Abstract
The epithelial surfaces of the upper respiratory tract are continuously exposed to a wide variety of commensal microorganisms. In addition to acting as a physical barrier, epithelial cells respond to specific microbial products with the generation of signals, such as cytokines, that trigger inflammation. Because they are common components of the nasopharyngeal flora that share the potential to cause disease, we investigated the effects of Haemophilus influenzae and Streptococcus pneumoniae, alone and in combination, on human respiratory epithelial cells in culture and in a murine model of nasopharyngeal colonization. Exposure of A549 or Detroit 562 epithelial cells to both S. pneumoniae and H. influenzae led to a synergistic increase in production of IL-8, the major neutrophil chemokine in the airway, through an NF-κB-dependent mechanism. Likewise, nasal cocolonization of mice caused a synergistic rise in local production of macrophage inflammatory protein 2 in nasal lavage fluid and subsequent recruitment of neutrophils. This synergistic effect depended on production of the pore-forming cytolytic toxin, pneumolysin, by S. pneumoniae and activation of host p38 mitogen-activated protein kinase. Although both H. influenzae and S. pneumoniae have ligands for Toll-like receptors (TLRs) TLR2 and TLR4, synergistic activation was TLR2- and TLR4-independent. Thus, epithelial surfaces are capable of amplifying proinflammatory responses during concurrent stimulation by multiple microbial species. These synergistic responses, demonstrated both in vitro and in vivo, may contribute to inflammation of heavily colonized mucosal barriers.
Keywords: Haemophilus influenzae, inflammation, nasopharyngeal colonization, Streptococcus pneumoniae
There is a growing body of evidence that local environmental factors can have marked effects on the response of epithelial cells to microbial stimuli. In the case of the respiratory tract, the epithelium senses the luminal contents of the airway and can transduce NF-κB-dependent proinflammatory signals, including production of the neutrophil chemokine IL-8 (1, 2). Interactions between bacteria and epithelial cells have generally been investigated by using single species in pure culture, although many mucosal surfaces are in constant contact with an array of diverse microbial species (3). The presence of commensal bacteria has been shown to attenuate the response of gastrointestinal epithelial cells to pathogenic Salmonella by stabilization of the NF-κB-inhibitory molecule IκB, suggesting that polymicrobial stimulation may dampen inflammatory responses (4). In contrast, the presence of proinflammatory cytokines may enhance epithelial responses to microbes (5). It appears, therefore, that epithelia function as complex signal integrators, assimilating information from combinations of microbes and the local cytokine environment to adjust their own proinflammatory response to a specific ecologic milieu.
In this study, Haemophilus influenzae and Streptococcus pneumoniae were used as model organisms to investigate the response of respiratory epithelial cells to simultaneous stimulation by multiple bacterial species. Both commonly exist as commensal organisms in the human nasopharynx and are capable of causing disease when they are able to gain access to other normally sterile sites. Asymptomatic carriage of each species may be stable over weeks or months and occurs in greater than half of some populations, especially young children (6). These factors imply that concurrent exposure of mucosal surfaces to these two species may not be a rare event. Constituents of both H. influenzae and S. pneumoniae have been described to stimulate respiratory epithelial cells to produce IL-8 (7, 8). However, little is known about the specific bacterial or host components involved in these processes, and the pattern of response to coinfection with H. influenzae and S. pneumoniae has not been investigated. As a consequence of the shared niche and high frequency of carriage of these two organisms, we postulated that the respiratory epithelium may have evolved mechanisms to respond to concurrent stimulation by H. influenzae and S. pneumoniae that might be qualitatively or quantitatively different from the response to either alone.
In this series of experiments, we describe synergistic (i.e., greater than additive) increases in production of the proinflammatory cytokines macrophage inflammatory protein (MIP)-2 (in the mouse nasopharynx) and IL-8 (in human epithelial cells) in response to costimulation with S. pneumoniae and H. influenzae. This finding is in contrast with previous studies (4) that noted a dampening effect of bacterial costimulation. Our findings suggest that proinflammatory polymicrobial stimulation may contribute to the protective function of multiply colonized epithelial surfaces.
Materials and Methods
Bacterial Strains and Culture Conditions. H. influenzae and S. pneumoniae strains were grown as described (9). Strains used in vitro included H. influenzae H233 (a nontypeable clinical isolate) and S. pneumoniae D39 (a type 2, capsulated clinical isolate) (9, 10). Strains used in vivo were selected because of their ability to colonize efficiently in the murine nasopharynx, and included H636 (a type b, constitutively phosphorylcholine-expressing mutant of strain Eagan that spontaneously developed resistance to streptomycin) and P1121 (a type 23F, capsulated isolate from the human nasopharynx) (11, 12). The pneumolysin (Ply)-deficient derivative D39ply was kindly provided by D. Briles (University of Alabama, Birmingham) (13). The Ply-deficient derivative of P1121 was constructed by transformation with genomic DNA from D39ply, followed by serial back-transformation.
Epithelial Cell Lines and Culture Conditions. A549 (ATCC CCL-185) and Detroit 562 (D562, ATCC CCL-138) epithelial cell lines were maintained as described (9). Human embryonic kidney (HEK)293 (ATCC CRL-1573) cells were kindly provided by D. Golenbock (University of Massachusetts Medical School, Worcester) and were grown in DMEM (Invitrogen) supplemented with 1 mM sodium pyruvate/10% FBS (HyClone)/100 units/ml penicillin/100 μg/ml streptomycin.
Mouse Model of Nasopharyngeal Colonization. Six-week-old C3H/HeOuJ [Toll-like receptor (TLR)4-sufficient] and C3H/HeJ (TLR4-deficient) mice were obtained from The Jackson Laboratory. Mice were inoculated intranasally with 107 colony-forming units (cfu) of PBS-washed, mid-log phase H. influenzae, S. pneumoniae, or a combination of the two applied separately to each naris. Twenty-four hours after inoculation, the animal was killed, the trachea was cannulated, and 200 μl of PBS was instilled. Lavage fluid was collected from the nasopharynx for determination of viable counts of bacteria in medium containing antibiotics to inhibit the growth of contaminants (100 μg/ml streptomycin to select for H. influenzae and 20 μg/ml neomycin to select for S. pneumoniae). In addition, the lavage fluid was assayed for the concentration of MIP-2 by ELISA (Pharmingen). These values were normalized to total protein (micro BCA protein assay, Pierce). In some experiments where indicated, C.B-17/lcrCrlscid (severe combined immunodeficient mice; Charles River Breeding Laboratories) or C57BL/6-TLR2-/- mice were used (14).
Bacterial Stimulation of Epithelial Cells and IL-8 ELISA. A549 or D562 cells were grown to confluence in 24-well plates (Costar) and then weaned from serum and antibiotics overnight. Bacteria were grown in liquid culture to mid-log phase (OD620 = 0.4), centrifuged, washed, and resuspended in DMEM without serum or antibiotics. Serial dilutions were plated to confirm bacterial density. A total of 1 × 107–8 cfu/ml H. influenzae and/or S. pneumoniae were added to the epithelial monolayer. Where indicated, purified Ply or its toxoid form (PdB), prepared as described (15), and the kind gift of J. Paton (University of Adelaide, Adelaide, Australia), was added to the cells (100 ng/ml) in place of S. pneumoniae. Plates were spun at 150 × g for 5 min to apply the bacteria to the epithelial cells and incubated at 37°C in 5% CO2 for 2 h. After stimulation, monolayers were washed four times with PBS, and MEM with 10 μg/ml ciprofloxacin was added overnight. The concentration of IL-8 in supernatants collected 18 h after stimulation was determined by ELISA (Pharmingen) according to the manufacturer's instructions. Cell viability as assessed by Trypan blue exclusion exceeded 95%. Because HEK cells adhere poorly if washed or weaned from serum, unweaned cells were exposed to heat-killed (70°C for 30 min) H. influenzae and/or S. pneumoniae for 18 h, and supernatants were assayed as above. Pretreatment of cells with 20 μM SB203580 (Calbiochem) or 20 μM N-p-tosyl-l-phenylalanine chloromethyl ketone (TPCK, Sigma) was for 30 min, and inhibitors were present until supernatants were collected for ELISA.
Plasmids, Transfection, and Luciferase Assays. D562 cells were grown to 50% confluence in 24-well dishes and transfected with 200 ng per well of pNF-κB-luc (Stratagene) and 20 ng per well of pRL-TK (Promega) by using FuGENE6 (Roche Applied Sciences) according to the manufacturer's instructions. Costimulation as described above was performed at 36–48 h after transfection, and relative luciferase activity was assessed 6 h later by using the dual luciferase assay kit (Promega) according to the manufacturer's instructions.
Western Blotting. Monolayers of serum-weaned A549 cells were stimulated for 30 min with 108 cfu/ml heat-killed H233, 100 ng/ml purified Ply, or the two together. After washing with PBS, cells were lysed in 1% Triton X-100 with protease and phosphatase inhibitor cocktails (Sigma), and separated by SDS/PAGE. Proteins were transferred to poly(vinylidene difluoride) membranes and probed by using the PhosphoPlus p38 mitogen-activated protein kinase (MAPK) (Thr-180/Tyr-182) antibody kit (Cell Signaling Technology, Beverly, MA) according to the manufacturer's instructions.
Statistical Analysis. Statistical comparisons among groups were made by using ANOVA with Bonferroni posttests as appropriate. For in vivo data, groups were compared with Mann–Whitney tests (graphpad prism v. 4).
Results
Coinfection of Respiratory Epithelial Cells with H. influenzae and S. pneumoniae Induces Synergistic Increases in Production of IL-8. We measured the concentration of IL-8, a major human neutrophil chemokine of the airway, in supernatants of the human respiratory epithelial cell line A549 after stimulation by H. influenzae strain H233, S. pneumoniae D39, or the two in combination. Exposure of the epithelial cells to bacteria for 2 h was required to induce a maximal response (data not shown). When similar numbers of organisms were applied, H233 was a more potent inducer of IL-8 production than D39 (Fig. 1A). When epithelial cell monolayers were exposed to the two organisms together, the production of IL-8 was significantly greater than additive (P < 0.001). Similar results were obtained by using D562 cells (data not shown).
Fig. 1.
H. influenzae and S. pneumoniae synergistically induce production of IL-8 by epithelial cells. (A) A549 respiratory epithelial cell monolayers were stimulated for 2 h with S. pneumoniae D39 (107 cfu/ml) and/or H. influenzae H233 (107–8 cfu/ml; the lower portion of the triangle represents 107 and the upper portion represents 108), and supernatants were assayed for IL-8 by ELISA 18 h later. Costimulation led to greater IL-8 production than exposure to either species alone (*, P < 0.001) or than the sum of individual species. Values here and in subsequent graphs are expressed as mean ± SEM (triplicate readings in at least three independent experiments). (B) C3H/HeOuJ mice were nasally colonized with 107 cfu of H. influenzae H636 and/or S. pneumoniae P1121 or its Ply-deficient derivative, P1121ply, and 24 h later, upper respiratory tract lavage samples were assayed for MIP-2 by ELISA, normalized to total protein. Cocolonization of H636 and P1121 led to synergistic induction of MIP-2 in lavage samples (*, P < 0.01; box-and-whiskers plot indicates median and interquartile ranges; n = 5 in each group), whereas cocolonization of H636 and Ply-deficient P1121ply did not. (C) Hematoxylin/eosin-stained sections (400×) of upper airways of C3H/HeOuJ mice 24 h after colonization with H636 (i), P1121 (ii), H636 and P1121 (iii, arrow indicates neutrophilic infiltrate), and H636 and P1121ply (iv). Mock-colonized mice had no detectable MIP-2 in nasal washes, and no inflammatory cells were seen in sections (data not shown).
Nasopharyngeal Cocolonization of Mice with H. influenzae and S. pneumoniae Leads to Increased Local Production of MIP-2 and Neutrophil Recruitment. To determine the in vivo relevance of synergistic cytokine induction, C3H/HeOuJ mice were colonized with S. pneumoniae or H. influenzae. Animals colonized with both bacterial species generated significantly higher local concentrations of MIP-2, the major murine neutrophil chemokine in the lung, than did those exposed to either organism alone (Fig. 1B; P < 0.01). Similar results were obtained by using nasal washes from severe combined immunodeficient mice colonized for 24 or 72 h (data not shown.) Correspondingly, histopathologic examination of the upper airways of colonized mice demonstrated a greater influx of inflammatory cells into the anterior nasal spaces of mice exposed to both bacterial species than those given either bacterium alone (Fig. 1C).
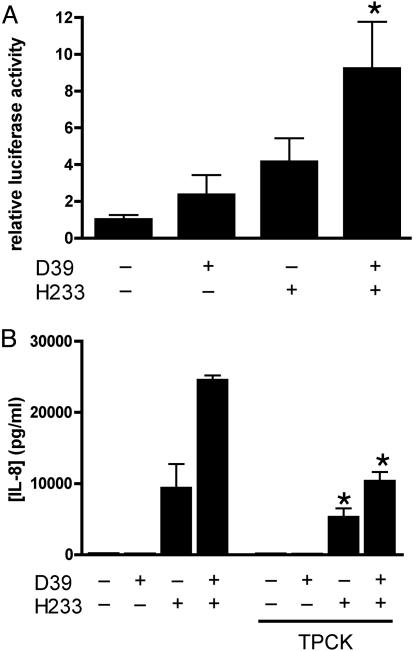
Synergistic Release of IL-8 from Epithelial Cells Is NF-κB-Dependent. The transcription factor NF-κB is crucial to the ability of respiratory epithelial cells to produce IL-8 in response to a variety of stimuli (16). To define whether the mechanism for synergistic proinflammatory response occurred in a pathway upstream of NF-κB, the relative activity of NF-κB was assessed in D562 cells transfected with luciferase reporter plasmid (NF-κB-luc). In accord with the data from the IL-8 ELISA, we demonstrated greater activation of NF-κB after exposure of the cells to H. influenzae and S. pneumoniae together than to either species alone (Fig. 2A) Treatment of cells with the proteasome inhibitor TPCK, which prevents nuclear translocation of NF-κB, significantly decreased production of IL-8 in response to bacterial stimulation (Fig. 2B).
Fig. 2.
Synergistic induction of IL-8 response in epithelial cells is NF-κB-dependent. (A) D562 cells were transfected with an NF-κB-dependent firefly luciferase construct and a control Renilla luciferase and stimulated with H. influenzae H233 and/or S. pneumoniae D39. Cell lysates were assayed for relative induction of luciferase activity. Values are expressed as fold induction of firefly luciferase activity normalized to levels of Renilla luciferase (*, P < 0.05). (B) Inhibition of NF-κB translocation with the proteasome inhibitor TPCK (20 μM) decreases IL-8 production by A549 cells treated for 2 h with S. pneumoniae D39 and H. influenzae H233 (*, P < 0.001 compared with conditions without inhibitor).
Synergistic IL-8 Production by Respiratory Epithelial Cells Does Not Require TLR2 or TLR4. Because signaling by means of TLR4 has been implicated in innate immunity to H. influenzae respiratory infection (17) and in the host response to the S. pneumoniae protein toxin Ply (18), we investigated the contribution of this system to the synergistic increase in production of IL-8. No difference in levels of MIP-2 in upper respiratory tract lavage samples taken from TLR4-sufficient (C3H/HeOuJ) and TLR4-deficient (C3H/HeJ) mice was observed (data not shown), indicating that host TLR4 is not an important factor in the generation of this cytokine in response to bacterial colonization or cocolonization. Similar findings in TLR2-/- mice confirmed that TLR2 is also not necessary for synergistic increases in cytokine production in vivo (data not shown). We further investigated the role of TLR signaling in vitro by stimulating HEK cells that lack endogenous TLR2 and TLR4, with combinations of bacteria (19, 20). A similar pattern of response, including a synergistic increase in production of IL-8, after coinfection with H. influenzae and S. pneumoniae was observed (Fig. 3A), confirming that this effect is preserved even in the absence of signaling through TLR2 and TLR4.
Fig. 3.
Ply is required for the pneumococcal contribution to synergistic IL-8 release. (A) HEK293 cells that lack TLR2 and TLR4 were stimulated with H. influenzae H233 and/or Ply-sufficient S. pneumoniae D39 or its Ply-deficient derivative, S. pneumoniae D39ply, and supernatants were assayed for IL-8 production after 18 h (*, P < 0.001). (B) Treatment of A549 cells with purified Ply toxin (100 ng/ml) recapitulates synergistic induction of IL-8 in the presence of 108 cfu/ml H. influenzae H233 (*, P < 0.001 compared with other groups), whereas PdB (100 ng/ml) has no effect.
Production of Ply Is Required for the Pneumococcal Contribution to Epithelial Stimulation. To investigate the contribution of Ply, a pore-forming toxin of S. pneumoniae, to the generation of a synergistic cytokine response, we used isogenic Ply-deficient strains of S. pneumoniae in both in vitro and in vivo experiments. Stimulation of HEK cells (Fig. 3A) or A549 (data not shown) demonstrated that costimulation with H. influenzae and a Ply-sufficient strain of S. pneumoniae is required for synergistic release of IL-8. Although the Ply-sufficient and -deficient strains colonized with equivalent efficiency at 24 h, no synergistic production of MIP-2 in nasal washes was observed in animals receiving Ply-deficient S. pneumoniae in combination with H. influenzae (Fig. 1B). Correspondingly, less inflammation was observed in the airways of mice cocolonized with H. influenzae and Ply-deficient S. pneumoniae than those treated with H. influenzae and Ply-sufficient S. pneumoniae (Fig. 1C). Moreover, treatment of epithelial cells with H. influenzae H233 and purified Ply was sufficient to synergistically enhance IL-8 production. PdB, which has <0.1% of the activity of the native toxin (15), failed to induce synergistic IL-8 production (Fig. 3B).
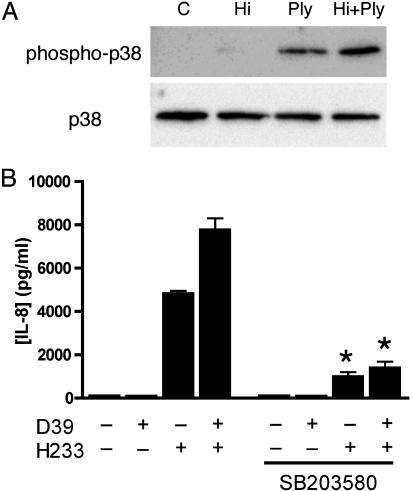
Host p38 MAPK Activity Is Required for Synergistic Epithelial Stimulation by H. influenzae and S. pneumoniae. Host cell activation of NF-κB in response to small cytoplasmic factors of H. influenzae has been previously described to require activity of p38 MAPK (21). Additionally, p38 MAPK is involved in H. influenzae-mediated up-regulation of mucin gene expression in epithelial cells (22). We investigated the activation of p38 MAPK after exposure of cells to H233, purified Ply, or the two in combination. Costimulation of cells with H. influenzae and Ply led to greater phosphorylation of p38 MAPK than either condition alone (Fig. 4A). The contribution of p38 MAPK activation to our observations of synergistic IL-8 production was investigated by using a specific inhibitor of p38 MAPK, SB203580. Pretreatment of A549 cells with SB203580 for 30 min reduced the response to H. influenzae H233 and suppressed the response to coinfection with S. pneumoniae (Fig. 4B), confirming the dependence of synergistic cytokine induction on p38 MAPK.
Fig. 4.
Activation of p38 MAPK is involved in the synergistic proinflammatory response. (A) A549 cells were treated for 30 min with 108 cfu/ml heat-killed H233 (Hi), 100 ng/ml purified Ply, or a combination of the two (Hi plus Ply). Control cells (C) received media alone. Western blotting was performed on whole-cell lysates by using antibodies specific for total or phosphorylated p38 MAPK. (B) Pretreatment of A549 cells with the p38 MAPK inhibitor SB203580 (20 μM for 30 min) suppresses synergistic IL-8 production in response to costimulation with H. influenzae H233 and S. pneumoniae D39 (*, P < 0.001 compared with conditions without inhibitor).
Discussion
We demonstrated synergistic induction of proinflammatory cytokine expression both in vitro and in vivo in response to two common constituents of the human nasopharyngeal flora. Epithelial surfaces in the upper airway face a dilemma similar to that in the gastrointestinal tract: how to preserve the ability to respond to microbial threats in the setting of constant exposure to multiple types of commensal organisms. Gastro-intestinal epithelial cells sequester some pattern-recognition receptors away from the luminal contents, and there are also data to suggest that certain members of the commensal flora may dampen epithelial responses to a variety of stimuli (4, 23). We investigated the pattern of response of respiratory epithelial cells exposed to two common organisms that exist as both commensal flora and upper respiratory pathogens in humans. The finding that exposure to microbes in combination can greatly alter the level of epithelial proinflammatory cytokine production and influx of neutrophils implies that the presence of certain bacterial species in the upper respiratory microenvironment may modulate the capacity of a host to respond to new challenges. Alternatively, acquisition of new bacterial species may prime inflammatory responses to preexisting commensals. Both of these possibilities are especially important in light of increasingly common medical interventions, such as antibiotic therapy or vaccination, that alter the composition of the nasopharyngeal flora (24, 25).
Generation of IL-8 by epithelial cells plays a central role in the initial control of respiratory tract infection, as it recruits neutrophils that contribute to bacterial killing and clearance to the airway lumen (26). Dysregulation of NF-κB activation and subsequent IL-8 production may play an important role in the pathogenesis of some inflammatory lung diseases such as cystic fibrosis (16). Synergistic production of IL-8 in response to S. pneumoniae and H. influenzae depends on an intact NF-κB signaling system, but interestingly, the response of NF-κB to costimulation as measured by a luciferase reporter system appeared to be additive rather than synergistic. Because of the presence of multiple NF-κB inhibitory molecules, including IκBα, complex relationships exist between the patterns of activation of NF-κB and the overall expression of downstream genes (27). In addition, the regulation of IL-8 production is multifactorial, involving both pre- and posttranscriptional regulatory steps. Our data do not exclude the possibility that other factors acting in concert with NF-κB contribute to synergistic epithelial production of IL-8. For example, phosphorylation of p38 MAPK, as demonstrated in this setting, can stabilize IL-8 mRNA, leading to prolongation of cytokine production (28).
Epithelial cells sense bacterial products through a variety of mechanisms, the best understood of which involve TLRs. Structures present on both H. influenzae and S. pneumoniae act as ligands for TLRs (17, 18, 29–33). In addition, TLR signaling appears to be important in innate immunity to both of these pathogens in vivo (34–37). Despite the evidence for interaction of both H. influenzae and S. pneumoniae with TLR2 and TLR4, it is clear that TLR-independent pathways of respiratory inflammation exist. For example, mice that lack either TLR2 or the crucial adapter molecule MyD88 still mount an immune response to S. pneumoniae in the lung (34, 35). Synergistic induction of IL-8 by S. pneumoniae and H. influenzae can occur in human cell lines and in mice that lack the capacity to signal through TLR2 or TLR4. This finding prompted us to investigate TLR2- and TLR4-independent means of epithelial stimulation by S. pneumoniae and H. influenzae.
Ply, the major protein toxin of S. pneumoniae, is a member of a family of Gram-positive cytotoxins that target cholesterol-containing membranes of eukaryotic cells and generate large pores (38). Ply-deficient strains of S. pneumoniae are attenuated in animal models of pneumonia and sepsis (13). In addition, purified toxin is sufficient to induce many of the features of pneumococcal lung disease, including neutrophil recruitment, just as it recapitulated the effect of whole S. pneumoniae in our experiments (39). Here, we demonstrate that Ply is necessary and sufficient for the pneumococcal contribution to synergistic epithelial activation, an effect that is independent of TLR4 (18). PdB has a single amino acid substitution (W433F) that decreases its cytotoxic activity >1,000-fold (15). The toxoid form, when presented in combination with H233, did not lead to synergistic enhancement of IL-8 production, implying that active pore-forming toxin is required for this effect.
H. influenzae is a potent activator of NF-κB-dependent genes in epithelial cells (5). In addition to its ligands for TLR2 and TLR4, an unknown constituent of the soluble cytoplasmic fraction (SCF) of H. influenzae causes activation of NF-κB in epithelial cells by means of a p38 MAPK-dependent mechanism (33). Because synergistic induction of IL-8 was noted even in the absence of functional TLR2 and TLR4, we investigated the contribution of host p38 MAPK. We demonstrated that p38 MAPK phosphorylation was increased in the setting of costimulation by H. influenzae and purified Ply. Synergistic IL-8 production by H. influenzae and S. pneumoniae could not occur in the setting of p38 MAPK inhibition. This finding is consistent with previous findings (21) that noted that p38 MAPK-dependent stimulation, largely due to components of the SCF, accounted for the majority of epithelial activation by H. influenzae. The dependence of synergistic IL-8 induction on both Ply and p38 MAPK raises the possibility that membrane pores created by Ply might serve to enhance delivery of SCF components to the cytoplasm of host epithelial cells, where they may stimulate innate immune responses. H. influenzae peptidoglycan, for example, has been shown to be a mediator of inflammation in vivo (40, 41). If H. influenzae peptidoglycan gains access to the cytoplasm through Ply pores, it may subsequently interact with the pattern recognition molecule Nod1, which senses Gram-negative peptidoglycan and initiates NF-κB-mediated responses (42). This suggested mechanism is reminiscent of cytolysin-mediated translocation in Streptocuccus pyogenes and of the delivery of peptidoglycan to the cytoplasm of epithelial cells by the type IV secretion system of Helicobacter pylori (43, 44).
Our observations represent a previously unrecognized phenomenon among members of the respiratory microflora: the action of a virulence factor from one microbe amplifying the host response to a second, unrelated species. Improved understanding of this synergistic activation and other epithelial responses to polymicrobial stimulation may provide insight into the molecular mechanisms impacting colonization.
Acknowledgments
We thank J. Paton, H. Shen (University of Pennsylvania), D. Briles, J. D. Li (House Ear Institute, Los Angeles), and D. Golenbock for providing reagents; M. Shchepetov for technical assistance, and J. Bergelson for comments on the manuscript. This work was supported by National Institute of Allergy and Infectious Disease Grant AI054647 (to J.N.W.) and the Pediatric Infectious Diseases Society–St. Jude Children's Research Hospital Fellowship Program (to A.J.R.)
Author contributions: A.J.R., E.S.L., and J.N.W. designed research; A.J.R., E.S.L., and M.N.P. performed research; A.J.R., E.S.L., M.N.P., and J.N.W. analyzed data; and A.J.R. and J.N.W. wrote the paper.
Abbreviations: TLR, Toll-like receptor; MAPK, mitogen-activated protein kinase; MIP, macrophage inflammatory protein; HEK, human embryonic kidney; cfu, colony-forming unit; Ply, pneumolysin; PdB, toxoid form of Ply; TPCK, N-p-tosyl-l-phenylalanine chloromethyl ketone.
References
- 1.DiMango, E., Zar, H. J., Bryan, R. & Prince, A. (1995) J. Clin. Invest. 96, 2204-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skerrett, S. J., Liggitt, H. D., Hajjar, A. M., Ernst, R. K., Miller, S. I. & Wilson, C. B. (2004) Am. J. Physiol. 287, L143-L152. [DOI] [PubMed] [Google Scholar]
- 3.Paster, B. J., Boches, S. K., Galvin, J. L., Ericson, R. E., Lau, C. N., Levanos, V. A., Sahasrabudhe, A. & Dewhirst, F. E. (2001) J. Bacteriol. 183, 3770-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neish, A. S., Gewirtz, A. T., Zeng, H., Young, A. N., Hobert, M. E., Karmali, V., Rao, A. S. & Madara, J. L. (2000) Science 289, 1560-1563. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe, T., Jono, H., Han, J., Lim, D. J. & Li, J. D. (2004) Proc. Natl. Acad. Sci. USA 101, 3563-3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neto, A. S., Lavado, P., Flores, P., Dias, R., Pessanha, M. A., Sousa, E., Palminha, J. M., Canica, M. & Esperanca-Pina, J. (2003) Microb. Drug Resist. 9, 99-108. [DOI] [PubMed] [Google Scholar]
- 7.Clemans, D. L., Bauer, R. J., Hanson, J. A., Hobbs, M. V., St. Geme, J. W., III, Marrs, C. F. & Gilsdorf, J. R. (2000) Infect. Immun. 68, 4430-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madsen, M., Lebenthal, Y., Cheng, Q., Smith, B. L. & Hostetter, M. K. (2000) J. Infect. Dis. 181, 1330-1336. [DOI] [PubMed] [Google Scholar]
- 9.Gould, J. M. & Weiser, J. N. (2002) J. Infect. Dis. 186, 361-371. [DOI] [PubMed] [Google Scholar]
- 10.Avery, O. T., MacLeod, C. M. & McCarty, M. (1944) J. Exp. Med. 79, 137-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lysenko, E., Richards, J. C., Cox, A. D., Stewart, A., Martin, A., Kapoor, M. & Weiser, J. N. (2000) Mol. Microbiol. 35, 234-245. [DOI] [PubMed] [Google Scholar]
- 12.McCool, T. L., Cate, T. R., Moy, G. & Weiser, J. N. (2002) J. Exp. Med. 195, 359-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berry, A. M., Yother, J., Briles, D. E., Hansman, D. & Paton, J. C. (1989) Infect. Immun. 57, 2037-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeuchi, O., Hoshino, K., Kawai, T., Sanjo, H., Takada, H., Ogawa, T., Takeda, K. & Akira, S. (1999) Immunity 11, 443-451. [DOI] [PubMed] [Google Scholar]
- 15.Paton, J. C., Lock, R. A., Lee, C. J., Li, J. P., Berry, A. M., Mitchell, T. J., Andrew, P. W., Hansman, D. & Boulnois, G. J. (1991) Infect. Immun. 59, 2297-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiMango, E., Ratner, A. J., Bryan, R., Tabibi, S. & Prince, A. (1998) J. Clin. Invest. 101, 2598-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang, X., Moser, C., Louboutin, J. P., Lysenko, E. S., Weiner, D. J., Weiser, J. N. & Wilson, J. M. (2002) J. Immunol. 168, 810-815. [DOI] [PubMed] [Google Scholar]
- 18.Malley, R., Henneke, P., Morse, S. C., Cieslewicz, M. J., Lipsitch, M., Thompson, C. M., Kurt-Jones, E., Paton, J. C., Wessels, M. R. & Golenbock, D. T. (2003) Proc. Natl. Acad. Sci. USA 100, 1966-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirschning, C. J., Wesche, H., Merrill Ayres, T. & Rothe, M. (1998) J. Exp. Med. 188, 2091-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nonnenmacher, C., Dalpke, A., Zimmermann, S., Flores-De-Jacoby, L., Mutters, R. & Heeg, K. (2003) Infect. Immun. 71, 850-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang, B., Cleary, P. P., Xu, H. & Li, J. D. (2003) Infect. Immun. 71, 5523-5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang, B., Lim, D. J., Han, J., Kim, Y. S., Basbaum, C. B. & Li, J. D. (2002) J. Biol. Chem. 277, 949-957. [DOI] [PubMed] [Google Scholar]
- 23.Gewirtz, A. T., Navas, T. A., Lyons, S., Godowski, P. J. & Madara, J. L. (2001) J. Immunol. 167, 1882-1885. [DOI] [PubMed] [Google Scholar]
- 24.Dagan, R., Muallem, M., Melamed, R., Leroy, O. & Yagupsky, P. (1997) Pediatr. Infect. Dis. J. 16, 1060-1064. [DOI] [PubMed] [Google Scholar]
- 25.Murphy, T. V., Pastor, P., Medley, F., Osterholm, M. T. & Granoff, D. M. (1993) J. Pediatr. (Berlin) 122, 517-523. [DOI] [PubMed] [Google Scholar]
- 26.Kunkel, S. L., Standiford, T., Kasahara, K. & Strieter, R. M. (1991) Exp. Lung Res. 17, 17-23. [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann, A., Levchenko, A., Scott, M. L. & Baltimore, D. (2002) Science 298, 1241-1245. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann, E., Dittrich-Breiholz, O., Holtmann, H. & Kracht, M. (2002) J. Leukocyte Biol. 72, 847-855. [PubMed] [Google Scholar]
- 29.Schroder, N. W., Morath, S., Alexander, C., Hamann, L., Hartung, T., Zahringer, U., Gobel, U. B., Weber, J. R. & Schumann, R. R. (2003) J. Biol. Chem. 278, 15587-15594. [DOI] [PubMed] [Google Scholar]
- 30.Yoshimura, A., Lien, E., Ingalls, R. R., Tuomanen, E., Dziarski, R. & Golenbock, D. (1999) J. Immunol. 163, 1-5. [PubMed] [Google Scholar]
- 31.Lazou Ahren, I., Bjartell, A., Egesten, A. & Riesbeck, K. (2001) J. Infect. Dis. 184, 926-930. [DOI] [PubMed] [Google Scholar]
- 32.Galdiero, M., Finamore, E., Rossano, F., Gambuzza, M., Catania, M. R., Teti, G., Midiri, A. & Mancuso, G. (2004) Infect. Immun. 72, 1204-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shuto, T., Xu, H., Wang, B., Han, J., Kai, H., Gu, X. X., Murphy, T. F., Lim, D. J. & Li, J. D. (2001) Proc. Natl. Acad. Sci. USA 98, 8774-8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koedel, U., Angele, B., Rupprecht, T., Wagner, H., Roggenkamp, A., Pfister, H. W. & Kirschning, C. J. (2003) J. Immunol. 170, 438-444. [DOI] [PubMed] [Google Scholar]
- 35.Knapp, S., Wieland, C. W., van 't Veer, C., Takeuchi, O., Akira, S., Florquin, S. & van der Poll, T. (2004) J. Immunol. 172, 3132-3138. [DOI] [PubMed] [Google Scholar]
- 36.Picard, C., Puel, A., Bonnet, M., Ku, C. L., Bustamante, J., Yang, K., Soudais, C., Dupuis, S., Feinberg, J., Fieschi, C., et al. (2003) Science 299, 2076-2079. [DOI] [PubMed] [Google Scholar]
- 37.Medvedev, A. E., Lentschat, A., Kuhns, D. B., Blanco, J. C., Salkowski, C., Zhang, S., Arditi, M., Gallin, J. I. & Vogel, S. N. (2003) J. Exp. Med. 198, 521-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchell, T. J. (2004) in The Pneumococcus, ed. Tuomanen, E. I. (Am. Soc. Microbiol., Washington, DC), pp. 61-74.
- 39.Feldman, C., Munro, N. C., Jeffery, P. K., Mitchell, T. J., Andrew, P. W., Boulnois, G. J., Guerreiro, D., Rohde, J. A., Todd, H. C., Cole, P. J., et al. (1991) Am. J. Respir. Cell Mol. Biol. 5, 416-423. [DOI] [PubMed] [Google Scholar]
- 40.Leake, E. R., Holmes, K., Lim, D. J. & DeMaria, T. F. (1994) J. Infect. Dis. 170, 1532-1538. [DOI] [PubMed] [Google Scholar]
- 41.Burroughs, M., Prasad, S., Cabellos, C., Mendelman, P. M. & Tuomanen, E. (1993) J. Infect. Dis. 167, 464-468. [DOI] [PubMed] [Google Scholar]
- 42.Girardin, S. E., Boneca, I. G., Carneiro, L. A., Antignac, A., Jehanno, M., Viala, J., Tedin, K., Taha, M. K., Labigne, A., Zahringer, U., et al. (2003) Science 300, 1584-1587. [DOI] [PubMed] [Google Scholar]
- 43.Viala, J., Chaput, C., Boneca, I. G., Cardona, A., Girardin, S. E., Moran, A. P., Athman, R., Memet, S., Huerre, M. R., Coyle, A. J., et al. (2004) Nat. Immunol. 5, 1166-1174. [DOI] [PubMed] [Google Scholar]
- 44.Madden, J. C., Ruiz, N. & Caparon, M. (2001) Cell 104, 143-152. [DOI] [PubMed] [Google Scholar]