Abstract
Objective/Background
Positive airway pressure (PAP) is highly efficacious treatment but non-adherence is prevalent with little improvement over the last 15 years. Tailored interventions show promise for promoting adherence to other treatments. The objective of this study was to examine the feasibility/acceptability of a tailored intervention to promote PAP adherence.
Participants
Convenience sample met inclusion criteria: newly-diagnosed OSA; treatment-naïve; ≥18 years. Exclusion criteria: previous OSA diagnosis/treatment; new psychiatric diagnosis; use of oxygen/bi-level PAP; secondary sleep disorder. Adults (n=118) were randomized to tailored intervention (TI; n=61) or usual care (UC; n=57); application of a priori exclusion criteria resulted in 30 participants/assignment who were middle-aged (51.3 ± 11.1 years) adults (70% male) with severe OSA (apnea hypopnea index [AHI], 35.9 ± 25.2).
Methods
Randomized, double-blind, single-site pilot controlled trial. A multi-phased tailored intervention targeting social cognitive perceptions of OSA/PAP treatment was delivered at four intervals. Descriptive analysis, group differences and self-efficacy change scores by t-test, and thematic analysis of acceptability data are reported.
Results
One-week PAP use among TI was 35 minutes greater than UC condition (p=0.20; Cohen’s d = 0.336). Treatment use decreased at 1-month and 3-months (NS). Per-protocol delivery of face-to-face intervention delivery was 100% but lower for telephone intervention delivery. Blinding was successful. Personalized approach was valued by participants.
Conclusions
A tailored intervention approach is acceptable to participants and feasibly implemented in a clinical sleep center setting. The intervention effect size at 1-week is consistent with other educational PAP adherence interventions but was not sustained; further pilot testing is warranted to address pilot RCT limitations.
Keywords: Intervention Studies, Nasal Continuous Positive Airway Pressure, Obstructive sleep apnea, Patient Adherence, Randomized Controlled Trial
INTRODUCTION
Obstructive sleep apnea (OSA) results from recurrent upper airway collapse during sleep (American Academy of Sleep Medicine Task Force, 1999). Such airway collapses cause repetitive nocturnal oxyhemoglobin desaturation and arousals from sleep (Punjabi, 2008). The immediate and deleterious consequences of OSA include excessive daytime sleepiness, impaired quality of life and function, mood/anxiety disorders, and cognitive impairment (Punjabi, 2008; Weaver & George, 2011). Longer term consequences of OSA include increased risk of cardiovascular disease (Somers et al., 2008), metabolic disease (Bonsignore, Borel, Machan, & Grunstein, 2013), cancer (Marshall, Wong, Cullen, Knuiman, & Grunstein, 2014; Nieto et al., 2012) and heightened accidental death risk (Mulgrew et al., 2008; Strohl et al., 2013). Approximately 9-15% of the U.S. adult population have moderate OSA (Young, Peppard, & Gottlieb, 2002), defined by the number of apneas and hypopneas per hour of sleep as ≥15 events per hour; five to 28% of adults worldwide are afflicted by diagnosable OSA, defined by ≥5 apneas and hypopneas per hour of sleep (Lee, Nagubadi, Kryger, & Mokhlesi, 2008). Recent epidemiological evidence suggests the prevalence and incidence of OSA is increasing, due in part to increased obesity rates and heightened awareness of the significance of OSA (Peppard et al., 2013).
The first-line treatment of OSA is with positive airway pressure therapy (PAP) (Epstein et al., 2009; Kushida et al., 2006). Adherence to PAP treatment is widely recognized as problematic with mean adherence rates range of 46-89% (Crawford, Espie, Bartlett, & Grunstein, 2013). Furthermore, approximately 25% of adults diagnosed with OSA refuse PAP treatment after diagnosis (Crawford et al., 2013). The overall prevalence and incidence of PAP non-adherence in the treatment of adult OSA is a significant problem limiting the effectiveness of this efficacious treatment modality.
Though the literature is replete with different operational definitions of PAP adherence, a commonly accepted definition of PAP adherence is ≥4hrs/night of use for a defined period of treatment exposure (e.g., 30 day clinical treatment period, study protocol period)(Crawford et al., 2013). This definition is now widely accepted in clinical practice as third party health payers in the U.S. are typically employing this criteria for reimbursement/payment for PAP treatment of OSA (Centers for Medicare and Medicaid Services, 2008). Studies of PAP adherence and important clinical outcomes suggest that less than four hours per night of PAP is associated with considerable risk of cardiovascular morbidity and mortality(Francisco Campos-Rodriguez et al., 2014; Campos-Rodriguez et al., 2005) and occupational and driving accidents (Ayas et al., 2006; Tan et al., 2008). Everyday OSA impairments are notably worse among adults who use PAP less than four hours per day; evidence suggests that more than four hours of PAP use per night is required for symptom response (Antic et al., 2011; Weaver et al., 2007; Zimmerman, Arnedt, Stanchina, Millman, & Aloia, 2006). PAP adherence less than four hours per night is also not cost-effective (Ayas et al., 2006; Guest, Helter, Morga, & Stradling, 2008; Tan et al., 2008). For these reasons, the public health problem of PAP adherence necessitates the development, testing, and translation of interventions to promote PAP adherence among adults with OSA.
Many intervention strategies have approached the problem of PAP non-adherence from a “one size fits all” perspective. In contrast, intervention approaches that account for individual variability in health and disease, including health behaviors, (i.e., precision health) are rapidly emerging as an opportunity to address persistent health and disease problems (Collins & Varmus, 2015). Studies of personalized intervention approaches necessarily examine not only the primary outcome of PAP usage but also other important secondary outcomes, such as participant experience/satisfaction, operational feasibility for translational capacity of the intervention, and potential moderating variables, interaction effects and contextual factors (Craig et al., 2008; Mohler, Bartoszek, Kopke, & Meyer, 2012). We conducted a pilot study to test a theoretically-derived, multi-component, tailored (i.e., personalized) intervention, designed to promote PAP adherence, in a randomized, double-blinded, parallel group controlled trial. The overall study aim was to determine feasibility and acceptability of the tailored intervention approach in a pilot trial, including recruitment and retention of the target population, blind effectiveness, intervention fidelity, and participant acceptability of the trial experience and exposure condition. Secondarily, the effect size of the complex tailored intervention on PAP adherence outcomes at one week (primary PAP use outcome), one month (secondary PAP use outcome), and three months (secondary PAP use outcome) was determined. The standard operational definition of PAP adherence was employed, using the usage threshold of ≥4 hours/night and the continuous outcome variable, mean hours use per night (± SD). For pilot study purposes, this outcome criterion was deemed appropriate in order that pilot study results could be compared with prior published intervention studies wherein this standard definition is consistently employed. Group differences and change scores of the tailored intervention critical indicators (i.e., risk perception, outcome expectancies, and treatment self-efficacy) derived from the theoretical constructs of Social Cognitive Theory (SCT)(Bandura, 2004) were also explored. All study participants received the established standard of care for diagnosis and PAP treatment of OSA (Epstein et al., 2009; Kushida et al., 2006). The exposure condition, tailored intervention group (TI), received standard of care plus an individualized introduction to OSA and PAP treatment delivered at a priori-determined protocol periods, or critical intervention periods, to potentially improve PAP adherence.
MATERIALS AND METHODS
Exposure Condition: Tailored Intervention
Decisions to use and persist with PAP treatment are multi-factorial, complex, and variable between individuals (Crawford et al., 2013). Therefore, tailored interventions that address the salient characteristics of patients’ experiences and are responsive to patients’ goals, preferences, and cognitive perceptions may improve PAP adherence outcomes. Tailored interventions are theory-based, customized interventions guided by and directed at particular characteristics of individual patients in order to improve the outcome of interest (Enderlin & Richards, 2006). Though tailored interventions are based on important individual indicators, these interventions are guided by a defined protocol. Tailored interventions may look similar within certain groups of individuals. In such cases, results of measured critical indicators, upon which tailoring of the intervention is founded, are consistent within this group of individuals. Yet, within-individual variance of the measured critical indicator permits differences in the delivered intervention across groups (Richards et al., 2007). The net result is a tailored intervention that provides opportunity to address differences across individuals while promoting a single end-point of interest.
Employ of a critical indicator in the current study, a central premise of the formal tailored intervention approach, differentiates the study’s intervention delivery approach from other tested interventions that have sought to improve PAP adherence by addressing the same psychological/cognitive perception constructs, primarily by motivational enhancement (Aloia, Arnedt, Riggs, Hecht, & Borrelli, 2004; Aloia, Arnedt, Strand, Millman, & Borrelli, 2013). By repeatedly measuring an intervention target construct(s) in real-time at the time of exposure, the delivered intervention content is varied across subjects and within-subjects. This formal tailored approach is essentially a measurable, construct-specific indicator to determine who needs what exposure content at what period of time. This approach, from an intervention design perspective, is closely aligned with stepped care models, wherein measurable, emergent patient-specific data guides “next steps” (Bower & Gilbody, 2005; Espie, 2009).
Intervention Design
The content of the intervention addressed cognitive perceptions of the diagnosis and treatment, outcome expectancies with PAP treatment and PAP treatment self-efficacy, all domains of SCT (Bandura, 2004). In addition, symptom response with PAP treatment observed by non-spousal, proximate social sources (i.e., friend, co-worker) was incorporated. The Self-efficacy Measure in Sleep Apnea (SEMSA), a validated instrument of disease-specific, OSA cognitive perceptions (Weaver et al., 2003), was employed as the critical indicator for intervention tailoring purposes. SEMSA sub-domains include risk perception, outcome expectancies, and treatment self-efficacy. SEMSA was collected at baseline (after randomization), prior to PAP titration polysomnogram (i.e., overnight, in-laboratory sleep study) when PAP was initially used under sleep laboratory conditions, immediately post-PAP titration polysomnogram, on day two of home PAP treatment, and between day 7-10 of home PAP treatment. Tailoring of the intervention was guided by a SEMSA sub-domain score of ≤3.0 for any one domain. In other words, if an exposure condition participant scored >3.0 on a SEMSA domain, the intervention activity addressing that domain was not addressed. For example, if SEMSA risk perception and outcome expectancy was scored at the critical indicator threshold at second intervention period (i.e., post-diagnostic PSG), comorbidity and health risk review was not addressed. If, however, only SEMSA risk perception was scored at the critical indicator threshold and outcome expectancy was scored ≤ 3.0, participant expectancies for outcomes with treatment associated with their own comordities were addressed without emphasis on the individual’s health risks associated with being diagnosed with OSA.
Everyday symptom responses to PAP treatment, including daytime sleepiness measured by the Epworth Sleepiness Scale (Johns, 1993) and functional outcomes measured by the Functional Outcomes of Sleep Questionnaire (Weaver, Laizner, et al., 1997) were also assessed and used during the tailored intervention to address treatment outcome expectancies with PAP use. Participant-identified proximate social sources were queried by participants during first week of treatment to offer observed treatment responses to participants. The tailored intervention was reviewed by a panel of experts prior to testing for intervention face content validity; the panel included experienced sleep providers, two sleep scientists, and a tailored intervention scientist.
Intervention Protocol
The intervention was delivered in four phases: pre-diagnosis, post-diagnosis (i.e., post diagnostic polysomnogram), immediately post-PAP titration polysomnogram, and with week one of home PAP treatment (Table 1). These critical intervention periods were purposefully determined as patients progressing from initial consultation to PAP initiation based on an in-laboratory procedure typically engage, face-to-face, with sleep center staff on this schedule; this approach potentially supports translational capacity of the intervention. Each intervention phase was 30-60 minutes in duration and occurred at the clinical research site (i.e., sleep center) in a private office/examination room or was delivered by telephone during the home PAP treatment period. The intervention was delivered by one research assistant (study interventionist, un-blinded, MV), a registered nurse without sleep specialty care experience, who was extensively trained to provide the tailored intervention. Study training, provided by the study PI (AMS), was conducted over a 1-month pre-study period for 20-hrs/week (total training time = 80hrs), with 40hrs of training specific to protocol operations and 40hrs of training specific to intervention delivery. The intervention delivery was guided by a protocol and script templates for specific exposure phases to minimize a potential interventionist effect. The study interventionist had no other responsibility for primary outcome data collection or cross-over with the comparison group to minimize risk of ascertainment bias.
Table 1.
Social Cognitive Intervention to Promote PAP Adherence: SCIP-PA Description
| Critical Intervention Period |
Critical Indicator (SEMSA*) Measurement |
Tailored Intervention Phase (Activity Participants) |
Tailored Intervention Description (Critical Indicator Domain Target*) |
|---|---|---|---|
| Pre- diagnosis |
1 | Phase I (Participant) |
|
| Post- diagnostic PSG† |
2 | Phase II (Participant; 1 social support resource) |
|
| Post-titration PSG |
3 | Phase III (Participant) |
|
| Day 1, Day 2, Day 5 Home CPAP Treatment Phone Follow Up & Day 8 Home Visit |
4 (Day 2) | Phase IV (Participant; social support resources) |
|
Notes. Theory Derived Intervention Components: RP – Risk Perception; OE – Outcome Expectations; SE – Self Efficacy; F – Facilitators; B – Barriers; G – Goals
Abbreviations: PSG, polysomnogram; SEMSA, Self Efficacy Measure in Sleep Apnea; ESS, Epworth Sleepiness Scale; FOSQ, Functional Outcomes of Sleep Questionnaire; PAP, Positive Airway Pressure
Comparison Condition: Usual Care
The comparison condition was usual care (UC), as defined by the established OSA management protocol at the accredited clinical research site and consistent with the current practice standards for the diagnosis and treatment of OSA in adults (Epstein et al., 2009; Kushida et al., 2006). UC protocol included sleep center provided informational brochures about OSA, diagnostic testing, and PAP prescription. In addition, access by telephone to sleep center staff for problems, questions, or concerns was provided during daytime and evening. The UC condition was delivered by sleep center providers/staff (initial consultation and exam, ordering/scheduling of diagnostic procedures, diagnosis, treatment recommendation, 30-45 day follow-up clinical visit) and one research assistant (study research assistant, un-blinded; JF) who was extensively trained to deliver the UC condition. Pre-study training by the study PI (AMS) was conducted over one month at 10 hours/week (total training time = 40hrs). The study research assistant had no responsibility for primary outcome data collection or cross-over with the intervention group to minimize ascertainment bias risk.
Study Design
A randomized, double-blind, parallel pilot controlled trial was conducted. The overall objective of the research was to examine feasibility and acceptability of the experimental condition and establish the intervention effect size. As a pilot study, statistical significance was not intended or anticipated. We estimated sample size based on prior published reports of pilot or preliminary studies of PAP adherence interventions and fully-powered randomized controlled trials of supportive, educational, cognitive behavioral, and mixed strategy interventions for PAP adherence (Weaver & Sawyer, 2010) as well as considerations for variability in acceptability outcomes. Prior published studies reported total sample sizes of 10 – 142 subjects, inclusive of two or three group randomization schemes with a primary outcome of objective PAP use. To address our study aims, we determined a priori that a sample size of 60 participants, randomized to two groups (n=30 per group), would provide sufficient variability for the primary and acceptability outcomes of the pilot trial.
Setting and Sample
The study was conducted at a suburban, tertiary, academic medical center where a large, American Academy of Sleep Medicine accredited clinical sleep center provided specialized sleep diagnostics and sleep disorder management. The study was approved by the Medical Center’s institutional review board and all participants were provided informed consent and gave authorization for use of identifiable, health-related data. In order that a participant blind was supported from the outset of the trial, IRB-approved consent modification was employed that specifically did not differentiate between study groups in terms of study activities or time commitment and a limited description of the overall study objective; this necessitated debriefing at study termination.
Potential participants were recruited from the clinical sleep center by an open letter of invitation to participate in the study. Any adult patient referred for a diagnostic polysomnogram was invited to participate in the study. Because patients were recruited prior to OSA diagnosis, a standardized pre-enrollment screening assessment was conducted by trained study personnel. The team conducted 431 screening calls with patients who agreed to be contacted by the researchers. Pre-enrollment screening criteria included: (1) no prior diagnosis with OSA or treatment exposure for OSA; and (2) scheduled for a diagnostic polysomnogram. To further enrich sampling of adults likely to have OSA, the Multivariate Apnea Prediction Index, MAP (Maislin et al., 1995), was also included in the pre-enrollment screening assessment. The MAP index is calculated based on a self-reported OSA symptom checklist and demographic characteristics (Maislin et al., 1995); the MAP index has been shown to have high sensitivity and specificity and high discriminatory power for OSA in general sleep center samples and commercial driver samples (Gurubhagavatula, Maislin, Nkwuo, & Pack, 2004; Maislin et al., 1995). An automated scoring program for a calculated MAP index score of ≥0.5 was employed.
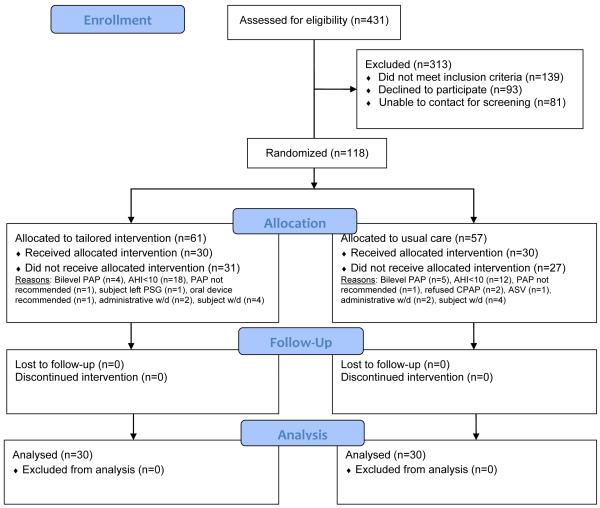
Convenience sampling was used with the following inclusion criteria: (1) newly-diagnosed, with OSA with AHI > 10 events/hour; (2) PAP-naïve; (3) males and females ≥ 18 years of age; and (4) able to read and speak English. Exclusion criteria were: (1) previous diagnosis and/or treatment of OSA; (2) medical record documented new psychiatric diagnosis within previous six months of study enrollment; (3) requirement of supplemental oxygen or bi-level PAP identified on PAP titration PSG suggesting diagnosis other than OSA; and (4) diagnosis of another sleep disorder in addition to OSA based on polysomnogram (i.e. Periodic Limb Movement Disorder [≥10 limb movements/hour of sleep with arousal], central sleep apnea [≥5/hr central apneas], insomnia, sleep hypoventilation syndrome, or narcolepsy). A total of 118 adults with suspected OSA were enrolled and consented for study participation (Figure 1). The trial period, recruitment/enrollment through collection of all outcome data was from December 2011 through March 2014.
Figure 1. CONSORT Flowchart for SCIP-PA Trial.
Notes. Abbreviations: PAP, Positive Airway Pressure; AHI, Apnea Hypopnea Index; PSG, polysomnogram; ASV, Auto-servo ventilation therapy; w/d, withdrawal.
Protocol
After pre-enrollment screening and informed consent, participants completed a demographic questionnaire and were randomized. Randomization employing random block sizes assigned participants to the exposure or comparison group and within each assignment level, 50% were randomly assigned to interview/no interview at study termination and debriefing. A randomization list was generated by the study biostatistician (TSK) and securely maintained at the clinical research site. Random assignment was concealed and completed by consecutive sealed envelope, opened sequentially by interventionist/study RA; sealed envelopes were secured, prepared and monitored by un-blinded study personnel (DAS).
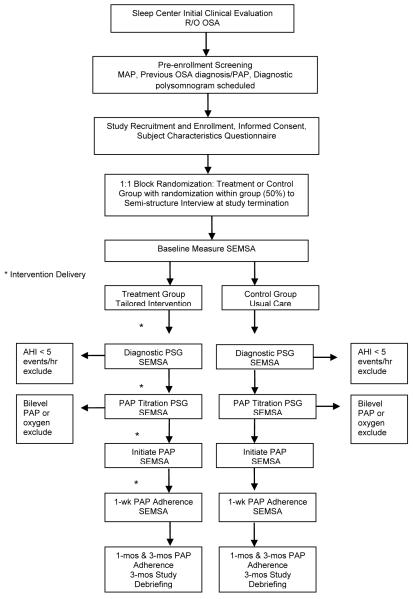
All participants completed a baseline SEMSA assessment after randomization. Thereafter, participants underwent diagnostic polysomnogram, PAP titration polysomnogram, and were initiated on home PAP treatment (Figure 2). PAP treatment was prescribed by the managing sleep provider and set-up by a health insurance-designated home equipment provider. All local home equipment providers used in the study provided standardized PAP education including familiarization with the device, demonstration of equipment use, a mask interface assessment, and access to home equipment company staff for emergencies or problems/questions. All PAP devices included a study-owned modem for daily PAP usage reporting to a study-specific, HIPAA compliant web-based server, accessible only to study personnel.
Figure 2. Study Protocol.
Notes. Abbreviations: OSA, obstructive sleep apnea; MAP, Multivariate Apnea Prediction index; PAP, positive airway pressure; SEMSA, Self-efficacy measure in sleep apnea; PSG, polysomnogram; AHI, apnea hypopnea index; wk, week; mos, months
Participants in the exposure condition completed telephone visits on day 2 and day 5 of home PAP treatment and a 1-week research visit with the study interventionist for intervention delivery. All participants, regardless of assignment, completed a study termination visit at three months with a blinded investigator (AMS) for blind evaluation and standardized debriefing guided by IRB-approved script template. Those participants randomized to a termination interview also completed the semi-structured interview at study termination. All three-month visit activities were guided by a protocol and IRB-approved script/interview guides.
The pilot RCT did not necessitate a data safety monitoring board (DSMB) per federal guidelines. Instead, an internal data safety monitoring committee (DSMC) was convened for monitoring adverse events and recruitment and enrollment progress. The DSMC included one co-investigator (AMK), study biostatistician/co-investigator (TSK), the project director (DAS), and the principal investigator (AMS). The DSMC met quarterly from trial start-up to completion of all data collection. To maintain blinding, no data that risked un-blinding was discussed unless a closed session was requested by the biostatistician or project director.
Measures
The following measures were employed in the study
Subject Characteristics Questionnaire
The questionnaire is an 18-item self-administered form for reporting patient characteristics data, including demographic and reason for seeking sleep care data.
Self-efficacy Measure in Sleep Apnea (SEMSA)
The SEMSA was employed to measure the participants’ risk perception, outcome expectancies, and perceived treatment self-efficacy (Weaver et al., 2003) and specifically as the critical indicator guiding tailoring of the intervention. The SEMSA is a 26-item Likert type scale with content validity, construct validity, internal consistency (Cronbach’s alpha = 0.92), and test-retest reliability for each subscale (r > 0.68)(Weaver et al., 2003). Alternate, non-repeating SEMSA forms were used at each measurement interval to reduce risk of response bias.
Diagnostic and PAP Titration Polysomnogram (PSG)
The overnight, in-laboratory diagnostic and PAP titration PSGs were performed and scored using standard criteria, including the alternate hypopnea definition (Iber, Ancoli-Israel, Chesson, & Quan, 2007). AHI was extracted from the diagnostic PSG. Therapeutic PAP pressure was extracted from the PAP titration PSG.
PAP Use (Adherence)
Objective nightly PAP use data was collected by PAP device and transmitted by modem to a secure study database. The data exported from PAP devices provided minutes of PAP use for every interval of use, operationalized as at-effective pressure when powered on for > 20 minutes. PAP use was quantified as minutes per 24 hours used at effective pressure (continuous outcome) and as a dichotomous outcome, <4 hours/night (non-adherence)/≥4 hours/night (adherence)(Kribbs et al., 1993).
Functional Outcomes of Sleep Questionnaire (FOSQ-30)
A 30-item version, self-report, disease-specific, valid and reliable measure of functional impairment for assessment of the impact of excessive sleepiness on functional status which is sensitive to PAP treatment was used in the study (Chasens, Ratcliffe, & Weaver, 2009; Weaver, Laizner, et al., 1997; Weaver et al., 2007). The FOSQ instrument has a Likert scale response (0-4) with lower scores indicating impaired function. The FOSQ was employed in the exposure condition as a symptom response to treatment assessment.
Epworth Sleepiness Scale (ESS)
A self-report, valid and reliable measure of daytime sleepiness commonly employed in OSA studies was used (Johns, 1993, 1994). The ESS is an 8-item instrument with Likert response scale (0-3) with higher scores suggesting presence of excessive daytime sleepiness. The ESS was employed in the exposure condition as a symptom response to treatment assessment.
Analysis
Standard descriptive statistics were used to describe PAP adherence outcomes and demographic and prognostic variables both overall and within each study group. Demographic variables were described at baseline, while adherence outcomes were described for PAP use at one week (primary PAP use outcome), one month (secondary PAP use outcome), and three months (secondary PAP use outcome). Change in PAP adherence over protocol outcome periods was statistically described. For continuous variables, means, medians, and ranges were produced while frequencies were produced for categorical variables. Graphical tests were used to test for normality of continuous variables and to guide the choice of transformation where warranted.
Variables were compared between the two study groups using appropriate tests, such as t-tests, Mann-Whitney U-test, and exact methods. PAP adherence (average number of hours per night of use) at one week, one month, and three months, as well as change in adherence, were compared between the treatment and control groups via Student’s t-test and used to calculate the effect sizes (Cohen’s d = (μ1- μ2)/SDpooled ; more conservatively, Glass’s Δ = (μ1-μ2)/SDcontrol ). Dichotomous outcomes (i.e. adherence yes/no using <4hours and ≥4hours per night of use) were compared using Fisher’s exact tests. To assess the influence of the hypothesized “active intervention components,” including the theory-derived constructs of social cognitive theory, SEMSA change scores over time were evaluated (time 2-time 1=Δ score) using descriptive statistics and compared between the exposure and control groups using repeated measures analysis of variance models. Baseline SEMSA scores were also accounted for in the repeated measures models. Time was defined by protocol interval, consistent with SEMSA measurement intervals (i.e., baseline, post-diagnostic PSG, post-PAP titration PSG, day 2 PAP home treatment, and day 7-10 PAP home treatment).
Standard descriptive statistics were used to evaluate the feasibility outcomes, including: (1) screening/recruitment/retention of participants; (2) per-protocol fidelity for exposure condition; (3) incomplete data; and (4) blinding assessment of participants. Qualitative descriptive analysis, identifying broad themes, was conducted using data from individual, semi-structured interviews with study participants at study termination to evaluate participant acceptability of the trial experience and exposure condition.
RESULTS
Sample Description
The sample (n=60) included middle-aged (51.3 ± 11.1 years), obese (mean BMI 38.0 [±9.2] kg/m2) men (70%) and women (30%). Participants were predominantly of white race (85%), non-Hispanic or Latino (92%), with severe OSA (AHI 35.9 ± 25.2 events/hour), and were subjectively sleepy (ESS, 19.6 ± 5.2). PAP use at one week, one month, and three months was 6.1 ± 1.72 hours/night, 5.1 ± 1.85 hours/night, and 4.8 ± 2.05 hours/night, respectively. There were no statistically significant differences for any characteristic or research variable between the TI and UC groups except SEMSA outcome expectancies (Table 2).
Table 2.
Sample Description
| Characteristic or Research Variable |
Overall Sample (n=60) [mean (SD) or n (%)] |
Tailored Intervention (n=30) |
Usual Care (n=30) |
P-value** |
|---|---|---|---|---|
| Gender | ||||
| Male | 42 (70%) | 19 (63%) | 23 (77%) | 0.26 |
| Female | 18 (30%) | 11 (37%) | 7 (23%) | |
| Age, yrs | 51.3 (11.1) | 52.5 (11.7) | 50.1 (10.5) | 0.41 |
|
Body Mass Index
(kg/m2) |
38.0 (9.2) | 37.0 (8.1) | 39.0 (10.3) | 0.41 |
| Race | ||||
| White | 51 (85%) | 24 (80%) | 27 (90%) | 0.71 |
| Black or African American |
7 (12%) | 5 (17%) | 2 (7%) | |
| Native Hawaiian/Other Pacific Islander |
0 (0%) | 0 (0%) | 0 (0%) | |
| Asian | 0 (0%) | 0 (0%) | 0 (0%) | |
| American Indian/Alaska Native |
2 (3%) | 1 (3%) | 1 (3%) | |
| Ethnicity | ||||
| Hispanic or Latino | 5 (8%) | 2 (7%) | 3 (10%) | 1.00 |
| Not Hispanic or Latino |
55 (92%) | 28 (93%) | 27 (90%) | |
| Marital Status | ||||
| Married | 45 (75%) | 20 (67%) | 25 (83%) | 0.37 |
| Single | 4 (7%) | 2 (7%) | 2 (7%) | |
| Separated or Divorced |
9 (15%) | 6 (20%) | 3 (10%) | |
| Widow(er) | 2 (3%) | 2 (7%) | 0 (0%) | |
| ESS Score | 19.6 (5.2) | 18.7 (5.1) | 20.4 (5.3) | 0.20 |
|
Polysomnography-
Diagnostics |
||||
| AHI (events/hr) | 35.9 (25.2) | 33.9 (21.3) | 38.0 (28.8) | 0.53 |
| Mild (5-15 events/hour) |
11 (18%) | 3 (10%) | 8 (27%) | 0.14 |
| Moderate (>15-30 events/hour) |
20 (33%) | 13 (43%) | 7 (23%) | |
| Severe (>30 events/hour) |
29 (48%) | 14 (47%) | 15 (50%) | |
| Time < 90% Oxygenation, minutes* |
35.7 (12.5, 112.5) | 34.4 (11.8, 91.5) | 37.4 (14.9, 135.8) | 0.49 |
| Percent of TST <90% Oxygenation* | 6.8 (2.7, 20.3) | 5.9 (2.5, 19.0) | 7.8 (3.1, 30.4) | 0.50 |
|
PAP use (mean
hours/night) |
||||
| 7 days (mean hours/night) |
6.1 (1.72) | 6.4 (2.01) | 5.8 (1.35) | 0.20 |
| <4 hours/night (non-adherers) |
6 (10%) | 4 (13%) | 2 (7%) | 0.67 |
| 30 days (mean hours/night) |
5.1 (1.85) | 5.1 (2.05) | 5.1 (1.66) | 0.90 |
| <4 hours/night (non-adherers) |
17 (28%) | 9 (30%) | 8 (27%) | 1.00 |
| 90 days (mean hours/night) |
4.8 (2.05) | 4.8 (2.27) | 4.7 (1.85) | 0.89 |
| <4 hours/night (non-adherers) |
21 (35%) | 11 (37%) | 10 (33%) | 1.00 |
| SEMSA-R | 2.6 (0.56) | 2.4 (0.5) | 2.7 (0.5) | 0.10 |
| SEMSA-OE | 3.0 (0.58) | 2.8 (0.5) | 3.1 (0.4) | 0.02 |
| SEMSA-SE | 3.1 (0.59) | 3.1 (0.6) | 3.2 (0.5) | 0.39 |
Notes. Abbreviations- SD, Standard Deviation; Yrs, Years; ESS, Epworth Sleepiness Scale; AHI, Apnea hypopnea index; TST, Total Sleep Time; PAP, Positive Airway Pressure; SEMSA-R, Self-efficacy Measure in Sleep Apnea risk perception domain; SEMSA-OE, Self-efficacy Measure in Sleep Apnea outcome expectancies domain; SEMSA-SE, Self-efficacy Measure in Sleep Apnea treatment self-efficacy domain
Median (Q1, Q3) reported
Fisher’s exact, t-test, or Wilcoxon rank sum test
Feasibility Outcomes
Screening, Recruitment and Retention
In an academic, clinical sleep center environment, there was ready-access to the target population; the target population was largely interested in participating in clinical research as 45% of adults with suspected OSA agreed to be contacted by a researcher for participation consideration. Enrolling adults with clinically suspicious OSA prior to diagnosis necessitated the use of a pre-enrollment screening protocol that resulted in 27.4% of willing patients identified as positive for study enrollment (n=118; negative screening rate of 72.6% [n=313]). Sampling criteria for apnea-hypopnea index (AHI) indicative of OSA with expectation to continue on to PAP treatment recommendation further reduced the enrolled participants (n=30, excluded based on AHI inclusion criteria). Thereafter, retention of enrolled participants was high, with attrition occurring among participants primarily for non-protocol related reasons (see Figure 1). Exclusions or withdrawals occurred for the following reasons: refused PAP (n=2), referred to other treatment (n=1), and titrated on other positive airway device for other sleep-related breathing disorders (n=10). Specific to the protocol, four participants did not complete titration PSG and eight participants requested to withdrawal due to personal (i.e., transportation, familial issues, work-related issues) or other pressing health problems. The remaining participants (n=60) completed the protocol; no attrition or loss of data for the PAP use outcome or feasibility/acceptability outcomes occurred. No study-related adverse or serious adverse events occurred.
Protocol periods included a pre-diagnosis visit, diagnostic PSG visit, titration PSG visit, research visits at one-week post-PAP home treatment and at three months post-PAP home treatment. The exposure condition was delivered at four phases. Time between phases were: pre-diagnosis visit (Phase I) to diagnostic PSG visit (Phase II) was 12.02 days (± 13.75 [SD]); diagnostic PSG visit (Phase II) to PAP titration PSG (Phase III) was 36.13 days (± 15.90); PAP titration PSG (Phase III) to home PAP treatment initiation (Phase IV) was 23.88 days (± 10.39).
Per-protocol Fidelity for Exposure Condition
Per-protocol fidelity was regularly assessed throughout the protocol period with weekly protocol audits by un-blinded study personnel (DAS). Two fidelity outcomes were monitored: (1) exposure condition delivery; and (2) duration of time between PAP titration polysomnogram and initiation of PAP home treatment. The exposure condition, TI, was delivered both face-to-face (FTF; phases I, II, and III) and by telephone (TELE; phase IV). Per-protocol delivery of FTF intervention activities was 100% for all participants who completed the protocol. Per-protocol delivery of TELE intervention activities, assessed for Day 2 and Day 5, was significantly lower; fidelity for Day 2 TELE intervention delivery was 43.3% while fidelity for Day 5 TELE intervention delivery was 23.3%.
Blind Assessment
An assessment of the blinding procedures was conducted at study termination (i.e., after 90 days of PAP treatment). Among all participants, 30% (n=18) correctly identified the group assignment (n=8, TI; n=10, UC); 22% (n=13) incorrectly identified the group assignment (n=7, TI; n=6, UC); and 48% (n=29) were unable or unwilling to guess the assignment (n=17, TI; n=14, UC). Among those who correctly identified their group assignment, subjective explanations for their responses included: (a) lots of information provided [to me]; (b) kept informed at every step of the process; (c) helped me understand the importance of using PAP every day; (d) [had a] modem on my PAP; (e) had help from first sleep study to after starting PAP at home; (f) helped me understand sleep study procedure and results and why PAP works for me; (g) only answered questionnaires and didn’t get or have to do much else; (h) no one told me anything about my sleep studies or PAP until I came back to see a sleep doctor after having PAP for a while. Among those who incorrectly identified their group assignment, subjective responses included: (a) just guessing because I have no idea; (b) got PAP quickly; (c) [study personnel] made sure I got PAP at home; (d) lots of communication between sleep center and research people. Among those who refused or were unwilling to guess the group assignment, the reasons offered were: “I don’t remember the group names,” “I don’t remember the differences between the groups to even try to guess,” or “I didn’t think about it since I started the study.”
Acceptability Outcome
The acceptability outcome was assessed at study debriefing after the 90-day period of home PAP treatment. An IRB-approved, semi-structured interview guide was employed to standardize the data collection procedure across randomly-assigned study participants. All participants were satisfied with the trial experience; no complaints about participation were offered. There were no official study complaints reported to non-study related personnel (i.e., IRB, sleep center personnel/providers). Analysis of comments elicited during the semi-structured individual interviews addressed several themes: (a) personalized attention; (b) personalized information about MY OSA and MY MEDICAL CARE; (c) knowing MY contact person who knew MY STORY and MY HEALTH; (d) preparing me for PAP before I had it at home; (e) getting others involved in MY OSA and MY TREATMENT helped me SEE MY OSA symptoms; and (f) answering questions about MY OSA and MY OSA WITH TREATMENT helped me see improvements.
Effect Size: SCIP-PA Tailored Intervention
PAP use as a continuous outcome variable was not significantly different between the TI and UC groups at any protocol interval (Table 2). One week PAP use among the TI group was 35 minutes greater than UC group (p=0.20; Cohen’s d = 0.336; Glass’s Δ = 0.427). At one month, PAP use among the TI group was 3.5 minutes less than UC group (p=0.90; Cohen’s d = −0.032; Glass’s Δ = −0.035). At three months, the TI group used PAP 4.4 minutes more than the UC group (p=0.89; Cohen’s d = 0.036; Glass’s Δ = 0.035). There was approximately 21% non-overlap in the distributions for one week PAP use for the tailored intervention versus usual care group; for all other outcome intervals, the non-overlap distribution was negligible. When PAP use was categorized as <4hrs/night and ≥4hrs/night, there were no differences between groups at 1 week, 1 month, or 3 months (all p > 0.05, Table 2). PAP use declined across the period of outcome assessments, from 1 week to 3 months, for all participants with a decrement from 6.1 (± 1.72) hrs/night at 1 week to 4.8 (± 2.05) hrs/night at 3 months; the attenuated use of PAP was not different between groups (all p > 0.05; Table 2).
Critical Indicator: SEMSA Change Scores and Differences by Group
Risk Perception SEMSA Domain
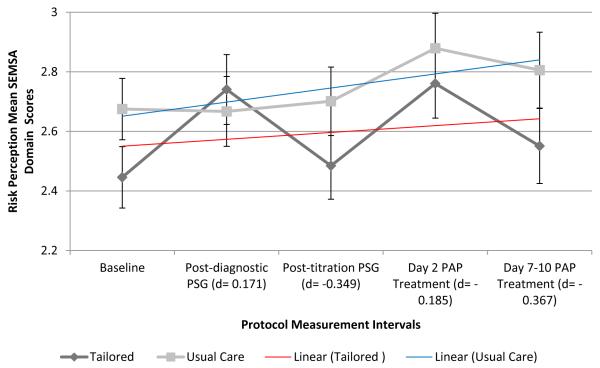
Risk perception scores (mean ± SD) increased across the protocol period among all participants (2.56 ± 0.54, baseline; 2.71 ± 0.63, post-diagnostic PSG; 2.59 ± 0.62, post-titration PSG; 2.83 ± 0.64, day 2 PAP treatment; and 2.68 ± 0.72, day 7-10 [week 1] PAP treatment). Within-group risk perception scores were highly variable among the TI group across the protocol period. Between-group risk perception change over time was different from baseline to post-diagnostic PSG (p=0.03, TI > UC) and from post-diagnostic PSG to post-titration PSG (p=0.04, UC > TI; Figure 3a). Effect sizes for risk perception at each protocol period were small, based on recommended interpretation categories (d=0.2 as small; d=0.5 as medium; d=0.8 as large)(Cohen, 1988). Overall between-group differences for risk perception change scores were not significant from baseline to primary outcome PAP use at week 1 of home treatment (p=0.90).
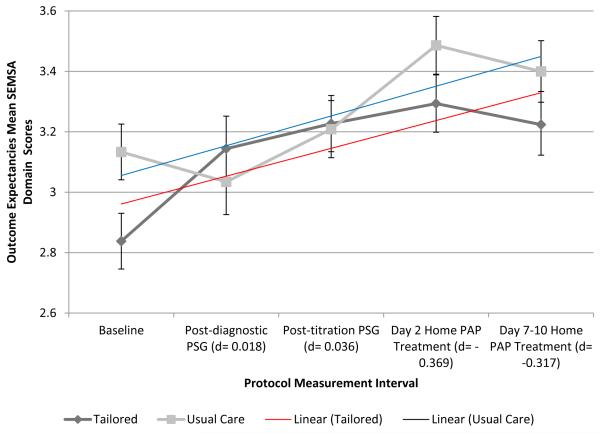
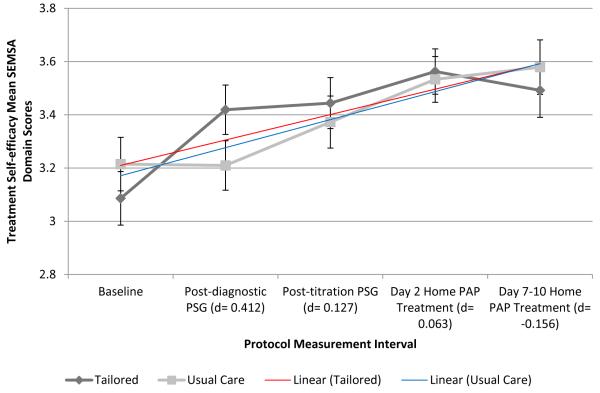
Figure 3. SEMSA Domain Scores Over Time by Group: Risk Perception (3a), Outcome Expectancies (3b), and Treatment Self-efficacy (3c).
Notes. SEMSA domain scores reported as group mean scores with standard errors by protocol measurement period. Effect sizes, Cohen’s d, between-group comparisons by SEMSA outcome at each protocol measurement interval. Linear trend lines by group, Blue = usual care; Red = tailored intervention. Abbreviations – SEMSA, Self-efficacy Measure in Sleep Apnea; PSG, polysomnogram; d, Cohen’s d effect size; PAP, Positive Airway Pressure
Outcome Expectancies SEMSA Domain
Outcome expectancies scores (mean ± SD) demonstrated a modest increase in scores across the protocol period among all participants (2.99 ± 0.49, baseline; 3.09 ± 0.58, post-diagnostic PSG; 3.23 ± 0.52, post-titration PSG; 3.38 ± 0.55, day 2 PAP treatment; and 3.31 ± 0.58, day 7-10 [week 1] PAP treatment). Within-group outcome expectancies were relatively stable among both groups with a modest increase in scores across the protocol period. Between-group outcome expectancies change over time was different from baseline to post-diagnostic PSG (p ≤ 0.001, TI > UC) and from post-diagnostic PSG to post-titration PSG (p=0.03, UC > TI; Figure 3b). Effect sizes for outcome expectancies were small at all protocol periods. Both groups demonstrated significant improvement (i.e., score increases) for outcome expectancies across the protocol period (TI, p ≤ 0.001; UC, p=0.02) without differences between groups (p=0.46).
Treatment Self-efficacy SEMSA Domain
Treatment self-efficacy mean scores (± SD) increased (i.e., improved) across the protocol period for all participants (3.15 ± 0.57, baseline; 3.32 ± 0.54, post-diagnostic PSG; 3.43 ± 0.52, post-titration PSG; 3.54 ± 0.44, day 2 PAP treatment; and 3.54 ± 0.51, day 7-10 [1 week] PAP treatment). Only baseline to post-diagnostic self-efficacy score changes were significantly different between groups (p=0.002, TI > UC; Figure 3c). Effect sizes were small for self-efficacy at all protocol periods with the exception of post-diagnostic PSG self-efficacy (d=0.412; Figure 3c). Within-group self-efficacy change score was significant across measurement periods, baseline to 1 week home treatment, for both TI (p=0.001) and UC groups (p=0.004).
DISCUSSION
PAP non-adherence is a well-recognized problem among adults with OSA and confers considerable health and function risks and impairments. The use of a formal tailoring intervention approach is new to the PAP adherence intervention literature. The results of this trial suggest that the tailored intervention is a feasible and acceptable approach to introducing OSA and PAP treatment to adults. Early PAP use (i.e., 1 week, primary PAP use outcome) was responsive to the tailored PAP adherence intervention approach, but this effect was attenuated over longer-term PAP use (i.e., 1 month and 3 months).
In the current study, participant acceptability of the tailored intervention suggests that personalized care approaches are highly desirable. Thematic analysis of individual semi-structured interview data at study debriefing suggests that participants had expectations for researchers to understand their individual experiences with OSA and PAP; tailored intervention approaches align with such expectations and are therefore likely to meet consumer needs and desires in this era of consumer-driven, person-centered healthcare and precision health.
We identified a small to moderate effect size for the tailored intervention on one week PAP use and a between-group mean difference of 0.58 hours/night of PAP use; this effect size was not persistent for longer-term secondary outcome assessments of PAP use (i.e., 1 month, 3 months). The one week effect size and PAP usage differences between the TI and UC groups identified in our pilot study are consistent with other educational intervention approaches, where the summary PAP use increase has been estimated to be 0.60 (95% CI 0.27-0.93)(Wozniak, Lasserson, & Smith, 2014). The one week effect size identified in the current study is also consistent with “practical support and encouragement” intervention approaches to PAP adherence, wherein the summary PAP use increase has been estimated to be 0.82 (95% CI 0.36-1.27) hours higher in intervention conditions versus comparison conditions (Wozniak et al., 2014). This consistency suggests that the tailored intervention employed in our study, if tested in a fully-powered trial, has potential to be an efficacious intervention strategy for PAP promotion at the outset of treatment among adult OSA patients who are newly-initiated on PAP therapy. Future testing will also address the operational definition of PAP adherence employed in the current study to better align with the overall personalized approach to PAP adherence; this would necessarily incorporate not only the standard operational definition of PAP adherence for comparative purposes, but also individualized treatment responses situated in PAP use (mean hrs/night), or a within-subjects/within-subgroup analysis.
The diminishing effect size over time and modest protocol fidelity for home treatment period intervention activities may indicate that “more” intervention is needed after the initial introduction to PAP therapy. Alternative mechanisms to engage PAP users in intervention activities during the early home treatment period to sustain intervention fidelity “outside the walls of the clinic” while also potentially bolstering the intervention effect size beyond one week of treatment should be considered. Such alternative approaches are now emerging in both clinical and research settings and include telehealth delivered interventions (Stepnowsky, et al., 2015) and mobile health solutions (Pulantara, Parmanto, & Germain, 2015). This recommendation is tempered by two considerations. First, intent-to-treat analyses, the standard recommended approach for clinical trials (Gluud, 2006; Piantadosi, 2005), were conducted to minimize risk of over-estimating effects; per-protocol analyses may have permitted a greater understanding of intervention fidelity effects on the PAP use outcome. Considering the nature of this pilot study, specifically the modest sample size, pursuing additional exploratory analyses that were not a priori-defined heighten the risk of type I error and thus were not pursued. Future testing of the intervention in an adequately powered clinical trial will ideally incorporate a primary intention-to-treat analysis and a per-protocol analysis to address intervention fidelity effects.
Second, we cannot exclude the possibility that usual care effects on the target construct, social cognitive perceptions of OSA and PAP, are not inferior to the effects of the tailored intervention. The primary outcome, PAP adherence, was higher for the tailored intervention group at one-week but these differences were attenuated at secondary outcome assessments; furthermore, the trajectory and change of social cognitive perceptions across the protocol period by group with overlap in standard errors suggest that usual care may be equally influential on the target construct and this may have moderated the attenuated effect on PAP use secondary outcome assessments. Among the tailored intervention group, we identified an increasing, or improving, trend in all SEMSA domains (i.e., risk perception, outcome expectancies, and treatment self-efficacy); similarly, the usual care group also demonstrated improvement for all SEMSA domains. Though this study was not powered to detect between-group statistical differences, these results suggest that by simply establishing an OSA diagnosis with in-laboratory polysomnography followed by PAP treatment exposure, adults gain cognitive insights to their sleep disorder and management of symptoms with PAP; this experiential insight, in and of itself, may result in cognitive perceptions changing over time.
The overall decrease in PAP use over time observed in our study is consistent with prior published observational studies of PAP use (Budhiraja et al., 2007; Weaver, Kribbs, et al., 1997) and randomized controlled trials of PAP interventions (Parthasarathy, Wendel, Haynes, Atwood, & Kuna, 2013). We observed a surprisingly high mean hours of nightly PAP use at one week for all participants (6.1 ± 1.72 hrs/night); PAP use decreased for the entire sample to 4.8 ± 2.05 hrs/night at three months without significant group differences. Ten percent of study participants at one week of PAP treatment were classified as non-adherent based on the commonly-employed non-adherence cut-point of <4hrs/night of use; at three months, 35% of the sample was classified as non-adherent. As prior evidence indicates that early PAP non-adherence (i.e., one week) is one of the most robust indicators of long-term PAP non-adherence (Aloia, Arnedt, Stanchina, & Millman, 2007; Budhiraja et al., 2007; Weaver, Kribbs, et al., 1997), future testing of this intervention approach will ideally consider enrolling adults with early demonstrated non-adherence to PAP or incorporate PAP non-adherence risk assessment as a sampling criteria to potentially minimize sampling bias risks (i.e., self-selection bias) that accompany convenience sampling methods and maximize intervention effects. This may be accomplished by real-time monitoring of early PAP use, adding intervention tailoring based on a PAP use critical indicator and enrolling those subjects with high likelihood, or risk, for PAP nonadherence. Identifying and enrolling newly-diagnosed OSA adults who are at high risk for PAP non-adherence also permits intervention testing in a sample with an identified need for PAP adherence promotion. Though PAP non-adherence risk stratification is relatively new to the field, there are stratification tools that preliminarily demonstrate good predictive validity (Balachandran, Yu, Wroblewski, & Mokhlesi, 2013; Sawyer et al., 2014).
Such an approach aligns with stepped care approaches that have been tested for other health behaviors (Rakowski et al., 2003) and for sleep disorders management, such as insomnia (Espie, 2009; Mack & Rybarczyk, 2011) and PAP non-adherence (Deng, Wang, Sun, & Chen, 2013). Stepped care is a care delivery framework that uses limited resources to greatest effect (Von Korff, Glasgow, & Sharpe, 2006) to conserve resources and appropriately allocate scarce resources while also ensuring individual patient healthcare needs are met.(Bower & Gilbody, 2005) Not unlike the tailored intervention approach we report, systematic assessment of key indicators provides baseline and subsequent data to guide decisions for stepped care, which may be optimized (increased, decreased, or maintained) based on repeated systematic assessment of the key indicators. This approach acknowledges that different individuals need different levels of care but such care delivery decisions are guided by systematic assessments, not unlike critical indicators in the tailored intervention approach.
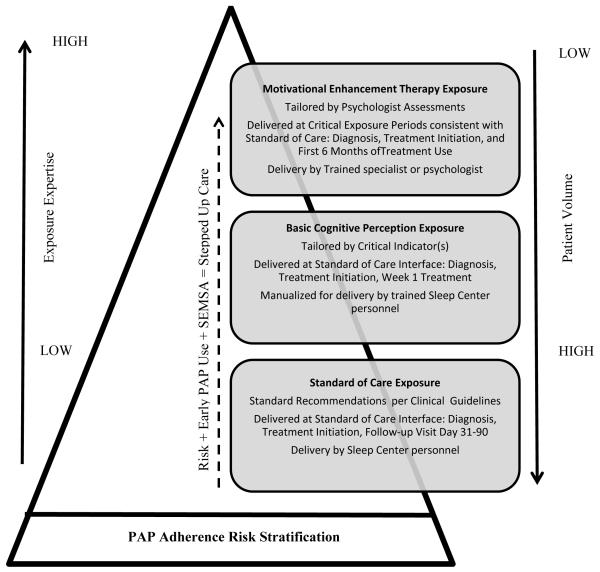
To our knowledge there are no published studies of formally-defined tailored interventions to promote PAP adherence, though there are studies that have approached the problem of PAP non-adherence from an individualized, or personalized, perspective and specifically targeted social cognitive constructs for behavior change (i.e., PAP adherence). This includes studies of motivational enhancement therapy (Aloia et al., 2013; Bakker et al., 2016; Lai, Fong, Lam, Weaver, & Ip, 2014), motivational interviewing (Olsen, Smith, Oei, & Douglas, 2012) and change stage-matched interventions (Deng et al., 2013). All such studies, including the study we report herein, are predicated on the value of personalized approaches to behavior change and provide theoretically-derived behavior change interventions that are quite similar. We suggest that key differences across these very similar PAP adherence interventions published to date are relative to (1) expertise required for exposure delivery; (2) time required for complete exposure; and (3) complexity of exposure conditions. If such differences are considered in the context of a stepped care approach, a model of personalized stepped care for PAP adherence can be readily envisioned (Figure 4).
Figure 4. A Stepped Care Model for PAP Adherence.
Notes. Abbreviations – PAP, positive airway pressure; SEMSA, Self-efficacy Measure in Sleep Apnea; Reprinted and adapted with permission from Espie CA (2009). “Stepped Care”: A health technology solution for delivering cognitive behavioral therapy as a first line insomnia treatment. Sleep 32(12): 1549-58.
Based on the pilot study results, there are opportunities to improve study design, methods, and protocol for subsequent testing including: (1) enrollment of high risk PAP non-adherers which necessitates a pre-enrollment risk stratification assessment; (2) employ an attention control comparator to improve participant blind effectiveness; (3) adding early PAP use (i.e., days 1-7) as a critical indicator for tailoring; and (4) development of intervention delivery strategies that support exposure to the condition during the first week of home PAP treatment. Further testing of the tailored intervention is predicated on using the feasibility and acceptability results of the current pilot study to potentiate a well-designed, high fidelity clinical trial that is adequately powered to test the overall efficacy of the tailored intervention to improve PAP adherence.
Acknowledgement
The investigators acknowledge the staff and providers at the Penn State Hershey Medical Center Sleep Center for their assistance and support during participant recruitment and conduct of the research.
Funding Sources
The project described was supported by Award Number R00NR011173 (Sawyer AM, PI) from the National Institute of Nursing Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health. The reported research was also supported by American Nurses Foundation and Sigma Theta Tau International (Sawyer AM, PI) and the American Sleep Apnea Association (sleep apnea educational materials). None of the funding sources contributed to study design, data collection, analysis and interpretation of the data, or dissemination of the study results.
Footnotes
Registration: NCT01454830
Disclosure of Conflicts of Interest
The following authors disclose no conflicts of interest: AM Sawyer, TS King, DA Sawyer, M Varrasse, J Franks, A Watach, A Kolanowski and KC Richards. TE Weaver discloses the following potential conflicts of interest: Research equipment from Philips Respironics; Investigator-initiated grant funding from TEVA, Inc. and Jazz Pharmaceuticals; License Agreements for the Functional Outcomes of Sleep Questionnaire with the following: Philips Respironics; Regent Medical, Inc.; Balance Therapeutics; Nyxoah; ResMed; ImThera Medical; Jazz Pharmaceuticals.
References
- Aloia MS, Arnedt JT, Riggs RL, Hecht J, Borrelli B. Clinical Management of Poor Adherence to CPAP: Motivational Enhancement. Behavioral Sleep Medicine. 2004;2(4):205–222. doi: 10.1207/s15402010bsm0204_3. [DOI] [PubMed] [Google Scholar]
- Aloia MS, Arnedt JT, Stanchina M, Millman RP. How early in treatment is PAP adherence established? Revisiting night-to-night variability. Behavioral Sleep Medicine. 2007;5:229–240. doi: 10.1080/15402000701264005. [DOI] [PubMed] [Google Scholar]
- Aloia MS, Arnedt JT, Strand M, Millman RP, Borrelli B. Motivational enhancement to improve adherence to positive airway pressure in patients with obstructive sleep apnea: a randomized controlled trial. Sleep. 2013;36(11):1655–1662. doi: 10.5665/sleep.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Sleep Medicine Task Force Sleep-related breathing disorders in adults: Recommendations for syndrome definitions and measurement techniques in clinical research. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- Antic NA, Catcheside P, Buchan C, Hensley M, Naughton M, Rowland S, McEvoy RD. The effect of CPAP on normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep. 2011;34:111–119. doi: 10.1093/sleep/34.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayas NT, FitzGerald JM, Fleetham JA, White DP, Schulzer M, Ryan F, Marra CA. Cost-effectiveness of continuous positive airway pressure therapy for moderate to severe obstructive sleep apnea/hypopnea. Archives of Internal Medicine. 2006;166:977–984. doi: 10.1001/archinte.166.9.977. [DOI] [PubMed] [Google Scholar]
- Bakker JP, Wang R, Weng J, Aloia MS, Toth C, Morrical MG, Redline S. Motivational Enhancement for Increasing Adherence to CPAP: A Randomized Controlled Trial. Chest. 2016;150(2):337–345. doi: 10.1016/j.chest.2016.03.019. http://dx.doi.org/10.1016/j.chest.2016.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran JS, Yu X, Wroblewski K, Mokhlesi B. A brief survey of patients' first impression after CPAP titration predicts future CPAP adherence: A pilot study. J Clin Sleep Med. 2013;9:199–205. doi: 10.5664/jcsm.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A. Health promotion by social cognitive means. Health Education & Behavior. 2004;31(2):143–164. doi: 10.1177/1090198104263660. [DOI] [PubMed] [Google Scholar]
- Bonsignore MR, Borel AL, Machan E, Grunstein R. Sleep apnoea and metabolic dysfunction. Eur Respir Rev. 2013;22(129):353–364. doi: 10.1183/09059180.00003413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower P, Gilbody S. Stepped care in psychological therapies: access, effectiveness and efficiency. Narrative literature review. Br J Psychiatry. 2005;186:11–17. doi: 10.1192/bjp.186.1.11. [DOI] [PubMed] [Google Scholar]
- Budhiraja R, Parthasarathy S, Drake CL, Roth T, Sharief I, Budhiraja P, Saunders V. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep. 2007;30:320–324. [PubMed] [Google Scholar]
- Campos-Rodriguez F, Martinez-Garcia MA, Reyes-Nuñez N, Caballero-Martinez I, Catalan-Serra P, Almeida-Gonzalez CV. Role of Sleep Apnea and CPAP therapy in the incidence of stroke or coronary heart disease in women. Am J Respir Crit Care Med. 2014;189(12):1544–1550. doi: 10.1164/rccm.201311-2012OC. [DOI] [PubMed] [Google Scholar]
- Campos-Rodriguez F, Pena-Grinan N, Reyes-Nunez F, De la Cruz-Moron I, Perez-Ronchel J, De la Vega-Gallardo F, Fernandez-Palacin A. Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure. Chest. 2005;128:624–633. doi: 10.1378/chest.128.2.624. [DOI] [PubMed] [Google Scholar]
- Centers for Medicare and Medicaid Services . Continuous Positive Airway Pressure (CPAP) Therapy for Obstructive Sleep Apnea (OSA) Centers for Medicare and Medicaid Services; 2008. http://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/mm6048.pdf: [Google Scholar]
- Chasens ER, Ratcliffe SJ, Weaver TE. Development of the FOSQ-10: A short version of the functional outcomes of sleep questionnaire. Sleep. 2009;32(7):915–919. doi: 10.1093/sleep/32.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Routledge Academic; New York: 1988. [Google Scholar]
- Collins FS, Varmus H. A New Initiative on Precision Medicine. New England Journal of Medicine. 2015;372(9):793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: new guidance. 2008 doi: 10.1136/bmj.a1655. Retrieved from www.mrc.ac.uk/complexinterventionsguidance. [DOI] [PMC free article] [PubMed]
- Crawford MR, Espie CA, Bartlett DJ, Grunstein RR. Integrating psychology and medicine in CPAP adherence - New concepts? Sleep Medicine Reviews. 2013 doi: 10.1016/j.smrv.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Deng T, Wang Y, Sun M, Chen B. Stage-matched intervention for adherence to CPAP in patients with obstructive sleep apnea: a randomized controlled trial. Sleep Breath. 2013;17(2):791–801. doi: 10.1007/s11325-012-0766-3. [DOI] [PubMed] [Google Scholar]
- Enderlin CA, Richards KC. Research testing of tailored interventions. Research and Theory for Nursing Practice: An International Journal. 2006;20(4):317–324. doi: 10.1891/rtnp-v20i4a007. [DOI] [PubMed] [Google Scholar]
- Epstein LJ, Kristo D, Strollo PJ, Jr., Friedman N, Malhotra A, Patil SP, Weinstein MD. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–276. [PMC free article] [PubMed] [Google Scholar]
- Espie CA. "Stepped care": a health technology solution for delivering cognitive behavioral therapy as a first line insomnia treatment. Sleep. 2009;32(12):1549–1558. doi: 10.1093/sleep/32.12.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluud LL. Bias in clinical intervention research. American Journal of Epidemiology. 2006;163(6):493–501. doi: 10.1093/aje/kwj069. [DOI] [PubMed] [Google Scholar]
- Guest JF, Helter MT, Morga A, Stradling JR. Cost-effectiveness of using continuous positive airway pressure in the treatment of severe obstructive sleep apnea/hypopnoea syndrome in the UK. Thorax. 2008;63:860–865. doi: 10.1136/thx.2007.086454. [DOI] [PubMed] [Google Scholar]
- Gurubhagavatula I, Maislin G, Nkwuo JE, Pack AI. Occupational screening for obstructive sleep apnea in commercial drivers. Am J Respir Crit Care Med. 2004;170(4):371–376. doi: 10.1164/rccm.200307-968OC. [DOI] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events. American Academy of Sleep Medicine; Westchester, IL: 2007. [Google Scholar]
- Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness Scale. Chest. 1993;103:30–36. doi: 10.1378/chest.103.1.30. [DOI] [PubMed] [Google Scholar]
- Johns MW. Sleepiness in different situations measured by the Epworth Sleepiness Scale. Sleep. 1994;17:703–710. doi: 10.1093/sleep/17.8.703. [DOI] [PubMed] [Google Scholar]
- Kribbs NB, Pack AI, Kline LR, Smith PL, Schwartz AR, Schubert NM, Dinges DF. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. American Reviews in Respiratory Diseases. 1993;147:887–895. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- Kushida CA, Littner MR, Kirshkowitz M, Morgenthaler TI, Alessi CA, Bailey D, Wise MS. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. 2006;29:375–380. doi: 10.1093/sleep/29.3.375. [DOI] [PubMed] [Google Scholar]
- Lai AY, Fong DY, Lam JC, Weaver TE, Ip MS. The efficacy of a brief motivational enhancement education program on CPAP adherence in OSA: a randomized controlled trial. Chest. 2014;146(3):600–610. doi: 10.1378/chest.13-2228. [DOI] [PubMed] [Google Scholar]
- Lee W, Nagubadi S, Kryger MH, Mokhlesi B. Epidemiology of Obstructive Sleep Apnea: a Population-based Perspective. Expert Rev Respir Med. 2008;2(3):349–364. doi: 10.1586/17476348.2.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack LJ, Rybarczyk BD. Behavioral treatment of insomnia: A proposal for a stepped-care approach to promote public health. Nature and Science of Sleep. 2011;3:87–99. doi: 10.2147/NSS.S12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maislin G, Pack A, Kribbs NB, Smith PL, Schwartz AR, Kline LR, Dinges D. A survey screen for prediction of apnea. Sleep. 1995;18:158–166. doi: 10.1093/sleep/18.3.158. [DOI] [PubMed] [Google Scholar]
- Marshall NS, Wong KK, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J Clin Sleep Med. 2014;10(4):355–362. doi: 10.5664/jcsm.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler R, Bartoszek G, Kopke S, Meyer G. Proposed criteria for reporting the development and evaluation of complext interventions in healthcare (CReDECI): guideline development. International Journal of Nursing Studies. 2012;49:40–46. doi: 10.1016/j.ijnurstu.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Mulgrew A, Nasvadi G, Butt A, Cheema R, Fox N, Fleetham J, Ayas N. Risk and severity of motor vehicle crashes in patients with obstructive sleep apnoea/hypopnoea. Thorax. 2008;63:536–541. doi: 10.1136/thx.2007.085464. [DOI] [PubMed] [Google Scholar]
- Nieto FJ, Peppard PE, Young T, Finn L, Hla KM, Farre R. Sleep-disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2012;186(2):190–194. doi: 10.1164/rccm.201201-0130OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen S, Smith SS, Oei TP, Douglas J. Motivational interviewing (MINT) improves continuous positive airway pressure (CPAP) acceptance and adherence: a randomized controlled trial. J Consult Clin Psychol. 2012;80(1):151–163. doi: 10.1037/a0026302. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S, Wendel C, Haynes PL, Atwood C, Kuna S. A pilot study of CPAP adherence promotion by peer buddies with sleep apnea. J Clin Sleep Med. 2013;9(6):543–550. doi: 10.5664/jcsm.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantadosi S. Clinical Trials: A methodological perspective. 2nd ed. John Wiley & Sons, Inc; Hoboken, New Jersey: 2005. Random error and bias; pp. 167–186. [Google Scholar]
- Pulantara W, Parmanto B, Germain A. The feasibility of mobile health (MHEALTH) applications in behavioral sleep interventions for military populations; Paper presented at the Sleep 2015; Seattle, Washington. 2015. [Google Scholar]
- Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proceedings of the American Thoracic Society. 2008;5:136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakowski W, Lipkus IM, Clark MA, Rimer BK, Ehrich B, Lyna PR. Reminder letter, tailored stepped-care and self-choice comparison for repeat mammography. American Journal of Preventive Medicine. 2003;25:308–314. doi: 10.1016/s0749-3797(03)00215-0. al, e. [DOI] [PubMed] [Google Scholar]
- Richards KC, Enderlin CA, Beck C, McSweeney JC, Jones TC, Roberson PK. Tailored behavioral interventions: A literature review and synthesis. Research and Theory for Nursing Practice: An International Journal. 2007;21(4):271–285. doi: 10.1891/088971807782428029. [DOI] [PubMed] [Google Scholar]
- Sawyer AM, King TS, Hanlon A, Richards KC, Sweer L, Rizzo A, Weaver TE. Risk assessment for CPAP nonadherence in adults with newly diagnosed obstructive sleep apnea: preliminary testing of the Index for Nonadherence to PAP (I-NAP) Sleep Breath. 2014;18(4):875–883. doi: 10.1007/s11325-014-0959-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Young T. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. 2008;52(8):686–717. doi: 10.1016/j.jacc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Strohl KP, Brown DB, Collop N, George C, Grunstein R, Han F, Wilson K. An official American Thoracic Society Clinical Practice Guideline: sleep apnea, sleepiness, and driving risk in noncommercial drivers. An update of a 1994 Statement. Am J Respir Crit Care Med. 2013;187(11):1259–1266. doi: 10.1164/rccm.201304-0726ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan MCY, Ayas NT, Mulgrew AT, Cortes L, FitzGerald JM, Fleetham JA, Marra CA. Cost-effectiveness of continuous positive airway pressure therapy in patients with obstructive sleep apnea-hypopnea in British Columbia. Canadian Respiratory Journal. 2008;15(3):159–165. doi: 10.1155/2008/719231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Korff M, Glasgow RE, Sharpe M. ABC of psychological medicine: Organising care for chronic illness. British Medical Journal. 2006;325:92–94. doi: 10.1136/bmj.325.7355.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver TE, George CFP. Cognition and performance in patients with obstructive sleep apnea. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 5th ed. Elsevier Saunders; St. Louis: 2011. pp. 1194–1205. [Google Scholar]
- Weaver TE, Kribbs NB, Pack AI, Kline LR, Chugh DK, Maislin G, Dinges DF. Night-to-night variability in CPAP use over first three months of treatment. Sleep. 1997;20:278–283. doi: 10.1093/sleep/20.4.278. [DOI] [PubMed] [Google Scholar]
- Weaver TE, Laizner A, Evans LK, Maislin G, Chugh DK, Lyons K, Dinges DF. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–843. [PubMed] [Google Scholar]
- Weaver TE, Maislin G, Dinges DF, Bloxham T, George CFP, Greenberg H. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30:711–719. doi: 10.1093/sleep/30.6.711. al., e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver TE, Maislin G, Dinges DF, Younger J, Cantor C, McCloskey S, Pack AI. Self-efficacy in sleep apnea: Instrument development and patient perceptions of obstructive sleep apnea risk, treatment benefit, and volition to use continuous positive airway pressure. Sleep. 2003;26:727–732. doi: 10.1093/sleep/26.6.727. [DOI] [PubMed] [Google Scholar]
- Weaver TE, Sawyer A. Adherence to continuous positive airway pressure treatment for obstructive sleep apnoea: Implications for future interventions. Indian Journal of Medical Research. 2010;131:245–258. [PMC free article] [PubMed] [Google Scholar]
- Wozniak D, Lasserson T, Smith I. Educational, supportive and behavioural interventions to improve usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database of Systematic Reviews. 2014;(1) doi: 10.1002/14651858.CD007736.pub2. [DOI] [PubMed] [Google Scholar]
- Young T, Peppard P, Gottlieb D. Epidemiology of obstructive sleep apnea: A population health perspective. American. Journal of Respiratory & Critical Care Medicine. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- Zimmerman ME, Arnedt T, Stanchina M, Millman RP, Aloia MS. Normalization of memory performance and positive airway pressure adherence in memory-impaired patients with obstructive sleep apnea. Chest. 2006;130:1772–1778. doi: 10.1378/chest.130.6.1772. [DOI] [PubMed] [Google Scholar]