Abstract
Showing modest efficacy, the RV144 HIV-1 vaccine clinical trial utilized a non-replicating canarypox viral vector and a soluble gp120 protein boost. Here we built upon the RV144 strategy by developing a novel combination of a replicating, but highly-attenuated Vaccinia virus vector, NYVAC-KC, and plant-produced HIV-1 virus-like particles (VLPs). Both components contained the full-length Gag and a membrane anchored truncated gp41 presenting the membrane proximal external region with its conserved broadly neutralizing epitopes in the pre-fusion conformation. We tested different prime/boost combinations of these components in mice and showed that the group primed with NYVAC-KC and boosted with both the viral vectors and plant-produced VLPs have the most robust Gag-specific CD8 T cell responses, at 12.7% of CD8 T cells expressing IFN-γ in response to stimulation with five Gag epitopes. The same immunization group elicited the best systemic and mucosal antibody responses to Gag and dgp41 with a bias towards IgG1.
Keywords: HIV-1, Vaccinia virus, Prime/boost, Virus-like particles, Tobacco mosaic virus, Electron microscopy, Live vector vaccines, Subunit vaccines
1. Introduction
Despite the success of antiretroviral treatment, human immunodeficiency virus (HIV) still causes millions of new infections every year, primarily in regions of the world with limited access to healthcare, making the need for a vaccine more apparent every year. To date, even the most successful HIV-1 vaccine clinical trial, the Phase III Thai Trial (RV144) had only modest and short-lived efficacy, thus leaving room for improvement (Rerks-Ngarm et al., 2009). The Thai Trial used a non-replicating canarypox viral vector (ALVAC) carrying recombinant genes for Gag, Pol and the surface subunit of the envelope (ENV) protein (gp120) followed by an AIDSVAX protein boost consisting of recombinant gp120 B/E produced in Chinese hamster ovary (CHO) cells. While ALVAC and AIDSVAX showed no protection when tested individually, their combination in RV144 was modestly protective and provided some valuable information on immune correlates of protection. The most direct immune correlates of protection pertained to (antibody) Ab responses against the V1-V2 loop of gp120 and revealed the importance of polyfunctional, non-neutralizing Abs (Haynes et al., 2012; Yates et al., 2014; Chung et al., 2014; Liao et al., 2013). This short-lived humoral response faded over time and did not provide long-lasting protective advantage over the placebo arm (Yates et al., 2014). The results of the RV144 trial indicated the strength of a prime-boost approach integrating on a live vector with a subunit protein vaccine. However, the modest efficacy of the trial also suggests that the particular combination of the specific antigens chosen can be further optimized (Haynes et al., 2012; Yates et al., 2014; Corey et al., 2015; McMichael and Koff, 2014; Prentice et al., 2015).
Because of its surface exposure, its immunogenicity, and the critical roles it plays during target-cell infection, gp120 has been a natural target for vaccine development since the early days of HIV-1 research (Burton and Mascola, 2015; Mascola and Haynes, 2013). This, however, has proven to be far from straightforward, because gp120 also functions as a highly mutable decoy with most of its functionally important immunogenic sites conformationally occluded or shielded by glycans. In fact, monomeric preparations of gp120, as well as various preparations aimed at presentation of gp120 trimers, have repeatedly failed to induce protective immune responses in animal models or humans, with the sole and modest exception of the Thai Trial (McGuire et al., 2014; Jacob et al., 2015; Moore et al., 2015; Haynes et al., 2016).
In contrast, far fewer studies have examined gp41. It contains highly immunogenic determinants that induce production of Abs that are among the first to arise during acute HIV-1 infection but are of very limited protective value, showing little or no antiviral activities (Burton and Mascola, 2015; Liao et al., 2011; Bonsignori et al., 2012). These immunodominant epitopes are located in a region of the protein spanning the two heptad repeat domains and in particular within the loop that connects them (Zolla-Pazner, 2004) and are exposed on the gp41 “stump” in its six-helical bundle conformation upon removal of the gp120 subunit (Burton and Mascola, 2015). Still, gp41 was found to be the target of a number of broadly neutralizing Abs (bnAbs) directed against conformational (35O22 binding at the gp41-gp120 interface) (Huang et al., 2014) and linear epitopes (2F5, 4E10, Z13 and 10E8 that recognize closely situated sites within the membrane proximal external region, MPER) (Parker et al., 2001; Cardoso et al., 2005; Nelson et al., 2007; Huang et al., 2012).
Beyond neutralization, anti-MPER Abs, including the above-mentioned bnAbs, were found to exhibit other potent anti-HIV-1 activities including transcytosis blockade (Tudor et al., 2012; Shen et al., 2010; Matoba et al., 2004, 2008, 2009), Ab-dependent cell-mediated cytotoxicity (ADCC) (Tudor and Bomsel, 2011; Hessell et al., 2007) and curbing of dendritic cell-mediated trans-infection (Tudor et al., 2012; Sagar et al., 2012; Magerus-Chatinet et al., 2007). Moreover, passive immunization with these bnAbs provided impressive protection against mucosal transmission of a simian-HIV hybrid (SHIV) in the macaque model (Baba et al., 2000; Hessell et al., 2010; Klein et al., 2013a). Anti-MPER Abs with anti-viral activities were also described in mucosal secretions of highly-exposed but seronegative individuals (Kaul et al., 1999, 2001; Pastori et al., 2000; Devito et al., 2000a, 2000b; Tudor et al., 2009). Lastly, anti-MPER Abs that were passively passed through breast milk from infected mothers to their uninfected babies were correlated with protection (Diomede et al., 2012; Pollara et al., 2015).
Despite their long-standing promise, anti-MPER bnAbs are naturally rare and are notoriously difficult to elicit. For example, less than 25% of HIV-1-infected patients develop broad and potent nAbs, which even if present require ~2–4 years to produce (Burton and Mascola, 2015; Mascola and Haynes, 2013; Haynes et al., 2016; Mascola and Montefiori, 2010). Several explanations have been suggested, including a lengthy maturation process involving many somatic mutations and extensive affinity maturation (Klein et al., 2013b; Kepler et al., 2014), and clonal deletion of the B-cell lines secreting such bnAbs that were shown to be partially autoreactive (Verkoczy et al., 2010, 2011, 2013). Interestingly, many of the autoreactivity targets are lipids that can be found in both the plasma membrane of the host cell and in the viral envelope (Haynes et al., 2005; Alam et al., 2007, 2009; Irimia et al., 2016).
It is widely accepted that the membrane milieu of the MPER region plays a major role in the functional immunogenicity of gp41 (Alam et al., 2009; Chen et al., 2014). Both the metastable native conformation of the gp120-gp41 trimer and the highly stable post-fusion conformations do not expose the neutralizing epitopes (Frey et al., 2008, 2010; Buzon et al., 2010; Pancera et al., 2014), and fail to efficiently elicit bnAbs (Williams et al., 2015; Sanders et al., 2015; Crooks et al., 2015; Davis et al., 2009; Decker et al., 2005; Labrijn et al., 2003) or engage the germline B cell precursors for specific bnAbs (McGuire et al., 2014; Hoot et al., 2013; Jardine et al., 2013).
An effective MPER-based antigen therefore requires presentation of the MPER within a context of a membrane and exposure of the region in a conformation mimicking the putative pre-fusion intermediate. For example, Bomsel and co-workers used a gp41 peptide (residues 649–684) spanning the MPER and part of the C-terminal heptad repeat to decorate liposomes (“virosome”) and demonstrated the ability of the immunogen to elicit systemic and mucosal antibodies with transcytosis-blocking activity in humans (Leroux-Roels et al., 2013) and nonhuman primates that were thereafter protected against a mucosal SHIV challenge (Bomsel et al., 2011). Together, these results indicate a strong potential for this antigenic region to be used as a vaccine component when presented correctly on a membrane.
Our laboratory developed an efficient production method for enveloped VLPs in the tobacco-relative Nicotiana benthamiana, consisting of Gag and deconstructed-gp41 (dgp41: MPER, transmembrane domain, and full-length cytoplasmic tail) (Kessans et al., 2013). Such VLPs display the MPER of gp41 without steric hindrance from gp120, without the immunodominant epitopes on both Env subunits, and with a higher antigen load per VLP than what exists in an HIV virion (Kessans et al., 2016). A similar construct has been shown by us to be a likely trimer with its bnAb epitopes exposed (Gong et al., 2015). When administered to mice, these VLPs elicit both serum IgG and mucosal IgA to Gag and dgp41 antigens (Kessans et al., 2016).
Live viral vectors are appealing as vaccine vehicles for the expression of HIV-1 antigens due to their proficiency for eliciting T cell responses. For example, a cytomegalovirus (CMV) vector was recently shown in nonhuman primates to clear an established SIV infection with dependency on CD8 T cells recognizing non-canonical epitopes on major histocompatibility complex (MHC) II instead of MHC I (Hansen et al., 2013a, 2013b). Poxviruses also stand out amongst other viral vectors [see reviews (Gomez et al., 2012a; Pantaleo et al., 2010; Jacobs et al., 2009)] as attested by the Thai Trial that employed a canarypox-based vector and was the first and only HIV-1 vaccine clinical trial to show efficacy (Rerks-Ngarm et al., 2009; Haynes et al., 2012). The canarypox-based viral vector used in the trial, ALVAC, is not capable of replication in mammalian cells (Taylor and Paoletti, 1988; Taylor et al., 1988). Inability to replicate in vivo in humans makes the virus safer to use as a vaccine or a vaccine vector. However, we hypothesized that replication could enhance immunogenicity by increasing antigen load as the virus replicates in vivo.
To test this hypothesis, we turned to a replicating vaccinia virus strain, NYVAC-KC. The virus was developed from the NYVAC strain previously rendered replication incompetent in human cells by deletion of 18 open reading frames from the genome of Copenhagen (Cop), its parental strain (Tartaglia et al., 1992a). NYVAC-KC contains a reinsertion of two host-range genes, K1L and C7L, which allows the virus to replicate in human tissue, thus improving immunogenicity while remaining highly attenuated (Kibler et al., 2011; Quakkelaar et al., 2011).
In this study, we describe the development of replicating vaccinia virus vectors based on NYVAC-KC that express Gag and dgp41 of HIV-1 as matching antigens to the plant-produced VLPs for prime/boosting purposes. We show that the viral vectors produce VLPs in vitro and test the viral vectors in different combinations with plant-produced VLPs in mice. The animal study revealed that these two vaccine components work in concert to elicit Gag-specific CD8 T cell responses and both systemic and mucosal antibodies to Gag and dgp41 peptides in the immunization group which most closely mimics the Thai Trial.
2. Materials and methods
2.1. Cloning pGNR plasmids for virus recombination
Construction of synthetic genes encoding Gag (from subtype C R5 HIV-1 isolate 1084i, GenBank #AY805330; synthetic construct GenBank #JX534517) and dgp41 (MPER derived from the B-clade MN isolate, GenBank #AF075722, transmembrane and C-terminal domains from the 1084i isolate #AY805330; synthetic construct # JX534518) was previously described (Kessans et al., 2013). The genes were cloned into the pGNR plasmid, which harbors a neoGFP selection cassette (neomycin resistance gene fused to GFP, known as pGNR) between homologous recombination arms for the vaccinia TK locus. Gag and dgp41 genes were amplified from pTM 488 (Gag) and pTM 602 (dgp41) [described in (Kessans et al., 2013)] into TOPO pCR-2.1 vectors (Invitrogen). Cloning was achieved using AccuStart Taq DNA polymerase HiFi PCR kit (Quanta Biosciences) with primers oTM 664 (5′-ACTAGTATGGGAGCTAGAGCCTCT-3″) and 665 1 (5′-CCCGGGTTATTGAGAGGAAG-3′) for Gag and oTM 666 (5′-ACTAGTATGGGATCTCAAACTCAACAA-3′) and 1667 (5′-CCCGGGTTATTGCAAAGCA G-3′) for dgp41 for the addition of 5′ flanking SpeI and 3′ flanking XmaI sites, denoted in italics. Ligation reactions were electroporated into DH5α Escherichia coli and plated onto LB +ampicillin plates. Gene insertion was confirmed by colony PCR using GoTaq Green Master Mix (Promega). Plasmids were extracted using the E.Z.N.A. mini-prep kit (Omega) and sequences verified using backbone-specific primers M13-forward and M13-reverse. The TOPO plasmids were designated pTM 813 (Gag) and pTM 814 (dgp41). Both pTM 813, 814, and pGNR were digested with SpeI and XmaI (NEB) and fragments separated via gel electrophoresis and extracted using QIAquick Gel Extraction Kit (Qiagen), then ligated into the pGNR backbone using T4 DNA ligase (Promega). Ampicillin resistant DH5α E. coli colonies were screened by colony PCR and plasmids extracted as above. Sequences were confirmed with pGNR backbone-specific primers oTM 686 (5′-CCCACCCGCTTTTTATAGTAA-3′) and 687 (5′-CGGTTTATCTAACGACACAACA-3′). Sequence-verified pGNR plasmids were named pTM 815 (Gag) and pTM 816 (dgp41).
2.2. Cell lines and viruses
Monkey kidney BSC-40 cells were grown in DMEM (Corning Cellgro) with 5% FBS plus gentamycin and 2 mM L-glutamine. Baby hamster kidney BHK cells were grown in MEM (Corning Cellgro) with 5% FBS plus gentamycin. Generation of the parental vaccinia virus (VACV) strain NYVAC-KC was described previously (Kibler et al., 2011).
2.3. In vivo recombination (IVR)
Simultaneous transfection/infection [in vivo recombination, IVR (Kibler et al., 1997; Brandt and Jacobs, 2001)] was performed with pTM 815 (Gag) and pTM 816 (dgp41) and NYVAC-KC to insert Gag and dgp41 into the vaccinia virus TK locus. 500 ng of plasmid DNA was transfected using Lipofectamine and Plus™ Reagent (Invitrogen) per manufacturer’s protocol. This was immediately followed by infection with NYVAC-KC at an MOI=0.01 in 35 mm2 dishes of BSC-40 cells. Recombination was allowed to proceed for 24 h followed by addition of G418 antibiotic (500 μg/mL). Cells were harvested and lysed at 48 h post infection (hpi). The IVR was used to infect 100 mm2 dishes of BSC-40 cells for selection of individual antibiotic-resistant plaques for subsequent expression screening (note: a mutation in the GFP gene prevented use of fluorescent screening). This process was repeated for multiple rounds of antibiotic selection before a > 98% pure virus was isolated as measured by immunoplaque assay.
2.4. Expression screening
Individual antibiotic-resistant plaques were grown in 60 mm2 dishes of BSC-40 cells to CPE and harvested in 1× SDS sample buffer [50 mM Tris-Cl pH 6.8, 2% SDS, 0.1% bromophenol blue, 10% glycerol, 100 mM β-mercaptoethanol, 1× protease inhibitor cocktail III (Research Products International Corp., Prospect, IL)] and then centrifuged through a QiaShredder (Qiagen) for NYVAC-KC-Gag plaques. For NYVAC-KC-dgp41, cells were lysed with RIPA lysis buffer [1% NP40, 0.1% SDS, 0.5% sodium deoxycholate, 1× protease inhibitor cocktail III, in 1× PBS without calcium or magnesium (Corning)]. RIPA lysates were mixed with an equal volume of 2× SDS sample buffer. Cell lysates were screened using SDS-PAGE as previously described (Kessans et al., 2013). Briefly, boiled samples were run on 12% polyacrylamide gels under denaturing conditions, transferred to nitrocellulose membranes (Bio-Rad) and probed with either Gag or dgp41 antibodies and anti-rabbit or anti-human IgG-HRP, respectively. Proteins were detected via chemiluminescence (ImmunoCruz Luminol Reagent, Santa Cruz).
2.5. Immunoplaque assays
Immunoplaque assays were performed in 6-well dishes by infecting BSC-40s with 50 pfu from cell lysates of individual plaques. Once plaques were visible, the cells were fixed with 1:1 acetone:methanol for 30 min at −20 °C, washed with PBS, then incubated with anti-p24 Gag polyclonal rabbit serum or 2F5 (obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: from Dr. Hermann Katinger) at 1:1000 in 3% FBS-PBS. Secondary biotinylated antibodies for anti-rabbit IgG or anti-human IgG from Vectastain ABC Kits (Vector Laboratories, Inc.) were used for detection with DAB peroxidase substrate (Vector Laboratories, Inc.) per manufacturer’s protocol. After counting positive plaques, cells were stained with Coomassie blue dye to count the number of negative plaques. Percentages were calculated by dividing positive plaques by total plaques per well. The plaque with the highest percentage of positives was used for further purification until a plaque with > 98% purity was identified and used to grow stocks.
2.6. Virus stocks
The final NYVAC-KC-Gag and NYVAC-KC-dgp41 plaques with > 98% purity determined by immunoplaque assay were grown to CPE in BHK cells in a 60 mm2 dish for the first passage (P1) stock. The P1 stock was titered in BSC-40s and used to infect five T150 flasks of BHK cells at an MOI=0.01 for the P2 stock. Cells were harvested at CPE, subjected to freeze-thaw and sonication, then cell lysates were placed on a 36% sucrose pad and centrifuged at 22,000 rpm for 80 min at 4 °C with an SW28 rotor to pellet virions. Partially purified virions were resuspended in 10 mM Tris-HCl pH 9.0, their titer determined in BSC-40s, and were stored in aliquots at −80 °C until further use.
2.7. Vaccinia in vitro VLP production
BSC-40 cells seeded in 100 mm2 dishes were infected with each of the vectors at an MOI=5 for a total MOI=10. At 24 hpi, medium and cells were harvested by centrifugation (700×g for 5 min). VLPs in the clarified medium were subjected to 40% ammonium sulfate precipitation and resuspended in 1× PBS (140 mM NaCl, 2 mM KCl, 10 mM Na2HPO4, 1 mM KH2PO4, pH 7.4). Aliquots were boiled and analyzed by SDS-PAGE for presence of Gag and dgp41 as described above.
2.8. Expression in plants of VLPs and their purification
HIV-1 Gag/dgp41 VLPs were expressed and purified as previously described (Kessans et al., 2013). Briefly, Gag transgenic N. benthamiana plants were infiltrated with Agrobacterium tumefaciens harboring pTM 602 with the dgp41 gene and leaf tissue was harvested 4–6 days post infiltration (dpi). Leaf tissue was crushed in a blender in extraction buffer (25 mM sodium phosphate, 100 mM NaCl, 1 mM EDTA, 50 mM sodium ascorbate, 1 mM PMSF, pH 7.8). VLPs were precipitated with 40% ammonium sulfate and resuspended in 1× PBS (140 mM NaCl, 2 mM KCl, 10 mM Na2HPO4, 1 mM KH2PO4, pH 7.4), then purified via iodixanol density gradient centrifugation. The VLP-containing 20% iodixanol fraction was concentrated on a 300 kDa molecular weight cut-off membrane (Sartorius) and quantified via immunoblot for immunizations as described in (Kessans et al., 2016). A typical total yield of a highly enriched VLP preparation in terms of its HIV-1 protein constituents was 275 mg/kg and 92 mg/kg for Gag and dgp41, respectively.
2.9. Antibodies
The following antibodies were used for the indicated assays: anti-p24 Gag polyclonal rabbit serum (Kessans et al., 2013); human anti-MPER 2F5 (AIDS Reagent Program and the kind gift of Morgane Bomsel); goat anti-human IgG-HRP (Sigma) for immunoblot; goat anti-rabbit IgG-HRP (Santa Cruz) for immunoblot; rabbit anti-human IgG-HRP (Santa Cruz) for ELISAs; rabbit anti-mouse IgG-HRP (Calbiochem) for ELISAs; mouse IgA-kappa (ICL Labs) for ELISAs; goat anti-mouse IgA (Sigma) for ELISAs.
2.10. Transmission electron microscopy (TEM)
BSC-40 cells grown in T75 flasks were infected with each of the vectors at an MOI=5 (for a total MOI=10) and harvested by trypsinization 24 hpi. Cells were pelleted between all wash steps at 700×g for 5 min at 4 °C until embedded in agarose prior to secondary fixation. Pelleted cells were washed twice in 1× PBS (140 mM NaCl, 2 mM KCl, 10 mM Na2HPO4, 1 mM KH2PO4, pH 7.4). For primary fixation, cells were placed in 2% glutaraldehyde in PBS for 15 min at room temperature followed by a second incubation with fresh fixative for 1 h at 4 °C. Fixed cells were resuspended in 1% agarose and washed three times in PBS for 30 min each at room temperature. Cells were then fixed with 1% osmium tetroxide in PBS for 1 h at room temperature followed by four 15-min washes in water. A 0.2% uranyl acetate solution in water was used to stain en bloc overnight at 4 °C followed by three 15-min washes in water. An ethanol series from 20% to 100% (anhydrous) was used for dehydration, increasing the ethanol concentration by 20% every 10 min with three incubations in anhydrous ethanol. Spurr’s resin was gradually introduced through 4- to 8-h incubations with 1:3, 1:1, and 3:1 ratios of resin:100% ethanol followed by three incubations in 100% resin. Cells were divided into several blocks of Spurr’s resin and polymerized at 60 °C for 36 h. Sections were cut at 70 nm thick, and were placed on formvar-coated copper slot grids followed by post-stain treatment with 2% uranyl acetate and 3% Sato’s lead citrate before imaging.
2.11. Mouse immunizations
All animal experiments were done with approval from the Arizona State University Institutional Animal Care and Use Committee.
Six-week-old C57BL/6 mice (Jackson Laboratories) were split into five groups. Three groups (n=5) received virus prime (VV) followed by two boosts of either mock (Group 1), VLP (Group 2), or VV+VLP (Group 3). Group 4 (n=3) received no virus prime and two VLP boosts and Group 5 served as the naïve control (n=1). NYVAC-KC-Gag and NYVAC-KC-dgp41 P2 stocks, each at a dose of 5×105 pfu, were mixed together for a total dose of 1×106 pfu per mouse. Virus immunizations prepared in Tris HCl pH 9.0 were injected intramuscularly (i.m.) into the thigh. Plant-produced, gradient-purified VLPs were prepared in 1× PBS pH 7.4 with 2 μg p24 and 1.2 μg MPER and mixed with Ribi adjuvant (Sigma) per manufacturer’s protocol to reach a final concentration of 2% oil [described previously in (Kessans et al., 2016)]. Mock mice were injected i.m. with Tris HCl pH 9.0 buffer in equivalent volumes. VACV and vehicle injections were delivered i.m. while VLPs were delivered intraperitoneally (i.p).
Three immunizations were given to mice at days 0, 45, and 90. Serum, fecal, and vaginal lavage samples were collected as previously described (Kessans et al., 2016) every 2 weeks at days 0, 14, 28, 42, 56, 80, and at the endpoint of day 97 for analysis of Ab production. One week post the final boost (day 97), spleens were collected for analysis of CD8 T-cell responses as described below.
Animals were monitored every 2–3 days for weight loss and other signs of illness, as a measure of vector safety.
2.12. ELISA detection of IgG and IgA
Antibody production was measured via ELISA as previously described (Kessans et al., 2016). Briefly, serum, fecal, and vaginal lavage samples were analyzed for IgG (serum) or IgA (mucosal sites) specific for Gag p24 or gp41 MPER regions. Samples were analyzed in threefold serial dilutions starting at 1:50, 1:2, and 1:5 for serum, fecal, and vaginal lavage samples, respectively. Endpoint titers were calculated as the reciprocal of the dilution factor at which OD 490 nm was equal to background levels (OD490 < 0.1) as previously described (Matoba et al., 2008; Kessans et al., 2016). IgG1 and IgG2a isotypes were detected with the same ELISA protocol, but with goat anti-mouse IgG1-HRP or goat anti-mouse IgG2a-HRP secondary antibodies (Santa Cruz Biotechnology) instead of total IgG. Purified mouse IgG1 kappa chain or mouse IgG2a kappa chain primary antibodies (Sigma) were used as controls.
For anti-VACV ELISAs, 96-well plates were coated with 107 pfu/well of sucrose-cushion purified NYVAC-KC in 1× PBS, and the plates were overnight at 37 °C. Wells were fixed in 2% paraformaldehyde in 1× PBS for 10 min at 4 °C to inactivate the virus. Wells were washed with buffer (150 mM NaCl, 0.5% Tween-20) then blocked with 5% nonfat milk in 1× PBST. Plates were overlaid with serially diluted serum samples and detected as previously described (Matoba et al., 2008; Kessans et al., 2016).
2.13. Intracellular cytokine staining and flow cytometry
Spleens were harvested in Hank’s medium (Corning Cellgro) and cells were strained through a 70 μm filter for resuspension in complete RPMI [cRPMI: 10% FBS, penicillin (100 units/mL), streptomycin (100 μg/mL), 2 mM L-glutamine]. Red blood cells were lysed with ACK Lysing Buffer (Gibco) and splenocytes were resuspended in cRPMI at a concentration of 2×107 cells/mL. Cells were plated at 1×106 cells/well and incubated for 5 h in the presence of Golgi Plug (BD Biosciences) and 1 μg of one of five different immunodominant ZM96 Gag CD8 epitopes: LRSLYNTV (LRS8), VIPMFTAL (VIP8), AMQMLKDT (AMQ8), YSPVSILDI (YSP9), EVKNWMTDTL (EVK10) (synthesized by GenScript and resuspended according to manufacturer’s protocol). Cells were stained with fluorescently conjugated antibodies: CD8-Pacific Blue, TNFα-FITC, and IFNγ-PE (BD Biosciences). Fixation and permeabilization was performed with a Cytofix/Cytoperm Kit (BD Biosciences) with final resuspension in FACS Buffer (1% FBS in 1× PBS). Samples were analyzed by flow cytometry on an LSR Fortessa. Data was analyzed using FlowJo Data Analysis Software (FlowJo, LLC; Ashland, OR).
2.14. Statistics
All statistical analyses were performed in GraphPad Prism Software (GraphPad Prism Software Inc.; La Jolla, CA). All data were analyzed using a one-way ANOVA multiple comparison with Dunn’s post-test. Significance cut-off was defined as p < 0.05.
3. Results
3.1. Generation of recombinant Vaccinia virus vectors
NYVAC-KC-Gag and NYVAC-KC-dgp41 viruses were generated through in vitro recombination (see Materials and Methods) (Fig. 1A and B). Each gene was under control of a synthetic early/late VACV promoter, ensuring the genes were expressed at all stages of viral replication to maximize antigen production. Second round individual plaques were screened for expression via immunoblot by probing for either Gag or dgp41 expression. Immunoplaque assays for either Gag or dgp41 were performed to identify the plaque with highest purity for further selection. This process was repeated until a plaque with > 98% purity was identified and then used to grow a P2 stock. The final sucrose-pad purified P2 stock show Gag and dgp41 expression in BSC-40 cell lysates at the proper size (Fig. 1C and D). It was noted during selection that viruses recombining with dgp41 plasmids had reduced plaque size and grew to much lower titers, suggesting that the HIV-1 gene makes the viral vector cytotoxic (data not shown).
Fig. 1. Generation of recombinant Vaccinia viruses.

Plasmids were cloned to contain either full-length HIV-1 Gag (A) or deconstructed-gp41 (B) as pTM 815 and 816, respectively. The genes are located between homologous recombination arms for the VACV TK locus under the control of a synthetic early/late (E/L) promoter. Also included is the neomycin resistance gene fused to GFP (GFP-neoR). (C and D) SDS-PAGE shows full-length Gag (C) or dgp41 (D) expression in BSC-40’s of NYVAC-KC-Gag (Lane 2) and dgp41 (Lane 3) from the final P2 virus stock used in animal experiments. Parental NYVAC-KC shows no expression of either protein (Lane 1). (E) SDS-PAGE of ammonium sulfate precipitations from media of BSC-40 cells infected with: NYVAC-KC (WT, Lane1), Gag (Lane 2), dgp41 (Lane 3), or a co-infection of Gag/dgp41 viruses (G/d, Lane 4). Samples were tested for both Gag (top) and dgp41 (bottom) expression.
Gag is known to be sufficient and necessary for HIV-1 VLP formation and budding into the medium (Garoff et al., 1998). To test whether BSC-40-infected cells support VLP formation and budding, culture medium was analyzed for the presence of Gag and dgp41. Indeed, Gag protein was detected in culture medium of cells either infected with NYVAC-KC-Gag or co-infected with NYVAC-KC-Gag and NYVAC-KC-dgp41 (Fig. 1E, Lanes 2 and 4). In contrast, dgp41 was detected only in the medium from co-infected cells, not in medium from cells that were infected with NYVAC-KC-dgp41 alone (Fig. 1E, Lane 4).
3.2. NYVAC-KC-Gag/dgp41 show cytotoxicity and in vitro VLP production
These results indicate that export of dgp41 to the medium depends on expression and export of Gag, suggesting that Gag VLPs and Gag/dgp41 VLPs are released, respectively, from cells that express Gag alone or co-express Gag and dgp41. We used TEM to test this possibility. BSC-40 cells that were infected (MOI=5) with NYVAC-KC, NYVAC-KC-Gag, NYVAC-KC-dgp41, or co-infected with Gag/dgp4, were processed for TEM at 24 hpi.
In cells infected with the parental NYVAC-KC strain, viral replication factories are clearly visible and all stages of viral replication are easily identified (Fig. 2A). Mature virions (indicated by white arrows) are seen both intracellularly (Fig. 2A-1) and extracellularly (Fig. 2A-2). Immature virions with and without incorporated genomes are also abundantly visible in this section (white and black triangles, respectively).
Fig. 2. Electron microscopy of NYVAC-KC infected BSC-40s.

BSC-40 cells were infected at an MOI of 5 and harvested 24 hpi and prepared for transmission electron microscopy. Cells were infected with either NYVAC-KC (A), NYVAC-KC-Gag (B), NYVAC-KC-dgp41 (C), or a co-infection with an MOI of 5 for each NYVAC-KC-Gag and NYVAC-KC-dgp41 (D). All stages of VACV replication are present: mature virions (white arrow), immature virions (black triangle), immature virions with genome incorporated (white triangle), crescent formation (black arrow), and ‘unfilled/empty’ virions (E). Budding HIV-1 Gag VLPs (black star) are also seen in those cells infected with NYVAC-KC-Gag. In certain infections, an accumulation of cytoplasmic ‘junk’ (J) is visible. Other cellular features: mitochondria (M), nucleus (N). Scale bar: 500 nm for all images, except (B-2) and (D-2) images are 100 nm for HIV-1 VLPs.
NYVAC-KC-Gag-, dgp41-, or co-infected cells have disorganized viral factories and mature viral particles are rare (Fig. 2B–D). Many early crescent formations are visible (black arrow), along with what appears to be “unfilled” or empty virion shells (indicated by “E”). The rarity of mature virus particles corresponds well to the smaller plaques typical of these strains and their reduced titers, as noted above. Instead, these infected cells appear to have large protein aggregates or precipitates in their cytoplasm (“junk” indicated by “J”), mimicking a phenotype associated with palmitate deficiency (Greseth and Traktman, 2014). Additionally, NYVAC-KC-dgp41-infected cells demonstrate nuclear condensation (Fig. 2C-2, “nucleus” indicated by “N”), a classic sign of apoptosis (Duprez et al., 2009). Furthermore, the mitochondria (indicated by “M”) in NYVAC-KC-Gag- and NYVAC-KC-dgp41-infected cells appear to be malformed, potentially indicating loss of structural integrity, which can also indicate occurrence of apoptosis (Li and Dewson, 2015). This phenotype seems far more drastic than that of NYVAC-KC-infected cells.
Importantly, in cells that were infected with NYVAC-KC-Gag (Fig. 2B-2) or co-infected with NYVAC-KC-Gag and NYVAC-KC-dgp41 (Fig. 2D-2), particles of approximately 100 nm in diameter are seen budding at the cell surface (black stars). Similar structures were not observed in any of the sections of cells expressing by itself dgp41. Based on their size, and appearance, the particles are likely to be HIV-1 Gag or Gag/dgp41 particles budding out of the cell. This identification is supported by our observation that Gag and dgp41 accumulated in the media of these infected cells (Fig. 1E).
3.3. Mouse immunizations
Six-week-old C57BL/6 mice were separated into five groups in order to assess different combinations of VACV and VLP immunization regimens (Fig. 3A). Mice were given a total of three immunizations separated by 45 days to allow for memory responses to develop prior to boosting. Three groups (n=5) were primed with the combination of NYVAC-KC-Gag and NYVAC-KC-dgp41 (denoted in all figures and text as VV), followed by two boosts with either vehicle (Group 1), VLPs alone (Group 2), or a combination of VV and VLPs (Group 3). A fourth group (n=3) received a mock (vehicle) prime followed by boosts with VLPs. The fifth group served as a naïve control (n=1).
Fig. 3. Mouse immunization schedule.
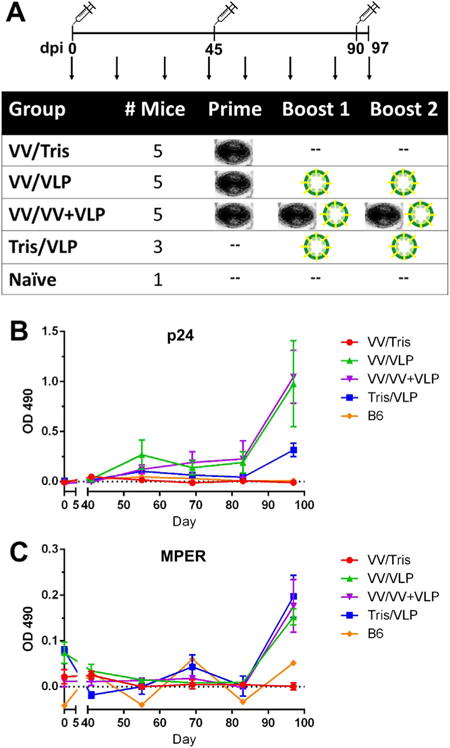
(A) Six-week-old C57BL/6 mice were immunized at days 0, 45, and 90 with different combinations of recombinant vaccinia virus and plant-produced HIV-1 VLPs, represented as an image of a poxvirus or green-enveloped particles, respectively. Vaccinia virus doses (VV) consisted of 1×106 pfu of equally mixed NYVAC-KC-Gag and NYVAC-KC-dgp41 delivered i.m. while plant-produced VLPs were mixed with Ribi adjuvant and delivered i.p. with 2 μg p24 and 1.2 μg MPER. Serum, fecal, and vaginal lavage samples were collected once every 2 weeks (indicated by arrows). Serum samples were analyzed for antigen-specific IgG to p24 Gag (B), or the MPER region of dgp41 (C), shown over the length of the experiment as the ELISA OD 490 nm for the 1:50 dilution (lowest tested).
Monitoring of weight revealed that none of the mice in any group lost weight (data not shown). This indicates little to no pathogenicity of the virus and is consistent with previous virulence data for NYVAC-KC (Kibler et al., 2011) and general safety of the plant-derived VLPs (Kessans et al., 2016).
3.4. Serum IgG responses to Gag and MPER
Antigen-specific serum IgG responses were measured by ELISA using p24 subunit or MPER peptide to detect Gag and dgp41 Ab responses, respectively. Throughout, OD 450 nm values for the lowest dilution tested (1:50) are shown both over time (Fig. 3B–C) and for individual animals at the endpoint, Day 97 (Fig. 4A). Additionally, endpoint titers were calculated for the final serum samples and are shown in Fig. 5A as the reciprocal of the dilution where the OD 450 nm fell below background levels (OD < 0.1).
Fig. 4. Systemic and mucosal antibody production.

Endpoint (Day 97) samples were analyzed for serum IgG (A), and mucosal IgA in fecal (B) and vaginal lavage (C) samples. Data is shown for both p24-specific (left) and MPER-specific (right) antibodies. Raw OD 490 nm values are shown for the lowest dilution factor tested (1:50). Statistically significant differences (p < 0.05) are indicated with an asterisk (*). Each marker represents a single animal.
Fig. 5. Endpoint serum IgG titers and isotyping.

(A) Endpoint titers were calculated as the reciprocal of the dilution factor that had background level of OD 490 nm (< 0.1). Isotypes were determined by ELISA for antigen-specific IgG1 (B) or IgG2a (C) for both p24 (left) and MPER (right) serum IgG. OD 490 nm readings are shown and clearly indicate a bias towards IgG1 production for both groups. Only endpoint (Day 97) serum samples were tested for these two groups because they had the highest responses. (*p < 0.05; **p < 0.01).
Serum IgG responses against both p24 and MPER antigens remained undetectable in all groups until the first sample (Day 55) after the first boost. Titers increased gradually and were boosted after the final immunization with VLPs (Fig. 3B–C), reaching significant levels at the endpoint analysis (Day 97) for both anti-p24 Gag and anti-MPER responses (Fig. 4A). Endpoint Gag responses were highest in the group boosted with a combination of VV and VLPs (VV/VV +VLPs), while the group boosted with VLPs alone (VV/VLPs) also showed significant titers which were more variable responses among animals (Fig. 5A). Anti-MPER serum IgG responses were lower than Gag titers but showed a similar trend, reaching significant levels in all groups receiving VLPs regardless of prime/boost regimen (Fig. 4A). However, the endpoint titer calculations showed no difference between groups, consistent with low Ab responses (Fig. 5A). It is interesting to note that plant-produced VLPs appear to be largely responsible for Ab stimulation because no detectable levels of antibodies were present in the first 45 days after a single dose of VV and only reached detectable levels after the second immunization in those groups that received VLPs.
3.5. Mucosal IgA responses to Gag and MPER
Gastrointestinal IgA responses to the dgp41 (MPER) antigen (as determined by measuring the secreted Ab levels in fecal samples) were low but detectable (Fig. 4B), whereas anti-MPER IgA levels in vaginal secretions were below our detection level irrespective of treatment (Fig. 4C). Fecal anti-MPER IgAs were higher in groups that were immunized using the prime/boost VV/VLPs regimen than groups that were vaccinated with either VV or VLPs alone. Contrasting the two groups, those mice that were boosted with a combination of VV and VLPs exhibited marginally higher levels of fecal IgAs than those boosted with VLPs alone, but their fecal IgA levels were significantly higher than the group that was primed with VV only. Interestingly, levels of fecal and vaginal anti-p24 Gag IgAs were too low for detection in all groups. This contrasts sharply with the generally much higher titers of serum anti-p24 Gag IgGs as compared to serum anti-MPER IgGs.
3.6. IgG isotyping
The two major IgG isotypes are IgG1 and IgG2a; the former is consistent with stimulation of a Th2 response for B cell activation, whereas the latter would be indicative of a Th1 response, which primarily results in inflammatory cytokine production and killing of intracellular pathogens (Snapper and Paul, 1987; Szabo et al., 2003). Our results so far indicate that the most important immunogens contributing to the elicitation of serum IgGs are the plant-derived VLPs. To further substantiate this conclusion, we determined the Ab isotypes contributing to this response, as protein-based vaccines (i.e. immune-complexes) usually elicit IgG1 (Mosser and Edwards, 2008; Martinez and Gordon, 2014).
To this end, serum samples from the endpoint for the two groups which showed the highest serum IgG responses (VV/VLP and VV/VV +VLP) were analyzed for their IgG isotype content by isotype-specific ELISA (Fig. 5A and B, respectively). Both groups show high levels of antigen-specific (either anti-p24 or anti-MPER) IgG1, but levels of IgG2a were too low for detection, in agreement with our hypothesis.
3.7. Anti-vector antibody responses
Having substantiated the contribution of the Gag/dgp41 VLPs for the elicitation of serum Ab responses against the two HIV-1 antigens, the question arose whether or not the viral vector induced production of anti-vector (i.e. anti-VV) serum Abs. Not surprisingly, mice belonging to Group 3 that received a total of three doses of VV (prime plus two boosts) showed significant levels of anti-VV serum IgG at the endpoint (Day 97, Fig. 6). However, a single dose of VV at Day 0 was not enough to induce a potent anti-vector response (Group 1).
Fig. 6. Anti-VACV responses in serum at endpoint.

Day 97 endpoint serum was analyzed by ELISA for anti-vector responses with endpoint titers calculated as for Gag and dgp41-specific serum IgG. The group which received a total of 3 doses of NYVAC-KC vectors shows significantly higher titers of VV-specific antibodies compared with any other group (purple triangles). (***p < 0.001; ****p < 0.0001).
3.8. Gag-specific CD8 T cell responses
A major rationale to include Gag in an HIV-1 vaccine, especially a live-vectored one, is its excellent ability to induce cellular responses. To test induction of CD8 T cell responses, we tested the ability of peptides corresponding to known Gag T-cell epitopes to stimulate proliferation of CD8 T-cells among splenocytes obtained from vaccinated animals. One week after the final boost, splenocytes were harvested and stimulated with five CD8 ZM96 Gag peptides that were previously determined to be dominant in C57BL/6 mice (Chowell et al., 2015). Peptides were assessed individually in order to obtain a better resolution of their responses. CD8 T cells were analyzed for IFN-γ or TNF-α production in response to peptide stimulation using cytokine staining and flow cytometry. Little to no TNF-α production was seen in the flow cytometry analysis (data not shown); however, IFN-γ production was detectable in several groups. The group boosted with both VV and VLPs showed significant production of IFN-γ with two of the five Gag peptides, AMQ8 and EVK10, at 2.6×105 CD8+ IFN-γ+ T cells (3.5% of CD8+ T cells) and 1.91×105 CD8+ IFN-γ+ T cells (2.4%), respectively (Fig. 7A and B). The other three peptides, LRS8, VIP8, and YSP9 showed a similar trend as AMQ8 and EVK10 but with slightly lower responses of CD8+ IFN-γ+ T cells at 1.73×105 (2.4%), 1.61×105 (2.1%), and 1.67×105 cells (2.3%), respectively, but failed to reach significance (Fig. 7C–E). When responses from all five peptides are added together, the VV/VV+VLPs group has a total CD8 Gag-specific mean response of 9.53×105 CD8+ IFN-γ+ T cells (12.7% of CD8 T cells) (Fig. 7F). The total Gag-specific CD8 responses of the other groups show minute differences between groups at 8.0% in Tris/VLP, 5.6% in VV/VLP, and 4.2% in VV/Tris. This suggests that plant-derived Gag/dgp41 VLPs may be providing some component of the T cell response and potentially boosting the initial VV-primed T cell responses as previously described for other Gag-containing VLPs (Williamson and Rybicki, 2015). Future experiments plan to distinguish the roles in these responses.
Fig. 7. Gag-specific CD8 T cell responses.

One week after the final immunization, peak CD8 T cell responses were measured by intracellular cytokine staining and flow cytometry for 5 immunodominant ZM96 Gag epitopes: AMQ8 (A), EVK10 (B), LRS8 (C), VIP8 (D), and YSP8 (E). Number of IFN-γ+ CD8+ T cells in the spleen is shown for each peptide individually and the total response in the spleen (F) was calculated by adding the individual responses together for each group. The group boosted with VV and VLPs (purple) was significantly higher than the VV/Tris group (red) for two peptides and the overall response. (* p < 0.05; ** p < 0.01).
4. Discussion
The RV144 Thai Trial demonstrated the strength of prime/boost vaccination approach by effectively combining two components that were previously shown to be ineffective on their own: a live (albeit non-replicating) canarypox viral vector (ALVAC) and a soluble protein boost (AIDSVAX). The low efficacy of the trial left much to be desired for widespread use as a vaccine while providing the conceptual basis for further improvement (Rerks-Ngarm et al., 2009). The vaccine design strategy presented here makes use of replicating, but highly attenuated, vaccinia virus NYVAC-KC and plant-produced HIV Gag/dgp41 VLPs.
Our approach reflects two hypotheses. First, we suggest that in addition to their excellent facility in eliciting T cell responses, replicating vectors are expected to increase antigen load, resulting in improved immunogenicity. Second we propose that VLPs would improve presentation of relevant neutralizing determinants to the immune system.
Live recombinant vectors based on viruses belonging to a wide range of families such as adenoviridae, poxviridae, and herpesviridae have been previously tested for their immunogenicity (Rerks-Ngarm et al., 2009; Hansen et al., 2013a; Huang et al., 2015; Buchbinder et al., 2008; Tartaglia et al., 1992b). Considering the relative success of ALVAC within the context of RV144, we and others have decided to focus on poxviruses [see reviews (Gomez et al., 2012a; Pantaleo et al., 2010; Jacobs et al., 2009)]. ALVAC is based on canarypox, which like other avipoxviruses is naturally attenuated in humans due to its restricted replication in non-avian cells (Taylor and Paoletti, 1988; Taylor et al., 1988) accounting for ALVAC’s high safety profile (Team AVEGP, 2001; Nitayaphan et al., 2004). Similarly, when developing vaccinia-based vaccine vectors, efforts were initially focused on strains that are non-replicating in human cells (e.g. MVA and NYVAC), due to safety concerns over potential complications with VACV infection in immune-compromised individuals.
NYVAC was originally designed as a highly attenuated vaccine vector by specifically deleting 18 open reading frames from its parental strain (Cop), thus preventing its replication in humans (Tartaglia et al., 1992b). NYVAC has been tested as an HIV vaccine vector and was shown to induce robust, polyfunctional CD4 and CD8 T cell responses to Env and Gag-Pol-Nef (GPN) antigens in vaccinated individuals (Harari et al., 2008, 2012). Because mucosal immune responses are thought to be particularly important in protection against HIV-1, it was significant to note that such responses could be detected in the gut of NYVAC-immunized patients (Perreau et al., 2011).
More recently, intensive research led to partial uncoupling of attenuation and replication in NYVAC-based vectors showing enhanced immunogenicity without compromising their safety. Specifically, reinsertion of two host-range genes yielded NYVAC-KC. This strain is capable of replicating in human cells and displays enhanced immune activation, yet remains highly attenuated in a mouse model of pathogenesis (Kibler et al., 2011; Quakkelaar et al., 2011), thus giving this particular vector many desirable traits of a vaccine vector and was chosen for use in the work presented here.
A large focus of HIV vaccine research is the HIV envelope protein (Env/gp120) and variations thereof. However, gp120 is not highly conserved and many critical neutralization targets are hidden or are only exposed upon conformational change during viral entry, thus limiting the effectiveness of any NAb response to this antigen (Pancera et al., 2014; Decker et al., 2005; Labrijn et al., 2003). Protein engineering has attempted to resolve many of these issues, including the design of SOSIP trimeric gp140 variants of Env to make a more structurally accurate target necessary for eliciting specific types of NAbs, including a design to specifically target nAb germline B cells (Jardine et al., 2013; Billington et al., 2007; Du et al., 2009; Sellhorn et al., 2012; Wan et al., 2009). Unfortunately, to date, no successful clinical trials incorporating an engineered gp140 antigen have been conducted. The HIV membrane protein gp41 contains the bnAb target known as the MPER. This region requires the context of a membrane in order to elicit the partially auto-reactive bnAbs found in HIV-infected patients (Verkoczy et al., 2010, 2013; Haynes et al., 2005; Zhang et al., 2016). In an effort to achieve such responses in vaccination platforms, VLPs offer desirable qualities of enveloped, native virion structure for displaying this important neutralization target.
VLPs are safe, yet immunogenic, components of several vaccines and candidate vaccines for multiple infectious diseases [for a review see (Kushnir et al., 2012)]. The potential for success of VLP-based vaccines is indicated by the widespread use of the human papillomavirus (HPV) vaccines Gardasil® (Merck and Co, 2006) and Cervarix® (GlaxoSmithKline, 2014). To date, many plant-produced vaccines have been tested in animal studies for immunogenicity for both human and veterinary diseases (Rybicki, 2010; Scotti and Rybicki, 2013). Our plant-produced HIV VLPs have the advantage of displaying the MPER of gp41 in the native context of a Gag matrix, providing an immunogenic platform for humoral responses to both antigens (Kessans et al., 2013, 2016). Additionally, HIV VLPs have been shown to boost T cell responses following a heterologous prime (Chapman et al., 2013; Chege et al., 2008; Pillay et al., 2010). CD8 T cell responses, often targeting the Gag protein, are known to be associated with reduced viral load, making this a key target for protective T cell immunity (Kiepiela et al., 2007; Koup et al., 1994; Mudd et al., 2012; Stephenson et al., 2012; Jiao et al., 2006).
Here we show that infecting susceptible cells with NYVAC-KC-Gag with or without NYVAC-KC-dgp41 releases, respectively, ~100 nm Gag or Gag/dgp41 VLPs into the extracellular medium (Fig. 1E). Such particles can be seen by TEM budding out of the cell (Fig. 2B-2 and D-2). Interestingly, dgp41 is detected in the medium only when co-expressed with Gag (Fig. 1E), a result compatible with the notion the two proteins assemble at the plasma membranes of cells in culture to form VLPs. Although HIV-1 VLPs produced by other VACV-based vectors were previously shown to elicit both humoral and cellular immunity (Chen et al., 2005; Goepfert et al., 2011; Perdiguero et al., 2015), in the experiments presented here, no antibodies were detectable in any group until after the first plant-derived VLP immunization (Fig. 3B–C). Among several potential explanations, the simplest one is that production of Gag/dgp41 VLPs in vivo necessitates co-infection of the same cell by the two viruses; this is achievable under cell culture conditions where high MOI can be ensured, but is unlikely to occur in an animal. We are currently pursuing VV vectors which co-express Gag and dgp41 from the same locus to ensure in vivo Gag/dgp41 VLP formation.
Another possible reason for the limited functionality of the dual vectors is the poor replication and/or spread in mice, potentially due to the cytotoxicity represented by protein precipitates in the cytoplasm and nuclear condensation in NYVAC-KC-Gag-, dgp41- or co-infected cells (Fig. 2B–D). The toxicity seen with TEM strongly correlates to reduced plaque sizes and lower viral titers noted during viral selection (data not shown). Gp41 is known to have a toxic cytoplasmic tail (Postler and Desrosiers, 2013; Micoli et al., 2006), and it is possible that this is responsible for the toxic effects seen in Fig. 2. The cytotoxicity of full-length gp160 has largely limited the use of gp41 in vaccine candidates despite the appealing, highly conserved target region of the MPER. Additionally, this is consistent with published data that NYVAC expressing HIV Gag-Pol-Nef induces extensive apoptosis (Gomez et al., 2007). Currently, we are attempting to resolve this issue by pursuing other VACV strains which are capable of limiting the toxic effects of the gp41 cytoplasmic tail in an effort to make it easier to produce vaccine candidates with full-length gp160 in its native, structurally accurate conformation.
Despite the poor priming capacity of the VV in terms of eliciting Ab responses against Gag and gp41, upon boosting with plant-derived VLPs at Day 45, Ab production spiked with another increase after the final immunization at Day 97 (Fig. 3B–C). Endpoint anti-p24 Ab titers reached significant levels for serum IgG in the group boosted with just VLPs (Group 2) or the combination of VV+VLPs (Group 3) (Fig. 5A), and all groups boosted with any combination of VV and VLPs (Groups 2–4) reached significance for MPER-specific serum IgG (Fig. 4A). As noted above, due to the lack of detectable Abs prior to boosting with VLPs, it seems that the plant-produced VLPs administered at a relatively low dose, are largely responsible for boosting the Ab responses following sub-responsive priming. In the groups with the highest responses, these antibodies were shown to be entirely IgG1 with no detectable IgG2a (Fig. 5B–C). This agrees with our yet unpublished data showing that plant-produced HIV VLPs stimulate a predominant Th2 response through activation of M2b macrophages, thus stimulating strong B cell Ab production.
Gag-specific CD8 T cell responses were highest in the group boosted with VV+VLPs (Group 3), reaching 12.7% of CD8 T cells expressing IFN-γ in response to the five Gag peptides (Fig. 7). While we do not have a non-replicating vector in this study, these responses are higher than MVA-induced Gag-specific CD8 T cells (< 0.5%) in humans after two doses (Keefer et al., 2011). Additionally, results here show higher Gag-specific CD8 T cell responses than seen in mice after DNA-NYVAC or DNA-MVA prime-boost regimen which elicit < 2% GPN/Env-specific CD8 T cells (< 0.5% Gag specific) and 12–15% GPN/Env-specific CD8 T cells (5.5% Gag-specific), respectively (Gomez et al., 2012b; Garcia-Arriaza et al., 2013). Taken together, this may suggest that replication plays a role in increasing T cell responses. Results from NYVAC HIV vaccines have shown proficiency for eliciting polyfunctional T cell responses (i.e. antigen-specific T cells expressing multiple cytokines) which are primarily effector memory T cells (Harari et al., 2008, 2012; Gomez et al., 2012b; Garcia-Arriaza et al., 2011). This is important to explore in future experiments involving replicating vectors such as NYVAC-KC due to the association of polyfunctional T cells in the periphery with prevention of HIV infection (Betts et al., 2006). Additionally, it is important to note than when Env is included in the MVA or NYVAC vector, T cell responses are skewed to be primarily Env-specific with minimal Gag recognition (Harari et al., 2008, 2012; Gomez et al., 2012b; Garcia-Arriaza et al., 2013; Garcia et al., 2011; Mooij et al., 2009). Env-specific cytotoxic T lymphocyte (CTL) responses were not protective in clinical trials nor correlated with improved disease in natural infection (McMichael and Koff, 2014), while Gag-specific responses have been correlated with improved CD4 count and reduced viral load (Kiepiela et al., 2007; Koup et al., 1994; Stephenson et al., 2012; Jiao et al., 2006; Ogg et al., 1998; Brander and Walker, 1999) and have epitopes which are less prone to CTL escape (Goulder and Watkins, 2004).
Here we have shown that the combination of NYVAC-KC-Gag/dgp41 replicating vaccinia virus vectors are immunogenic in mice and elicit the best responses when administered together in a regimen closely mimicking the RV144 clinical trial (VV/VV+VLPs). The NYVAC-KC vector is a replicating, but safe, live viral vector with which may induce higher Gag-specific CD8 T cell responses than with non-replicating poxviral vaccine vectors, although a direct comparison would be required to validate this point. Plant-produced VLPs show proficiency for eliciting high Ab titers and may boost T cell responses primed by the NYVAC-KC vectors, though this is the subject of future studies. These vaccine candidates show promise as a cost-effective, scalable vaccine production platform to take forward in an effort to build upon the known success of the RV144 clinical trial to find the elusive HIV vaccine.
Acknowledgments
We would like to thank the Arizona State University vivarium DACT team for contributing to animal care and Dave Lowry in the School of Life Sciences Electron Microscopy Facility for training and supervising TEM imaging. Furthermore, we would like to thank Megan McAfee for helping with processing mouse samples and analyzing CD8 T cell responses and Trung Hyunh for training in the generation of recombinant vaccinia virus vectors. Work was supported in part by the National Institute of Allergy and Infectious Diseases (NIAID, U19 AI062150, TSM, Project 2). LRM was supported by a Graduate Research Fellowship from the National Science Foundation (NSF, GRFP DGE-0802261).
References
- Alam SM, McAdams M, Boren D, Rak M, Scearce RM, Gao F, Camacho ZT, Gewirth D, Kelsoe G, Chen P, Haynes BF. The role of antibody polyspecificity and lipid reactivity in binding of broadly neutralizing anti-HIV-1 envelope human monoclonal antibodies 2F5 and 4E10 to glycoprotein 41 membrane proximal envelope epitopes. J Immunol. 2007;178:4424–4435. doi: 10.4049/jimmunol.178.7.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam SM, Morelli M, Dennison SM, Liao HX, Zhang R, Xia SM, Rits-Volloch S, Sun L, Harrison SC, Haynes BF, Chen B. Role of HIV membrane in neutralization by two broadly neutralizing antibodies. Proc Natl Acad Sci USA. 2009;106:20234–20239. doi: 10.1073/pnas.0908713106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini LA, Posner MR, Katinger H, Stiegler G, Bernacky BJ, Rizvi TA, Schmidt R, Hill LR, Keeling ME, Lu Y, Wright JE, Chou TC, Ruprecht RM. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billington J, Hickling TP, Munro GH, Halai C, Chung R, Dodson GG, Daniels RS. Stability of a receptor-binding active human immunodeficiency virus type 1 recombinant gp140 trimer conferred by intermonomer disulfide bonding of the V3 loop: differential effects of protein disulfide isomerase on CD4 and coreceptor binding. J Virol. 2007;81:4604–4614. doi: 10.1128/JVI.02138-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomsel M, Tudor D, Drillet AS, Alfsen A, Ganor Y, Roger MG, Mouz N, Amacker M, Chalifour A, Diomede L, Devillier G, Cong Z, Wei Q, Gao H, Qin C, Yang GB, Zurbriggen R, Lopalco L, Fleury S. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity. 2011;34:269–280. doi: 10.1016/j.immuni.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Bonsignori M, Alam SM, Liao HX, Verkoczy L, Tomaras GD, Haynes BF, Moody MA. HIV-1 antibodies from infection and vaccination: insights for guiding vaccine design. Trends Microbiol. 2012;20:532–539. doi: 10.1016/j.tim.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brander C, Walker BD. T lymphocyte responses in HIV-1 infection: implications for vaccine development. Curr Opin Immunol. 1999;11:451–459. doi: 10.1016/S0952-7915(99)80076-4. [DOI] [PubMed] [Google Scholar]
- Brandt TA, Jacobs BL. Both carboxy- and amino-terminal domains of the vaccinia virus interferon resistance gene, E3L, are required for pathogenesis in a mouse model. J Virol. 2001;75:850–856. doi: 10.1128/JVI.75.2.850-856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, McElrath MJ, Casimiro DR, Gottesdiener KM, Chodakewitz JA, Corey L, Robertson MN, Step Study Protocol, T. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DR, Mascola JR. Antibody responses to envelope glycoproteins in HIV-1 infection. Nat Immunol. 2015;16:571–576. doi: 10.1038/ni.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzon V, Natrajan G, Schibli D, Campelo F, Kozlov MM, Weissenhorn W. Crystal structure of HIV-1 gp41 including both fusion peptide and membrane proximal external regions. PLoS Pathog. 2010;6:e1000880. doi: 10.1371/journal.ppat.1000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso RM, Zwick MB, Stanfield RL, Kunert R, Binley JM, Katinger H, Burton DR, Wilson IA. Broadly neutralizing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusion-associated motif in gp41. Immunity. 2005;22:163–173. doi: 10.1016/j.immuni.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Chapman R, Stutz H, Jacobs W, Jr, Shephard E, Williamson AL. Priming with recombinant auxotrophic BCG expressing HIV-1 Gag, RT and Gp120 and boosting with recombinant MVA induces a robust T cell response in mice. PLoS One. 2013;8:e71601. doi: 10.1371/journal.pone.0071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chege GK, Shephard EG, Meyers A, van Harmelen J, Williamson C, Lynch A, Gray CM, Rybicki EP, Williamson AL. HIV-1 subtype C Pr55gag virus-like particle vaccine efficiently boosts baboons primed with a matched DNA vaccine. J Gen Virol. 2008;89:2214–2227. doi: 10.1099/vir.0.83501-0. [DOI] [PubMed] [Google Scholar]
- Chen J, Frey G, Peng H, Rits-Volloch S, Garrity J, Seaman MS, Chen B. Mechanism of HIV-1 neutralization by antibodies targeting a membrane-proximal region of gp41. J Virol. 2014;88:1249–1258. doi: 10.1128/JVI.02664-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Rock MT, Hammonds J, Tartaglia J, Shintani A, Currier J, Slike B, Crowe JE, Jr, Marovich M, Spearman P. Pseudovirion particle production by live poxvirus human immunodeficiency virus vaccine vector enhances humoral and cellular immune responses. J Virol. 2005;79:5537–5547. doi: 10.1128/JVI.79.9.5537-5547.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowell D, Krishna S, Becker PD, Cocita C, Shu J, Tan X, Greenberg PD, Klavinskis LS, Blattman JN, Anderson KS. TCR contact residue hydrophobicity is a hallmark of immunogenic CD8+ T cell epitopes. Proc Natl Acad Sci USA. 2015;112:E1754–E1762. doi: 10.1073/pnas.1500973112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung AW, Ghebremichael M, Robinson H, Brown E, Choi I, Lane S, Dugast AS, Schoen MK, Rolland M, Suscovich TJ, Mahan AE, Liao L, Streeck H, Andrews C, Rerks-Ngarm S, Nitayaphan S, de Souza MS, Kaewkungwal J, Pitisuttithum P, Francis D, Michael NL, Kim JH, Bailey-Kellogg C, Ackerman ME, Alter G. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci Transl Med. 2014;6:228ra238. doi: 10.1126/scitranslmed.3007736. [DOI] [PubMed] [Google Scholar]
- Corey L, Gilbert PB, Tomaras GD, Haynes BF, Pantaleo G, Fauci AS. Immune correlates of vaccine protection against HIV-1 acquisition. Sci Transl Med. 2015;7:310rv317. doi: 10.1126/scitranslmed.aac7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks ET, Tong T, Chakrabarti B, Narayan K, Georgiev IS, Menis S, Huang X, Kulp D, Osawa K, Muranaka J, Stewart-Jones G, Destefano J, O’Dell S, LaBranche C, Robinson JE, Montefiori DC, McKee K, Du SX, Doria-Rose N, Kwong PD, Mascola JR, Zhu P, Schief WR, Wyatt RT, Whalen RG, Binley JM. Vaccine-elicited tier 2 HIV-1 neutralizing antibodies bind to quaternary epitopes involving glycan-deficient patches proximal to the CD4 binding site. PLoS Pathog. 2015;11:e1004932. doi: 10.1371/journal.ppat.1004932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KL, Gray ES, Moore PL, Decker JM, Salomon A, Montefiori DC, Graham BS, Keefer MC, Pinter A, Morris L, Hahn BH, Shaw GM. High titer HIV-1 V3-specific antibodies with broad reactivity but low neutralizing potency in acute infection and following vaccination. Virology. 2009;387:414–426. doi: 10.1016/j.virol.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker JM, Bibollet-Ruche F, Wei X, Wang S, Levy DN, Wang W, Delaporte E, Peeters M, Derdeyn CA, Allen S, Hunter E, Saag MS, Hoxie JA, Hahn BH, Kwong PD, Robinson JE, Shaw GM. Antigenic conservation and immunogenicity of the HIV coreceptor binding site. J Exp Med. 2005;201:1407–1419. doi: 10.1084/jem.20042510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devito C, Hinkula J, Kaul R, Lopalco L, Bwayo JJ, Plummer F, Clerici M, Broliden K. Mucosal and plasma IgA from HIV-exposed seronegative individuals neutralize a primary HIV-1 isolate. AIDS. 2000a;14:1917–1920. doi: 10.1097/00002030-200009080-00006. [DOI] [PubMed] [Google Scholar]
- Devito C, Broliden K, Kaul R, Svensson L, Johansen K, Kiama P, Kimani J, Lopalco L, Piconi S, Bwayo JJ, Plummer F, Clerici M, Hinkula J. Mucosal and plasma IgA from HIV-1-exposed uninfected individuals inhibit HIV-1 transcytosis across human epithelial cells. J Immunol. 2000b;165:5170–5176. doi: 10.4049/jimmunol.165.9.5170. [DOI] [PubMed] [Google Scholar]
- Diomede L, Nyoka S, Pastori C, Scotti L, Zambon A, Sherman G, Gray CM, Sarzotti-Kelsoe M, Lopalco L. Passively transmitted gp41 antibodies in babies born from HIV-1 subtype C-seropositive women: correlation between fine specificity and protection. J Virol. 2012;86:4129–4138. doi: 10.1128/JVI.06359-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du SX, Idiart RJ, Mariano EB, Chen H, Jiang P, Xu L, Ostrow KM, Wrin T, Phung P, Binley JM, Petropoulos CJ, Ballantyne JA, Whalen RG. Effect of trimerization motifs on quaternary structure, antigenicity, and immunogenicity of a noncleavable HIV-1 gp140 envelope glycoprotein. Virology. 2009;395:33–44. doi: 10.1016/j.virol.2009.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprez L, Wirawan E, Vanden Berghe T, Vandenabeele P. Major cell death pathways at a glance. Microbes Infect. 2009;11:1050–1062. doi: 10.1016/j.micinf.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Frey G, Peng H, Rits-Volloch S, Morelli M, Cheng Y, Chen B. A fusion-intermediate state of HIV-1 gp41 targeted by broadly neutralizing antibodies. Proc Natl Acad Sci USA. 2008;105:3739–3744. doi: 10.1073/pnas.0800255105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey G, Chen J, Rits-Volloch S, Freeman MM, Zolla-Pazner S, Chen B. Distinct conformational states of HIV-1 gp41 are recognized by neutralizing and non-neutralizing antibodies. Nat Struct Mol Biol. 2010;17:1486–1491. doi: 10.1038/nsmb.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia F, Bernaldo de Quiros JC, Gomez CE, Perdiguero B, Najera JL, Jimenez V, Garcia-Arriaza J, Guardo AC, Perez I, Diaz-Brito V, Conde MS, Gonzalez N, Alvarez A, Alcami J, Jimenez JL, Pich J, Arnaiz JA, Maleno MJ, Leon A, Munoz-Fernandez MA, Liljestrom P, Weber J, Pantaleo G, Gatell JM, Plana M, Esteban M. Safety and immunogenicity of a modified pox vector-based HIV/AIDS vaccine candidate expressing Env, Gag, Pol and Nef proteins of HIV-1 subtype B (MVA-B) in healthy HIV-1-uninfected volunteers: a phase I clinical trial (RISVAC02) Vaccine. 2011;29:8309–8316. doi: 10.1016/j.vaccine.2011.08.098. [DOI] [PubMed] [Google Scholar]
- Garcia-Arriaza J, Arnaez P, Gomez CE, Sorzano CO, Esteban M. Improving adaptive and memory immune responses of an HIV/AIDS vaccine candidate MVA-B by deletion of vaccinia virus genes (C6L and K7R) blocking interferon signaling pathways. PLoS One. 2013;8:e66894. doi: 10.1371/journal.pone.0066894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Arriaza J, Najera JL, Gomez CE, Tewabe N, Sorzano CO, Calandra T, Roger T, Esteban M. A candidate HIV/AIDS vaccine (MVA-B) lacking vaccinia virus gene C6L enhances memory HIV-1-specific T-cell responses. PLoS One. 2011;6:e24244. doi: 10.1371/journal.pone.0024244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H, Hewson R, Opstelten DJ. Virus maturation by budding. Microbiol Mol Biol Rev. 1998;62:1171–1190. doi: 10.1128/mmbr.62.4.1171-1190.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GlaxoSmithKline I. Product Monograph – Cervarix 2014 [Google Scholar]
- Goepfert PA, Elizaga ML, Sato A, Qin L, Cardinali M, Hay CM, Hural J, DeRosa SC, DeFawe OD, Tomaras GD, Montefiori DC, Xu Y, Lai L, Kalams SA, Baden LR, Frey SE, Blattner WA, Wyatt LS, Moss B, Robinson HL, National Institute of A, Infectious Diseases HIVVTN Phase 1 safety and immunogenicity testing of DNA and recombinant modified vaccinia Ankara vaccines expressing HIV-1 virus-like particles. J Infect Dis. 2011;203:610–619. doi: 10.1093/infdis/jiq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez CE, Perdiguero B, Garcia-Arriaza J, Esteban M. Poxvirus vectors as HIV/AIDS vaccines in humans. Hum Vaccin Immunother. 2012a;8:1192–1207. doi: 10.4161/hv.20778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez CE, Perdiguero B, Najera JL, Sorzano CO, Jimenez V, Gonzalez-Sanz R, Esteban M. Removal of vaccinia virus genes that block interferon type I and II pathways improves adaptive and memory responses of the HIV/AIDS vaccine candidate NYVAC-C in mice. J Virol. 2012b;86:5026–5038. doi: 10.1128/JVI.06684-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez CE, Najera JL, Jimenez EP, Jimenez V, Wagner R, Graf M, Frachette MJ, Liljestrom P, Pantaleo G, Esteban M. Head-to-head comparison on the immunogenicity of two HIV/AIDS vaccine candidates based on the attenuated poxvirus strains MVA and NYVAC co-expressing in a single locus the HIV-1BX08 gp120 and HIV-1(IIIB) Gag-Pol-Nef proteins of clade B. Vaccine. 2007;25:2863–2885. doi: 10.1016/j.vaccine.2006.09.090. [DOI] [PubMed] [Google Scholar]
- Gong Z, Martin-Garcia JM, Daskalova SM, Craciunescu FM, Song L, Dorner K, Hansen DT, Yang JH, LaBaer J, Hogue BG, Mor TS, Fromme P. Biophysical characterization of a vaccine candidate against HIV-1: the transmembrane and membrane proximal domains of HIV-1 gp41 as a maltose binding protein fusion. PLoS One. 2015;10:e0136507. doi: 10.1371/journal.pone.0136507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulder PJ, Watkins DI. HIV and SIV CTL escape: implications for vaccine design. Nat Rev Immunol. 2004;4:630–640. doi: 10.1038/nri1417. [DOI] [PubMed] [Google Scholar]
- Greseth MD, Traktman P. De novo fatty acid biosynthesis contributes significantly to establishment of a bioenergetically favorable environment for vaccinia virus infection. PLoS Pathog. 2014;10:e1004021. doi: 10.1371/journal.ppat.1004021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SG, Sacha JB, Hughes CM, Ford JC, Burwitz BJ, Scholz I, Gilbride RM, Lewis MS, Gilliam AN, Ventura AB, Malouli D, Xu G, Richards R, Whizin N, Reed JS, Hammond KB, Fischer M, Turner JM, Legasse AW, Axthelm MK, Edlefsen PT, Nelson JA, Lifson JD, Fruh K, Picker LJ. Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science. 2013b;340:1237874. doi: 10.1126/science.1237874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SG, Piatak M, Jr, Ventura AB, Hughes CM, Gilbride RM, Ford JC, Oswald K, Shoemaker R, Li Y, Lewis MS, Gilliam AN, Xu G, Whizin N, Burwitz BJ, Planer SL, Turner JM, Legasse AW, Axthelm MK, Nelson JA, Fruh K, Sacha JB, Estes JD, Keele BF, Edlefsen PT, Lifson JD, Picker LJ. Immune clearance of highly pathogenic SIV infection. Nature. 2013a;502:100–104. doi: 10.1038/nature12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harari A, Rozot V, Cavassini M, Bellutti Enders F, Vigano S, Tapia G, Castro E, Burnet S, Lange J, Moog C, Garin D, Costagliola D, Autran B, Pantaleo G, Bart PA. NYVAC immunization induces polyfunctional HIV-specific T-cell responses in chronically-infected, ART-treated HIV patients. Eur J Immunol. 2012;42:3038–3048. doi: 10.1002/eji.201242696. [DOI] [PubMed] [Google Scholar]
- Harari A, Bart PA, Stohr W, Tapia G, Garcia M, Medjitna-Rais E, Burnet S, Cellerai C, Erlwein O, Barber T, Moog C, Liljestrom P, Wagner R, Wolf H, Kraehenbuhl JP, Esteban M, Heeney J, Frachette MJ, Tartaglia J, McCormack S, Babiker A, Weber J, Pantaleo G. An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces reliable, polyfunctional, and long-lasting T cell responses. J Exp Med. 2008;205:63–77. doi: 10.1084/jem.20071331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes BF, Shaw GM, Korber B, Kelsoe G, Sodroski J, Hahn BH, Borrow P, McMichael AJ. HIV-host interactions: implications for vaccine design. Cell Host Microbe. 2016;19:292–303. doi: 10.1016/j.chom.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, Robinson J, Scearce RM, Plonk K, Staats HF, Ortel TL, Liao HX, Alam SM. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308:1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell AJ, Rakasz EG, Tehrani DM, Huber M, Weisgrau KL, Landucci G, Forthal DN, Koff WC, Poignard P, Watkins DI, Burton DR. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J Virol. 2010;84:1302–1313. doi: 10.1128/JVI.01272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW, Marx PA, Burton DR. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- Hoot S, McGuire AT, Cohen KW, Strong RK, Hangartner L, Klein F, Diskin R, Scheid JF, Sather DN, Burton DR, Stamatatos L. Recombinant HIV envelope proteins fail to engage germline versions of anti-CD4bs bNAbs. PLoS Pathog. 2013;9:e1003106. doi: 10.1371/journal.ppat.1003106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, Imamichi H, Bailer RT, Chakrabarti B, Sharma SK, Alam SM, Wang T, Yang Y, Zhang B, Migueles SA, Wyatt R, Haynes BF, Kwong PD, Mascola JR, Connors M. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature. 2012;491:406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Kang BH, Pancera M, Lee JH, Tong T, Feng Y, Imamichi H, Georgiev IS, Chuang GY, Druz A, Doria-Rose NA, Laub L, Sliepen K, van Gils MJ, de la Pena AT, Derking R, Klasse PJ, Migueles SA, Bailer RT, Alam M, Pugach P, Haynes BF, Wyatt RT, Sanders RW, Binley JM, Ward AB, Mascola JR, Kwong PD, Connors M. Broad and potent HIV-1 neutralization by a human antibody that binds the gp41-gp120 interface. Nature. 2014;515:138–142. doi: 10.1038/nature13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Follmann D, Nason M, Zhang L, Huang Y, Mehrotra DV, Moodie Z, Metch B, Janes H, Keefer MC, Churchyard G, Robb ML, Fast PE, Duerr A, McElrath MJ, Corey L, Mascola JR, Graham BS, Sobieszczyk ME, Kublin JG, Robertson M, Hammer SM, Gray GE, Buchbinder SP, Gilbert PB. Effect of rAd5-vector HIV-1 preventive vaccines on HIV-1 acquisition: a participant-level meta-analysis of randomized trials. PLoS One. 2015;10:e0136626. doi: 10.1371/journal.pone.0136626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irimia A, Sarkar A, Stanfield RL, Wilson IA. Crystallographic identification of lipid as an integral component of the epitope of HIV broadly neutralizing antibody 4E10. Immunity. 2016;44:21–31. doi: 10.1016/j.immuni.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob RA, Moyo T, Schomaker M, Abrahams F, Grau Pujol B, Dorfman JR. Anti-V3/glycan and Anti-MPER neutralizing antibodies, but not anti-V2/glycan site antibodies, are strongly associated with greater anti-HIV-1 neutralization breadth and potency. J Virol. 2015;89:5264–5275. doi: 10.1128/JVI.00129-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Langland JO, Kibler KV, Denzler KL, White SD, Holechek SA, Wong S, Huynh T, Baskin CR. Vaccinia virus vaccines: past, present and future. Antivir Res. 2009;84:1–13. doi: 10.1016/j.antiviral.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine J, Julien JP, Menis S, Ota T, Kalyuzhniy O, McGuire A, Sok D, Huang PS, MacPherson S, Jones M, Nieusma T, Mathison J, Baker D, Ward AB, Burton DR, Stamatatos L, Nemazee D, Wilson IA, Schief WR. Rational HIV immunogen design to target specific germline B cell receptors. Science. 2013;340:711–716. doi: 10.1126/science.1234150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Xie J, L T, Han Y, Qiu Z, Zuo L, Wang A. Correlation between gag-specific CD8 T-cell responses, viral load, and CD4 count in HIV-1 infection is dependent on disease status. J Acquir Immune Defic Syndr. 2006;42:263–268. doi: 10.1097/01.qai.0000221692.00091.a2. [DOI] [PubMed] [Google Scholar]
- Kaul R, Plummer F, Clerici M, Bomsel M, Lopalco L, Broliden K. Mucosal IgA in exposed, uninfected subjects: evidence for a role in protection against HIV infection. AIDS. 2001;15:431–432. doi: 10.1097/00002030-200102160-00026. [DOI] [PubMed] [Google Scholar]
- Kaul R, Trabattoni D, Bwayo JJ, Arienti D, Zagliani A, Mwangi FM, Kariuki C, Ngugi EN, MacDonald KS, Ball TB, Clerici M, Plummer FA. HIV-1-specific mucosal IgA in a cohort of HIV-1-resistant Kenyan sex workers. AIDS. 1999;13:23–29. doi: 10.1097/00002030-199901140-00004. [DOI] [PubMed] [Google Scholar]
- Keefer MC, Frey SE, Elizaga M, Metch B, De Rosa SC, Barroso PF, Tomaras G, Cardinali M, Goepfert P, Kalichman A, Philippon V, McElrath MJ, Jin X, Ferrari G, Defawe OD, Mazzara GP, Montefiori D, Pensiero M, Panicali DL, Corey L, Network NHVT A phase I trial of preventive HIV vaccination with heterologous poxviral-vectors containing matching HIV-1 inserts in healthy HIV-uninfected subjects. Vaccine. 2011;29:1948–1958. doi: 10.1016/j.vaccine.2010.12.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepler TB, Liao HX, Alam SM, Bhaskarabhatla R, Zhang R, Yandava C, Stewart S, Anasti K, Kelsoe G, Parks R, Lloyd KE, Stolarchuk C, Pritchett J, Solomon E, Friberg E, Morris L, Karim SS, Cohen MS, Walter E, Moody MA, Wu X, Altae-Tran HR, Georgiev IS, Kwong PD, Boyd SD, Fire AZ, Mascola JR, Haynes BF. Immunoglobulin gene insertions and deletions in the affinity maturation of HIV-1 broadly reactive neutralizing antibodies. Cell Host Microbe. 2014;16:304–313. doi: 10.1016/j.chom.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessans SA, Linhart MD, Matoba N, Mor T. Biological and biochemical characterization of HIV-1 Gag/dgp41 virus-like particles expressed in Nicotiana benthamiana. Plant Biotechnol J. 2013;11:681–690. doi: 10.1111/pbi.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessans SA, Linhart MD, Meador LR, Kilbourne J, Hogue BG, Fromme P, Matoba N, Mor TS. Immunological characterization of plant-based HIV-1 Gag/Dgp41 virus-like particles. PLoS One. 2016;11:e0151842. doi: 10.1371/journal.pone.0151842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibler KV, Shors T, Perkins K, Zeman C, MP B, Biesterfeldt J, Langland JO, Jacobs BL. Double-stranded RNA is a trigger for apoptosis in vaccinia virus-infected cells. J Virol. 1997;71:1992–2003. doi: 10.1128/jvi.71.3.1992-2003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibler KV, Gomez CE, Perdiguero B, Wong S, Huynh T, Holechek S, Arndt W, Jimenez V, Gonzalez-Sanz R, Denzler K, Haddad EK, Wagner R, Sekaly RP, Tartaglia J, Pantaleo G, Jacobs BL, Esteban M. Improved NYVAC-based vaccine vectors. PLoS One. 2011;6:e25674. doi: 10.1371/journal.pone.0025674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiepiela P, Ngumbela K, Thobakgale C, Ramduth D, Honeyborne I, Moodley E, Reddy S, de Pierres C, Mncube Z, Mkhwanazi N, Bishop K, van der Stok M, Nair K, Khan N, Crawford H, Payne R, Leslie A, Prado J, Prendergast A, Frater J, McCarthy N, Brander C, Learn GH, Nickle D, Rousseau C, Coovadia H, Mullins JI, Heckerman D, Walker BD, Goulder P. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med. 2007;13:46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- Klein F, Mouquet H, Dosenovic P, Scheid JF, Scharf L, Nussenzweig MC. Antibodies in HIV-1 vaccine development and therapy. Science. 2013a;341:1199–1204. doi: 10.1126/science.1241144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein F, Diskin R, Scheid JF, Gaebler C, Mouquet H, Georgiev IS, Pancera M, Zhou T, Incesu RB, Fu BZ, Gnanapragasam PN, Oliveira TY, Seaman MS, Kwong PD, Bjorkman PJ, Nussenzweig MC. Somatic mutations of the immunoglobulin framework are generally required for broad and potent HIV-1 neutralization. Cell. 2013b;153:126–138. doi: 10.1016/j.cell.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, Farthing C, Ho DD. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnir N, Streatfield SJ, Yusibov V. Virus-like particles as a highly efficient vaccine platform: diversity of targets and production systems and advances in clinical development. Vaccine. 2012;31:58–83. doi: 10.1016/j.vaccine.2012.10.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrijn AF, Poignard P, Raja A, Zwick MB, Delgado K, Franti M, Binley J, Vivona V, Grundner C, Huang CC, Venturi M, Petropoulos CJ, Wrin T, Dimitrov DS, Robinson J, Kwong PD, Wyatt RT, Sodroski J, Burton DR. Access of antibody molecules to the conserved coreceptor binding site on glycoprotein gp120 is sterically restricted on primary human immunodeficiency virus type 1. J Virol. 2003;77:10557–10565. doi: 10.1128/JVI.77.19.10557-10565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux-Roels G, Maes C, Clement F, van Engelenburg F, van den Dobbelsteen M, Adler M, Amacker M, Lopalco L, Bomsel M, Chalifour A, Fleury S. Randomized phase I: safety, immunogenicity and mucosal antiviral activity in young healthy women vaccinated with HIV-1 Gp41 P1 peptide on virosomes. PloS One. 2013;8:e55438. doi: 10.1371/journal.pone.0055438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MX, Dewson G. Mitochondria and apoptosis: emerging concepts. F1000Prime Rep. 2015;7:42. doi: 10.12703/P7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao HX, Chen X, Munshaw S, Zhang R, Marshall DJ, Vandergrift N, Whitesides JF, Lu X, Yu JS, Hwang KK, Gao F, Markowitz M, Heath SL, Bar KJ, Goepfert PA, Montefiori DC, Shaw GC, Alam SM, Margolis DM, Denny TN, Boyd SD, Marshal E, Egholm M, Simen BB, Hanczaruk B, Fire AZ, Voss G, Kelsoe G, Tomaras GD, Moody MA, Kepler TB, Haynes BF. Initial antibodies binding to HIV-1 gp41 in acutely infected subjects are polyreactive and highly mutated. J Exp Med. 2011;208:2237–2249. doi: 10.1084/jem.20110363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao HX, Bonsignori M, Alam SM, McLellan JS, Tomaras GD, Moody MA, Kozink DM, Hwang KK, Chen X, Tsao CY, Liu P, Lu X, Parks RJ, Montefiori DC, Ferrari G, Pollara J, Rao M, Peachman KK, Santra S, Letvin NL, Karasavvas N, Yang ZY, Dai K, Pancera M, Gorman J, Wiehe K, Nicely NI, Rerks-Ngarm S, Nitayaphan S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Sinangil F, Kim JH, Michael NL, Kepler TB, Kwong PD, Mascola JR, Nabel GJ, Pinter A, Zolla-Pazner S, Haynes BF. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity. 2013;38:176–186. doi: 10.1016/j.immuni.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magerus-Chatinet A, Yu H, Garcia S, Ducloux E, Terris B, Bomsel M. Galactosyl ceramide expressed on dendritic cells can mediate HIV-1 transfer from monocyte derived dendritic cells to autologous T cells. Virology. 2007;362:67–74. doi: 10.1016/j.virol.2006.11.035. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola JR, Montefiori DC. The role of antibodies in HIV vaccines. Annu Rev Immunol. 2010;28:413–444. doi: 10.1146/annurev-immunol-030409-101256. [DOI] [PubMed] [Google Scholar]
- Mascola JR, Haynes BF. HIV-1 neutralizing antibodies: understanding nature’s pathways. Immunol Rev. 2013;254:225–244. doi: 10.1111/imr.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoba N, Kajiura H, Cherni I, Doran JD, Bomsel M, Fujiyama K, Mor TS. Biochemical and immunological characterization of the plant-derived candidate human immunodeficiency virus type 1 mucosal vaccine CTB-MPR. Plant Biotechnol J. 2009;7:129–145. doi: 10.1111/j.1467-7652.2008.00381.x. [DOI] [PubMed] [Google Scholar]
- Matoba N, Magerus A, Geyer BC, Zhang Y, Muralidharan M, Alfsen A, Arntzen CJ, Bomsel M, Mor TS. A mucosally targeted subunit vaccine candidate eliciting HIV-1 transcytosis-blocking Abs. Proc Natl Acad Sci USA. 2004;101:13584–13589. doi: 10.1073/pnas.0405297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoba N, Griffin TA, Mittman M, Doran JD, Alfsen A, Montefiori DC, Hanson CV, Bomsel M, Mor TS. Transcytosis-blocking abs elicited by an oligomeric immunogen based on the membrane proximal region of HIV-1 gp41 target non-neutralizing epitopes. Curr HIV Res. 2008;6:218–229. doi: 10.2174/157016208784324994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire AT, Glenn JA, Lippy A, Stamatatos L. Diverse recombinant HIV-1 Envs fail to activate B cells expressing the germline B cell receptors of the broadly neutralizing anti-HIV-1 antibodies PG9 and 447-52D. J Virol. 2014;88:2645–2657. doi: 10.1128/JVI.03228-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael AJ, Koff WC. Vaccines that stimulate T cell immunity to HIV-1: the next step. Nat Immunol. 2014;15:319–322. doi: 10.1038/ni.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merck, Co. I. Prescribing Information – Gardasil 2006 [Google Scholar]
- Micoli KJ, Mamaeva O, Piller SC, Barker JL, Pan G, Hunter E, McDonald JM. Point mutations in the C-terminus of HIV-1 gp160 reduce apoptosis and calmodulin binding without affecting viral replication. Virology. 2006;344:468–479. doi: 10.1016/j.virol.2005.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooij P, Balla-Jhagjhoorsingh SS, Beenhakker N, van Haaften P, Baak I, Nieuwenhuis IG, Heidari S, Wolf H, Frachette MJ, Bieler K, Sheppard N, Harari A, Bart PA, Liljestrom P, Wagner R, Pantaleo G, Heeney JL. Comparison of human and rhesus macaque T-cell responses elicited by boosting with NYVAC encoding human immunodeficiency virus type 1 clade C immunogens. J Virol. 2009;83:5881–5889. doi: 10.1128/JVI.02345-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PL, Williamson C, Morris L. Virological features associated with the development of broadly neutralizing antibodies to HIV-1. Trends Microbiol. 2015;23:204–211. doi: 10.1016/j.tim.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd PA, Martins MA, Ericsen AJ, Tully DC, Power KA, Bean AT, Piaskowski SM, Duan L, Seese A, Gladden AD, Weisgrau KL, Furlott JR, Kim YI, Veloso de Santana MG, Rakasz E, Capuano S, 3rd, Wilson NA, Bonaldo MC, Galler R, Allison DB, Piatak M, Jr, Haase AT, Lifson JD, Allen TM, Watkins DI. Vaccine-induced CD8+ T cells control AIDS virus replication. Nature. 2012;491:129–133. doi: 10.1038/nature11443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JD, Brunel FM, Jensen R, Crooks ET, Cardoso RM, Wang M, Hessell A, Wilson IA, Binley JM, Dawson PE, Burton DR, Zwick MB. An affinity-enhanced neutralizing antibody against the membrane-proximal external region of human immunodeficiency virus type 1 gp41 recognizes an epitope between those of 2F5 and 4E10. J Virol. 2007;81:4033–4043. doi: 10.1128/JVI.02588-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitayaphan S, Pitisuttithum P, Karnasuta C, Eamsila C, de Souza M, Morgan P, Polonis V, Benenson M, VanCott T, Ratto-Kim S, Kim J, Thapinta D, Garner R, Bussaratid V, Singharaj P, el-Habib R, Gurunathan S, Heyward W, Birx D, McNeil J, Brown AE, Thai AVEG Safety and immunogenicity of an HIV subtype B and E prime-boost vaccine combination in HIV-negative Thai adults. J Infect Dis. 2004;190:702–706. doi: 10.1086/422258. [DOI] [PubMed] [Google Scholar]
- Ogg GS, Jin X, Bonhoeffer S, Dunbar PR, Nowak MA, Monard S, Segal JP, Cao Y, Rowland-Jones SL, Cerundolo V, Hurley A, Markowitz M, Ho DD, Nixon DF, McMichael AJ. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- Pancera M, Zhou T, Druz A, Georgiev IS, Soto C, Gorman J, Huang J, Acharya P, Chuang GY, Ofek G, Stewart-Jones GB, Stuckey J, Bailer RT, Joyce MG, Louder MK, Tumba N, Yang Y, Zhang B, Cohen MS, Haynes BF, Mascola JR, Morris L, Munro JB, Blanchard SC, Mothes W, Connors M, Kwong PD. Structure and immune recognition of trimeric pre-fusion HIV-1 Env. Nature. 2014;514:455–461. doi: 10.1038/nature13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G, Esteban M, Jacobs B, Tartaglia J. Poxvirus vector-based HIV vaccines. Curr Opin HIV AIDS. 2010;5:391–396. doi: 10.1097/COH.0b013e32833d1e87. [DOI] [PubMed] [Google Scholar]
- Parker CE, Deterding LJ, Hager-Braun C, Binley JM, Schulke N, Katinger H, Moore JP, Tomer KB. Fine definition of the epitope on the gp41 glycoprotein of human immunodeficiency virus type 1 for the neutralizing monoclonal antibody 2F5. J Virol. 2001;75:10906–10911. doi: 10.1128/JVI.75.22.10906-10911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastori C, Barassi C, Piconi S, Longhi R, Villa ML, Siccardi AG, Clerici M, Lopalco L. HIV neutralizing IgA in exposed seronegative subjects recognise an epitope within the gp41 coiled-coil pocket. J Biol Regul Homeost Agents. 2000;14:15–21. [PubMed] [Google Scholar]
- Perdiguero B, Gomez CE, Cepeda V, Sanchez-Sampedro L, Garcia-Arriaza J, Mejias-Perez E, Jimenez V, Sanchez C, Sorzano CO, Oliveros JC, Delaloye J, Roger T, Calandra T, Asbach B, Wagner R, Kibler KV, Jacobs BL, Pantaleo G, Esteban M. Virological and immunological characterization of novel NYVAC-based HIV/AIDS vaccine candidates expressing clade C trimeric soluble gp140(ZM96) and Gag(ZM96)-Pol-Nef(CN54) as virus-like particles. J Virol. 2015;89:970–988. doi: 10.1128/JVI.02469-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreau M, Welles HC, Harari A, Hall O, Martin R, Maillard M, Dorta G, Bart PA, Kremer EJ, Tartaglia J, Wagner R, Esteban M, Levy Y, Pantaleo G. DNA/NYVAC vaccine regimen induces HIV-specific CD4 and CD8 T-cell responses in intestinal mucosa. J Virol. 2011;85:9854–9862. doi: 10.1128/JVI.00788-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay S, Shephard EG, Meyers AE, Williamson AL, Rybicki EP. HIV-1 sub-type C chimaeric VLPs boost cellular immune responses in mice. J Immune Based Ther Vaccin. 2010;8:7. doi: 10.1186/1476-8518-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollara J, McGuire E, Fouda GG, Rountree W, Eudailey J, Overman RG, Seaton KE, Deal A, Edwards RW, Tegha G, Kamwendo D, Kumwenda J, Nelson JA, Liao HX, Brinkley C, Denny TN, Ochsenbauer C, Ellington S, King CC, Jamieson DJ, van der Horst C, Kourtis AP, Tomaras GD, Ferrari G, Permar SR. Association of HIV-1 envelope-specific breast milk IgA responses with reduced risk of postnatal mother-to-child transmission of HIV-1. J Virol. 2015;89:9952–9961. doi: 10.1128/JVI.01560-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postler TS, Desrosiers RC. The tale of the long tail: the cytoplasmic domain of HIV-1 gp41. J Virol. 2013;87:2–15. doi: 10.1128/JVI.02053-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice HA, Tomaras G, Geraghty DE, Apps R, Fong Y, Ehrenberg PK, Rolland M, Kijak GH, Krebs SJ, Nelson W, DeCamp A, Shen X, Yates NL, Zolla-Pazner S, Nitayaphan S, Rerks-Ngarm S, Kaewkungwal J, Pitisuttihum P, Ferrari G, McElrath MJ, Montefiori D, Bailer RT, Koup RA, O’Connell RJ, Robb ML, Michael NL, Gilbert PB, Kim JH, Thomas R. HLA class II genes modulates vaccine-induced antibody responses to affect HIV-1 acquisition. Sci Transl Med. 2015;7:296ra112. doi: 10.1126/scitranslmed.aab4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quakkelaar ED, Redeker A, Haddad EK, Harari A, McCaughey SM, Duhen T, Filali-Mouhim A, Goulet JP, Loof NM, Ossendorp F, Perdiguero B, Heinen P, Gomez CE, Kibler KV, Koelle DM, Sekaly RP, Sallusto F, Lanzavecchia A, Pantaleo G, Esteban M, Tartaglia J, Jacobs BL, Melief CJ. Improved innate and adaptive immunostimulation by genetically modified HIV-1 protein expressing NYVAC vectors. PLoS One. 2011;6:e16819. doi: 10.1371/journal.pone.0016819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael L, Kunasol P, Kim JH. Vaccination with ALVAC and AIDSVAX to Prevent HIV-1 Infection in Thailand. N Engl J Med. 2009 doi: 10.1056/NEJMoa0908492. (doi:NEJMoa0908492)(pii) (10.1056/NEJMoa0908492) [DOI] [PubMed] [Google Scholar]
- Rybicki EP. Plant-made vaccines for humans and animals. Plant Biotechnol J. 2010;8:620–637. doi: 10.1111/j.1467-7652.2010.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar M, Akiyama H, Etemad B, Ramirez N, Freitas I, Gummuluru S. Transmembrane domain membrane proximal external region but not surface unit-directed broadly neutralizing hiv-1 antibodies can restrict dendritic cell-mediated HIV-1 trans-infection. J Infect Dis. 2012;205:1248–1257. doi: 10.1093/infdis/jis183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders RW, van Gils MJ, Derking R, Sok D, Ketas TJ, Burger JA, Ozorowski G, Cupo A, Simonich C, Goo L, Arendt H, Kim HJ, Lee JH, Pugach P, Williams M, Debnath G, Moldt B, van Breemen MJ, Isik G, Medina-Ramirez M, Back JW, Koff WC, Julien JP, Rakasz EG, Seaman MS, Guttman M, Lee KK, Klasse PJ, LaBranche C, Schief WR, Wilson IA, Overbaugh J, Burton DR, Ward AB, Montefiori DC, Dean H, Moore JP. HIV-1 VACCINES. HIV-1 neutralizing antibodies induced by native-like envelope trimers. Science. 2015;349:aac4223. doi: 10.1126/science.aac4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotti N, Rybicki EP. Virus-like particles produced in plants as potential vaccines. Expert Rev Vaccin. 2013;12:211–224. doi: 10.1586/erv.12.147. [DOI] [PubMed] [Google Scholar]
- Sellhorn G, Kraft Z, Caldwell Z, Ellingson K, Mineart C, Seaman MS, Montefiori DC, Lagerquist E, Stamatatos L. Engineering, expression, purification, and characterization of stable clade A/B recombinant soluble heterotrimeric gp140 proteins. J Virol. 2012;86:128–142. doi: 10.1128/JVI.06363-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen R, Drelichman ER, Bimczok D, Ochsenbauer C, Kappes JC, Cannon JA, Tudor D, Bomsel M, Smythies LE, Smith PD. GP41-specific antibody blocks cell-free HIV type 1 transcytosis through human rectal mucosa and model colonic epithelium. J Immunol. 2010;184:3648–3655. doi: 10.4049/jimmunol.0903346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper CM, Paul WE. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Stephenson KE, Li H, Walker BD, Michael NL, Barouch DH. Gag-specific cellular immunity determines in vitro viral inhibition and in vivo virologic control following SIV challenges of vaccinated monkeys. Retrovirology. 2012;9:245. doi: 10.1128/JVI.00996-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- Tartaglia J, Cox WI, Taylor J, Perkus M, Riviere M, Meignier B, Paoletti E. Highly attenuated poxvirus vectors. AIDS Res Hum Retrovir. 1992b;8:1445–1447. doi: 10.1089/aid.1992.8.1445. [DOI] [PubMed] [Google Scholar]
- Tartaglia J, Perkus ME, Taylor J, Norton EK, Audonnet JC, Cox WI, Davis SW, van der Hoeven J, Meignier B, Riviere M, et al. NYVAC: a highly attenuated strain of vaccinia virus. Virology. 1992a;188:217–232. doi: 10.1016/0042-6822(92)90752-b. [DOI] [PubMed] [Google Scholar]
- Taylor J, Paoletti E. Fowlpox virus as a vector in non-avian species. Vaccine. 1988;6:466–468. doi: 10.1016/0264-410x(88)90091-6. [DOI] [PubMed] [Google Scholar]
- Taylor J, Weinberg R, Languet B, Desmettre P, Paoletti E. Recombinant fowlpox virus inducing protective immunity in non-avian species. Vaccine. 1988;6:497–503. doi: 10.1016/0264-410x(88)90100-4. [DOI] [PubMed] [Google Scholar]
- Team AVEGP. Cellular and humoral immune responses to a canarypox vaccine containing human immunodeficiency virus type 1 Env, Gag, and Pro in combination with RGP120. J Infect Dis. 2001;183:563–570. doi: 10.1086/318523. [DOI] [PubMed] [Google Scholar]
- Tudor D, Bomsel M. The broadly neutralizing HIV-1 IgG 2F5 elicits gp41-specific antibody-dependent cell cytotoxicity in a FcgammaRI-dependent manner. AIDS. 2011;25:751–759. doi: 10.1097/QAD.0b013e32834507bd. [DOI] [PubMed] [Google Scholar]
- Tudor D, Derrien M, Diomede L, Drillet AS, Houimel M, Moog C, Reynes JM, Lopalco L, Bomsel M. HIV-1 gp41-specific monoclonal mucosal IgAs derived from highly exposed but IgG-seronegative individuals block HIV-1 epithelial transcytosis and neutralize CD4(+) cell infection: an IgA gene and functional analysis. Mucosal Immunol. 2009;2:412–426. doi: 10.1038/mi.2009.89. [DOI] [PubMed] [Google Scholar]
- Tudor D, Yu H, Maupetit J, Drillet AS, Bouceba T, Schwartz-Cornil I, Lopalco L, Tuffery P, Bomsel M. Isotype modulates epitope specificity, affinity, and antiviral activities of anti-HIV-1 human broadly neutralizing 2F5 antibody. Proc Natl Acad Sci USA. 2012;109:12680–12685. doi: 10.1073/pnas.1200024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkoczy L, Diaz M, Holl TM, Ouyang YB, Bouton-Verville H, Alam SM, Liao HX, Kelsoe G, Haynes BF. Autoreactivity in an HIV-1 broadly reactive neutralizing antibody variable region heavy chain induces immunologic tolerance. Proc Natl Acad Sci USA. 2010;107:181–186. doi: 10.1073/pnas.0912914107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkoczy L, Chen Y, Bouton-Verville H, Zhang J, Diaz M, Hutchinson J, Ouyang YB, Alam SM, Holl TM, Hwang KK, Kelsoe G, Haynes BF. Rescue of HIV-1 broad neutralizing antibody-expressing B cells in 2F5 VH × VL knockin mice reveals multiple tolerance controls. J Immunol. 2011;187:3785–3797. doi: 10.4049/jimmunol.1101633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkoczy L, Chen Y, Zhang J, Bouton-Verville H, Newman A, Lockwood B, Scearce RM, Montefiori DC, Dennison SM, Xia SM, Hwang KK, Liao HX, Alam SM, Haynes BF. Induction of HIV-1 broad neutralizing antibodies in 2F5 knock-in mice: selection against membrane proximal external region-associated autoreactivity limits T-dependent responses. J Immunol. 2013;191:2538–2550. doi: 10.4049/jimmunol.1300971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y, Liu L, Wu L, Huang X, Ma L, Xu J. Deglycosylation or partial removal of HIV-1 CN54 gp140 V1/V2 domain enhances env-specific T cells. AIDS Res Hum Retrovir. 2009;25:607–617. doi: 10.1089/aid.2008.0289. [DOI] [PubMed] [Google Scholar]
- Williams WB, Liao HX, Moody MA, Kepler TB, Alam SM, Gao F, Wiehe K, Trama AM, Jones K, Zhang R, Song H, Marshall DJ, Whitesides JF, Sawatzki K, Hua A, Liu P, Tay MZ, Seaton KE, Shen X, Foulger A, Lloyd KE, Parks R, Pollara J, Ferrari G, Yu JS, Vandergrift N, Montefiori DC, Sobieszczyk ME, Hammer S, Karuna S, Gilbert P, Grove D, Grunenberg N, McElrath MJ, Mascola JR, Koup RA, Corey L, Nabel GJ, Morgan C, Churchyard G, Maenza J, Keefer M, Graham BS, Baden LR, Tomaras GD, Haynes BF. HIV-1 VACCINES. Diversion of HIV-1 vaccine-induced immunity by gp41-microbiota cross-reactive antibodies. Science. 2015;349:aab1253. doi: 10.1126/science.aab1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson AL, Rybicki EP. Justification for the inclusion of Gag in HIV vaccine candidates. Expert Rev Vaccin. 2015 doi: 10.1586/14760584.2016.1129904:1-14. [DOI] [PubMed]
- Yates NL, Liao HX, Fong Y, deCamp A, Vandergrift NA, Williams WT, Alam SM, Ferrari G, Yang ZY, Seaton KE, Berman PW, Alpert MD, Evans DT, O’Connell RJ, Francis D, Sinangil F, Lee C, Nitayaphan S, Rerks-Ngarm S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Pinter A, Zolla-Pazner S, Gilbert PB, Nabel GJ, Michael NL, Kim JH, Montefiori DC, Haynes BF, Tomaras GD. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med. 2014;6:228ra239. doi: 10.1126/scitranslmed.3007730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Verkoczy L, Wiehe K, Munir Alam S, Nicely NI, Santra S, Bradley T, Pemble C Wt, Zhang J, Gao F, Montefiori DC, Bouton-Verville H, Kelsoe G, Larimore K, Greenberg PD, Parks R, Foulger A, Peel JN, Luo K, Lu X, Trama AM, Vandergrift N, Tomaras GD, Kepler TB, Moody MA, Liao HX, Haynes BF. Initiation of immune tolerance-controlled HIV gp41 neutralizing B cell lineages. Sci Transl Med. 2016;8:336ra362. doi: 10.1126/scitranslmed.aaf0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolla-Pazner S. Identifying epitopes of HIV-1 that induce protective antibodies. Nat Rev Immunol. 2004;4:199–210. doi: 10.1038/nri1307. [DOI] [PMC free article] [PubMed] [Google Scholar]


