Abstract
We aimed to determine if serum measures of impaired glucose homeostasis (glucose concentrations or glycated serum protein, GSP) or systemic inflammation (high-sensitivity C-reactive protein, CRP) are related to incident typical knee osteoarthritis (KOA) or incident accelerated KOA. We conducted a case-control study using the Osteoarthritis Initiative's baseline and first 4 annual visits. All participants had no radiographic KOA at baseline (Kellgren-Lawrence [KL]<2). We classified 3 groups: 1) incident accelerated KOA: ≥1 knee developed advance-stage KOA (KL Grade 3 or 4) within 48 months, 2) incident typical KOA: ≥1 knee increased in radiographic scoring within 48 months (excluding those with accelerated KOA), and 3) No KOA: no change in KL grade by 48 months. We matched on sex. A laboratory blinded to group assignment used baseline serum samples to conduct assays for CRP, GSP, and glucose. Due to nonlinear relationships, we used 3 piece-wise multinomial logistic regression models to determine if baseline CRP, GSP, or glucose were associated with incident typical KOA or accelerated KOA compared with no KOA. We adjusted for age, body mass index, and sex. We analyzed 54 adults/group. Lower and higher GSP concentrations were associated with incident typical KOA compared with adults with concentrations (log) closer to 5.7 (lnGSP<5.7: OR=0.28, 95%CI=0.08-0.93; lnGSP>5.7: OR=3.21, 95%CI=1.07-9.62). Glucose, GSP, and CRP were not significantly associated with incident accelerated KOA. Glucose homeostasis may predict individuals at risk of incident typical KOA but not accelerated KOA, which may indicate accelerated KOA is a distinct disorder from typical KOA.
Keywords: glycated serum protein, inflammation, C-reactive protein, glucose concentrations, knee osteoarthritis
Greater age and body mass index are associated with knee osteoarthritis, which has generated significant interest in the role of inflammation and impaired glucose homeostasis in knee osteoarthritis. Aging – and an array of age-related chronic diseases – are associated with elevated inflammation (1; 2). Furthermore, older individuals may be more susceptible to an enhanced cytokine response after performing highly repetitive tasks (3). In addition to aging, obese individuals are 2 to 6 times more likely than normal-weight individuals to have elevated C-reactive protein (CRP), a commonly used clinical marker of systemic inflammation (4). The association between obesity and CRP may be partly influenced by adipose tissue producing interleukin-6 (5), which in turns stimulates the production of CRP by the liver and adipose tissue (6; 7). CRP may also be associated with osteoarthritis; specifically, it may be more associated with osteoarthritis symptoms than radiographic progression (8). Recently, investigators from the Rotterdam study reported that CRP was not associated with the prevalence of osteoarthritis but was predictive of incident knee osteoarthritis (9). Inflammation may play a greater role in some individuals with osteoarthritis. For example, 57% of adults with advanced-stage knee or hip osteoarthritis have inflammatory infiltrates in their synovial membrane and these adults have greater concentrations of plasma CRP than other adults with osteoarthritis (10). It may be advantageous to explore the role of systemic inflammation among well-defined subsets of individuals with knee osteoarthritis.
Glycation pathways are also a plausible pathway for the association of obesity and osteoarthritis. Osteoarthritic chondrocytes exposed to high glucose concentrations are unable to down-regulate glucose transporter 1, which leads to elevated reactive oxidative species and catabolic processes associated with osteoarthritis (11). There is conflicting evidence that impaired glucose homeostasis may be associated with incident knee osteoarthritis (12; 13). Some of the discrepancies in the literature may be attributable to the heterogeneity within a population of individuals with knee osteoarthritis. It may be advantageous to explore the role of inflammation and glucose homeostasis among well-defined subsets of individuals with knee osteoarthritis.
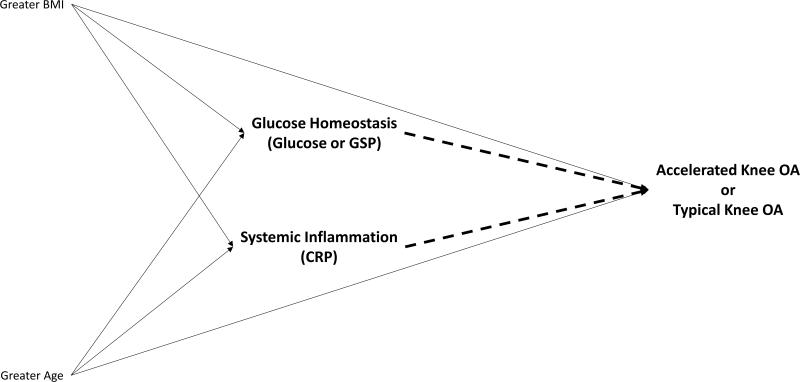
We recently characterized a group of individuals who develop an accelerated form of knee osteoarthritis, which we defined as a knee without radiographic osteoarthritis that develops incident advance-stage disease in less than 4 years and often in just 12 months (14-18). Besides an accelerated rate of disease onset, individuals with accelerated knee osteoarthritis are more likely to be older (14), report greater knee pain (16), and experience a recent knee injury (14; 17) than their peers who develop a typical (gradual) onset of knee osteoarthritis. Similar to individuals with a typical onset of knee osteoarthritis, adults with accelerated knee osteoarthritis are more likely to be overweight than individuals without knee osteoarthritis (14; 17). The characteristics of this group raise questions about whether greater inflammation or impaired glucose homeostasis may lead someone to be more susceptible to accelerated knee osteoarthritis (Figure 1). Hence, we aimed to determine if serum measures of impaired glucose homeostasis or inflammation (high-sensitivity CRP) are related to incident typical knee osteoarthritis or incident accelerated knee osteoarthritis compared with no knee osteoarthritis. To assess impaired glucose homeostasis, we assessed glucose concentrations and glycated serum protein (GSP), which provides a stable marker of glucose homeostasis over the prior 14 to 21 days. By separating these two subsets of adults with incident knee osteoarthritis, we may be able to clarify the association between these biomarkers and knee osteoarthritis.
Figure 1.
Conceptual diagram of a priori hypotheses about associations between glucose homeostasis, systemic inflammation, and incident knee osteoarthritis. Dashed lines represent the hypothesized associations being tested in these analyses.
METHODS
To assess the association between serum measures of impaired glucose homeostasis or inflammation with incident typical knee osteoarthritis or accelerated knee osteoarthritis we conducted a case-control study using data and images from baseline and the first four years of follow-up in the Osteoarthritis Initiative (OAI). The OAI is an observational study of individuals with or at risk for knee osteoarthritis at four clinical sites in the United States: Memorial Hospital of Rhode Island, The Ohio State University, University of Maryland and Johns Hopkins University, and the University of Pittsburgh. The study staff enrolled 4,796 men and women (45 to 79 years of age) between February 2004 and May 2006. Descriptions of the eligibility criteria and the OAI protocol are publicly available on the OAI website (19). Institutional review boards at each OAI clinical site and the coordinating center (University of California, San Francisco) approved the OAI study. All participants provided informed consent.
Case and Control Definitions
We assessed 3 groups, which we defined based on radiographic criteria of knee osteoarthritis. For our primary analyses, all participants had no radiographic knee osteoarthritis at baseline (Kellgren-Lawrence [KL] grade < 2). The first group of cases was individuals with incident accelerated knee osteoarthritis. We defined accelerated knee osteoarthritis as someone with at least 1 knee that developed advanced-stage knee osteoarthritis (KL grade 3 or 4, developed a definite osteophyte and joint space narrowing) within 48 months (n = 54). The second group of cases was individuals with incident typical knee osteoarthritis (gradual onset) who had no radiographic knee osteoarthritis in either knee at baseline (Kellgren-Lawrence [KL] grade < 2) and at least 1 knee that increased in radiographic scoring within 48 months (excluding those who developed a KL grade > 2; n = 187). Hence, individuals with typical knee osteoarthritis could meet this definition three ways: progression from KL 0 to 1 (development of possibly osteophyte), KL 0 to 2 (development of definite osteophyte), and KL 1 to 2 (development of definite osteophyte). Finally, the control group was individuals without knee osteoarthritis who had no radiographic knee osteoarthritis at baseline in both knees and no change in KL grade in either knee from baseline to 48-month follow-up (n = 1,325).
To ensure feasibility of performing the biospecimens assays, we did 1:1:1 matching for the three groups based on sex. Matching was completed at random. Each group had 54 participants. For the cases, the index knee was the knee that first met the definition of incident typical knee osteoarthritis or accelerated knee osteoarthritis. For individuals without knee osteoarthritis (controls), we defined the index to be the same knee as that person's matched member of the accelerated knee osteoarthritis group.
The serum measurements of impaired glucose homeostasis and inflammation represent a person-level risk factor. Therefore, our primary analyses focused on the 54 individuals with accelerated knee osteoarthritis who had no knee osteoarthritis in either knee at baseline as well as their matched participants with incident typical knee osteoarthritis and no knee osteoarthritis. For secondary analyses, we included 71 more people with incident accelerated knee osteoarthritis who had radiographic osteoarthritis in the contralateral knee at baseline. We excluded one person with accelerated knee osteoarthritis because they had a hemolyzed serum sample. In the secondary analyses, the 70 included people with incident accelerated knee osteoarthritis also had matched participants with incident typical knee osteoarthritis and no knee osteoarthritis. Hence, in our secondary analyses we had 124 adults per group.
Knee radiographs and semi-quantitative grading
The study sites obtained bilateral weight-bearing, fixed-flexion posteroanterior knee radiographs at baseline and the first 4 annual follow-up visits. Central readers were blinded to the order of follow-up radiographs. They scored the images for KL grades (0 to 4). The agreement for these readings (read–reread) was good (weighted κ [intra-rater reliability] = 0.70–0.80). These KL grades are publicly available (files: kXR_SQ_BU##_SAS [versions 0.6, 1.6, 3.5, 5.5, and 6.3]) (19).
Acquisition of Biospecimens
Study staff performed blood draws at the OAI baseline visit at least one hour after the participant had awoken. Participants were advised to fast for at least 8 hours prior to the study visit. Immediately after the blood draw, study staff inverted the serum sample tubes and then kept the tubes at room temperature for 30 minutes (maximum = 60 minutes). They then centrifuged the tubes at 4°C (30,000 g-minutes) and then immediately transferred the biospecimens to cryovials. Within 15 minutes of being placed in the cryovials the samples were placed in a −70°C freezer for at least 30 minutes prior to being shipped to Fisher Bioservices (Rockville, MD) for long-term storage. In September 2015, Fisher Bioservices shipped the biospecimens to Temple University School of Medicine. The full protocols for the biospecimens collection and processing are available on the OAI website (19).
Biochemical assays
All assays were performed in duplicate at Temple University School of Medicine within 2 months of delivery. The samples were de-identified to ensure the laboratory staff was blinded. To assess serum high-sensitivity CRP we used an enzyme-linked immunosorbent assay (ELISA, Novex by Life Technology, Carlsbad, CA, USA; range = 18.75 to 1200 pg/mL, sensitivity = 18.75 pg/mL, coefficient of variation <7.52% for intra-assay precision, and coefficient of variation <10% for inter-assay precision). To assess GSP we used an ELISA kit (MyBioSource, San Diego, CA, USA; range = 78.125 nmol/mL to 1000nmol/mL, sensitivity = 48.875 nmol/mL, and coefficient of variation <10%). Samples used for glucose analyses were deproteinized prior to analysis with 10kD Spin Columns (Abcam, Cambridge, MA, USA). For fasting glucose levels we used Abcam Glucose Assay Kits (Cambridge, MA, USA; range = 1 - 1000 μM, sensitivity = 1 μM, and coefficient of variation <2%).
Clinical Data
Demographic, anthropometric, and other characteristics, which we selected a priori for descriptive statistics or potential covariates, were acquired based on a standard protocol. The data and protocol are publicly available (19). We extracted several key baseline variables: sex, age, body mass index, presence of diabetes, and fasting time prior to blood draw. All data are publicly available on the OAI website (files: enrollees [version 19] and allclinical00 [version 0.2.2]).
Statistical Analysis
To assess the relationship between serum measurements (predictors) and incident typical knee osteoarthritis or accelerated knee osteoarthritis (outcomes) compared with no knee osteoarthritis (reference outcome) we planned to perform multinomial logistic regression analyses adjusted for sex (matching factor), age, and body mass index. Prior to performing the primary analyses, we calculated descriptive statistics; explored the distribution of CRP, GSP, and glucose concentrations; and checked for a linear relationship between the three predictors and the log odds for incident typical knee osteoarthritis and accelerated knee osteoarthritis. The distribution of GSP was not normally distributed. Hence, we calculated the natural log (ln) of GSP concentrations. We also detected nonlinear relationships. Therefore, we used 3 piece-wise multinomial logistic regression models to determine if baseline CRP, GSP, or glucose were associated with incident typical knee osteoarthritis or incident accelerated knee osteoarthritis compared with no knee osteoarthritis. We selected the relatively robust cutpoints for CRP, GSP, and glucose based on restricted cubic splines using different number of knots. We also explored in separate models if body mass index had an interaction with CRP, GSP, or glucose concentrations. A priori we defined the subset of individuals with no knee osteoarthritis in either knee at baseline as the primary analyses (n = 54/group). For a secondary analysis, we included the full study sample (n = 124/group), which included individuals with incident accelerated knee osteoarthritis in one knee and prevalent radiographic knee osteoarthritis in the contralateral knee at baseline. Results are reported as means (standard deviation) and odds ratio (OR) with 95% confidence intervals (95 CI%). We conducted all analyses in SAS 9.4 (Cary, NC, USA) and defined statistical significance based on p ≤ 0.05.
RESULTS
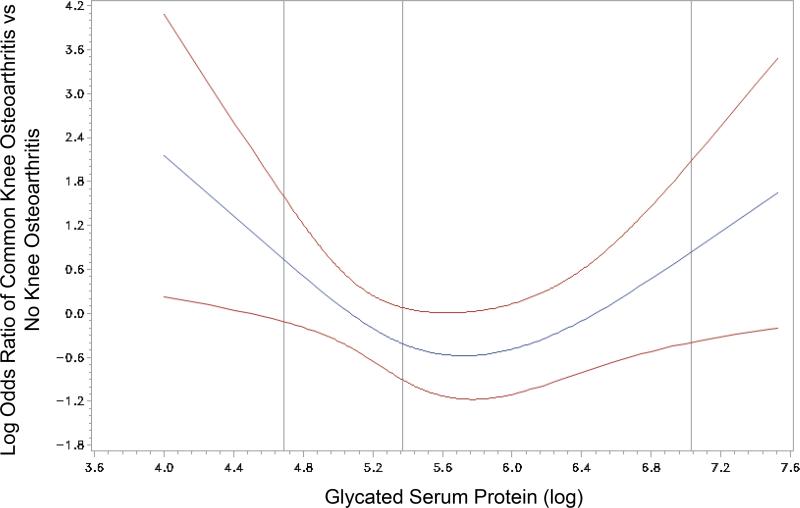
For our primary analysis, we analyzed 54 adults/group and described their characteristics in Table 1. Lower and higher GSP concentrations were associated with incident typical knee osteoarthritis compared with adults with concentrations (log) closer to 5.7 (Table 2, Figure 2). We found no statistical association between CRP and glucose with incident typical knee osteoarthritis. Furthermore, baseline CRP, GSP, and glucose were not statistically significantly associated with incident accelerated knee osteoarthritis (Table 2). We found no significant interactions between body mass index and CRP, GSP, or glucose concentrations.
Table 1.
No Significant Baseline Differences Among Those with Accelerated Knee Osteoarthritis (AKOA), Gradual Onset of Knee Osteoarthritis (KOA), and no KOA
| Baseline variable | No KOA (n = 54) | KOA (n = 54) | AKOA (n = 54) |
|---|---|---|---|
| Females (n, %) | 24 (63%) | 24 (63%) | 24 (63%) |
| Presence of Diabetes (n, %) | 1 (2%) | 0 (0%) | 4 (8%) |
| Age (years) | 59 (8) | 57 (8) | 62 (9) |
| Body Mass Index (kg/m2) | 27.3 (4.9) | 27.9 (4.6) | 28.9 (4.7) |
| Fasting Time (hrs) | 12 (2) | 12 (3) | 12 (2) |
| C-reactive Protein (mg/L) | 3.53 (1.09) | 3.16 (1.15) | 3.70 (1.05) |
| Glycated Serum Protein (ln) | 5.47 (0.58) | 5.51 (0.83) | 5.48 (0.77) |
| Glucose (mg/dL) | 111.25 (24.34) | 106.38 (24.90) | 107.27 (32.16) |
Frequencies were analyzed with Chi-Square test and group means with paired-sample t-tests.
Table 2.
Baseline Glycated Serum Protein Concentrations are Associated with Incident Knee Osteoarthritis (KOA)
| Predictor | KOA (n = 54) vs No KOA (n = 54) Adjusted ORa | Accelerated KOA (n = 54) vs No KOA (n = 54) Adjusted ORa |
|---|---|---|
| C-reactive Protein < 3.4 mg/L | 0.70 (0.37,1.34) | 1.27 (0.58,2.79) |
| C-Reactive Protein > 3.4 mg/L | 0.69 (0.33,1.45) | 0.91 (0.46,1.79) |
| Glycated Serum Protein (ln) < 5.7 | 0.28 (0.08, 0.93) | 0.40 (0.12,1.37) |
| Glycated Serum Protein (ln) > 5.7 | 3.21 (1.07,9.62) | 2.01 (0.64,6.33) |
| Glucose <115 mg/dL | 0.99 (0.97,1.02) | 0.98 (0.96,1.00) |
| Glucose >115 mg/dL | 0.99 (0.95,1.03) | 1.02 (0.98,1.05) |
All analyses are based on piece-wise regression models with cut points defined by graphing splines. All analyses are adjusted for age, body mass index, and sex (matching variable). There were no significant interactions with body mass index.
Odds ratios are per one unit increase of CRP, GSP, or glucose.
Figure 2.
Spline analysis highlights the nonlinear relationship between glycated serum protein and common knee osteoarthritis
Our secondary analyses with 124 adults/group led to similar results; however, the association between GSP and incident typical knee osteoarthritis was attenuated (lnGSP < 5.7: OR = 0.56, 95% CI = 0.28 to 1.13, p = 0.11; lnGSP > 5.7: OR = 2.52, 95% CI = 1.22 to 5.21, p = 0.01).
DISCUSSION
By dividing cases of incident knee osteoarthritis into those with typical or accelerated knee osteoarthritis we found that glucose homeostasis, based on GSP concentrations, may predict individuals at risk for a typical onset of knee osteoarthritis but not accelerated knee osteoarthritis. Specifically, we found a U-shaped relationship between incident typical knee osteoarthritis and GSP. This may indicate that there are two clinically relevant subsets at risk for typical knee osteoarthritis – those with low GSP concentrations and others with high GSP concentrations. Furthermore, our findings support the notion that accelerated knee osteoarthritis may be a distinct disorder from typical knee osteoarthritis.
Our findings highlight the complex relationship between glucose homeostasis and incident knee osteoarthritis. Within a higher range of GSP, our results support prior findings that hemoglobin A1c (HbA1c) was greater in women who developed incident knee osteoarthritis than women who did not (12). Interestingly, we found that people with very low levels of GSP were also more likely to develop incident typical knee osteoarthritis. It is imperative that future studies account for a possible U-shaped relationship between incident typical knee osteoarthritis and measures of glucose homeostasis. Furthermore, measures of glucose homeostasis that reflect stability over the prior weeks (e.g., GSP, HbA1c) may be more informative than fasting-glucose concentrations, which may not predict incident knee osteoarthritis (13).
We failed to confirm our hypothesis that systemic inflammation relates with incident knee osteoarthritis (typical or accelerated). Most of the prior research examining the association between CRP and osteoarthritis have been cross-sectional or case-control designs (8). Authors of a recent systematic review concluded that systemic inflammation may be more strongly related to osteoarthritis-related symptoms than radiographic osteoarthritis (8). However, only a few studies have examined the association between CRP and incident knee osteoarthritis. Among 40 individuals with and without knee osteoarthritis, CRP was associated with radiographic progression over 8 years but not over 5 years (20). Furthermore, in the Rotterdam study CRP was predictive of incident knee osteoarthritis (9). Investigators using the Rotterdam study (9) may have detected an association because it is a population-based sample. In contrast, men over 129.3 kg and women over 113.4 kg were excluded because the OAI required everyone to undergo magnetic resonance imaging. Furthermore, adults in the OAI without knee osteoarthritis at baseline were selected because they had other risk factors for knee osteoarthritis. This may limit the generalizability of the OAI; however, the OAI is advantageous because it represents the ideal population to screen for risk of knee osteoarthritis. Furthermore, the extensive data and imaging of the OAI enabled us to characterize people with accelerated knee osteoarthritis.
We found that accelerated knee osteoarthritis was not associated with any of the biochemical measures. Hence, these biomarkers may be less than ideal prognostic indicators of who will develop accelerated knee osteoarthritis. Future research needs to explore if changes in inflammation or glucose homeostasis relate with the development of accelerated knee osteoarthritis and represent biomarkers of disease processes.
These findings may also highlight that accelerated knee osteoarthritis may be a distinct pathology from typical knee osteoarthritis. Glucose homeostasis may play a greater role in the etiology of typical knee osteoarthritis than accelerated knee osteoarthritis. In contrast, biomechanical factors may play a greater role in the etiology of accelerated knee osteoarthritis. For example, we previously demonstrated that individuals with a greater coronal tibial slope and static malalignment or a knee injury, especially if the injury compromises the subchondral bone or menisci, are at greater risk for accelerated knee osteoarthritis (15; 21).
The OAI offers a unique opportunity to characterize people with accelerated knee osteoarthritis and explore potential risk factors; however, there are some important limitations. Firstly, we could not measure Hba1c because fresh whole blood was not collected in the OAI. Despite this limitation, GSP is an indicator of non-enzymatic glycation and measures fructosamine levels in serum protein, which provides a stable indicator of glucose homeostasis over the prior 2 to 3 week. Secondly, we had a limited sample (n = 54/group in primary and n = 124/group in secondary analyses) but we were able to decrease the heterogeneity among the cases by identifying well-characterized subsets of incident knee osteoarthritis. The sample size may limit our ability to explore interactions and this should be followed up in future studies. The sample size also limited our ability to explore other potential covariates. However, we focused on key covariates (i.e., age and body mass index) and omitted potential baseline factor that we previously found were not associated with incident accelerated knee osteoarthritis in this cohort (e.g., comorbidities, medications, physical activity) (17). Finally, the sample size of people with accelerated knee osteoarthritis limits our ability to perform secondary analyses to explore subsets of accelerated knee osteoarthritis. We previously demonstrated that our definition of accelerated knee osteoarthritis may be ideal because it represents a comprehensive definition that requires the development of osteophytes and joint space narrowing (18); however, future studies may consider more stringent definitions.
In conclusion, glucose homeostasis may predict individuals at risk for incident typical knee osteoarthritis but not accelerated knee osteoarthritis. Furthermore, we detected a nonlinear relationship, where individuals with very low or very high levels of GSP were more likely to develop typical knee osteoarthritis. Within the context of our prior work, we found that impaired glucose homeostasis is associated with typical knee osteoarthritis while biomechanical factors (e.g., coronal tibial slope and femorotibial alignment angle)(21) and certain injuries may relate with incident accelerated knee osteoarthritis(15). This study offers further evidence that accelerated knee osteoarthritis may be a distinct disorder from typical knee osteoarthritis.
ACKNOWLEDGEMENTS
This work was supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (Grant Number R01-AR065977). The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258, N01-AR-2-2259, N01-AR-2-2260, N01-AR-2-2261, N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use data set and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners. This work was also supported in part by the Houston Veterans Affairs Health Services Research and Development Center of Excellence (HFP90-020). The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Footnotes
Author Contributions Statement: All authors drafted the paper or revised it critically as well as read and approved the final submitted version. Driban, Price, Lu, Lo, and McAlindon also significantly contributed to the research design as well as analysis and interpretation of data. Eaton, Amin, and Barbe also significantly contributed to the research design as well as acquisition, analysis, and interpretation of data. Stout also significantly contributed interpretation of data.
REFERENCES
- 1.Wu T-L, Tsao K-C, Chang CPY, et al. Development of ELISA on microplate for serum C-reactive protein and establishment of age-dependent normal reference range. Clin. Chim Acta. 2002;322:163–168. doi: 10.1016/s0009-8981(02)00172-9. [DOI] [PubMed] [Google Scholar]
- 2.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 3.Xin DL, Harris MY, Wade CK, et al. Aging enhances serum cytokine response but not task-induced grip strength declines in a rat model of work-related musculoskeletal disorders. BMC Musculoskelet Disord. 2011;12:63. doi: 10.1186/1471-2474-12-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Visser M, Bouter LM, McQuillan GM, et al. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 5.Mohamed-Ali V, Goodrick S, Rawesh A, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 6.Yap SH, Moshage HJ, Hazenberg BPC, et al. Tumor necrosis factor (TNF) inhibits interleukin (IL)-1 and/or IL-6 stimulated synthesis of C-reactive protein (CRP) and serum amyloid A (SAA) in primary cultures of human hepatocytes. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1991;1091:405–408. doi: 10.1016/0167-4889(91)90207-e. [DOI] [PubMed] [Google Scholar]
- 7.Anty R, Bekri S, Luciani N, et al. The Inflammatory C-Reactive Protein is Increased in Both Liver and Adipose Tissue in Severely Obese Patients Independently from Metabolic Syndrome, Type 2 Diabetes, and NASH. Am J Gastroenterol. 2006;101:1824–1833. doi: 10.1111/j.1572-0241.2006.00724.x. [DOI] [PubMed] [Google Scholar]
- 8.Jin X, Beguerie JR, Zhang W, et al. Circulating C reactive protein in osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74:703–710. doi: 10.1136/annrheumdis-2013-204494. [DOI] [PubMed] [Google Scholar]
- 9.Saberi Hosnijeh F, Siebuhr AS, Uitterlinden AG, et al. Association between biomarkers of tissue inflammation and progression of osteoarthritis: evidence from the Rotterdam study cohort. Arthritis Res Ther. 2016;18:1–10. doi: 10.1186/s13075-016-0976-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearle AD, Scanzello CR, George S, et al. Elevated high-sensitivity C-reactive protein levels are associated with local inflammatory findings in patients with osteoarthritis. Osteoarthritis Cartilage. 2007;15:516–523. doi: 10.1016/j.joca.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Rosa SC, Gonçalves J, Judas F, et al. Impaired glucose transporter-1 degradation and increased glucose transport and oxidative stress in response to high glucose in chondrocytes from osteoarthritic versus normal human cartilage. Arthritis Res Ther. 2009;11:R80. doi: 10.1186/ar2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshimura N, Muraki S, Oka H, et al. Accumulation of metabolic risk factors such as overweight, hypertension, dyslipidaemia, and impaired glucose tolerance raises the risk of occurrence and progression of knee osteoarthritis: a 3-year follow-up of the ROAD study. Osteoarthritis Cartilage. 2012;20:1217–1226. doi: 10.1016/j.joca.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Engstrom G, Gerhardsson de Verdier M, Rollof J, et al. C-reactive protein, metabolic syndrome and incidence of severe hip and knee osteoarthritis. A population-based cohort study. Osteoarthritis Cartilage. 2009;17:168–173. doi: 10.1016/j.joca.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Driban JB, Eaton CB, Lo GH, et al. Overweight older adults, particularly after an injury, are at high risk for accelerated knee osteoarthritis: data from the Osteoarthritis Initiative. Clin Rheumatol. 2016;35:1071–1076. doi: 10.1007/s10067-015-3152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Driban JB, Ward RJ, Eaton CB, et al. Meniscal extrusion or subchondral damage characterize incident accelerated osteoarthritis: Data from the Osteoarthritis Initiative. Clin Anat. 2015;28:792–799. doi: 10.1002/ca.22590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Driban JB, Price LL, Eaton CB, et al. Individuals with incident accelerated knee osteoarthritis have greater pain than those with common knee osteoarthritis progression: data from the Osteoarthritis Initiative. Clin Rheumatol. 2015;35:1565–1571. doi: 10.1007/s10067-015-3128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Driban JB, Eaton CB, Lo GH, et al. Association of knee injuries with accelerated knee osteoarthritis progression: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2014;66:1673–1679. doi: 10.1002/acr.22359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Driban JB, Stout AC, Lo GH, et al. Best performing definition of accelerated knee osteoarthritis: Data from the Osteoarthritis Initiative. Ther Adv Musculoskelet Dis. 2016;8:165–171. doi: 10.1177/1759720X16658032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. [December 23, 2016];The Osteoarthritis Initiative. http://oai.epi-ucsf.org/.
- 20.Sharif M, Shepstone L, Elson CJ, et al. Increased serum C reactive protein may reflect events that precede radiographic progression in osteoarthritis of the knee. Ann Rheum Dis. 2000;59:71–74. doi: 10.1136/ard.59.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Driban JB, Stout AC, Duryea J, et al. Coronal tibial slope is association with accelerated knee osteoarthritis: Data from the Osteoarthritis Initiative. BMC Musculoskelet Disord. 2016;17:299. doi: 10.1186/s12891-016-1158-9. [DOI] [PMC free article] [PubMed] [Google Scholar]