Abstract
Appraisal of muscle mass is important when considering the serious consequences of sarcopenia in an aging society. However, the associations between sarcopenia and its clinical outcomes might vary according to the method applied in its diagnosis. We compared the relationships between cardiometabolic risk parameters and sarcopenia defined according to three different diagnostic methods using dual-energy X-ray absorptiometry (DXA) and computed tomography (CT). Appendicular skeletal muscle mass (ASM) adjusted by height squared and BMI (ASM/height2 and ASM/BMI) measured using DXA and thigh muscle cross-sectional area (tmCSA) adjusted by weight (tmCSA/weight) measured using CT were used as indices of muscle mass. Sarcopenia was defined as two standard deviations below either the mean ASM/height2, ASM/BMI, or tmCSA/weight of a young reference group. ASM/BMI and tmCSA/weight showed a negative relationship with several components of metabolic syndrome and HOMA-IR, whereas ASM/height2 was positively associated with theses cardiometabolic risk factors. Logistic regression analyses demonstrated that ASM/BMI-defined sarcopenia was significantly associated with increased HOMA-IR (P = 0.01) and prevalence of visceral obesity (P = 0.03) and metabolic syndrome (P = 0.025), while ASM/height2- and tmCSA/weight-defined sarcopenia were not. ASM/BMI-defined sarcopenia exhibits a closer relationship with cardiometabolic risk factors than does ASM/height2- or tmCSA/weight-defined sarcopenia.
Introduction
The epidemiological trend that characterizes our generation is an aging society. Aging results in a progressive loss of muscle mass and strength, called sarcopenia, which is a Greek word meaning ‘poverty of flesh1’. Sarcopenia is related to physiological and metabolic deterioration, as well as functional impairment2, 3. Although its underlying mechanisms have not been fully clarified, several cellular and molecular mechanisms, such as inflammation, insulin resistance, vitamin D deficiency, and decreased physical activity, have been reported to play roles in the development of sarcopenia4–6. Interestingly, mechanisms associated with sarcopenia have been established as important risk factors for cardiometabolic diseases.
Various techniques have been used to assess muscle mass. Although bioelectrical impedance (BIA) and measurement of mid-arm muscle circumference or calf circumference may be easily applicable in the clinical field, they are often considered too inaccurate to provide reliable information7. In contrast, dual energy X-ray absorptiometry (DXA) determines appendicular and total body lean mass, and computed tomography (CT) estimates muscle cross sectional area (CSA).
DXA is a well-defined technique for analyzing body composition and is currently the procedure of choice for assessment of bone mineral density. Based on studies indicating that the amount of appendicular skeletal muscle mass (ASM) could be estimated using the bone-free and fat-free masses of the arms and legs as assessed with DXA8, 9, the most commonly used definition of sarcopenia with DXA has been proposed by Baumgartner et al.10. ASM is divided by height squared (ASM/height2) to adjust for body size, and sarcopenia is defined as a reduction in ASM/height2 relative to the values in a younger reference group. The International Working Groups on Sarcopenia (IWGS)11 and the European Working Group on Sarcopenia in Older People (EWGSOP)12 adopted this criterion based on DXA to define sarcopenia. Older men and women with low muscle mass by DXA-derived skeletal muscle mass adjusted by squared body height were shown to be three to four times more likely to report disability10.
However, these diagnostic criteria for sarcopenia were based on expert opinions and have not been validated with large data sets. Recently, to address this important gap, the Foundation for the National Institutes of Health (FNIH) Sarcopenia Project has developed new consensus criteria for a clinical diagnosis of sarcopenia13. They proposed body mass index (BMI)-adjusted appendicular skeletal muscle mass (ASM/BMI) as a muscle mass index. Hirani et al. reported that incident disability and mortality are associated with sarcopenia, as defined by the FNIH criteria, in elderly Australian men14. In our collaborative study with Australian researchers, appendicular lean mass normalized to BMI was differentially associated with metabolic syndrome, according to ethnicity15.
In contrast, CT and MRI are considered gold standards for clinical research, although they are expensive and difficult to adopt on a large scale7. A 14.7% decline in thigh muscle CSA (tmCSA), assessed by CT, was observed in older men over a period of 12 years16. Visser et al. indicated that smaller mid-thigh muscle area, evaluated by CT, was associated with poorer lower extremity performance in well-functioning older men and women17. Since tmCSA showed a strong association with body weight rather than with body height, tmCSA was corrected by body weight (tmCSA/weight) as a sarcopenic index of body weight burden thigh muscle mass18. In Japanese men, tmCSA/weight was significantly and negatively associated with carotid intima-media thickness and arterial stiffness, reliable indicators of atherosclerosis18. Moreover, quadriceps CSA/weight was related to postural instability, independent of age and sex19.
Despite the associations between sarcopenia and various important health outcomes, there has been very limited research comparing the associations between cardiometabolic risk factors and sarcopenia defined by DXA or CT. Therefore, the aim of the current study was to compare the relationships between sarcopenia indices estimated by DXA and CT (ASM/height2, ASM/BMI, or tmCSA/weight) and cardiometabolic risk factors known to be associated with sarcopenia, such as high sensitivity C-reactive protein (hsCRP), Homeostasis Model Assessment as an index of insulin resistance (HOMA-IR), visceral obesity, and components of metabolic syndrome, in an apparently healthy Asian population.
Methods
Study subjects
The current study included participants from the Korean Sarcopenic Obesity Study (KSOS), a prospective observational cohort study designed to examine the prevalence of sarcopenia and sarcopenic obesity in Korean adults with (diabetic KSOS cohort) or without diabetes (non-diabetic KSOS cohort) and to evaluate their effects on metabolic disorders and health outcomes20, 21. The non-diabetic cohort of adults aged ≥20 years consisted of 526 subjects (198 men and 328 women). Eligible participants in the non-diabetic KSOS cohort had no history of any type of diabetes, CVD (myocardial infarction, unstable angina, stroke, or cardiovascular revascularization), stage 2 hypertension (resting blood pressure, ≥160/100 mmHg), malignant disease, or severe renal or hepatic disease. Medical histories and lifestyle information were collected by personal interview using a detailed questionnaire22. All participants provided written informed consent, and the study was approved by the Korea University Institutional Review Board (IRB No.: MD0715) conforming to the Declaration of Helsinki of the World Medical Association. All methods were performed in accordance with the nationally approved guidelines and regulations.
We analyzed the data of 490 participants who had complete body compositional analysis data by a combination of both DXA and CT to define sarcopenia. Therefore, this study included 125 healthy young adults aged between 20 and 39 years (our reference group) and 365 participants aged 40 years or older (the population of interest).
Clinical and laboratory measurements
BMI was calculated as the weight/height2 (kg/m2), and waist circumference was measured at the midpoint between the lower border of the rib cage and the iliac crest. All blood samples were obtained in the morning after a 12-hour overnight fast and were immediately stored at −80 °C for subsequent assays. Serum triglycerides and high-density lipoprotein (HDL) cholesterol levels were determined enzymatically using a chemistry analyzer (Hitachi 747; Tokyo, Japan). Low-density lipoprotein (LDL) cholesterol levels were calculated according to the Friedewald formula23. A glucose oxidase method was used to measure fasting plasma glucose (FPG), and an immunoradiometric assay (DIAsource Diagnostics, Nivelles, Belgium) was used to measure insulin levels. Insulin resistance was calculated using HOMA-IR24. hsCRP levels were measured by Latex-enhanced Turbidometric Immunoassay (HiSens hsCRP LTIA; HBI Co., Ltd.) with an interassay coefficient of variation of 7.2%. Serum 25[OH]D levels were measured using radioimmunoassay kits (DIAsource Diagnostics, Nivelles, Belgium) with quality control materials provided by the manufacturer. Metabolic syndrome was defined according to criteria established by the National Cholesterol Education Program Adult Treatment Panel III, using the adjusted waist circumference for Asians25. Accordingly, participants with three or more of the following five criteria were defined as having metabolic syndrome: (i) abdominal obesity based on waist circumference (defined as Asian specific waist circumference cut-off values ≥90 cm for men and ≥80 cm for women), (ii) systolic blood pressure ≥130 mmHg, diastolic blood pressure ≥85 mmHg, or use of antihypertensive medication, (iii) elevated FPG (≥5.6 mmol/L), (iv) hypertriglyceridemia (≥1.7 mmol/L), and (v) low serum HDL–cholesterol <1.03 mmol/L in men and <1.29 mmol/L in women.
Measurement of body composition
A whole body DXA scan was performed for each patient to measure regional lean mass (kg), total body fat (kg), and total body fat percentage (%) using fan-beam technology (Hologic Discovery A, Hologic; Bedford, MA, USA). ASM (kg) was defined as the sum of the lean soft tissue mass for the arms and legs, following the method of Heymsfield et al.8. In addition, the tmCSA was quantified by CT (Brilliance 64, Philips Medical Systems, Cleveland, OH). A cross-sectional scan of the left thigh, halfway between the pubic symphisis and the inferior condyle of the femur, was performed, and the mid-thigh muscle CSA was obtained, as previously described26. Skeletal muscle was determined by measuring the mean value of the pixels within an attenuation range of 0 to 100 Hounsfield units to exclude most of the intermuscular, or “marbled,” adipose tissue in the analysis. However, omission of −29 to 0 HU range may fail to account for some significant proportion of the total muscle cross-sectional area in some individuals. Meanwhile, visceral fat area (VFA) was calculated from a 10-mm CT slice scan image between the fourth and fifth lumbar vertebrae, obtained during suspended respiration. VFA was quantified by delineating the intra-abdominal cavity at the internal aspect of the abdominal and oblique muscle walls surrounding the cavity and the posterior aspect of the vertebral body. Fat attenuation was determined by measuring the mean value of the pixels within a range of −190 to −30 Hounsfield units (HU).
Definitions of sarcopenia and visceral obesity
Sarcopenia was defined using three criteria (two criteria by DXA and one criterion by CT). The first criterion was an ASM/height2 less than two standard deviations (SDs) below the sex-specific young adult reference value27. The sex-specific cut-off points of ASM/height2 were 7.45 kg/m2 in men and 5.23 kg/m2 in women, which were slightly different from our previous results due to the exclusion of subjects without CT data. The second criterion was an ASM/BMI less than two SDs below the gender-specific mean value of the young reference group. The ASM/BMI cut-off points for sarcopenia were 0.90 and 0.63 for men and women, respectively. Last, CT-defined sarcopenia was defined as tmCSA/weight less than two SDs below the tmCSA/weight distribution in a young reference group for both men and women19. The cut-off values of tmCSA/weight were 1.58 cm2/kg for men and 1.25 cm2/kg for women. On the other hand, visceral obesity was defined as a VFA ≥100 cm2, which is known to be highly associated with metabolic impairment28.
Statistical analysis
Numerical data are expressed as means ± standard deviation or median [inter-quartile range]. Categorical variables are presented as percentage. Differences in quantitative variables between two groups were investigated using the independent two-sample t-test or the Mann-Whitney U-test, and Pearson’s Chi-square test was used to test for differences in the distribution of categorical variables. Cohen’s κ coefficient is used to assess the degree of agreement between different sarcopenia definitions. We specified sarcopenia as binary categories and estimate the gender-specific coefficients29. A value of 0 implies no agreement beyond chance, whereas a value of 1 corresponds to a perfect agreement between the two methods. Spearman’s partial correlation analysis adjusting for age and gender was performed to determine the relationships between metabolic parameters and sarcopenia indices, such as ASM/height2, ASM/BMI, and tmCSA/weight. Multiple linear regression analysis using each of the sarcopenia indices as a dependent variable was conducted to determine the relative contributions made by each variable to the outcome variable. Age, gender, alcohol consumption, smoking status, physical activity, systolic and diastolic blood pressure, total cholesterol, triglycerides, HDL-cholesterol, HOMA-IR, hsCRP, and 25[OH]D were used as independent variables. Statistically significant independent variables were chosen by means of a stepwise variable selection approach. Logistic regression models were used to evaluate the relationship between sarcopenia defined by DXA or CT as a dependent variable and each of the following independent variables, expressed as odds ratio (OR) with 95% confidence intervals (CI): individual metabolic syndrome components, presence of visceral obesity and metabolic syndrome, hsCRP, HOMA-IR, and 25[OH]D levels after controlling for age, gender, smoking status, alcohol consumption, and physical activity. Finally, factors related to the presence of metabolic syndrome were analyzed by multiple logistic regression analyses with a stepwise backward procedure. All statistical outcomes based on two-sided tests were obtained using SPSS for Windows (Version 12.0, SPSS Inc., Chicago, IL, USA). A P-value < 0.05 was regarded as significant.
Results
In this cohort of 365 subjects aged 40 years and older (136 men and 229 women), the average age was 58.5 ± 9.6 years, and there were no significant age differences between men and women (58.6 ± 10.7 vs 58.5 ± 9.6, P = 0.976). The prevalence of sarcopenia defined by ASM/height2 index was 1.9% and was higher among men (4.4%) than among women (0.4%). In contrast, when defined by ASM/BMI, there was no significant difference in the prevalence of sarcopenia (9.6% in men, 7.9% in women, and 8.5% across both groups). Applying tmCSA/weight, the prevalence of sarcopenia was 2.9% in men, 1.7% in women, and 2.2% in total (Fig. 1). The Cohen’s κ coefficient between the two DXA-defined criteria (ASM/height2 and ASM/BMI) was 0.38 in men, 0.09 in women, and 0.24 in total. By comparing the three different criteria, the degree of consensus among them demonstrated similar trends of low values in females and moderate values in males.
Figure 1.
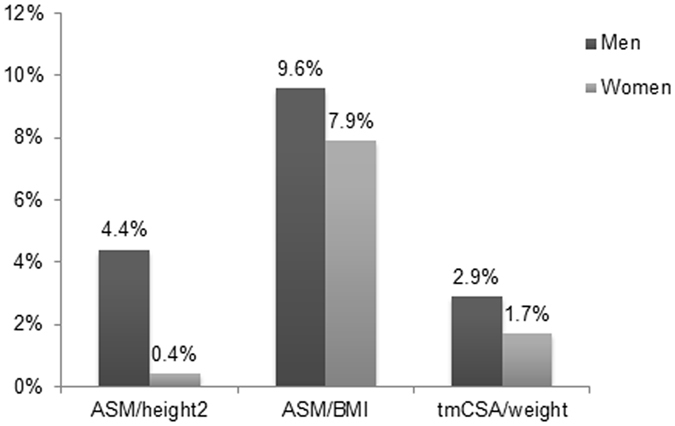
Prevalence (%) of ASM/height2-, ASM/BMI-, and tmCSA/weight-defined sarcopenia according to gender.
Table 1 shows the clinical and metabolic parameters according to the three categories of DXA- and CT-defined sarcopenia. DXA and CT, as well as height and weight adjustments, identified sarcopenic populations with different characteristics; although not statistically significant, subjects fulfilling DXA-defined and the square of height-adjusted sarcopenia criteria (ASM/height2) were older and had lower BMI, waist circumference, and prevalence of metabolic syndrome compared with subjects without sarcopenia. In contrast, subjects fulfilling ASM/BMI-defined or tmCSA/weight-defined sarcopenia criteria had higher BMI and lower HDL-cholesterol than those without sarcopenia. Additionally, serum 25[OH]D level tended to be lower in subjects with ASM/BMI-defined or tmCSA/weight-defined sarcopenia compared to those without sarcopenia. Defining sarcopenia based on ASM/BMI of DXA-defined sarcopenia criteria, HOMA-IR (2.3 [2.0, 3.3] vs. 1.9 [1.4, 2.6], P = 0.001) and serum hsCRP level (0.9 [0.3, 1.7] vs. 0.4 [0.2, 0.9], P = 0.001) were significantly higher in subjects with sarcopenia than in those without sarcopenia. Only subjects who fulfilled the criteria of ASM/BMI-defined sarcopenia had significantly higher prevalence of visceral obesity (P = 0.008) and metabolic syndrome (P = 0.005) relative to those without sarcopenia.
Table 1.
Clinical characteristics of study subjects stratified by sarcopenia defined using DXA and CT.
| Tool | DXA | CT | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Index of sarcopenia | ASM/height2 | ASM/BMI | tmCSA/weight | |||||||||||||
| Clinical variables | No sarcopenia (n = 358) | Sarcopenia (n = 7) | P-value | No sarcopenia (n = 334) | Sarcopenia (n = 31) | P-value | No sarcopenia (n = 357) | Sarcopenia (n = 8) | P-value | |||||||
| Age (years) | 58.4 | ± 9.5 | 63.7 | ± 15.3 | 0.151 | 58.1 | ± 9.7 | 63.6 | ± 8.2 | 0.001 | 58.4 | ± 9.6 | 64.5 | ± 8.2 | 0.074 | |
| Male gender (n (%)) | 130 | (36.3) | 6 | (85.7) | 0.007 | 122 | (36.5) | 13 | (41.9) | 0.551 | 131 | (36.7) | 4 | (50.0) | 0.441 | |
| Alcohol consumption (%) | 196 | (54.7) | 5 | (71.4) | 0.380 | 175 | (52.4) | 26 | (83.9) | 0.001 | 194 | (54.3) | 7 | (87.5) | 0.062 | |
| Current smoker (%) | 67 | (18.7) | 3 | (42.9) | 0.108 | 65 | (19.5) | 5 | (16.1) | 0.652 | 68 | (19.0) | 2 | (25.0) | 0.672 | |
| Physical activity (%) | 233 | (65.1) | 5 | (71.4) | 0.727 | 218 | (65.3) | 20 | (64.5) | 0.933 | 235 | (65.8) | 3 | (37.5) | 0.096 | |
| Body mass index (kg/m2) | 24.6 | ± 3.2 | 22.0 | ± 3.7 | 0.108 | 24.3 | ± 2.9 | 27.5 | ± 4.4 | <0.001 | 24.5 | ± 3.1 | 27.9 | ± 4.0 | 0.003 | |
| Waist circumference (cm) | 85.2 | ± 8.1 | 81.6 | ± 8.9 | 0.324 | 84.7 | ± 7.8 | 89.8 | ± 9.5 | 0.007 | 85.0 | ± 8.0 | 90.8 | ± 10.3 | 0.160 | |
| Systolic BP (mmHg) | 124.4 | ± 12.8 | 127.9 | ± 10.4 | 0.419 | 124.4 | ± 12.9 | 125.5 | ± 11.3 | 0.624 | 124.5 | ± 12.6 | 124.8 | ± 17.3 | 0.966 | |
| Diastolic BP (mmHg) | 80.7 | ± 9.9 | 85.1 | ± 10.1 | 0.291 | 80.9 | ± 9.9 | 79.6 | ± 9.7 | 0.481 | 80.9 | ± 9.9 | 77.1 | ± 10.3 | 0.342 | |
| Total cholesterol (mmol/L) | 4.9 [4.3, 5.4] | 5.1 [4.7, 5.9] | 0.315 | 4.9 [4.3, 5.4] | 5.0 [4.5, 5.3] | 0.703 | 4.9 [4.3, 5.4] | 4.7 [3.5, 5.9] | 0.508 | |||||||
| HDL cholesterol (mmol/L) | 1.4 [1.2, 1.7] | 1.3 [1.2, 1.5] | 0.429 | 1.4 [1.2, 1.7] | 1.3 [1.2, 1.5] | 0.029 | 1.4 [1.2, 1.7] | 1.2 [1.1, 1.3] | 0.034 | |||||||
| Triglycerides (mmol/L) | 1.3 [0.9, 1.8] | 1.2 [1.2, 1.7] | 0.493 | 1.3 [0.9, 1.8] | 1.6 [1.2, 1.9] | 0.063 | 1.3 [0.9, 1.8] | 1.7 [1.3, 1.9] | 0.284 | |||||||
| FPG (mmol/L) | 5.2 [4.9, 5.8] | 5.2 [5.1, 5.7] | 0.532 | 5.2 [4.9, 5.7] | 5.3 [5.0, 6.1] | 0.128 | 5.2 [4.9, 5.8] | 5.4 [5.2, 6.2] | 0.238 | |||||||
| HOMA-IR | 1.9 [1.4, 2.6] | 1.9 [1.7, 2.9] | 0.547 | 1.9 [1.4, 2.6] | 2.3 [2.0, 3.3] | 0.001 | 1.9 [1.4, 2.6] | 2.5 [1.9, 3.0] | 0.098 | |||||||
| hsCRP (mg/L) | 0.4 [0.2, 1.0] | 0.9 [0.3, 2.7] | 0.217 | 0.4 [0.2, 0.9] | 0.9 [0.3, 1.7] | 0.001 | 0.4 [0.2, 1.0] | 0.8 [0.3, 1.6] | 0.259 | |||||||
| 25(OH)D (mmol/L) | 28.9 [20.9, 42.2] | 30.4 [24.0, 44.5] | 0.858 | 29.9 [21.2, 43.2] | 25.2 [18.0, 35.8] | 0.071 | 30.0 [21.2, 42.6] | 21.6 [17.3, 27.3] | 0.098 | |||||||
| Presence of MetS (%) | 118 | (33.0) | 0 | (0) | 0.065 | 101 | (30.2) | 17 | (54.8) | 0.005 | 115 | (32.2) | 3 | (37.5) | 0.752 | |
| Presence of visceral obesity (%) | 235 | (65.6) | 4 | (57.1) | 0.639 | 212 | (63.5) | 27 | (87.1) | 0.008 | 232 | (65.0) | 7 | (87.5) | 0.185 | |
| ASM (kg) | 21.3 | ± 4.7 | 16.8 | ± 2.8 | 0.005 | 21.4 | ± 4.7 | 18.7 | ± 3.4 | 0.002 | 21.2 | ± 4.7 | 20.6 | ± 3.0 | 0.603 | |
| ASM/height2 (kg/m2) | 8.2 | ± 1.2 | 6.6 | ± 0.8 | 0.002 | 8.2 | ± 1.2 | 7.8 | ± 1.3 | 0.112 | 8.2 | ± 1.2 | 7.8 | ± 0.9 | 0.276 | |
| ASM/BMI (kg/kg/m2) | 0.87 | ± 0.17 | 0.79 | ± 0.20 | 0.347 | 0.88 | ± 0.17 | 0.69 | ± 0.12 | <0.001 | 0.87 | ± 0.17 | 0.75 | ± 0.11 | 0.023 | |
| tmCSA/weight (cm2/kg) | 1.8 | ± 0.3 | 1.8 | ± 0.3 | 0.999 | 1.9 | ± 0.3 | 1.7 | ± 0.3 | 0.001 | 1.9 | ± 0.3 | 1.3 | ± 0.2 | <0.001 | |
25[OH]D, 25-hydroxyvitamin D; ASM, appendicular skeletal muscle mass; BMI, body mass index; BP, blood pressure; FPG, fasting plasma glucose; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; hsCRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; MetS, metabolic syndrome; tmCSA, thigh muscle cross-sectional area.
Data are presented as mean ± SD, median [inter-quartile range] or n (%), as appropriate.
P-values represent differences between sarcopenia and non-sarcopenia as determined by Student’s t-test or Wilcoxon rank test for continuous variables, and Fisher’s exact test or Pearson’s Chi-square test for categorical variables.
After adjusting for age and gender, ASM/height2 of DXA-derived muscle mass indices was positively correlated with BMI (r = 0.67, P < 0.001), whereas DXA-derived ASM/BMI and CT-derived index of muscle mass (tmCSA/weight) were negatively correlated with BMI (r = −0.42, P < 0.001 and r = −0.44, P < 0.001, respectively). Moreover, ASM/height2 was positively correlated with measures of adiposity, systolic and diastolic blood pressure, triglycerides, FPG, and HOMA-IR, while ASM/BMI and tmCSA/weight were negatively correlated with waist circumference, serum triglycerides level, HOMA-IR, and hsCRP and positively correlated with HDL cholesterol (Supplementary Table 1). Interestingly, ASM/BMI index was positively correlated with all of muscle indices such as ASM, tmCSA, ASM/height2 and tmCSA/weight, whereas tmCSA/weight index was not correlated with ASM and ASM/height2 among muscle indices.
Multiple regression analyses were performed using each index of muscle mass defined by DXA or CT as a dependent variable and clinical and metabolic parameters as independent variables (Table 2). ASM/height2 was independently and negatively associated with age (P < 0.001) and positively associated with systolic blood pressure (P < 0.001) and serum triglycerides (P < 0.001). In contrast, CT-derived index of muscle mass (tmCSA/weight) was independently and negatively associated with alcohol consumption (P = 0.001), systolic blood pressure (P < 0.001), and serum triglycerides (P = 0.010) and positively associated with 25[OH]D level (P = 0.023). HOMA-IR and physical activity were independently associated with both ASM/BMI and tmCSA/weight, while age was negatively associated with ASM/BMI, but not with tmCSA/weight.
Table 2.
Multiple linear regression analysis to identify clinical and metabolic variables associated with ASM/height2, ASM/BMI, or tmCSA/weight.
| DXA | CT | |||||
|---|---|---|---|---|---|---|
| ASM/height2 | ASM/BMI | tmCSA/weight | ||||
| Coefficient (SE) | P | Coefficient (SE) | P | Coefficient (SE) | P | |
| Age (years) | −0.022 (0.005) | <0.001 | −0.003 (0.001) | <0.001 | −0.002(0.001) | 0.084 |
| Gender (M/F) | 1.424 (0.100) | <0.001 | 0.268 (0.013) | <0.001 | 0.315 (0.027) | <0.001 |
| Physical activity | 0.042 (0.011) | <0.001 | 0.079 (0.023) | <0.001 | ||
| Alcohol consumption | −0.021 (0.013) | 0.103 | −0.071 (0.026) | 0.007 | ||
| Systolic blood pressure (mmHg) | 0.015 (0.004) | <0.001 | −0.001 (0.001) | 0.085 | −0.002 (0.001) | 0.007 |
| Total cholesterol (mmol/L) | −0.128 (0.058) | 0.027 | ||||
| Triglycerides (mmol/L) | 0.224 (0.058) | <0.001 | −0.034 (0.012) | 0.005 | ||
| HOMA-IR | −0.009 (0.002) | <0.001 | −0.020 (0.005) | <0.001 | ||
| HDL-cholesterol (mmol/L) | 0.245 (0.153) | 0.110 | ||||
| 25(OH)D (mmol/L) | 0.002 (0.001) | 0.023 | ||||
| Adjusted R 2 | 47.6% | 67.4% | 48.0% | |||
The following covariates were considered independent variables prior to stepwise variable selection approach: age, gender, alcohol consumption, smoking status, physical activity, systolic blood pressure, total cholesterol, triglycerides, HDL-cholesterol, HOMA-IR, hsCRP, 25(OH)D values.
To investigate whether DXA- or CT-defined sarcopenia was related independently to markers of inflammation and insulin resistance and the presence of visceral obesity and metabolic syndrome, multiple logistic regression analysis was performed (Table 3). After adjusting for age, gender, current smoking, alcohol consumption, and physical activity, ASM/BMI-defined sarcopenia was independently and positively associated with BMI (P < 0.001), HOMA-IR (P = 0.010), waist circumference (0.004) and presence of visceral obesity (P = 0.030), and was negatively associated with ASM (P < 0.001). Although ASM/BMI defined sarcopenia was not independently associated with each metabolic syndrome components, it was independently associated with presence of metabolic syndrome (OR 2.454, CI 1.118, 5.388, P = 0.025). In contrast, although BMI was independently and negatively associated with ASM/height2-sarcopenia and was positively associated with tmCSA/weight, ASM/height2-defined and tmCSA/weight-defined sarcopenia were not independently associated with presence of visceral obesity or metabolic syndrome or with HOMA-IR, hsCRP, or 25[OH]D value.
Table 3.
Logistic analysis to evaluate relationships between sarcopenia defined by DXA or CT and clinical, metabolic, and body composition variables including presence of visceral obesity and metabolic syndrome.
| DXA-defined sarcopenia | CT-defined sarcopenia | ||||||
|---|---|---|---|---|---|---|---|
| ASM/height2 | ASM/BMI | tmCSA/weight | |||||
| OR (95% CI)† | P | OR (95% CI)† | P | OR (95% CI)† | P | ||
| BMI | 0.691 (0506–0.945) | 0.021 | 1.340 (1.181–1.520) | <0.001 | 1.256 (1.034–1.526) | 0.022 | |
| Waist circumference | 0.892 (0.798–0.997) | 0.045 | 1.073 (1.022–1.125) | 0.004 | 1.061 (0.975–1.154) | 0.168 | |
| ASM | 0.088 (0.008–0.939) | 0.044 | 0.682 (0.574–0.810) | <0.001 | 0.912 (0.709–1.173) | 0.472 | |
| Systolic blood pressure | 1.004 (0.941–1.072) | 0.902 | 0.991 (0.959–1.023) | 0.565 | 0.983 (0.926–1.044) | 0.575 | |
| Triglycerides | 0.820 (0.302–2.225) | 0.697 | 1.207 (0.787–1.852) | 0.388 | 0.858 (0.307–2.395) | 0.769 | |
| HDL-cholesterol | 1.703 (0.143–20.206) | 0.673 | 0.476 (0.131–1.732) | 0.260 | 0.173 (0.009–3.176) | 0.237 | |
| Fasting plasma glucose | 0.735 (0.241–2.243) | 0.589 | 1.174 (0.859–1.604) | 0.313 | 1.248 (0.641–2.432) | 0.515 | |
| HOMA-IR | 0.926 (0.582–1.474) | 0.747 | 1.226 (1.050–1.431) | 0.010 | 1.146 (0.991–1.324) | 0.066 | |
| hsCRP | 1.103 (0.953–1.277) | 0.189 | 1.036 (0.889–1.208) | 0.650 | 1.007 (0.739–1.372) | 0.964 | |
| 25(OH)D | 0.995 (0.938–1.056) | 0.877 | 0.976 (0.947–1.005) | 0.108 | 0.949 (0.879–1.024) | 0.179 | |
| Presence of visceral obesity | 0.357 (0.068–1.881) | 0.224 | 3.443 (1.126–10.523) | 0.030 | 2.431 (0.274–21.558) | 0.425 | |
| Presence of Metabolic syndrome | 0 | 0.943 | 2.454 (1.118–5.388) | 0.025 | 0.981 (0.216–4.462) | 0.980 | |
CI, confidence interval; OR, odds ratio.
†Each of the independent variables is included in the logistic regression model after adjusting for age, gender, current smoking, alcohol consumption, and physical activity.
Because few participants with sarcopenia defined by ASM/height2 and tmCSA/weight may limit statistical power, we reanalyzed the relationship between each of three different sarcopenia and cardiometabolic risk factors using 1 SD below sex-specific mean for young reference group as another cutoff value of sarcopenia. Like ASM/BMI-defined sarcopenia, tmCSA/weight-defined sarcopenia was independently associated with obesity indices such as BMI and waist circumference, HOMA-IR and presence of visceral obesity especially in women (Supplementary Table 2), while the association between cardiometabolic risk factors and sarcopenia was non-significant in men when sarcopenia was defined by tmCSA/weight (Supplementary Table 3). However, contrary to our expectations, tmCSA/weight-defined sarcopenia was associated with higher ASM in women. In men, tmCSA/weight-defined sarcopenia was not significantly associated with ASM. Whereas ASM/BMI-defined sarcopenia was significantly associated with higher BMI and lower ASM in each sex using ASM/BMI-1SD of a sex-specific young reference group as a cut-off point of sarcopenia. Meanwhile, multiple logistic regression analysis showed that ASM/BMI defined sarcopenia was a risk factor for the presence of metabolic syndrome both in addition to systolic blood pressure, triglycerides, HDL-cholesterol, FPG, and ASM both in men and in women (Supplementary Table 4).
Discussion
Sarcopenia is known to be associated with increased mortality, as well as functional decline and disability30, 31. Assessment of muscle mass has pivotal clinical importance in the context of increasing awareness of the far-reaching consequences of sarcopenia. Aim of the present cohort study was to compare the relation between sarcopenia as defined by three different methods and cardiometabolic risk factors. We found that sarcopenia as defined by DXA-based appendicular muscle mass adjusted for BMI (ASM/BMI) was associated with insulin resistance, visceral obesity and metabolic syndrome while sarcopenia defined by DXA based ASM adjusted for height2 (ASM/height2) and CT-defined sarcopenia adjusted for weight (tmCSA/weight) were not. Moreover, the prevalence of sarcopenia varied considerably within the same cohort when we applied different indices to define sarcopenia. We observed that the prevalence of sarcopenia was highest when defined by ASM/BMI.
Previous studies about the impact of sarcopenia on cardiometabolic risk factors have not shown consistent results. Aubertin-Leheudre et al. found that obese women without sarcopenia had increased cardiometabolic risk factors, such as a worse lipid profile and increased visceral fat, than did obese women with sarcopenia defined using the DXA method and adjusting for height squared32. Therefore, they concluded that sarcopenia appears to be associated with lower risk factors predisposing cardiovascular disease in obese postmenopausal women32. However, reduced muscle mass assessed using CT and adjusting for weight (tmCSA/weight) was reported to be independently associated with arterial stiffness in a general male population18. This finding suggests that sarcopenia diagnosed based on tmCSA using CT may lead to higher cardiovascular risk. In the present study, tmCSA/weight index was significantly correlated with metabolic syndrome related parameters including BMI even after adjusting for age and gender. In addition, when tm/CSA/weight-1SD of a sex-specific young reference group was used as a cut-off point of sarcopenia, tmCSA/weight-defined sarcopenia was significantly associated with higher ASM as well as higher BMI and HOMA-IR especially in women. These results suggest that tmCSA/weight may be more of an index of obesity rather than one of muscle in women. Although the FNIH definition with ASM/BMI is the most recent evidence-based criterion, and low ASM/BMI may have predictive value for mortality13, 33, little is known about the association between ASM/BMI-defined sarcopenia and metabolic derangement. In the present study, we examined an apparently healthy population without diabetes or cardiovascular disease, adjusting for body size simultaneously using height squared, BMI, or weight and using accurate methods of DXA and CT to define sarcopenia. We compared three different indices of sarcopenia using the cutoff points recommended by several major working groups on sarcopenia (two SDs below the mean levels of a young reference group) with regard to cardio-metabolic risk in this study.
Recently, there has been much debate regarding the relative strengths and weaknesses of the varying definitions of sarcopenia. Baumgartner et al. were the first to define sarcopenia as DXA-derived ASM/height2 two SD below the mean of a young reference group10. Although Baumgartner’s index (ASM/height2) has been used extensively to estimate total or regional skeletal muscle mass in adults and has been validated for this application in older subjects34, Newman et al. indicated that the ASM/height2 index primarily identified individuals with low BMI as sarcopenic and could have the limitation of underestimating sarcopenia in overweight or obese individuals35. In the present study, ASM/height2 of DXA-derived indices of muscle mass showed a positive relationship with metabolic syndrome components, including BMI, waist circumference, systolic and diastolic blood pressure, and triglycerides, even after adjusting for age and gender. As a result, the ASM/height2 index exhibits a positive association with cardiovascular risk factors, which is consistent with previous cross-sectional studies35–37. In contrast, our data indicated that among a set of cardiometabolic risk factors, insulin resistance, metabolic syndrome and visceral obesity appeared as independent predictors of sarcopenia as identified by ASM/BMI.
The etiology of sarcopenia is multifactorial and not fully understood. Many explanations for sarcopenia have been proposed, such as a reduction in anabolic hormone, dysregulation of cytokine secretions, a modification in the inflammatory state, insulin resistance, vitamin D deficiency, and decreased physical activity38, 39. Insulin resistance is associated with metabolic syndrome, which leads to type 2 diabetes and cardiovascular disease. In our previous study, type 2 diabetes was independently associated with increased risk of sarcopenia21. HOMA-IR, as a marker for insulin resistance, was independently and negatively associated with both muscle mass indices (ASM/BMI and tmCSA/weight) after correcting for potential confounding factors. However, HOMA-IR was independently implicated only with ASM/BMI-defined sarcopenia rather than with tmCSA/weight-defined sarcopenia in Korean adults. In addition, we observed an association between ASM/BMI-defined sarcopenia and the presence of metabolic syndrome, whereas ASM/height2- and tmCSA/weight-defined sarcopenia did not show such an independent relationship. Visceral obesity induces dysregulation of inflammatory cytokines, such as TNF-α and interleukin-6 (IL-6), which have catabolic effects on skeletal muscle40. We reported that visceral obesity was related to future loss of skeletal muscle mass in a longitudinal study41.
Physical activity is a pivotal factor associated with both sarcopenia and cardiometabolic indices. Muscle mass, which is increased by a high degree of physical activity, may be a proxy for lifestyle variables. This study found that physical activity is independently and positively associated with ASM/BMI and tmCSA/weight of muscle mass indices as a continuous variable although physical activity was not independently associated with the presence of sarcopenia as a category. In contrast, many previous studies have reported that muscle mass itself is significantly associated with disability and chronic metabolic disorders, even after adjusting for confounding variables, including lifestyle factors10, 21. The present study also demonstrated that different associations with risk factors according to the method of definition persisted after adjusting for lifestyle variables, including physical activity.
There are several limitations to this study. First, we used baseline data from an ongoing prospective cohort study, which did not allow us to determine causality. Analysis of follow-up data might provide appropriate information about causal relationships. Sarcopenia is associated with physical inactivity and exacerbate obesity-associated insulin resistance, and therefore could promote the development of metabolic syndrome12, 40, 42. We found that sarcopenia and metabolic syndrome are deeply interwined and could cause adverse effects on each other. Second, our cohort was composed of relatively well-functioning Asian elderly adults, which limits the ability to generalize our study results to other ethnic groups or populations with different characteristics. Third, few participants with sarcopenia defined tmCSA/weight and ASM/height2 can hinder the ability to detect associations between sarcopenia and cardiovascular risk factors. In addition, statistical analyses were not performed separately for women and men in this study. Therefore, for trending, we performed multiple logistic regression analysis on the association of each of three different indices-defined sarcopenia with cardiometabolic risk factors separately in women and men using 1 SD below sex-specific mean for young reference group. Ideally, our results should be validated in study populations with larger numbers of participants with sarcopenia. Lastly, potentially relevant muscle quality measurements, such as muscle strength and gait speed, were not performed as part of this study. However, a strength of this study is that that we assessed muscle mass using the most precise techniques for detecting sarcopenia, DXA and CT, and simultaneously adjusted for body size in different ways. Furthermore, we also evaluated the potential risk factors for sarcopenia and cardiometabolic diseases, such as components of metabolic syndrome, HOMA-IR, hsCRP, vitamin D level, and presence of visceral obesity assessed by abdominal CT. Although the determination of which operational methods are most appropriate for determining the degree of low muscle mass that actually contributes to clinical outcomes for sarcopenia, including weakness and slowness, in given individuals remains inconclusive, our study revealed that ASM/BMI, the FNIH recommended muscle mass index, is more capable of showing the effects of age and cardiovascular risk factors on the presence of sarcopenia.
In conclusion, the present study demonstrates that sarcopenia diagnosed using the various muscle mass indices of ASM/height2, ASM/BMI, and tmCSA/weight show considerably different relationships with cardiometabolic risk factors known to be associated with the pathogenesis of sarcopenia. Cardiovascular disease risk factors, including insulin resistance index, visceral obesity, and metabolic syndrome, were closely related to ASM/BMI-defined sarcopenia rather than to ASM/height2- or tmCSA/weight-defined sarcopenia.
Electronic supplementary material
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Education, Science, and Technology (NRF-2015R1D1A1A09057389) (K.M.C) and by the Priority Research Centers Program through the NRF, funded by the Ministry of Education, Science, and Technology (2010-0020224) (T.N.K.).
Author Contributions
T.N.K. and K.M.C researched data, contributed to the discussion, and wrote and reviewed/edited the manuscript. M.S.P., E.J.L., H.S.C., H.J.Y., H.J.K., W.S., and S.H.B. researched data, contributed to the discussion, and reviewed/edited the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-06831-7
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rosenberg IH, Roubenoff R. Stalking sarcopenia. Ann Intern Med. 1995;123:727–728. doi: 10.7326/0003-4819-123-9-199511010-00014. [DOI] [PubMed] [Google Scholar]
- 2.Dutta C. Significance of sarcopenia in the elderly. J Nutr. 1997;127:992S–993S. doi: 10.1093/jn/127.5.992S. [DOI] [PubMed] [Google Scholar]
- 3.Ryall JG, Schertzer JD, Lynch GS. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology. 2008;9:213–228. doi: 10.1007/s10522-008-9131-0. [DOI] [PubMed] [Google Scholar]
- 4.Roubenoff R. Physical activity, inflammation, and muscle loss. Nutr Rev. 2007;65:S208–212. doi: 10.1301/nr.2007.dec.S208-S212. [DOI] [PubMed] [Google Scholar]
- 5.Kim, T. N. et al. Relationships between Sarcopenic Obesity and Insulin Resistance, Inflammation, and Vitamin D Status:The Korean Sarcopenic Obesity Study (KSOS). Clin Endocrinol (Oxf) (2012). [DOI] [PubMed]
- 6.Abbatecola AM, Paolisso G. Is there a relationship between insulin resistance and frailty syndrome? Curr Pharm Des. 2008;14:405–410. doi: 10.2174/138161208783497750. [DOI] [PubMed] [Google Scholar]
- 7.Abellan van Kan G, Houles M, Vellas B. Identifying sarcopenia. Curr Opin Clin Nutr Metab Care. 2012;15:436–441. doi: 10.1097/MCO.0b013e328356bbf4. [DOI] [PubMed] [Google Scholar]
- 8.Heymsfield SB, et al. Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am J Clin Nutr. 1990;52:214–218. doi: 10.1093/ajcn/52.2.214. [DOI] [PubMed] [Google Scholar]
- 9.Wang ZM, et al. Skeletal muscle mass: evaluation of neutron activation and dual-energy X-ray absorptiometry methods. J Appl Physiol. 1996;80:824–831. doi: 10.1152/jappl.1996.80.3.824. [DOI] [PubMed] [Google Scholar]
- 10.Baumgartner RN, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 11.Fielding RA, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cruz-Jentoft AJ, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Studenski SA, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirani V, et al. Sarcopenia Is Associated With Incident Disability, Institutionalization, and Mortality in Community-Dwelling Older Men: The Concord Health and Ageing in Men Project. J Am Med Dir Assoc. 2015;16:607–613. doi: 10.1016/j.jamda.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Scott D, et al. Associations of Low Muscle Mass and the Metabolic Syndrome in Caucasian and Asian Middle-aged and Older Adults. J Nutr Health Aging. 2016;20:248–255. doi: 10.1007/s12603-015-0559-z. [DOI] [PubMed] [Google Scholar]
- 16.Frontera WR, et al. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol. 2000;88:1321–1326. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- 17.Visser M, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 18.Ochi M, et al. Arterial stiffness is associated with low thigh muscle mass in middle-aged to elderly men. Atherosclerosis. 2010;212:327–332. doi: 10.1016/j.atherosclerosis.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 19.Ochi M, et al. Quadriceps sarcopenia and visceral obesity are risk factors for postural instability in the middle-aged to elderly population. Geriatr Gerontol Int. 2010;10:233–243. doi: 10.1111/j.1447-0594.2010.00610.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim TN, et al. Prevalence of sarcopenia and sarcopenic obesity in Korean adults: the Korean sarcopenic obesity study. Int J Obes (Lond) 2009;33:885–892. doi: 10.1038/ijo.2009.130. [DOI] [PubMed] [Google Scholar]
- 21.Kim TN, et al. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: the Korean Sarcopenic Obesity Study (KSOS) Diabetes Care. 2010;33:1497–1499. doi: 10.2337/dc09-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salti I, et al. A population-based study of diabetes and its characteristics during the fasting month of Ramadan in 13 countries: results of the epidemiology of diabetes and Ramadan 1422/2001 (EPIDIAR) study. Diabetes Care. 2004;27:2306–2311. doi: 10.2337/diacare.27.10.2306. [DOI] [PubMed] [Google Scholar]
- 23.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 24.Matthews DR, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 25.Grundy SM, et al. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summary. Cardiol Rev. 2005;13:322–327. [PubMed] [Google Scholar]
- 26.Bernard S, et al. Peripheral muscle weakness in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158:629–634. doi: 10.1164/ajrccm.158.2.9711023. [DOI] [PubMed] [Google Scholar]
- 27.Baumgartner RN. Body composition in healthy aging. Annals of the New York Academy of Sciences. 2000;904:437–448. doi: 10.1111/j.1749-6632.2000.tb06498.x. [DOI] [PubMed] [Google Scholar]
- 28.Cabre A, et al. Fatty acid binding protein 4 is increased in metabolic syndrome and with thiazolidinedione treatment in diabetic patients. Atherosclerosis. 2007;195:e150–158. doi: 10.1016/j.atherosclerosis.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 29.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 30.Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci. 2002;57:B359–365. doi: 10.1093/gerona/57.10.B359. [DOI] [PubMed] [Google Scholar]
- 31.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 32.Aubertin-Leheudre M, Lord C, Goulet ED, Khalil A, Dionne IJ. Effect of sarcopenia on cardiovascular disease risk factors in obese postmenopausal women. Obesity (Silver Spring) 2006;14:2277–2283. doi: 10.1038/oby.2006.267. [DOI] [PubMed] [Google Scholar]
- 33.Moon JH, et al. Predictive Values of the New Sarcopenia Index by the Foundation for the National Institutes of Health Sarcopenia Project for Mortality among Older Korean Adults. PLoS One. 2016;11:e0166344. doi: 10.1371/journal.pone.0166344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansen RD, Raja C, Aslani A, Smith RC, Allen BJ. Determination of skeletal muscle and fat-free mass by nuclear and dual-energy x-ray absorptiometry methods in men and women aged 51-84 y (1-3) Am J Clin Nutr. 1999;70:228–233. doi: 10.1093/ajcn.70.2.228. [DOI] [PubMed] [Google Scholar]
- 35.Newman AB, et al. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–1609. doi: 10.1046/j.1532-5415.2003.51534.x. [DOI] [PubMed] [Google Scholar]
- 36.Kim TN, et al. Skeletal muscle mass to visceral fat area ratio is associated with metabolic syndrome and arterial stiffness: The Korean Sarcopenic Obesity Study (KSOS) Diabetes Res Clin Pract. 2011;93:285–291. doi: 10.1016/j.diabres.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 37.Lim S, et al. Sarcopenic obesity: prevalence and association with metabolic syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA) Diabetes Care. 2010;33:1652–1654. doi: 10.2337/dc10-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boirie Y. Physiopathological mechanism of sarcopenia. J Nutr Health Aging. 2009;13:717–723. doi: 10.1007/s12603-009-0203-x. [DOI] [PubMed] [Google Scholar]
- 39.Visser M, Deeg DJ, Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88:5766–5772. doi: 10.1210/jc.2003-030604. [DOI] [PubMed] [Google Scholar]
- 40.Choi KM. Sarcopenia and sarcopenic obesity. Endocrinol Metab (Seoul) 2013;28:86–89. doi: 10.3803/EnM.2013.28.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim TN, et al. Impact of visceral fat on skeletal muscle mass and vice versa in a prospective cohort study: the Korean Sarcopenic Obesity Study (KSOS) PLoS One. 2014;9:e115407. doi: 10.1371/journal.pone.0115407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PLoS One. 2010;5:e10805. doi: 10.1371/journal.pone.0010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


