Abstract
Background
The relationship between atrial electrogram (EGM) characteristics in atrial fibrillation (AF) and those in sinus rhythm (SR) are generally unknown. The activation rate and direction may affect EGM characteristics. We examined characteristics of left atrial (LA) EGMs obtained during pacing from different sites.
Methods
The study included 10 patients undergoing pulmonary vein isolation for AF. Atrial EGMs were recorded from a 64-pole basket catheter placed in the LA, and bipolar EGM amplitudes from the distal electrode pair (1–2) and proximal electrode pair (6–7) from 8 splines were averaged. The high right atrium (HRA), proximal coronary sinus (CSp), and distal coronary sinus (CSd) were paced at 600 ms and 300 ms.
Results
When the LA voltage at SR was ≥1.5 mV, bipolar voltages of the HRA were greater than those of the CSp, which were greater than those of the CSd, regardless of the pacing cycle length. The shorter pacing cycle length resulted in a reduction of the LA EGM voltage at sites of SR voltage ≥1.5 mV, but no significant difference was seen at sites where the SR EGM amplitude was between >0.5 and <1.5 mV. No significant differences were seen in intra-basket conduction times between pacing cycle lengths of 600 ms and 300 ms at any pacing site.
Conclusion
The rate and direction-dependent reduction of the amplitude of atrial EGMs may explain, in part, the voltage discordance during SR and AF.
Keywords: Atrial fibrillation, Atrial pacing, Left atrial voltage, Pacing rate
1. Introduction
Although pulmonary vein isolation is a well-established treatment for paroxysmal atrial fibrillation (PAF) [1], the success rate for persistent AF (PerAF) is less than satisfactory [2]. pulmonary vein isolation plus ablation of complex fractionated atrial electrogram (CFAE) sites during AF identified by time domain analysis or high dominant frequency (DF) sites identified by frequency domain analysis during AF has been shown by some studies to improve acute and long-term success rates in patients with PerAF [3], [4]. However, the added CFAE ablation has been shown by other studies to be of limited long-term efficacy [5], [6], [7]. Recordings of low voltage, fractionated electrograms (EGMs) from the left atrium (LA) during sinus rhythm (SR) in patients with AF [8] has led to individualized atrial substrate modification based on low-voltage areas and a decreased likelihood of AF recurrence, regardless of the AF type [9], [10], [11], [12]. Electroanatomical voltage mapping can be used to estimate the degree of myocardial fibrosis in the atria [13]. However, the amplitude of the atrial EGMs may depend on the atrial rhythm and dominant cycle length in cases of AF. Left atrial voltage discordance during tachycardia versus SR has also been described [14], [15], but the underlying mechanism is not understood. In previous studies, we showed that most CFAE sites and high DF sites identified during AF do not correspond to high DF sites identified during SR, and we also showed, by spectral analysis of atrial EGMs obtained during SR, that DF and high DF (>70 Hz) are affected by the direction of conduction but not by the pacing rate [16], [17]. Herein, we describe a study in which we examined the effects of the direction and rate of activation on LA bipolar EGM voltage.
2. Material and methods
2.1. Study patients
The study included 10 consecutive patients (10 men, mean age: 59.4±9.1 years) scheduled for their first catheter ablation of AF. The clinical characteristics of these patients are shown in Table 1. One had PAF (AF lasting less than 7 days), and 9 had PerAF (AF lasting 7 days or more). No patient with cardiomyopathy, valvular heart disease, or congenital heart disease was included in the study. Adequate oral anticoagulation therapy was given for at least 1 month before the ablation procedure, and all antiarrhythmic drugs were discontinued for at least 5 half-lives before the procedure. Transesophageal echocardiography and transthoracic echocardiography were performed upon admission, and the following baseline echocardiographic values were obtained: LA dimension, maximum LA volume by the prolate ellipsoid method, and left ventricular ejection fraction by the Teichholz method. The study protocol was approved by the Institutional Review Board of Nihon University Itabashi Hospital (December 7, 2012; RK-121109-5), and all patients provided written informed consent.
Table 1.
Characteristics of the study patients.
| N=10 | |
|---|---|
| Age (years) | 59.4±9.1 |
| Male sex | 10 (100) |
| BMI (kg/m2) | 24.9±2.5 |
| Hypertension | 3 (30) |
| Diabetes mellitus | 2 (20) |
| Hyperlipidemia | 3 (30) |
| Heart failure | 4 (40) |
| AF duration (months) | 36.0 (6.5–60) |
| LVEF (%) | 63.6±9.7 |
| LAD (mm) | 43.5±5.2 |
| LAV (mL) | 64.7±23.1 |
Data are presented as n (%), mean±SD, or mean (range).
Abbreviations: AF, atrial fibrillation; BMI, body mass index; LAD, left atrial dimension; LAV, left atrial volume; LVEF, left ventricular ejection fraction.
2.2. Electrophysiologic study
Electrophysiologic study was performed in all patients under conscious sedation achieved with dexmedetomidine, propofol, and fentanyl, as described previously [8], [9]. After vascular access was obtained, a single transseptal puncture was performed, and intravenous heparin was administered to maintain an activated clotting time of more than 300 s. After 2 long sheaths (1 SL0 sheath and 1 Agilis sheath; St. Jude Medical, Inc., St. Paul, MN, USA) were inserted into the LA via transseptal puncture, the 3-dimensional geometry of the LA and the pulmonary veins (PVs) was reconstructed with the use of an EnSite NavX Classic mapping system (St. Jude Medical, Inc.) and a 64-pole basket catheter (Constellation, EP Technologies/Boston Scientific Corporation, San Jose, CA, USA), which consisted of 8 splines (A–H), each with 8 electrodes 4 mm in length. The basket catheter was deployed in the LA, and the distal end was placed at the left PV antrum (Fig. 1). A basket catheter of adequate size (38 mm with interelectrode spacing of 3 mm, 48 mm with interelectrode spacing of 4 mm, or 60 mm with interelectrode spacing of 5 mm) was chosen for consistent contact with the LA endocardium. We recorded multiple bipolar signals (filter setting: 30–300 Hz) simultaneously during SR. A duo-decapolar catheter (BeeAT, Japan Lifeline Co., Tokyo, Japan) was placed in the coronary sinus (CS) through the right internal jugular vein. If the patient was in AF, SR EGMs were recorded after internal atrial cardioversion was achieved by a single delivery of biphasic energy shocks of 15–20 J. Cardioversion was performed only once, and the electrophysiologic study was conducted after a 30-min waiting period.
Fig. 1.
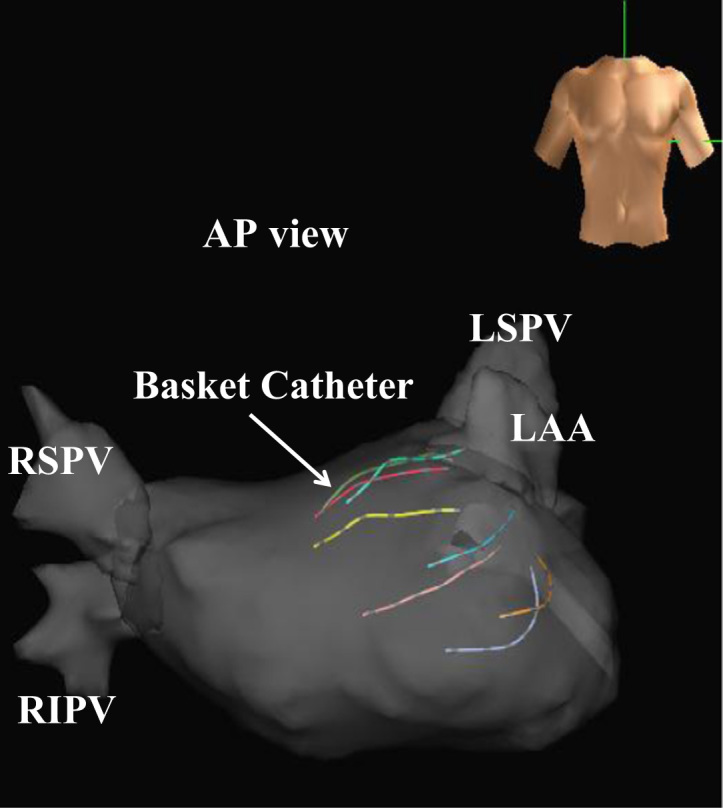
Position of the basket catheter in the left atrium. Abbreviations: AP, antero-posterior; LSPV, left superior pulmonary vein; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
2.3. Bipolar signal recordings
Because of the limited number of electrodes that can be recorded by the EnSite NavX Classic system, signals from 1 proximal electrode of each spline of the basket catheter were not recorded. Thus, EGMs were recorded from 6 of the 7 bipolar electrode pairs along each spline for a total of 48 bipolar EGMs (6 pairs×8 splines) and entered into the analysis. With the basket catheter sitting in a stable position, baseline bipolar signals were recorded from each of the 48 bipoles and stored in the NavX mapping system. DFs were calculated from EGMs obtained during SR and EGMs obtained during pacing from the high right atrium (HRA), proximal CS (CSp), and distal CS (CSd) at pacing rates of 600 ms and 300 ms. The conduction time to each bipolar pair of electrodes on the basket catheter was calculated as the difference observed between the earliest activation and latest activation from the pacing spike. To ensure stability and reliability of the bipolar signals, low voltage (≤0.5 mV) bipolar signals recorded during SR were excluded from the analysis. Overall, 12.7±8.4 electrode sites per patient were excluded. We classified the LA bipolar voltages during SR as normal (≥1.5 mV) or borderline (>0.5 mV and <1.5 mV) to examine the effect of pre-existing voltage.
2.4. Statistical analysis
Values are shown as mean±SD. Mean bipolar voltages and conduction times obtained at pacing rates of 600 ms and 300 ms were compared between the HRA, CSp, and CSd, and the values obtained at 600 ms and 300 ms were also compared. All statistical analyses were performed with JMP 8 software (SAS Institute, Cary, NC, USA), and a P value <0.05 was considered significant.
3. Results
3.1. LA bipolar voltages during pacing at the HRA, CSp, and CSd
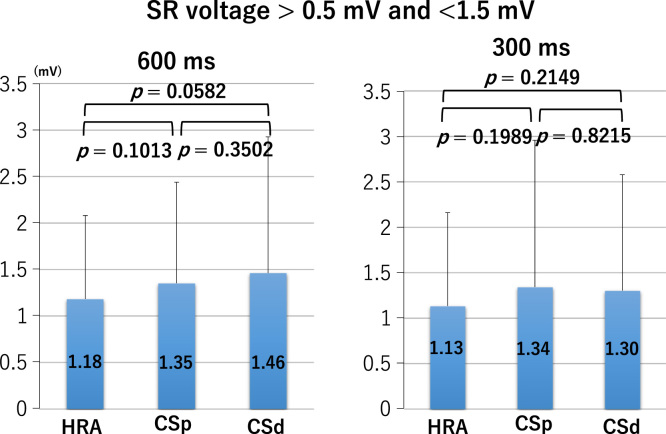
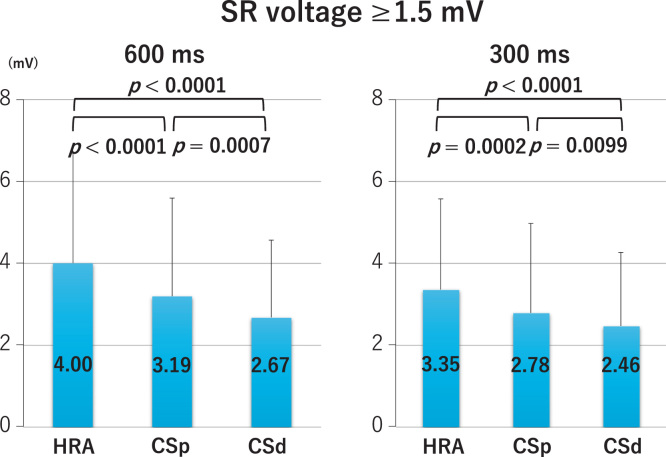
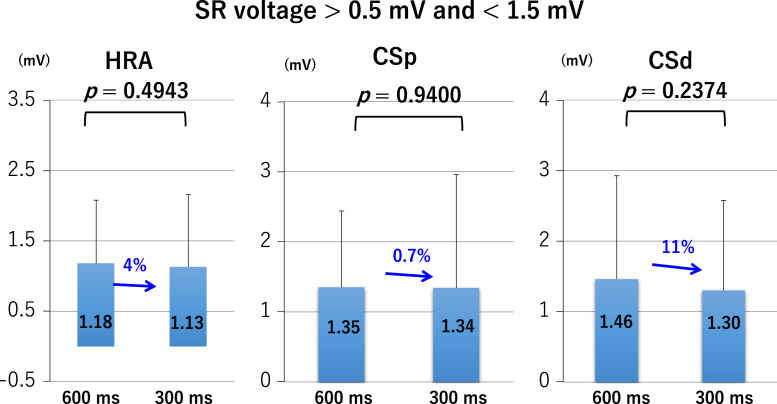
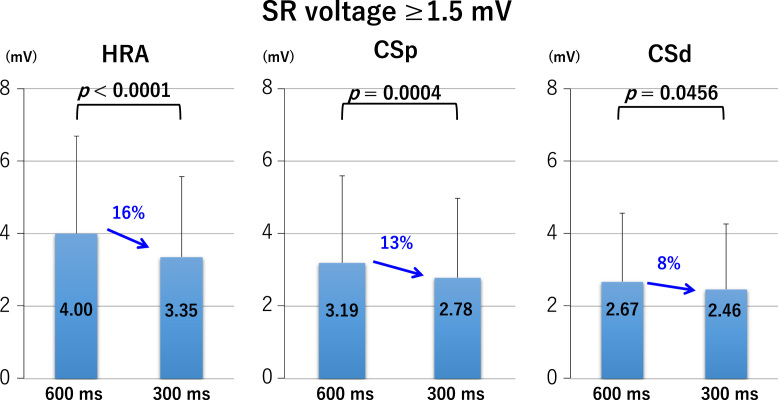
LA bipolar voltages recorded at sites of borderline SR voltage, regardless of the pacing cycle length, did not differ significantly between the HRA, CSp, and CSd (Fig. 2 lower panel and Fig. 3). At sites of normal SR voltage, LA bipolar voltages differed significantly between the HRA, CSp, and CSd. At these sites, bipolar voltages of the HRA were greater than those of the CSp, which were greater than those of the CSd (Fig. 2 upper panel and Fig. 4), regardless of the pacing cycle length. LA bipolar voltages recorded during HRA, CPs, and CSd pacing at cycle lengths of 600 ms and 300 ms are shown in Table 2A. LA bipolar voltages recorded at sites of borderline SR voltage did not differ significantly between pacing cycle lengths of 600 ms and 300 ms, whether in the HRA, CSp, or CSd (Fig. 5), but LA bipolar voltages recorded at sites of normal SR voltage at pacing cycle lengths of 300 ms were significantly lower than those recorded at cycle lengths of 600 ms (Fig. 6).
Fig. 2.
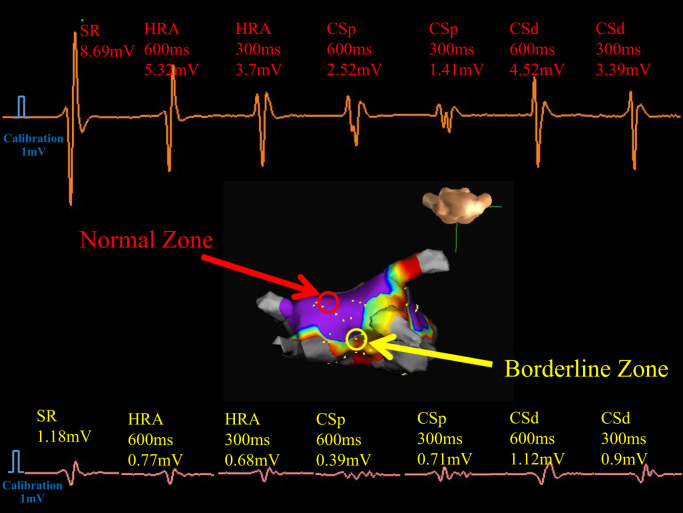
Representative electrograms in a patient with persistent atrial fibrillation. The upper panel shows a normal electrogram (electrogram amplitude more than 1.5 mV during sinus rhythm). The lower panel shows a borderline electrogram (electrogram amplitude between >0.5 mV and <1.5 mV during sinus rhythm) with changes in bipolar electrograms during sinus rhythm (SR), high right atrium (HRA), proximal coronary sinus (CSp), and distal coronary sinus (CSd) pacing at pacing cycle lengths of 600 and 300 ms. The middle panel shows 3-dimensional distributions of bipolar electrograms where the blue color shows an electrogram amplitude >1.5 mV.
Fig. 3.
Left atrial voltage with sinus rhythm (SR) electrogram amplitude between >0.5 mV and <1.5 mV during pacing from the high right atrium (HRA), proximal coronary sinus (CSp), and distal coronary sinus (CSd) at cycle lengths of 600 ms (left panel) and 300 ms (right panel).
Fig. 4.
Left atrial voltage with sinus rhythm (SR) electrogram amplitude more than 1.5 mV during pacing from the high right atrium (HRA), proximal coronary sinus (CSp), and distal coronary sinus (CSd) at cycle lengths of 600 ms (left panel) and 300 ms (right panel).
Table 2A.
Bipolar voltages during HRA, CSp, and CSd pacing.
| Pacing site | Cycle length (ms) | Voltage (mV) | P value⁎ | |
|---|---|---|---|---|
| HRA | 0.5 mV<SR<1.5 mV | 600 | 1.18±0.90 | |
| 300 | 1.13±1.03 | 0.4943 | ||
| SR≥1.5 mV | 600 | 4.00±2.69 | ||
| 300 | 3.35±2.22 | <0.0001 | ||
| CSp | 0.5 mV<SR<1.5 mV | 600 | 1.35±1.09 | |
| 300 | 1.34±1.62 | 0.9400 | ||
| SR≥1.5 mV | 600 | 3.19±2.40 | ||
| 300 | 2.78±2.19 | 0.0004 | ||
| CSd | 0.5 mV<SR<1.5 mV | 600 | 1.46±1.47 | |
| 300 | 1.30±1.28 | 0.2374 | ||
| SR≥1.5 mV | 600 | 2.67±1.89 | ||
| 300 | 2.46±1.80 | 0.0456 |
Abbreviations: CSd, distal coronary sinus; CSp, proximal coronary sinus; HRA, high right atrium; SR, sinus rhythm.
Versus 600 ms.
Fig. 5.
Left atrial voltage at pacing cycle lengths of 600 ms and 300 ms with sinus rhythm (SR) electrogram amplitude between during pacing from the high right atrium (HRA), proximal coronary sinus (CSp), and distal coronary sinus (CSd).
Fig. 6.
Left atrial voltage at pacing cycle lengths of 600 ms and 300 ms with sinus rhythm (SR) electrogram amplitude more than 1.5 mV during pacing from the high right atrium (HRA), proximal coronary sinus (CSp), and distal coronary sinus (CSd).
3.2. LA conduction times at pacing cycle lengths of 600 ms and 300 ms
Mean LA conduction times to each bipolar pair of electrodes on the basket catheter during HRA, CSp, and CSd pacing did not differ significantly between cycle lengths of 600 ms and 300 ms (Table 2B).
Table 2B.
Intra-basket conduction time during HRA, CSp, and CSd pacing.
| Pacing site | Cycle length (ms) | Conduction time (ms) | P value ⁎ |
|---|---|---|---|
| HRA | 600 | 74.3±17.4 | 0.5337 |
| 300 | 77.3±12.3 | ||
| CSp | 600 | 68.8±25.9 | 0.2657 |
| 300 | 66.1±22.2 | ||
| CSd | 600 | 66.6±13.0 | 0.3310 |
| 300 | 73.7±24.8 |
Abbreviations: CSd, distal coronary sinus; CSp, proximal coronary sinus; HRA, high right atrium; SR, sinus rhythm.
Versus 600 ms.
4. Discussion
4.1. Major findings
In this study, we compared bipolar voltages between the HRA, CSp, and CSd at pacing sites where the voltage fell within the borderline range during SR and between the HRA, CSp, and CSd at pacing sites where the voltage fell within the normal range during SR. We also examined differences at pacing cycle lengths of 600 ms and 300 ms, and we examined whether conduction times varied in relation to the bipolar voltages we recorded during pacing. First, we found that the bipolar voltages recorded at sites of normal SR voltage differed significantly between the HRA, CSp, and CSd, regardless of whether the pacing was performed at a cycle length of 600 ms or 300 ms. However, the bipolar voltages at the sites of borderline SR voltage did not differ between the HRA, CSP, and CSd, regardless of the pacing cycle length. Second, we found that the bipolar voltages at sites of normal SR voltage, but not at sites of borderline voltage, were significantly lower when pacing at a cycle length of 300 ms was performed than when pacing at a cycle length of 600 ms was performed. Intra-basket catheter conduction times did not differ significantly, regardless of the pacing site or the cycle length. We excluded the bipolar voltage data during SR≤0.5 mV because a previous study demonstrated that the currently available basket catheter leaves nearly 50% of the endocardial LA surface unmapped, and many electrodes do not make sufficient contact [18].
4.2. Voltage of the LA bipolar EGMs during SR and AF
Individualized substrate ablation based on low-voltage areas has been shown to be effective for PerAF [9], [10], [11], [12]. However, the criteria used to define low voltage differed between the published studies (Table 3). In addition, LA voltage has been shown to be significantly reduced during AF in comparison with that during SR [19], [20]. Because immediate recurrence of AF often occurs after cardioversion in patients with PerAF, it is important that consistent criteria be followed for assessment of AF in patients. In a previous study, we found that when we defined low voltage in SR as ≤0.5 mV, areas of low voltage in SR corresponded to areas of 0.2 mV in AF [21].
Table 3.
Definitions of left atrial normal and low voltages used in other studies.
| Rolf et al. [9] |
|
|
|
|
|
| Kottkamp et al. [10] |
|
|
|
|
|
| Yang et al. [12] |
|
|
|
|
|
| Jadidi et al. [11] |
|
|
|
|
Voltage: peak-to-peak amplitude of the bipolar electrograms.
Abbreviations: AF, atrial fibrillation; SR, sinus rhythm.
4.3. Direction-dependent changes in the LA bipolar EGM
Markides et al. showed that the LA endocardium has complex but characteristic patterns of activation during SR, during pacing, and during AF initiated by ectopic beats from PVs, patterns that are determined largely by the functional properties of the atrial musculature [22]. Wong et al. showed that differing wavefront directions caused changes in conduction velocity, biatrial activation time, site-specific conduction delay, and voltage in patients with lone AF and in reference patients, but these direction-dependent abnormalities were amplified in the lone AF patients in comparison to those in the reference patients. The AF patients exhibited greater slowing of conduction velocities, prolongation of biatrial activation time, increases in the number and length of lines of conduction block, increase in the proportion of fractionated EGMs, and decrease in voltage during distal CS pacing [23], [24]. In a study by Jadidi et al., LA EGM fractionation did not match between SR and CS-paced rhythm at 70±10% sites, and activation maps obtained during SR and CS-paced rhythm showed that wave collision and regional slow conduction caused the EGM fractionation [25].
We have shown that the mean DF and the percentage of high DF sites in the LA increase more during left-to-right atrial activation than during right-to-left atrial activation [26]. Therefore, the mechanism underlying the increase in DF during distal CS pacing might in part be explained by anisotropic conduction within the LA leading to increases in the number and length of lines of conduction block and to an increase in the proportion of fractionated EGMs. In the present study, we showed site-dependent differences in LA bipolar voltage amplitudes, and no cycle-dependent differences in LA bipolar EGM amplitudes. Qureshi et al. showed that shortening the pacing cycle length resulted in a reduction in LA unipolar voltages, and they also showed a difference in voltage during an activation wavefront from the opposite direction across the entire cycle length [27]. Havranek et al. showed that both LA bipolar and unipolar EGM voltages are rate dependent, and they also showed a relative reduction in voltage (up to 50% of the reference value) for short-coupled extra stimuli [28]. Our findings were consistent with these previously reported findings, which were based on a limited number of EGMs from 1 or 2 pacing sites. We speculated that the different results between the normal zone voltage and borderline zone voltage on the direction-dependent changes in bipolar voltages in this study could be caused by diseased fibrotic atrial myocardium in the borderline zone; therefore, these areas might be less affected by the direction of activation.
4.4. Clinical implications
The magnitude of the reduction in rate-dependent LA EGM voltage varies widely, depending on the pacing site and SR voltage. Therefore, further reductions can be expected at shorter atrial cycle lengths in the setting of AF. The LA voltage during pacing might also be affected by the voltage during SR.
4.5. Study limitations
Our study was limited by the small number of patients; therefore, we did not examine the data obtained from PAF patients and PerAF patients separately. In addition, we did not include the LA bipolar EGM data for EGM amplitude ≤0.5 mV because it would have been difficult to discriminate a true low voltage from poor contact with the LA wall. Even in the border zone, it was difficult to distinguish between true diseased myocardium and poor contact with the LA wall because we did not examine the pacing threshold from the border zone electrograms. Further, we did not include control patients without AF because of the need for transseptal puncture and placement of a basket catheter in the LA. In addition, we used the NavX system-automated algorithm to define and detect fractionation intervals and DFs. We cannot rule out the possibility that use of a different automated algorithm with a different mapping system or use of a mapping catheter with different interelectrode spacing could have yielded different results. Our study included patients with PAF and patients with PerAF, and the left ventricular function was preserved and cardioversion prior to ablation was feasible in all of the patients. Thus, our findings may not necessarily apply to the broader AF population. Furthermore, the currently available basket catheter leaves nearly 50% of the endocardial LA surface unmapped, and many electrodes do not make sufficient contact. Therefore, high-density mapping of the whole LA chamber is needed to clarify the relationship between the direction of conduction, the wavefront collision sites, and the LA EGM amplitudes.
5. Conclusion
Left atrial EGM voltage is rate- and direction-dependent at the normal (≥1.5 mV) voltage zone. This implies that voltages during SR are difficult to predict from voltages during atrial tachycardia/fibrillation and vice versa.
Source of funding
This study was supported by departmental resources only.
Conflict of interest
All authors declare no conflict of interest related to this study.
References
- 1.Bhargva M., Di Biase L., Mohanty P. Impact on type of atrial fibrillation and repeat catheter ablation on the long-term freedom from atrial fibrillation: results of a multicenter study. Heart Rhythm. 2009;6:1403–1412. doi: 10.1016/j.hrthm.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Oral H., Knight B.P., Tada H. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation. 2002;105:1077–1081. doi: 10.1161/hc0902.104712. [DOI] [PubMed] [Google Scholar]
- 3.Hunter R.J., Berriman T.J., Diab I. Long-term efficacy of catheter ablation for atrial fibrillation: impact of additional targeting of fractionated electrograms. Heart. 2010;96:1372–1378. doi: 10.1136/hrt.2009.188128. [DOI] [PubMed] [Google Scholar]
- 4.Kumagai K., Nakano M., Kutsuzawa D. The efficacy of ablation based on the combined use of the dominant frequency and complex fractionated atrial electrograms for non-paroxysmal atrial fibrillation. J Cardiol. 2016;67:545–550. doi: 10.1016/j.jjcc.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Bassiouny M., Saliba W., Hussein A. Randomized study of persistent atrial fibrillation ablation: ablate in sinus rhythm versus ablate complex-fractionated atrial electrograms in atrial fibrillation. Circ Arrhythm Electrophysiol. 2016;9:e003596. doi: 10.1161/CIRCEP.115.003596. [DOI] [PubMed] [Google Scholar]
- 6.Verma A., Jiang C.Y., Betts T.R. STAR AF II Investigators. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–1822. doi: 10.1056/NEJMoa1408288. [DOI] [PubMed] [Google Scholar]
- 7.Vogler J., Willems S., Sultan A. Pulmonary vein isolation versus defragmentation: the CHASE-AF clinical trial. J Am Coll Cardiol. 2015;66:2743–2752. doi: 10.1016/j.jacc.2015.09.088. [DOI] [PubMed] [Google Scholar]
- 8.Stiles M.K., John B., Wong C.X. Paroxysmal lone atrial fibrillation is associated with abnormal atrial substrate characterizing the “second factor”. J Am Coll Cardiol. 2009;53:1182–1191. doi: 10.1016/j.jacc.2008.11.054. [DOI] [PubMed] [Google Scholar]
- 9.Rolf S., Kircher S., Arya A. Tailored atrial substrate modification based on low-voltage areas in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7:825–833. doi: 10.1161/CIRCEP.113.001251. [DOI] [PubMed] [Google Scholar]
- 10.Kottkamp H., Berg J., Bender R. Box isolation of fibrotic areas (BIFA): a patient-tailored substrate modification approach for atrial fibrillation. J Cardiovasc Electrophysiol. 2016;27:22–30. doi: 10.1111/jce.12870. [DOI] [PubMed] [Google Scholar]
- 11.Jadidi AS, Lehmann H, Keyl D, etal. Ablation of persistent atrial fibrillation targeting low-voltage areas with selective activation characteristics. Circ Arrhythm Electrophysiol 2017, 10.1016/j.joa.2016.10.001[inpress] [inpress]. [DOI] [PubMed]
- 12.Yang G, Yang B, Wei Y, et al. Catheter ablation of nonparoxysmal atrial fibrillation using electrophysiologically guided substrate modification during sinus rhythm after pulmonary vein isolation. Circ Arrhythm Electrophysiol 2017, 10.1161/CIRCEP.115.002962. [in press]. [DOI] [PubMed]
- 13.Malcolme-Lawes L.C., Juli C., Karim R. Automated analysis of atrial late gadolinium enhancement imaging that correlates with endocardial voltage and clinical outcomes: a 2-center study. Heart Rhythm. 2013;10:1184–1191. doi: 10.1016/j.hrthm.2013.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemery R., Birnie D., Tang A.S. Normal atrial activation and voltage during sinus rhythm in the human heart: an endocardial and epicardial mapping study in patients with a history of atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:402–408. doi: 10.1111/j.1540-8167.2007.00762.x. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi Y., O’Neill M.D., Hocini M. Effects of stepwise ablation of chronic atrial fibrillation on atrial electrical and mechanical properties. J Am Coll Cardiol. 2007;49:1306–1314. doi: 10.1016/j.jacc.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 16.Kofune M., Okumura Y., Watanabe I. Comparative distribution of complex fractionated atrial electrograms, high dominant frequency (HDF) sites during atrial fibrillation and HDF sites during sinus rhythm. J Interv Card Electrophysiol. 2013;36:297–306. doi: 10.1007/s10840-012-9748-4. [DOI] [PubMed] [Google Scholar]
- 17.Laughner J., Shome S., Child N. Practical considerations of mapping persistent atrial fibrillation with whole-chamber basket catheters. J Am Coll Cardiol EP. 2016;2:55–65. doi: 10.1016/j.jacep.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Okumura Y., Watanabe I., Kofune M. Characteristics and distribution of complex fractionated atrial electrograms and the dominant frequency during atrial fibrillation: relationship to the response and outcome of circumferential pulmonary vein isolation. J Interv Card Electrophysiol. 2012;34:267–275. doi: 10.1007/s10840-011-9637-2. [DOI] [PubMed] [Google Scholar]
- 19.Ndrepepa G., Schneider M.A., Karch M.R. Impact on atrial fibrillation on the voltage of bipolar signals acquired from the left and right atria. Pacing Clin Electrophysiol. 2003;26:862–869. doi: 10.1046/j.1460-9592.2003.t01-1-00151.x. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki N., Watanabe I., Okumura Y. Complex fractionated atrial electrograms, high dominant frequency regions, and left atrial voltages during sinus rhythm and atrial fibrillation. J Arrhythmia. 2017 doi: 10.1016/j.joa.2016.10.001. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi K, Okumura Y, Watanabe I, et al. Impact of atrial fibrillation on the voltage of bipolar signals from the left atrium. In: Proceedings of the 63rd annual meeting of the Japanese arrhythmia and electrocardiology. (abstract) O-43.
- 22.Markides V., Schilling R.J., Ho S.Y. Characterization of left atrial activation in the intact human heart. Circulation. 2003;107:733–739. doi: 10.1161/01.cir.0000048140.31785.02. [DOI] [PubMed] [Google Scholar]
- 23.Wong C.X., Stiles M.K., John B. Direction-dependent conduction in lone atrial fibrillation. Heart Rhythm. 2010;7:1192–1199. doi: 10.1016/j.hrthm.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 24.Wong C.X., John B., Brooks A.G. Direction-dependent conduction abnormalities in the chronically stretched atria. Europace. 2012;14:954–961. doi: 10.1093/europace/eur428. [DOI] [PubMed] [Google Scholar]
- 25.Jadidi A.S., Duncan E., Miyazaki S. Functional nature of electrogram fractionation demonstrated by left atrial high-density mapping. Circ Arrhythm Electrophysiol. 2012;5:32–42. doi: 10.1161/CIRCEP.111.964197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iso K., Watanabe I., Kogawa R. Influence of direction of conduction on the sinus rhythm dominant frequency of left atrium in patients with atrial fibrillation. J Nihon Univ Med Assoc. 2017 [in press] [Google Scholar]
- 27.Qureshi N.A., Sau A., Kin S. Wavefront direction and cycle length influence unipolar endocardial tissue voltage independent of late-gadolinium enhanced cardiac MRI-defined scar. Heart Rhythm. 2013;11(Issue 5S):S227. [Google Scholar]
- 28.Havranek S., Fingrova Z., Boucek T. Atrial rhythm and atrial electrogram amplitude. Europace. 2015;17(Suppl. 3):P574. [Google Scholar]