Abstract
Objective
To evaluate the relationship between chondrocalcinosis and pain or synovitis in knee joints by examining data from the Osteoarthritis Initiative (OAI).
Methods
Data were obtained from the OAI public use data sets. The relationship between chondrocalcinosis on baseline knee radiograph and pain at baseline and at 4 years was examined. Analyses were adjusted for age, gender, body mass index, and Kellgren-Lawrence (KL) grade and the correlation between two knees in a subject was controlled using generalized estimating equations. The relationship between chondrocalcinosis and synovitis on MRI was examined by comparing knees with chondrocalcinosis at baseline and age, gender, KL grade-matched knees with no chondrocalcinosis. We read MRIs of a subset of knees for synovitis using MRI Osteoarthritis Knee Score (MOAKS) on baseline and 4-year MRI.
Results
Knees with chondrocalcinosis (n=162) more often had pain compared to knees without chondrocalcinosis (n=2030) at baseline and had higher Western Ontario and McMaster Universities Arthritis Index (WOMAC) pain scores both at baseline (2.4 (95% CI 1.9,2.9) vs 1.8 (1.7,1.9)) and at 4 years (2.5 (1.9,3.1) vs 1.6 (1.5,1.8)) as well as higher Intermittent and Constant Osteoarthritis Pain (ICOAP) intermittent pain scores at 4 years. There was no difference in MOAKS synovitis scores at baseline and at 4 years between the chondrocalcinosis group (n=102) and the control group (n=99).
Conclusion
Knees with chondrocalcinosis had increased pain and did not have higher synovitis scores on MRI compared to knees without chondrocalcinosis. The mechanisms by which chondrocalcinosis is associated with increased pain remain to be determined.
Calcium-containing crystals, including calcium pyrophosphate dihydrate (CPP) crystals and basic calcium phosphate (BCP) crystals, are commonly deposited in hyaline cartilage and meniscal fibrocartilage in joints and are detected radiographically as linear calcifications, termed chondrocalcinosis. Chondrocalcinosis is associated with osteoarthritis (OA) (1) and several studies have found an association between the presence of CPP crystals in synovial fluid and radiographic severity of OA (2, 3). However, others have suggested that chondrocalcinosis is not associated with joint space width on knee radiographs (4). Furthermore, chondrocalcinosis was not associated with increased cartilage loss or OA progression in a prospective, longitudinal magnetic resonance imaging (MRI) study (5).
CPP crystals are known to induce inflammatory reactions and synovitis and can cause acute or chronic crystal-induced inflammatory arthritis (6). CPP crystals induce the production of proinflammatory and catabolic mediators including nitric oxide (NO), matrix metalloproteinase-13 (MMP-13) and prostaglandin E2 (PGE2) in human chondrocytes and synoviocytes in vitro (7). Injections of CPP crystals into meniscectomized rabbit knee results in worsening of OA (8). Additionally, BCP crystals induce interleukin-1β (IL-1β) secretion from macrophages through the NLRP3 inflammasome in vitro (9) and intra-articular injection of BCP crystals elicits synovial inflammation and cartilage degradation in mice (10). However, the significance of these crystals in human joints is not completely understood. Particularly, it is unknown whether calcium-containing crystals seen as chondrocalcinosis induce synovitis or contribute to pathogenesis of OA.
Synovial volume as measured in non-contrast enhanced MRI has been found to change as knee pain severity changes (11) and has been found to predict both cartilage loss (12) and the development of OA (13). This evidence suggests that it is a validated marker for the amount of synovitis in the knee.
In this study, we evaluated whether chondrocalcinosis was associated with knee pain or synovitis measured on non-contrast enhanced MRI by examining data from the Osteoarthritis Initiative (OAI).
Patients and Methods
Study design and subjects
OAI is an NIH-initiated multi-center, prospective, observational study of knee OA which establishes and maintains a database for OA that includes clinical evaluation data, radiological images, and a biospecimen repository from 4796 participants. We obtained data from the OAI public use data sets. The study rationale and general inclusion criteria for the OAI (e.g., men and women ages 45–79 with symptoms of and/or knee radiographic OA or risk factors for developing knee OA) have been described previously and are publicly available (http://oai.epi-ucsf.org/datarelease/). All subjects were examined between 2004 and 2010. For the purpose of this study, we included subjects who were 60 years and older and knees with Kellgren-Lawrence (KL) grade 0, 1, or 2 on baseline radiographs (14).
We examined the relationship between chondrocalcinosis at baseline and knee pain at baseline and at the 4 year follow-up. In OAI, the presence of knee pain was assessed by using three measures (knee pain more than half the days of a month in the past 12 months, any knee pain in the past 30 days, and knee pain more than half the days in the past 30 days). The severity of knee pain was assessed by visual analog scale (VAS), Western Ontario and McMaster Universities Arthritis Index (WOMAC) (15), and Intermittent and Constant Osteoarthritis Pain (ICOAP) scores (16). ICOAP is a questionnaire designed to assess intermittent and constant pain for the past week with the scores ranging from 0 to 100.
Next, we examined the relationship between chondrocalcinosis and synovitis on MRI by comparing knees with chondrocalcinosis at baseline and age, gender, and KL grade-matched knees with no chondrocalcinosis. We selected controls by randomly choosing subjects who met the criteria in the list. Subjects who did not have MRI at the 4 year time point were excluded from the MRI analyses.
Given that reading MRIs involves time and effort, we tried to select knees that would be most informative in addressing the questions of interest. All the knees with KL grade 1 or 2 that had chondrocalcinosis and met the inclusion criteria were read for synovitis on MRI. Among knees with KL grade 0, there were very few cases of chondrocalcinosis and the first 13 knees with chondrocalcinosis that were randomly selected were read for synovitis on MRI. If the subject had chondrocalcinosis and the same KL grade in bilateral knees, we read one knee (left knee) per person in large part because we have found much symmetry in MRI findings when knees appear identical on radiographs and it would not be efficient to read both knees in this circumstance. If the subject had chondrocalcinosis and the knees differed in KL grade, we read both knees.
Knee radiography
Flexed, weight-bearing posteroanterior (PA) radiographs of knee were obtained for all subjects in OAI. Each knee was graded for OA on the KL scale and for the presence of chondrocalcinosis at baseline or during 48-month follow-up. Chondrocalcinosis was defined as being present if there was definite linear cartilage calcification on the PA view in a compartment-specific manner (5). Radiographs of knees with KL grade 2 were read for chondrocalcinosis by central readers at Boston University. Radiographs with KL grade 0 or 1 in bilateral knees were read for chondrocalcinosis at the Cooper Medical Center. There was 100% agreement between the readers in the diagnosis of chondrocalcinosis in a sample of 25 films (with and without chondrocalcinosis) shared between the readers at the two sites.
Knee MRI grading of synovitis
MRI of the knee was obtained with a 3 Tesla scanner from subjects in OAI (17). MRI scans were graded for synovitis by one experienced musculoskeletal radiologist, blinded to clinical data, using a validated method, MRI Osteoarthritis Knee Score (MOAKS) (18). MOAKS synovitis score includes Hoffa synovitis and effusion-synovitis scores. Hoffa synovitis consists of signal changes in the Hoffa fat pad representing chronic synovitis and scored from 0 to 3 (19). These hyperintense signal changes are found within the fat pad on T2-weighted or PD-weighted fat saturated images of the mid-slices of sagittal plane (11). Effusion-synovitis scores are based on the maximal distention (mm) of intra-articular hyperintensity on T2-weighted or PD-weighted images of axial planes and represent a composite of effusion and synovial hypertrophy (20). In this study, the intra-rater intraclass correlation coefficients were 0.804 for Hoffa synovitis and 0.852 for effusion-synovitis.
Statistical Analysis
We used logistic and linear regression to assess whether baseline chondrocalcinosis was associated with the presence of knee pain at baseline and at 4 years. Linear regression was performed to compare pain severity, WOMAC scores and ICOAP scores at baseline and at 4 years, and the change between knees with and without chondrocalcinosis at baseline. Age, gender, BMI, and KL grade were adjusted and the correlation between two knees in a subject was controlled using generalized estimating equations. For the matched control study of synovitis on MRI, analysis of variance for repeated measures on normalized ranks was used for pair-wise comparisons. Data was transformed to normalized ranks prior to analysis to account for non-normal data. Results were considered to be significant for two tailed comparison at p < 0.05.
Results
Comparison of knee pain in knees with and without chondrocalcinosis
We compared pain between knees with chondrocalcinosis (n=162) and knees with no chondrocalcinosis (n=2030) on baseline radiographs. Baseline characteristics are described in Table 1. Compared with knees that did not have chondrocalcinosis, those with chondrocalcinosis had a significantly higher prevalence of frequent pain at baseline assessed by all three pain questions (knee pain more than half the days of a month in the past 12 months, any knee pain in the past 30 days, and knee pain more than half the days in the past 30 days) after adjustment for age, gender, BMI, and KL grade. At 4 years, only any knee pain in the past 30 days was more prevalent in the chondrocalcinosis group (Table 2).
Table 1.
Baseline characteristics
| Baseline characteristics | Subjects eligible at baseline (n=1380) |
Subjects with MRI reading (n=201) |
|||
|---|---|---|---|---|---|
| CC+ | CC− | CC+ | CC− | ||
| Subject level, n | 114 | 1266 | 84 | 82 | |
| Age, mean (SD), year | 70.1 (5.4) | 68.3 (5.3) | 68.8 (5.4) | 69.0 (5.2) | |
| BMI, mean (SD), kg/m2 | 28.0 (3.9) | 28.9 (4.5) | |||
| Female, N(%) | 65 (57.0) | 803 (63.4) | 47 (56.0) | 46 (56.1) | |
| Knee level, n | 162 | 2030 | 102 | 99 | |
| KL grade, N(%) | 0 | 26 (16.1) | 379 (18.7) | 13 (12.7) | 12 (12.1) |
| 1 | 29 (17.9) | 438 (21.6) | 28 (27.5) | 28 (28.3) | |
| 2 | 107 (66.1) | 1213 (59.8) | 61 (59.8) | 59 (59.6) | |
Table 2.
Baseline chondrocalcinosis and knee pain (yes vs no) at year 0 and year 4
| CC at baseline | knee pain n(%) | Crude model | Adjusted model* | ||
|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| knee pain more than half the days of a month, past 12 month | |||||
| at year 0 | |||||
| CC− (n=2020) | 584 (28.9) | 1 | 1 | ||
| CC+ (n=161) | 60 (37.3) | 1.4 (1.0,2.0) | 0.08 | 1.5 (1.0,2.1) | 0.05 |
| at year 4 | |||||
| CC− (n=1894) | 546 (28.8) | 1 | 1 | ||
| CC+ (n=151) | 48 (31.8) | 1.2 (0.8,1.8) | 0.29 | 1.3 (0.9,1.9) | 0.21 |
|
| |||||
| any knee pain, past 30 days | |||||
| at year 0 | |||||
| CC− (n=2027) | 1156 (57.0) | 1 | 1 | ||
| CC+ (n=162) | 106 (65.4) | 1.4 (1.0,2.0) | 0.07 | 1.5 (1.0,2.1) | 0.04 |
| at year 4 | |||||
| CC− (n=1895) | 1069 (56.4) | 1 | 1 | ||
| CC+ (n=151) | 95 (62.9) | 1.3 (0.9,1.9) | 0.13 | 1.4 (1.0,2.1) | 0.07 |
|
| |||||
| knee pain more than half the days, past 30 days | |||||
| at year 0 | |||||
| CC− (n=2024) | 534 (26.4) | 1 | 1 | ||
| CC+ (n=162) | 61 (37.7) | 1.6 (1.1,2.3) | <0.01 | 1.7 (1.2,2.4) | <0.01 |
| at year 4 | |||||
| CC− (n=1889) | 477 (25.3) | 1 | 1 | ||
| CC+ (n=151) | 45 (29.8) | 1.3 (0.9,1.9) | 0.19 | 1.3 (0.9,2.0) | 0.15 |
Adjusting for age, gender, BMI, and KL grade at baseline
The knees with chondrocalcinosis at baseline had more severe knee pain assessed by VAS in the past 30 days at baseline compared to the knees with no chondrocalcinosis. At baseline and at 4 years, WOMAC knee pain subscale scores were higher in the knees with chondrocalcinosis. There was no difference in change of VAS or WOMAC scores over 4 years in the two groups (table 3). In addition, ICOAP intermittent pain was significantly higher in knees with baseline chondrocalcinosis at 4 years. ICOAP constant knee pain was not different between the two groups at any time point (Table 4).
Table 3.
Baseline chondrocalcinosis and knee pain (continuous measurement) at year 0 and year 4, and change of knee pain
| CC at baseline | Crude model | Adjusted model* | ||
|---|---|---|---|---|
| mean (95% CI) | p-value | adjusted mean (95% CI) | p-value | |
| knee pain severity, past 30 days (0–10) | ||||
| at year 0 | ||||
| CC− (n=2026) | 2.3 (2.2,2.4) | 0.04 | 2.0 (1.9,2.2) | 0.02 |
| CC+ (n=162) | 2.8 (2.3,3.3) | 2.6 (2.2,3.1) | ||
| at year 4 | ||||
| CC− (n=1895) | 2.2 (2.1,2.4) | 0.19 | 2.0 (1.8,2.1) | 0.11 |
| CC+ (n=151) | 2.6 (2.1,3.1) | 2.4 (1.9,2.9) | ||
| change from year 0 to year 4 | ||||
| CC− (n=1891) | 0.0 (−0.2,0.1) | 0.47 | −0.1 (−0.2,0.1) | 0.50 |
| CC+ (n=151) | −0.3 (−0.8,0.3) | −0.3 (−0.9,0.3) | ||
|
| ||||
| WOMAC knee pain subscale (0–20) | ||||
| at year 0 | ||||
| CC− (n=2030) | 2.1 (1.9,2.2) | 0.08 | 1.8 (1.7,1.9) | 0.03 |
| CC+ (n=162) | 2.6 (2.0,3.1) | 2.4 (1.9,2.9) | ||
| at year 4 | ||||
| CC− (n=1898) | 1.8 (1.7,2.0) | 0.01 | 1.6 (1.5,1.8) | <0.01 |
| CC+ (n=151) | 2.6 (2.0,3.2) | 2.5 (1.9,3.1) | ||
| change from year 0 to year 4 | ||||
| CC− (n=1898) | −0.2 (−0.3,0.0) | 0.63 | −0.1 (−0.3,0.0) | 0.68 |
| CC+ (n=151) | 0.0 (−0.7,0.6) | 0.0 (−0.6,0.6) | ||
Adjusting for age, gender, BMI, and KL grade at baseline
Table 4.
Baseline chondrocalcinosis and intermittent, constant knee pain (continuous measurements) at year 4
| CC at baseline | Crude model | Adjusted model* | ||
|---|---|---|---|---|
| mean (95% CI) | p-value | adjusted mean (95% CI) | p-value | |
| ICOAP Knee Intermittent Pain Score at Year 4 (0–100) | ||||
| CC− (n=1760) | 9.2 (8.3,10.0) | 0.05 | 8.2 (7.4,9.1) | 0.02 |
| CC+ (n=138) | 12.0 (9.2,14.9) | 11.7 (8.7,14.6) | ||
|
| ||||
| ICOAP Knee Constant Pain Score at Year 4 (0–100) | ||||
| CC− (n=1760) | 1.9 (1.4,2.4) | 0.20 | 1.9 (1.4,2.4) | 0.12 |
| CC+ (n=138) | 3.2 (1.2,5.2) | 3.5 (1.4,5.5) | ||
|
| ||||
| ICOAP Knee Intermittent and Constant Pain Total Score at Year 4 (0–100) | ||||
|
| ||||
| CC− (n=1759) | 5.8 (5.3,6.4) | 0.03 | 5.3 (4.8,5.9) | 0.01 |
| CC+ (n=138) | 8.1 (6.1,10.1) | 8.0 (6.0,10.1) | ||
Adjusting for age, gender, BMI, and KL grade at baseline
Comparison of synovitis on knee MRI
We assessed MRIs of 201 knees (102 chondrocalcinosis, 99 controls) at baseline and at 4 years. Baseline characteristics are described in Table 1.
There was no difference in Hoffa synovitis score and effusion-synovitis score at baseline and at 4 years between the chondrocalcinosis group and the control group (Table 5). We also compared subjects within each KL grade and found there was no association with synovitis.
Table 5.
Baseline chondrocalcinosis and synovitis at year 0 and year 4
| CC at baseline | Hoffa synovitis (0–3) | Effusion-synovitis (mm) | ||
|---|---|---|---|---|
| mean (95% CI) | p-value | mean (95% CI) | p-value | |
| All KL grades | ||||
| at year 0 | ||||
| CC− (n=99) | 0.7 (0.6, 0.8) | 0.30 | 3.4 (2.9, 3.9) | 0.67 |
| CC+ (n=102) | 0.8 (0.7, 0.9) | 3.7 (3.2, 4.3) | ||
| at year 4 | ||||
| CC− (n=99) | 0.9 (0.8, 1.0) | 0.37 | 3.7 (3.2, 4.2) | 0.94 |
| CC+ (n=102) | 0.9 (0.8, 1.0) | 3.6 (3.1, 4.1) | ||
|
| ||||
| KL grade 0 | ||||
| at year 0 | ||||
| CC− (n=12) | 0.4 (0.1, 0.7) | 0.22 | 3.1 (1.3, 4.8) | 0.44 |
| CC+ (n=13) | 0.6 (0.2, 1.0) | 2.9 (1.6, 4.2) | ||
| at year 4 | ||||
| CC− (n=12) | 0.7 (0.3, 1.1) | 0.49 | 2.5 (1.3, 3.5) | 0.97 |
| CC+ (n=13) | 0.8 (0.4, 1.1) | 3.0 (1.8, 4.3) | ||
|
| ||||
| KL grade 1 | ||||
| at year 0 | ||||
| CC− (n=28) | 0.8 (0.5, 1.0) | 0.63 | 3.1 (2.3, 3.9) | 0.12 |
| CC+ (n=28) | 0.8 (0.6, 1.0) | 3.5 (2.5, 4.4) | ||
| at year 4 | ||||
| CC− (n=28) | 0.9 (0.6, 1.1) | 0.31 | 3.5 (2.7, 4.4) | 0.51 |
| CC+ (n=28) | 1.0 (0.8, 1.1) | 3.3 (2.4, 4.2) | ||
|
| ||||
| KL grade 2 | ||||
| at year 0 | ||||
| CC− (n=59) | 0.8 (0.6, 0.9) | 0.84 | 3.6 (3.0, 4.3) | 0.58 |
| CC+ (n=61) | 0.8 (0.6, 0.9) | 4.1 (3.4, 4.7) | ||
| at year 4 | ||||
| CC− (n=59) | 0.9 (0.7, 1.1) | 0.99 | 4.0 (3.4, 4.6) | 0.52 |
| CC+ (n=61) | 0.9 (0.8, 1.1) | 3.9 (3.2, 4.5) | ||
We examined whether there were differences in synovitis scores between baseline and year 4 and found there were no differences (data not shown). There was a weak but statistically significant correlation between Hoffa synovitis and effusion synovitis (r=0.23, p < 0.01).
Discussion
In these analyses, we demonstrated that chondrocalcinosis in knee joints was modestly associated with increased knee pain and was not associated with synovitis on knee MRI. After the results were adjusted for possible confounders including age, gender, BMI and KL grade, knees with chondrocalcinosis had increased pain by several different measures, including VAS, WOMAC and ICOAP for various time periods. Although there was no single primary outcome, several validated measures were used to assess pain. Knee OA pain can fluctuate over time and we showed consistently increased pain in knees with chondrocalcinosis. Our findings were consistent with a previous report which showed chondrocalcinosis is independently associated with pain and disability in knee OA patients (21)
In view of the association of CPP and BCP crystals with inflammation, we hypothesized that the calcium-containing crystals in chondrocalcinosis contribute to pain by inducing synovitis. However, our results do not support this hypothesis. In a subset of 201 knees assessed in this study, we observed that knees with chondrocalcinosis did not have increased MOAKS synovitis scores on MRI compared to knees without chondrocalcinosis. These synovitis scores include Hoffa synovitis and effusion synovitis scores and there were no differences in either score between the two groups at year 0 and year 4.
Synovial volume on MRI correlates with pain severity in OA (11, 22). Yet, recent work has suggested that fluctuating inflammatory changes in synovium, such as those that may be induced by intermittent release of crystals in the joint space, are likely to be related to synovial perfusion and vascularity which may be better assessed with dynamic rather than static imaging of synovial volume done either with or without contrast (23). Further, while synovitis is likely to be an important cause of pain, certain molecular mediators of the pain signal such as C-C motif chemokine 2(CCL2) (24) or brain-derived neurotropic factor(BDNF) (25), may contribute to pain independently of synovitis.
It has been reported that chondrocalcinosis is not associated with increased histological synovitis or angiogenesis in synovium specimens obtained from patients undergoing total knee joint replacement for OA and our findings support these results (26). One possible explanation is that calcium-containing crystals identified as chondrocalcinosis on radiographs may represent crystals embedded within cartilage (27) and these crystals are normally protected from interacting with synovial macrophages (28). CPP crystal shedding may occur only under certain uncommon circumstances including a rapid decrease of serum calcium levels (29) and this intermittent release may trigger fluctuations of synovial perfusion which would not be detected by a measure of synovial volume (23).
Additionally, it has been observed that calcium-containing crystal formation is regulated by extracellular matrix changes (30). We speculate that calcium-containing crystal deposition is a marker of a knee osteoarthritis phenotype with degenerative changes in extracellular matrix and increased pain, rather than a contributor to synovitis. Histologic studies have shown that calcification of cartilage correlates with the expression of type X collagen, a marker of chondrocyte hypertrophy (31). Furthermore, type II collagen suppresses the ability of ATP to stimulate calcification, while a combination of type II and type I collagen increase the effect of ATP and beta-glycerophosphate on calcification in vitro. Proteoglycans suppress calcium-containing crystal formation when added to either type I or type II collagen (32).
We selected subjects who were 60 years and older, and knees with KL grade 0, 1, or 2. Patients rarely develop chondrocalcinosis at a younger age, and it was not efficient to examine those younger than 60 years. We excluded knees with KL grade 3 or 4 because cartilage and meniscus are often eroded in knees with advanced OA and, as a consequence, chondrocalcinosis which is present in these tissues may be no longer visible. Further, subjects with KL grade 3 or 4 have more background pain and synovitis from their OA and it might be difficult to tease out the impact of chondrocalcinosis in this population.
Our study has several limitations. We assessed synovitis on non-contrast enhanced MRIs in the OAI database which may not be as accurate for evaluating synovitis as contrast-enhanced MRI (33), but as noted above, images without contrast have been successful in demonstrating the relation of synovitis with pain fluctuation and with cartilage loss (11, 12, 13). The OAI is a large cohort of subjects in the community and it would have been difficult to justify obtaining contrast-enhanced MRI for this study due to safety concerns related to the use of contrast. Secondly, variability in pain and synovitis of knee OA in a given subject over time could have potentially affected our results. However, these analyses showed consistent findings of pain and synovitis confirmed by using various measures at year 0 and at year 4.
In summary, knees with chondrocalcinosis had increased knee pain but did not have higher synovitis scores on MRI compared to knees without chondrocalcinosis. These results suggest that calcium-containing crystals seen as chondrocalcinosis in knee joints do not appear to induce synovitis under usual circumstances. The mechanisms by which chondrocalcinosis is associated with increased pain remain to be determined.
Figure 1.
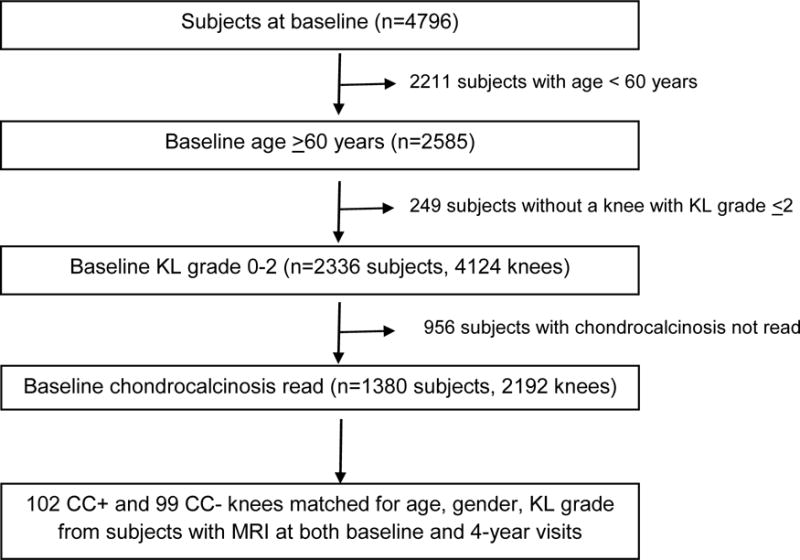
Flowchart
Significance and innovation.
This study examined the association of chondrocalcinosis in knee joints with pain and synovitis using the Osteoarthritis Initiative (OAI) database.
Chondrocalcinosis was significantly associated with knee pain assessed by various questionnaires including Western Ontario and McMaster Universities Arthritis Index (WOMAC) pain scores and Intermittent and Constant Osteoarthritis Pain (ICOAP) intermittent pain scores.
Chondrocalcinosis was not associated with either Hoffa synovitis or effusion-synovitis measured on non-contrast enhanced magnetic resonance imaging (MRI).
Acknowledgments
Grants: The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use data set and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners. Drs. Felson and Niu are supported by NIH AR47785.
Footnotes
Conflict of interests: Nothing to disclose
Contributions: BKH and DF contributed to the conception and design of the study. BKH, WK, SB and VB contributed to acquisition of the data. BKH, JN, JG, CW, SK and DF contributed to analysis and interpretation of the data. All authors contributed to drafting or revising the article and approved the final version to be submitted. BKH takes responsibility for the integrity of the work as a whole, from inception to finished article.
Contributor Information
Bobby Kwanghoon Han, Division of Rheumatology, University of Washington School of Medicine. Seattle, WA. hank4@uw.edu.
Woojin Kim, Division of Musculoskeletal Imaging, Department of Radiology, Perelman School of Medicine at the University of Pennsylvania. Philadelphia, PA. woojin.kim@uphs.upenn.edu.
Jingbo Niu, Clinical Epidemiology Research and Training Unit, Boston University School of Medicine, Boston, MA. niujp@bu.edu.
Shristi Basnyat, Division of Rheumatology, Cooper Medical School of Rowan University. Voorhees, NJ. basnyat-shristi@cooperhealth.edu.
Veniamin Barshay, Department of Radiology, Cooper Medical School of Rowan University. Camden, NJ. barshay-veniamin@cooperhealth.edu.
John P Gaughan, Cooper Research Institute, Cooper Medical School of Rowan University, Camden, NJ. gaughan-john@cooperhealth.edu.
Charlene Williams, Department of Biomedical Sciences, Cooper Medical School of Rowan University. Camden, NJ. williamsch@rowan.edu.
Sharon L Kolasinski, Division of Rheumatology, Perelman School of Medicine, University of Pennsylvania. Philadelphia, PA. sharon.kolasinski@uphs.upenn.edu.
David T Felson, Clinical Epidemiology Research and Training Unit, Boston University School of Medicine, Boston, MA. dfelson@bu.edu.
References
- 1.Felson DT, Anderson JJ, Naimark A, Kannel W, Meenan RF. The prevalence of chondrocalcinosis in the elderly and its association with knee osteoarthritis: the Framingham Study. J Rheumatol. 1989;16:1241–5. [PubMed] [Google Scholar]
- 2.Pattrick M, Hamilton E, Wilson R, Austin S, Doherty M. Association of radiographic changes of osteoarthritis, symptoms, and synovial fluid particles in 300 knees. Ann Rheum Dis. 1993;52:97–103. doi: 10.1136/ard.52.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nalbant S, Martinez JA, Kitumnuaypong T, Clayburne G, Sieck M, Schumacher HR., Jr Synovial fluid features and their relations to osteoarthritis severity: new findings from sequential studies. Osteoarthritis Cartilage. 2003;11:50–4. doi: 10.1053/joca.2002.0861. [DOI] [PubMed] [Google Scholar]
- 4.Neame RL, Carr AJ, Muir K, Doherty M. UK community prevalence of knee chondrocalcinosis: evidence that correlation with osteoarthritis is through a shared association with osteophyte. Ann Rheum Dis. 2003;62:513–8. doi: 10.1136/ard.62.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neogi T, Nevitt M, Niu J, LaValley MP, Hunter DJ, Terkeltaub R, et al. Lack of association between chondrocalcinosis and increased risk of cartilage loss in knees with osteoarthritis: results of two prospective longitudinal magnetic resonance imaging studies. Arthritis Rheum. 2006;54:1822–8. doi: 10.1002/art.21903. [DOI] [PubMed] [Google Scholar]
- 6.Zhang W, Doherty M, Bardin T, Barskova V, Guerne PA, Jansen TL, et al. European League Against Rheumatism recommendations for calcium pyrophosphate deposition. Part I: terminology and diagnosis. Ann Rheum Dis. 2011;70:563–70. doi: 10.1136/ard.2010.139105. [DOI] [PubMed] [Google Scholar]
- 7.Liu YZ, Jackson AP, Cosgrove SD. Contribution of calcium-containing crystals to cartilage degradation and synovial inflammation in osteoarthritis. Osteoarthritis Cartilage. 2009;17:1333–40. doi: 10.1016/j.joca.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 8.Fam AG, Morava-Protzner I, Purcell C, Young BD, Bunting PS, Lewis AJ. Acceleration of experimental lapine osteoarthritis by calcium pyrophosphate microcrystalline synovitis. Arthritis Rheum. 1995;38:201–10. doi: 10.1002/art.1780380208. [DOI] [PubMed] [Google Scholar]
- 9.Pazár B, Ea HK, Narayan S, Kolly L, Bagnoud N, Chobaz V, et al. Basic calcium phosphate crystals induce monocyte/macrophage IL-1β secretion through the NLRP3 inflammasome in vitro. J Immunol. 2011;186:2495–502. doi: 10.4049/jimmunol.1001284. [DOI] [PubMed] [Google Scholar]
- 10.Ea HK, Chobaz V, Nguyen C, Nasi S, van Lent P, Daudon M, et al. Pathogenic role of basic calcium phosphate crystals in destructive arthropathies. PLoS One. 2013;8:e57352. doi: 10.1371/journal.pone.0057352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill CL, Hunter DJ, Niu J, Clancy M, Guermazi A, Genant H, et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007;66:1599–603. doi: 10.1136/ard.2006.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayral X, Pickering EH, Woodworth TG, Mackillop N, Dougados M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis – results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage. 2005;13:361–7. doi: 10.1016/j.joca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Felson DT, Niu J, Neogi T, Goggins J, Nevitt MC, Roemer F, et al. Synovitis and the risk of knee osteoarthritis: the MOST Study. Osteoarthritis Cartilage. 2016;24:458–64. doi: 10.1016/j.joca.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Annals of the rheumatic diseases. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–40. [PubMed] [Google Scholar]
- 16.Hawker GA, Davis AM, French MR, Cibere J, Jordan JM, March L, et al. Development and preliminary psychometric testing of a new OA pain measure–an OARSI/OMERACT initiative. Osteoarthritis Cartilage. 2008;16:409–14. doi: 10.1016/j.joca.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16:1433–41. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score) Osteoarthritis Cartilage. 2011;19:990–1002. doi: 10.1016/j.joca.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez-Madrid F, Karvonen RL, Teitge RA, Miller PR, An T, Negendank WG. Synovial thickening detected by MR imaging in osteoarthritis of the knee confirmed by biopsy as synovitis. Magn Reson Imaging. 1995;13:177–83. doi: 10.1016/0730-725x(94)00119-n. [DOI] [PubMed] [Google Scholar]
- 20.Roemer FW, Kassim Javaid M, Guermazi A, Thomas M, Kiran A, Keen R, et al. Anatomical distribution of synovitis in knee osteoarthritis and its association with joint effusion assessed on non-enhanced and contrast-enhanced MRI. Osteoarthritis Cartilage. 2010;18:1269–74. doi: 10.1016/j.joca.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Musacchio E, Ramonda R, Perissinotto E, Sartori L, Hirsch R, Punzi L, et al. The impact of knee and hip chondrocalcinosis on disability in older people: the ProVA Study from northeastern Italy. Ann Rheum Dis. 2011;70:1937–43. doi: 10.1136/ard.2011.150508. [DOI] [PubMed] [Google Scholar]
- 22.Baker K, Grainger A, Niu J, Clancy M, Guermazi A, Crema M, et al. Relation of synovitis to knee pain using contrast-enhanced MRIs. Ann Rheum Dis. 2010;69:1779–83. doi: 10.1136/ard.2009.121426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gait AD, Hodgson R, Parkes MJ, Hutchinson CE, O’Neill TW, Maricar N, et al. Synovial volume vs synovial measurements from dynamic contrast enhanced MRI as measures of response in osteoarthritis. Osteoarthritis Cartilage. 2016;24:1392–8. doi: 10.1016/j.joca.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White FA, Sun J, Waters SM, Ma C, Ren D, Ripsch M, et al. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc Natl Acad Sci U S A. 2005;102:14092–7. doi: 10.1073/pnas.0503496102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein K, Aeschlimann A, Jordan S, Gay R, Gay S, Sprott H. ATP induced brain-derived neurotrophic factor expression and release from osteoarthritis synovial fibroblasts is mediated by purinergic receptor P2X4. PLoS One. 2012;7:e36693. doi: 10.1371/journal.pone.0036693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walsh DA, Bonnet CS, Turner EL, Wilson D, Situ M, McWilliams DF. Angiogenesis in the synovium and at the osteochondral junction in osteoarthritis. Osteoarthritis Cartilage. 2007;15:743–51. doi: 10.1016/j.joca.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 27.Grassi W, Meenagh G, Pascual E, Filippucci E. “Crystal clear”-sonographic assessment of gout and calcium pyrophosphate deposition disease. Semin Arthritis Rheum. 2006;36:197–202. doi: 10.1016/j.semarthrit.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Lioté F, Ea HK. Recent developments in crystal-induced inflammation pathogenesis and management. Curr Rheumatol Rep. 2007;9:243–50. doi: 10.1007/s11926-007-0039-5. [DOI] [PubMed] [Google Scholar]
- 29.Bennett RM, Lehr JR, McCarty DJ. Factors affecting the solubility of calcium pyrophosphate dihydrate crystals. J Clin Invest. 1975;56:1571–9. doi: 10.1172/JCI108239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalya S, Rosenthal AK. Extracellular matrix changes regulate calcium crystal formation in articular cartilage. Curr Opin Rheumatol. 2005;17:325–9. doi: 10.1097/01.bor.0000160783.14798.10. [DOI] [PubMed] [Google Scholar]
- 31.Fuerst M, Bertrand J, Lammers L, Dreier R, Echtermeyer F, Nitschke Y, et al. Calcification of articular cartilage in human osteoarthritis. Arthritis Rheum. 2009;60:2694–703. doi: 10.1002/art.24774. [DOI] [PubMed] [Google Scholar]
- 32.Jubeck B, Gohr C, Fahey M, Muth E, Matthews M, Mattson E, et al. Promotion of articular cartilage matrix vesicle mineralization by type I collagen. Arthritis Rheum. 2008;58:2809–17. doi: 10.1002/art.23762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayashi D, Roemer FW, Katur A, Felson DT, Yang SO, Alomran F, et al. Imaging of synovitis in osteoarthritis: current status and outlook. Semin Arthritis Rheum. 2011;41:116–30. doi: 10.1016/j.semarthrit.2010.12.003. [DOI] [PubMed] [Google Scholar]


