Abstract
In this exploratory study, gait analysis and quantitative MRI (QMRI) were used to assess biomechanical differences in patients that present with cyclops lesions at 12 months after ACL-reconstruction (ACLR). Thirty ACLR patients without and 10 ACLR patients with cyclops lesions underwent 3T MR T1ρ mapping of the reconstructed knee joint prior to ACLR and at 12 months after ACLR, as well as a gait assessment during a fixed walking speed at 12 months after ACLR. Both external sagittal and frontal plane knee joint moments and joint moment impulses were calculated and assessed throughout the stance phase of gait. ACLR patients with cyclops lesions demonstrated a significantly greater (34% larger, p=0.03) first peak knee flexion moment (KFM) and KFM impulse (42% larger, p=0.05), compared to those without cyclops lesions, which may suggest an increased load during the loading response phase of gait. There were no differences (p>0.05) in knee extension or adduction joint moments or moment impulses. ACLR patients with cyclops lesions demonstrated a significantly increased change in T1ρ (ΔT1ρ = 4.7ms, p=0.03), over 12 months, within the central medial tibia. The results of the study suggest that ACLR patients with cyclops lesions demonstrate altered sagittal plane loading patterns which may be related to an increased rate of medial tibiofemoral cartilage degeneration at 12 months after ACLR. The first peak external KFM may be an important target for intervention programs in ACLR patients with cyclops lesions in order to possibly slow the onset or progression of medial tibiofemoral cartilage degeneration.
Keywords: Cyclops Lesions, ACL-reconstruction, Gait, Cartilage Degeneration, T1ρ
Introduction
The anterior cruciate ligament (ACL) is a primary mechanical stabilizer that prevents excessive anterior tibial translation and provides mediolateral and rotational stability of the knee joint1–3. ACL injury causes lack of knee stability and frequently requires ACL-reconstruction (ACLR) in order to restore functional and anatomical joint stability4. Despite the restoration of knee joint stability after ACLR, altered knee joint mechanics and loading patterns exist and may lead to knee cartilage degeneration2,5–7. ACLR has been shown to accelerate cartilage degeneration and promote possible onset of knee joint osteoarthritis (OA)7 but the exact causes need to be investigated and understood in order to prevent or slow down the onset of knee joint OA.
Previous biomechanical analyses demonstrated altered knee joint mechanics after ALCR during gait8–11. Compared to the contralateral limb, patients demonstrated similar knee joint moments at 6 months after ACLR during walking8 yet at 5 years post-ACLR, those with radiographic medial knee OA demonstrated reduced external peak knee flexion moments compared to those ACLR patients without medial knee OA9. Also, it has been suggested that the peak external knee flexion moment, in addition to the peak external knee adduction moment, should be considered when evaluating knee joint loading during gait11,12. Previous work has shown that associations exist between altered knee joint mechanics and quantitative magnetic resonance imaging (QMRI) based measurements of knee joint articular cartilage composition, indicating that altered knee joint mechanics in ACLR patients are associated with a greater risk of knee OA onset or progression10,13. The use of MR-based assessments such as QMRI (i.e. T1ρ mapping) of knee OA, instead of radiographic based assessments, may prove to be more beneficial in understanding the potential links between knee joint mechanics after ACLR and knee joint pathologies, as QMRI was able to detect changes in knee joint cartilage composition as early as one year after ACLR7. Also, T1ρ mapping might be more sensitive in detecting early cartilage damage, compared to other QMRI techniques such as T2 mapping, due to the ability of T1ρ to better detect loss of proteoglycan14.
Besides graft impingement, another cause of reduced range of motion after ACLR is the presence of cyclops lesions15–22. Cyclops lesions are focal nodules of fibrocartilaginous tissue that are tightly connected to the anterior portion of the ACL graft, most frequently located within the intercondylar notch19,22 and occur in approximately 10 – 25% of ACLR patients23–25. The formation of cyclops lesions may be due to repetitive microtrauma to the ACL graft15,20 or by debris that is present within the knee joint from the drilling and preparation of the tibial tunnel during ACLR21. The consistency (i.e. soft or hard) of the cyclops lesion may be a factor in whether or not loss of knee joint extension or worsening of clinical symptoms occurs after ACLR22. The “cyclops syndrome”, defined as a mechanical block that prevents full extension of the knee joint due to the presence of a cyclops lesion, is relatively rare (0 – 2%)15,26. However, the presence of cyclops lesions may cause altered knee joint mechanics in asymptomatic ACLR patients as well15,16,22.
Despite the interest in the presence/development of cyclops lesions after ACLR15–22, the effect of cyclops lesions on lower extremity mechanics and the onset or progression of knee cartilage degeneration in ACLR patients has yet to be investigated. Therefore, the purpose of the current study was to assess the association between knee joint mechanics and medial tibiofemoral cartilage composition in ACLR patients with and without cyclops lesions. We hypothesized that ACLR patients with cyclops lesions will demonstrate altered knee joint mechanics during walking and will also exhibit an increased rate of medial tibiofemoral cartilage degeneration over a 12 month period after ACLR compared to ACLR patients without cyclops lesions.
METHODS
Study Participants
This case-control study (Level III) was approved by the Committee on Human Research. Each participant provided written informed consent prior to any type of testing. A total of 40 ACLR patients that sustained acute, unilateral ACL injury, with no previous history of knee trauma or disease, were enrolled in the current study. Of these 40 ACLR patients, 10 patients (Cyclops; 6 males, 28.6±8.8 years, 22.7±2.3 kg·m−2) presented with MRI-based findings of a cyclops lesion at 12 months after isolated ACLR and 30 patients did not present with cyclops lesions (Non-Cyclops; 20 males, 33.6±7.8 years, 24.1±2.7 kg·m−2).
All ACLR patients in the current study demonstrated both clinical and radiological (MRI) based indications of acute complete ACL rupture. All patients underwent anatomic single bundle ACLR using either hamstrings allograft or autograft or a posterior tibialis allograft. All ACLRs were performed by 1 of 4 sports fellowship trained orthopaedic surgeons at the same institution using anatomic single bundle ACLR with the femoral tunnels drilled using anteromedial portal drilling. All patients underwent a standard protocol of postoperative rehabilitation. Patients were excluded from this study if they demonstrated prior history of OA, previous surgery on the tested limb, required surgical intervention for other ligamentous injuries (i.e. collateral ligaments and posterior cruciate ligaments) or meniscal repair.
MRI Acquisition
All ACLR patients underwent MR-imaging of the tested knee joint after injury but prior to ACLR (baseline) and at 12-months after ACLR (12M). All MR-imaging was performed using a 3-Tesla GE MR Scanner (General Electric Healthcare, Milwaukee, WI) and an 8-channel phase array knee coil (InVivo, Gainesville, FL). Two imaging protocols were implemented in the current study. A high resolution 3D fast spin echo (FSE) (TR=1500ms; TE=26.69ms; 384×384 matrix; FOV=16cm; slice thickness of 0.5mm; echo train length of 32; BW=50kHz; NEX=0.5) was used to assess for the presence of cyclops lesions. A 3D sagittal plane combined T1ρ/T2 sequence27 (T1ρ spin-lock times (TSL) of 0/10/40/80ms; spin-lock frequency of 500Hz; FOV=14cm; 256×128 matrix; slice thickness of 4mm) was used to assess cartilage matrix composition.
MRI Assessment of Cyclops Lesions
Cyclops lesions were defined as soft tissue masses with a convex anterior border located in the intercondylar notch, characterized by varying signal intensities on intermediate-weighted images (3D FSE), as previously described28. Previous studies have demonstrated a good sensitivity, specificity, and accuracy of MRI in revealing cyclops lesions (sensitivity 85.0%, specificity 84.6%, and accuracy 84.8%)23,24. In addition, a diameter of 5mm in each plane was used as the minimum required size to avoid false-positive cases23,24.
The volumes of the cyclops lesions (VCL, mm3) were measured. Similar to previous studies23,25, two perpendicular diameters (a⊥, b⊥) of the lesion were measured on the sagittal 3D FSE sequence and a third measurement (c‖) was made on the axial reconstruction where the lesion was more conspicuous (Fig. 1). All the measurements were performed on a picture archiving and communication system (Agfa IMPAX 6, Agfa Healthcare, Mortsel, Belgium). Using the semi-axes measurements derived from the measurements described above, the VCL was approximated to be the volume of an ellipsoid:
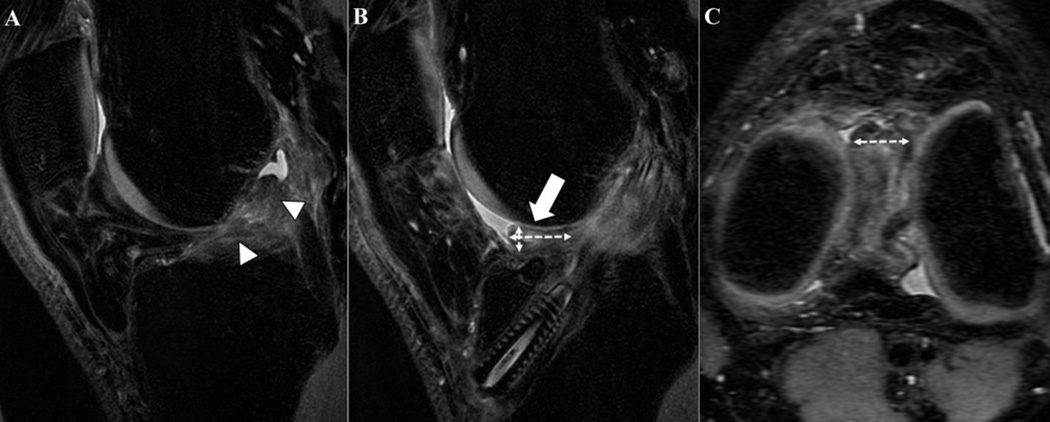
Figure 1.
Sagittal image at baseline (Figure 1A) and the corresponding sagittal and axial 3D fast spin-echo weighted images at 12 months after ACL-reconstruction (ACLR) (Figures 1B and 1C) of a 30-year-old male patient. In Figure 1A, a complete tear of the ACL is shown (arrowheads). In Figures 1B (sagittal) and 1C (axial), a well-defined mass (arrow) in the intercondylar notch, localized anteriorly to the anterior cruciate ligament graft, with a convex border and intermediate signal is present at 12 months after ACLR. The 3 axes (sagittal: a⊥, b⊥; transverse: c‖) used to measure the volume of the cyclops lesion (VCL) are represented by the dashed double-headed arrows.
Image Processing
All image post-processing was performed using in-house programs written in MATLAB (The Mathworks, Natick, MA) according to previously described methods29,30. Briefly, high-resolution sagittal 3D FSE images were rigidly registered to the first echo of the T1ρ weighted image (TSL=0ms) and were used for knee cartilage segmentation. A semi-automatic segmentation was used to identify the medial femoral (MF) and medial tibial (MT) condyle compartments in all slices. The MF and MT were then sub-divided into 6 sub-compartments (anterior, central and posterior) within the edges of the menisci, in order to further assess the load bearing conditions within each of these sub-compartments. Regions of interest (ROIs) were created around each of these sub-compartments and were used to analyze T1ρ relaxation times within each of these sub-compartments. During the course of this longitudinal study, the GE 3T HDx Long Bore MRI used during the first year of this study was replaced with a GE 3T MR750 Wide Bore system. Potential variations in T1ρ values related to different MR systems were assessed by scanning phantoms and study subjects27,30. A linear regression model was used to adjust T1ρ values between the two systems as described in our previous work30. In order to ensure that the same anatomical regions of the tibial and femoral cartilage layers were analyzed, all T1ρ echoes of the follow-up MR-scans were registered to the first echo of the T1ρ image of the injured knee adopting a pyramidal multi-resolution bi-spline non-rigid registration using Mattes Mutual Information as a measure of similarity31. The image registration procedure was implemented using the elastix ITK library32,33. T1ρ relaxation times were estimated using a pixel by pixel two-parameter exponential fit with the T1ρ relaxation times values of each sub-region computed as the mean of all pixels within that particular ROI. Changes in T1ρ relaxation times in each of the 6 sub-compartments, from baseline to one-year post ACLR, were computed for both groups.
Gait Analysis
Each participant underwent a 3D gait analysis at 12 months follow-up. Three dimensional position data were collected at 250Hz using a 10-camera motion capture system (Vicon, Oxford, UK) and ground reaction (GRF) data were collected simultaneously at 1000Hz using two in-ground force plates (AMTI, Watertown, MA). Forty-one retro-reflective markers were used to collect three-dimensional position data. Calibration markers were placed bilaterally at the greater trochanters, medial and lateral femoral epicondyles, medial and lateral malleoli, first and fifth metatarsal heads for estimation of joint centers and segment lengths. Additional markers used for segment tracking were placed at the anterior superior iliac spines, iliac crests and at the L5/S1 joint. Rigid marker clusters were placed bilaterally on the thighs, shanks and heel shoe counters. A one-second standing calibration trial was performed and the calibration markers were removed from the participant.
Each participant was asked to perform 3 successful gait trials at a controlled speed of 1.35 m·s−1. This speed was chosen as it is the mean of the average walking speeds of male and female adults on a smooth surface34. A gait trial was considered successful if the participant’s entire foot made a clean strike on one of the two force plates and was within 5% (0.7 m·s−1) of the controlled speed. All raw marker position and GRF data were filtered using a low-pass 4th order Butterworth filter with cut-off frequencies of 6Hz and 50Hz, respectively. The standing calibration trial was used to create a seven segment musculoskeletal model, using Visual3D (C-motion, Rockville, MD) and consisted of the pelvis, bilateral thighs, shanks and feet. Local joint coordinate systems were created and segment position and orientation were described using an unweighted least squares method35. Joint rotations were resolved using a Cardan sequence of X-Y’-Z”, representing the medial-lateral, anterior-posterior and superior-inferior directions, respectively. All joint angles were normalized to the standing calibration trial. Initial contact was defined when the foot made contact with a force plate and the vertical GRF exceeded a 20 Newton threshold. The stance phase of gait was defined as initial contact to toe-off and normalized to 101 points (0 – 100% stance). External sagittal and frontal plane knee joint moments were calculated using inverse dynamics and were normalized by body mass (Nm·kg−1) with knee extension and adduction described as positive joint moments.
Knee flexion (KFM) and extension (KEM) moment impulses (Nm·ms·kg−1) were calculated as the integral of the negative and positive portions, respectively, of the sagittal plane knee joint moment with respect to time. The knee adduction moment (KAM) impulse was calculated as the integral of the positive portion of the frontal plane knee joint moment with respect to time. Variables of interest include the peak KEM, KFM and KAM joint moments and moment impulses during the first 50% and second 50% of the stance phase. Averaged data from 3 successful trials were used for statistical analysis.
Statistical Analysis
Demographics and biomechanical gait parameters at 12 months post ACLR as well as the changes in medial tibiofemoral T1ρ relaxation times from baseline to one year post ACLR were compared between the non-cyclops and cyclops lesion groups using an analysis of covariance (ANCOVA) with covariates of age, gender, body mass index (BMI) and weeks from baseline scan to ACL-reconstruction. Partial eta-squared () values were to assess the strength of the relationship between these dependent variables and the cyclops lesion pathology with values of 0.02, 0.13 and 0.26 corresponding to small, medium and large effect sizes, respectively. Statistical analyses were performed using SPSS (v23, IBM, Armonk, NY) and alpha was set a priori at the 0.05 level.
Results
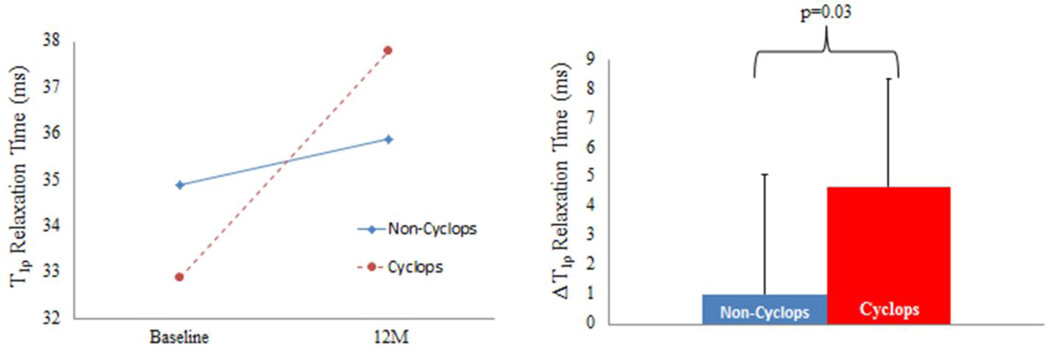
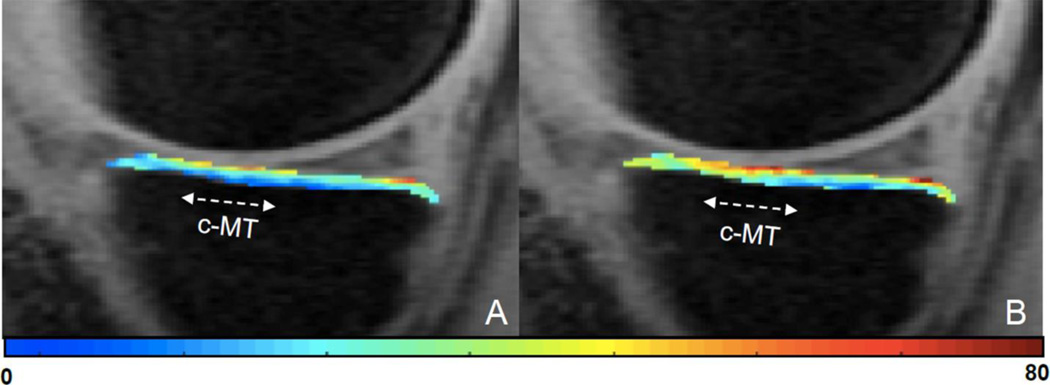
Both groups demonstrated similar demographics (Table 1). The mean volume of the cyclops lesions (VCL) at 12 months post ACLR was 723.4±796.2mm3. At one year post ACLR, the cyclops lesion group demonstrated a significantly larger change in T1ρ relaxation times in the central MT compared to the Non-Cyclops group (Cyclops: 4.7±3.7ms, Non-Cyclops: 1.0±4.1ms, p = 0.03, ) (Fig. 2). There were no significant differences in the sub-compartmental changes in T1ρ relaxation times of the MF at one year post ACLR. A representative T1ρ color map for an ACLR patient at baseline and one year after ACLR is shown in Figure 3.
Table 1.
Participant Demographics at 12 Months Post ACL-Reconstruction
| Non-Cyclops (N=30) | Cyclops (N=10) | p-value | |
|---|---|---|---|
| Age (years)a | 33.6±7.92 | 29.8±8.32 | 0.20 |
| Genderb | 0.57 | ||
| Male | 19 (63%) | 6 (60%) | - |
| Female | 11 (37%) | 4 (40%) | - |
| BMI (kg·m−2)a | 24.1±2.76 | 22.8±2.31 | 0.21 |
| Time to surgery (weeks)a | 10.8±7.97 | 8.3±3.89 | 0.34 |
| ACL graftb | |||
| Autograft Hamstring | 24 (80%) | 6 (60%) | - |
| Allograft Posterior Tibialis | 5 (17%) | 4 (10%) | - |
| Allograft Hamstring | 1 (3%) | 0 (0%) | - |
| VCL (mm3)a | - | 723.4±796.2 | - |
VCL: Volume of cyclops lesion;
Data expressed as mean±standard deviation;
Data expressed as count (%),
Figure 2.
The T1ρ relaxation times for the central (left) medial tibia (MT) for both groups at baseline and 12 months post ACL-reconstruction (12M) as well as the change in T1ρ relaxation times (ΔT1ρ) for the central MT from baseline to 12M post ACL-reconstruction (right) are shown.
Figure 3.
A T1ρ color map of the central medial tibia, at baseline (Figure 3A) and 12 months after ACLR (Figure 3B), in a 25 year old male ACL-reconstruction (ACLR) patient with a cyclops lesion. The colormap indicates an increase in the T1ρ relaxation time within the central medial tibia (c-MT) at 12 months after ACLR.
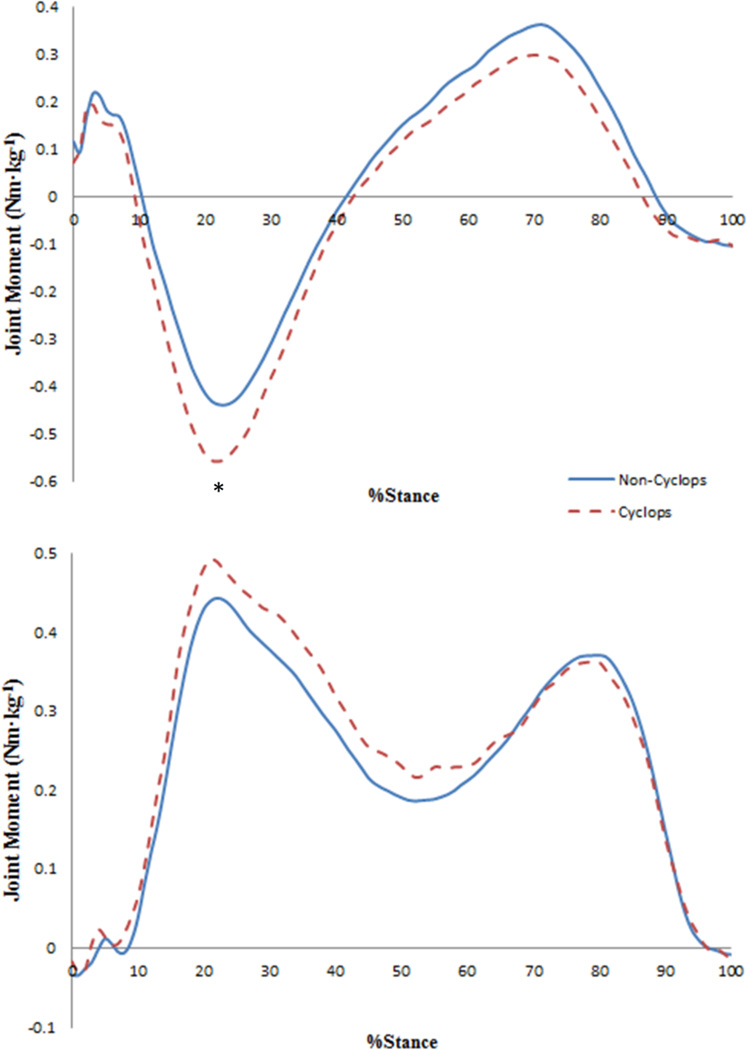
The cyclops lesion group demonstrated a greater first peak KFM (Cyclops: −0.63±0.20Nm·kg−1, Non-Cyclops: −0.47±0.20Nm·kg−1, p = 0.03, ) and first KFM impulse (Cyclops: 88.6±44.1Nm·ms·kg−1, Non-Cyclops: 62.5±30.9Nm·ms·kg−1, p = 0.05, ) during the loading response phase of gait (Fig. 4). Both groups demonstrated similar first and second peak KEM and KAM values and impulses (Table 2).
Figure 4.
External knee sagittal (top) and frontal (bottom) plane joint moments (Nm·kg−1) during the stance phase of gait for the non-cyclops (solid) and cyclops lesion (dashed) groups. Positive values represent knee extension and adduction. An asterisk indicates a statistically significant difference (p<0.05).
Table 2.
External Knee Sagittal and Frontal Plane Peak Joint Moments and Joint Moment Impulses during Fixed Speed Gait at 12 Months Post ACL-Reconstruction. Results are Reported as the Mean±Standard Deviation with corresponding effect sizes().
| Non-Cyclops | Cyclops | p-value | |||
|---|---|---|---|---|---|
| Joint Moment (Nm·kg−1) | |||||
| 1st Peak Extension | 0.29±0.09 | 0.25±0.09 | 0.23 | 0.04 | |
| 1st Peak Flexion* | −0.47±0.20 | −0.63±0.20 | 0.03 | 0.13 | |
| 1st Peak Adduction | 0.48±0.15 | 0.52±0.16 | 0.83 | <0.01 | |
| 2nd Peak Extension | 0.40±0.17 | 0.32±0.14 | 0.10 | 0.08 | |
| 2nd Peak Flexion | −0.14±0.06 | −0.13±0.04 | 0.72 | <0.01 | |
| 2nd Peak Adduction | 0.40±0.17 | 0.38±0.13 | 0.36 | 0.02 | |
| Joint Moment Impulse (Nm·ms·kg−1) | |||||
| 1st Peak Extension | 11.6±5.11 | 8.64±3.72 | 0.15 | 0.06 | |
| 1st Peak Flexion* | 62.5±30.9 | 88.6±44.1 | 0.05 | 0.11 | |
| 1st Peak Adduction | 86.9±36.1 | 96.6±25.8 | 0.78 | <0.01 | |
| 2nd Peak Extension | 75.6±34.4 | 59.2±31.4 | 0.12 | 0.07 | |
| 2nd Peak Flexion | 48.5±24.6 | 68.3±44.5 | 0.12 | 0.07 | |
| 2nd Peak Adduction | 85.2±36.1 | 93.7±27.5 | 0.83 | <0.01 | |
An * Indicates a Statistically Significant Difference (p ≤ 0.05).
Discussion
In this exploratory study, the presence of cyclops lesions was shown to be associated with altered knee joint mechanics during gait and may lead to a greater rate of knee joint cartilage degeneration at 12 months after ACLR. More specifically, a greater first peak KFM and first peak KFM impulse were present during gait in ACLR patients with cyclops lesions, compared to ACLR patients without cyclops lesions and may help to explain the increased change in T1ρ relaxation times in the central medial tibia of ACLR patients with cyclops lesions at 12 months after ACLR. Although, peak KAM and KAM impulse were suggested to be related to an increased risk of onset or progression of medial knee joint OA10,11,36–38, the patients in the current study did not demonstrate any differences in knee frontal plane joint mechanics during gait. These results suggest that ACLR patients with cyclops lesions possess altered sagittal plane loading patterns during the loading response phase of gait, which may place those with cyclops lesions at a higher risk of medial knee joint cartilage degeneration compared to ACLR patients without cyclops lesions.
Previous work has shown that T1ρ relaxation times were able to detect changes in the knee joint cartilage matrix composition as early as one year after ACLR7. When compared to healthy controls at one year after surgical reconstruction, ACLR patients demonstrated increased T1ρ relaxation times in the weight bearing region of the medial knee joint7. The results by Li et al (2011) may suggest that after ACLR, patients are already at a higher risk of medial knee joint OA and combined with the results of the current study, those ACLR patients with cyclops lesions may be at an increased risk of medial knee joint OA due to the association between the presence of a cyclops lesion and greater knee joint loading during gait.
Previous work demonstrated a reduced external peak KFM during gait in ACLR patients with radiographic medial knee joint OA at 5 years after ACLR9, as well as a higher peak KAM which corresponded to elevated MR-relaxation times in the medial knee joint10. Also, a previous study demonstrated a longitudinal relationship between knee joint biomechanics, as measured by loaded MRI, at 6 months after ACLR with altered cartilage matrix composition at one year after ACLR13. In the current study, patients with cyclops lesions demonstrated a greater first peak KFM and first peak KFM impulse during the loading phase of gait, as well as an increased rate of medial knee joint cartilage degeneration in the central portion of the medial tibia over a one year period after ACLR. The greater first peak KFM demonstrated by the ACLR patients with cyclops lesions may indicate a larger peak joint load during gait and may help to further support the notion that the peak KFM is an important contributor to medial knee joint loading11,12. The ACLR patients with cyclops lesions exhibited a greater first peak KFM impulse during the loading response phase of gait. The larger first KFM impulse in ACLR patients with cyclops lesions may suggest a greater external knee flexor load throughout the loading response phase of gait. More specifically, the greater first peak KFM and KFM impulse in ACLR patients with cyclops lesions may expose certain regions of the articular cartilage, such as the central medial tibia, to longer durations and larger intensities of loading, over multiple stride cycles and thereby induce the cartilage of the central medial tibia to degenerate at a faster rate compared to other regions of the tibiofemoral joint. In addition, a lack of differences in peak KAM or KAM impulse between ACLR patients with and without cyclops lesions may indicate that the peak KAM may not be the sole contributor to higher medial compartment loads in the knee joint9. This result is consistent to the emerging evidence in recent literature that suggested that both the peak KFM and KAM should be considered when evaluating knee joint loads from external knee moments9,11,12. The use of the KFM impulse may also prove to be an important variable in assessing medial knee joint loading in the ACLR population. Both the first KFM and KFM impulse in ACLR patients with cyclops lesions were found to be highly correlated, as a post-hoc Pearson’s (ρ) correlation analysis demonstrated a significant negative correlation (ρ = −0.97, p < 0.01), which in the current study suggests that as the first KFM increases so does the first KFM impulse. The results of this study suggest that the first peak KFM and KFM impulse may be strong contributors to the overall degeneration of the medial knee joint in those with cyclops lesions and may be a potential target for intervention programs in ACLR patients with cyclops lesions. Although a difference exists in first peak KFM between ACLR patients with and without cyclops lesions, it is important to note that these metrics do not reflect the exact magnitude of the peak medial compartment load that occurs during gait. Therefore, similar to previous studies9,11, future studies should incorporate electromyography or musculoskeletal simulations in order to evaluate muscle activations, co-contractions and forces during gait that may potentially affect the peak medial compartment load in ACLR patients with cyclops lesions.
Limitations exist within the current study and should be considered when interpreting the results presented in this work. Baseline (pre-injury) knee joint mechanics were not available in these patients, thereby making it difficult to assess any causal effects of cyclops lesions on knee joint loading and osteoarthritis. The cohort size used in this study was small and a future study using a larger cohort size would prove beneficial. Also, a longer follow-up would provide for a better understanding of the long-term effects of cyclops lesions on medial knee joint loading patterns and associated cartilage degeneration. Future studies involving gait analysis should investigate the causal mechanics between hip and ankle joint compensations and cartilage degeneration in ACLR patients with cyclops lesions. Although walking was chosen as the task of interest in the current study, future studies should assess how the presence of cyclops lesions affects knee joint mechanics during more dynamic tasks such as a sidestep cut or drop landing, in which the knee joint is exposed to larger joint loads. It should be noted that all patients in involved in this study did not demonstrate obvious loss of knee joint motion upon physical examination and when possible, the orthopaedic surgeons at our University aim to preserve the ACL remnant during surgical reconstruction. The longitudinal effects of cyclops lesions on knee joint health may be limited to surgeries that employ this ACL preservation technique and therefore, orthopaedic surgeons should highly consider the method of ACL preservation used. Also, it would be of interest to understand whether or not the surgical excision of the cyclops lesion restores normal knee joint mechanics during gait and possibly slows the onset or progression of medial knee joint cartilage degeneration.
In conclusion, ACLR patients with cyclops lesions demonstrated higher sagittal plane knee joint loading during gait compared to ACLR patients without cyclops lesions. The ACLR patients with cyclops lesions demonstrated significantly higher increases in T1ρ within the medial tibia during the first year after ACLR, compared to ACLR patients without cyclops lesions, which suggests more rapid degeneration of the medial tibial cartilage. The results of this exploratory study suggest that the presence of a cyclops lesion after ACLR may be an important factor in the development of medial knee joint OA. It is important to note that the clinical implication of this work remains inconclusive. These data suggest that changes in biomechanics in concurrence with a cyclops lesion may reveal the mechanism by which knee OA manifests following ACLR in these patients. Future studies should include a larger cohort size and longer follow-ups in order to fully understand the causal link between the presence of cyclops lesions, altered knee joint loading and cartilage degeneration.
Acknowledgments
This study was supported by funding from the NIH/NIAMS P50-060752 and the American Orthopaedic Society for Sports Medicine (AOSSM). The contents of this study are sole responsibility of the authors and do not necessarily represent the official views of the NIH or the AOSSM.
Footnotes
All authors have made significant contributions to the project design, data analysis/interpretation, drafting and revising of the manuscript.
All authors read and approved the final version of this manuscript.
REFERENCES
- 1.Andersen NH, Dyhre-Poulsen P. The anterior cruciate ligament does play a role in controlling axial rotation in the knee. Knee Surgery, Sports Traumatology, Arthroscopy. 1997;5:145–149. doi: 10.1007/s001670050042. [DOI] [PubMed] [Google Scholar]
- 2.Chaudhari A, Briant P, Bevill S, et al. Knee kinematics, cartilage morphology, and osteoarthritis after ACL injury. Medicine and Science in Sports and Exercise. 2008;40:215–222. doi: 10.1249/mss.0b013e31815cbb0e. [DOI] [PubMed] [Google Scholar]
- 3.Markolf KL, Burchfield DM, Shapiro MM, et al. Combined knee loading states that generate high anterior cruciate ligament forces. Journal of Orthopaedic Research. 1995;13:930–935. doi: 10.1002/jor.1100130618. [DOI] [PubMed] [Google Scholar]
- 4.Marder RA, Raskind JR, Carroll M. Prospective evaluation of arthroscopically assisted anterior cruciate ligament reconstruction: Patellar tendon versus semitendinosus and gracilis tendons. The American Journal of Sports Medicine. 1991;19:478–484. doi: 10.1177/036354659101900510. [DOI] [PubMed] [Google Scholar]
- 5.Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: New insights. Part 1: The disease and its risk factors. Ann Intern Med. 2000;133:635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 6.Daniel D, Stone M, Dobson B, et al. Fate of the ACL-injured patient. A prospective outcome study. The American Journal of Sports Medicine. 1994;22:632–644. doi: 10.1177/036354659402200511. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Kuo D, Theologis A, et al. Cartilage in anterior cruciate ligament–reconstructed knees: MR imaging T1ρ and T2—initial experience with 1-year follow-up. Radiology. 2011;258:505–514. doi: 10.1148/radiol.10101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hooper D, Morrissey M, Drechsler W, et al. Gait analysis 6 and 12 months after anterior cruciate ligament reconstruction surgery. Clinical Orthopaedics and Related Research. 2002;403:168–178. doi: 10.1097/00003086-200210000-00025. [DOI] [PubMed] [Google Scholar]
- 9.Khandha A, Manal K, Wellsandt E, et al. Gait mechanics in those with/without medial compartment knee osteoarthritis 5 years after anterior cruciate ligament reconstruction. Journal of Orthopaedic Research. 2016 doi: 10.1002/jor.23261. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar D, Kothari A, Souza RB, et al. Frontal plane knee mechanics and medial cartilage MR relaxation times in individuals with ACL reconstruction : A pilot study. The Knee. 2014;21:881–885. doi: 10.1016/j.knee.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manal K, Gardinier E, Buchanan TS, et al. A more informed evaluation of medial compartment loading: The combined use of the knee adduction and flexor moments. Osteoarthritis and Cartilage. 2015;23:1107–1111. doi: 10.1016/j.joca.2015.02.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creaby MW. It's not all about the knee adduction moment: The role of the knee flexion moment in medial knee joint loading. Osteoarthritis and Cartilage. 2015;23:1038–1040. doi: 10.1016/j.joca.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 13.Amano K, Pedoia V, Su F, et al. Persistent biomechanical alterations after ACL reconstruction are associated with early cartilage matrix changes detected by quantitative MR. Orthopaedic Journal of Sports Medicine. 2016;4 doi: 10.1177/2325967116644421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dijkgraaf LC, de Bont LGM, Boering G, et al. The structure, biochemistry, and metabolism of osteoarthritic cartilage: A review of the literature. Journal of Oral and Maxillofacial Surgery. 1995;53:1182–1192. doi: 10.1016/0278-2391(95)90632-0. [DOI] [PubMed] [Google Scholar]
- 15.Jackson D, Schaefer R. Cyclops syndrome: Loss of extension following intra-articular anterior cruciate ligament reconstruction. Arthroscropy. 1990;6:171–178. doi: 10.1016/0749-8063(90)90072-l. [DOI] [PubMed] [Google Scholar]
- 16.Papakonstantinou O, Chung C, Chanchairujira K, et al. Complications of anterior cruciate ligament reconstruction: MR imaging. European Radiology. 2003;13:1106–1107. doi: 10.1007/s00330-002-1622-9. [DOI] [PubMed] [Google Scholar]
- 17.Hettrich CM, Dunn WR, Reinke EK, et al. The rate of subsequent surgery and predictors after anterior cruciate ligament reconstruction: Two- and 6-year follow-up results from a multicenter cohort. The American Journal of Sports Medicine. 2013;41:1534–1540. doi: 10.1177/0363546513490277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marzo J, Bowen M, Warren R, et al. Intrarticular fibrous nodule as a cause of loss of extension following anterior cruciate ligament reconstruction. Arthroscopy. 1992;8:10–18. doi: 10.1016/0749-8063(92)90129-y. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Ao Y. Analysis of different kinds of cyclops lesions with or without extension loss. Arthroscopy. 2009;25:626–631. doi: 10.1016/j.arthro.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Mariani PP, Ferretti A, Conteduca F, et al. Arthroscopic treatment of flexion deformity after acl reconstruction. Arthroscopy. 1992;8:517–521. doi: 10.1016/0749-8063(92)90018-7. [DOI] [PubMed] [Google Scholar]
- 21.Delcogliano A, Franzese S, Branca A, et al. Light and scan electron microscopic analysis of cyclops syndrome: Etiopathogenic hypothesis and technical solutions. Knee Surgery, Sports Traumatology, Arthroscopy. 1996;4:194–199. doi: 10.1007/BF01567962. [DOI] [PubMed] [Google Scholar]
- 22.Muellner T, Kdolsky R, GroBschmidt K, et al. Cyclops and cyclopoid formation after anterior cruciate ligament reconstruction: Clinical and histomorphological differences. Knee Surgery, Sports Traumatology, Arthroscopy. 1999;7:284–289. doi: 10.1007/s001670050165. [DOI] [PubMed] [Google Scholar]
- 23.Bradley DM, Bergman AG, Dillingham MF. MR imaging of cyclops lesions. American Journal of Roentgenology. 2000;174:719–726. doi: 10.2214/ajr.174.3.1740719. [DOI] [PubMed] [Google Scholar]
- 24.Cha J, Choi S-H, Kwon JW, et al. Analysis of cyclops lesions after different anterior cruciate ligament reconstructions: A comparison of the single-bundle and remnant bundle preservation techniques. Skeletal Radiology. 2012;41:997–1002. doi: 10.1007/s00256-011-1347-4. [DOI] [PubMed] [Google Scholar]
- 25.Facchetti L, Schwaiger BJ, Gersing AS, et al. Cyclops lesions detected by MRI are frequent findings after ACL surgical reconstruction but do not impact clinical outcome over 2 years. European Radiology. 2016:1–10. doi: 10.1007/s00330-016-4661-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tonin M, Saciri V, Veselko M, et al. Progressive loss of knee extension after injury: Cyclops syndrome due to a lesion of the anterior cruciate ligament. The American Journal of Sports Medicine. 2001;29:545–549. doi: 10.1177/03635465010290050401. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Wyatt C, Rivoire J, et al. Simultaneous acquisition of T1ρ and T2 quantification in knee cartilage: Repeatability and diurnal variation. Journal of Magnetic Resonance Imaging. 2014;39:1287–1293. doi: 10.1002/jmri.24253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Recht MP, Piraino DW, Cohen MA, et al. Localized anterior arthrofibrosis (cyclops lesion) after reconstruction of the anterior cruciate ligament: MR imaging findings. American Journal of Roentgenology. 1995;165:383–385. doi: 10.2214/ajr.165.2.7618562. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Han ET, Busse RF, et al. In vivo T1ρ mapping in cartilage using 3d magnetization-prepared angle-modulated partitioned k-space spoiled gradient echo snapshots (3D MAPSS) Magnetic Resonance in Medicine. 2008;59:298–307. doi: 10.1002/mrm.21414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, Pedoia V, Kumar D, et al. Cartilage T1ρ and T2 relaxation times: Longitudinal reproducibility and variations using different coils, mr systems and sites. Osteoarthritis and Cartilage. 2015;23:2214–2223. doi: 10.1016/j.joca.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pedoia V, Li X, Su F, et al. Fully automatic analysis of the knee articular cartilage T1ρ relaxation time using voxel-based relaxometry. Journal of Magnetic Resonance Imaging. 2016;43:970–980. doi: 10.1002/jmri.25065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klein S, Staring M, Murphy K, et al. Elastix: A toolbox for intensity-based medical image registration. IEEE Transactions on Medical Imaging. 2010;29:196–205. doi: 10.1109/TMI.2009.2035616. [DOI] [PubMed] [Google Scholar]
- 33.Shamonin DP, Bron EE, Lelieveldt BPF, et al. Fast parallel image registration on cpu and gpu for diagnostic classification of alzheimer's disease. Frontiers in Neuroinformatics. 2014;7 doi: 10.3389/fninf.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perry J, Burnfield J. Gait analysis: Normal and pathological function. Thorofare, New Jersey: Asist Group; 2010. p. 576. [Google Scholar]
- 35.Spoor CW, Veldpaus FE. Rigid body motion calculated from spatial co-ordinates of markers. J Biomech. 1980;13:391–393. doi: 10.1016/0021-9290(80)90020-2. [DOI] [PubMed] [Google Scholar]
- 36.Chang AH, Moisio KC, Chmiel JS, et al. External knee adduction and flexion moments during gait and medial tibiofemoral disease progression in knee osteoarthritis. Osteoarthritis and Cartilage. 2015;23:1099–1106. doi: 10.1016/j.joca.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bennell KL, Bowles K-A, Wang Y, et al. Higher dynamic medial knee load predicts greater cartilage loss over 12 months in medial knee osteoarthritis. Annals of the Rheumatic Diseases. 2011;70:1770–1774. doi: 10.1136/ard.2010.147082. [DOI] [PubMed] [Google Scholar]
- 38.Butler RJ, Minick KI, Ferber R, et al. Gait mechanics after ACL reconstruction: Implications for the early onset of knee osteoarthritis. British Journal of Sports Medicine. 2009;43:366–370. doi: 10.1136/bjsm.2008.052522. [DOI] [PubMed] [Google Scholar]