Fossil records suggest that bacteria developed the ability to photosynthesize ≈3,500 million years ago (mya), initiating a very slow accumulation of atmospheric oxygen (1). Recent geochemical models suggest that atmospheric oxygen did not accumulate to levels conducive for aerobic life until 500–1,000 mya (2, 3). The oxygenation of Earth's atmosphere resulted in the emergence of aerobic organisms followed by a great diversification of biological species and the eventual evolution of humans.
Oxygen: A Gas to Love and Fear
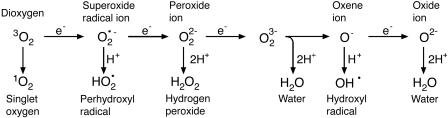
Although oxygen is thought to have been responsible for the expansion of life on Earth, there are two sides to this molecule: life giving and life taking. Oxygen in the air we breathe is a relatively nonreactive chemical (4). However, when oxygen is exposed to high-energy or electron-transferring chemical reactions, it can be converted to various highly reactive chemical forms (Fig. 1) collectively designated “reactive oxygen species” (ROS; ref. 5). ROS are toxic to biological organisms because they oxidize lipids, proteins, DNA, and carbohydrates, resulting in the breakdown of normal cellular, membrane, and reproductive functions. Ultimately, toxic levels of ROS cause a chain reaction of cellular oxidation, resulting in disease and lethality (5–7). ROS are unavoidable byproducts of biochemical pathways, such as glycolysis and photosynthesis, that are central to energy production and storage strategies of aerobic microbes, animals, and plants (4, 5). As a result, aerobic organisms have evolved enzymatic and nonenzymatic antioxidation mechanisms to eliminate ROS and avoid oxidative destruction. The growth and reproduction of all aerobic prokaryotes and eukaryotes require a balance between the generation of ROS and the capacity of antioxidation systems to eliminate them. It is noteworthy when novel antioxidation systems are identified. In this issue of PNAS, Chen and Dickman (8) demonstrate that the amino acid proline is a potent scavenger of ROS. Unlike other amino acids, proline has a cyclized amino nitrogen that has significant influence on the conformation of polypeptides (9). Proline is also a major component of structural proteins in animals and plants and a known osmoprotectant capable of mitigating the impacts of drought, salt, and temperature stress in plants. Based on the work of Chen and Dickman, proline can now be added to an elite list of nonenzymatic antioxidants that microbes, animals, and plants require to mitigate the impacts of ROS.
Fig. 1.
Generation of different ROS by energy transfer or sequential univalent reduction of ground-state triplet oxygen (from ref. 5).
The amino acid proline is a potent scavenger of reactive oxygen species.
The ROS story is complicated by the fact that plants and animals also have evolved mechanisms that capitalize on the toxic property of ROS to combat pathogens. For example, when plants are exposed to microbial pathogens, they produce ROS that induce programmed cell death in the plant cells surrounding the infection site to effectively “wall off” the pathogen and terminate the disease process (5). ROS may also be transmitted through the phloem to distant plant tissues signaling a pathogen attack (10). In these examples, ROS act locally as toxin and distantly as signaling molecules. However, it appears that ROS have a number of other potential biochemical functions such as biochemical signaling, gene expression, protein inhibition, environmental sensing, and activation of transcription factors (4–7, 11).
When organisms are exposed to abiotic stresses such as temperature extremes, dehydration, salt, UV light, ozone, and heavy metals, ROS are produced (11). In fact, the generation of ROS is the only event known to be common among such divergent stresses. When an abiotic stress induces an oxidative environment, organisms produce antioxidation systems to decrease the concentration of toxic intracellular ROS. Chen and Dickman (8) demonstrate that the ROS scavenging property of proline prevents the induction of programmed cell death by ROS generated during nutritional stress. In addition, proline protects fungal cells against other abiotic stresses such as UV light, heat, salt, and hydrogen peroxide. There may be functional roles such as signaling or sensing for ROS during exposure to abiotic stress, but none have been confirmed.
Insight into the Evolution of Symbiosis
One of the more interesting aspects of the work by Chen and Dickman (8) is the possibility that it may reveal an elusive mechanism responsible for the ability of symbiotic fungi to protect plants against abiotic and biotic stress. Fossil records indicate that fungi have been associated with plants for >400 mya (12–14), and it is theorized that these recordings represent early symbiotic interactions that were responsible for the establishment of land plants (15). Since the first description of plant/fungal symbiosis (16, 17), all plants studied in natural ecosystems have been found to be symbiotic with fungi (18). Fungal symbionts may express a variety of lifestyles, including parasitism, mutualism, or commensalism that decrease, increase, or have no effect on host fitness, respectively (19). However, in natural ecosystems, pathogenic symbioses are the exception, and nonpathogenic symbioses are the rule. Fitness benefits conferred to host plants by fungal mutualists include tolerance to abiotic and biotic stresses such as temperature extremes, dehydration, salt, UV light, heavy metals, and pathogen attack (20–24). However, the mechanism(s) responsible for symbiotically conferred stress tolerance are poorly defined, and none focus on the antioxidation property of proline. All of the abiotic stresses listed above result in the production of ROS, and Chen and Dickman demonstrate that proline protects fungi against these stresses (dehydration and pathogen attack were not tested). Therefore, symbiotically conferred stress tolerance may be based on ROS generation, the one common aspect of stress. Mutualistic fungi allow symbiotic plants to perceive stress more quickly than nonsymbiotic plants, resulting in the rapid and strong activation of plant biochemical reactions that mitigate the impacts of stress (23, 25). It is possible that symbiotic fungi prompt plants to activate the biosynthesis of proline to scavenge ROS generated by stress.
The ramifications of the work by Chen and Dickman (8) go beyond the realm of fungi, because it addresses a fundamental aspect of evolution, how cells balance the generation, and elimination of ROS. Based on this work, the role of proline as a ROS scavenger, and its ability to mitigate the impacts of abiotic stress should be evaluated across all evolutionary lineages.
See companion article on page 3459.
References
- 1.Schopf, J. W. (1993) Science 260, 640-646. [DOI] [PubMed] [Google Scholar]
- 2.Kah, L. C., Lyons, T. W. & Frank, T. D. (2004) Nature 431, 834-838. [DOI] [PubMed] [Google Scholar]
- 3.Canfield, D.E. & Teske, A. (1996) Nature 382, 127-132. [DOI] [PubMed] [Google Scholar]
- 4.Cadens, E. (1989) Annu. Rev. Biochem. 58, 79-110. [DOI] [PubMed] [Google Scholar]
- 5.Apel, K. & Hirt, H. (2004) Annu. Rev. Plant Biol. 55, 373-399. [DOI] [PubMed] [Google Scholar]
- 6.Valko, M., Izakovic, M., Mazur, M., Rhodes, C. J. & Telser, J. (2004) Mol. Cell. Biochem. 266, 37-56. [DOI] [PubMed] [Google Scholar]
- 7.Harper, M. E., Bevilacqua, L., Hagopian, K., Weindruch, R. & Ramsey, J. J. (2004) Acta Physiol. Scand. 182, 321-331. [DOI] [PubMed] [Google Scholar]
- 8.Chen, C. & Dickman, M. B. (2005) Proc. Natl. Acad. Sci. USA 102, 3459-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bright, J. N. & Sansom, M. S. P. (2002) J. Phys. Chem. B. 107, 627-636. [Google Scholar]
- 10.Van Bel, A. J. E. & Gaupels, F. (2004) Mol. Plant Pathol. 5, 495-504. [DOI] [PubMed] [Google Scholar]
- 11.Mittler, R & Zilinskas, B. A. (2004) in Molecular Ecotoxicology of Plants, ed. Sandermann, H. (Springer, Berlin), pp. 51-73.
- 12.Simon, L., Bousquet, J., Levesque, R. C. & Lalonde, M. (1993) Nature 363, 67-69. [Google Scholar]
- 13.Remy, W., Taylor, T. N., Hass, H. & Kerp, H. (1994) Proc. Nat. Acad. Sci. 91, 11841-11843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redecker, D., Kodner, R. & Graham, L. E. (2000) Science 289, 1920-1921. [DOI] [PubMed] [Google Scholar]
- 15.Pirozynski, K. A. & Malloch, D. W. (1975) BioSystems 6, 153-164. [DOI] [PubMed] [Google Scholar]
- 16.De Bary, A. (1879) in Vortrag auf der Versammlung der Naturforscher und Artze zu Cassel, ed. Trubner, K. J. (Strassburg, Germany), pp. 1-30.
- 17.Hertig, M., Taliaferro, W. H. & Schwartz, B. (1937) J. Parasitol. 23, 326-329. [Google Scholar]
- 18.Petrini, O. (1986) in Microbiology of the Phyllosphere, eds. Fokkema, N. J. & van den Heuvel, J. (Cambridge Univ. Press, Cambridge, U.K.), pp. 175-187.
- 19.Lewis, D. H. (1985) in The Biology of Mutualism, ed. Boucher, D. H. (Croom Helm, London), pp. 29-39.
- 20.Stone, J. K., Bacon, C. W. & White, J. F. (2000) in Microbial Endophytes, eds. Bacon, C.W. & White, J. F. J. (Dekker, New York), pp. 3-30.
- 21.Read, D. J. (1999) in Mycorrhiza, eds. Varma, A. & Hock, B. (Springer, Berlin), pp. 3-34.
- 22.Schardl, C. L., Leuchtmann, A. & Spiering, M. J. (2004) Annu. Rev. Plant Biol. 55, 315-340. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez, R., Redman, R. & Henson, J. M. (2004) Mitigation Adapt. Strategies Global Change 9, 261-272. [Google Scholar]
- 24.Redman, R. S., Sheehan, K. B., Stout, R. G., Rodriguez, R. J. & Henson, J. M. (2002) Science 298, 1581. [DOI] [PubMed] [Google Scholar]
- 25.Redman, R. S., Freeman, S., Clifton, D. R., Morrel, J., Brown, G. & Rodriguez, R. J. (1999) Plant Physiol. 119, 795-804. [DOI] [PMC free article] [PubMed] [Google Scholar]