Abstract
Prostaglandin D2 (PGD2) released by degranulating mast cells is believed to play a key role in orchestrating mechanisms of inflammation in allergies and asthma. The biological effects of PGD2 are mediated by D-prostanoid (DP1), CRTH2 (DP2), and thromboxane prostanoid (TP) receptors. The CRTH2 receptor is involved in induction of migration and activation of T helper type 2 (Th2) lymphocytes, eosinophils, and basophils; up-regulation of adhesion molecules; and promotion of pro-inflammatory Th2-type cytokines (interleukin [IL]-4, 5, 13), whereas the DP receptor is associated with relaxation of smooth muscles, vasodilation, inhibition of cell migration, and apoptosis of eosinophils. A number of CRTH2/PGD2 receptor antagonists have been investigated in asthma and allergic diseases. The CRTH2 antagonist (OC000459) or dual CRTH2 and TP receptor antagonist (ramatroban) were effective in reducing eosinophilia, nasal mucosal swelling, and clinical symptoms of allergic rhinitis, with the latter drug registered for clinical use in this indication. OC000459 and setipiprant reduced the late but not early phase of response in an allergen challenge in atopic asthmatics. In persistent asthma, some molecules induced limited improvement in lung function, quality of life, and asthma symptoms (OC000459, BI671800), but in other trials with AMG 853 and AZ1981 these findings were not confirmed. The clear discrepancy between animal studies and clinical efficacy of CRTH2 antagonism in allergic rhinitis, and lack of efficacy in a general cohort of asthmatics, highlight the issue of patient phenotyping. There is no doubt that the PGD2/CATH2/DP1 pathway plays a key role in allergic inflammation and further studies with selective or combined antagonisms in well defined cohorts of patients are needed.
Key Points
| Several in vitro and in vivo studies in animal models of allergic inflammation confirmed the pivotal role of prostaglandin D2 (PGD2) and signaling via CRTH2 and D-prostanoid (DP) receptors, suggesting a possible role of the antagonism of those receptors in the management of allergic diseases in humans. |
| A number of CRTH2 and/or PGD2 receptor antagonists, including CRTH2 antagonist (OC000459), dual CRTH2 and thromboxane prostanoid receptor antagonist (ramatroban, BI671800), AMG 853, and AZ1981, have been investigated in asthma and allergic diseases. |
| The PGD2/CRTH2/DP1 pathway plays a key role in allergic inflammation and further studies with selective or combined antagonisms in well defined cohorts of patients are needed. |
Introduction
Several biologically active lipid mediators derived from arachidonic acid, including prostaglandins synthesized along the cyclooxygenase (COX) pathways, play a key role in orchestrating mechanisms of inflammation in allergies and asthma. Two functional COX isoforms have been identified: COX 1, which is constitutively expressed in most tissues and involved in physiological regulation of homeostatic function, and COX 2, the inducible form upregulated in inflammation. The primary product of the COX pathway, prostaglandin H2, represents a substrate for specific isomerases that catalyze biosynthesis of prostaglandins and thromboxane A2. Of these, prostaglandin D (PGD) synthase is responsible for the production of prostaglandin D2 (PGD2). Prostaglandins, like other eicosanoids, are rapidly metabolized, which is usually associated with a significant decrease in biological activity. PGD2 is metabolized to 9a,11b-PGF2 (which can be measured in plasma and urine) and also has a major urinary tetranor metabolite, PGDM (11,15-dioxo-9-hydroxy-2,3,4,5-tetranorprostane-1,20-dioic acid) [1].
PGD2 is mainly produced by activated mast cells following allergen exposure and antigen cross-linking with the high-affinity receptor for immunoglobulin (Ig) E (FcεRI). PGD2 is also released in significant amounts by dendritic cells, macrophages, eosinophils, T helper type 2 (Th2) cells, and endothelial cells. The biological effects of PGD2 may be mediated by three different receptors: D-prostanoid (DP1), DP2 (CRTH2), and thromboxane prostanoid (TP) [2, 3], and are probably highly dependent on the balance between expression and agonistic effect (or potentially antagonisms) of different receptors. PGD2 can also bind to peroxisome proliferator-activated receptor (PPAR)-c and stimulate transcription of target genes. PGD2 seems to be an important mediator both in the early and the late phases of allergic reaction. It enhances eosinophilic lung inflammation and cytokine release, including leukotriene C4 (LTC4) production by eosinophils [4, 5]. PGD2 has been found in broncho-alveolar lavage fluid (BAL) in a mouse model of asthma [6]. PGD2 is released into human airways during acute allergen challenge and increased levels of PGD2 have been detected in patients with severe asthma [7]. Studies involving exogenous PGD2 or overexpression of human PGD2 synthase have demonstrated an increase in Th2 cytokine production and enhanced eosinophil accumulation into the airways after allergen challenge [8]. In an allergen challenge model in asthmatic patients, it has been found that combined antagonisms of leukotrienes (zafirlukast) and histamine (loratadine) resulted in approximately 75% inhibition of both early and late phase response. Thus, it has been hypothesized by Roquet et al. that the remaining 25% may be mediated by PGs, in particular PGD2 [9]. The imbalance between PGE2 and PGD2 has been proposed to play an important role in the development of asthma and nasal polyps in aspirin hypersensitivity syndrome [10]. Taking into account these findings, PGD2 seems to be a potentially important mediator in several diseases such as asthma, allergic rhinitis, conjunctivitis, and atopic dermatitis, thus representing a promising target in the treatment of allergic inflammation. The aim of the paper is to introduce the PGD2/CRTH2/DP1 signaling pathway and its role in asthma/allergic disorders, and to briefly summarize the preclinical evidence indicating that blocking PGD2 signaling via receptor antagonists may offer a therapeutic benefit (Tables 1, 2).
Table 1.
Comparison between CRTH2 and DP receptors (based on Nagata and Hirai [14])
| CRTH2 | DP | |
|---|---|---|
| Main agonist | PGD2, PGJ2 | PGD2, PGJ2 |
| Selective agonist | Δ12-PGD2, Δ12-PGJ2, DK-PGD2, 15d-PGJ2, 15d-PGJ2, indomethacin L888,607 |
BW245C |
| G protein coupled | Gi | Gs |
| Intracellular changes upon activation | Decrease in cAMP Increase in Ca2+ |
Increase in cAMP |
| Biological effect | Increase in cell migration, chemotaxis, shape change activation of eosinophils, basophils, and Th2 cells, promotion of Th2 inflammation and Th2-type cytokine production (IL-4, 5, 13), upregulation of adhesion molecules | Inhibition of platelet aggregation, vasodilation, relaxation of smooth muscles, inhibition of cell migration, inhibition of apoptosis |
| Selective antagonists | AM211, AM156, ARRY-502, TASP0376377, MK-7246, AZD1981a, OC000459a, setipipranta, BI 671800a | Laropipranta |
| Dual antagonist for CRTH2 and DP | AMG853a | |
| Dual antagonist for CRTH2 and TP | Ramatrobanb | |
cAMP cyclic adenosine monophosphate, CRTH2 chemoattractant receptor-homologous molecule on T helper type 2 cells, DP D-prostanoid, TP thromboxane prostanoid
aCurrently under clinical evaluation
bApproved for allergic rhinitis in Japan
Table 2.
Summary of key clinical studies on molecules targeting the PGD2/CRTH2/DP1 signaling pathway in asthma and allergic disease
| References | Drug and dosing | Indications and no. of subjects | Key results | Comments |
|---|---|---|---|---|
| Dual CRTH2 and thromboxane prostanoid (TP) receptor antagonist (ramatroban, BAY u 3405) | ||||
| Terada et al. [55] | Ramatroban 150 mg bid | 10 patients with perennial allergic rhinitis (allergen challenge model) | Nasal cavity volume (measured by acoustic rhinometry) decrease was significantly inhibited by ramatroban pretreatment | |
| Terada et al. [58] | Ramatroban 150 mg bid for 4 weeks | 11 patients with perennial allergic rhinitis (allergen challenge model) | Significant decrease in eosinophil counts and eosinophil cationic protein levels, the degree of nasal cavity reactivity to histamine decreased | |
| Johnston et al. [59] | Ramatroban 20 mg single oral dose | 10 men with allergic rhinitis, PGD2 nasal insufflation model | No protection against PGD2-induced nasal blockage | |
| Aizawa et al. [60] | Ramatroban 75 mg bid for 2 weeks vs placebo | 12 adult asthmatics | Significant decrease in bronchial hyperreactivity in methacholine challenge model | |
| Selective DP receptor antagonist (laropiprant) | ||||
| Philip et al. [61] | ||||
| Asthma sub-study | Laropiprant 300 mg daily or placebo | 100 patients with severe asthma | No significant differences in FEV1 or asthma symptoms were reported for the active treatment | Montelukast alone, used as a positive control, did demonstrate significant improvements versus placebo |
| Rhinitis sub-study | Laropiprant 25 mg daily, 100 mg daily, cetirizine 10 mg daily or placebo for 2 weeks | 767 patients with seasonal allergic rhinitis | Only cetirizine but not laropiprant demonstrated an improvement in daytime nasal symptoms | Laropiprant safety profile similar to that of placebo |
| Dual CRTH2 and DP receptor antagonist (AMG853) | ||||
| Busse et al. [65] | 5, 25, or 100 mg of oral AMG 853 bid or 200 mg AMG 853 od vs placebo for 12 weeks as add-on treatment to inhaled corticosteroids | 397 patients, moderate-to-severe asthma | No effect over placebo in the primary endpoint (change in ACQ) No differences in secondary endpoints: lung function (FEV1), symptom score, rescue medication use, exacerbations |
Good safety profile |
| CRTH2 antagonists | ||||
| Singh et al. [68] | OC000459 (200 mg bid) for 16 days, vs placebo | 16 allergic asthma patients (allergen challenge model) | Significant reduction in the AUC for FEV1 in the late allergic response but not in the early phase | |
| Horak et al. [69] | OC000459, 200 mg bid for 8 days | 35 patients with allergic rhinitis, Vienna Challenge Chamber model | Significant reduction in nasal and ocular symptoms was found on only the 2nd day of treatment | Good safety profile |
| Barnes et al. [70] | OC000459, 200 mg bid, 4 weeks of treatment | 132 adult asthmatics with moderate persistent asthma | Improvement in the quality of life and night symptoms score, reduction in sputum eosinophils from 2.1 to 0.7 % (not significant over placebo) | In the per protocol population the mean change in FEV1 reached 9.2 vs 1.8 % (placebo) (p < 0.05) |
| Pettipher et al. [71] | OC000459 25 mg OD, 200 mg OD or 100 mg bid, vs placebo for 12 weeks | Adult asthmatics 476 subjects completed |
Significant improvement in FEV1 | Better improvement in phenotype of atopic eosinophilic subjects with uncontrolled asthma |
| Kuna et al. [72] | ||||
| Phase I | AZD1981 1000 mg bid or placebo | 1113 patients with stable asthma withdrawn from ICS | Primary endpoint (change in PEF) not reached | Study drug well tolerated |
| Phase II | AZD1981 50, 400, or 1000 mg bid or placebo | 2368 patients with uncontrolled asthma despite ICS therapy | Primary endpoint (change in PEF) not reached Improvement in asthma control |
|
| Hall et al. [73] | ||||
| Trial 1 | BI 671800 (50, 200, or 400 mg) and fluticasone propionate (220 μg) bid vs placebo | 388 symptomatic controller-naïve adults with asthma | Moderate but statistically significant improvement in FEV1 | |
| Trial 2 | BI 671800 400 mg bid vs montelukast 10 mg vs placebo | 243 patients with asthma receiving inhaled fluticasone (88 μg bid) | Moderate but statistically significant improvement in FEV1 | |
| Diamant et al. [75] | Setipiprant 1000 mg or placebo bid for 5 d | 15 allergic asthmatics (allergen challenge model) | Significant reduction in the allergen-induced late asthmatic response defined as decrease in AUC3–10 h for FEV1 but not in the early asthmatic response | Significant reduction in airway hyperresponsiveness to methacholine |
AUC area under the curve, ACQ Asthma Control Questionnaire, bid twice daily, CRTH2 chemoattractant receptor-homologous molecule on T helper type 2 cells, DP D-prostanoid, FEV 1 forced expiratory volume in 1 s, ICS inhaled corticosteroid, od once daily, PEF peak expiratory flow, PGD 2 prostaglandin D2
DP and CRTH2 Receptors
D-prostanoid receptor (DP) is widely expressed on several cell types. The DP receptor stimulates adenylyl cyclase (AC) through the Gs protein which leads to an increase in cyclic adenosine monophosphate (cAMP) levels. An increase in cAMP level is believed to be linked to inhibition of target cell function, thus PGD2 via the DP receptor inhibits chemotaxis of eosinophils, basophils, and Th2 cells, decreases degranulation of basophils and mast cells, and lowers migration of dendritic cells and production of several cytokines (interleukin [IL]-4, 5, 10, and 13) [11].
CRTH2 was identified in 2001 as a Th2 selective surface molecule, and later discovered to be a novel PGD2 receptor with different functions from DP [12]. Monneret et al. [13], in the same year, described a new receptor on eosinophils, calling it DP2. It is now widely accepted that DP2 receptor, in regards to ligand selectivity and functions, is identical to CRTH2, and due to some major differences with other prostanoid receptors, the name ‘chemoattractant receptor-homologous molecule on T helper type 2 cells’ (CRTH2) is commonly used. Details regarding structure, properties, and function of CRTH2 have been widely reviewed elsewhere [14]. In short, human CRTH2 receptor is composed of 395 amino acids with seven transmembrane domains. The structure of the receptor is unique among other prostanoid receptors and is more akin to the chemoattractant receptors such as the N-Formylmethionyl-leucyl-phenylalanine (FMLP) receptor, C3a receptor, and leukotriene B4 receptor (BLT2) [14]. The CRTH2 gene is located on the long arm (q) of chromosome 11 (11q12). Interestingly, some genetic studies linked the CRTH2 gene with traits of allergic diseases and asthma [15, 16]. CRTH2 mRNA has been detected in virtually all tissues of the human body. In regard to leukocytes, CRTH2 has been found on mature CD4+ and CD8+ T cells, with a typical Th2-type cytokine profile produced on stimulation, including IL-4, IL-5, and no interferon (IFN)-γ release. CRTH2 has been found also on cytotoxic Tc cells, mast cells, osteoblasts, monocytes, eosinophils, and basophils. The highest binding affinity of CRTH2 is seen with PGD2 and its metabolites including 13,14-dihydro-15-keto-PGD2 (DK-PGD2) and PGJ2 [17]. 15-deoxy-Δ12,14-PGD2 is a selective agonist for CRTH2 but not DP receptor. In general, CRTH2 inhibits AC through the Giα protein, which decreases the intracellular cAMP levels. The Gβγ complex, upon activation, stimulates phospholipase Cβ which generates inositol triphosphate (IP3) and induces mobilization of Ca2+ from endoplasmic reticulum stores [11]. The increase in intracellular Ca2+ levels is associated with immune-cell activation, migration, shape change, chemotaxis, and degranulation.
DP and CRTH2 in Allergic Inflammation
The physiological actions of PGD2 include vasodilation, increase in microvascular permeability, relaxation of smooth muscles, and inhibition of platelet activation, eosinophil infiltration and goblet cell depletion. The function of DP and CRTH2 receptors in inflammation can be regarded as antagonistic. In general, CRTH2 mediates pro-inflammatory and pro-stimulatory effects, while DP, in contrast, may limit the effect of CRTH2 activation upon exposure to PGD2 [11]. The mechanisms of action of PDG2 in allergic inflammation are outlined in Fig. 1. Murray et al. [18] found that levels of PGD2 increase in airways of asthmatic patients 150-fold after challenge with a specific allergen. Recently, it has been found that PGD2 production was the highest in patients with severe asthma. Simultaneously, CRTH2 mRNA studies and immunochemistry assays revealed the highest expression of this receptor in severe asthmatics [19].
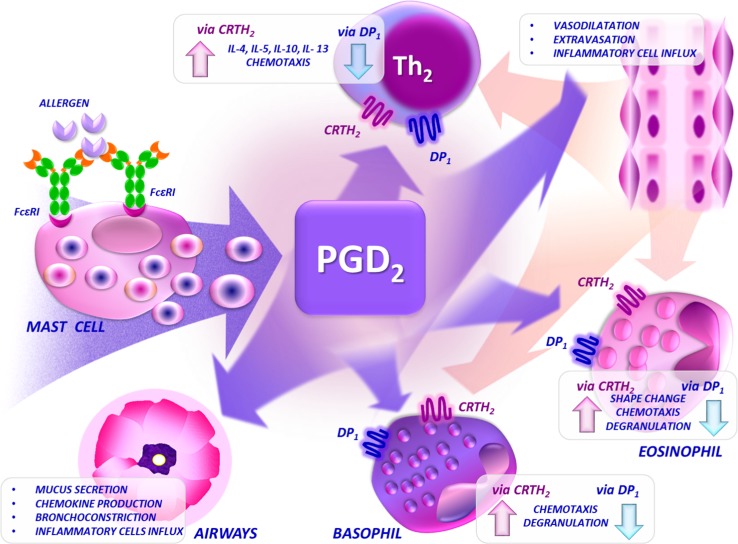
Fig. 1.
Mechanisms of action of PGD2 in allergic inflammation. The function of DP and CRTH2 receptors in inflammation can be regarded as antagonistic. In general, CRTH2 mediates the pro-inflammatory and pro-stimulatory effects of PDG2, while DP may limit the effect of CRTH2 activation upon exposure to PGD2. CRTH2 chemoattractant receptor-homologous molecule on T helper type 2 cells, DP D-prostanoid, FcεRI high-affinity receptor for immunoglobulin E, IL interleukin, PGD 2 prostaglandin D2. The figure was drawn by Łukasz Błażowski MD, PhD
Nagata et al. [20] found that CRTH2 was expressed only in a small subset of activated (CD25+) CD4+ T cells in fresh peripheral blood mononuclear cells (PBMCs) in humans. Moreover, an allergen-induced proliferative response in PBMCs was significantly reduced by subtracting CRTH2+ cells. The production of Th2-type cytokines was higher in CRTH2 cells in comparison with CRTH2– cells, suggesting a possible role of CRTH2 in cytokine production as proposed by the authors or, which seems to be more probable, in Th2 cell activation. Xue et al. [21, 22] proved that PGD2 causes induction of pro-inflammatory Th2 cytokines (IL4, 5, 13) through the CRTH2-dependent mechanism. In another study, the same group [23] found that 13-14-dihydro-15-keto-PGD2, a selective CRTH2 agonist, has an important antiapoptotic role in human Th2 cells. It has been reported [24] that CRTH2 is highly expressed on human peripheral blood basophils and eosinophils. Stimulation of cord blood-derived mast cells with an anti-FcεRI antibody induced Ca2+ mobilization in CRTH2-expressing cells. Gervais et al. [25] demonstrated that PGD2 leads to a rapid change in human eosinophil morphology, promotes eosinophil degranulation, and increases chemokine production. The described effects were induced by 13-14-dihydro-15-keto-PGD2, which is a selective CRTH2 agonist, but not by the DP-selective agonist BW245C. Moreover, BW245C may delay the onset of apoptosis in cultured eosinophils. Interestingly, several active PGD2 metabolites, including Δ12-PGJ2, have been shown to cause eosinophil mobilization from the bone marrow and prime eosinophils for chemotaxis through the CRTH2 receptor [26, 27]. PGD2 via CRTH2 not only induces cell migration but is also involved in up-modulation of adherent molecules [28]. A plausible scenario of PGD2 action and cooperation of DP and CRTH2 receptors in inflammation has been proposed by Hirai et al. [12]. PGD2 produced by mast cells upon allergen stimulation induces local vasodilation via DP, which enhances migration of leucocytes including activated (via CRTH2 receptor) Th2 cells, eosinophils, and basophils. Several other cytokines and chemokines, including but not limited to IL4, 5 and 13 (produced by activated Th2 cells?), eotaxin, and RANTES, are also involved in this process. In vivo studies suggest that PGD2 is a dominant mast cell mediator involved in the activation and induction of migration of Th2 cells through CRTH2-dependent signaling [8]. Immunohistochemical studies revealed that anti-CRTH2 antibody-labeled eosinophils, macrophages, mast cells, T lymphocytes, epithelial cells, and submucosal glands can be found in the nasal mucosa in humans, and that expression of the CRTH2 receptor is upregulated in allergic compared with non-allergic subjects [29, 30].
It has been discovered that the COX inhibitor indomethacin is a potent CRTH2 agonist. Experimental research with this nonsteroidal anti-inflammatory drug (NSAID) improved our understanding of PGD2 action and the role of the CRTH2 receptor. Hirai et al. [28] reported that indomethacin in submicromolar concentrations, in contrast to other NSAIDs, induced Ca2+ mobilization and migration of Th2 cells, eosinophils, and basophils via the CRTH2 receptor, as anti-CRTH2 monoclonal antibodies (mAb) blocked this effect. In another study, Stubbs et al. [31] confirmed that indomethacin causes a PGD2-like and eotaxin-like selective response in eosinophils and basophils. Selective synthetic agonists of the CRTH2 receptor have been synthesized (L-888,607) and may represent a suitable tool for further investigations of the in vivo function of the PGD2/CRTH2 signaling pathway [32].
Of particular interest is the report of Shiraishi et al. [33], which evaluated the effect of double-stranded (ds)RNA, a nucleotide synthesized during viral replication, on airway inflammation and bronchial hyperresponsiveness in a murine model of asthma. It has been found that intratracheal installation of dsRNA induces PGD2 synthesis in the lungs, particularly in alveolar macrophages, and increases airway eosinophilia and bronchial hyperresponsiveness. Pretreatment by ramatroban, a dual CRTH2 and thromboxane A2 (TXA2) antagonist, but not by a selective TXA2 antagonist, nearly completely abolished the dsRNA and allergen-induced airway eosinophilia. This experimental model mimics the pathological and physiological changes seen in the airways of asthmatic patients during acute exacerbations induced by viral infection and suggest that the PGD2/CRTH2 pathway plays a key role in this setting.
In conclusion, results of several in vitro studies presented here suggest that blockade of PGD2-mediated effects with selective CRTH2 or DP antagonists may represent an attractive approach in the management of allergic inflammation.
Animal Models of DP and CRTH2 Antagonisms
Preliminary studies on the effect of antagonism of the prostaglandin D2 receptors DP and CRTH2 on airway inflammation have been performed in animal models. Mice deficient in DP receptor were found to develop significantly decreased asthmatic response in an ovalbumin-induced asthma model [34, 35]. In guinea pigs, prostaglandin D2 induces contraction of smooth muscles through the activation of TP receptor, however this effect is less potent than U46619 (stable TXA2 analog) [36]. In another guinea pig model, a selective DP antagonist, S-5751, reduced antigen-induced nasal blockage, plasma exudation in the conjunctiva, and inflammatory cell infiltration into the upper and lower airways [37]. Hirai et al. [38] identified the mouse CRTH2 homolog of human CRTH2, which is similar in gene structure and mainly expressed in the eosinophils from IL5-transgenic mice, thus suggesting that animal models would add to our understanding of CRTH2 function in allergic diseases. Uller et al. [39] compared affinity of specific CRTH2 antagonist, TM30089, and ramatroban—the dual TP/CRTH2 antagonist. TM30089 was found to display high selectivity and potency on mouse CRTH2 but not TP and several other receptors including anaphylotoxin C3a and C5a receptors. TM30089, similarly to ramatroban, inhibited several typical asthma pathology findings in vivo including reduction of peribronchial eosinophilia and mucus cell hyperplasia. In other in vivo studies, CRTH2 was involved in eosinophil mobilization from guinea-pig bone marrow [27], promotion of eosinophilia in mouse models of allergic asthma and atopic dermatitis [40], and eosinophil influx into the airways after intratracheal administration of PGD2 or a selective CRTH2 agonist [41, 42]. Stebbins et al. [43] evaluated the therapeutic efficacy of AM156 in a murine model of allergic rhinitis and house-dust-mite-induced pulmonary inflammation. AM156 was found to inhibit sneezing and nasal rubs in a model of rhinitis and inhibited pulmonary inflammation and mucus hypersecretion induced by inhalation of house dust mite. Interestingly, AM156 influenced the inflammation when administered during the sensitization phase (priming) but not during the challenge (effector) phase, which suggest that CRTH2 antagonism may prevent polarization of Th0 cells into Th2 cells and may be disease modifying; on the other hand, the effect may be less pronounced in chronic inflammation. In other studies, ramatroban has been showed to attenuate sneezes and nasal rubs induced by exposure to cedar pollen, decreased nasal airway resistance, and eosinophil infiltration in guinea pigs [43]. TM30089-attenuated ovalbumin induced lung tissue eosinophilia and mucus cell metaplasia [43]. Yet another potent and selective CRTH2 antagonist, namely MK-7246, has been studied [44] and was found to present high affinity for the human and animal CRTH2 receptor. The molecule exhibited selectivity over other prostanoid receptors, demonstrated good oral bioavailability and stability, and significantly blocked antigen-induced, late phase bronchoconstriction and airway hyper-responsiveness in sheep. Similarly, in a recent paper, Lukacs et al. [45] confirmed that a highly potent and specific CRTH2 antagonist (coded in the study as compound A) ameliorated inflammation caused by acute or sub-chronic sensitization with the cockroach antigen, reduced mRNA levels of proinflammatory cytokines associated with the Th2-type response, and decreased antigen-specific IgE levels, mucus deposition, and leukocyte infiltration into the large airways.
In conclusion, animal models seem to confirm the pivotal role of PGD2 and CRTH2 receptors in allergic inflammation. Interestingly, as it has been reviewed previously [39], mice that lack a functional CRTH2 receptor and thus are incapable of signaling through CRTH2 have been showed to present confusingly increased or reduced features of allergic inflammation in models of asthma and atopic dermatitis [46–48]. This may suggest that the PGD2/DP/CRTH2 pathway is neither the only, nor probably the most important axis of regulation of Th2 cell activation and eosinophilic influx in allergic inflammation. The limitations of animal models, with specific regard to the scarce translational possibility towards pathomechanisms of allergic diseases and asthma in humans should be highlighted. This in part may explain a clear discrepancy between animal studies and clinical efficacy of CRTH2 antagonism in allergic rhinitis and lack of efficacy in a general cohort of asthmatics (for details see below).
Isolated Human Bronchi Model
Yet another interesting model of potential action of the PGD2/CRTH2/DP1 signaling pathway is represented by isolated human bronchi. Safholm et al. [49] showed that low concentrations of prostaglandin E2 (PGE2) (0.01–1 µmol/L) relaxed the bronchi pre-contracted by histamine. Higher concentrations of PGE2 (10–100 µmol/L) contracted the small airways, however; the TP receptor agonist U-46619, PGF2α, and PGD2 were more potent than PGE2. Norel et al. [50] found that U-46619 was a more potent contractile agonist than PGD2, PGF2α, or histamine in human isolated bronchial and pulmonary arterial muscle preparations. BAY u3405 (ramatroban, dual CRTH2, and TP receptor antagonist) attenuated the contractions induced by U46619, PGF2α, and PGD2 in both experimental settings. In another study [51], the contracted human bronchial preparations were significantly relaxed by PGD2 or by BW245C, a DP-receptor agonist. However, these responses did not exceed 40% of the relaxation induced by papaverine. In addition, the relaxations induced by PGD2 were inhibited by pretreatment with BWA868C. Authors concluded that the relaxation of human isolated bronchial preparations induced by prostanoids involved IP, EP2, and to a lesser extent DP receptors but not EP4 receptor. The CRTH2 (DP2) receptor has no effect on smooth muscle but can activate epithelial cells. Due to this fact, there are no extensive studies reported on selective CRTH2 antagonism in this model.
Clinical Evaluations of DP and CRTH2 Antagonism
Dual CRTH2 and Thromboxane Prostanoid (TP) Receptor Antagonist
Ramatroban (Baynas, BAY u3405, Cayman Chemical), a dual antagonist against TP receptor and CRTH2, is marketed in Japan for allergic rhinitis. Thromboxane A2 (TXA2), an arachidonic acid metabolite, plays an important role in inflammation, and thus in the pathomechanisms of allergic diseases and asthma. Thromboxane produced by platelets, mast cells, and eosinophils induces vasoconstriction and bronchoconstriction, and increases vascular permeability and airway hypersensitivity. TXA2 is believed to be involved in pathogenesis of allergic rhinitis since increased levels of TXB2, a metabolite of TXA2, have been found in the lavage fluid collected after antigen challenge in sensitized guinea pigs and after allergen provocation in patients with allergic rhinitis [52–55]. Increased permeability of capillaries in the nasal epithelium, leading to mucosal edema and nasal obstruction, may be induced by TXA2. The effect of a thromboxane A2 receptor antagonist BAY-u-3405 in experimental allergic reactions included inhibition of an antigen-induced increase in respiratory resistance in guinea pigs, allergen-induced biphasic increase in respiratory resistance and airway inflammation in mice, and antibody-mediated skin reactions in mice [56]. Sugimoto et al. [57] reported that ramatroban has a potent and selective antagonistic activity against CRTH2. In vivo ramatroban has been found to significantly reduce local eosinophilia and nasal mucosal swelling in patients with allergic rhinitis [55, 58]. However, it has been hypothesized that the burdensome symptoms of nasal blockade as a result of vasodilation of venous sinusoids induced by PGD2 may be mediated by the DP receptor rather than CRTH2, as ramatroban did not provide a protective effect on PGD2-induced nasal blockade in humans [59]. It has been shown [60] that ramatroban 75 mg/day administered orally reduces bronchial hyperresponsiveness to methacholine in asthmatic patients.
Selective DP Receptor Antagonist
The pharmacokinetic and pharmacodynamic properties of laropiprant (MK-0524), a selective and potent DP antagonist, have been reported [61]. In humans, pretreatment with laropiprant at a dose of 25 or 100 mg daily for 3 days inhibited nasal congestion induced by installation of PGD2 [62]. In genetic studies it has been found that specific variants in the prostaglandin D receptor gene (PTGDR) for DP (at least one copy of the promoter haplotype with low transcriptional efficiency) are associated with lower risk of asthma [63]. Philip et al. [61] reported results of clinical studies of selective DP antagonism in asthma and allergic rhinitis. One hundred patients with persistent asthma were randomized in a double-blind, crossover study to receive placebo or laropiprant 300 mg daily alone for 3 weeks or with montelukast 10 mg daily for 2 weeks. No significant differences in FEV1 (forced expiratory volume in 1 s) or asthma symptoms were reported for the active treatment alone or in combination with montelukast. Interestingly, montelukast alone, serving as a positive control, did demonstrate significant improvements versus placebo (p < 0.005), showing that the study design and the selection of patients should be sufficient to demonstrate the efficacy of laropiprant. For allergic rhinitis, 767 patients with seasonal symptoms were allocated to receive laropiprant 25 mg daily, 100 mg daily, cetirizine 10 mg daily, or placebo for 2 weeks in a double-blind, parallel study. Similarly, only cetirizine and not laropiprant demonstrated an improvement in daytime nasal symptoms (p < 0.005). Laropiprant was well tolerated, with a safety profile similar to that of placebo. In a genetic sub-study, variations in PTGDR were not related to baseline asthma severity or treatment response. The authors concluded that, in this clinical setting, laropiprant did not demonstrate any efficacy in patients with asthma or allergic rhinitis, suggesting that targeting DP for airway disease therapy does not add much to the current options for management.
Dual CRTH2 and DP Receptor Antagonist
AMG 853, a CRTH2 and DP dual antagonist, has been identified [64] and preliminary data on its pharmacokinetic and pharmacodynamic properties were reported as abstracts. Safety and efficacy of AMG 853 were evaluated in moderate-to-severe asthma patients [65]. A total of 397 patients were randomized to receive placebo, 5, 25, or 100 mg of oral AMG 853 twice daily or 200 mg of AMG 853 once daily for 12 weeks as add-on treatment to their inhaled corticosteroids. There was no effect over placebo in the primary endpoint, which was change in Asthma Control Questionnaire (−0.492 vs −0.44 to −0.555, placebo vs range of AMG 853 groups, p > 0.05). No significant differences were found in secondary endpoints including lung function (FEV1), symptom score, rescue medication use (short-acting β2-agonist), and exacerbations. The studied drug was safe as no differences were reported in regards to adverse events between the active and placebo arms, with the most common adverse events including asthma, upper respiratory tract infections, and headache. Authors of the study concluded that AMG 853 as an add-on to inhaled corticosteroids did not demonstrate any effect in improving asthma symptoms or lung function in patients with inadequately controlled moderate-to-severe asthma. What should be pointed out are the characteristics of the study cohort. The majority of the included patients were obese, atopic subjects (91–95%), with long duration of the disease (24–28 years), FEV1 of 66–68% of predicted, on inhaled corticosteroid (ICS) treatment (409–484 µg of fluticasone), with day and night asthma symptoms, and relatively high rescue medication use (2.7–3.6 puffs daily). It is debatable whether, in such a cohort with chronic disease and probably advanced remodeling, an agent that is known to act mainly in the early stages of the development of allergic inflammation would show any significant effects. On the other hand, as fairly stressed by the authors, patients with asthma whose symptoms are not controlled, despite ICS therapy with or without additional long-acting β2-agonists (LABAs), represent the upper limit for demonstrating efficacy with additional therapies.
Clinical Studies of CRTH2 Antagonisms
Several potent CRTH2 receptor antagonists, including isoquinoline derivatives (TASP0376377) [66], substituted indole-1-acetic acids (AZD1981) [67], and other molecules (OC000459, AM211, setipiprant, ARRY-502, MK-7246) have been synthesized and studied.
OC000459
The preliminary studies on efficacy of OC000459, a CRTH2 antagonist, were performed in a model of allergen challenge in asthmatic patients. Singh et al. [68] reported results of a randomized, double blind, placebo-controlled trial of 16 days treatment with OC000459 (200 mg twice daily) on the early (EAR) and late (LAR) asthmatic response after allergen challenge. A significant reduction in the area under the curve (AUC) for FEV1 in the LAR (25.4%, p = 0.018 vs placebo) but not in the EAR was found. Sputum eosinophil counts at day 1 after the challenge were significantly lower in the treatment arm (p = 0.002). Similarly, PGD2-induced blood eosinophil shape change evaluated ex vivo was lower in the OC000459 group (−33.6%, p = 0.048). In the discussion, the authors concluded that OC000459 had no effect on the early phase of the response, which suggests that activation via the CRTH2 receptor does not play a significant role in acute bronchoconstriction after allergen exposure as those effects of PGD2 are probably mediated via the thromboxane (TP) receptor. PGD2/CRTH2 signaling seems to be involved in the Th2-driven inflammatory cell influx during the late phase of the reaction. Interestingly, the authors suggested that combining CRTH2 and TP antagonism may thus have a significantly greater effect on the antigen response in allergic subjects.
Horak et al. [69] evaluated the effect of OC000459 on nasal and ocular symptoms in allergic patients exposed to grass pollen in a randomized, cross-over, placebo controlled, double-blind trial. Thirty five patients treated for 8 days with OC000459, 200 mg twice daily, were exposed to grass pollen (≥1400 grains/m3) after 2 and 8 days in the Vienna Challenge Chamber. A significant reduction in both nasal and ocular symptoms (p = 0.003) was found on only the 2nd day of treatment and was even more pronounced on the 8th day of administration (p = 0.011). The therapeutic effect of OC000459 seemed to persist in the second treatment period (placebo, after cross-over), despite the 3-week washout phase. The profile of adverse events reported was similar in the treatment and placebo arms, suggesting good safety of the evaluated molecule. Authors concluded that the effect of OC000459 on nasal symptoms is at least as good as observed with levocetirizine, fexofenadine, and nasal fluticasone furoate [69], and that reduction of ocular symptoms seems to exceed effects of antihistamines and intranasal steroids. The clinical effects were quick, noticeable after only 2 days of treatment, which may be due to inhibition of basophil and eosinophil recruitment. The long-term effect (3–4 weeks after the end of the treatment phase), exceeding possible pharmacokinetic half-life (12–15 h in humans) is probably related to inhibition of Th2 inflammation by induction of apoptosis and clearance of Th2 lymphocytes.
Efficacy of OC000459 in moderate persistent asthma was evaluated by Barnes et al. [70]. In this study, 132 adult asthmatics were randomized to receive oral OC000459 or placebo. After 4 weeks of treatment, lung function and induced sputum eosinophil count were studied. In the analysis of the whole study group, mean change in FEV1 was 7.1% in the treatment arm versus 4.3% in the placebo group, and did not reach statistical significance (p > 0.05). However, in the per protocol population, the mean change in FEV1 reached 9.2 and 1.8%, respectively (p = 0.037). OC000459 significantly improved the quality of life and night symptoms score (p < 0.05). The sputum eosinophil count was reduced from 2.1 to 0.7%, but this effect was not significant when compared with placebo (p = 0.37). The safety profile was similar in patients receiving OC000459 and placebo with respiratory infections less common in the treatment arm. In the discussion, when analyzing decreases in the common cold, the authors concluded that PGD2/CRTH2 signaling via induction of Th2 response and cytokines may increase the expression of the inter-cellular adhesion molecule 1 (ICAM-1), which is a receptor for rhinovirus on the bronchial epithelial cells. These mechanisms have been confirmed in animal models (see Sect. 4) [33] and may play an important role in the prevention of viral exacerbations in asthmatic patients.
In another trial, Pettipher et al. [71] aimed to determine the effect of OC000459 in asthmatic subjects and to try to define the phenotype of patients potentially most responsive to the treatment. Adult asthmatics were randomized to receive OC000459 25 mg once daily, 200 mg once daily, 100 mg twice daily, or placebo for 12 weeks. The study drug induced a significant improvement in FEV1 at all studied doses (mean change in FEV1 95 mL vs placebo, p = 0.024). In the post hoc analysis of atopic eosinophilic subjects with uncontrolled asthma, a mean increase in FEV1 reached 220 mL (p = 0.005 vs placebo). This effect was even more pronounced in younger subjects (defined as aged ≤40 years) and ended in 355 mL compared with placebo (p = 0.007). In all cohorts, including the whole study group and the atopic eosinophilic subgroup, an improvement in asthma control and quality of life was found. There was a significantly lower incidence of exacerbations and respiratory infections in patients treated with OC000459. No drug-related serious adverse events were reported. This is the first trial of a CRTH2 antagonist showing the importance of phenotyping asthma patients and suggesting a potentially better clinical effect of CRTH2 antagonism in a subgroup of eosinophilic, atopic, Th2-type asthma.
A detailed search in the ClinicalTrials.gov database revealed that, with regards to molecules with potential impact on the PGD2/CRTH2/DP1 signaling pathway in asthma and allergies, there are only two ongoing and recruiting studies with OC000459 in severe eosinophilic asthma (NCT02560610) and in rhinovirus challenge in asthma (NCT02660489). This information should be taken with caution, considering possible limitations in reporting and updating information in the ClinicalTrials.gov database.
AZD1981
AZD1981 is an orally available, selective CRTH2 receptor antagonist which proved to inhibit DK-PGD2-induced CD11b expression in human eosinophils [67]. Kuna et al. [72] reported results of two phase II randomized trials with AZD1981 in adults with asthma. In study 1, 113 patients with stable asthma (FEV1 65–110%) were withdrawn from their ICS (<400 µg/day) and were randomized to 1000 mg of AZD1981 twice daily or placebo. In study 2, 368 patients with uncontrolled asthma (FEV1 40–85%) despite ICS therapy (≥500 µg/day) received 50, 400, or 1000 mg AZD1981 twice daily or placebo. The primary endpoint of both studies was morning peak expiratory flow (PEF) change after 4 weeks of treatment. At the end of the trial there was a nonsignificant increase in PEF in study 1 (9.5 L/min vs placebo, p = 0.086) and study 2 (12 L/min vs placebo, p = 0.16). In study 2, active treatment improved asthma control as defined by improvement in Asthma Control Questionnaire (5-item version; ACQ-5) score (0.26–0.3 units vs placebo, p = 0.01–0.022); however, the observed effect was not dose dependent. The study drug was well tolerated. Authors of the report underlined that improvements in ACQ-5 and FEV1 were seen in the majority of atopic patients.
BI 671800
Recently, efficacy of BI 671800, an oral CRTH2 antagonist, in poorly controlled asthma as a sole controller and in the presence of ICS treatment was reported [73]. BI 671800 (50, 200, or 400 mg) and fluticasone propionate (220 μg), all given twice daily, were compared with placebo in symptomatic controller-naïve adults with asthma (Trial 1); and BI 671800 400 mg twice daily compared with montelukast 10 mg once daily, and matching placebo, was studied in patients with asthma receiving inhaled fluticasone (88 μg twice daily) (Trial 2). After 6 weeks of treatment in Trial 1, improvement in FEV1 (% predicted) reached 3.08, 3.59, and 3.98% for BI 671800 50, 200 and 400 mg twice daily, respectively, and 8.62% for fluticasone 220 μg twice daily (p = 0.0311, p = 0.0126, p = 0.0078, and p < 0.0001, respectively). In Trial 2, adjusted mean FEV1 treatment differences compared with placebo were 3.87% for BI 671800 400 mg twice daily and 2.37% for montelukast (p = 0.0050 and p = 0.0657, respectively). The authors concluded that results of their study support the hypothesis that BI 671800, by blocking the CRTH2 receptor, may improve lung function in both symptomatic controller-naïve patients and asthmatic subjects already on ICS.
Setipiprant
Setipiprant, an orally active and selective CRTH2 antagonist, has been evaluated in a single- and multiple-dose tolerability and pharmacokinetic study in healthy male subjects [74]. Setipiprant was rapidly absorbed and followed a biphasic elimination pattern with a half-life of 10–18 h. The steady-state condition was reached after 2–3 days and the drug was well tolerated, with headache reported as the most frequent adverse event. The efficacy of setipiprant was evaluated in a model of a standardized allergen challenge [75]. Fifteen allergic asthmatics were randomized to setipiprant 1000 mg or placebo twice daily for 5 days in a cross-over design with 3 weeks of washout. The study drug significantly reduced the allergen-induced LAR, defined as a decrease in AUC3–10 h for FEV1 by on average 25.6% (p = 0.006), but did not influenced the EAR. Setipiprant significantly reduced airway hyperresponsiveness to methacholine (p = 0.0029). There were no differences in exhaled nitric oxide (eNO) levels between the study groups. Setipiprant was well tolerated and no clinically adverse events were reported. There is a striking similarity in the mode of action and the potency of setipiprant to another CRTH2 antagonist, OC000459, evaluated in a similar model by Singh et al. [68] (see Sect. 6.4.1), which supports the hypothesis that described effects are common for the PGD2/CRTH2 pathway and may be of clinical significance.
Final Remarks and Future Perspectives
Prostaglandin D2 (PGD2) released by degranulating mast cells upon allergen exposure is believed to play a key role in orchestrating mechanisms of inflammation in allergies and asthma. The biological effects of PGD2 are mediated by three receptors, DP (DP1), CRTH2 (DP2), and TP (thromboxane receptor). The CRTH2 receptor is involved in induction of migration and activation of Th2 lymphocytes, eosinophils, and basophils, up-regulation of adhesion molecules, and promotion of pro-inflammatory Th2-type cytokines (IL-4, 5, 13), whereas the DP receptor is associated with relaxation of smooth muscles, vasodilation, inhibition of cell migration, and apoptosis of eosinophils. Several in vitro and in vivo studies in animal models of allergic inflammation confirmed the pivotal role of PGD2 and signaling via CRTH2 and DP receptors, suggesting a possible role of those receptors’ antagonism in management of allergic diseases in humans. A CRTH2 antagonist (OC000459) or dual CRTH2 and TP receptor antagonist (ramatroban) were effective in reduction of eosinophilia, nasal mucosal swelling, and clinical symptoms of allergic rhinitis, with the latter drug registered for clinical use in this indication. OC000459 and setipiprant reduced the late but not early phase of response in an allergen challenge in atopic asthmatics. In persistent asthma, some molecules induced limited improvement in lung function, quality of life, and asthma symptoms (OC000459, BI671800), but in other trials with AMG 853 and AZ1981 these findings were not confirmed. There is a strikingly clear discrepancy between animal studies and clinical efficacy of CRTH2 antagonism in allergic rhinitis and lack of efficacy in a general cohort of asthmatics. There are several plausible explanations for these findings. Certainly, some differences in pathomechanisms of allergic inflammation between rodents and humans, including the role of PGD2, CRTH2, and DP receptors, may be of importance. Possibly, inhibition of only one pathway (PGD2/CRTH2/DP signaling) in a complex milieu of allergic inflammation may not grant sufficient clinical efficacy, as other compensatory or simultaneous mechanisms may play a role here. Thus, comparison of CRTH2/DP antagonisms to glucocorticosteroids with known pluripotent anti-inflammatory mechanisms of action should be done with caution. On the other hand, there is no doubt that glucocorticosteroids belong to the mainstream controller medications in allergic rhinitis and asthma, and provide a valid reference method of treatment. As proposed by Roquet et al. [9], a combined action, for example antagonisms of leukotrienes, histamine, and PGD2 (via CRTH2 receptor), could be more effective. On one hand, the combined CRTH2 and TP receptor antagonist (ramatroban) is highly effective in the treatment of allergic rhinitis [55, 58]; on the other hand, this approach has not been successful in dual CRTH2 and DP antagonisms (AMG 853) [65] or in a DP antagonist (laropiprant) combined with an anti-leukotriene (montelukast) study [61].
Yet another important aspect may be timing of treatment in a course of chronic inflammation. It has been suggested by the results of AM156 studies in an animal model [43] that CRTH2 antagonism may impact the inflammation when administered during the sensitization phase (priming), but not during the challenge (effector) phase. These findings support the hypothesis that PGD2/CRTH2 antagonism may prevent polarization of Th0 cells into Th2 cells and may be disease modifying, but on the other hand this effect may be less pronounced in chronic inflammation, especially in persistent asthma with significant bronchial wall remodeling. This seems to be further confirmed by high efficacy of CRTH2 antagonists in models of allergen exposure both in allergic rhinitis and atopic asthma. Some studies, both in animal models and in a clinical setting, showed possible impact of PGD2/CRTH2 antagonism on bronchial hyperreactivity, which is a key characteristic of asthma. It could be hypothesized that such an intervention may influence airway smooth muscle contractility and proliferation, thus possibly reducing remodeling in the long term. Further, long-term studies may improve our understanding of those pathways in the airways of patients with asthma.
Regarding the aforementioned studies in asthma, several possible limitations may be raised, including selection of primary endpoint (lung function) [70], short duration of studies, and inclusion of the general asthma cohort without any further phenotyping. Taking into account results of clinical studies, especially the effort of Pettipher et al. [71], who aimed to identify a phenotype of asthma responsive to CRTH2 antagonisms, it could be concluded that patients with allergic, eosinophilic, Th2-type asthma with early onset of the disease may represent the target group for further studies. It could be suggested that some biomarkers of Th2-type allergic inflammation (eosinophils in induced sputum or peripheral blood, eNO levels, periostin [76]) may help in phenotyping and selection of appropriate cohort for further trials. Possibly primary endpoints other than lung function should be prioritized, including asthma control, quality of life, medication use, and exacerbation rate. Some animal models [33] and clinical studies [70] suggested that PGD2/CRTH2 antagonism may be of exceptional efficacy in reducing the burden of exacerbations induced either by viral infections or allergen exposure.
Conclusions
In conclusion, CRTH2 antagonism seems to be the most potent in allergic, eosinophilic inflammation in allergic rhinitis and atopic early onset asthma. What should be pointed out is the fact that in all studies, CRTH2 and DP receptor antagonists were safe, with the adverse event profiles similar to that of placebo. There is no doubt that the PGD2/CRTH2/DP1 signaling pathway plays a key role in allergic inflammation and further studies with selective or combined antagonisms in well defined cohorts of patients are needed.
Acknowledgements
We would like to thank Łukasz Błażowski MD, PhD for his editorial help and drawing of Fig. 1.
Compliance with Ethical Standards
Conflicts of interest
MK declares no conflict of interest with regards to the manuscript. PK participated in a clinical study with AZD1981 sponsored by Astra Zeneca.
Funding
No funding has been received for the preparation of this manuscript.
References
- 1.Kupczyk M, Lundstrom S, Dahlen B, et al. Lipid mediators in severe asthma. Eur Respir Mon. 2011;51:218–235. [Google Scholar]
- 2.Pettipher R, Hansel TT. Antagonists of the prostaglandin D2 receptor CRTH2. Drug News Perspect. 2008;21:317–322. doi: 10.1358/dnp.2008.21.6.1246831. [DOI] [PubMed] [Google Scholar]
- 3.Schuligoi R, Sturm E, Luschnig P, et al. CRTH2 and D-type prostanoid receptor antagonists as novel therapeutic agents for inflammatory diseases. Pharmacology. 2010;85:372–382. doi: 10.1159/000313836. [DOI] [PubMed] [Google Scholar]
- 4.Fujitani Y, Kanaoka Y, Aritake K, et al. Pronounced eosinophilic lung inflammation and Th2 cytokine release in human lipocalin-type prostaglandin D synthase transgenic mice. J Immunol. 2002;168:443–449. doi: 10.4049/jimmunol.168.1.443. [DOI] [PubMed] [Google Scholar]
- 5.Mesquita-Santos FP, Vieira-de Abreu A, Calheiros AS, et al. Cutting edge: prostaglandin D2 enhances leukotriene C4 synthesis by eosinophils during allergic inflammation: synergistic in vivo role of endogenous eotaxin. J Immunol. 2006;176:1326–1330. doi: 10.4049/jimmunol.176.3.1326. [DOI] [PubMed] [Google Scholar]
- 6.Herrerias A, Torres R, Serra M, et al. Activity of the cyclooxygenase 2—prostaglandin E prostanoid receptor pathway in mice exposed to house dust mite aeroallergens, and impact of exogenous prostaglandin E2. J Inflamm. 2009;6:30. doi: 10.1186/1476-9255-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balzar S, Fajt ML, Comhair SA, et al. Mast cell phenotype, location, and activation in severe asthma: data from the severe asthma research program. Am J Respir Crit Care Med. 2011;183:299–309. doi: 10.1164/rccm.201002-0295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gyles SL, Xue L, Townsend ER, et al. A dominant role for chemoattractant receptor-homologous molecule expressed on T helper type 2 (Th2) cells (CRTH2) in mediating chemotaxis of CRTH2+ CD4+ Th2 lymphocytes in response to mast cells supernatants. Immunology. 2006;119:362–368. doi: 10.1111/j.1365-2567.2006.02440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roquet A, Dahlen B, Kumlin M, et al. Combined antagonism of leukotrienes and histamine produces predominant inhibition of allergen-induced early and late phase airway obstruction in asthmatics. Am J Respir Crit Care Med. 1997;155:1856–1863. doi: 10.1164/ajrccm.155.6.9196086. [DOI] [PubMed] [Google Scholar]
- 10.Pierzchalska M, Szabo Z, Sanak M, et al. Deficient prostaglandin E2 production by bronchial fibroblasts of asthmatic patients with special reference to aspirin-induced asthma. J Allergy Clin Immunol. 2003;111:1041–1048. doi: 10.1067/mai.2003.1491. [DOI] [PubMed] [Google Scholar]
- 11.Kostenis E, Ulven T. Emerging roles of DP and CRTH2 in allergic inflammation. Trends Mol Med. 2006;12:148–158. doi: 10.1016/j.molmed.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Hirai H, Tanaka K, Yoshie O, et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J Exp Med. 2001;193:255–261. doi: 10.1084/jem.193.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monneret G, Gravel S, Diamond M, et al. Prostaglandin D2 is a potent chemoattractant for human eosinophils that acts via a novel DP receptor. Blood. 2001;98:1942–1948. doi: 10.1182/blood.V98.6.1942. [DOI] [PubMed] [Google Scholar]
- 14.Nagata K, Hirai H. The second PGD2 receptor CRTH2: structure, properties, and functions in leukocytes. Prostaglandins Leukot Essent Fatty Acids. 2003;69:169–177. doi: 10.1016/S0952-3278(03)00078-4. [DOI] [PubMed] [Google Scholar]
- 15.Cookson W. The alliance of genes and environment in asthma and allergy. Nature. 1999;402:B5–B11. doi: 10.1038/35037002. [DOI] [PubMed] [Google Scholar]
- 16.Hsu SC, Chen LC, Kuo ML, et al. Novel SNPs in a candidate gene, CRTH2, for allergic diseases. Genes Immun. 2002;3:114–116. doi: 10.1038/sj.gene.6363826. [DOI] [PubMed] [Google Scholar]
- 17.Gazi L, Gyles S, Rose J, et al. Δ12-Prostaglandin D2 is a potent and selective CRTH2 receptor agonist and causes activation of human eosinophils and Th2 lymphocytes. Prostaglandins Other Lipid Mediat. 2005;75:153–167. doi: 10.1016/j.prostaglandins.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Murray JJ, Tonnel AB, Brash AR, et al. Release of prostaglandin D2 into human airways during acute antigen challenge. N Engl J Med. 1986;315:800–804. doi: 10.1056/NEJM198609253151304. [DOI] [PubMed] [Google Scholar]
- 19.Fajt ML, Gelhaus SL, Freeman B, et al. Prostaglandin D2 pathway upregulation: relation to asthma severity, control and TH2 inflammation. J Allergy Clin Immunol. 2013;131:1504–1512. doi: 10.1016/j.jaci.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagata K, Tanaka K, Ogawa K, et al. Selective expression of a novel surface molecule by human Th2 cells in vivo. J Immunol. 1999;162:1278–1286. [PubMed] [Google Scholar]
- 21.Xue L, Gyles SL, Wettey FR, et al. Prostaglandin D2 causes preferential induction of proinflammatory Th2 cytokine production through an action on chemoattractant receptor-like molecule expressed on Th2 cells. J Immunol. 2005;175:6531–6536. doi: 10.4049/jimmunol.175.10.6531. [DOI] [PubMed] [Google Scholar]
- 22.Xue L, Barrow A, Pettipher R. Interaction between prostaglandin D2 and chemoattractant receptor-homologous expressed n Th2 cells mediates cytokine production by Th2 lymphocytes in response to activated mast cells. Clin Exp Immunol. 2009;156:126–133. doi: 10.1111/j.1365-2249.2008.03871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xue L, Barrow A, Pettipher R. Novel function of CRTH2 in preventing apoptosis of human Th2 cells through activation of the phosphatidylinositol 3-kinase pathway. J Immunol. 2009;182:7580–7586. doi: 10.4049/jimmunol.0804090. [DOI] [PubMed] [Google Scholar]
- 24.Nagata K, Hirai H, Tanaka K, et al. CRTH2, an orphan receptor of T-helper-2 cells, is expressed on basophils and responds to mast cell-derived factor(s) FEBS Lett. 1999;459:195–199. doi: 10.1016/S0014-5793(99)01251-X. [DOI] [PubMed] [Google Scholar]
- 25.Gervais FG, Cruz RPG, Chateauetneuf A, et al. Selective modulation of chemokinesis, degranulation, and apoptosis through the PGD2 receptors CRTH2 and DP. J Allergy Clin Immunol. 2001;108:982–988. doi: 10.1067/mai.2001.119919. [DOI] [PubMed] [Google Scholar]
- 26.Schroder R, Xue L, Konya V, et al. PGH1, the precursor for the anti-inflammatory prostaglandins of the 1-series, is a potent activator of the pro-inflammatory receptor CRTH2/DP2. PLoS One. 2012;7(3):e33329. doi: 10.1371/journal.pone.0033329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinemann A, Schuligoi R, Sabroe I, et al. Δ12-prostaglandin J2, a plasma metabolite of prostaglandin D2, causes eosinophil mobilization form the bone marrow and primes eosinophils for chemotaxis. J Immunol. 2003;170:4752–4758. doi: 10.4049/jimmunol.170.9.4752. [DOI] [PubMed] [Google Scholar]
- 28.Hirai H, Tanaka K, Takano S, et al. Cutting edge: agonistic effect of indomethacin on a prostaglandin D2 receptor, CRTH2. J Immunol. 2002;168:981–985. doi: 10.4049/jimmunol.168.3.981. [DOI] [PubMed] [Google Scholar]
- 29.Shirasaki H, Kikuchi M, Kanaizumi E, et al. Accumulation of CRTH2-positive leukocytes in human allergic nasal mucosa. Ann Allergy Asthma Immunol. 2009;102:110–115. doi: 10.1016/S1081-1206(10)60239-6. [DOI] [PubMed] [Google Scholar]
- 30.Nantel F, Fong C, Lamontagne S, et al. Expression of prostaglandin D synthase and the prostaglandin D2 receptors DP and CRTH2 in human nasal mucosa. Prostaglandins Other Lipid Mediat. 2004;73:87–101. doi: 10.1016/j.prostaglandins.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Stubbs VEL, Schratl P, Hartnell A, et al. Indomethacin causes prostaglandin D2-like and eotaxin-like selective responses in eosinophils and basophils. J Biol Chem. 2002;277(29):26012–26020. doi: 10.1074/jbc.M201803200. [DOI] [PubMed] [Google Scholar]
- 32.Gervais FG, Morello JP, Beaulieu C, et al. Identification of a potent and selective synthetic agonist at the CRTH2 receptor. Mol Pharmacol. 2005;67(6):1834–1839. doi: 10.1124/mol.104.009068. [DOI] [PubMed] [Google Scholar]
- 33.Shiraishi Y, Asano K, Niimi K, et al. Cyclooxygenase-2/prostaglandin D2/CRTH2 pathway mediates double-stranded RNA-induced enhancement of allergic airway inflammation. J Immunol. 2008;180:541–549. doi: 10.4049/jimmunol.180.1.541. [DOI] [PubMed] [Google Scholar]
- 34.Matsuoka T, Hirata M, Tanaka H, et al. Prostaglandin D2 as a mediator of allergic asthma. Science. 2000;287:2013–2017. doi: 10.1126/science.287.5460.2013. [DOI] [PubMed] [Google Scholar]
- 35.Kabashima K, Narumiya S. The DP receptor, allergic inflammation and asthma. Prostaglandins Leukot Essent Fatty Acids. 2003;69:187–194. doi: 10.1016/S0952-3278(03)00080-2. [DOI] [PubMed] [Google Scholar]
- 36.Larsson AK, Hagfjard A, Dahlen SE, Adner M. Prostaglandin D2 induces contractions through activation of TP receptors in peripheral lung tissue from the guinea pig. Eur J Pharmacol. 2011;669(1–3):136–142. doi: 10.1016/j.ejphar.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 37.Arimura A, Yasui K, Kishino J, et al. Prevention of allergic inflammation by a novel prostaglandin receptor antagonist, S-5751. J Pharmacol Exp Ther. 2001;29:411–419. [PubMed] [Google Scholar]
- 38.Hirai H, Abe H, Tanaka K, et al. Gene structure and functional properties of mouse CRTH2, a prostaglandin D2 receptor. Biochem Biophys Res Commun. 2003;307:797–802. doi: 10.1016/S0006-291X(03)01266-X. [DOI] [PubMed] [Google Scholar]
- 39.Uller L, Mathiesen JM, Alenmyr L, et al. Antagonism of the prostaglandin D2 receptor CRTH2 attenuates asthma pathology in mouse eosinophilic airway inflammation. Respir Res. 2007;8:16–25. doi: 10.1186/1465-9921-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spik I, Brenuchon C, Angeli V, et al. Activation of the prostaglandin D2 receptor DP2/CRTH2 increases allergic inflammation in mouse. J Immunol. 2005;174(6):3703–3708. doi: 10.4049/jimmunol.174.6.3703. [DOI] [PubMed] [Google Scholar]
- 41.Shichijo M, Sugimoto H, Nagao K, et al. Chemoattractant receptor-homologous molecule expressed on Th2 cells activation in vivo increases blood leukocyte counts and its blockade abrogates 13,14-dihydro-15-keto-prostaglandin D2-induced eosinophilia in rats. J Pharmacol Exp Ther. 2003;307(2):518–525. doi: 10.1124/jpet.103.055442. [DOI] [PubMed] [Google Scholar]
- 42.Shiraishi Y, Asano K, Nakajima T, et al. Prostaglandin D2-induced eosinophilic airway inflammation is mediated by CRTH2 receptor. J Pharmacol Exp Ther. 2005;312(3):954–960. doi: 10.1124/jpet.104.078212. [DOI] [PubMed] [Google Scholar]
- 43.Stebbins KJ, Broadhead AR, Correa LD, et al. Therapeutic efficacy of AM 156, a novel prostanoid DP2 receptor antagonist, in murine models of allergic rhinitis and house dust mite-induced pulmonary inflammation. Eur J Pharmacol. 2010;638:142–149. doi: 10.1016/j.ejphar.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 44.Gervais FG, Sawyer N, Stocco R, et al. Pharmacological characterization of MK-7246, a potent and selective CRTH2 (chemoattractant receptor-homologous molecule expressed on T-helper type 2 cells) antagonist. Mol Pharmacol. 2011;79(1):69–76. doi: 10.1124/mol.110.068585. [DOI] [PubMed] [Google Scholar]
- 45.Lukacs NW, Berlin AA, Franz-Bacon K, et al. CRTH2 antagonism significantly ameliorates airway hyperreactivity and downregulates inflammation-induced genes in a mouse model of airway inflammation. Am J Physiol Lung Cell Mol Physiol. 2008;295(5):L767–L779. doi: 10.1152/ajplung.90351.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chevalier E, Stock J, Fisher T, et al. Cutting edge: chemoattractant receptor-homologous molecule expressed on TH2 cells plays a restricting role on IL-5 production and eosinophil recruitment. J Immunol. 2005;175(4):2056–2060. doi: 10.4049/jimmunol.175.4.2056. [DOI] [PubMed] [Google Scholar]
- 47.Satoh T, Moroi R, Aritake K, et al. Prostaglandin D2 plays an essential role in chronic allergic inflammation of the skin via CRTH2 receptor. J Immunol. 2006;177(4):2621–2629. doi: 10.4049/jimmunol.177.4.2621. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalo J, Qiu Y, Coyle AJ, Hodge MR. CRTH2(DP2) and not the DP1 receptor mediate allergen induced mucus production and airway hyperresponsiveness. Am J Respir Crit Care Med. 2005;163(5):A811. [Google Scholar]
- 49.Safholm J, Manson ML, Bood J, et al. Prostaglandin E2 inhibits mast cell-dependent bronchoconstriction in human small airways through the E prostanoid subtype 2 receptor. J Allergy Clin Immunol. 2015;136(5):1232–1239. doi: 10.1016/j.jaci.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 50.Norel X, Labat C, Gardiner PJ, Brink C. Inhibitory effects of BAY u3405 on prostanoid-induced contractions in human isolated bronchial and pulmonary arterial muscle preparations. Br J Pharmacol. 1991;104:591–595. doi: 10.1111/j.1476-5381.1991.tb12474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Norel X, Walch L, Labat C, et al. Prostanoid receptors involved in the relaxation of human bronchial preparations. Br J Pharmacol. 1999;126:867–872. doi: 10.1038/sj.bjp.0702392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dogne JM, de Leval X, Benoit P, et al. Thromboxane A2 inhibition. Therapeutic potential in bronchial asthma. Am J Respir Med. 2002;1(1):11–17. doi: 10.1007/BF03257158. [DOI] [PubMed] [Google Scholar]
- 53.Ishizuka T, Matsui T, Okamoto Y, et al. Ramatroban (BAY u 3405): a novel dual antagonist of TXA2 receptor and CRTh2, a newly identified prostaglandin D2 receptor. Cardiovasc Drug Rev. 2004;22(2):71–90. doi: 10.1111/j.1527-3466.2004.tb00132.x. [DOI] [PubMed] [Google Scholar]
- 54.Narita S, Asakura K, Kataura A. Effects of thromboxane A2 receptor antagonist (Bay u 3405) on nasal symptoms after antigen challenge in sensitized guinea pigs. Int Arch Allergy Immunol. 1996;109:161–166. doi: 10.1159/000237215. [DOI] [PubMed] [Google Scholar]
- 55.Terada N, Yamakoshi T, Hasegawa M, et al. The effect of ramatroban (BAY u 3405), a thromboxane A2 receptor antagonist, on nasal cavity volume and minimum cross-sectional area and nasal mucosal hemodynamics after nasal mucosal allergen challenge in patients with perennial allergic rhinitis. Acta Oto-Laryngol. 2009;118(537):32–37. doi: 10.1080/00016489850182323. [DOI] [PubMed] [Google Scholar]
- 56.Nagai H, Takeda H, Yamaguchi S, et al. The effect of a thromboxane A2 receptor antagonist BAY-u-3405 on experimental allergic reactions. Prostaglandins. 1995;50:75–87. doi: 10.1016/0090-6980(95)00111-5. [DOI] [PubMed] [Google Scholar]
- 57.Sugimoto H, Shichijo M, Iino T, et al. An orally bioavailable small molecule antagonist of CRTH2, ramatroban (BAY u3405), inhibits prostaglandin D2-induced eosinophil migration in vitro. J Pharmacol Exp Ther. 2003;305:347–352. doi: 10.1124/jpet.102.046748. [DOI] [PubMed] [Google Scholar]
- 58.Terada N, Yamakoshi T, Hasegawa M, et al. Effect of a thromboxane A2 receptor antagonist, ramatroban (BAY u3405), on inflammatory cells, chemical mediators and non-specific nasal hyperreactivity after allergen challenge in patients with perennial allergic rhinitis. Allergol Int. 1998;47:59–67. doi: 10.2332/allergolint.47.59. [DOI] [PubMed] [Google Scholar]
- 59.Johnston SL, Smith S, Harrison J, et al. The effect of BAY u 3405, a thromboxane receptor antagonist, on prostaglandin D2-induced nasal blockage. J Allergy Clin Immunol. 1993;91:903–909. doi: 10.1016/0091-6749(93)90348-J. [DOI] [PubMed] [Google Scholar]
- 60.Aizawa H, Shigyo M, Nogami H, et al. BAY u3405, a thromboxane A2 antagonist, reduces bronchial hyperresponsiveness in asthmatics. Chest. 1996;109(2):338–342. doi: 10.1378/chest.109.2.338. [DOI] [PubMed] [Google Scholar]
- 61.Philip G, van Adelsberg J, Loeys T, et al. Clinical studies of the DP1 antagonist laropiprant in asthma and allergic rhinitis. J Allergy Clin Immunol. 2009;124:942–948. doi: 10.1016/j.jaci.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 62.Van Hecken A, Depre M, De Lepeleire I, et al. The effect of MK-0524, a prostaglandin D(2) receptor antagonist, on prostaglandin D2-induced nasal airway obstruction in healthy volunteers. Eur J Clin Pharmacol. 2007;63:135–141. doi: 10.1007/s00228-006-0211-2. [DOI] [PubMed] [Google Scholar]
- 63.Oguma T, Palmer LJ, Birben E, et al. Role of prostanoid DP receptor variants in susceptibility to asthma. N Engl J Med. 2004;351:1752–1763. doi: 10.1056/NEJMoa031785. [DOI] [PubMed] [Google Scholar]
- 64.Liu J, Li A-R, Wang Y, et al. Discovery of AMG 853, a CRTH2 and DP dual antagonist. ACS Med Chem Lett. 2011;2:326–330. doi: 10.1021/ml1002234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Busse WW, Wenzel SE, Meltzer EO, et al. Safety and efficacy of the prostaglandin D2 receptor antagonist AMG 853 in asthmatic patients. J Allergy Clin Immunol. 2013;131:339–345. doi: 10.1016/j.jaci.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 66.Nishikawa-Shimoto R, Sekiguchi Y, Koami T, et al. Isoquinoline derivatives as potent CRTH2 receptor antagonists: synthesis and SAR. Bioorgan Med Chem Lett. 2012;22:3305–3310. doi: 10.1016/j.bmcl.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 67.Luker T, Bonnert R, Brough S, et al. Substituted indole-1-acetic acids as potent and selective CRTH2 antagonists—discovery of AZD1981. Bioorgan Med Chem Lett. 2011;21:6288–6292. doi: 10.1016/j.bmcl.2011.08.124. [DOI] [PubMed] [Google Scholar]
- 68.Singh D, Cadden P, Hunter M, et al. Inhibition of the asthmatic allergen challenge response by the CRTH2 antagonist OC000459. Eur Respir J. 2013;41:46–52. doi: 10.1183/09031936.00092111. [DOI] [PubMed] [Google Scholar]
- 69.Horak F, Zieglmayer P, Zieglmayer R, et al. The CRTH2 antagonist OC000459 reduces nasal and ocular symptoms in allergic subjects exposed to grass pollen, a randomised, placebo-controlled, double-blind trial. Allergy. 2012;67:1572–1579. doi: 10.1111/all.12042. [DOI] [PubMed] [Google Scholar]
- 70.Barnes N, Pavord I, Chuchalin A, et al. A randomized, double-blind, placebo-controlled study of the CRTH2 antagonist OC000459 in moderate persistent asthma. Clin Exp Allergy. 2011;42:38–48. doi: 10.1111/j.1365-2222.2011.03813.x. [DOI] [PubMed] [Google Scholar]
- 71.Pettipher R, Hunter MG, Perkins CM, et al. Heightened response of eosinophilic asthmatic patients to the CRTH2 antagonist OC000459. Allergy. 2014;69(9):1223–1232. doi: 10.1111/all.12451. [DOI] [PubMed] [Google Scholar]
- 72.Kuna P, Bjermer L, Tornling G. Two phase II randomized trials on the CRTH2 antagonist AZD 1981 in adults with asthma. Drug Des Dev Ther. 2016;10:1–12. doi: 10.2147/DDDT.S105142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hall IP, Fowler AV, Gupta A, et al. Efficacy of BI 671800, an oral CRTH2 antagonist, in poorly controlled asthma as sole controller and in the presence of inhaled corticosteroid treatment. Pulm Pharmacol Ther. 2015;32:37–44. doi: 10.1016/j.pupt.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 74.Sidharta PN, Diamant Z, Dingemanse J. Single- and multiple-dose tolerability and pharmacokinetics of the CRTH2 antagonist setipiprant in healthy male subjects. Fundam Clin Pharmacol. 2014;28:690–699. doi: 10.1111/fcp.12079. [DOI] [PubMed] [Google Scholar]
- 75.Diamant Z, Sidharta PN, Singh D, et al. Setipiprant, a selective CRTH2 antagonist, reduces allergen-induced airway responses in allergic asthmatics. Clin Exp Allergy. 2014;44:1044–1052. doi: 10.1111/cea.12357. [DOI] [PubMed] [Google Scholar]
- 76.Jia G, Erickson RW, Choy DF, et al. Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J Allergy Clin Immunol. 2012;130:647–654. doi: 10.1016/j.jaci.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]