better disentangle the relationships among patient perceptions, negative and depressive symptoms, and functioning. The study of biological pathways that link depression and schizophrenia spectrum disorders (Frick et al., 2013; Jun et al., 2012; Ray et al., 2014; Walton et al., 2014; Yu et al., 2013) could also be important for improving our understanding of the role of depression in functional outcomes in schizophrenia.
Cognitive performance was not a consistent predictor of current functional status. Better working memory was associated with current paid employment, but slower number sequencing was associated with current close friendships, and worse visual learning was associated with recreational engagement. History of prior heavy stimulant use was also an inconsistent predictor of current functional status in various domains. Unlike a recent study (Depp et al., 2015), we did not find that smoking status was associated with functional outcomes in our multivariate models. It should be noted that the multivariate models predicting variance in current functional status did not reveal strong relationships in general; the highest percentage of variance explained across functional domains was 11%.
Regarding predictors of variance in functional decline, we found few significant predictors in multivariate models. Female sex was protective against decline in paid employment and close friendships, possibly due to the later age of onset of psychosis in women (Lindamer et al., 1997). Worse planning performance and lower premorbid IQ were associated with less decline in employment status. This relationship was an unexpected finding and requires replication with future longitudinal studies. It is possible that better executive functioning is related to successful navigation of the disability entitlement system, which could lead to lower rates of employment. However, further studies are needed to examine this hypothesis since very few study participants maintained employment across their lifespan. It is also possible that those with better cognitive performance had better pre-psychosis functioning and therefore more room to decline. Poorer verbal learning performance was associated with less decline in recreational engagement. On the other hand, better cognition in the domains of motor speed and attention/vigilance was associated with less decline in the domains of living independence and romantic relationships, respectively. It should be noted that our multivariate models explained less than 30% of the variance in functional decline across ALFA domains. Since longitudinal symptom severity ratings were unavailable, we did not include psychiatric symptom severity predictors in our post-psychosis decline models. Therefore, it is possible that psychiatric symptom severity over the lifespan is the largest contributor to functional decline. Indeed, a recent large-scale study found that amotivation, along with neurocognition, predicted functional change over time (Fervaha et al., 2014).
Our study has several limitations that must be considered. Our sample size was sufficient for the measurement of current functional status, but significantly smaller for indices of functional decline. Therefore, our results regarding functional decline require replication in a larger sample. The ALFA is a self-report measure (Joseph et al., 2015) and may be prone to inaccurate reporting (Kendler et al., 1996; Shiffman, 2000) due to recall bias. Gathering information from long-term close contacts could add significantly to our understanding of variation in lifespan functioning, when reliable informants are available (Keefe and Fenton, 2007; Sabbag et al., 2011). In addition, our findings could not be corroborated with patient medical records since factors relevant to our study were not available in these reports. Therefore, there is a great need to include self-report lifespan measures to supplement patient medical records and informant reports in schizophrenia research studies. The ALFA measures functional domains dichotomously to reduce recall error, rather than allowing for a finer grained analysis of functioning (e.g., hours worked per week, number of close friends).We cannot interpret the direction of causality, especially for our subset of findings related to post psychosis decline in functioning, with a cross-sectional study. Unmeasured factors [e.g., metacognitive ability (Lysaker et al., 2013; Lysaker et al., 2010)] may also contribute to functional outcomes. We were only able to examine functional decline in adulthood, in a subset of outpatients with adult-onset psychosis. Thus, our results may not generalize to inpatients or individuals with childhood-onset psychosis. Finally, cross-sectional assessment of cognitive predictors may not capture periods of significant cognitive deterioration (Harvey, 2014) that could influence everyday functioning in schizophrenia.
This report represents the first assessment of predictors and correlates of adult lifespan functioning in schizophrenia using a quantitative scale. Our study included a measure of functional capacity, but future quantitative assessments of lifespan functioning may benefit from incorporation of real world performance factors (Gupta et al., 2012). While symptoms influence many functional outcomes, it will be important to replicate non-symptom modifiers, as symptom remission is not a consistent predictor functional recovery (Harvey et al., 2012). Although we found no consistent modifiable predictors of variance in functional decline across ALFA domains, depressive symptom severity was associated with current functional status in two of the five ALFA domains, and has previously been associated with both objective and subjective quality of life in people with schizophrenia (Narvaez et al., 2008). Increased assessment and treatment of comorbid depressive symptoms may improve functional outcomes and quality of life in people with schizophrenia.
Supplementary Material
Acknowledgments
The authors would like to thank all of the study participants and Barbara Johnson for her assistance with study data collection and management.
Funding
This work was supported by grants from NIMH (R01MH081861 to M.T.T., R01MH080150 to E.W.T., R01MH085521 to S.J.G. and T32MH018399 to J.J.), NIA (R01AG018386 to W.S.K. and C.E.F), the Gerber Foundation (S.J.G.), the Sidney R. Baer, Jr. Foundation (S.J.G.), and the Brain and Behavior Research Foundation (S.J.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
E.W.T. designed the study, oversaw data analyses, and edited the manuscript. J. J. conducted literature searches, analyses and interpretation of data, and wrote the initial draft of the manuscript. J.v.d.L. assisted with statistical analyses and edited the manuscript. W.S.K., C.E.F., and S.J.G. provided assistance with data interpretation and edited the manuscript. S.D.C. and M.T.T. edited the manuscript.
Conflict of Interest
The authors report no conflicts of interest.
References
- Addington D, Addington J, Atkinson M. A Psychometric Comparison of the Calgary Depression Scale for Schizophrenia and the Hamilton Depression Rating Scale. Schizophrenia research. 1996;19(2–3):205–212. doi: 10.1016/0920-9964(95)00070-4. [DOI] [PubMed] [Google Scholar]
- Addington D, Addington J, Schissel B. A Depression Rating Scale for Schizophrenics. Schizophrenia research. 1990;3(4):247–251. doi: 10.1016/0920-9964(90)90005-r. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) University of Iowa: American Psychological Association, Iowa City; 1983. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) University of Iowa: American Psychological Association, Iowa City; 1984. [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) fourth. Washington, DC: 2000. (text revision) ed. [Google Scholar]
- Barnes TRE, Leeson VC, Mutsatsa SH, Watt HC, Hutton SB, Joyce EM. Duration of untreated psychosis and social function: 1-year follow-up study of first-episode schizophrenia. The British Journal of Psychiatry. 2008;193(3):203–209. doi: 10.1192/bjp.bp.108.049718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie CR, Depp C, McGrath JA, Wolyniec P, Mausbach BT, Thornquist MH, Luke J, Patterson TL, Harvey PD, Pulver AE. Prediction of real-world functional disability in chronic mental disorders: a comparison of schizophrenia and bipolar disorder. American Journal of Psychiatry. 2010;167(9):1116–1124. doi: 10.1176/appi.ajp.2010.09101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor-Graae E, Selten J-P. Schizophrenia and Migration: A Meta-Analysis and Review. American Journal of Psychiatry. 2005;162(1):12–24. doi: 10.1176/appi.ajp.162.1.12. [DOI] [PubMed] [Google Scholar]
- Carpenter WT, Buchanan RW. Schizophrenia. New England Journal of Medicine. 1994;330(10):681–690. doi: 10.1056/NEJM199403103301006. [DOI] [PubMed] [Google Scholar]
- Conklin HM, Curtis CE, Katsanis J, Iacono WG. Verbal working memory impairment in schizophrenia patients and their first-degree relatives: evidence from the digit span task. The American journal of psychiatry. 2000;157(2):275–277. doi: 10.1176/appi.ajp.157.2.275. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Li TK. Quantifying the risks associated with exceeding recommended drinking limits. Alcoholism: Clinical & Experimental Research. 2005;29(5):902–908. doi: 10.1097/01.alc.0000164544.45746.a7. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan executive functioning system (D-KEFS) Psychological Corporation. 2001 [Google Scholar]
- Depp CA, Bowie CR, Mausbach BT, Wolyniec P, Thornquist MH, Luke JR, McGrath JA, Pulver AE, Patterson TL, Harvey PD. Current smoking is associated with worse cognitive and adaptive functioning in serious mental illness. Acta Psychiatrica Scandinavica. 2015;131(5):333–341. doi: 10.1111/acps.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fervaha G, Foussias G, Agid O, Remington G. Motivational and neurocognitive deficits are central to the prediction of longitudinal functional outcome in schizophrenia. Acta Psychiatrica Scandinavica. 2014;130(4):290–299. doi: 10.1111/acps.12289. [DOI] [PubMed] [Google Scholar]
- Frick LR, Williams K, Pittenger C. Microglial dysregulation in psychiatric disease. Clinical and Developmental Immunology. 2013:1–10. doi: 10.1155/2013/608654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JI, Harvey PD, Coleman T, Moriarty PJ, Bowie C, Parrella M, White L, Adler D, Davis KL. Six-year follow-up study of cognitive and functional status across the lifespan in schizophrenia: a comparison with Alzheimer’s disease and normal aging. American Journal of Psychiatry. 2001;158(9):1441–1448. doi: 10.1176/appi.ajp.158.9.1441. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophrenia bulletin. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophrenia research. 2004;72(1):41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Gupta M, Bassett E, Iftene F, Bowie CR. Functional outcomes in schizophrenia: understanding the competence-performance discrepancy. Journal of psychiatric research. 2012;46(2):205–211. doi: 10.1016/j.jpsychires.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A Rating Scale for Depression. Journal of Neurology, Neurosurgery & Psychiatry. 1960;23(1):56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD. What is the evidence for changes in cognition and functioning over the lifespan in patients with schizophrenia? The Journal of clinical psychiatry. 2014;75(suppl 2):34–38. doi: 10.4088/JCP.13065su1.08. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Heaton RK, Carpenter WT, Green MF, Gold JM, Schoenbaum M. Functional impairment in people with schizophrenia: Focus on employability and eligibility for disability compensation. Schizophrenia research. 2012;140(1–3):1–8. doi: 10.1016/j.schres.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD, Howanitz E, Parrella M, White L, Davidson M, Mohs RC, Hoblyn J, Davis KL. Symptoms, Cognitive Functioning, and Adaptive Skills in Geriatric Patients With Lifelong Schizophrenia: A Comparison Across Treatment Sites. American Journal of Psychiatry. 1998;155(8):1080–1086. doi: 10.1176/ajp.155.8.1080. [DOI] [PubMed] [Google Scholar]
- Janssen I, Krabbendam L, Bak M, Hanssen M, Vollebergh W, Graaf Rd, Os Jv. Childhood abuse as a risk factor for psychotic experiences. Acta Psychiatrica Scandinavica. 2004;109(1):38–45. doi: 10.1046/j.0001-690x.2003.00217.x. [DOI] [PubMed] [Google Scholar]
- Joseph J, Kremen WS, Glatt SJ, Franz CE, Chandler SD, Liu X, Johnson BK, Tsuang MT, Twamley EW. Assessment of Lifespan Functioning Attainment (ALFA) scale: A quantitative interview for self-reported current and functional decline in schizophrenia. Journal of psychiatric research. 2015;65:102–107. doi: 10.1016/j.jpsychires.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun H, Mohammed Qasim Hussaini S, Rigby MJ, Jang M-H. Functional role of adult hippocampal neurogenesis as a therapeutic strategy for mental disorders. Neural Plasticity. 2012:1–20. doi: 10.1155/2012/854285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalache SM, Mulsant BH, Davies SJ, Liu AY, Voineskos AN, Butters MA, Miranda D, Menon M, Kern RS, Rajji TK. The Impact of Aging, Cognition, and Symptoms on Functional Competence in Individuals With Schizophrenia Across the Lifespan. Schizophrenia bulletin. 2014;41(2):374–381. doi: 10.1093/schbul/sbu114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RS, Fenton WS. How should DSM-V criteria for schizophrenia include cognitive impairment? Schizophrenia bulletin. 2007;33(4):912–920. doi: 10.1093/schbul/sbm046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Thacker L, Walsh D. Self-report measures of schizotypy as indices of familial vulnerability to schizophrenia. Schizophrenia bulletin. 1996;22(3):511–520. doi: 10.1093/schbul/22.3.511. [DOI] [PubMed] [Google Scholar]
- Kolakowska T, Williams AO, Ardern M, Reveley MA, Jambor K, Gelder MG, Mandelbrote BM. Schizophrenia with good and poor outcome. I: Early clinical features, response to neuroleptics and signs of organic dysfunction. The British Journal of Psychiatry. 1985;146(3):229–239. doi: 10.1192/bjp.146.3.229. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Jacobsen KC, Xian H, Eisen SA, Eaves LJ, Tsuang MT, Lyons MJ. Genetics of verbal working memory processes: a twin study of middle-aged men. Neuropsychology. 2007;21(5):569–580. doi: 10.1037/0894-4105.21.5.569. [DOI] [PubMed] [Google Scholar]
- Kring AM, Gur RE, Blanchard JJ, Horan WP, Reise SP. The Clinical Assessment Interview for Negative Symptoms (CAINS): final development and validation. The American journal of psychiatry. 2013;170(2):165–172. doi: 10.1176/appi.ajp.2012.12010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz MM. Neurocognitive impairment across the lifespan in schizophrenia: an update. Schizophrenia research. 2005;74(1):15–26. doi: 10.1016/j.schres.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Leifker FR, Patterson TL, Bowie CR, Mausbach BT, Harvey PD. Psychometric properties of performance-based measurements of functional capacity: test-retest reliability, practice effects, and potential sensitivity to change. Schizophrenia research. 2010;119(1–3):246–252. doi: 10.1016/j.schres.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifker FR, Patterson TL, Heaton RK, Harvey PD. Validating measures of real-world outcome: the results of the VALERO expert survey and RAND panel. Schizophrenia bulletin. 2011;37(2):334–343. doi: 10.1093/schbul/sbp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle PF. Schizophrenic syndromes, cognitive performance and neurological dysfunction. Psychological medicine. 1987;17(1):49–57. doi: 10.1017/s0033291700012976. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Friston KJ, Frith CD, Hirsch SR, Jones T, Frackowiak RS. Patterns of cerebral blood flow in schizophrenia. The British Journal of Psychiatry. 1992;160(2):179–186. doi: 10.1192/bjp.160.2.179. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Morris DL. Schizophrenic syndromes and frontal lobe performance. The British journal of psychiatry : the journal of mental science. 1991;158:340–345. doi: 10.1192/bjp.158.3.340. [DOI] [PubMed] [Google Scholar]
- Lindamer LA, Lohr JB, Harris MJ, Jeste DV. Gender, estrogen, and schizophrenia. Psychopharmacology bulletin. 1997;33(2):221–228. [PubMed] [Google Scholar]
- Lysaker PH, Gumley A, Luedtke B, Buck KD, Ringer JM, Olesek K, Kukla M, Leonhardt BL, Popolo R, Dimaggio G. Social cognition and metacognition in schizophrenia: evidence of their independence and linkage with outcomes. Acta Psychiatrica Scandinavica. 2013;127(3):239–247. doi: 10.1111/acps.12012. [DOI] [PubMed] [Google Scholar]
- Lysaker PH, Shea AM, Buck KD, Dimaggio G, Nicolo G, Procacci M, Salvatore G, Rand KL. Metacognition as a mediator of the effects of impairments in neurocognition on social function in schizophrenia spectrum disorders. Acta Psychiatrica Scandinavica. 2010;122(5):405–413. doi: 10.1111/j.1600-0447.2010.01554.x. [DOI] [PubMed] [Google Scholar]
- Marwaha S, Johnson S. Schizophrenia and employment. Social psychiatry and psychiatric epidemiology. 2004;39(5):337–349. doi: 10.1007/s00127-004-0762-4. [DOI] [PubMed] [Google Scholar]
- Milev P, Ho BC, Arndt S, Andreasen NC. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. The American journal of psychiatry. 2005;162(3):495–506. doi: 10.1176/appi.ajp.162.3.495. [DOI] [PubMed] [Google Scholar]
- Narvaez JM, Twamley EW, McKibbin CL, Heaton RK, Patterson TL. Subjective and objective quality of life in schizophrenia. Schizophrenia research. 2008;98(1):201–208. doi: 10.1016/j.schres.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ, Gold JM, Goldberg T, Heaton RK, Keefe RS, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. The American journal of psychiatry. 2008;165(2):203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Archives of general psychiatry. 1994;51(11):849–859. doi: 10.1001/archpsyc.1994.03950110009002. discussion 863–844. [DOI] [PubMed] [Google Scholar]
- Palmer BW, Dawes SE, Heaton RK. What do we know about neuropsychological aspects of schizophrenia? Neuropsychol Rev. 2009;19(3):365–384. doi: 10.1007/s11065-009-9109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson T, Goldman S. The UCSD Performance-Based Skills Assessment Administration Manual (UPSA-2) 2005 [Google Scholar]
- Ray MT, Shannon Weickert C, Webster MJ. Decreased BDNF and TrkB mRNA expression in multiple cortical areas of patients with schizophrenia and mood disorders. Translational Psychiatry. 2014;4(5):e389. doi: 10.1038/tp.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbag S, Twamley EM, Vella L, Heaton RK, Patterson TL, Harvey PD. Assessing everyday functioning in schizophrenia: not all informants seem equally informative. Schizophrenia research. 2011;131(1–3):250–255. doi: 10.1016/j.schres.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbag S, Twamley EW, Vella L, Heaton RK, Patterson TL, Harvey PD. Predictors of the accuracy of self assessment of everyday functioning in people with schizophrenia. Schizophrenia research. 2012;137(1–3):190–195. doi: 10.1016/j.schres.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebat J, Levy DL, McCarthy SE. Rare structural variants in schizophrenia: one disorder, multiple mutations; one mutation, multiple disorders. Trends Genetics. 2009;25(12):528–535. doi: 10.1016/j.tig.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd S, Depp CA, Harris G, Halpain M, Palinkas LA, Jeste DV. Perspectives on schizophrenia over the lifespan: a qualitative study. Schizophrenia bulletin. 2012;38(2):295–303. doi: 10.1093/schbul/sbq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S. Real-time self-report of momentary states in the natural environment: Computerized ecological momentary assessment. The science of self-report: Implications for research and practice. 2000:277–296. [Google Scholar]
- Silverstein SM, Kovács I, Corry R, Valone C. Perceptual organization, the disorganization syndrome, and context processing in chronic schizophrenia. Schizophrenia research. 2000;43(1):11–20. doi: 10.1016/s0920-9964(99)00180-2. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Rujescu D, Cichon S, Pietiläinen OP, Ingason A, Steinberg S, Fossdal R, Sigurdsson E, Sigmundsson T, Buizer-Voskamp JE, Hansen T, Jakobsen KD, Muglia P, Francks C, Matthews PM, Gylfason A, Halldorsson BV, Gudbjartsson D, Thorgeirsson TE, Sigurdsson A, Jonasdottir A, Bjornsson A, Mattiasdottir S, Blondal T, Haraldsson M, Magnusdottir BB, Giegling I, Möller HJ, Hartmann A, Shianna KV, Ge D, Need AC, Crombie C, Fraser G, Walker N, Lonnqvist J, Suvisaari J, Tuulio-Henriksson A, Paunio T, Toulopoulou T, Bramon E, Di Forti M, Murray R, Ruggeri M, Vassos E, Tosato S, Walshe M, Li T, Vasilescu C, Mühleisen TW, Wang AG, Ullum H, Djurovic S, Melle I, Olesen J, Kiemeney LA, Franke B, Sabatti C, Freimer NB, Gulcher JR, Thorsteinsdottir U, Kong A, Andreassen OA, Ophoff RA, Georgi A, Rietschel M, Werge T, Petursson H, Goldstein DB, Nöthen MM, Peltonen L, Collier DA, St Clair D, Stefansson K GROUP. Large recurrent microdeletions associated with schizophrenia. Nature. 2008;455(7210):232–236. doi: 10.1038/nature07229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagman AM. Deficit and nondeficit forms of schizophrenia: the concept. The American journal of psychiatry. 1988;145:578–583. doi: 10.1176/ajp.145.5.578. [DOI] [PubMed] [Google Scholar]
- Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, Nord AS, Kusenda M, Malhotra D, Bhandari A. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320(5875):539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- Walton NM, de Koning A, Xie X, Shin R, Chen Q, Miyake S, Tajinda K, Gross AK, Kogan JH, Heusner CL, Tamura K, Matsumoto M. Gastrin-releasing peptide contributes to the regulation of adult hippocampal neurogenesis and neuronal development. Stem Cells. 2014;32(9):2454–2466. doi: 10.1002/stem.1740. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-III: Wechsler adult intelligence scale. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- Wilkinson G., 3rd . The Wide Range Achievement Test: Manual. Wilmington DE: 1993. [Google Scholar]
- Williams LM, Gordon E, Wright J, Bahramali H. Late component ERPs are associated with three syndromes in schizophrenia. Int J Neurosci. 2000;105(1–4):37–52. doi: 10.3109/00207450009003264. [DOI] [PubMed] [Google Scholar]
- Wölwer W, Lowe A, Brinkmeyer J, Streit M, Habakuck M, Agelink MW, Mobascher A, Gaebel W, Cordes J. Repetitive Transcranial Magnetic Stimulation (rTMS) Improves Facial Affect Recognition in Schizophrenia. Brain Stimuli. 2014;7(4):559–563. doi: 10.1016/j.brs.2014.04.011. [DOI] [PubMed] [Google Scholar]
- Yu Y, Shen H, Zeng L-L, Ma Q, Hu D. Convergent and divergent functional connectivity patterns in schizophrenia and depression. PloS one. 2013;8(7):e68250. doi: 10.1371/journal.pone.0068250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit S, Allebeck P, David AS, Dalman C, Hemmingsson T, Lundberg I, Lewis G. A longitudinal study of premorbid IQ score and risk of developing schizophrenia, bipolar disorder, severe depression, and other nonaffective psychoses. Archives of general psychiatry. 2004;61(4):354–360. doi: 10.1001/archpsyc.61.4.354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.