Abstract
AK032317 is the GenBank accession no. of a full-length RIKEN mouse cDNA. It encodes a putative variant of the C3-type TRPC (transient receptor potential channel) that differs from the previously cloned murine TRPC3 cDNA in that it has a 5′ extension stemming from inclusion of an additional exon (exon 0). The extended cDNA adds 62 aa to the sequence of the murine TRPC3. Here, we report the cloning of a cDNA encoding the human homologue of this extended TRPC3 having a highly homologous 73-aa N-terminal extension, referred to as hTRPC3a. A query of the GenBank genomic database predicts the existence of a similar gene product also in rats. Transient expression of the longer TRPC3a in human embryonic kidney (HEK) cells showed that it mediates Ca2+ entry in response to stimulation of the Gq–phospholipase C β pathway, which is similar to that mediated by the shorter hTRPC3. However, after isolation of HEK cells expressing hTRPC3 in stable form, TRPC3a gave rise to Ca2+-entry channels that are not only activated by the Gq–phospholipase C β pathway (receptor-activated Ca entry) but also by thapsigargin triggered store depletion. In conjunction with findings from our and other laboratories that TRPC1, TRPC2, TRPC4, TRPC5, and TRPC7, can each mediate store-depletion-activated Ca2+ entry in mammalian cells, our findings with hTRC3a support our previous proposal that TRPCs form capacitative Ca-entry channels.
Keywords: capacitative Ca entry, cation channel, store-operated Ca entry
TRPCs (transient receptor potential channels) are the founding members of the superfamily of TRP channels. Other subfamilies include the TRPV (TRP, vanilloid subfamily) and TRPM (TRP, melastatin subfamily) channels (1). TRPs encode cation channels with widely varying cation selectively and widely differing mechanisms of activation. TRPCs have been expressed in reporter cells, such as HEK 293 and CHO cells, and found to be activated by protocols that lead to activation of the Gq–phospholipase C (PLC) signaling pathway that leads to formation of inositol 1,4,5-trisphosphate (IP3) and diacylglycerol. Current evidence supports the hypothesis that activated IP3 receptors both release Ca2+ from intracellular stores by virtue of their function as Ca2+ release channels and activate TRPC channels at the plasma membrane by virtue of a TRPC-activating function. This latter function requires protein–protein interaction between IP3 receptors and TRPCs, which has been shown to occur (2).
In addition to agonist-activated Ca2+ influx that accompanies the IP3 receptor-mediated cascade described above, mammalian cells exhibit the phenomenon of capacitative Ca2+ entry (CCE), also store-depletion-activated Ca entry, and TRPCs have been proposed to be the molecular units that form CCE channels (2, 3).
It has been a matter of discussion whether some, all, or none of the TRPC class of ion channels form store-operated CCE channels. CCE channels are activated without coactivation of PLC and formation of IP3 and diacylglycerol. The reason for the uncertainty lies in the fact that, for the most part, TRPCs overexpressed in reporter cells are not activated by mere store depletion, such as elicited by treating cells with the intracellular Ca2+ pump-inhibitor thapsigargin. Yet, there are several reports in support of the hypothesis that TRPCs form CCE channels. Zitt et al. (4) and Liu et al. (5) reported augmented thapsigargin-induced activity in cells transfected with TRPC1. TRPC2 was activated by thapsigargin in transiently transfected human embryonic kidney (HEK) cells (6), and sperm cell thapsigargin-activated Ca2+ influx was inactivated by a TRPC2-selective antibody (7). Philipp et al. (8) and Freichel et al. (9) reported store-depletion-induced activation of TRPC4, and Philipp et al. (10) were able to activate TRPC5 in transfected cells. Riccio et al. (11) and, more recently, Livremont et al. (12) have reported activation by store depletion of TRPC7 expressed in cultured cells. In contrast, studying TRPC3, Schultz and coworkers (13), Kamouchi et al. (14), and we (unpublished data) have been unable to activate this channel by inhibition of endogenous sarcoplasmic-endoplasmic reticulum Ca2+ pumps with thapsigargin without concurrent activation of PLC or addition of IP3.
Recent studies suggest that among the variables affecting the characteristics of TRP-type ion channels is the level at which TRPC-type channels are expressed. This was alluded to in a report by Yue et al. (15), who invoked current density as a factor relevant to the permeation properties of TRPV6, and it was clearly shown to be an important variable by Schindl et al. (16). The possibility that expression level may be an important variable in need of control was further emphasized by Vazquez et al. (17) working with chicken DT40 cells. These authors observed differing susceptibility to regulation by store depletion depending on the “strength” of the promoter used to express TRPC3. TRPC3 expressed under control of a weak promoter in the avian cells was activated by store depletion, whereas expression under control of a strong chicken actin promoter, led to TRPC3 channels insensitive to thapsigargin. Their work raised the possibility that “store-depletion-insensitive” TRPC3 channels may nevertheless be able to form store-depletion-activated channels also in mammalian cells if expression levels were controlled. In agreement with this hypothesis, Schulz and coworkers reported in a short communication that a rat TRPC3 cDNA generated thapsigargin-activated Ca2+ entry but only if tested at early times after transfection (18).
Studies on the mechanism of TRPC channels activation are complex, not only because of the nature of the putative regulatory mechanisms that may be involved but also because of existence of splice variants. Splice variants have been described for TRPC1 (19), TRPC2 (6, 20), TRPC4 (21), TRPC5 (10), and TRPC3 (22), for which a shorter version in the rat (TRPC3sv), that lacks nucleotides that would code for two of the three N-terminal ankyrin repeats. TRPC3sv was shown to be a Ca2+-activated nonselective cation (CAN) channel that could also be activated by depolarization and ionomycin but was insensitive to thasigargin (22). In this context, it was of interest that the RIKEN genome sequencing consortium deposited into the GenBank database what appeared to be an additional TRPC3 splice variant (GenBank accession no. AK032317) cloned from a mouse olfactory-bulb cDNA library. This cDNA predicts an N terminally extended form of the channel for which no functional studies have been reported. Below, we report that upon stable expression of the human homologue of this splice variant, which we call TRPC3a, it behaves as a store-operated Ca2+-entry channel.
Materials and Methods
Fura-2–acetoxymethyl ester and thapsigargin were obtained from Molecular Probes; arginine vasopressin was obtained from Sigma–Aldrich, and tissue culture reagents were obtained from GIBCO. All other reagents were obtained from standard sources, and they were of the highest available chemical grade.
Recombinant DNA Techniques. Standard procedures as described in Sambrook et al. (23) were followed to manipulate plasmid DNAs and PCR products. The human homologue of the mouse TRPC3 cDNA (GenBank accession no. AK032317) was cloned by using standard PCR techniques and primers designed on the basis of the mRNA predicted from analyzing of the genomic human sequence of TRPC3a (GenBank accession no. AC108930). PCR products were cloned into pCR2.1-TOPO (Invitrogen) and assembled to yield the full-length cDNA, which was transferred into pCDNA3 (Invitrogen) downstream of the CMV promotor and pKNH downstream of the SV40 replication origin/promoter/enhancer (24, 25).
Tissue Culture. Standard tissue-culture procedures and reagents (GIBCO) were used throughout. HEK 293 cells were grown in DMEM with 1% l-glutamine, 10% heat-inactivated FCS, and 1% each of penicillin and streptomycin (GIBCO). Transfections to express TRPC cDNAs in transient or stable fashion were performed as described (26, 27) by using 60-mm dishes with HEK 293 cells that had been freshly plated at a density of 1 × 106 cells per plate at 24 h before transfection and Lipofectamine 2000 (Invitrogen) as the DNA carrier. For transient expressions, the cDNAs were under the control of the CMV promoter of pCDNA3 and were cotransfected with 1/10th the amount of the V1a vasopressin receptor cDNA (28) also in pCDNA3. Total DNA in the transfection mixture added to 60-mm dishes was 5–7 μg. HEK cell lines expressing human TRPC3a in stable form were obtained by transfecting the cDNA cloned into either pCDNA3 downstream of the CMV promoter (Invitrogen) or pKNH downstream of the SV40 promoter/enhancer (24, 25). Both vectors carry the neomycin-resistance marker. Cells that had incorporated the plasmid DNA in stable form were selected by addition of G418 at 600 μg of active principle per ml (pCDNA3) or 400 μg of active principle per ml (pKNH) in DMEM. Clonal cell lines were derived from initial sets of G418-resistant colonies by limiting dilution into the wells of 96-well plates, and they are referred to as t3a cells. The t3a-12 clonal cell lines were obtained by transfecting TRPC3a under the control of the CMV promoter of pCDNA3; and the t3a-10.5 clonal cell line was obtained by transfecting TRPC3a under the control of the SV40 early promoter/enhancer of pKNH.
Quantitative Ratiometric Fluorescence Imaging of Cytosolic Ca ([Ca2+]i) Using Fura-2. A Nikon TS-100 fitted with an InCyt Im2 imaging system (Intracellular Imaging, Cincinnati) was used to follow changes in intracellular Ca. Cells were plated onto 40 × 40-mm polylysine-coated cover glasses that were placed in a Teflon holding frame to form the bottom of an incubation chamber to which solutions were added with the additions shown in the figures. Cells to be tested for changes in intracellular Ca2+ ([Ca2+]i) were plated onto the coverslips the day before the experiments at a density of ≈300,000. Immediately before the experiments, the cells were loaded with the fluorescent Ca2+ indicator dye Fura-2 by incubation with 2 μM Fura-2–acetoxymethyl ester in culture medium for 20 min at 37°C in a 5% CO2 incubator. The cells were then washed twice with HPSS buffer (116 mM NaCl/5.4 mM KCl/1.13 mM MgCl2/10 mM glucose/20 mM Hepes, pH 7.4) containing 2 mM CaCl2 and allowed to equilibrate at room temperature for 10 min before the beginning of the imaging experiments (shown in Figs. 1, 2, 3, 4, 5). Individual cells were chosen for monitoring fluorescence changes by using a ×20 Plan Fluor objective (numerical aperture, 0.5; working distance, 2.1 mm). The software for data acquisition and analysis of dual wavelength fluorescence intensities was obtained from Intracellular Imaging. On average, a 40 × 40-mm cover glass provided 15–20 cells for a single imaging experiment. Therefore, 80 cells denote the analysis of cells from at least four cover glasses. Data for the experiments shown on each figure were obtained on a single day. Further details on data processing were as described in Zhu et al. (26) and Boulay et al. (2).
Fig. 1.
TRPC3a amino acid sequence alignments. The human TRPC3a splice variant (t3_hum), cloned in the present work, the TRPC3a amino acid sequence encoded in the mouse cDNA (t3_mus; GenBank accession no. AK032317) and the amino acid sequence deduced from a predicted rat TRPC3a cDNA (t3_rat) are compared. The rat TRPC3a cDNA was predicted by blast analysis of the mouse nucleotide sequence (GenBank accession no. AK032317) against the rat genomic sequence. This search returned predicted exon sequences that, upon manual refinement, led to the in silico construction of the rat intron–exon structure, with the corresponding TRPC3a coding sequence that predicts amino acid sequence shown above as t3_rat. The alignments compare the rat and mouse amino acid sequences to that of the human. –, Amino acid identical to that of the human sequence differences are identified;., gap; |, intron–exon boundaries; *, stop. N-terminal exon 0 sequences are shown in bold at the beginning of the alignments. The number of amino acids encoded in the three ORFs are listed at the end the sequences, which are the number of amino acids encoded in the human TRPC3 (GenBank accession no. U4750), mouse TRPC3 (GenBank accession no. AF190645), and rat TRPC3 (GenBank accession no. AB022331) cDNAs.
Fig. 2.
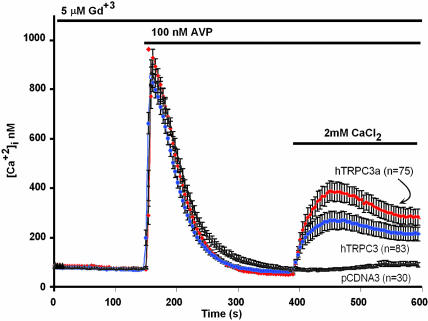
Transiently expressed hTRPC3a mediates agonist-activated Ca2+ entry. HEK cells were cotransfected with the vasopressin 1a receptor (V1aR) and pCDNA3 carrying or not the indicated cDNAs. TRPC3 (26) was expressed as a point of reference. To suppress Ca2+ entry through endogenous Ca2+-entry channels, 5 μM GdCl3 was present throughout. Error bars indicate SEM of [Ca2+]i averaged over the indicated number of cells.
Fig. 3.
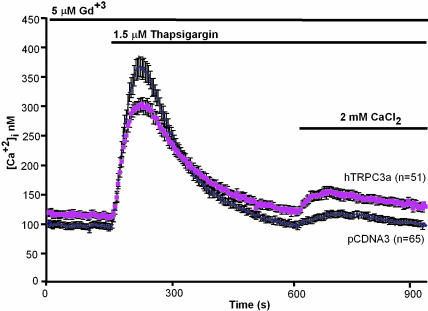
Transiently expressed hTRPC3a is activated poorly, if at all, by thapsigargin-induced store depletion. HEK cells were transfected with pCDNA3 carrying or not the TRPC3a cDNA and tested for thapsigargin-induced Ca2+ entry. Error bars indicate SEM of [Ca2+]i averaged over the indicated number of cells.
Fig. 4.
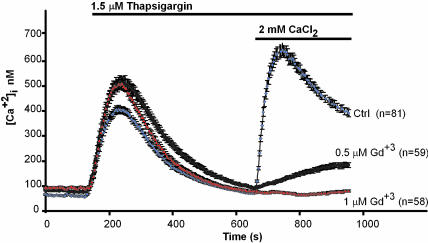
At 1 μM, Gd3+ fully inhibits agonist-activated Ca2+ entry in HEK cells. HEK cells were tested for thapsigargin-activated Ca2+ entry in the presence of the indicated concentrations of GdCl3. Error bars indicate SEM of [Ca2+]i averaged over the indicated number of cells.
Fig. 5.
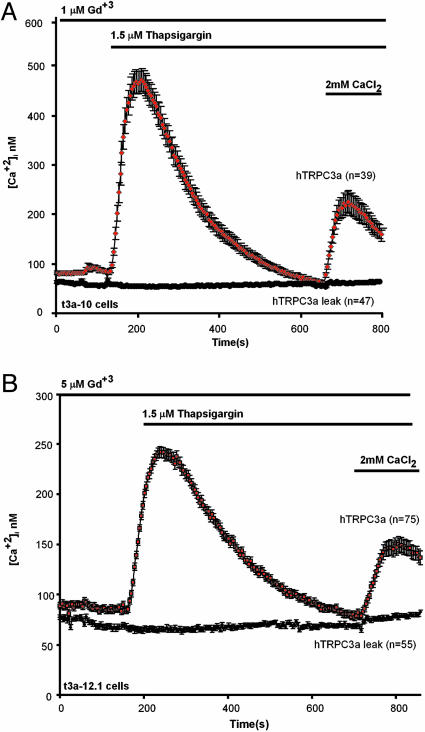
Response to store depletion of clonal cell HEK lines expressing TRPC3a in stable form. HEK t3a-10 cells expressing TRPC3 under the control of the SV40 promoter enhancer of pKNH were plated onto 4 × 4-cm cover glasses, mounted for videomicroscopy imaging, loaded with Fura-2, and tested for their response to store depletion, as described in Materials and Methods;1(A) or 5 (B) μM Gd3+ were present throughout for t3a-10 and t3a-12.1 cells, respectively. As controls, t3a cells were treated the same way, except thapsigargin was omitted. The difference highlights that TRPC3a is capable of forming store-depletion-activated Ca2+-entry channels. Error bars indicate SEM of [Ca2+]i averaged over the indicated number of cells.
Informatics. Nucleotide and amino acid sequence analysis and similarity searches were performed either with the gcg wisconsin package (version 10.3; Accelerys, San Diego) or software provided on the web site of the National Center for Biotechnology Information (http://ncbi.nlm.nih.gov; National Library of Medicine, National Institutes of Health).
Results
The Mouse, Human, and Rat Genomes Encode an Additional Exon Encoding an N-Terminal Extension of TRPC3. Translation of the ORF encoded in the nucleotide sequence of a full-length cDNA cloned by the RIKEN Fantom consortium (GenBank accession no. AK032317), yields a polypeptide of 921 aa, which is 73 aa longer than the murine TRPC3 (GenBank accession no. AF190645). The nucleotides encoding the additional amino acids preceded those encoding the known TRPC3 ORF. A search for the location of these nucleotides in the mouse genome showed them to be located, as a single block, 18,196 nt 5′ of the TRPC3 initiator ATG codon (GenBank accession no. NW_000184). We refer to this nucleotide set as exon 0 of TRPC3, and we refer to the extended version of TRPC3 as TRPC3a. A search of the human genome for the existence of a homologous exon 0 returned a highly similar block of nucleotides located 18,428 nt 5′ to the human TRPC3 initiator ATG (GenBank accession no. NT_0165354). By using primers based on the nucleotide sequence of the predicted human exon 0, we amplified and assembled a cDNA encoding the human homologue of the mouse TRPC3a, hTRPC3a. A query of the rat genome revealed the existence of a highly homologous rat TRPC3a sequence, with conserved intron–exon boundaries (GenBank accession no. NW_047625). The predicted amino acid sequences of human, mouse, and rat TRPC3a are shown and compared in Fig. 1, which also highlights the intron–exon boundaries. The human, mouse, and rat TRPC3a ORFs encode proteins of 921, 911, and 910 aa, respectively, compared with 848, 836, and 835 aa for the shorter TRPC3 versions. Rat and mouse TRPC3a coding sequences differ in 15 of 911 positions (98% identical), whereas human and mouse differ by 56 of 921 positions, including 10 gaps (93% identical).
Expression of hTRPC3a Reveals a Ca2+ Entry Function Similar to That of hTRPC3. We subjected cells transiently transfected with TRPC3a to the standard test for mediation of agonist-activated Ca2+ entry. In this test, Fura-2-loaded cells are first challenged with agonist in the absence of external Ca2+, allowed to extrude the IP3-stimulated Ca2+ released from internal stores into the external medium, and then exposed to external Ca2+ to reveal activation (or not) of a Ca2+-entry path. As shown in Fig. 2, TRPC3a resembles TRPC3 in that upon transient expression in a mammalian cell it forms classical TRPC-type Ca2+-entry channels.
Transiently Expressed hTRPC3 Shows Little if Any Responsiveness to Thapsigargin-Induced Store Depletion. Next, we tested whether transiently expressed TRPC3a could be activated by store depletion in the absence of concomitant activation of the PLC–IP3–IP3 receptor pathway. Fig. 3 shows the most optimistic response that we obtained in several tests. As is the case with TRPC3, TRPC3a also shows a degree of spontaneous activity, which can be revealed as “leak” influx in cells subjected to the media exchanges intrinsic to the test protocol, but omitting thapsigargin. Overall, when averaged over several experiments, the small influx of Ca2+ observed after readdition of Ca2+ to the external medium was not significantly different from leak entry.
Clonal HEK 293 Cell Lines That Stably Express TRPC3a Reveal an Intrinsic Responsiveness to Store Depletion. Given the recent findings with avian DT40 cells (17) showing that responsiveness to store depletion may be interfered with by channel overexpression, we next sought to isolate cells with varying TRPC3a expression levels by taking advantage of the phenomenon that upon clonal isolation of cells expressing cDNAs in stable manner, the isolated cell clones express the cDNA inserts at randomly varying levels (28). Thus, two transfections were carried out, followed by selection of clonal cell lines. In the first, we transfected the TRPC3a cDNA cloned into pCDNA3, where it would be expressed under the control of the CMV promoter. We obtained one colony, t3a-12, from which several clonal cell lines (t3a-12.1 to t3a-12.7) that all responded to thapsigargin with a Gd+3-resistant CCE. In the second transfection, the TRPC3a cDNA was carried by a different expression plasmid, pKNH. In pKNH, the inserted cDNA is under the control of the weaker (compared with CMV) SV40 early promoter, which normally controls the expression of the T antigen and includes an enhancer element and the SV40 origin of replication. This expression plasmid was developed in the laboratory of S. Numa (University of Kyoto, Kyoto) in Japan in the late 1980s (25). An initial screen of 33 independent clonal cell lines, carried out as before in the presence of 5 μM Gd3+ to suppress endogenous CCE, yielded no clones exhibiting a Gd3+-resistant CCE. We reasoned that if indeed we were mimicking a true CCE channel, it might also be more sensitive to inhibition by Gd3+ than expected from the resistance of overexpressed TRPC3a. A test of the sensitivity of endogenous Ca2+ influx channels showed that 1 μM of the lanthanide would suffice to screen out the endogenous Ca2+ influx activities from interfering with the test for TRTPC3a ability to mediate CCE (Fig. 4). Thus, the 33 clonal cell lines that had stably integrated the TRPC3a cDNA inserted into pKNH were retested in the presence of 1 μM, instead of 5 μM, Gd3+. We obtained one cell clone, t3a-10, that showed robust Gd3+-resistant thapsigargin-induced CCE and several other cell clones that showed a weaker Gd3+-resistant CCE. Fig. 5 shows thapsigargin-stimulated Ca2+ entry into HEK cells stably expressing TRPC3a under the SV40 (t3a-10 cells; A) or CMV (t3a-12.1 cells; B) promoter.
Discussion
The experiments described above, in which the concentration of Gd3+ had to be reduced to uncover the responsiveness of TRPC3a to store-depletion signal, highlight an intrinsic difficulty in research designed to unravel the molecular makeup of CCE channels. CCE channels are ubiquitous and present in all cell types studied so far, whether they are excitable (such as muscle and neuronal cells), nonexcitable (such as hepatocytes, endothelial, or epithelial cells), or dedifferentiated fibroblasts kept in culture for decades (such as mouse tk-L cells) (29). Many of these cells, including HEK 293 cells, are positive for TRPC3 mRNA, as assessed by RT-PCR (ref. 30 and E.Y. and L.B., unpublished data), and presumably also positive for TRPC3 protein and TRPC3 channel activity. However, all of the endogenous capacitative and agonist-activated Ca2+-entry channels are highly sensitive to Gd3+. Thus, we reason that, the closer artificially expressed channel molecules mimic the natural channels, the more sensitive they are likely to be to inhibition by Gd3+, which is the putative “screen” used to eliminate interference by endogenous channels and facilitate visualization of the expressed cDNAs. To avoid this catch-22 situation, studies should be done without the use of the Gd3+ visualization aid.
In conclusion, the demonstration that TRPCs can be activated by store depletion, as shown in the present report for TRPC3a, and in other reports for TRPC1, TRPC2, TRPC4 and TRPC5 (vide supra), clearly indicates that TRPCs have the intrinsic characteristics that make them prime candidates for forming CCE channels. However, true CCE channels, which in addition to being activated by store depletion also exhibit the highly Ca2+ selective permeation characteristics, have yet to be recapitulated in in vitro transfection experiments. One reason for this failure, set forth immediately after discovering the existence of multiple mammalian TRPCs (3), may be that CCE channels are heteromultimers, and instead of expressing single TRPCs, it is necessary to express the correct combination of TRPCs. Another reason may be whatever lies at the core of the different properties of TRPC channels expressed at differing levels. One possibility is that CCE channel activity requires a limiting cofactor and that overexpression would leave TRPCs wanting for this factor. Whatever this factor may be, if it exists, would have to form stable complexes with the endogenous channels, because factor depletion would otherwise be predicted to render the endogenous channels Gd3+-resistant. Another possibility is that the difference between overexpressed and endogenous channels depends on the intracellular location of the protein, such that endogenous channels and overexpressed channels do not mix. The recent report by Singh et al. (31) that TRPC3 interacts with proteins from the exocytotic machinery, VAMP2 and αSNAP, and the fact that stimulation of the cellular Gq–PLC–IP3 pathway is associated with an increase of transfected TRPC3 molecules on the cell surface lends support to the idea that subcellular compartmentalization of CCE channels is a relevant variable to consider. In this context, if overexpressed TRPC channels and CCE channels distribute in different compartments, they could also have distinct properties. Studies are needed to address these questions.
Acknowledgments
We thank Joel Abramowitz for advice and careful reading of the manuscript and Will Williams for invaluable help with its electronic submission. This research was carried out in partial fulfillment of the requirements for a Ph.D. degree for E.Y. in Molecular, Cell, and Developmental Biology (College of Letters and Science, University of California, Los Angeles).
Author contributions: E.Y. and L.B. designed research; E.Y. and B.T.K. performed research; E.Y., B.T.K., and L.B. analyzed data; E.Y. and L.B. wrote the paper.
Abbreviations: HEK, human embryonic kidney; CCE, capacitative Ca2+ entry; IP3, inositol 1,4,5-trisphosphate; PLC, phospholipase C.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AY865574, for the human TRPC3a clone).
References
- 1.Montell, C., Birnbaumer, L. & Flockerzi, V. (2002) Cell 108, 595-598. [DOI] [PubMed] [Google Scholar]
- 2.Boulay, G., Brown, D. M., Qin, N., Jiang, M., Dietrich, A., Zhu, M. X., Chen, Z., Birnbaumer, M., Mikoshiba, K. & Birnbaumer, L. (1999) Proc. Natl. Acad. Sci. USA 96, 14955-14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birnbaumer, L., Zhu, X., Jiang, M., Boulay, G., Peyton, M., Vannier, B., Brown, D., Platano, D., Sadeghi, H., Stefani, E. & Birnbaumer, M. (1996) Proc. Natl. Acad. Sci. USA 93, 15195-15202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zitt, C., Zobel, A., Obukhov, H., Kalkbrenner, F., Lückhoff, A. & Schultz, G. (1996) Neuron 16, 1189-1196. [DOI] [PubMed] [Google Scholar]
- 5.Liu, X., Wang, W., Singh, B. B., Lockwich, T., Jadlowiec, J., O'Connell, B., Wellner, R., Zhu, M. X. & Ambudkar, I. S. (2000) J. Biol. Chem. 275, 3403-3411. [DOI] [PubMed] [Google Scholar]
- 6.Vannier, B., Peyton, M., Boulay, G., Brown, D., Qin, N., Jiang, M., Zhu, X. & Birnbaumer, L. (1999) Proc. Natl. Acad. Sci. USA 96, 2060-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jungnickel, M. K., Marrero, H., Birnbaumer, L., Lémos, J. R. & Florman, H. M. (2000) Nat. Cell Biol. 3, 499-502. [DOI] [PubMed] [Google Scholar]
- 8.Philipp, S., Cavalie, A., Freichel, M., Wissenbach, U., Zimmer, S., Trost, C., Marquart, A., Murakami, M. & Flockerzi, V. (1996) EMBO J. 15, 6166-6171. [PMC free article] [PubMed] [Google Scholar]
- 9.Freichel, M., Suh, S. H., Pfeifer, A., Schweig, U., Trost, C. P., Weissgerber, P., Biel, M., Philipp, S., Freise, D., Droogmans, G., et al. (2001) Nat. Cell Biol. 3, 121-127. [DOI] [PubMed] [Google Scholar]
- 10.Philipp, S., Hambrecht, J., Braslavski, L., Schroth, G., Freichel, M., Murakami, M., Cavalie, A. & Flockerzi, V. (1998) EMBO J. 17, 4274-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riccio, A., Mattei, C., Kelsell, R. E., Medhurst, A. D., Calver, A. R., Randall, A. D., Davis, J. B., Benham, C. D. & Pangalos, M. N. (2002) J. Biol. Chem. 277, 12302-12309. [DOI] [PubMed] [Google Scholar]
- 12.Lievremont, J. P., Bird, G. S. & Putney, J. W., Jr., (2004) Am. J. Physiol. 287, C1709-C1716. [DOI] [PubMed] [Google Scholar]
- 13.Zitt, C., Obukhov, A. G., Strubing, C., Zobel, A., Kalkbrenner, F., Lückhoff, A. & Schultz, G. (1997) J. Cell. Biol. 138, 1333-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamouchi, M., Philipp, S., Flockerzi, V., Wissenbach, U., Mamin, A., Raeymaekers, L., Eggermont, J., Droogmans, G. & Nilius, B. (1999) J. Physiol. 2, 345-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yue, L., Peng, J. B., Hediger, M. A. & Clapham, D. E. (2001) Nature 410, 705-709. [DOI] [PubMed] [Google Scholar]
- 16.Schindl, R., Kahr, H., Graz, I., Groschner, K. & Romanin, C. (2002) J. Biol. Chem. 277, 26950-26958. [DOI] [PubMed] [Google Scholar]
- 17.Vazquez, G., Wedel, B. J., Trebak, M., Bird, G. S. & Putney, J. W, Jr., (2003) J. Biol. Chem. 278, 21649-21654. [DOI] [PubMed] [Google Scholar]
- 18.Preuss, K. D., Noeller, J. K., Krause, E., Goebel, A. & Schulz, I. (1997) Biochem. Biophys. Res. Commun. 240, 167-170. [DOI] [PubMed] [Google Scholar]
- 19.Zhu, X., Chu, P. B., Peyton, M. & Birnbaumer, L. (1995) FEBS Lett. 373, 193-198. [DOI] [PubMed] [Google Scholar]
- 20.Yildirim, E., Dietrich, A. & Birnbaumer, L. (2003) Proc. Natl. Acad. Sci. USA 100, 2220-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mery, L., Magnino, F., Schmidt, K., Krause, K. H. & Dufour, J. F. (2001) FEBS Lett. 487, 377-383. [DOI] [PubMed] [Google Scholar]
- 22.Ohki, G., Miyoshi, T., Murata, M., Ishibashi, K., Imai, M. & Suzuki, M. (2000) J. Biol. Chem. 275, 39055-39060. [DOI] [PubMed] [Google Scholar]
- 23.Sambrook, J., Fritsch, E. F. & Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Lab. Press, Woodbury, NY), 2nd Ed.
- 24.Takeshima, H., Nishimura, S., Matsumoto, T., Ishida, H., Kangawa, K., Minamino, N., Matsuo, H., Ueda, M., Hanaoka, M., Hirose, T. & Numa, S. (1989) Nature 339, 439-445. [DOI] [PubMed] [Google Scholar]
- 25.Fukuda, K., Higashida, H., Kubo, T., Maeda, A., Akiba, I., Bujo, H., Mishina, M. & Numa, S. (1988) Nature 335, 355-358. [DOI] [PubMed] [Google Scholar]
- 26.Zhu, X., Jiang, M., Peyton, M. J., Boulay, G., Hurst, R., Stefani., E. & Birnbaumer, L. (1996) Cell 85, 661-671. [DOI] [PubMed] [Google Scholar]
- 27.Zhu, X., Jiang, M. & Birnbaumer, L. (1998) J. Biol. Chem. 273, 133-142. [DOI] [PubMed] [Google Scholar]
- 28.Innamorati, G., Lolait, S. J. & Birnbaumer, M. (1996) Biochem. J. 314, 710-711. [PMC free article] [PubMed] [Google Scholar]
- 28.Levy, F. O., Zhu, X., Kaumann, A. J. & Birnbaumer, L. (1993) Proc. Natl. Acad. Sci. USA 90, 10798-10802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao, C. F., Schilling, W. P., Birnbaumer, M. & Birnbaumer, L. (1990) J. Biol. Chem. 265, 11273-11284. [PubMed] [Google Scholar]
- 30.Riccio, A., Medhurst, A. D., Mattei, C., Kelsell, R. E., Calver, A. R., Randall, A. D., Benham, C. D. & Pangalos, M. N. (2002b) Brain Res. Mol. Brain Res. 109, 95-104. [DOI] [PubMed] [Google Scholar]
- 31.Singh, B. B., Lockwich, T. P., Bandyopadhyay, B. C., Liu, X., Bollimuntha, S., Brazer, S. C., Combs, C., Das, S., Leenders, A. G., Sheng, Z. H., et al. (2004) Mol. Cell 15, 635-646. [DOI] [PubMed] [Google Scholar]