Abstract
The present explorative study was initiated to evaluate the clinical value of 18F-FES PET/CT in monitoring the change of estrogen receptor (ER) expression and potential predictive value in metastatic breast cancer patients. Twenty-two pathology-confirmed breast cancer patients were prospectively enrolled and randomly divided into two groups (T: docetaxel, n = 14 and TF: docetaxel + fulvestrant, n = 8). The percentage of patients without disease progression after 12 months (PFS > 12 months) was 62.5% in group TF compared with 21.4% in group T (P = 0.08). According to 18F-FES PET/CT scans, the SUVmax (maximum standard uptake value) of all the metastatic lesions decreased in group TF after 2 cycles of treatment (6 weeks ± 3 days). However, 6 of 9 patients in group T had at least one lesion with higher post-treatment SUVmax. There was a significant difference in the reduction of ER expression between these two groups (P = 0.028). In group TF, the patients with PFS > 12 months had significantly greater SUVmax changes of 18F-FES than those with PFS < 12 months (PFS > 12 months: 91.0 ± 12.0% versus PFS < 12 months: 20.7 ± 16.2%; t = −4.64, P = 0.01). Our preliminary study showed that 18F-FES PET/CT, as a noninvasive method to monitor ER expression, could be utilized to predict prognosis based on changes in SUVmax.
Introduction
Breast cancer, as one of the most common cancers in women, was estimated to account for 15% of newly diagnosed cancers in China in the year of 20151. Estrogen receptor (ER) plays a key role in the development and progression of breast cancers. Approximately 65–70% of women with breast cancer are ER positive (ER+)2, 3. Preclinical evidence and clinical evidence have both suggested that ER + breast cancers are less responsive to chemotherapy than ER-negative (ER−) tumors, indicating that ER might interfere with factors determining the sensitivity to chemotherapy4–7.
Massive studies have been undertaken to explain the mechanism of ER-mediated drug resistance to find new strategies to reverse resistance. Chemoresistance might be caused by ER itself or by ER modulation of the levels of factors8–17. Since the expression of ERα is associated with decreased sensitivity to chemotherapy, inhibition of the ER pathway should naturally reverse ER-mediated chemoresistance. However, previous in vitro and clinical data have demonstrated an antagonistic effect between tamoxifen and chemotherapy12–14. A possible explanation is that tamoxifen also has estrogen-like agonist activity.
Fulvestrant, which is a new type of selective ER down-regulator, can block ER-mediated transcriptional activity through binding ER and inducing ER degradation18. Preclinical evidence has proved that fulvestrant can dramatically reverse resistance to various cytotoxic agents (doxorubicin, paclitaxel, docetaxel, vinorelbine, and 5-fluorouracil), especially with docetaxel, suggesting a novel strategy for reversing ER-mediated chemoresistance12, 19–22.
Docetaxel, with a response rate of 30–40%, is considered one of the most effective single agent chemotherapies for breast cancer and was shown to have synergistic effects on inhibiting tumor growth when combined with fulvestrant in vivo 22. Given the promising preclinical evidence, combination treatment of fulvestrant and chemotherapeutic agents might be beneficial.
With the advent of molecular imaging, positron emission tomography (PET) with ER-targeting radiopharmaceuticals has emerged as a noninvasive method for simultaneously measuring the in vivo delivery and binding of estrogen, and thus of ER expression, at multiple sites. Previous studies have successfully validated that 18F-FES PET uptake correlates well with immunohistochemical (IHC) scoring for ER23–28. Thus, we hypothesized that we could use 18F-FES PET to monitor the change in ER during combination treatment, with the potential to predict prognosis.
Materials and Methods
Patients
The inclusion criteria were: women between 18 and 70 years old with histologically confirmed hormone receptor (HR)-positive, HER2-negative metastatic breast cancer; an Eastern Cooperative Oncology Group performance status <2; life expectancy ≥3 months; adequate hematologic, hepatic, renal and cardiac function; and at least one measurable site according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria, version 1.1. Patients included in this study had to have failed previous endocrine therapy (adjuvant therapy or first line therapy for advanced disease) or have rapidly progressive disease needing disease control. Premenopausal women were required to receive ovarian suppression. The enrollment had to occur at least 4 weeks after any previous treatment.
Exclusion criteria were: had previously been treated with fulvestrant, uncontrolled infection or diabetes mellitus, central nervous system metastases, pre-existing ≥ grade 2 peripheral neuropathy, pregnancy or lactation, and any chemotherapy in metastatic settings. Additionally, to avoid pretreatment 18F-FES false-negative results, ER antagonists were discontinued for a minimum of 5 weeks before the study.
This study was approved by the Fudan University Shanghai Cancer Center Ethic Committee for Clinical Investigation and all of the methods were performed in accordance with the relevant guidelines and regulations. All of the patients signed written informed consent forms before randomization.
Treatment and study design
In this single center, open-label, phase II clinical trial (NCT02137083, registration date: 6 May, 2014; details at https://clinicaltrials.gov), patients were randomly assigned to receive docetaxel 75 mg/m2 D1 every 21 days (group T) or docetaxel 75 mg/m2 D2 every 21 days plus fulvestrant 500 mg D1, 15 and 29 and every 28 days thereafter (group TF). Treatment continued until disease progression, intolerable toxicity, or consent withdrawal.
The primary endpoint of this trial was progression free survival (PFS); secondary endpoints included overall response rate, overall survival and the value of 18F-FES PET in monitoring the expression changes of ER. This analysis mainly focused on the clinical value of 18F-FES PET; results of other end points were not discussed in this article.
Synthesis of 18F-FES, 18F-FDG and quality control
18F-FES was synthesized as described by Mori et al.29 and modified by us, as reported in our previous study30, 31. The total preparation time was approximately 100 min, and the corrected radiochemical yield was approximately 40% (at the end of synthesis). After final purification, the radiochemical purity was >99%, and the specific activity was 1–10 Ci/μmol at the time of injection.
18F-FDG was produced routinely and automatically by cyclotron (Siemens CTI RDS Eclips ST, Knoxville, Tennessee, USA) using an Explora FDG4 module in our center. The radiochemical purity was greater than 95%.
PET/CT procedure
The patients underwent both 18F-FES and 18F-FDG PET/CT before and after two cycles of treatment (6 weeks ± 3 days) in our center. The interval between 18F-FES and 18F-FDG PET/CT was within 7 days.
All of the patients were requested to fast for more than 4 h prior to 18F-FES PET/CT scans to eliminate the excretion of 18F-FES from the hepatobiliary system and the gastrointestinal tract, which might interfere with image interpretation in the pelvic cavity. An average dose of 222 MBq (6 mCi) of 18F-FES was injected over 1–2 minutes. Scanning consisted of a whole-body PET/CT examination (2–3 min per table position) from the proximal thighs to the head and was initiated 1 h after administration of the tracer on a Siemens biograph 16HR PET/CT scanner (Knoxville, Tennessee, USA). The transaxial intrinsic spatial resolution was 4.1 mm (full width at half maximum) in the center of the field of view. PET image data sets were reconstructed iteratively by applying the CT data for attenuation correction, and co-registered images were displayed on a workstation.
Regarding 18F-FDG PET/CT scans, all of the subjects fasted at least 6 h, and they had to present blood glucose level less than 10 mmol/L at the time of tracer injection (dosage: 7.4 MBq/kg). Before and after injection, they were kept lying comfortably in a quiet, dimly lit room. The parameters for PET/CT were the same as for 18F-FES PET/CT scans.
Image interpretation
A multimodality computer platform (Syngo, Siemens, Knoxville, Tennessee, USA) was utilized for image review and manipulation. Two experienced board-certified nuclear medicine physicians evaluated the images independently and reached a consensus in cases of discrepancy.
Semi-quantitative analysis of tumor metabolic activity was obtained using the standardized uptake value (SUV) normalized to body weight. Lesions on 18F-FES PET/CT scans were identified using paired 18F-FDG PET/CT images. When there was no 18F-FES uptake was detected in suspicious metastatic lesions, we used other conventional methods (bone scan, ultrasound, CT and MRI) for reference. The maximum SUV (SUVmax) for each metastatic lesion was recorded for further analysis by manually placing an individual region of interest (ROI) on co-registered and fused transaxial PET/CT images. In reference to other 18F-FES PET studies and our previous experiences, we used a cut-off value of 1.5 to dichotomize the results into ER positive and negative32–35.
The change in SUVmax was defined as the lesion with the largest difference before and after treatment in a patient-based analysis. However, if a patient had higher SUVmax of either 18F-FDG or 18F-FES after treatment, we used this value subtracted from the pretreatment SUVmax as the change.
Lesions smaller than 1.5 cm were excluded because of partial-volume limitation and resolution restriction. In addition, liver lesions were not included in the 18F-FES PET/CT analysis due to their high physiological uptake. In patients with widespread bone metastasis, up to 5 of the largest 18F-FES PET lesions corresponding to the most 18F-FDG avid lesions, were tabulated for each of 5 areas: skull, thorax (including sternum, scapula, clavicle and ribs), long bones, spine and pelvis.
Assessments
Radiologic evaluation, including spiral CT or MRI scans, was performed at baseline, every 2 cycles (6 weeks ± 3 days) to confirm treatment efficacy and every 3 months during follow-up until disease progression or death. Tumor responses were confirmed by the investigators according to the RECIST 1.1 criteria. Adverse events (AEs) were monitored throughout the study and were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Statistical analysis
Data are expressed as the mean ± SD. Normality tests of quantitative data were performed with the Kolmogorov Smirnov two-tailed one sample test.
PFS was defined as the time from random assignment to disease progression or death. Statistical analyses for PFS were performed using the Kaplan-Meier method and were compared between treatment groups using log-rank test.
The change in 18F-FES uptake before and after treatment in groups T and TF were compared by Fisher’s exact test. The differences in SUVmax changes between PFS > 12 months and PFS < 12 months in the patients in each group were tested by independent t tests. In group TF, for the comparison of pretreatment SUVmax between PFS > 12 months and PFS < 12 months in patients in the lesion-based analysis, we also utilized independent t tests. The data were analyzed by the SPSS software packages, version 20.0 (IBM Corporation, Armonk, New York, USA). All of the analyses were two sided. A P value less than 0.05 was taken to indicate a statistically difference.
Results
Patients and treatment outcomes
From May 2014 to April 2016, 22 women with HR + /HER2- metastatic breast cancer were enrolled, including 8 patients treated with docetaxel and fulvestrant and 14 patients treated with docetaxel monotherapy. The baseline characteristics were well balanced between the two treatment groups (Table 1).
Table 1.
Patients and tumor characteristics.
| Characteristic | TF (n = 8) | T (n = 14) | P-value | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, years | |||||
| Median | 46 | 55 | |||
| Range | 37–68 | 35–73 | |||
| Hormone receptor and Her2 status | |||||
| ER positive | 8 | 100 | 14 | 100 | |
| PR positive | 6 | 75 | 14 | 100 | |
| HER-2 negative | 8 | 100 | 14 | 100 | |
| Menopausal status | |||||
| Postmenopausal | 5 | 62.5 | 12 | 85.7 | 0.31 |
| Premenopausal | 3 | 37.5 | 2 | 14.3 | |
| LHRHa | 1 | 12.5 | 0 | 0 | |
| Oothecectomy | 2 | 25.0 | 1 | 7.1 | |
| Radical Surgery | |||||
| Yes | 7 | 87.5 | 11 | 78.6 | 1.00 |
| No | 1 | 12.5 | 3 | 21.4 | |
| Disease-free interval | |||||
| <24 m | 2 | 25.0 | 3 | 21.4 | 1.00 |
| >24 m | 5 | 62.5 | 8 | 57.1 | |
| No. of metastatic sites | |||||
| 1 | 1 | 12.5 | 1 | 7.1 | 0.91 |
| 2 | 2 | 25.0 | 4 | 28.6 | |
| ≥3 | 5 | 62.5 | 9 | 64.3 | |
| Metastatic sites | |||||
| Lung | 3 | 37.5 | 9 | 64.3 | 0.38 |
| Liver | 2 | 25.0 | 4 | 28.6 | 1.00 |
| Bone | 5 | 62.5 | 7 | 50 | 0.68 |
| Visceral disease | 5 | 62.5 | 12 | 85.7 | 0.31 |
This trial was terminated early due to slow enrollment, so that the sample size was not sufficiently powered to detect significant differences of PFS. The primary endpoint, was met by 5 patients (62.5%) in the TF group and 9 patients (64.3%) in the T group by the time of the analysis (Table 2), with a median PFS numerically longer in the TF group than that in the T group (12.3 vs. 9.9 months, Fig. 1). The percentage of patients without disease progression after 12 months (PFS > 12 months) was 62.5% in the combination arm compared with 21.4% in the single-agent docetaxel arm (P = 0.08).
Table 2.
Main clinical outcomes.
| Outcomes | TF group* | T group | P value | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Partial response | 5 | 71.4 | 11 | 78.6 | |
| Stable disease | 2 | 28.6 | 3 | 21.4 | |
| PFS, months | |||||
| Median | 12.3 | 9.9 | |||
| 95%CI | 5.0–20.0 | 5.8–14.1 | |||
| PFS > 12 months | 5 | 62.5 | 3 | 21.4 | 0.08 |
*Included 7 patients with evaluable responses.
Figure 1.
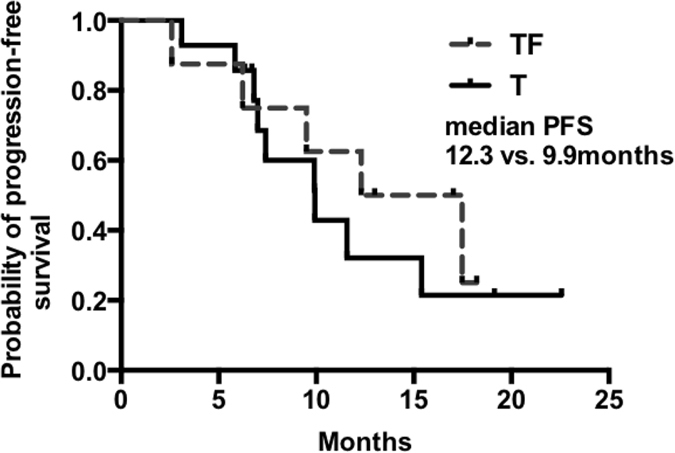
Kaplan-Meier estimates of the progression-free survival of patients treated with docetaxel plus fulvestrant (TF) and with docetaxel monotherapy (T).
18F-FES and 18F-FDG PET/CT Results
5 patients in group T and 2 patients in group TF did not undergo pre- or post-treatment 18F-FES PET/CT for various reasons. Therefore, 9 patients in group T and 6 patients in group TF were included for further PET/CT analysis (Table 3). At baseline, a total of 159 metastatic lesions were detected. Lesions were located in lymph nodes (n = 76), bones (n = 32), lungs (n = 22), soft tissue (n = 17), and the liver (n = 12).
Table 3.
Pre- and post- 18F-FES, FDG results and clinical outcomes of each patient. +ongoing.
| No. | Group | Tumor sites | lesions (n) | ER expression | Baseline FES SUV | Follow-up FES SUV | %Change FES SUV | Baseline FDG SUV | Follow-up FDG SUV | %Change FDG SUV | PFS (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | T | liver, lung, lymph nodes, chest wall | 21 | Increased | 1.81 | 2.98 | 65 | 2.88 | 3.07 | 7 | 3.1 |
| 2 | T | lung | 1 | Decreased | 6 | 0 | −100 | 2.84 | 0 | −100 | 9.9 |
| 3 | T | liver, lung, bone, lymph nodes | 17 | Decreased | 2.76 | 0 | −100 | 8.11 | 0 | −100 | 7 |
| 4 | T | lung, lymph nodes | 5 | Increased | 2.27 | 2.65 | 17 | 3.93 | 0 | −100 | 9.93 |
| 5 | T | lung, bone, lymph nodes | 17 | Decreased | 2.33 | 0 | −100 | 6.78 | 0 | −100 | 11.57 |
| 6 | T | lung, lymph nodes | 12 | Increased | 1.51 | 1.76 | 17 | 8.08 | 0 | −100 | 6.8 |
| 7 | T | bone, lymph nodes, contralateral breast | 13 | Increased | 2.05 | 2.2 | 7 | 11.14 | 0 | −100 | 19.13+ |
| 8 | T | liver, lung, bone, lymph nodes | 18 | Increased | 10.18 | 17.38 | 71 | 5.8 | 0 | −100 | 15.4 |
| 9 | T | lymph nodes, pleural membrane | 7 | Increased | 2.49 | 4.67 | 86 | 4.03 | 0 | −100 | 22.57+ |
| 10 | TF | lung, lymph nodes | 9 | Decreased | 1.78 | 1.65 | −7 | 5.16 | 1.21 | −77 | 2.6 |
| 11 | TF | liver, bone | 7 | Decreased | 2.37 | 2.12 | −10 | 2.49 | 3.65 | 47 | 6.23 |
| 12 | TF | lymph nodes | 2 | Decreased | 2.68 | 1.46 | −45 | 2.63 | 3.03 | 15 | 9.5 |
| 13 | TF | bone, lymph nodes, pleural membrane, breast | 7 | Decreased | 9.9 | 2.72 | −73 | 5.29 | 3.02 | −43 | 18.23+ |
| 14 | TF | lymph nodes, breast, chest wall | 13 | Decreased | 1.8 | 0 | −100 | 15.46 | 0 | −100 | 17.47 |
| 15 | TF | lung, bone, pleural membrane | 10 | Decreased | 1.98 | 0 | −100 | 4.69 | 0 | −100 | 17.03+ |
All of these metastatic lesions were FDG avid, with SUVmax values ranging from 1.3 to 15.46. In 18F-FES analysis, 145 lesions were included (12 liver lesions and 2 lung lesions adjacent to the liver were excluded; SUVmax = 0.73–20.15). Using a cut-off value of SUVmax = 1.5, 35 lesions were 18F-FES negative. Most of the patients (9/15) had both 18F-FES-positive and -negative lesions, showing conspicuous heterogeneity of ER expression in these recurrent breast cancer cases.
After 2 cycles of treatment (6 weeks ± 3 days), the 18F-FDG uptakes of the majority of lesions decreased (n = 89) or was absent (n = 63); only 7 lesions had higher SUVmax. On 18F-FES analysis, 60 lesions showed decreases in ER expression, 59 lesions were absent, and 26 lesions had a higher SUVmax values.
Fulvestrant reduced ER expression
According to the 18F-FES PET/CT scans, the SUVmax values of all of these lesions decreased in group TF after 2 cycle of treatment. However, 6 of 9 patients in group T had at least one lesion with a higher post-treatment SUVmax value (Fig. 2). There was a significant difference in the reduction of ER expression between two groups (P = 0.028). The data demonstrated that fulvestrant did reduce the ER expression in metastatic breast cancer patients.
Figure 2.
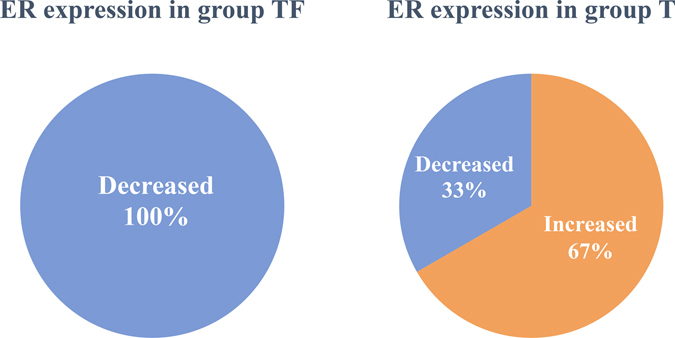
The ER expression changes in patients treated with docetaxel or docetaxel plus fulvestrant.
The change in ER expression showed potential to predict PFS: patient-based analysis
In group TF, the patients with PFS > 12 months had significantly greater SUVmax changes in 18F-FES than those with PFS < 12 months (PFS > 12 months: 91.0 ± 12.0% versus PFS < 12 months: 20.7 ± 16.2%; t = -4.64, P = 0.01; Figs 3 and 4). However, the change in 18F-FDG uptake could not differentiate the patients with better prognosis (PFS > 12 months: 81.0 ± 25.2% versus PFS < 12 months: 5.0 ± 48.0%; t = -1.821, P = 0.143; Figs 5 and 6).
Figure 3.
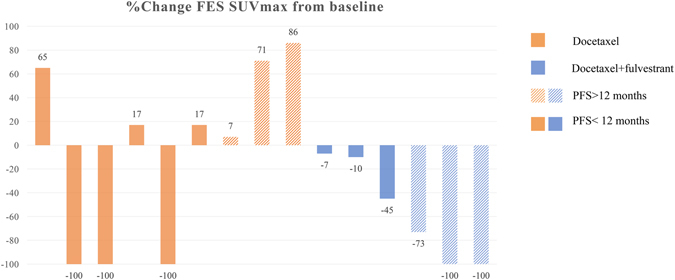
Waterfall plot showing the relative changes in tumor FES uptake in individual patients treated with docetaxel or docetaxel plus fulvestrant on follow-up scans, compared with baseline.
Figure 4.
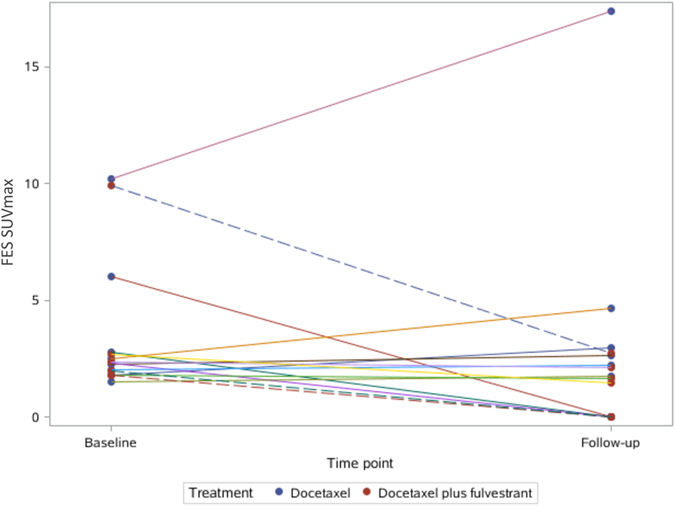
The spaghetti plot of 18F-FES changes.
Figure 5.
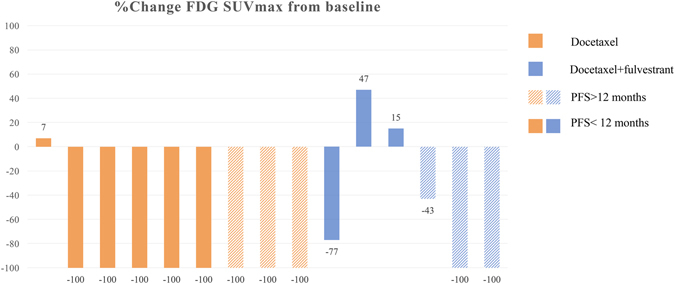
Waterfall plot showing the relative changes in tumor FDG uptake in individual patients treated with docetaxel or docetaxel plus fulvestrant on follow-up scans, compared with baseline.
Figure 6.
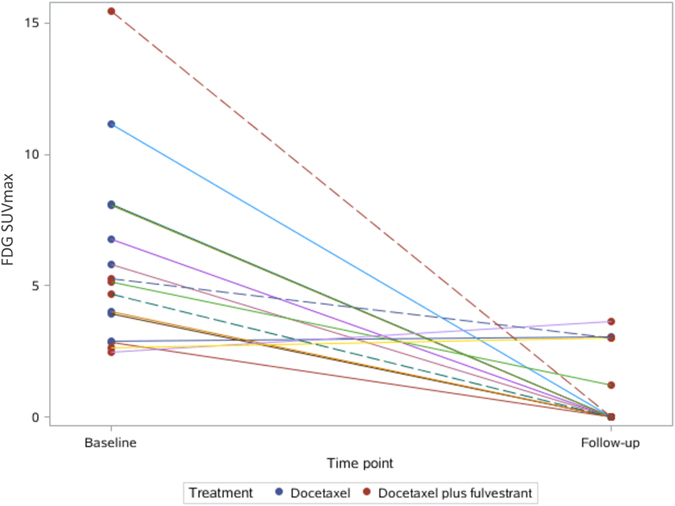
The spaghetti plot of 18F-FDG changes.
In group T, the SUVmax changes with neither 18F-FES nor 18F-FDG showed significant differences between the patients with PFS > 12 months and those with PFS < 12 months (P > 0.05).
Pretreatment 18F-FES SUVmax might predict PFS in group TF: lesion-based analysis
In group TF, there were a total of 48 metastatic lesions detected. Among them, 41 lesions were included for further 18F-FES analysis (PFS > 12 months: n = 28; PFS < 12 months: n = 13; 5 liver lesions and 2 lung lesions adjacent to the liver were excluded). The pretreatment 18F-FES SUVmax of the metastatic lesions in patients with PFS > 12 months was obviously greater than in patients with PFS < 12 months (PFS > 12 months: 4.1 ± 5.2 versus PFS < 12 months: 1.9 ± 0.5; t = 2.175, P = 0.038; Fig. 7). Regarding the results mentioned above, the data suggested that fulvestrant might help metastatic breast cancer patients with ER+ lesions to increase their chemosensitivity by reducing ER expression.
Figure 7.
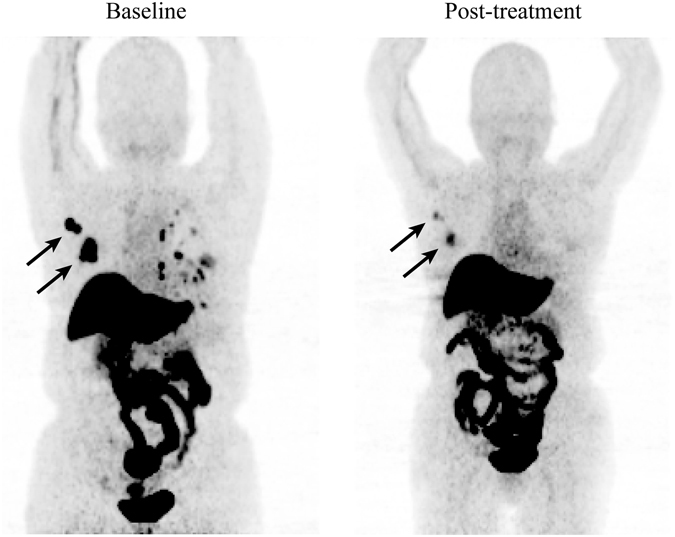
A 68-year-old female breast cancer patient, pretreatment with 18F-FES PET/CT (A) showed high uptake in these metastatic lesions (SUVmax = 4.8–20.15). After two cycles of combination treatment (group TF), these lesions had obvious decreases in 18F-FES uptake (SUVmax = 2.31–7.26, B). The greatest change was observed in the right axillary lymph node (>70%, arrow). The patient had a PFS > 12 months.
On pretreatment 18F-FDG SUVmax analysis, however, no significant difference was observed (PFS > 12 months: n = 30, SUVmax = 6.3 ± 3.5 versus PFS < 12 months: n = 18, SUVmax = 5.0 ± 1.7; t = 1.678, P = 0.1).
Discussion
As far as we know, this study was the first preliminary study to investigate the feasibility of docetaxel and fulvestrant in HR + /HER2- metastatic breast cancer patients. Our results showed that the addition of fulvestrant to docetaxel improved PFS from 9.9 months to 12.3 months, although no significant difference was observed due to the small sample size. This tendency was consistent with preclinical findings that the combination of fulvestrant and docetaxel had synergistic effect on inhibiting tumor growth22.
Because ER plays such important role in chemoresistance, the serial detection of ER during treatment could be useful. Contemporary assessments of ER expression in breast cancer have traditionally conducted in vitro assays of biopsied tissue using IHC staining quantitatively or qualitatively. Nevertheless, the presence of ER by IHC does not necessarily guarantee patient benefit from endocrine therapy36. Hence, it is far from satisfactory. The reasons could be explained as follows. First, the technique is semi-quantitative. There existed high and consistent rates of both intra- and inter-laboratory variability, and ER scoring also depends on the antibody used and the delay-to-fixation time37, 38. It was reported in a systematic review that as much as 20% of all IHC determinations worldwide were inaccurate, according to the American Society of Clinical Oncology and the College of American Pathologists39. Second, there was intratumoral heterogeneity of receptor content within the same lesions, as well as variations in ER expression among the primary and metastatic sites40, 41. Barry et al. suggested that the importance of understanding the role of tumor heterogeneity in measurements of tumor behavior, and they developed approaches and data sets to test the precision of their algorithms42. Therefore, we need noninvasive, ER-targeted molecular imaging to observe serial ER expression accurately in clinical practice.
18F-FES PET/CT has been evaluated in numerous breast cancer clinical studies as a promising method for assessing in vivo ER expression, predicting response (to hormone therapy and adjuvant chemotherapy), evaluating effective ER blockade and assisting in individualized treatment strategy decisions43–47. Several previous works, including our own study, have showed that 18F-FES PET detected a high occurrence of heterogeneity in recurrent breast cancer patients48, 49. Additionally, van Kruchten et al.50 utilized serial 18F-FES to observe tumor estrogen uptake, and it could successfully provide insight into the dose needed for ER antagonists to abolish ER completely. All of the studies mentioned above suggested that 18F-FES PET/CT was a useful technique for acquiring ER information sequentially and accurately in vivo.
Here, we conducted the first study to use 18F-FES PET/CT to observe the changes in ER expression during combination treatment. Our preliminary results were inspiring. According to 18F-FES PET/CT scans, the SUVmax values of all of the metastatic lesions decreased in group TF after 2 cycles of treatment. The data demonstrated that fulvestrant did reduce the ER expression in metastatic breast cancer patients. Furthermore, patients with PFS > 12 months had significantly greater SUVmax changes in 18F-FES than with PFS < 12 months. All of these findings reflected the potential of 18F-FES PET/CT to predict prognosis.
Due to slow enrollment, the trial was terminated early. However, one of the greatest obstacles for enrollment was the high cost of fulvestrant. If we had experimental evidence to select appropriate patients who might benefit from such an expensive drug, it might have been possible for us to recruit patients more easily. Based on the preliminary results with 18F-FES PET/CT, we considered that it might be a potential tool for our physicians to make treatment decisions. Further studies could be designed.
In our study, there are several limitations worth mentioning. The first was the small sample. Given the character of the study, we enrolled only 22 patients. We noticed a tendency, but no significant difference in PFS was observed. In addition, only 15 patients underwent both pre- and post-treatment 18F-FES and 18F-FDG PET/CT. Therefore, we could not demonstrate our results sufficiently. Second, a well-established optimal dose of fulvestrant has not been demonstrated in clinical practice, and it is unknown whether our current dose of fulvestrant, was sufficient for maximal ER downregulation and affording metastatic breast cancer patients the most benefit from the treatment. However, as a noninvasive method, 18F-FES PET/CT might guide physicians in choosing the appropriate dose of fulvestrant according to changes in SUVmax in the near future. Third, all of our patients were Chinese; the consequences might be different when compared with other races, thus limiting the generalizability of the results.
Conclusion
Our preliminary study showed that 18F-FES PET/CT, as a noninvasive method to monitor ER expression, could be utilized to observe serial ER regulation during treatment in vivo and that it has the potential to predict prognosis; therefore, an individualized treatment strategy could be recommended.
Acknowledgements
This study was funded by the National Science Foundation of China (Grant No. 81302300), the Shanghai Committee of Science and Technology Fund (15ZR1407600) and Shanghai Engineering Research Center of Molecular Imaging Probes (14DZ2251400). We thanked all of the physicians, nurses and technicians who have participated in the clinical trials in our department.
Author Contributions
Conception and design: Zhongyi Yang, Biyun Wang, Chengcheng Gong. Acquiring data, or analyzing and interpreting data: Zhongyi Yang, Chengcheng Gong, Yifei Sun, Jian Zhang, Chunlei Zheng, Leiping Wang, Yongping Zhang, Jing Xue, Zhifeng Yao and Herong Pan. Writing and revising the manuscript: Zhongyi Yang and Chengcheng Gong. Figures plotting: Chengcheng Gong (Figures 1–6), and Zhongyi Yang (Figure 7). Critically contributing to or revising the manuscript: Zhongyi Yang and Biyun Wang. Enhancing its intellectual: Zhongyi Yang, Yingjian Zhang and Biyun Wang.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhongyi Yang, Email: yangzhongyi21@163.com.
Biyun Wang, Email: pro_wangbiyun@163.com.
References
- 1.Chen W, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Lim E, Metzger-Filho O, Winer EP. The natural history of hormone receptor-positive breast cancer. Oncology (Williston Park). 2012;26(688-694):96. [PubMed] [Google Scholar]
- 3.Paik S, Hartmann DP, Dickson RB, Lippman ME. Antiestrogen resistance in ER positive breast cancer cells. Breast Cancer Res Treat. 1994;31:301–307. doi: 10.1007/BF00666162. [DOI] [PubMed] [Google Scholar]
- 4.Lippman ME, et al. The relation between estrogen receptors and response rate to cytotoxic chemotherapy in metastatic breast cancer. N Engl J Med. 1978;298:1223–1228. doi: 10.1056/NEJM197806012982203. [DOI] [PubMed] [Google Scholar]
- 5.Faneyte IF, et al. Breast cancer response to neoadjuvant chemotherapy: predictive markers and relation with outcome. Br J Cancer. 2003;88:406–412. doi: 10.1038/sj.bjc.6600749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Early Breast Cancer Trialists’ Collaborative G Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 7.Berry DA, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006;295:1658–1667. doi: 10.1001/jama.295.14.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller WR, Larionov A. Changes in expression of oestrogen regulated and proliferation genes with neoadjuvant treatment highlight heterogeneity of clinical resistance to the aromatase inhibitor, letrozole. Breast Cancer Res. 2010;12:R52. doi: 10.1186/bcr2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burow ME, Weldon CB, Tang Y, McLachlan JA, Beckman BS. Oestrogen-mediated suppression of tumour necrosis factor alpha-induced apoptosis in MCF-7 cells: subversion of Bcl-2 by anti-oestrogens. J Steroid Biochem Mol Biol. 2010;78:409–448. doi: 10.1016/S0960-0760(01)00117-0. [DOI] [PubMed] [Google Scholar]
- 10.Tabuchi Y, et al. Resistance to paclitaxel therapy is related with Bcl-2 expression through an estrogen receptor mediated pathway in breast cancer. Int J Oncol. 2009;34:313–319. [PubMed] [Google Scholar]
- 11.Li. JJ, et al. Estrogen mediates Aurora-A overexpression, centrosome amplification, chromosomal instability, and breast cancer in female ACI rats. Proc Natl Acad Sci USA. 2004;101:18123–18128. doi: 10.1073/pnas.0408273101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ikeda H, et al. The estrogen receptor influences microtubule-associated protein tau (MAPT) expression and the selective estrogen receptor inhibitor fulvestrant downregulates MAPT and increases the sensitivity to taxane in breast cancer cells. Breast Cancer Res. 2010;12:R43. doi: 10.1186/bcr2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woods KE, Randolph JK, Gewirtz DA. Antagonism between tamoxifen and doxorubicin in the MCF-7 human breast tumor cell line. Biochem Pharmacol. 1994;47:1449–1452. doi: 10.1016/0006-2952(94)90346-8. [DOI] [PubMed] [Google Scholar]
- 14.Albain KS, et al. Adjuvant chemotherapy and timing of tamoxifen in postmenopausal patients with endocrine-responsive, node-positive breast cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374:2055–2563. doi: 10.1016/S0140-6736(09)61523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frasor J, et al. Selective estrogen receptor modulators: discrimination of agonistic versus antagonistic activities by gene expression profiling in breast cancer cells. Cancer Res. 2004;64:1522–1533. doi: 10.1158/0008-5472.CAN-03-3326. [DOI] [PubMed] [Google Scholar]
- 16.Mutoh K, Tsukahara S, Mitsuhashi J, Katayama K, Sugimoto Y. Estrogen-mediated post transcriptional down-regulation of P-glycoprotein in MDR1-transduced human breast cancer cells. Cancer Sci. 2006;97:1198–1204. doi: 10.1111/j.1349-7006.2006.00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alli E, Bash-Babula J, Yang JM, Hait WN. Effect of stathmin on the sensitivity to antimicrotubule drugs in human breast cancer. Cancer Res. 2002;62:6864–6869. [PubMed] [Google Scholar]
- 18.Osborne CK, Wakeling A, Nicholson RI. Fulvestrant: an oestrogen receptor antagonist with a novel mechanism of action. Br J Cancer. 2004;90:S2–6. doi: 10.1038/sj.bjc.6601629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sui M, Huang Y, Park BH, Davidson NE, Fan W. Estrogen receptor alpha mediates breast cancer cell resistance to paclitaxel through inhibition of apoptotic cell death. Cancer Res. 2007;67:5337–5344. doi: 10.1158/0008-5472.CAN-06-4582. [DOI] [PubMed] [Google Scholar]
- 20.Sui M, Jiang D, Hinsch C, Fan W. Fulvestrant (ICI 182,780) sensitizes breast cancer cells expressing estrogen receptor alpha to vinblastine and vinorelbine. Breast Cancer Res Treat. 2010;121:335–345. doi: 10.1007/s10549-009-0472-4. [DOI] [PubMed] [Google Scholar]
- 21.Scott SM, Brown M, Come SE. Emerging data on the efficacy and safety of fulvestrant, a unique antiestrogen therapy for advanced breast cancer. Expert Opin Drug Saf. 2011;10:819–826. doi: 10.1517/14740338.2011.595560. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda H, et al. Combination treatment with fulvestrant and various cytotoxic agents (doxorubicin, paclitaxel, docetaxel, vinorelbine, and 5-fluorouracil) has a synergistic effect in estrogen receptor-positive breast cancer. Cancer Sci. 2001;102:2038–2042. doi: 10.1111/j.1349-7006.2011.02050.x. [DOI] [PubMed] [Google Scholar]
- 23.McGuire AH, et al. Positron tomographic assessment of 16 alpha-[18F] fluoro-17 beta-estradiol uptake in metastatic breast carcinoma. J Nucl Med. 1991;32:1526–1531. [PubMed] [Google Scholar]
- 24.Mintun MA, et al. Breast cancer: PET imaging of estrogen receptors. Radiology. 1988;169:45–48. doi: 10.1148/radiology.169.1.3262228. [DOI] [PubMed] [Google Scholar]
- 25.Peterson LM, et al. Quantitative imaging of estrogen receptor expression in breast cancer with PET and 18F-fluoroestradiol. J Nucl Med. 2008;49:367–374. doi: 10.2967/jnumed.107.047506. [DOI] [PubMed] [Google Scholar]
- 26.Gemignani ML, et al. Feasibility and predictability of perioperative PET and estrogen receptor ligand in patients with invasive breast cancer. J Nucl Med. 2013;54:1697–1702. doi: 10.2967/jnumed.112.113373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dehdashti F, et al. Positron tomographic assessment of estrogen receptors in breast cancer: comparison with FDG-PET and in vitro receptor assays. J Nucl Med. 1995;36:1766–1774. [PubMed] [Google Scholar]
- 28.van Kruchten M, et al. Assessment of estrogen receptor expression in epithelial ovarian cancer patients using 16α-18F-fluoro-17β-estradiol PET/CT. J Nucl Med. 2015;56:50–55. doi: 10.2967/jnumed.114.147579. [DOI] [PubMed] [Google Scholar]
- 29.Mori T, et al. Automatic synthesis of 16 alpha-[(18)F]fluoro-17beta-estradiol using a cassette-type [(18)F]fluorodeoxyglucose synthesizer. Nucl Med Biol. 2006;33:281–286. doi: 10.1016/j.nucmedbio.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, et al. Fully automated synthesis of 16α [18F]fluoro-17β-estrogen using Explora GN/LC dual module. Chin J Nucl Med. 2011;31:196–200. [Google Scholar]
- 31.Wang M, Zhang Y, Zhang Y, Yuan H, Gao Z. Automated synthesis of 16α-[~(180F)fluoro-17β-estrogen as estrogen receptor imaging probe of breast cancer. Nuclear Techniques. 2009;32:839–844. [Google Scholar]
- 32.van Kruchten M, et al. PET imaging of estrogen receptors as a diagnostic tool for breast cancer patients presenting with a clinical dilemma. J Nucl Med. 2012;53:182–190. doi: 10.2967/jnumed.111.092734. [DOI] [PubMed] [Google Scholar]
- 33.Gemignani ML, et al. Feasibility and predictability of perioperative PET and estrogen receptor ligand in patients with invasive breast cancer. J Nucl Med. 2013;54:1697–1702. doi: 10.2967/jnumed.112.113373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Z, et al. Can fluorine-18 fluoroestradiol positron emission tomography-computed tomography demonstrate the heterogeneity of breast cancer in vivo? Clin Breast Cancer. 2013;13:359–363. doi: 10.1016/j.clbc.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 35.Sun Y, et al. The preliminary study of 16α-[18F]fluoroestradiol PET/CT in assisting the individualized treatment decisions of breast cancer patients. PLoS One. 2015;10:e0116341. doi: 10.1371/journal.pone.0116341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeSombre ER, et al. Prognostic usefulness of estrogen receptor immunocytochemical assays for human breast cancer. Cancer Res. 1986;46:4256s–4264s. [PubMed] [Google Scholar]
- 37.Sharangpani GM, et al. Semi-automated imaging system to quantitate estrogen and progesterone receptor immunoreactivity in human breast cancer. J Microsc. 2007;226:244–255. doi: 10.1111/j.1365-2818.2007.01772.x. [DOI] [PubMed] [Google Scholar]
- 38.Pusztai L, Mazouni C, Anderson K, Wu Y, Symmans WF. Molecular classification of breast cancer: limitations and potential. Oncologist. 2006;11:868–877. doi: 10.1634/theoncologist.11-8-868. [DOI] [PubMed] [Google Scholar]
- 39.Hammond ME, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castagnetta L, et al. Do multiple oestrogen receptor assays give significant additional information for the management of breast cancer? Br J Cancer. 1989;59:636–638. doi: 10.1038/bjc.1989.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Netten JP, et al. Cellular distribution patterns of estrogen receptor in human breast cancer. Eur J Cancer Clin Oncol. 1988;24:1899–1901. doi: 10.1016/0277-5379(88)90105-8. [DOI] [PubMed] [Google Scholar]
- 42.Barry WT, et al. Intratumor heterogeneity and precision of microarray-based predictors of breast cancer biology and clinical outcome. J Clin Oncol. 2010;28:2198–2206. doi: 10.1200/JCO.2009.26.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Linden HM, et al. Fluoroestradiol positron emission tomography reveals differences in pharmacodynamics of aromatase inhibitors, tamoxifen, and fulvestrant in patients with metastatic breast cancer. Clin Cancer Res. 2011;17:4799–4805. doi: 10.1158/1078-0432.CCR-10-3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liao GJ, Clark AS, Schubert EK, Mankoff DA. 18F-Fluoroestradiol PET: Current Status and Potential Future Clinical Applications. J Nucl Med. 2016;57:1269–1275. doi: 10.2967/jnumed.116.175596. [DOI] [PubMed] [Google Scholar]
- 45.van Kruchten M, et al. PET imaging of oestrogen receptors in patients with breast cancer. Lancet Oncol. 2013;14:e465–475. doi: 10.1016/S1470-2045(13)70292-4. [DOI] [PubMed] [Google Scholar]
- 46.Sun Y, et al. The preliminary study of 16α-[18F]fluoroestradiol PET/CT in assisting the individualized treatment decisions of breast cancer patients. PLoS One. 2015;10:e0116341. doi: 10.1371/journal.pone.0116341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang Z, et al. Can positron emission tomography/computed tomography with the dual tracers fluorine-18 fluoroestradiol and fluorodeoxyglucose predict neoadjuvant chemotherapy response of breast cancer?—A pilot study. PLoS One. 2013;8:e78192. doi: 10.1371/journal.pone.0078192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang Z, et al. Can fluorine-18 fluoroestradiol positron emission tomography-computed tomography demonstrate the heterogeneity of breast cancer in vivo? Clin Breast Cancer. 2013;13:359–363. doi: 10.1016/j.clbc.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 49.Kurland BF, et al. Between-patient and within-patient (site-to-site) variability in estrogen receptor binding, measured in vivo by 18F-fluoroestradiol PET. J Nucl Med. 2011;52:1541–1549. doi: 10.2967/jnumed.111.091439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Kruchten M, et al. Measuring residual estrogen receptor availability during fulvestrant therapy in patients with metastatic breast cancer. Cancer Discov. 2015;5:72–81. doi: 10.1158/2159-8290.CD-14-0697. [DOI] [PubMed] [Google Scholar]


