Abstract
Background
The present study aimed to elucidate the safety and effectiveness of a noble and unique airway management technique in which a pediatric intubation tube is used in adult patients with atrial fibrillation (AF) undergoing catheter ablation (CA) under continuous deep sedation.
Methods
In total, 246 consecutive patients with AF (mean age, 65±10 years; 60 women) underwent CA under dexmedetomidine-based continuous deep sedation. A 4-mm pediatric intubation tube guided by a 10-French intratracheal suction tube was inserted smoothly, and the tip of the tube was located at the base of the epiglottis. The maximum shifting distance of the heart (MSDH) was measured with the 3D mapping system (Ensite NavX system) before and after inserting the pediatric intubation tube.
Results
At baseline, the MSDH of patients under continuous deep sedation was 23±14 mm. The pediatric intubation tube reduced the MSDH to 13±6 mm (mean reduction from baseline, 38.4±21.7%; P<0.0001). In contrast, oxygen saturation was significantly increased from 89±8% to 95±3% (P<0.0001). The mean distance between the nostril and base of the epiglottis was 16.6±0.5 mm. Major periprocedural complications occurred in 9 (3.6%) patients including 3 (1.2%) cardiac tamponade and 6 (2.4%) phrenic nerve injury cases. Larger MSDH (odds ratio, 1.13; 95% confidence interval, 1.04–1.25; P=0.007) was a significant predictor of major periprocedural complications. No major airway complications occurred, except in 3 patients (1.2%) who had minor nasal bleeding.
Conclusion
This unique airway management technique using a pediatric intubation tube for CA procedures performed in adult patients with AF under continuous deep sedation was easy, safe, and effective.
Keywords: Atrial fibrillation, Catheter ablation, Complication, Intubation, Sedation
1. Introduction
Catheter ablation (CA) is a standard therapy for treating patients with atrial fibrillation (AF) [1], [2], [3]. In contrast to CA for other less complex arrhythmias, CA of AF requires a relatively long procedural time, resulting in patient restlessness, which interferes with the procedure. Furthermore, the application of radiofrequency (RF) energy during the procedure causes considerable pain to patients. During the procedure, patients are expected to lie still on the operation table for an extended period. Hence, it is necessary to provide patients with adequate sedation and analgesia to avoid restlessness, movement, and pain, and to stabilize their respiration. Several sedation methods are used during CA including conscious sedation with an intravenous anesthetic and an analgesic, deep sedation with or without airway support, and general anesthesia [4]. While each method has advantages and disadvantages, no direct comparison has been reported. Conscious sedation with an intravenous anesthetic and an analgesic is widely used during CA for other arrhythmias and requires a relatively short procedural time. Although conscious sedation has the advantage of avoiding respiratory and hemodynamic instability, it may be insufficient to prevent restless body movements, relieve pain, and stabilize respiration during relatively long procedures such as CA of AF. In addition, this type of sedation may be not applied in institutions where repetitive intra-cardiac defibrillation is required during AF ablation procedures. General anesthesia could meet all these requirements, but it requires the support of an anesthesiologist and intubation of the patient. Recently, deep sedation with intravenous anesthetics and analgesics was reported to be effective during pulmonary vein isolation (PVI) [5]. In other studies, sedation with a propofol infusion administered by cardiologists with or without assisted ventilation was proven to be safe, effective, and feasible for use in AF ablation [4], [6]. Deep sedation, however, suppresses respiration and/or sometimes results in airway obstruction by a retracted tongue root, causing a decrease in arterial oxygen saturation. Further, it leads to unstable respiration with considerable respiratory variations that interfere with the CA procedure of AF, including catheter positioning. In the present study, we assessed the safety and efficacy of a unique method of airway support using a 4-mm pediatric intubation tube guided by a 10-French (Fr) intratracheal suction tube.
2. Methods
2.1. Study population
In total, 246 consecutive patients (mean age, 65 years; women, n=60) were enrolled in this study. The patients were referred to our institution for an initial CA to treat AF refractory to antiarrhythmic drugs. AF was defined as paroxysmal when it terminated spontaneously within 7 days, and as long-standing persistent when it continued for >12 months according to the guideline [7]. All patients provided written informed consent, and our institutional review board approved the study protocol (IRB approval on 2014/03/18; IRB no. 14-28).
2.2. Sedation
All procedures were performed with patients under deep sedation. Sedation levels were determined using the Ramsay sedation scale [8] and targeted to level 6 on the scale. The goal of sedation was to keep the patient in deep sedation throughout the procedure while maintaining spontaneous ventilation and cardiovascular hemodynamic stability. After patients were positioned supine on the catheter laboratory table, a pentazocine bolus of 15–30 mg was intravenously administered for analgesia. An introduction dose (3 µg/kg/h) of dexmedetomidine was administered for 20–30 min, followed by a maintenance dose (0.5 µg/kg/h). Low-dose propofol (0.08–0.2 mg/kg/h) was also administered from the beginning of the procedure if required to achieve stable sedation. The dose was carefully titrated to achieve the desired sedation level and to prevent side effects such as bradycardia or hypotension. Additional 2-mL boluses of 2% propofol were given as required to maintain the state of deep sedation. Oxygen was administered via a face mask starting at 5 L/min. Vital signs were continuously monitored throughout the procedure. Heart rate was monitored with a 12-lead electrocardiogram, and arterial pressure was measured invasively through a 4-Fr sheath in the femoral artery. Ventilator function was monitored by regular observation, and by auscultation of the patient, if necessary; oxygenation was continuously monitored by pulse oximetry. The patient׳s level of consciousness was examined continuously throughout the procedure.
2.3. Insertion of the pediatric intubation tube
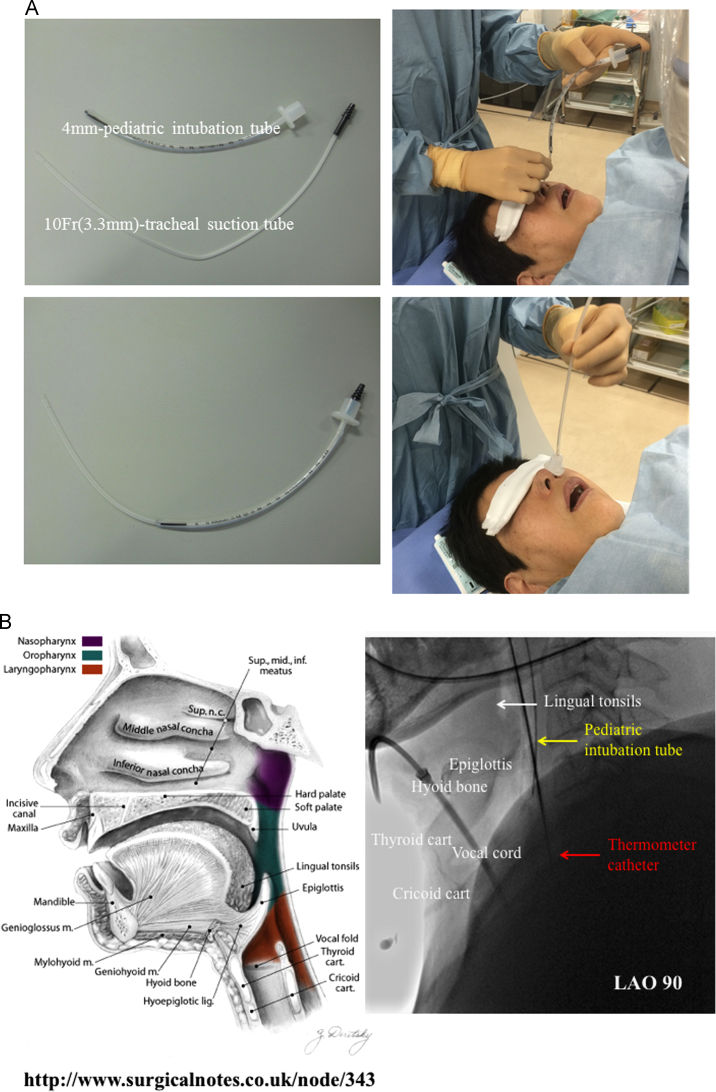
After lidocaine hydrochloride jelly was injected through the nasal cavity, a 4-mm pediatric intubation tube was smoothly inserted by a physician or a nurse using a 10-Fr (3.3 mm) tracheal suction tube. The location of the tip of the tube was determined at the point below the epiglottis and beyond the vocal cord. As the vocal cord was not visible under fluoroscopy, we positioned the tip of the tube at the base of the epiglottis as shown in Fig. 1, so we could achieve sufficient airway support and avoid vocal cord damage. If required, the proximal side of the tube was cut to adjust the tube length.
Fig. 1.
Simple items and method for the insertion of pediatric intubation tube (a), and anatomy (left) and fluoroscopy (right) of the pharynx (b). The tip of the intubation tube is placed just below the level of the epiglottis. Sufficient airway support is achieved, and vocal cord damage is avoided. LAO, left anterior oblique.
2.4. Maximum shifting distance of the heart (MSDH) using the EnSite NavX System
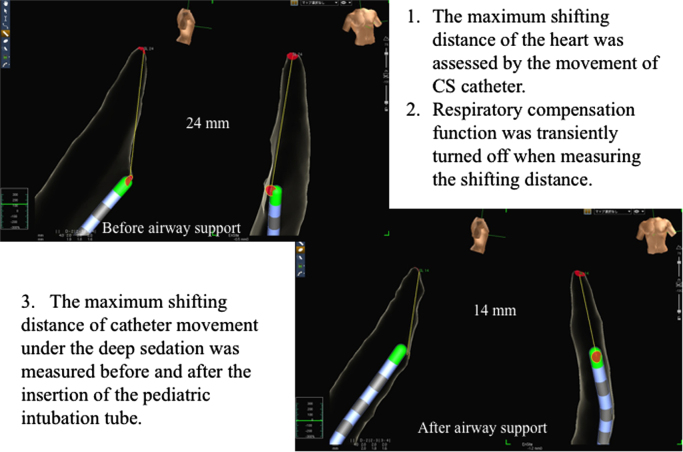
As shown in Fig. 2, the MSDH was defined as the shifting distance of the catheter in the coronary sinus compared to the initial catheter location displayed in the 3-dimensional mapping system (Ensite NavX, St. Jude Medical, St. Paul, MN, USA). The MSDH was defined as the maximum shifting distance between the location where patients breathe in and that where they breathe out in a stable respiratory state. The distance was measured twice, and the mean value was applied for the study. The MSDH was measured after automatic geometry acquisition without the respiratory compensation function. The MSDH under deep sedation was measured before and after inserting the pediatric intubation tube during the stable state of respiration.
Fig. 2.
Measurement of the maximum shifting distance of the heart. CS, coronary sinus.
2.5. Electrophysiological study
Antiarrhythmic drugs were discontinued for >7 days (amiodarone was discontinued for >1 month) before the ablation. All patients were also effectively anticoagulated for >1 month. A 7-Fr, 20- or 14-pole, two-site mapping catheter (Irvine Biomedical, Irvine, CA, USA) was inserted through the right jugular vein and positioned in the coronary sinus for pacing, recording, and internal cardioversion.
2.6. CA technique
The strategy of extensive pulmonary vein isolation was previously described [9]. An activated clotting time of >300 s was maintained with a continuous infusion of heparin during the procedure. After a transseptal puncture, two long sheaths (SL0, AF Division, St Jude Medical, Minneapolis, MN, USA) were introduced into both superior pulmonary veins (PVs) via the same transseptal hole, and pulmonary venography and contrast esophagography were performed to determine the anatomical relationships of the PV ostia, left atrium, and esophagus. Two circular mapping catheters were placed in the superior and inferior PVs, and the left and right ipsilateral PVs were circumferentially and extensively ablated under fluoroscopic and electrophysiological guidance. RF energy was delivered with a non-irrigated 8-mm-tip ablation catheter (Japan Lifeline., Tokyo, Japan) using an RF power of 35–40 W under a temperature limit of 55 °C, or an irrigated 4-mm-tip ablation catheter (Cool Path Duo, St. Jude Medical, St. Paul, MN, USA or EZ Steer Thermocool NAV, Biosense Webster, Diamond Bar, CA, USA) with an RF power of 25–30 W and a cut-off temperature of 45 °C. The endpoint of this procedure was complete PVI, which was defined as the disappearance of all PV potentials recorded by multipolar circular catheters (Inquiry TM A-focus TM II, St. Jude Medical, St. Paul, MN, USA or Lasso, Biosense Webster, Diamond Bar, CA, USA) (entrance block) and a loss of capture of the left atrium by circumferential pacing from circular catheters placed at the PV ostium (exit block). Adenosine triphosphate (20–40 mg) was injected to unmask any dormant conduction ≥30 min after completing PVI, and the reconnected conduction was disconnected [10]. A cavotricuspid isthmus line was created with an endpoint of a bidirectional conduction block [11]. If AFs were reproducibly initiated from non-PV foci, they were focally ablated [12]. When non-PV foci were located in the superior vena cava (SVC), the SVC was electrically isolated [13], [14]. Before SVC isolation, pacing was performed with an output of 5 mA and pulse width of 2.0 ms to avoid phrenic nerve injury. Linear ablations (i.e., the left atrial roof and/or bottom and/or mitral isthmus lines) were performed only when AFs from undetermined origins or macroreentrant atrial tachycardia spontaneously occurred, with an endpoint of a bidirectional conduction block [15], [16]. On completion of the procedure, the endpoints of extensive PVI, SVC isolation, and linear ablations were re-confirmed.
2.7. Statistical analysis
The data are expressed as mean (standard deviation) for continuous variables, or frequencies and percentages for categorical variables. To compare the MSDH and oxygen saturation at baseline during continuous deep sedation and after insertion of the pediatric intubation tube, the paired t test was used. To clarify the predictors of major periprocedural complications, univariate analysis was performed first. Sequentially, all variables with P-values <0.3 in the univariate analysis were included in multiple logistic regression analysis, and odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. P-values <0.05 were considered statistically significant.
3. Results
3.1. Patients’ characteristics and procedure details
The baseline characteristics of the 246 patients are shown in Table 1. Eighteen patients (7.3%) were obese (body mass index ≥30 kg/m2). 193 (78.5%) patients were CA-naive, among whom 85 (44.0%) underwent the procedure for non-paroxysmal AF. The pediatric intubation tube was successfully inserted in all patients through the tracheal suction tube within 3±2 min, and it was placed in the appropriate location as confirmed by fluoroscopy. Among patients who received the initial CA, PVI was completely achieved in all patients; focal ablation, including SVC isolation, was performed in 93 of 193 (48.1%) patients, and linear ablation was also performed in 35 of 193 (18.1%) patients. However, in 53 patients, the procedures were for the redo session, and 32 (60.4%) patients underwent the procedure for non-paroxysmal AF. Of those, focal ablation, including SVC isolation, was performed in 40 of 53 (75.4%) patients, and linear ablation was also performed in 32 of 53 (60.4%) patients. During the procedure, neither the shifting of the electro-anatomical map nor the dislocation of the reference electrode due to patient movements was observed. The mean procedure time was 190±51 min. Although 5 (2.0%) patients had hypotension (blood pressure, <90 mmHg), no patients required any inotropic drugs during the procedure. Further, according to the post-procedural questionnaire, none of the patients had severe pain, and 239 (97.2%) did not remember anything during the procedure. Regarding major complications, 3 (1.2%) patients had cardiac tamponade and required pericardiocentesis (Table 2). In addition, 6 (2.4%) patients had phrenic nerve injury; the symptom of dyspnea improved within 6 months in all patients, but the diaphragmatic function was not completely recovered in 1 patient. Regarding airway complications, 3 (1.2%) patients had minor nasal bleeding without a decrease of the hemoglobin level. Among the 9 patients with major complications, 5 (55.6%) had a large MSDH >15 mm (Table 2a, Table 2b).
Table 1.
Baseline patient characteristics.
| N=246 | |
|---|---|
| Age, years | 65±10 |
| Sex, female | 60 (24.4) |
| BMI, kg/m2 | 24.4±3.4 |
| First session | 190 (77.2) |
| AF type | |
| Paroxysmal AF | 129 (52.4) |
| Persistent AF | 45 (18.3) |
| Long-standing persistent AF | 72 (29.3) |
| Congestive heart failure | 30 (12.2) |
| Hypertension | 134 (54.7) |
| Diabetes | 29 (11.8) |
| Stroke | 20 (8.2) |
| CHADS2 score | 1.1±1.0 |
| LAV, mL | 51.4±20.1 |
| LVEF, % | 62±9 |
Data are presented as n (%) or mean (standard deviation).
AF, atrial fibrillation; BMI, body mass index; LAV, left atrial volume; LVEF, left ventricular ejection fraction.
Table 2a.
Periprocedural complications.
| Periprocedural complications | n (%) |
|---|---|
| Major complications | 9 (3.6) |
| Cardiac tamponade | 3 (1.2) |
| Phrenic neve injury | 6 (2.4) |
| Thromboembolism | 0 (0) |
| Vagal nerve injury | 0 (0) |
| Vascular injury | 0 (0) |
| Airway complications | 3 (1.2) |
| Nasal bleeding (minor) | 3 (1.2) |
| Laryngospasm | 0 (0) |
| Pharyngospasm | 0 (0) |
| Vocal cord paralysis | 0 (0) |
| Laryngeal trauma | 0 (0) |
| Pharyingeal trauma | 0 (0) |
| Aspiration pneumonia | 0 (0) |
Table 2b.
Details of patients with major complications (N=9).
| Pt. No | Complication | Sex | Age | BMI | Session no. | Type of AF | LAV | Operation | MSDH (B) | MSDH (UDS) | MSDH (AS) | SAT (B) | SAT (UDS) | SAT (AS) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CT | m | 78.0 | 31.9 | 1 | PAF | 34.90 | PVI | 9 | 18 | 13 | 100 | 90 | 96 |
| 2 | CT | m | 68.0 | 23.2 | 1 | PAF | 25.40 | PVI, CTI | 7 | 25 | 23 | 99 | 94 | 97 |
| 3 | CT | f | 64.0 | 22.4 | 1 | PAF | 33.50 | PVI,CTI | 12 | 19 | 16 | 99 | 88 | 98 |
| 4 | PI | m | 76.0 | 27.0 | 1 | PAF | 36.00 | PVI,CTI,SVCI | 15 | 48 | 50 | 98 | 90 | 95 |
| 5 | PI | m | 54.0 | 23.9 | 1 | PAF | 56.00 | PVI,CTI,SVCI | 8 | 17 | 9 | 100 | 93 | 100 |
| 6 | PI | f | 69.0 | 21.4 | 1 | PAF | 48.60 | PVI,CTI,SVCI,FA | 11 | 12 | 6 | 99 | 97 | 98 |
| 7 | PI | m | 55.0 | 21.0 | 1 | PAF | 25.10 | PVI,CTI | 9 | 24 | 17 | 99 | 90 | 95 |
| 8 | PI | m | 67.0 | 22.5 | 1 | PEF | 37.80 | PVI,SVCI, FA | 9 | 18 | 13 | 99 | 93 | 97 |
| 9 | PI | m | 78.0 | 27.3 | 2 | LPEF | 42.60 | PVI,CTI,LA | 8 | 25 | 16 | 98 | 74 | 94 |
AF, atrial fibrillation; BMI, body mass index; CT, cardiac tamponade; CTI, cavotricuspid isthmus ablation; FA, focal ablation; LA, linear ablation; LAV, left atrial volume; LPEF, long standing persistent atrial fibrillation; MSDH (AS), maximum shifting distance of the heart under the airway support; MSDH (B), MSDH at baseline; MSDH (UDS), MSDH under the deep sedation; PAF, paroxysmal atrial fibrillation; PEF, persistent atrial fibrillation; PI, phrenic nerve injury; PVI, pulmonary vein isolation; SAT (AS), saturation under the airway support; SAT (B), saturation at baseline; SAT (UDS), saturation under the deep sedation.
3.2. MSDH before and after inserting the pediatric intubation tube
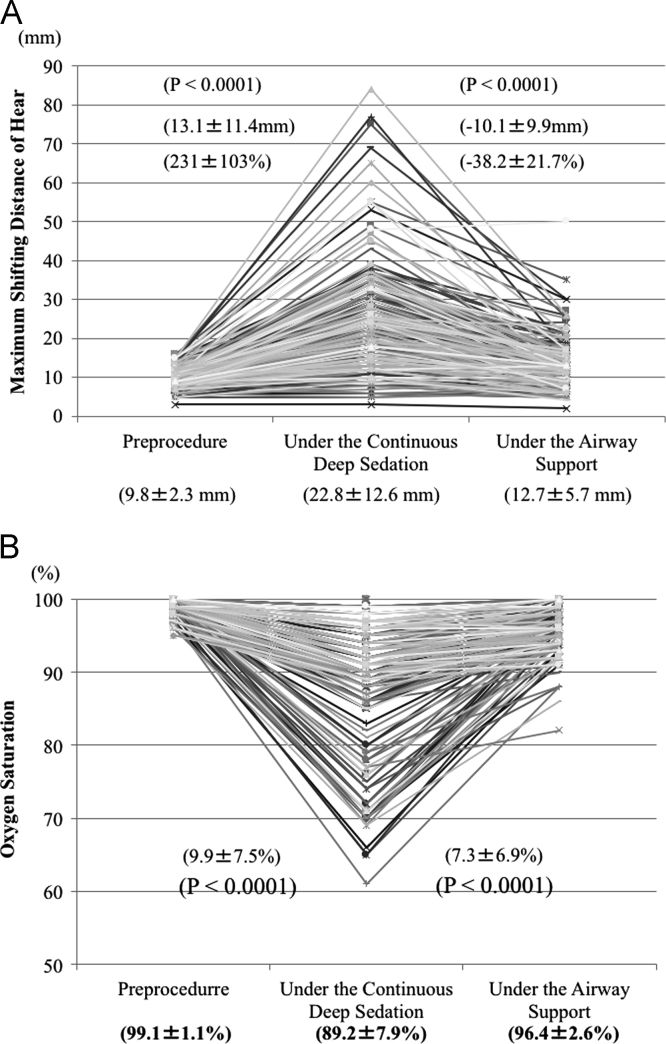
The MSDH is shown in Fig. 3a. At baseline, the mean MSDH was 9.8±2.3 (median, 10 mm; range, 3–16 mm). Under continuous deep sedation, the mean MSDH significantly increased to 22.8±12.6 mm (median, 20 mm; range, 3–84 mm). However, the pediatric intubation tube reduced it to 12.7±5.7 mm (median, 12 mm; range, 2–50 mm). Under airway support, 23 (9.3%) patients still had a large MSDH ≥20 mm. The mean distance between the nasal cavity and the back of the epiglottis was 16.9±0.4 mm in men and 15.8±0.3 mm in women.
Fig. 3.
Maximum shifting distance of the heart (a) and oxygen saturation (b) before sedation, under continuous deep sedation, and under airway support.
3.3. Oxygen saturation before and after inserting the pediatric intubation tube
The shift of oxygen saturation is shown in Fig. 3b. At baseline, the mean oxygen saturation was 99.1±1.1 (median, 99% mm; range, 95–100%). Under continuous deep sedation, the mean oxygen saturation significantly decreased to 89.2±7.9% (median, 90%; range, 61–100%). Oxygen saturation decreased <90% due to an airway obstruction by the tongue root in 91 (34.0%) patients. However, the pediatric intubation tube improved the oxygen saturation to 96.4±52.6% (median, 97%; range, 82–100%).
3.4. Predictors of major complications
Univariate Cox proportional analysis showed that a smaller left atrial volume (LAV) (P=0.028) and larger MSDH (P=0.005) were significantly associated with the occurrence of major complications as shown in Table 3. In addition, these two parameters were both associated with those complications in multivariate analysis (LAV: OR, 0.95; 95% CI, 0.91–1.00; P=0.06 and MSDH: OR, 1.13; 95% CI, 1.04–1.25; P=0.007).
Table 3.
Predictors of major complications.
| Univariate | Multivariate | OR | 95% CI | |
|---|---|---|---|---|
| Age, years | 0.44 | |||
| Sex, female | 0.39 | |||
| BMI | 0.67 | |||
| Non-paroxysmal AF | 0.21 | 0.53 | 0.57 | 0.07–3.03 |
| First session | 0.53 | |||
| Congestive heart failure | 0.91 | |||
| Hypertension | 0.33 | |||
| Diabetes | 0.26 | 0.19 | 3.6 | 0.47–20.23 |
| Stroke | 0.65 | |||
| CHADS2 score | 0.69 | |||
| LAV | 0.028 | 0.06 | 0.95 | 0.91–1.00 |
| LVEF | 0.9 | |||
| Focal ablation | 0.55 | |||
| SVCI | 0.29 | 0.47 | 1.77 | 0.35–8.57 |
| Substrate modifications | 0.37 | |||
| MSDH | 0.0047 | 0.0073 | 1.13 | 1.04–1.25 |
AF, atrial fibrillation; BMI, body mass index; CA, catheter ablation; CI, confidence interval; LAV, left atrial volume; LVEF, left ventricular ejection fraction; MSDH, maximum shifting distance of the heart; OR, odds ratio; SVCI, superior vena cava isolation.
4. Discussion
4.1. Main findings
In the present study, we objectively demonstrated the effectiveness of our simple and unique method and elucidated the following: (1) continuous deep sedation with dexmedetomidine and low-dose propofol significantly increased heart movement and decreased the oxygen saturation; (2) the airway management technique using a pediatric intubation tube was, simple, safe, and effective for supporting the airway during CA procedures in adult patients with AF under continuous deep sedation; and (3) a larger MSDH under the continuous deep sedation and a smaller LAV were significantly associated with the occurrence of major complications.
4.2. Continuous deep sedation during AF ablation
Several studies reported the effectiveness and safety of continuous deep sedation during AF CA. However, heart movement and desaturation caused by unstable respiration with considerable respiratory variations and airway obstruction due to a retracted tongue root are the main problems during the procedure. To avoid these issues, some studies recommended assisted ventilation or an anesthetist in immediate attendance during the procedures [6], [17], [18], [19]. However, these services require money, time, and the support of an anesthesiologist, and not all institutions can afford these preparations. In contrast, providing airway support under continuous deep sedation with a pediatric intubation tube was a simple, safe, and effective method.
The nasopharyngeal airway can be another possible choice. However, Stoneham et al. studied 120 anesthetized adult patients and demonstrated that the nasopharyngeal airways were frequently misplaced, 60% positioned beyond the tip of the epiglottis and 13% positioned in the vallecular [20]. They reported that the mean distances between the nostril to the vocal cord were 20.9 cm in men and 18.0 cm in women, whereas those between the nostril and the tip of the epiglottis were 15.9 cm and 14.0 cm, respectively [20]. Considering this evidence, the location of the tip of the tube in the present study seemed to be ideal; the mean distances between the nostril and back of the epiglottis were 16.9±0.4 mm in men and 15.8±0.3 mm in women. In addition, insertion of the tube through the nasopharynx is reported to be associated with a risk of epistaxis [21], and a severe case of epistaxis requires abrasion of the nasal mucosa, including Kiesselbach׳s area [22]. In addition, a unique characteristic of AF ablation is that patients are usually anticoagulated and considerably heparinized during the procedure. For this reason, we sometimes experience very severe epistaxis requiring blood transfusion. From the results of the present study, we believe that the risk of epistaxis is remarkably low when inserting a pediatric tube through the tracheal suction tube. Although the pediatric intubation tube bore may be too narrow, it prevents glossoptosis because the air not only passes through the tube, but also in the space around the tube without any severe airway complications. From the economical point of view, the combination of a pediatric tube and a tracheal suction tube costs ¥770 (USD $ 6.67), which is more reasonable than a nasal airway tube ¥1070 (USD $ 9.27).
In this study, we quantitatively demonstrated the effectiveness of a pediatric intubation tube in adult patients with AF. However, a larger MSDH after airway support remained an independent predictor of major complications, including cardiac tamponade and phrenic nerve injury. During our procedure, we performed high-output pacing from the ablation catheter just before RF applications at the SVC and the anterior wall of the right PVs, confirmed the location of the phrenic nerve, and prevented its injury. However, we did not keep pacing during the applications. As a result, the location of the ablation catheter may have moved during deep respiration, and phrenic nerve injury would have occurred in patients with a larger MSDH. In these patients, assisted ventilation or an anesthetist in immediate attendance may reduce complications. Further examination should be performed to elucidate this.
Although some studies have reported hypotension as a side effect of deep sedation during AF ablation, it is not prolonged and usually controllable [23]. In the present study, the sedation level was appropriate, and no patients required inotropic drugs for severe hemodynamic instability.
4.3. Limitations
The present study has some limitations. First, this was an observational study by design, patients were not randomized against a comparison group with a nasopharyngeal tube, and assisted ventilation was not used in this study. However, we believe that our results have clearly shown that the use of a pediatric intubation tube is at least a safe and feasible way to support the airway without causing any severe airway complications. Second, although the result of the study showed that a larger MSDH was a predictor of the major complication, there were few complications. Further study, preferably with a randomized design and a larger population, is warranted. Third, because of the short follow-up period, the AF recurrence rate was not discussed in this study. Thus, the impact of airway support using a pediatric intubation tube on the AF recurrence rate should be clarified in a further study.
5. Conclusions
A unique airway management technique using a pediatric intubation tube was safe and effective for performing CA procedures in adult patients with AF under continuous deep sedation. However, in patients with a large MSDH, even under airway support with a pediatric intubation tube, the CA procedure should be carefully performed to avoid complications.
Source of funding
None.
Conflict of interest
All authors declare no conflict of interest related to this study.
Acknowledgments
None.
References
- 1.Haïssaguerre M., Jaïs P., Shah D.C. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 2.Bhargava M., Di Biase L., Mohanty P. Impact of type of atrial fibrillation and repeat catheter ablation on long-term freedom from atrial fibrillation: results from a multicenter study. Heart Rhythm. 2009;6:1403–1412. doi: 10.1016/j.hrthm.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Ouyang F., Bänsch D., Ernst S. Complete isolation of left atrium surrounding the pulmonary veins: new insights from the double-Lasso technique in paroxysmal atrial fibrillation. Circulation. 2004;110:2090–2096. doi: 10.1161/01.CIR.0000144459.37455.EE. [DOI] [PubMed] [Google Scholar]
- 4.Murakami T., Yamaji H., Numa K. Adaptive-servo ventilation combined with deep sedation is an effective strategy during pulmonary vein isolation. Europace. 2013;15:951–956. doi: 10.1093/europace/eut007. [DOI] [PubMed] [Google Scholar]
- 5.Kottkamp H., Hindricks G., Eitel C. Deep sedation for catheter ablation of atrial fibrillation: a prospective study in 650 consecutive patients. J Cardiovasc Electrophysiol. 2011;22:1339–1343. doi: 10.1111/j.1540-8167.2011.02120.x. [DOI] [PubMed] [Google Scholar]
- 6.Salukhe T.V., Willems S., Drewitz I. Propofol sedation administered by cardiologists without assisted ventilation for long cardiac interventions: an assessment of 1000 consecutive patients undergoing atrial fibrillation ablation. Europace. 2012;14:325–330. doi: 10.1093/europace/eur328. [DOI] [PubMed] [Google Scholar]
- 7.Calkins H., Brugada J., Packer D.L. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm. 2007;4:816–861. doi: 10.1016/j.hrthm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Ramsay M.A., Savege T.M., Simpson B.R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;22:656–659. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takigawa M., Takahashi A., Kuwahara T. Long-term follow-up after catheter ablation of paroxysmal atrial fibrillation: the incidence of recurrence and progression of atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7:267–273. doi: 10.1161/CIRCEP.113.000471. [DOI] [PubMed] [Google Scholar]
- 10.Hachiya H., Hirao K., Takahashi A. Clinical implications of reconnection between the left atrium and isolated pulmonary veins provoked by adenosine triphosphate after extensive encircling pulmonary vein isolation. J Cardiovasc Electrophysiol. 2007;18:1–7. doi: 10.1111/j.1540-8167.2006.00753.x. [DOI] [PubMed] [Google Scholar]
- 11.Miyazaki S., Takahashi A., Kuwahara T. Randomized comparison of the continuous vs. point-by-point radiofrequency ablation of the cavotricuspid isthmus for atrial flutter. Circ J. 2007;71:1922–1926. doi: 10.1253/circj.71.1922. [DOI] [PubMed] [Google Scholar]
- 12.Lin W.S., Tai C.T., Hsieh M.H. Catheter ablation of paroxysmal atrial fibrillation initiated by non–pulmonary vein ectopy. Circulation. 2003;107:3176–3183. doi: 10.1161/01.CIR.0000074206.52056.2D. [DOI] [PubMed] [Google Scholar]
- 13.Tsai C.F., Tai C.T., Hsieh M.H. Initiation of atrial fibrillation by ectopic beats originating from the superior vena cava: electrophysiological characteristics and results of radiofrequency ablation. Circulation. 2000;102:67–74. doi: 10.1161/01.cir.102.1.67. [DOI] [PubMed] [Google Scholar]
- 14.Higuchi K., Yamauchi Y., Hirao K. Superior vena cava as initiator of atrial fibrillation: factors related to its arrhythmogenicity. Heart Rhythm. 2010;7:1186–1191. doi: 10.1016/j.hrthm.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 15.Jais P., Hocini M., Hsu L.F. Technique and results of linear ablation at the mitral isthmus. Circulation. 2004;110:2996–3002. doi: 10.1161/01.CIR.0000146917.75041.58. [DOI] [PubMed] [Google Scholar]
- 16.Hocini M., Jaïs P., Sanders P. Techniques, evaluation, and consequences of linear block at the left atrial roof in paroxysmal atrial fibrillation: a prospective randomized study. Circulation. 2005;112:3688–3696. doi: 10.1161/CIRCULATIONAHA.105.541052. [DOI] [PubMed] [Google Scholar]
- 17.Wutzler A., Rolf S., Huemer M. Safety aspects of deep sedation during catheter ablation of atrial fibrillation. Pacing Clin Electrophysiol. 2012;35:38–43. doi: 10.1111/j.1540-8159.2011.03260.x. [DOI] [PubMed] [Google Scholar]
- 18.Elkassabany N., Garcia F., Tschabrunn C. Anesthetic management of patients undergoing pulmonary vein isolation for treatment of atrial fibrillation using high-frequency jet ventilation. J Cardiothorac Vasc Anesth. 2012;26:433–438. doi: 10.1053/j.jvca.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Di Biase L., Conti S., Mohanty P. General anesthesia reduces the prevalence of pulmonary vein reconnection during repeat ablation when compared with conscious sedation: results from a randomized study. Heart Rhythm. 2011;8:368–372. doi: 10.1016/j.hrthm.2010.10.043. [DOI] [PubMed] [Google Scholar]
- 20.Stoneham M.D. The nasopharyngeal airway. Assessment of position by fibreoptic laryngoscopy. Anaesthesia. 1993;48:575–580. doi: 10.1111/j.1365-2044.1993.tb07119.x. [DOI] [PubMed] [Google Scholar]
- 21.Sugiyama K., Manabe Y., Kohjitani A. A styletted tracheal tube with a posterior-facing bevel reduces epistaxis during nasal intubation: a randomized trial. Can J Anaesth. 2014;61:417–422. doi: 10.1007/s12630-014-0156-3. [DOI] [PubMed] [Google Scholar]
- 22.Sim W.S., Chung I.S., Chin J.U. Risk factors for epistaxis during nasotracheal intubation. Anaesth Intensive Care. 2002;30:449–452. doi: 10.1177/0310057X0203000408. [DOI] [PubMed] [Google Scholar]
- 23.Wutzler A., Loehr L., Huemer M. Deep sedation during catheter ablation for atrial fibrillation in elderly patients. J Interv Card Electrophysiol. 2013;38:115–121. doi: 10.1007/s10840-013-9817-3. [DOI] [PubMed] [Google Scholar]