Abstract
Background
Large-scale investigations on the use of oral anticoagulants including direct oral anticoagulants (DOACs) in patients with atrial fibrillation (AF) have not included Japanese patients.
Methods
We established the multicenter SAKURA AF Registry to support prospective observational research on the status of anticoagulation treatment, especially with DOAC, for AF in Japan. We enrolled 3266 AF patients treated with warfarin (n=1577) or any of 4 DOACs (n=1689) from 63 institutions (2 cardiovascular centers, 13 affiliated hospitals or community hospitals, and 48 private clinics) in the Tokyo area.
Results
We conducted our first analysis of the registry data, and although we found equivalent mean age between the DOAC and warfarin users (71.8±9.5 vs. 72.3±9.4 years, p=0.2117), we found a slightly lower risk of stroke (CHADS2 score of 0 or 1 [46.9% vs. 39.4%, p<0.0001]) and significantly better creatinine clearance in DOAC users (70.4±27 vs. 65.6±25.7 mL/min, p<0.0001). Importantly, we documented under-dosing in 32% of warfarin users and inappropriate-low-dosing in 19.7–27.6% of DOAC users.
Conclusions
Our initial analysis of the SAKURA AF Registry data clarified the real-world use of anticoagulants, which includes DOACs and warfarin in Japan. The DOAC users were at a lower risk for stroke than the warfarin users. In 20–30% of DOAC users, the dose was inappropriately reduced.
Keywords: Atrial fibrillation, Direct oral anticoagulants, Warfarin, SAKURA AF Registry
1. Introduction
Atrial fibrillation (AF), the most common arrhythmia in the elderly, affects approximately 0.6% of the Japanese population, with its prevalence expected to increase to over 1 million persons by 2050 [1]. AF is an independent risk factor for stroke and death. Although anticoagulation with warfarin provides effective stroke prophylaxis in patients with AF, its use can be troublesome because of issues such as food-drug interactions, a narrow therapeutic window, and the need for frequent monitoring of the prothrombin-international normalized ratio (PT-INR). To overcome the limitations of warfarin, direct oral anticoagulants (DOACs) have been developed.
The benefits of DOACs over warfarin in reducing the risk of vascular events and bleeding complications in patients with AF have been substantiated in randomized clinical trials (RCTs) [2], [3], [4], [5]. Although there have been several “real-world” larger-scale registries in Japan such as the J-RHYTHM Registry [6], [7], Fushimi AF Registry [8], [9], and SHINKEN database [10], these registries included very few DOAC users. As such, no large-scale studies on the use of DOACs in Japan have been conducted to clarify the clinical characteristics of the AF patients for whom they are prescribed or to confirm the efficacy and safety of these drugs. We therefore established the multicenter SAKURA AF Registry to support prospective observational research on the status of anticoagulation treatment, i.e., treatment with DOACs and warfarin and to clarify the associated long-term outcomes in terms of strokes and bleeding complications in Japanese patients with AF (SAKURA AF Registry: UMIN Clinical Trials Registry: UMIN000014420). The study described herein stands as the first analysis of the SAKURA AF Registry data and was designed specifically to characterize DOAC and warfarin users separately.
2. Methods
2.1. The SAKURA AF Registry
The SAKURA AF Registry was set up to track the results of the follow-up examinations and clinical events of AF patients for at least 1 year and up to 3 years after their enrollment. Recruitment began in September 2013 and ceased in December 2015. The participating institutions consisted of 2 cardiovascular centers (Nihon University Itabashi Hospital and Nihon University Hospital), 13 affiliated or community hospitals, and 48 private clinics, all located mainly in the capital city of Tokyo or a Tokyo suburb. The analysis of the registry data was approved by our institutional review board (IRB) and individual hospital IRBs. All enrollees provided written informed consent for participation in the registry.
2.2. SAKURA AF Registry population
Patients enrolled in the registry were those aged ≥20 years in whom AF was diagnosed by 12-lead electrocardiograms (ECGs), 24-hour Holter ECGs, or event-activated ECGs, and who had been given warfarin or DOACs as stroke prophylaxis. Patients with rheumatic mitral valve disease, history of prosthetic valve replacements, active infective endocarditis, or who failed to provide written informed consent were not enrolled.
2.3. Data collection
Baseline data collected for the registry included the following: patient clinical characteristics, including the age, sex, body weight, and height; type of AF (paroxysmal AF [AF lasting ≤7 days], persistent AF [AF lasting >7 days and ≤1 year], or long-standing persistent AF [AF lasting >1 year]); current medications used, including antiarrhythmic, anticoagulant, and antiplatelet agents; co-morbidities and/or risk factors including hypertension, diabetes, strokes or transient ischemic attacks, coronary heart disease, and congestive heart failure, and whether the patients smoked or consumed alcohol at the time of enrollment. Any prior major bleeding events were also recorded. The CHADS2[11] and CHA2DS2-VASc [12] scores (for stroke risk) and HAS-BLED [13] score (for bleeding risk) were calculated and recorded. If available in the patient clinical records, the N-terminal pro-natriuretic peptide (NT-proBNP) levels were obtained. If available, the BNP was converted to NT-proBNP (NT-proBNP=BNP1.341−15). The PT-INR was recorded for warfarin users. Hypertension, diabetes, dyslipidemia, and heart failure were diagnosed as previously reported. [9] Creatinine clearance (CrCl) was calculated according to the Cockcroft-Gault formula [14].
2.4. Data management
A website created for the SAKURA AF Registry was used to collect all patient data through a web-based registration system. Each participating investigator was trained on how to use the study website, and received a personal ID and password for access. The patients’ baseline clinical data were entered into online forms and saved to the website. The data entry was checked by clinical research coordinators at the general registry office.
2.5. Study goals and factors analyzed
In addition to ascertaining the characteristics of the total patient population enrolled in the SAKURA AF Registry and then characterizing the warfarin and DOAC users, we explored whether warfarin and DOACs were being appropriately prescribed. In analyzing the warfarin administration, we looked at the PT-INR and accepted 1.6–2.6 as the optimal therapeutic range for those aged ≥70 years and 2.0–3.0 for those aged ≤69 years [7]. “Overdosing” was defined as a warfarin-related PT-INR above the therapeutic range, and “under-dosing” as that below the therapeutic range. In analyzing the DOAC administration, “appropriate-standard-dosing” and “appropriate-low-dosing” were defined as an administration according to a standard or low-dose regimen, respectively. The definition of a low-dose regimen for each DOAC is shown in Table 1. Inappropriate-low-dosing was defined as administering low-dose DOACs despite the standard dosage criteria being met. Inappropriate-standard-dosing was defined as administering standard-dose DOACs despite the low-dose regimen criteria being met. Dabigatran was considered to be contraindicated if the patient׳s CrCl was <30 mL/min; the other DOACs were considered to be contraindicated if the patient׳s CrCl was <15 mL/min.
Table 1.
Low-dose regimen for each of the direct oral anticoagulants.
| Dabigatran 110 mg bid (vs. a standard dosage of 150 mg bid) | Rivaroxaban 10 mg od (vs. a standard dosage of 15 mg od) | Apixaban 2.5 mg bid (vs. a standard dosage of 5 mg bid) | Edoxan 30 mg od (vs. a standard dosage of 60 mg od) |
| If CrCl is 30–50 mL/min, age is ≥70 years, or the patient has a prior bleeding history. | If CrCl is 15–50 mL/min. | Two of the following characteristics: ≥80 years, body weight <60 kg, or serum Cr level ≥1.5 mg/dL. | If CrCl is 15–50 mL/min or body weight is ≤60 kg.a |
CrCl, creatinine clearance.
The use of P-plycoprotein inhibitors (verapamil and quinidine or short-term azithromycin, clarithromycin, cyclosporine, or ketoconazole) was not available in this study.
2.6. Statistical analysis
Continuous variables are expressed as the mean±SD or median value and interquartile range for the warfarin user group and each DOAC user group. Differences in the clinical characteristics between warfarin and DOAC users were analyzed by unpaired Student t-test, Mann–Whitney test, or chi-square test, as appropriate. The results of the 4 types of DOAC users were compared using an analysis of variance (ANOVA) or chi-square test, followed by the Tukey׳sHSD (honest significant difference) multiple comparison test or a residual analysis. Multiple logistic regression analyses were performed to identify the clinical characteristics associated with DOAC inappropriate-low-dosing, and odds ratios were calculated for the relationship between the identified characteristics and inappropriate-low-dosing. The appropriate standard DOAC doses were used for reference. Between-group differences in the categorical variables were analyzed by chi-square tests. All statistical analyses were performed with JMP software version 11.0.2 (SAS Institute Inc., Cary, NC, USA), with a p<0.05 considered as statistically significant.
3. Results
3.1. Total enrollment
A total of 3266 patients with AF were enrolled in the registry between September 1, 2013 and December 31, 2015: 1200 (36.7%) from cardiovascular centers, 1310 (40.1%) from affiliated or community hospitals, and 756 (23.1%) from private clinics (see the Appendix). Regionally, 2094 (64.1%) patients were from Tokyo city, 763 (23.4%) from Saitama prefecture, 281 (8.6%) from Kanagawa prefecture, and 47 (1.4%) from other prefectures of Tokyo suburbs. The majority of the patients (1277 [39.1%] patients) were from the northern (referred to as “Johoku”) region of Tokyo city, and in particular, one-third of the registered patients (1051 [32.2%] patients) came from Itabashi-Ku, Tokyo.
3.2. Characteristics of the SAKURA AF patients
The characteristics of the registry patients are summarized in Table 2. Of the total of 3266 patients, 1577 (48.3%) were on warfarin while 1689 (51.7%) were on a DOAC (dabigatran, n=456 [14.0%], rivaroxaban, n=766 [23.5%], apixaban, n=437 [13.4%], or edoxaban, n=30 [0.9%]). The total patients’ mean age was 72.0 years, and 840 (25.7%) were women. The mean body weight was 63.8 kg. AF was paroxysmal in 1210 (37.0%) patients, persistent in 723 (22.1%), and long-standing persistent in 1301 (39.8%); the type of AF was not recorded in the remaining 32 patients. Although there was no difference between the warfarin and DOAC groups in the age, body mass index, smoking status, or drinking status, DOAC users were more likely than warfarin users to be female and have paroxysmal AF. Overall, the co-morbidities associated with strokes, such as hypertension and heart failure, tended to be less prevalent among DOAC users than among warfarin users. The average CHADS2 and CHA2DS2-VASc scores were 1.80 and 2.74, respectively. The CHADS2, CHA2DS2-VASc, and HAS-BLED scores were significantly lower among DOAC users than among warfarin users.
Table 2.
Patient clinical characteristics upon enrollment in the SAKURA AF Registry.
| Total patients | DOAC users | Warfarin users | P value⁎ | |
|---|---|---|---|---|
| (n=3266) | (n=1689) | (n=1577) | ||
| Age (years) | 72.0±9.4 | 71.8±9.5 | 72.2±9.3 | 0.2117 |
| Female sex | 840 (25.7) | 477 (28.2) | 363 (23.0) | 0.0006 |
| Height (cm) | 162.5±9.5 | 162.1±9.6 | 162.7±9.4 | 0.0202 |
| Weight (kg) | 63.8±13.0 | 63.7±13.4 | 64.0±12.5 | 0.4487 |
| BMI (kg/m2) | 24.1±3.7 | 24.1±3.9 | 24.0±3.6 | 0.6238 |
| AF type | ||||
| Paroxysmal AF | 1210 (37.0) | 716 (42.4) | 494 (31.3) | <0.0001 |
| Persistent AF | 723 (22.1) | 355 (21.0) | 368 (23.3) | |
| LS-AF | 1301 (39.8) | 602 (35.6) | 699 (44.3) | |
| Not reported | 32 (1.0) | 16 (1.0) | 16 (1.0) | |
| Systolic BP (mmHg) | 127.5±16.1 | 128.3±16.1 | 126.8±16.0 | 0.0089 |
| Diastolic BP (mmHg) | 74.6±11.3 | 74.9±11.6 | 74.2±11.0 | 0.0601 |
| Heart rate (bpm) | 73.7±15.4 | 73.5±15.9 | 73.9±14.8 | 0.4911 |
| Current smoking status | 398 (12.2) | 210 (12.4) | 188 (11.9) | 0.4744 |
| Current alcohol use | 1877 (57.5) | 981 (58.1) | 896 (56.8) | 0.1147 |
| Institution type | ||||
| High-volume center | 1200 (36.7) | 591 (35.0) | 609 (38.6) | <0.0001 |
| Hospital | 1310 (40.1) | 650 (38.5) | 660 (41.9) | |
| Clinic | 756 (23.1) | 448 (26.5) | 308 (19.5) | |
| Clinical history | ||||
| Hypertension | 2330 (71.3) | 1169 (69.1) | 1161 (73.6) | 0.0053 |
| Diabetes mellitus | 744 (22.8) | 362 (21.4) | 382 (24.2) | 0.0574 |
| Dyslipidemia | 1263 (38.7) | 618 (36.6) | 654 (40.9) | 0.0115 |
| Hyperuricemia | 657 (20.1) | 314 (18.6) | 343 (21.8) | 0.0244 |
| Heart failure | 722 (22.1) | 320 (19.0) | 402 (25.5) | <0.0001 |
| Stroke/TIA | 368 (11.2) | 176 (10.4) | 192 (12.2) | 0.1130 |
| CAD | 313 (9.6) | 149 (8.8) | 164 (10.4) | 0.1316 |
| Major bleeding | 30 (0.9) | 15 (0.9) | 15 (1.0) | 0.8502 |
| AF ablation | 287 (8.8) | 159 (9.4) | 128 (8.1) | 0.1907 |
| CHADS2 score | 1.80±1.15 | 1.72±1.14 | 1.90±1.16 | <0.0001 |
| 0 | 364 (11.1) | 211 (12.5) | 153 (9.7) | 0.0007 |
| 1 | 1050 (32.2) | 581 (34.4) | 469 (29.7) | |
| 2 | 1056 (32.3) | 533 (31.6) | 523 (33.2) | |
| 3 | 529 (16.2) | 240 (14.2) | 289 (18.3) | |
| 4 | 202 (6.2) | 93 (5.5) | 109(6.9) | |
| 5 | 59 (1.8) | 28 (1.7) | 31 (2.0) | |
| 6 | 6 (0.2) | 3 (0.2) | 3 (0.2) | |
| CHA2DS2-VASc score | 2.74±1.38 | 2.63±1.36 | 2.85±1.40 | <0.0001 |
| 0 | 129 (4.0) | 72 (4.3) | 57 (3.6) | 0.0023 |
| 1 | 470 (14.4) | 272 (16.1) | 198 (12.6) | |
| 2 | 862 (26.4) | 470 (27.8) | 392 (24.9) | |
| 3 | 929 (28.4) | 465 (27.5) | 464 (29.4) | |
| 4 | 547 (16.7) | 265 (15.7) | 282 (17.9) | |
| 5 | 223 (6.8) | 100 (5.9) | 123 (7.8) | |
| 6 | 83 (2.5) | 34 (2.0) | 49 (3.1) | |
| 7 | 23 (0.7) | 11 (0.7) | 12 (0.8) | |
| ≥ 8 | 0 (0) | 0 (0) | 0 (0) | |
| HAS-BLED score | 1.44±0.85 | 1.28±0.78 | 1.62±0.88 | <0.0001 |
| 0 | 309 (9.5) | 212 (12.6) | 97 (6.2) | |
| 1 | 1602 (49.1) | 914 (54.1) | 688 (43.7) | |
| 2 | 1015 (31.1) | 452 (26.7) | 563 (35.7) | |
| 3 | 286 (8.7) | 100 (5.9) | 186 (11.8) | <0.0001 |
| 4 | 51 (1.6) | 11 (0.7) | 40 (2.5) | |
| 5 | 3 (0.1) | 0 (0.0) | 3 (0.2) | |
| 6 | 0 (0) | 0 (0) | 0 (0) |
Values are shown as the mean±SD or n (%). AF atrial fibrillation; BMI, body mass index; BP, blood pressure; CAD, coronary artery disease; CHADS2, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus and stroke; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke, vascular disease, age 65–74 years and sex category; Center, cardiovascular center; DOAC, direct oral anticoagulant; HAS-BLED, uncontrolled hypertension (baseline systolic blood pressure ≥160 mmHg), abnormal renal function (serum creatinine clearance ≥2.26 mg/dL)/liver function (chronic hepatic disease [e.g., cirrhosis] or aspartate aminotransferase and/or alanine aminotransferase >3x normal range), stroke, prior major bleeding, elderly (age ≥65 years), drugs (anti-platelet drugs or non-steroidal anti-inflammatory drugs)/alcohol (160 g/day or more), Labile INR (overdosing shown by baseline PT-INR in warfarin users); LS-AF, long-standing persistent AF, Hospital, affiliated hospital or community hospital; TIA, transient ischemic attack.
Per the Student t-test or chi-square test, as appropriate.
3.3. Medications used, laboratory test results, and dosing in the warfarin and DOAC groups
The medications used by the patients at the time of enrollment are summarized in Table 3. Importantly, only 5.1% of warfarin group patients were new users (oral anticoagulant administered within 3 months before the enrollment date), compared to 33.4% of the DOAC group. The use of antiplatelet drugs was relatively low (15.7% of patients) and less common among DOAC users than among warfarin users. The DOAC users were preferentially given rhythm control rather than rate control drugs, whereas warfarin users were preferentially given rate control rather than rhythm control drugs.
Table 3.
Patient use of medications and laboratory test results upon enrollment in the SAKURA AF Registry.
| Total patients | DOAC users | Warfarin users | P value⁎ | |
|---|---|---|---|---|
| (n=3266) | (n=1689) | (n=1577) | ||
| New use | 645 (19.7) | 564 (33.4) | 81 (5.1) | <0.0001 |
| Antiplatelet drugs | 513 (15.7) | 207 (12.3) | 306 (19.4) | <0.0001 |
| Aspirin | 385 (11.8) | 151 (8.9) | 234 (14.8) | <0.0001 |
| Ticlopidine | 8 (0.2) | 0 (0.0) | 8 (0.5) | 0.0034 |
| Clopidogrel | 88 (2.7) | 40 (2.4) | 48 (3.0) | 0.2335 |
| Prasugrel | 19 (0.6) | 7 (0.4) | 12 (0.8) | 0.0001 |
| Cilostazol | 31 (0.9) | 10 (0.6) | 21 (1.3) | 0.0294 |
| Other | 25 (0.8) | 16 (1.0) | 9 (0.6) | 0.2172 |
| NSAID | 56 (1.7) | 34 (2.0) | 22 (1.4) | 0.1740 |
| Rate control drugs | 1979 (60.6) | 958 (56.7) | 1021 (64.7) | <0.0001 |
| Digitalis | 436 (13.4) | 172 (10.2) | 264 (16.7) | <0.0001 |
| Ca channel blocker | 436 (13.4) | 214 (12.7) | 222 (14.1) | 0.2374 |
| Beta blocker | 1450 (44.4) | 719 (42.6) | 731 (46.4) | 0.0296 |
| Rhythm control drugs | 740 (22.7) | 407 (24.2) | 333 (21.1) | 0.0382 |
| Class I | 422 (12.9) | 238 (14.1) | 184 (11.7) | 0.0391 |
| Bepridil | 322 (9.9) | 173 (10.3) | 149 (9.4) | 0.4466 |
| Amiodarone | 30 (0.9) | 11 (0.7) | 19 (1.2) | 0.0975 |
| Laboratory test results | ||||
| CrCl (mL/min) | 68.1±26.7 | 70.5±27.4 | 65.6±25.6 | |
| >80 | 901 (27.6) | 495 (29.3) | 406 (25.7) | <0.0001 |
| >50 to 80 | 1535 (47.0) | 810 (47.8) | 725 (46.0) | |
| >30 to 50 | 640 (19.6) | 320 (18.9) | 320 (20.3) | |
| ≤30 | 152 (4.7) | 43 (2.5) | 109 (6.9) | <0.0001 |
| Not reported | 38 (1.2) | 21 (1.2) | 17 (1.1) | |
| NT-proBNP (pg/mL) | 503 (195–1082) | 474 (153–1111) | 524 (238–1063) | 0.0121 |
| Not reported | 834 (25.5) | 462 (27.3) | 372 (23.6) |
Values are shown as the mean±SD, median and interquartile range, or n (%). AST, aspartate aminotransferase; ALT, alanine aminotransferase; BUN, blood urea nitrogen; NT-proBNP, N-terminal pro brain natriuretic peptide; CrCl, creatinine clearance; new use, warfarin or DOAC starting within 3 months after the registration date; Hb, hemoglobin; HDL, high-density lipoprotein; LDH, lactate dehydrogenase; LDL, low-density lipoprotein; NSAID: non-steroidal anti-inflammatory drug; TC, total cholesterol.
Per the Student t-test, Mann–Whitney test, or chi-square test, as appropriate.
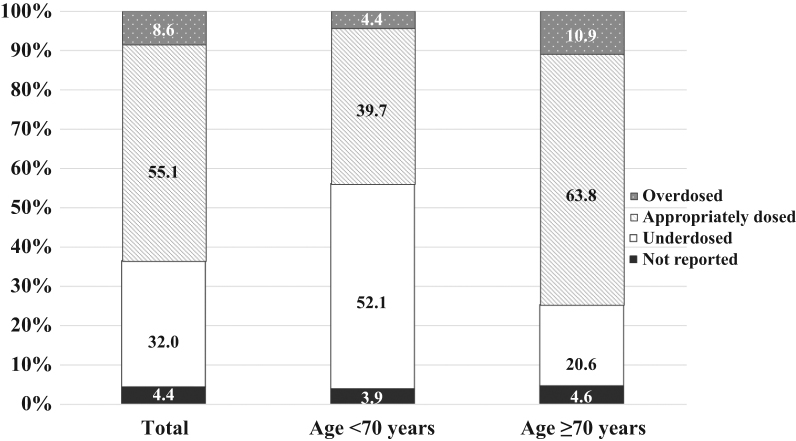
The CrCl rate was significantly higher and NT-proBNP level significantly lower in the DOAC group than in the warfarin group. The mean PT-INR in the warfarin group was 1.99±0.56 and did not differ significantly between warfarin users aged<70 years and those aged ≥70 years (2.00±0.55 vs. 1.99±0.57, p=0.7942). Only 55.1% of warfarin users had a PT-INR within the optimal therapeutic range; 32% had a PT-INR below the therapeutic range and 8.6% above the therapeutic range. Under-dosing of warfarin was more prevalent among patients aged <70 years than those aged ≥70 years (Fig. 1).
Fig. 1.
The percentages of warfarin users with a PT-INR in the therapeutic range upon enrollment, shown per age categories (<70 years and ≥70 years). Refer to the text for the definition of each category.
3.4. Clinical characteristics of the dabigatran, rivaroxaban, apixaban, and edoxaban users
The characteristics of the DOAC users are shown per subgroup (i.e., according to the specific DOAC they were taking) in Table 4. Patients taking apixaban were significantly older than those taking dabigatran and rivaroxaban. Apixaban users were significantly more likely to be female and have a significantly lower height and weight than dabigatran users. The prevalence of persistent AF and long-lasting AF was significantly lower and higher among rivaroxaban users than among apixaban users, respectively. Patients on dabigatran had a significantly higher CrCl than rivaroxaban and apixaban users. The percentage of patients who were new dabigatran users was significantly lower than the percentage of rivaroxaban users. Although not statistically significant, apixaban users were at relatively higher risk for a stroke.
Table 4.
Clinical characteristics of the dabigatran, rivaroxaban, apixaban, and edoxaban users.
| Dabigatran users | Rivaroxaban users | Apixaban users | Edoxaban users | P value | |
|---|---|---|---|---|---|
| (n=456) | (n=766) | (n=437) | (n=30) | ||
| Age (years) | 70.9±9.5⁎ | 71.5±9.1⁎ | 73.1±10.0 | 74.0±7.8 | 0.0014 |
| Female | 111 (24.3)⁎ | 201 (26.2) | 155 (35.5) | 10 (33.3) | 0.0009 |
| Height (cm) | 163.5±9.0⁎ | 162.1±9.5 | 160.7±10.2 | 160.6±10.6 | 0.0002 |
| Weight (kg) | 65.1±13.3⁎ | 63.7±13.5 | 62.6±13.5 | 58.7±11.6 | 0.0077 |
| BMI (kg/m2) | 24.2±3.6 | 24.1±4.0 | 24.1±4.0 | 22.6±3.0 | 0.1843 |
| AF type | |||||
| Paroxysmal AF | 193 (42.3) | 311 (40.6) | 199 (45.5) | 13 (43.3) | 0.0029 |
| Persistent AF | 99 (21.7) | 137 (17.9)⁎ | 109 (24.9) | 10 (33.3) | |
| LS-AF | 162 (35.5) | 308 (40.2)⁎ | 125 (28.6) | 7 (23.3) | |
| Institution type | |||||
| High-volume center | 136 (29.8)§ | 282 (36.8) | 156 (35.7) | 17 (56.7) | <0.0001 |
| Hospital | 217 (47.6)† | 267 (34.9) | 156 (35.7) | 10 (33.3) | |
| Clinic | 103 (22.6) | 217 (28.3) | 125 (28.6) | 3 (10.0) | |
| CHADS2 score | 1.70±1.17 | 1.67±1.12 | 1.81±1.14 | 1.60±1.22 | 0.2060 |
| CHA2DS2-VASc score | 2.57±1.37 | 2.61±1.35 | 2.76±1.37 | 2.57±1.36 | 0.1763 |
| New use | 57 (12.5)⁎,§ | 252 (32.9) | 231 (52.9) | 24 (80.0) | <0.0001 |
| PT-INR | NA | 1.41±0.42⁎ | 1.26±0.38 | 1.36±0.39 | 0.0063 |
| APTT (sec) | 44.0±9.9 | NA | NA | NA | |
| CrCl (mL/min) | 74.6±30.2⁎,† | 70.5±26.1 | 66.5±26.1 | 65.8±24.3 | 0.0001 |
Values are shown as the mean±SD or n (%). APTT, activated partial thromboplastin time; NA, not applicable; PT-INR, prothrombin time-international normalized ratio. Other abbreviations are as in Table 2, Table 3. P value by an ANOVA or chi-square test, as appropriate.
P<0.05 vs. apixaban users.
P<0.05 vs. rivaroxaban users.
P<0.05 vs. edoxaban in the HSD Tukeypost-hoc analysis or residual analysis.
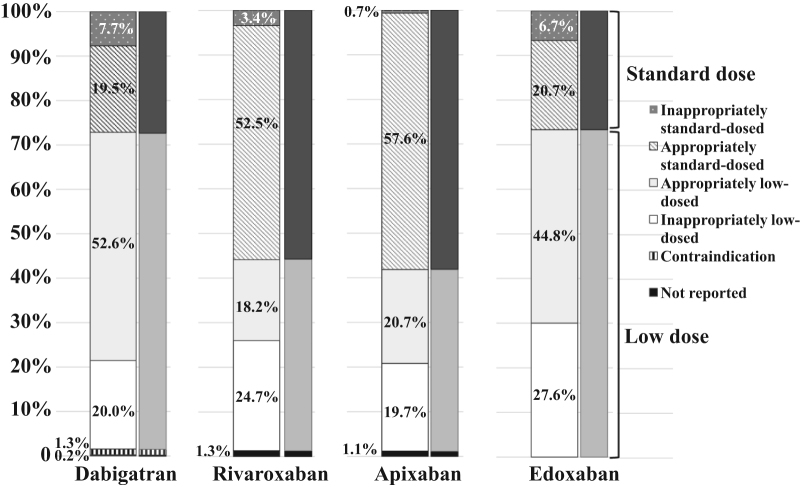
Low drug doses were prescribed for 72.6% of dabigatran users and 72.4% of the edoxaban users, in contrast to only 42.9% of rivaroxaban users and 40.4% of apixaban users. Notably, an inappropriate dose reduction was observed in 19.7–27.6% of DOAC users. Inappropriate-standard-dosing of DOACs occurred in only 0.7–7.7% (Fig. 2). The characteristics of DOAC patients who were prescribed an appropriate standard dose and those who were prescribed an inappropriate low dose are shown in Table 5. Patients on inappropriately low doses of DOACs were significantly older than those taking appropriately-standard-doses and were also more likely to be female. Inappropriately low-dosed DOAC users also had significantly lower body weight, CHADS2 score, hemoglobin level, and CrCl. Fewer inappropriately low-dosed DOAC users consumed alcohol than appropriately-standard-dosed DOAC users. New use of DOACs was less common among inappropriately low-dosed DOAC users than among appropriately-standard-dosed DOAC users. After multivariate adjustment, age ≥75 years, non-drinking status, CrCl ≤50 mL/min, and use of dabigatran or edoxaban were strongly associated with inappropriately low-dosed DOAC use (Table 5).
Fig. 2.
The percentages of DOAC users appropriately dosed, inappropriately-standard-dosed, inappropriately low-dosed, and for whom the drug was contraindicated. Percentages are shown for the 4 different DOACs prescribed. Refer to the text for the definition of each category.
Table 5.
Results of the univariate and multivariate analyses for factors predictive of DOAC inappropriate-low-dosing.
| Appropriate-standard-dose users | Inappropriate-low-dose users | P value⁎ | Factors predictive of inappropriate-low-dosing |
||
|---|---|---|---|---|---|
| (n=746) | (n=374) | Odds ratio(95% CI) | P value | ||
| Age (years) | 66.8±9.0 | 71.2±8.1 | <0.0001 | ||
| <65 | 256 (34.3) | 73 (19.5) | 1 | ||
| 65 to <75 | 345 (46.3) | 161 (43.1) | <0.0001 | 1.48 (1.00–2.20) | 0.0489 |
| ≥75 | 145 (19.4) | 140 (37.4) | 3.57 (2.21–5.81) | <0.0001 | |
| Female sex | 157 (21.1) | 109 (29.1) | 0.0027 | 1.19 (0.82–1.71) | 0.3504 |
| Weight (kg) | 68.6±13.3 | 64.6±12.5 | <0.0001 | ||
| ≤60 kg | 198 (26.5) | 141 (37.7) | 0.0001 | 1.16 (0.84–1.59) | 0.6257 |
| Paroxysmal AF | 334 (44.8) | 166 (44.4) | 0.9022 | ||
| Current smoking status | 113 (15.2) | 53 (14.1) | 0.5797 | ||
| Current alcohol use | 515 (69.0) | 204 (54.4) | <0.0001 | 0.58 (0.43–0.77) | 0.0010 |
| Institution type | |||||
| Center | 321 (43.0) | 123 (32.9) | 0.76 (0.53–1.09) | 0.1364 | |
| Hospital | 236 (31.6) | 152 (40.6) | 0.0020 | 1.02 (0.71–1.45) | 0.9320 |
| Clinic | 189 (25.3) | 99 (26.5) | |||
| Clinical history | |||||
| Hypertension | 509 (68.2) | 262 (70.1) | 0.5345 | ||
| Diabetes | 166 (22.3) | 79 (21.1) | 0.6664 | ||
| Dyslipidemia | 304 (40.8) | 143 (38.2) | 0.4175 | ||
| Hyperuricemia | 144 (19.3) | 55 (14.7) | 0.0577 | ||
| Heart failure | 121 (16.2) | 68 (18.2) | 0.4083 | ||
| Stroke/TIA | 70 (9.4) | 28 (7.5) | 0.2894 | ||
| CAD | 59 (7.9) | 41 (11.0) | 0.0910 | ||
| Major bleeding | 4 (0.5) | 5 (1.3) | 0.1569 | ||
| AF ablation | 93 (12.5) | 42 (11.2) | 0.5489 | ||
| CHADS2 score | 1.45±1.06 | 1.62±1.09 | 0.0129 | ||
| CHADS2 ≥2 | 303 (40.7) | 181 (48.4) | 0.0146 | 0.91 (0.67–1.25) | 0.5708 |
| DOAC agent | |||||
| Dabigatran | 89 (11.9) | 91 (24.3) | <0.0001 | 9.88 (6.05–16.37) | <0.0001 |
| Rivaroxaban | 400 (53.6) | 188 (50.3) | 2.21 (1.54–3.21) | <0.0001 | |
| Apixaban | 251 (33.7) | 86 (23.0) | 1 | ||
| Edoxaban | 6 (0.8) | 9 (2.4) | 7.31 (2.31–24.62) | 0.0008 | |
| New use | 296 (39.7) | 122 (32.6) | 0.0213 | 0.93 (0.69–1.27) | 0.6486 |
| Antiplatelet drugs | 85 (11.4) | 49 (13.1) | 0.4063 | ||
| CrCl (mL/min) | 84.3±27.4 | 70.1±21.3 | <0.0001 | ||
| >80 | 353 (47.3) | 99 (26.5) | 1 | ||
| >50 to 80 | 375 (50.3) | 243 (65.0) | <0.0001 | 1.95 (1.38–2.78) | 0.0001 |
| ≤50 | 19 (2.6) | 34 (9.1) | 7.84 (3.79–16.6) | < 0.0001 | |
Values are shown as the mean±SD or n (%).
CrCl, creatinine clearance.
AF atrial fibrillation; BMI, body mass index; BP, blood pressure; CAD, coronary artery disease; CHADS2, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus and stroke; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke, vascular disease, age 65–74 years and sex category; Center, cardiovascular center; DOAC, direct oral anticoagulant; HAS-BLED, uncontrolled hypertension (baseline systolic blood pressure ≥160 mmHg), abnormal renal function (serum creatinine clearance ≥2.26 mg/dL)/liver function (chronic hepatic disease [e.g., cirrhosis] or aspartate aminotransferase and/or alanine aminotransferase >3x normal range), stroke, prior major bleeding, elderly (age ≥65 years), drugs (anti-platelet drugs or non-steroidal anti-inflammatory drugs)/alcohol (160 g/day or more), Labile INR (overdosing shown by baseline PT-INR in warfarin users); LS-AF, long-standing persistent AF, Hospital, affiliated hospital or community hospital; TIA, transient ischemic attack.
Per the Student t-test or chi-square test, as appropriate.
4. Discussion
The SAKURA AF Registry is a large-scale registry designed to gather data that can be used for prospective evaluation of AF patients in Japan who are treated with a DOAC or warfarin. The main findings were as follows: (1) although there was no difference between the warfarin and DOAC groups regarding the age and body mass index, the DOAC users were at significantly lower risk for a stroke than warfarin users; (2) we documented under-dosing in 32% of warfarin users and inappropriately low dosing in 19.7–27.6% of DOAC users; (3) inappropriately low dosing of DOACs was strongly associated with age ≥75 years, non-drinking status, CrCl ≤50 mL/min, and use of dabigatran or edoxaban.
AF patients enrolled in the SAKURA AF Registry were being treated at cardiovascular centers (36.7%), affiliated or community hospitals (40.1%), and general practice clinics (23.2%) in Tokyo or a Tokyo suburb. One-third (1051 patients) of the registry patients were in Itabashi-Ku, which is located in the northern region of Tokyo city. The population of Itabashi-Ku is approximately 540,000 people. Based on an epidemiological prevalence of AF in the Japanese population of 0.6% [1], the number of AF patients in Itabashi-Ku is estimated to be approximately 3240. This number is larger than the number enrolled from Itabashi-Ku in this registry; however, the AF patients in this registry were assumed to partially reflect a typical ward in Tokyo city.
In contrast, patients enrolled in the J-RHYTHM Registry, a previously established, well-known registry in Japan [6], [7], were from cardiovascular centers throughout Japan. This large registry was further expanded to the J-RHYTHM 2 Registry [16]. The FUSHIMI AF Registry [8], [9], and most of the patients enrolled in that Registry, were from general practice clinics in Fushimi-ku, Kyoto. The SAKURA AF Registry clarified the differences in the patient characteristics of these registries. For example, the mean age of the SAKURA AF Registry patients was 72.0 years; this was older than that of the J-RHYTHM Registry (69 years) [6], [7] and J-RHYTHM 2 Registry (70 years) [16], but younger than that of the FUSHIMI AF Registry patients (74 years) [8], [9]. The CHADS2 score of 1.8 for the SAKURA AF Registry patients was higher than that of both the J-RHYTHM and J-RHYTHM 2 Registry patients (1.7 each) and lower than that of the FUSHIMI AF Registry patients (2.1). Based on the data from the Statistics Bureau, Ministry of Internal Affairs and Communications in Japan, the mean age and its distribution in Fushimi-Ku, Kyoto were similar to those in Itabashi-ku, Tokyo (mean age, 44.2 years vs. 44.5 years; age ≥65 years in 22.1% vs. 21.3%, respectively). Therefore, the differences in patient characteristics may be due to the number of patients enrolled from general practice clinics between these registries, rather than the different regions in Japan.
Patients in the SAKURA AF Registry were enrolled very recently (in 2015); therefore, the SAKURA AF Registry is a better reflection of the current status of AF treatment in Japan. Recent studies from the J-RHYTHM and SHINKEN database reported increases in DOAC administration, from 6.1% of patients in 2012 to 20.4% in 2014 [16], and from none in 2007–2009 to 25.5% in 2010–2012 [17]. In fact, the data from our SAKURA AF Registry included the largest cohort of DOAC users in Japan, totaling at 1689 (51.7%) users, compared to none in the J-RHYTHM Registry, 66 (2.1%) in the FUSHIMI AF Registry, and 923 (14.0%) in the J-RHYTHM 2 Registry.
We were able to characterize Japanese DOAC and warfarin users in terms of the clinical characteristics, use of particular medications, and initial laboratory test results. DOAC users did not differ from warfarin users in age, but they were at a lower risk for stroke and major bleeding. DOAC users were preferentially given rhythm control drugs rather than rate control drugs, probably because paroxysmal AF was more prevalent in this group than in the warfarin group. The relatively good health of the DOAC users was reflected by the laboratory test results, i.e., slightly higher CrCl and lower NT-proBNP levels. Physicians tended to be less familiar with DOACs than with warfarin, but DOACs are superior to warfarin in terms of patient management, as they do not require PT-INR monitoring and have are fewer drug-drug interactions. One limitation is that impaired renal function may alter the metabolism of DOACs [2], [3], [4], [5], [14], [15]. These features may have motivated physicians (especially those practicing in small clinics) to prescribe DOACs to patients at low risk for strokes and bleeding and with relatively normal kidney function. Apixaban and edoxaban are thought to be safer than the other DOACs for the elderly and patients with moderate renal impairment [2], [3], [4], [5], and, as was expected, our registry data showed a tendency for these DOACs to be prescribed to older patients with moderate renal impairment.
We found that 32% of warfarin users were under-dosed. The suboptimal PT-INR was pronounced in patients <70 years of age; the Japanese guidelines strongly recommend a PT-INR between 2.0 and 3.0 for patients in this age group. The relatively low PT-INR therapeutic ranges aimed at preventing future bleeding episodes are well in line with the J-RHYTHM Registry [6] and FUSHIMI AF Registry [8] data. Most importantly, our registry-based study identified inappropriate-low-dosing in 19.7–27.6% of DOAC users. Inappropriately low-dosed DOAC users were older than appropriately standard-dosed DOAC users; furthermore, they had no history of alcohol abuse and only moderate renal impairment. Age >75 years and impaired renal function are known risk factors for stroke and bleeding [2], [3], [4], [5], [14]. Physicians may have reduced the dosages as a precaution against future bleeding events. The low doses were preferentially prescribed to dabigatran and edoxaban users, and inappropriate-low-dosing of these drugs was proportionally greater than appropriate-low-dosing. This trend toward inappropriate-low-dosing may be due to the reported evidence of the effectiveness of these 2 drugs at low doses [2], [5]; however, the ROCKET AF and ARISTOTLE trials had too few patients in the low-dose DOAC groups to establish superiority, equivalence, or non-inferiority [3], [4].
4.1. Study limitations
Our study has several limitations. First, although this was a large-scale prospective observational study, the registry incorporated only selected institutions in a limited geographical area. Therefore, we cannot conclude that the data reflected all of Japan. Second, the registry did not include patients without anticoagulant drugs. This was because the annual incidence of strokes and bleeding had already been well evaluated in Japanese AF patients without anticoagulants [10]. Third, because edoxaban was approved last in Japan, the enrollment of edoxaban users was small, which may potentially distort the statistical analysis. Nonetheless, this effect was probably negligible, given the small number of edoxaban users in this study. Fourth, we defined “inappropriate-low-dosing” of DOACs according to the low-dose regimen shown in Table 1 to characterize the use of DOACs. However, inappropriate-low-dosing of dabigatran and edoxaban may not have exactly reflected the same meaning as that of rivaroxaban or apixaban, as the effectiveness and safety of low doses of dabigatran and edoxaban are well-established, even in patients that fulfilled those low-dose regimens in the clinical trials [2], [5]. Finally, the follow-up data to explore the incidences of strokes and bleeding and the time in therapeutic range (TTR) for warfarin were not available. Analysis of the full registry follow-up data will resolve any remaining questions regarding the clinical efficacy and safety of DOACs and warfarin, as well as its impact on the TTR in Japanese AF patients.
5. Conclusions
The SAKURA AF Registry data characterized Japanese AF patients recently treated with DOACs and warfarin. The DOAC users were at lower risk for strokes than the warfarin users. Twenty to thirty percent of DOACs were inappropriately low-dosed, while 30% of warfarin users were under-dosed.
Funding sources
The study was supported by scholarship funds from Astellas Pharma, Bayer Healthcare, Boehringer Ingelheim, Bristol-Meyers Squibb, Daiichi-Sankyo, Eisai, MSD, Nihon Medi-Physics, Pfizer, and Sumitomo Dainippon Pharma.
Conflict of interest
Drs. Hirayama and Matsumoto have each received research funding from several of the following: Astellas Pharma, Bayer Healthcare, Boehringer Ingelheim, Boston Scientific, Bristol-Meyers Squibb, Daiichi-Sankyo, Eisai, Hokushin Medical, MSD, Nihon Medi-Physics, Otsuka Pharmaceutical, Pfizer, and Sumitomo Dainippon Pharma. Dr. Hirayama has accepted remuneration from Astellas Pharma, Bayer Healthcare, Bristol-Meyers Squibb, Daiichi-Sankyo, Eisai, Sanofi, and Takeda Pharmaceutical, and Dr. Matsumoto has accepted remuneration from Biosensors Interventional Technologies Japan, FUJIFILM RI Pharma, and Nihon Medi-Physics.
Acknowledgments
The authors thank Ms. Wendy Alexander-Adams and Mr. John Martin for encouragement and assistance with the reporting of our findings in English.
Appendix
The key personnel and institutions participating in the registry are as follows:
Chief investigator: Hirayama A (Division of Cardiology, Department of Medicine, Nihon University School of Medicine)
Vice-chief investigator: Okumura Y (Division of Cardiology, Department of Medicine, Nihon University School of Medicine)
Steering Committee: Kunimoto S, Okumura Y, Kato M (Division of Cardiology, Department of Medicine, Nihon University School of Medicine)
Statistical analysis: Udagawa S (Department of Mathematics, Nihon University School of Medicine)
Participating institutions: Division of Cardiology, Department of Medicine, Nihon University School of Medicine (Hirayama A, Okumura Y, Watanabe I, Hiro T, Takayama T, Kunimoto S, Nakai T, Kato M); Department of Cardiology, Nihon University Hospital (Yokoyama K, Matsumoto N); Kawaguchi Municipal Medical Center (Tachibana E, Kuronuma K); Yokohama Chuo Hospital (Oiwa k); Sekishindo Hospital (Kojima T); Asakadai Central General Hospital (Hanada S); Tokyo Rinkai Hospital (Nomoto K); Kasukabe Municipal Hospital (Arima K); Yasuda Hospital (Takahashi F) Itou Cardiovascular Clinic (Itoh T); Makita General Hospital (Kotani T); Itabashi Medical Association Hospital (Ikeya Y); Kondo Clinic (Kondo K); Ukima Central Hospital (Fukushima S); Keiai Clinic (Chiku M); Ohno Medical Clinic (Ohno Y); Onikura Clinic (Onikura M); Kobari General Clinic (Kobari C); Chikuma Central Hospital (Tokai K); Kondo Clinic (Kondo K); Yamada Clinic (Osamura Y); Higashi Saitama General Hospital (Fukuda Y, Nakahara S); Zengyo-danchi Ishikawa Clinic (Ishikawa N); Tokutake Clinic (Tokutake E); Sugino Clinic (Sugino K); Yokohama Sotetsu Bldg. Clinic (Mori H); Suzuki Clinic (Suzuki Y); Fujita Clinic (Fujita M); Yumikura Clinic (Yumikura S); Keiai Hospital (Ando H); Sekimachi Clinic (Shin I); Tokiwadai Surgical Hospital (Mochizuki R); Hikarigaoka Clinic (Tokuyasu Y); Osuga Clinic (Osuga E); Nakai Clinic (Nakai K); Kurumatani Clinic (Kurumatani H); Horinaka Hospital (Sato C); Nasu Red Cross Hospital (Akabane M); Sato Clinic (Sato K); Kofune Clinic (Kofune T); Ikebukuro Okubo Clinic (Yamane A); Minami Machida Hospital (Horie T); Ishii Clinic (Ishii N); Akabane Central Hospital (Ominato M); Hirose Clinic (Hirose S); Sekimoto Memorial Hospital (Sekimoto M); Matsumura Clinic (Matsumura K); Tanaka Clinic (Tanaka M); Nakadai Clinic (Ogawa T); Naruse Clinic (Naruse K); Narimune Clinic (Kato A); Miyata Clinic (Miyata H); Aoyama Clinic (Aoyama H); Hara Clinic (Hara K); Yano Clinic (Yano F); Sekino Hospital (Sekino H); Kurokawa Clinic (Kurokawa H); Ishii Clinic (Ishii T); Abe Clinic (Abe Y); Kikuchi Clinic (Kikuchi T); Imamoto Clinic (Imamoto S); Nirenoki Clinic (Nagai C); Kachidoki View Tower Clinic (Sugino K).
References
- 1.JCS Joint Working Group Guidelines for pharmacotherapy of atrial fibrillation (JCS 2013)–digest version. Circ J. 2014;78:997–2021. doi: 10.1253/circj.cj-66-0092. [DOI] [PubMed] [Google Scholar]
- 2.Connolly S.J., Ezekowitz M.D., Yusuf S. RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 3.Patel M.R., Mahaffey K.W., Garg J. ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 4.Granger C.B., Alexander J.H., McMurray J.J. ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 5.Giugliano R.P., Ruff C.T., Braunwald E. ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 6.Atarashi H., Inoue H., Okumura K. J-RHYTHM Registry Investigators. Present status of anticoagulation treatment in Japanese patients with atrial fibrillation: a report from the J-RHYTHM Registry. Circ J. 2011;75:1328–1333. doi: 10.1253/circj.cj-10-1119. [DOI] [PubMed] [Google Scholar]
- 7.Inoue H., Atarashi H., Okumura K. Thromboembolic events in paroxysmal vs. permanent non-valvular atrial fibrillation. Subanalysis of the J-RHYTHM Registry. Circ J. 2014;78:2388–2393. doi: 10.1253/circj.cj-14-0507. [DOI] [PubMed] [Google Scholar]
- 8.Akao M., Chun Y.H., Wada H. Fushimi AF Registry Investigators: current status of clinical background of patients with atrial fibrillation in a community-based survey: the Fushimi AF Registry. J Cardiol. 2013;61:260–266. doi: 10.1016/j.jjcc.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Akao M., Chun Y.H., Esato M. Fushimi AF Registry Investigators. Inappropriate use of oral anticoagulants for patients with atrial fibrillation. Circ J. 2014;78:2166–2172. doi: 10.1253/circj.cj-14-0344. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki S., Yamashita T., Okumura K. Incidence of ischemic stroke in Japanese patients with atrial fibrillation not receiving anticoagulation therapy--pooled analysis of the Shinken Database, J-RHYTHM Registry, and Fushimi AF Registry. Circ J. 2015;79:432–438. doi: 10.1253/circj.CJ-14-1131. [DOI] [PubMed] [Google Scholar]
- 11.Gage B.F., Waterman A.D., Shannon W. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 12.Lip G.Y., Nieuwlaat R., Pisters R. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 13.Pisters R., Lane D.A., Nieuwlaat R. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in atrial fibrillation patients: the Euro Heart Survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 14.Maccallum P.K., Mathur R., Hull S.A. Patient safety and estimation of renal function in patients prescribed new oral anticoagulants for stroke prevention in atrial fibrillation: a cross-sectional study. BMJ Open. 2013;3:e003343. doi: 10.1136/bmjopen-2013-003343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eikelboom J.W., Wallentin L., Connolly S.J. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation. 2011;123:2363–2372. doi: 10.1161/CIRCULATIONAHA.110.004747. [DOI] [PubMed] [Google Scholar]
- 16.Kodani E., Atarashi H., Inoue H. J-RHYTHM Registry Investigators. Beneficial effect of non-vitamin K antagonist oral anticoagulants in patients with nonvalvular atrial fibrillation–results of the J-RHYTHM Registry 2. Circ J. 2016;80:843–851. doi: 10.1253/circj.CJ-16-0066. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki S., Otsuka T., Sagara K. Nine-year trend of anticoagulation use, thromboembolic events, and major bleeding in patients with non-valvular atrial fibrillation–Shinken Database analysis. Circ J. 2016;80:639–649. doi: 10.1253/circj.CJ-15-1237. [DOI] [PubMed] [Google Scholar]