Abstract
Mullerian inhibiting substance (MIS) inhibits breast cancer cell growth in vitro. To extend the use of MIS to treat breast cancer, it is essential to test the responsiveness of mammary tumor growth to MIS in vivo. Mammary tumors arising in the C3(1) T antigen mouse model expressed the MIS type II receptor, and MIS in vitro inhibited the growth of cells derived from tumors. Administration of MIS to mice was associated with a lower number of palpable mammary tumors compared with vehicle-treated mice (P = 0.048), and the mean mammary tumor weight in the MIS-treated group was significantly lower compared with the control group (P = 0.029). Analysis of proliferating cell nuclear antigen (PCNA) expression and caspase-3 cleavage in tumors revealed that exposure to MIS was associated with decreased proliferation and increased apoptosis, respectively, and was not caused by a decline in T antigen expression. The effect of MIS on tumor growth was also evaluated on xenografted human breast cancer cell line MDA-MB-468, which is estrogen receptor- and retinoblastoma-negative and expresses mutant p53, and thus complements the C3(1)Tag mouse mammary tumors that do not express estrogen receptor and have functional inactivation of retinoblastoma and p53. In agreement with results observed in the transgenic mice, MIS decreased the rate of MDA-MB-468 tumor growth and the gain in mean tumor volume in severe combined immunodeficient mice compared with vehicle-treated controls (P = 0.004). These results suggest that MIS can suppress the growth of mammary tumors in vivo.
Keywords: proliferation, apoptosis, simian virus 40 large T antigen
Mullerian inhibiting substance (MIS) is a member of the TGF-β family, a class of molecules that govern a myriad of cellular processes including growth, differentiation, and apoptosis. Synthesis of MIS demonstrates a sexually dimorphic pattern and is produced by Sertoli cells of the fetal and adult testis and granulosa cells of the postnatal ovary. In male embryos, MIS causes regression of the Mullerian duct, the anlagen of the Fallopian tubes, uterus, and upper vagina (1). However, a postnatal role for MIS in males and females has yet to be defined. Signaling by MIS is propagated by binding of MIS to the MIS type II receptor, a transmembrane serine, threonine kinase expressed at high levels in the Mullerian duct, Sertoli cells, and granulosa cells of the embryonic and adult gonads and in the uterus (2–4). The MIS-bound type II receptor subsequently recruits a type I receptor. Activin-like kinase 2 (ALK2), ALK3, and ALK6 have been implicated in mediating MIS signaling in cells (5–9).
We recently demonstrated the presence of MIS receptors in mammary tissue and breast cancer cell lines, suggesting that the mammary gland is a likely target for MIS (6, 10, 11). In the rat mammary gland, expression of the MIS type II receptor is suppressed during puberty when the ductal system branches and invades the adipose stroma and during massive expansion at pregnancy and lactation, but is up-regulated during involution, a time of tissue regression (11, 12). The decline in MIS type II receptor expression during various stages of postnatal mammary growth suggested a growth-suppressive role for MIS in the mammary gland. Consistent with this concept, MIS inhibited the growth of both estrogen receptor-positive and -negative breast cancer cells by inducing cell cycle arrest and apoptosis (10). Moreover, injection of MIS into female mice induced apoptosis in the epithelium of mammary tissue compared with vehicle-injected control animals (11).
To evaluate whether MIS may be useful in breast cancer therapy, we determined whether the growth inhibitory effect of MIS observed in vitro would be recapitulated in vivo. Assaying the effect of MIS on mammary tumor models in vivo is critical for determining whether MIS could act as an antitumor agent in a milieu replete with several growth factors that promote tumor growth. The C3(1)Tag transgenic mouse model carries the simian virus 40 (SV40) large T antigen targeted to the epithelium of the mammary and prostate glands. The transgenic female mice spontaneously develop atypical ductal hyperplasia by 8 weeks, nodular atypical hyperplasia by 12 weeks, and invasive carcinomas by 16–20 weeks. Disease progression in this model occurs within a relatively short period and correlates well with progressive stages of human breast cancer (13), and has been used in several studies to test novel therapeutic strategies on various stages of mammary tumor progression (13, 14).
The oncogenic SV40 large T antigen-induced tumorigenesis involves functional inactivation of the tumor suppressor genes retinoblastoma (Rb) and p53 (15, 16), and invasive carcinomas arising in this model are estrogen-independent. Mutations in Rb are prevalent in 20% of human breast cancers and p53 mutations/alterations are detected in ≈50% of primary human breast tumors (17, 18), suggesting that inactivation of these two tumor suppressors may be critical in human breast tumorigenesis. The estrogen receptor-negative human breast cancer cell line MDA-MB-468 is Rb negative, harbors mutant p53, overexpresses the EGF receptor (19), and is highly responsive to MIS treatment in vitro (20). Thus testing the efficacy of MIS in severe combined immunodeficient (SCID) mice bearing MDA-MB-468 tumors would validate the antitumor studies in the C3(1)Tag model for spontaneous mammary carcinoma. In this study, we evaluated whether MIS can inhibit the growth of mammary tumors in the C3(1)Tag model as well as MDA-MB-468 xenografts established in SCID mice. Our results demonstrate that MIS suppresses the growth of mammary tumors in vivo in both experimental systems.
Methods
Cell Lines, Reagents, and Growth Inhibition Assays. The M6 tumor cells established from C3(1)Tag mice and MDA-MB-468 cells were grown in DMEM supplemented with 10% female FBS, glutamine, and penicillin/streptomycin. To measure inhibition of M6 cell proliferation by MIS, cells were plated in a 24-well plate at a density of 2,500 cells per well and treated with 1, 5, and 10 μg/ml of MIS for 4 days. Cell numbers were quantified by using a hemocytometer. Recombinant human MIS was immunoaffinity-purified from CHO cells transfected with the human MIS gene (21) followed by desalting and concentration by centricon (Millipore) and quantification by the BioRad method.
Antibodies and Western Blot Analyses. The rabbit MIS type II receptor antibody has been described (22). The anti-SV40 large Tag antibody was purchased from Pharmingen, and the anti-cleaved caspase-3 antibody was from Cell Signaling Technology, Beverly, MA. Western analysis was performed as described (23).
Animal Studies. C3(1)Tag Mice. All animals were cared for and experiments were performed at The Wellman Animal Facility, Massachusetts General Hospital under American Association for Laboratory Animal Science guidelines using protocols approved by the Institutional Review Board-Institutional Animal Care and Use Committee of the Massachusetts General Hospital. A pair of young homozygous male and female C3(1)Tag mice were used to build a mouse colony. Twenty-five 10-week-old female mice (consisting of seven separate litters born 2–3 days apart) were randomized into two groups to receive PBS (vehicle control, 13 animals) and 20 μg of MIS per animal per day (12 animals) by i.p. injections. Animals were injected for 5 days with 2 days of treatment-free interval for 6 weeks. Mice were monitored daily for evidence of toxicity and found to be healthy and active during the entire course of treatment. None of the animals had externally visible tumors at the commencement of treatment. Three weeks after treatment began palpable tumors emerged in some animals. At the end of the experiment, animals were killed, and tumors were excised, weighed, and snap-frozen or fixed for histologic and biochemical evaluation.
MIS ELISA (24) to determine MIS concentration in blood collected from mice at the end of the experiment was analyzed in duplicate at six serial dilutions by using a standard curve constructed with four-parameter logistical curve fitting Delta-Soft II (BioMetallics, Princeton, NJ). Assay sensitivity was 0.5 ng/ml; and the intraassay and interassay coefficients of variation were 9% and 15% respectively. The ELISA did not recognize luteinizing hormone, follicle-stimulating hormone, activin, inhibin, TGF-β, or bovine or rodent MIS.
SCID mice. MDA-MB-468 xenografts were established by bilaterally injecting 4 × 106 cells per site in 50 μl of DMEM s.c. into the dorsal flanks of 10 6-week-old female SCID mice maintained in the Edwin L. Stelle Laboratory for Tumor Biology, Boston. Mice were ear-tagged to monitor the kinetics of tumor growth at each site. After ≈4 weeks palpable tumors were observed in 8 of 10 animals. Some developed tumors bilaterally (7/10), whereas another had just one tumor (1/10). The eight animals with palpable tumors were divided randomly into two treatment groups. The PBS-treated group had four animals, three of which had two tumors and one of which had one tumor. The MIS treatment group had four animals, each of which had two tumors.
Treatment of both groups began at the same time. The tumor volumes in the animals in the two groups were comparable. Mice were injected daily i.p. with PBS (100 μl) or 20 μg MIS per animal for 5 days a week with a treatment-free interval of 2 days. Tumors were measured by using calipers just before treatment began and at regular intervals throughout the treatment period. Volume was calculated as L × W2 (L, length; W, width). Serum MIS concentration was measured at the end of the experiment by MIS-ELISA.
Serum MIS Measurement. Blood was collected by cardiac puncture from PBS- and MIS-treated mice and placed in 1.5-ml microcentrifuge tubes to facilitate clot formation. The clots were centrifuged, and serum was removed to measure serum MIS concentrations as described (25).
Statistical Analyses. The number of measurable tumors in each group on the last day of treatment was compared by using one-sided Fisher's exact test. Differences were considered to be significant when P < 0.05.
The mean tumor weights at the end of the experiment in PBS- and MIS-treated C3(1)Tag mice were compared by using the Kruskal–Wallis test, and differences were considered to be significant when P < 0.05.
Because each mouse is an experimental unit our calculations are based on total tumor volume per animal. In MDA-MB-468 tumor-bearing SCID mice, tumor volumes were comparable between sites and were summed to obtain total tumor volume per animal. The gain in tumor volume per mouse, at the end of the experiment, was calculated as {tumor volumeFinal - tumor volumeInitial ÷ tumor volumeInitial}. Statistical analysis was performed by using two-sided Student's t test. Differences were considered to be significant when P < 0.05.
Immunohistochemical Analyses. Tissues were fixed in formalin and embedded in paraffin. Sections of 5-μ thickness were stained with hematoxylin and eosin. To detect apoptosis, sections were immunostained with Cleaved Caspase-3 antibody (Asp-175, Cell Signaling Technology) according to the manufacturer's instructions. Briefly, sections were deparaffinized, treated with citrate buffer at subboiling temperature to retrieve antigen, and cooled, and peroxidase quench was added. The slides were washed and blocked, and primary antibody was added and incubated overnight. After washing three times, slides were incubated with secondary antibody for 30 min, and Avidin Biotin solution was added. Color was developed with substrate chromagen, and sections were counterstained with hematoxylin.
Proliferation in tumors was assessed by staining sections with the proliferating cell nuclear antigen (PCNA) staining kit (Zymed). Sections were treated with hydrogen peroxide to inhibit endogenous peroxidase, and antigen was retrieved by microwaving the samples in citrate buffer. Slides were stained with a biotinylated PCNA mAb (clone PC10) followed by streptavidin-peroxidase as a signal generator and diaminobenzidine as chromogen. Sections were counterstained with hematoxylin.
Results
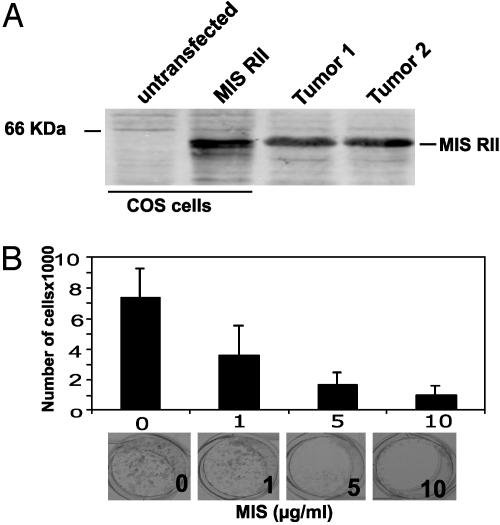
MIS Inhibits the in Vitro Growth of Cells Established from Mammary Tumors Arising in C3(1)Tag Mice. We had demonstrated that MIS inhibits the growth of breast cancer cells in vitro (10). To confirm these observations in vivo, the effect of MIS on the growth of mammary tumors in the C3(1)Tag mice was tested. Western blot analysis of proteins isolated from mammary tumors in the C3(1)Tag mice demonstrated the expression of MIS type II receptor. Proteins extracted from vector and MIS type II receptor-transfected COS cells were used as negative and positive controls, respectively (Fig. 1A).
Fig. 1.
MIS inhibits M6 cell growth in vitro. (A) MIS type II receptor expression in C3(1)Tag mouse mammary tumors. Total protein from tumors was analyzed by Western blot. Untransfected and MIS type II receptor-transfected COS cells were used as negative and positive controls, respectively. Position of the MIS type II receptor protein is shown. (B) Equal numbers of M6 cells were treated with increasing concentrations of MIS. The number of cells in each well after 4 days of MIS treatment and a representative view of the wells is shown.
Before testing the effect of MIS on mammary tumor growth in vivo, we first determined whether MIS could inhibit the in vitro growth of M6 cells established from C3(1)Tag mouse tumors. M6 cells were treated with 1, 5, and 10 μg/ml of MIS for 4 days, and cell numbers were quantified. As shown in Fig. 1B, MIS inhibited the growth of M6 cells by 51%, 74%, and 86%, respectively (P < 0.001 by two-sided Student's t test), suggesting that these mammary tumor cells are responsive to the growth inhibitory effects of MIS. Consistent with this observation, staining the cells for PCNA demonstrated that MIS suppressed the proliferation of M6 cells in culture (data not shown).
MIS Inhibits the Growth of Spontaneously Arising Mammary Tumors in C3(1)Tag Mice in Vivo. Two groups of 10-week-old mice were injected with either PBS or 20 μg of MIS daily for 5 days with 2 days of treatment-free interval for six cycles. The PBS- and the MIS-injected groups consisted of 13 and 12 mice, respectively. Externally palpable tumors were not observed in any of the animals at the commencement of treatment. One mouse in the MIS group was removed from the experiment within a week of treatment because of a large nonmammary tumor, which caused discomfort to the animal. Except for this animal, there was neither weight loss nor any other discernible adverse effects in the animals within the two groups.
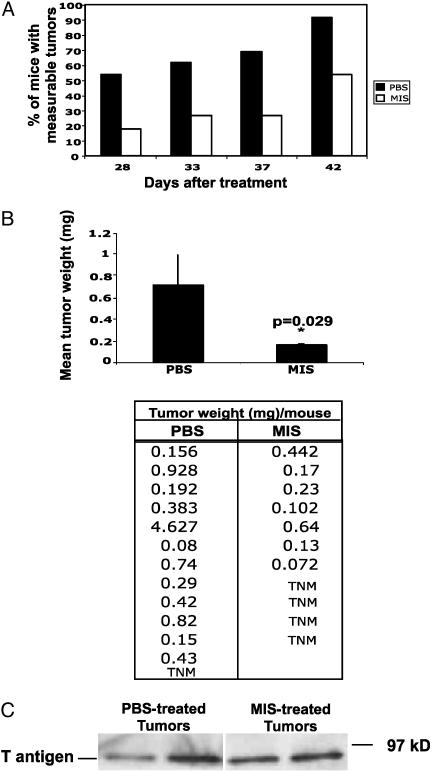
During the course of treatment, 10 animals in the PBS-treated group and 6 animals in the MIS-injected group developed externally palpable tumors. Of the 10 animals that developed palpable tumors in the PBS-injected group, 7 presented with tumors on day 28, 1 on day 32, 1 on day 37, and another on day 42 of treatment. At death, an additional two animals in this group were found to have measurable tumors in their mammary gland. In the MIS-treated group, two animals presented with externally palpable tumors on day 28 of treatment, one presented on day 32, and an additional three animals presented with tumors on day 42. Interestingly the animals in this group, which did not present with externally palpable tumors, did not have any large tumor masses at death. These results indicate that by 42 days of treatment MIS exposure is associated with animals having fewer palpable tumors (Fig. 2A; P < 0.048 by one-sided Fisher's Exact test).
Fig. 2.
MIS treatment is associated with a decrease in the number of palpable tumors in animals. (A) Ten-week-old C3(1)Tag mice were injected with either PBS (n = 13) or MIS (n = 11), and animals were monitored for palpable tumors. The graph shows the percentage of animals with measurable tumors in each group vs. the days of treatment. On the 42nd day after treatment, the total number of measurable tumors was lower in the MIS group compared with the PBS group. P < 0.05 by one-sided Fisher's Exact test. (B) At the end of the experiment, animals were killed, and tumors were excised and weighed. The tumor weight in each animal is given. TNM (tumor not measurable) represents animals in which tumors could not be detected by palpation. The graph shows the mean tumor weight ± standard error in the PBS- and MIS-injected groups. (C) Proteins extracted from tumors in PBS- and MIS-treated animals were immunoblotted with an anti-SV40Tag antibody. The position of the SV40 large T antigen is shown.
At the end of the experiment, animals were killed and tumors were excised and weighed. The tumor weights in the PBS-treated control animals ranged from 0.08 to 4.63 mg with a mean tumor weight of 0.71 mg and a median of 0.38 mg. The tumor weights in the MIS-treated animals ranged from 0.07 to 0.64 mg with a mean weight of 0.16 mg and a median of 0.10 mg. The lowest tumor (0.07 mg) in this group represents the total weight of micronodules of tumor that could not be excised free of normal tissue. The mean tumor weight in animals was significantly lower in the MIS-treated group compared with controls (Fig. 2B; P = 0.029 by Kruskal–Wallis test). The statistical analysis was repeated excluding the largest tumor (4.627 mg) present in a mouse in the PBS-treated group and indicated that the difference in tumor weights between the two groups was still significant (P = 0.048). To ensure that the decrease in tumor growth in the MIS-treated animals was not caused by suppression of SV40 T antigen in tumors, tumor samples from PBS- and MIS-treated mice were analyzed by Western blot. As demonstrated in Fig. 2C, MIS treatment did not alter the expression of SV40 T antigen in tumors.
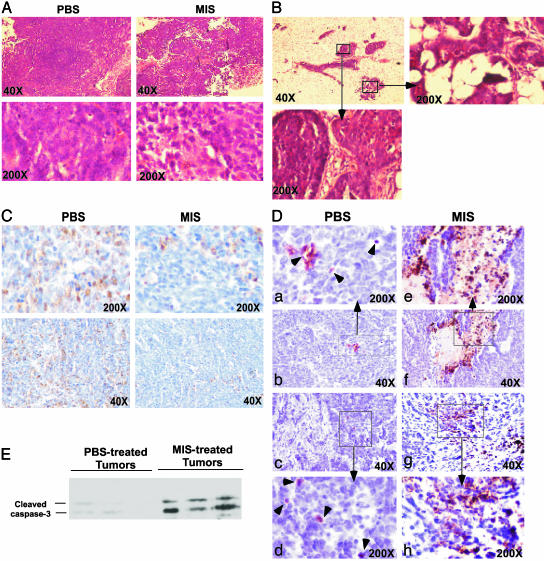
MIS Suppresses Proliferation and Induces Apoptosis in Mammary Tumors in Vivo. Both PBS- and MIS-treated animals with grossly palpable tumors had well developed invasive adenocarcinomas (Fig. 3A). Histological evaluation of tissues demonstrated that the mammary glands of MIS-treated animals that did not have externally palpable tumors had nodular atypical hyperplasia and mammary intraepithelial neoplasia, in which neoplastic cells filled the lumens of the duct, but did not present with invasive carcinomas (Fig. 3B). To determine whether suppression/delay in mammary tumors observed in MIS-treated mice was caused by decreased proliferation and/or increased apoptosis compared with that observed in PBS-treated controls, tumors were stained with antibodies against PCNA, a marker of proliferation, and cleaved caspase-3, a marker of early-stage apoptosis. The extent of PCNA staining in the mammary adenocarcinomas resected from PBS-treated animals was uniform throughout the tumors, whereas the MIS-treated adenocarcinomas demonstrated PCNA-positive regions interspersed with PCNA-negative patches (Fig. 3C). The nodular atypical hyperplasia and mammary intraepithelial neoplasia in the mammary glands of MIS-treated mice also demonstrated patchy PCNA staining. These results indicate that mammary tumors exposed to MIS undergo less proliferation compared with those in PBS-treated controls.
Fig. 3.
MIS decreases proliferation and increases apoptosis in mammary tumors. (A) Hematoxylin and eosin staining of palpable mammary tumors excised from the MIS- and PBS-treated animals. Histology of a representative tumor is shown. (B) Histological analysis of the mammary glands of MIS-treated mice, which did not present with palpable tumors. The higher magnifications (Insets) demonstrate regions of atypical hyperplasia (Right) and mammary intraepithelial neoplasia (Lower Left). (C) PCNA expression in tumors resected from PBS- and MIS-treated mice. A representative tumor from each group is shown. (D) Caspase-3 cleavage in PBS- and MIS-treated tumors. (b and c) Tumors from two PBS-treated animals. (a and d) Higher magnifications of b and c Insets are shown in a and d, respectively. Arrowheads show cells positive for caspase-3 cleavage. (f and g) Tumors from two MIS-treated animals. (e and h) Higher magnifications of f and g Insets are shown in e and h, respectively. (E) Proteins extracted from PBS- and MIS-treated tumors were analyzed by Western blot using an antibody against cleaved caspase-3.
Staining the tumors for activated caspase-3 revealed a marked increase in the number of apoptotic cells in the MIS-treated tumors compared with PBS-injected controls (Fig. 3D), suggesting that exposure to MIS induces apoptosis in the mammary tumors in vivo. This idea was also confirmed by immunoblotting tumor proteins for cleaved caspase-3 (Fig. 3E).
MIS Suppresses MDA-MB-468 Tumor Growth in SCID Mice. The human breast cancer cell line MDA-MB-468 is estrogen receptor-negative and Rb-negative and harbors mutant p53. MIS inhibits the growth of MDA-MB-468 cells in vitro. To validate and confirm the relevance of the results obtained in transgenic mice, we tested the growth inhibitory effects of MIS on MDA-MB-468 xenografts established in SCID mice. This experimental system closely complements the C3(1)Tag mouse mammary tumor model in which the tumors are estrogen-independent (13) and arise because of functional inactivation of Rb and p53 by the oncogenic T antigen (15, 16).
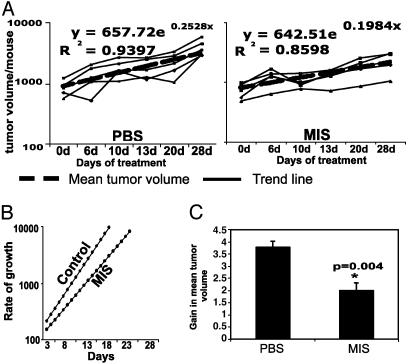
MDA-MB-468 xenografts were grown s.c. and bilaterally in the dorsal flanks of 6-week-old female SCID mice. After ≈4 weeks, the eight animals with palpable tumors were randomized into two groups with four animals in the PBS control group and four mice in the MIS treatment group. Both groups were treated at the same time with either PBS or 20 μg MIS per animal for 5 days a week with a treatment-free interval of 2 days for 4 weeks. Volume was calculated as L × W2 (L, length; W, width) at regular intervals (Fig. 4A). Analysis of the rate of mean growth during the treatment demonstrated that tumors in the PBS group were growing more rapidly compared with tumors in the MIS-treated group (Fig. 4B). The gain in tumor volume over the course of treatment was calculated as volume at the end of treatment - volume at the beginning of treatment/volume at the beginning of treatment. The PBS group had a higher gain in tumor volume than the MIS group (Fig. 4C; P = 0.004 by two-tailed Student's t test).
Fig. 4.
MIS decreases the growth of MDA-MB-468 tumor xenografts established in SCID mice. (A) MDA-MB-468 tumor xenografts were established in mice. Animals with palpable tumors were treated with PBS (n = 4) or MIS (n = 4), and tumor volumes were measured. The graphs demonstrate changes in total tumor volume in each animal during the course of treatment in the two groups. The thick line represents the mean gain in tumor volume and the hatched line represents the trend line derived from the means, assuming that tumor growth is exponential. (B) The rates of tumor growth within the two groups were calculated based on the equations derived from trend lines. (C) The mean of the gain in tumor volume in each group ± standard error is shown; the gain in the MIS-treated group was significantly different from the PBS-treated group (P < 0.005 by two-sided Student's t test).
Discussion
The presence of MIS in the serum well after regression and differentiation of the Mullerian duct in males and females, respectively, (24, 26) suggests that MIS may have a postnatal role in adults. Moreover, the expression of MIS receptors in nongonadal tissues such as the mammary and prostate glands (10–12, 27) suggests additional functions for this hormone besides the induction of apoptotic regression of the Mullerian duct. We had demonstrated that MIS inhibits breast cancer cell growth in vitro by preventing cell cycle progression and inducing apoptosis (10). In this article, using two in vivo model systems, we demonstrate that administration of MIS suppresses mammary tumor growth in mice. We had previously injected a single dose of 100 μg of MIS into female mice and tested the induction of IEX-1, a MIS-inducible gene, in the mammary glands of mice. These results demonstrated that MIS at this high dose could induce the expression of IEX-1 (11). Subsequently, Stephen et al. (28), tested the efficacy of MIS against ovarian cancer cell lines in vivo and reported that daily injections of 10 μg of purified exogenous recombinant human MIS suppressed tumor growth in immune-suppressed mice. Based on these results a comparable dose of MIS (20 μg per animal per day) was used in these experiments.
In the C3(1)Tag model, fewer animals in the MIS-treated group developed palpable tumors compared with PBS-injected controls. Although the measurable tumors in both groups progressed to adenocarcinomas, histological analyses of tumors indicated that tumors in the MIS-treated group were less dense compared with those in the PBS-treated group. This observation is consistent with the remarkable increase in apoptosis and curtailed proliferation in MIS-treated tumors compared with the PBS-injected controls. The presence of nodular atypical hyperplasia and mammary intraepithelial neoplasia in the MIS-treated mice that did not present with palpable tumors suggests that MIS may not block neoplastic transformation by the SV40 large tumor antigen but suppresses or delays tumor progression, resulting in the overall delay in the appearance of palpable tumors in the MIS-treated group.
This concept is further supported by the results observed in the MD-MB-468 xenograft model, in which administering MIS to animals with established tumors decreased the rate of tumor growth compared with vehicle-treated controls. Although the mean tumor weight at the end of the experiment was higher in the PBS group than in the MIS group (0.51 vs. 0.33 mg), this difference was not statistically significant (P = 0.13 by two-sided Student's t test). This finding was surprising given that the gain in mean tumor volume during the course of the experiment was significantly higher in the PBS-treated animals (P < 0.004) than in the MIS-treated mice. However, this result could reflect variations in initial tumor weights, which could not be measured.
The ability of MIS to inhibit MDA-MB-468 tumor growth in SCID mice is likely to occur directly at the cellular level because SCIDs harbor a mutation that severely impairs the development of T and B lymphocytes and MIS can inhibit MDA-MB-468 cell growth in vitro. Although MIS has no known immune modulatory effects, whether its inhibitory effect on mammary tumors arising in the immune-competent transgenic mouse model involves enhancement of host immune function remains to be determined. We recently demonstrated that MIS signaling intersects with the IFN-γ pathway and enhances IFN-γ induced expression of downstream target genes such as IRF-1 and CEACAM1. Furthermore, a combination of MIS and IFN-γ led to a greater degree of growth inhibition of breast cancer cells compared with either agent alone because of enhanced apoptosis rather than a combinatorial effect on cell cycle progression (20). The C3(1)Tag mice would provide an excellent experimental system to confirm these observations in vivo because regression of mammary tumors in response to cytokine treatment in this model has been reported to correlate with an increase in serum concentration of IFN-γ (14). Our preliminary results demonstrate that IFN-γ administration to mice can suppress mammary tumor growth in both experimental systems (data not shown). Although the antitumor effect of IFN-γ in vivo has been well documented, toxicity associated with exposure to IFN-γ has diminished its utility in treatment (29). Whether MIS may prove to be beneficial in harnessing the antitumor effects of this cytokine remains to be determined.
Our results demonstrate that MIS can suppress the growth of spontaneously arising mouse mammary tumors and established human breast cancer xenografts in immune-competent and immune-compromised mice, respectively. These tumors are estrogen-independent and lack functional p53 and Rb mutations, alterations of which have been detected in human breast cancers (17, 18). The growth inhibitory effects of MIS could be beneficial in the treatment/prevention of these hormone refractory mammary tumors, especially because high levels of MIS have not shown any harmful effects in humans (30), and the serum levels used here are well below those sustained in normal healthy postnatal to prepubertal boys (26). These data support expansion to larger studies to validate these findings before progressing to studies in humans.
Acknowledgments
We thank Drs. Jose Teixeira and Leif Ellisen for critically reading this manuscript and Rakesh Jain for access to the Edwin L. Steele Laboratory for Tumor Biology. This work was supported by Department of Defense Breast Cancer Research Grant DAMD17-03-1-0407 (to V.G.), National Institutes of Health/National Institute of Child Health and Human Development Grant HD32112 and National Institutes of Health/National Cancer Institute Grant CA17393 (to P.K.D.), and National Institutes of Health/National Cancer Institute Grant CA089138-04 (to S.M.).
Author contributions: V.G., P.K.D., D.T.M., and S.M. designed research; V.G., J.L.C., and H.K. performed research; J.E.G., P.K.D., and D.T.M. contributed new reagents/analytic tools; V.G., A.M., P.K.D., D.T.M., and S.M. analyzed data; V.G. and S.M. wrote the paper; and J.E.G. generated the mice and helped with experimental design.
Abbreviations: MIS, Mullerian inhibiting substance; PCNA, proliferating cell nuclear antigen; Rb, retinoblastoma; SCID, severe combined immunodeficient; SV40, simian virus 40.
References
- 1.Teixeira, J., Maheswaran, S. & Donahoe, P. K. (2001) Endocr. Rev. 22, 657-674. [DOI] [PubMed] [Google Scholar]
- 2.Baarends, W. M., van Helmond, M. J., Post, M., van der Schoot, P. J., Hoogerbrugge, J. W., de Winter, J. P., Uilenbroek, J. T., Karels, B., Wilming, L. G., Meijers, J. H., et al. (1994) Development (Cambridge, U.K.) 120, 189-197. [DOI] [PubMed] [Google Scholar]
- 3.di Clemente, N., Wilson, C., Faure, E., Boussin, L., Carmillo, P., Tizard, R., Picard, J. Y., Vigier, B., Josso, N. & Cate, R. (1994) Mol. Endocrinol. 8, 1006-1020. [DOI] [PubMed] [Google Scholar]
- 4.Teixeira, J., He, W. W., Shah, P. C., Morikawa, N., Lee, M. M., Catlin, E. A., Hudson, P. L., Wing, J., Maclaughlin, D. T. & Donahoe, P. K. (1996) Endocrinology 137, 160-165. [DOI] [PubMed] [Google Scholar]
- 5.Gouedard, L., Chen, Y. G., Thevenet, L., Racine, C., Borie, S., Lamarre, I., Josso, N., Massague, J. & di Clemente, N. (2000) J. Biol. Chem. 275, 27973-27978. [DOI] [PubMed] [Google Scholar]
- 6.Jamin, S. P., Arango, N. A., Mishina, Y. & Behringer, R. R. (2002) in The Genetics and Biology of Sex Determination, Novartis Foundation Symposium Series, eds. Chadwick, D. & Goode, J. (Wiley, New York), pp. 157-164.
- 7.Jamin, S. P., Arango, N. A., Mishina, Y., Hanks, M. C. & Behringer, R. R. (2002) Nat. Genet. 32, 408-410. [DOI] [PubMed] [Google Scholar]
- 8.Visser, J. A., Olaso, R., Verhoef-Post, M., Kramer, P., Themmen, A. P. & Ingraham, H. A. (2001) Mol. Endocrinol. 15, 936-945. [DOI] [PubMed] [Google Scholar]
- 9.Clarke, T. R., Hoshiya, Y., Yi, S. E., Liu, X., Lyons, K. M. & Donahoe, P. K. (2001) Mol. Endocrinol. 15, 946-959. [DOI] [PubMed] [Google Scholar]
- 10.Segev, D. L., Ha, T. U., Tran, T. T., Kenneally, M., Harkin, P., Jung, M., MacLaughlin, D. T., Donahoe, P. K. & Maheswaran, S. (2000) J. Biol. Chem. 275, 28371-28379. [DOI] [PubMed] [Google Scholar]
- 11.Segev, D. L., Hoshiya, Y., Stephen, A. E., Hoshiya, M., Tran, T. T., MacLaughlin, D. T., Donahoe, P. K. & Maheswaran, S. (2001) J. Biol. Chem. 276, 26799-26806. [DOI] [PubMed] [Google Scholar]
- 12.Hoshiya, Y., Gupta, V., Segev, D. L., Hoshiya, M., Carey, J. L., Sasur, L. M., Tran, T. T., Ha, T. U. & Maheswaran, S. (2003) Mol. Cell Endocrinol. 211, 43-49. [DOI] [PubMed] [Google Scholar]
- 13.Green, J. E., Shibata, M. A., Yoshidome, K., Liu, M. L., Jorcyk, C., Anver, M. R., Wigginton, J., Wiltrout, R., Shibata, E., Kaczmarczyk, S., et al. (2000) Oncogene 19, 1020-1027. [DOI] [PubMed] [Google Scholar]
- 14.Wigginton, J. M., Park, J. W., Gruys, M. E., Young, H. A., Jorcyk, C. L., Back, T. C., Brunda, M. J., Strieter, R. M., Ward, J., Green, J. E. & Wiltrout, R. H. (2001) J. Immunol. 166, 1156-1168. [DOI] [PubMed] [Google Scholar]
- 15.Mietz, J. A., Unger, T., Huibregtse, J. M. & Howley, P. M. (1992) EMBO J. 11, 5013-5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dyson, N., Buchkovich, K., Whyte, P. & Harlow, E. (1989) Cell 58, 249-255. [DOI] [PubMed] [Google Scholar]
- 17.Lemoine, N. R. (1994) Ann. Oncol. 5, Suppl. 4, 31-37. [DOI] [PubMed] [Google Scholar]
- 18.Ziyaie, D., Hupp, T. R. & Thompson, A. M. (2000) Breast 9, 239-246. [DOI] [PubMed] [Google Scholar]
- 19.Yin, F., Giuliano, A. E., Law, R. E. & Van Herle, A. J. (2001) Anticancer Res. 21, 413-420. [PubMed] [Google Scholar]
- 20.Hoshiya, Y., Gupta, V., Kawakubo, H., Brachtel, E., Carey, J. L., Sasur, L., Scott, A., Donahoe, P. K. & Maheswaran, S. (2003) J. Biol. Chem. 278, 51703-51712. [DOI] [PubMed] [Google Scholar]
- 21.Ragin, R. C., Donahoe, P. K., Kenneally, M. K., Ahmad, M. F. & MacLaughlin, D. T. (1992) Protein Expression Purif. 3, 236-245. [DOI] [PubMed] [Google Scholar]
- 22.Barbie, T. U., Barbie, D. A., MacLaughlin, D. T., Maheswaran, S. & Donahoe, P. K. (2003) Proc. Natl. Acad. Sci. USA 100, 15601-15606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ha, T. U., Segev, D. L., Barbie, D., Masiakos, P. T., Tran, T. T., Dombkowski, D., Glander, M., Clarke, T. R., Lorenzo, H. K., Donahoe, P. K. & Maheswaran, S. (2000) J. Biol. Chem. 275, 37101-37109. [DOI] [PubMed] [Google Scholar]
- 24.Hudson, P. L., Dougas, I., Donahoe, P. K., Cate, R. L., Epstein, J., Pepinsky, R. B. & MacLaughlin, D. T. (1990) J. Clin. Endocrinol. Metab. 70, 16-22. [DOI] [PubMed] [Google Scholar]
- 25.Stephen, A. E., Masiakos, P. T., Segev, D. L., Vacanti, J. P., Donahoe, P. K. & MacLaughlin, D. T. (2001) Proc. Natl. Acad. Sci. USA 98, 3214-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee, M. M., Donahoe, P. K., Hasegawa, T., Silverman, B., Crist, G. B., Best, S., Hasegawa, Y., Noto, R. A., Schoenfeld, D. & MacLaughlin, D. T. (1996) J. Clin. Endocrinol. Metab. 81, 571-576. [DOI] [PubMed] [Google Scholar]
- 27.Segev, D. L., Hoshiya, Y., Hoshiya, M., Tran, T. T., Carey, J. L., Stephen, A. E., MacLaughlin, D. T., Donahoe, P. K. & Maheswaran, S. (2002) Proc. Natl. Acad. Sci. USA 99, 239-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stephen, A. E., Pearsall, L. A., Christian, B. P., Donahoe, P. K., Vacanti, J. P. & MacLaughlin, D. T. (2002) Clin. Cancer Res. 8, 2640-2646. [PubMed] [Google Scholar]
- 29.Borden, E. C., Lindner, D., Dreicer, R., Hussein, M. & Peereboom, D. (2000) Semin. Cancer Biol. 10, 125-144. [DOI] [PubMed] [Google Scholar]
- 30.Gustafson, M. L., Lee, M. M., Scully, R. E., Moncure, A. C., Hirakawa, T., Goodman, A., Muntz, H. G., Donahoe, P. K., MacLaughlin, D. T. & Fuller, A. F., Jr. (1992) N. Engl. J. Med. 326, 466-471. [DOI] [PubMed] [Google Scholar]