Abstract
Klebsiella pneumoniae (KP) resistance to broad-spectrum cephalosporin (BSC) in meningitis is important because of limited therapeutic options. To investigate the antibiotic resistance, virulence and epidemiology of KP in meningitis, we conducted a retrospective study for 33 non-metastatic isolates, including primary meningitis (n = 20) and post-craniotomy meningitis (n = 13) collected from 1999 to 2013. BSC resistance was found in 9 (27.3%) isolates, all from post-craniotomy meningitis, harboring bla SHV-5 (n = 6), bla CMY-2 (n = 2), bla DHA-1 (n = 2), and bla TEM-1B (n = 1). Positive virulence factors were hypermucoviscosity (n = 22), larger bacterial size (n = 24), virulent capsule serotypes (n = 24, K2, 11; K1, 5; K57, 3; K5, 2; K20, 2 and K54, 1), rmpA (n = 23), rmpA 2 (n = 20), aerobactin gene (n = 22) and high-grade serum resistance (n = 23, 69.7%). Higher mouse lethality (LD50 < 106) was found in 16 isolates (48.5%). Post-craniotomy isolates were significantly less virulent than primary meningitis isolates, except for similar serum resistance capability. The pulsotype and sequence typing (ST) results were diverse. A minor cluster with pulsotype C and ST23 (n = 5) was identified in primary meningitis isolates. In conclusion, virulence factors and BSC resistance corresponded to about 70% and 30% of KP meningitis isolates respectively. BSC remains appropriate for treating primary meningitis, whereas meropenem is indicated for post-craniotomy meningitis empirically.
Introduction
Klebsiella pneumoniae causes different types of community-acquired and healthcare-associated infections, including pneumonia, bloodstream infection, surgical site infections, liver abscess and meningitis1–4. The clinical spectrum of K. pneumoniae meningitis can be categorized into 3 distinct forms: first, metastatic meningitis, particularly from the distant liver abscesses1; second, post-craniotomy meningitis following neurosurgical procedures for brain lesions or head injury; and third, primary or spontaneous meningitis, usually among elderly patients or those with underlying immunocompromised conditions5.
In Taiwan, K. pneumoniae has remained the leading causative pathogen with prevalence rates about 25% to 40% among adult patients with bacterial meningitis6–10. Besides, K. pneumoniae was responsible for 50% of severe meningitis in adult patients who required intensive care and up to 68% among a subpopulation of adult diabetic patients11, 12, with high mortality rates ranging from 48.5% to 66%5, 13.
The pathogenesis of K. pneumoniae for being capable of invasion into the central nervous system is not well elucidated. The meningeal metastatic isolates frequently arising from the liver abscess have been recognized as “hypervirulent” K. pneumoniae (hvKP), which is commonly hypermucoviscous on blood agar plates2, 4. Distinct from hvKP, less virulent “classic” K. pneumoniae (cKP) that does not exhibit the hypermucoviscosity (HV) phenotype usually causes various infections in hospitals14. We previously reported that the rmpA (regulator of mucoid phenotype) gene was highly prevalent among both primary meningitis and liver abscess isolates15. Nonetheless, the detailed profiles of virulence, virulence determinant factors and relationship with antibiotic resistance of K. pneumoniae from non-metastatic meningitis isolates have not been fully characterized. We hypothesized that post-craniotomy meningitis could be caused by cKP through disruption of the central nervous system barrier, whereas primary meningitis might commonly be caused by hvKP.
Therefore, we aimed to investigate the molecular epidemiology and representative virulence factors in terms of capsule sizes, capsule serotypes, HV phenotype, serum resistance, pathogenic genes, including plasmid-borne rmpA, rmpA2, aerobactin gene and chromosomal rmpA (c-rmpA), kfu (responsible for an iron uptake system) and allS (associated with allantoin metabolism), as well as mouse lethality capability for K. pneumoniae strains isolated from cerebrospinal fluid (CSF) among adult patients without liver or distant abscesses. Antibiotic resistance profiles and virulence factors between non-metastatic K. pneumoniae isolates of primary meningitis and post-craniotomy meningitis were compared.
Materials and Methods
Bacterial isolates
We previously collected 33 K. pneumoniae isolates from CSF of non-metastatic meningitis patients without liver or distant abscess, including isolates from Taipei Veterans General Hospital in Taipei City, northern Taiwan, 2003–2006 (n = 7); National Cheng-Kung University Hospital in Tainan City, southern Taiwan, 1999–2007 (n = 10); and Chi-Mei Medical Center in Tainan City, southern Taiwan, 2005–2013 (n = 16). The isolates included primary meningitis (n = 20) and post-craniotomy meningitis (n = 13). These isolates were obtained as part of routine care on clinical indication.
Antimicrobial susceptibility testing
The minimum inhibitory concentrations (MICs) of antimicrobial agents were determined by using the agar dilution method16. The tested compounds included ampicillin, aztreonam, cefotaxime, ceftazidime, cefepime, piperacillin-tazobactam, meropenem, ciprofloxacin, colistin sulfate (Sigma Chemical Company, St. Louis, MO, USA) and tigecycline (Wyeth, Puerto Rico).
According to the British Society for Antimicrobial Chemotherapy Standing Committee for Antimicrobial Susceptibility Testing (Version 14.0, 2015), the susceptible MIC breakpoint for colistin against Enterobacteriaceae is ≦2 μg/mL and it is considered resistant if MIC > 2 μg/mL. The breakpoints for tigecycline against Enterobacteriaceae are ≦1μ g/mL as susceptible and >2 μg/mL as resistant.
Phenotypic detection of extended-spectrum β-lactamase (ESBL)
ESBL double-disk synergy test was performed with disks containing ceftazidime or cefepime with clavulanic acid on Mueller-Hinton agar plates, and the results were interpreted as described previously17. Control experiments were assured by testing E. coli ATCC 25922 and K. pneumoniae 700603.
DNA manipulation and PCR amplification
Genomic DNA was extracted using QIAamp DNA Mini Kit (Qiagen, Hilden, Germany). Plasmid DNA was also extracted by QIAprep Spin Miniprep (Qiagen, Hilden, Germany). PCR amplifications were performed by using specific primers as previous described.
PCR detection and sequencing of antibiotic resistance genes
Plasmid DNA as templates, PCR was used to amplify the ESBL genes (bla TEM, bla SHV, bla CTX-M), ampC genes (bla DHA-1 and bla CMY-2) and to screen the representative carbapenemase gene (bla KPC-2) using specific primers as previously published18. Amplicons of β-lactamase genes were purified with PCR clean-up kits (Roche Diagnostics, GmbH, Penzberg, Germany) and were sequenced on an ABI PRISM 3730 sequencer analyzer (Applied Biosystems, Foster City, CA, USA).
PCR detection of virulence factors
Genomic DNA as templates, PCR was used to amplify the K capsule serotype-specific genes (including 6 liver abscess-associated capsule serotypes K1, K2, K5, K20, K54, and K57), c-rmpA, kfu and allS genes. Plasmid DNA as templates, PCR was used to amplify the rmpA, rmpA2 and aerobactin gene. Specific primers were used as previously described19–23.
Phenotypic detection of virulence factors with HV phenotype and capsule size
The HV phenotype was defined positive as a viscous string of >5 mm of the colony on blood agar plate24. We measured bacterial size to represent capsule size of the bacteria. The microscopic images of bacterial size in short transverse diameter randomly counted from 100 bacterial cells per single isolate were analyzed by using the image analysis software cellSens standard version 1.8 (Olympus Optical Co. Ltd., Tokyo, Japan).
Phenotypic detection of virulence factor with serum resistance
The susceptibility of the K. pneumoniae isolates to human serum was analyzed as described previously25. In brief, twenty-five microliters of the bacterial suspension (about 2 × 106 CFU) were mixed with 75 μl of pooled normal human serum in microtiter plates and then incubated at 37 °C for a period of 3 h. The test was performed in triplicate and the number of recovered bacteria was determined and graded. Resistance grading was defined from grade 1 to grade 6, with grade 6 (viable counts at 1, 2 and 3 h >100% and increasing throughout the 3-h period) considered to be the most serum resistant. Grades 5 and 6 were regarded as high-grade serum resistance.
Mouse lethality experiment
Determination of the virulence of K. pneumoniae in mouse lethality tests and the median lethal dose (LD50) was performed as described previously22. In brief, female BALB/c mice (6–7 weeks old) were obtained from the National Laboratory Animal Center (NLAC) (Tainan, Taiwan). Mice were maintained under standard conditions of temperature, light and feeding according to NLAC guidelines and the Chi Mei Medical Center Animal Care and Use Committee approved-protocols (Permit Number: 100120771). Each dose was injected intraperitoneally with 0.1 mL of bacterial suspension into four mice. After 14 days, calculations were based on the number of survivors. The degree of virulence was read as highly virulent for an LD50 of <103 CFU; moderate virulence for an LD50 of 104–105 CFU; low virulence for an LD50 of 106–107 CFU; and no virulence for an LD50 of >108 CFU.
Molecular genotyping of isolates
Pulsed-field gel electrophoresis (PFGE)
PFGE, as a major strain typing method, was used to confirm genetic relatedness of isolates as described by Pfaller et al.26. Whole chromosomal DNA in agarose was digested with XbaI (Bio-Rad Laboratories, CA., USA), and the restriction fragments were separated in a CHEF Mapper XA System (Bio-Rad Laboratories, CA., USA). Cluster analysis was performed by the Dice similarity coefficients and unweighted-pair group matching algorithm (UPGMA) with a tolerance of 1.0% and 1.0% optimization using the BioNumerics program. All bands had to match exactly to classify isolates as indistinguishable. Isolates were designated nontypeable if repeated attempts to prepare DNA failed.
Multilocus sequence typing (MLST)
MLST was performed with 7 housekeeping genes as previously described27. Multiple sequences alignment analysis was conducted by using Institute Pasteur (http://bigsdb.web.pasteur.fr/klebsiella/klebsiella.html).
Data collection
The imaging studies and following main data relevant to patient characteristics were collected: gender, age, comorbidities, use of ventilator, CSF profiles, and random blood sugar obtained on the day of CSF collection. Outcome was described as in-hospital mortality within the same hospital episode.
Statistical Analysis
The Fisher’s exact test was used for categorical variables and Student’s t-test was used for continuous variables, where appropriate. A two-tailed p value < 0.05 was considered statistically significant. All statistics were performed using Stata version 12.1 (Stata Press, College Station, TX, USA).
Ethical approval
The study and waiver from the inform consent process were approved by the Institutional Review Board (IRB) of the Chi Mei Medical Center, Tainan city, Taiwan (IRB Serial number 10603-013).
Results
MIC
All 20 primary meningitis isolates were susceptible to cefotaxime and ceftazidime. Nine of 13 post-craniotomy meningitis isolates were resistant to cefotaxime and ceftazidime, but were all susceptible to meropenem, colistin and tigecycline. These 9 isolates were of multi-resistant phenotype. In contrast, all the 20 primary meningitis isolates were only resistant to ampicillin (Table 1).
Table 1.
MIC profiles of cerebrospinal fluid Klebsiella pneumoniae isolates from post-craniotomy and primary meningitis.
| MIC (μg/mL) of antibiotic compounds | Post-craniotomy meningitis (n = 13) | Primary meningitis (n = 20) | Total (n = 33) |
|---|---|---|---|
| Ampicillin | |||
| MIC50 | >16 | >16 | >16 |
| MIC90 | >16 | >16 | >16 |
| MIC range | >16 | >16 | >16 |
| Aztreonam | |||
| MIC50 | >16 | ≤1 | ≤1 |
| MIC90 | >16 | ≤1 | >16 |
| MIC range | ≤1–>16 | ≤1 | ≤1–>16 |
| Cefotaxime | |||
| MIC50 | >32 | ≤1 | ≤1 |
| MIC90 | >32 | ≤1 | >32 |
| MIC range | ≤1–>32 | ≤1–2 | ≤1–>32 |
| Ceftazidime | |||
| MIC50 | >16 | ≤1 | ≤1 |
| MIC90 | >16 | ≤1 | >16 |
| MIC range | ≤1–>16 | ≤1 | ≤1–>16 |
| Cefepime | |||
| MIC50 | 2 | ≤1 | ≤1 |
| MIC90 | 16 | ≤1 | 8 |
| MIC range | ≤1–>16 | ≤1 | ≤1–>16 |
| Piperacillin-tazobactam | |||
| MIC50 | >64 | ≤4 | ≤4 |
| MIC90 | >64 | 8 | >64 |
| MIC range | ≤4–>64 | ≤4–16 | ≤4–>64 |
| Meropenem | |||
| MIC50 | ≤0.25 | ≤0.25 | ≤0.25 |
| MIC90 | ≤0.25 | ≤0.25 | ≤0.25 |
| MIC range | ≤0.25 | ≤0.25 | ≤0.25 |
| Ciprofloxacin | |||
| MIC50 | 2 | ≤0.06 | ≤0.06 |
| MIC90 | >2 | 0.12 | 2 |
| MIC range | ≤0.06–>2 | ≤0.06–025 | ≤0.06–>2 |
| Colistin | |||
| MIC50 | 1 | 1 | 1 |
| MIC90 | 2 | 1 | 1 |
| MIC range | ≤0.5–2 | ≤0.5–1 | ≤0.5–2 |
| Tigecycline | |||
| MIC50 | 0.5 | 0.5 | 0.5 |
| MIC90 | 1 | 1 | 1 |
| MIC range | 0.5–1 | ≤0.25–2 | ≤0.25–2 |
ESBL and AmpC phenotypes
Resistance to cefotaxime and ceftazidime was found in 9 isolates. Among them, ESBL phenotype was detected in 7 isolates and AmpC phenotype was suspicious for 2 isolates (KP-004 and KP-042).
ESBL and AmpC β-lactamase genes
PCR amplifications for β-lactamase genes were performed for all 33 isolates. ESBL genes were detected in 7 isolates (6 with bla SHV-5 and 1 with bla TEM-1B). AmpC genes were detected in 4 isolates (2 with bla CMY-2 and 2 with bla DHA-1), including 2 isolates with dual ESBL and AmpC genes (1 with bla SHV-5 plus bla DHA-1 and 1 with bla TEM-1B plus bla CMY-2). KPC-2 gene was not identified. Overall, 9 of 13 (69.2%) post-craniotomy meningitis isolates produced ESBL and/or AmpC β-lactamases (Table 2).
Table 2.
Epidemiology and virulence profiles of Klebsiella pneumoniae isolates from CSF of post-craniotomy and primary meningitis.
| CSF Strains | PFGE | MLST | ESBL | AmpC | Virulence factors | LD50 (log) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacterial size (μm) | HV (mm) | Capsule K | rmpA | rmpA2 | c-rmpA | Kfu | allS | aerobactin | SR | ||||||
| Post-craniotomy meningitis (n = 13) | |||||||||||||||
| KP-004a | A | 17 | − | CMY-2 | 1.11 ± 0.21 | <0.5 | x | − | − | − | − | − | − | G2 | >6.50 |
| KP1050b | O | 107 | SHV-5 | − | 1.42 ± 0.16 | <0.5 | x | − | − | − | + | − | − | G6 | >6.84 |
| KP1051b | H1 | 17 | SHV-5 | − | 1.44 ± 0.11 | <0.5 | x | − | − | − | − | − | − | G2 | >6.72 |
| KP1052b | N1 | 15 | SHV-5 | DHA-1 | 1.72 ± 0.07 | <0.5 | x | − | − | − | − | − | − | G6 | >6.42 |
| KP1053b | G | 54 | SHV-5 | − | 1.28 ± 0.15 | <0.5 | x | − | − | − | − | − | − | G6 | >6.55 |
| KP1054b | I1 | 86 | SHV-5 | − | 2.98 ± 0.41 | <0.5 | 2 | + | + | − | − | − | + | G5 | >6.88 |
| KP-042c | unidentified | 185 | − | DHA-1 | 1.31 ± 0.18 | <0.5 | x | − | − | − | − | − | − | G4 | >6.52 |
| KP-023c | D | undefined | TEM-1B | CMY-2 | 0.92 ± 0.16 | <0.5 | x | − | − | − | + | − | − | G3 | >6.36 |
| KP-201c | E2 | 65 | SHV-5 | − | 4.62 ± 0.65 | >2 | 2 | + | + | − | − | − | + | G6 | 4.13 |
| KP-001a | J1 | 373 | − | − | 4.66 ± 0.66 | >2 | 2 | + | − | − | − | − | + | G3 | 5.83 |
| KP-007a | S | 661 | − | − | 1.04 ± 0.08 | <0.5 | x | − | − | − | − | − | − | G2 | >6.84 |
| KP-232c | E1 | 65 | − | − | 3.95 ± 0.46 | >2 | 2 | + | + | − | − | − | + | G5 | 5.05 |
| KP-960b | N2 | 15 | − | − | 1.27 ± 0.17 | <0.5 | 5 | − | − | − | + | − | − | G6 | >6.71 |
| Primary meningitis (n = 20) | |||||||||||||||
| KP-002a | C4 | 23 | − | − | 2.42 ± 0.33 | >2 | 1 | + | − | − | + | + | + | G5 | 4.83 |
| KP-003a | C5 | 23 | − | − | 10.73 ± 1.11 | >2 | 1 | + | + | − | + | + | + | G6 | 4.50 |
| KP-005a | M | 268 | − | − | 7.24 ± 0.76 | >2 | 20 | + | + | − | − | − | + | G5 | >6.49 |
| KP-006a | Q | 65 | − | − | 3.67 ± 0.42 | 0.5–1 | 2 | + | + | − | − | − | + | G6 | 4.41 |
| KP-064c | R1 | 218 | − | − | 3.74 ± 0.49 | >2 | 57 | + | + | − | − | − | + | G2 | >6.45 |
| KP-088c | P2 | 373 | − | − | 4.77 ± 0.46 | >2 | 2 | + | + | − | − | − | + | G6 | 1.59 |
| KP-477c | C3 | 23 | − | − | 7.75 ± 1.37 | >2 | 1 | + | + | + | + | + | + | G5 | 4.41 |
| KP-600c | C1 | 23 | − | − | 2.33 ± 0.24 | <0.5 | x | − | − | − | − | − | − | G2 | >6.45 |
| KP-777c | C2 | 23* | − | − | 5.30 ± 0.71 | >2 | 1 | + | + | + | + | + | + | G6 | 4.98 |
| KP-164c | R1 | 218 | − | − | 3.04 ± 0.48 | >2 | 57 | + | + | − | − | − | − | G3 | >6.15 |
| KP-552b | B | 23 | − | − | 7.05 ± 0.72 | >2 | 1 | + | + | − | + | + | + | G5 | 5.35 |
| KP-780b | P1 | 373 | − | − | 6.92 ± 0.53 | >2 | 2 | + | + | − | − | − | + | G6 | >6.44 |
| KP-783b | P1 | 373 | − | − | 3.06 ± 0.43 | >2 | 2 | + | + | − | − | − | + | G6 | 5.4 |
| KP-957b | K | 65 | − | − | 4.56 ± 0.56 | >2 | 2 | + | + | − | − | − | + | G6 | 3.94 |
| KP-958b | I2 | 86* | − | − | 3.45 ± 0.49 | >2 | 2 | + | + | − | − | − | + | G5 | 1.56 |
| KP-959b | unidentified | 1049 | − | − | 4.71 ± 0.89 | >2 | 5 | + | + | − | + | - | + | G6 | >6.50 |
| KP-961b | J2 | 373 | − | − | 4.58 ± 0.39 | >2 | 2 | + | + | − | − | − | + | G6 | 3.77 |
| KP-962b | F | 1544 | − | − | 5.09 ± 1.06 | 0.5–1 | 20 | + | + | − | + | − | + | G6 | 5.14 |
| KP-963b | H2 | 29 | − | − | 4.51 ± 0.53 | >2 | 54 | + | + | − | − | − | + | G6 | 5.41 |
| KP-966b | L | 592 | − | − | 2.75 ± 0.45 | 0.5–1 | 57 | + | − | − | − | − | + | G2 | >6.59 |
NOTE. CSF, cerebrospinal fluid; PFGE, Pulsed-field gel electrophoresis; MLST, multilocus sequence typing (*one nucleotide change); SR, serum resistance (G: grade); ESBL, extended-spectrum β-lactamase; HV, hypermucoviscosity phenotype. x, unidentified, non-virulent capsule K types (i.e., not for K1, K2, K5, K20, K54, K57 types). aFrom Taipei Veterans General Hospital in Taipei City, northern Taiwan, 2003–2006 (n = 7); bfrom Chi-Mei Medical Center in Tainan City, southern Taiwan, 2005–2013 (n = 16); cfrom National Cheng-Kung University Hospital in Tainan City, southern Taiwan, 1999–2007 (n = 10).
Hypermucoviscosity (HV) phenotype
The prevalence of HV phenotype was 66.7% (22/33) of K. pneumoniae CSF isolates in general, and was specifically in the subgroups of the isolates as follows: (1), 95.0% (19/20) of primary meningitis isolates; (2), 23.1% (3/13) of post-craniotomy isolates and (3), 11.1% (1/9) of ESBL/AmpC-producing isolates (Table 2). Primary meningitis isolates were significantly more hypermucoviscous than post-craniotomy meningitis isolates (p < 0.0001), in accordance with our hypothesis that hvKP was the main etiology of primary KP meningitis, while cKP was mainly responsible for post-craniotomy meningitis (Table 3). The cKP group has higher prevalence of ESBL/AmpC production than hvKP group (9/11 vs. 0/22, p < 0.0001, Table 2).
Table 3.
Different virulence profiles between cerebrospinal fluid Klebsiella pneumoniae (KP) isolates from post-craniotomy and primary meningitis.
| Cerebrospinal fluid KP isolates (n = 33) | Post-craniotomy meningitis (n = 13) | Primary meningitis (n = 20) | p |
|---|---|---|---|
| ESBL/AmpC genotypes | 9 | 0 | <0.0001* |
| Bacterial (capsule) size | |||
| >2 mm (n = 24) | 4 | 20 | <0.0001* |
| Hypermucoviscosity | |||
| Phenotype (n = 22) | 3 | 19 | <0.0001* |
| rmpA (n = 23) | 4 | 19 | 0.0002* |
| rmpA2 (n = 20) | 3 | 17 | 0.0007* |
| c-rmpA (n = 2) | 0 | 2 | 0.5076 |
| Capsule K serotype | |||
| Virulent K types (K1, K2, K5, K20, K54, K57) | 5 | 19 | 0.0007* |
| K1/K2 | 4 | 12 | 0.1571 |
| K1 | 0 | 5 | 0.1310 |
| K2 | 4 | 7 | 1.0000 |
| K5 | 1 | 1 | 1.0000 |
| K20 | 0 | 2 | 0.5076 |
| K54 | 0 | 1 | 1.0000 |
| K57 | 0 | 3 | 0.2614 |
| Kfu (n = 10) | 3 | 7 | 0.7006 |
| allS (n = 5) | 0 | 5 | 0.1310 |
| Aerobactin gene (n = 22) | 4 | 18 | 0.0007* |
| Serum resistance | |||
| Grades 5–6 (n = 23) | 7 | 16 | 0.1393 |
| Grades 3–4 | 3 | 1 | 0.2757 |
| Grades 1–2 | 3 | 3 | 0.6588 |
| LD50 (log) | |||
| >6 (n = 17) | 10 | 7 | 0.0324* |
| 4–6 | 3 | 9 | 0.2775 |
| <4 | 0 | 4 | 0.1359 |
Bacterial (capsule) size
By measuring the short transverse diameter, mean bacterial size of each bacterium ranged from 0.92 ± 0.16 μm to 10.73 ± 1.11 μm. All 20 isolates recovered from primary meningitis had mean bacterial sizes of more than 2 μm. All nine strains with a mean bacterial size of <2 μm were post-craniotomy meningitis isolates, which were significantly different to primary meningitis isolates (p < 0.0001). In addition, only two of the nine isolates with ESBL/AmpC β-lactamase production had a mean bacterial size more than 2 μm (Tables 2 and 3).
The HV phenotype was highly prevalent in the isolates with mean bacterial size of >2 mm (91.7%, 22/24), whereas none of the nine isolates with mean capsule size of <2 mm had the HV phenotype (p < 0.0001).
Capsule K genotype
The common liver abscess-associated capsule serotypes were identified in 24 (72.7%) isolates by PCR methods, including capsule K2 (11 strains), K1 (5 strains), K57 (3 strains), 2 strains each for K5 and K20, and one K54 respectively. These virulent K serotypes were highly prevalent in primary meningitis isolates than in post-craniotomy isolates (19/20 vs. 5/13, p = 0.0007). However, specific K serotype (such as K1 or K2) was not statistically different between the two groups (Table 3).
Meanwhile, the isolates with virulent K serotypes had higher prevalence of >2 μm bacterial size (23/24 vs. 1/9, p < 0.0001), higher proportion of HV phenotype (22/24 vs 0/9, p < 0.0001), and lower prevalence of ESBL/AmpC production (2/24 vs. 7/9, p = 0.0003) than those with undefined capsule K serotypes (Table 2).
Prevalence of virulence-associated genes
Among the isolates of K. pneumoniae recovered from CSF, the prevalence of rmpA was 69.7% (23/33); rmpA2, 60.6% (20/33); c-rmpA2, 6.1% (2/33); kfu, 30.3%, (10/33); alls, 15.6% (5/33); and aerobactin gene, 66.7% (22/33). In general, the rmpA, rmpA2 and aerobactin gene were highly prevalent in primary meningitis isolate than in post-craniotomy isolates (Table 3).
The HV phenotype was highly prevalent in those with rmpA-positive vs. rmpA-negative isolates (22/23 vs. 0/10, p < 0.0001), with rmpA2-positive vs. rmpA2-negative isolates (19/20 vs. 3/13, p < 0.0001) and with aerobactin-positive vs. aerobactin-negative isolates (21/22 vs. 1/11, p < 0.0001). In similar, the isolates positive for rmpA, rmpA2 and aerobactin gene had larger bacterial size (≥2 μm) and were highly prevalent in the isolates with virulent K serotypes (such as K1, K2, K5, K20, K54 and K57).
Serum resistance
In general, the distribution of isolates in the grading of serum resistance were 23 strains in grades 5 and 6; grade 4 (1 strain), grade 3 (3 strains); and grade 2 (6 strains) (Table 3). Unexpectedly, the prevalence of high serum resistance (grades 5 and 6) could not reach statistically significant difference between primary meningitis isolates and post-craniotomy isolates (16/20 vs. 7/13, p = 0.1393), which was distinct from the above-mentioned virulence factors between groups (Table 3).
However, the 23 isolates with high serum resistance (grades 5–6) were still significantly associated with virulence K serotypes (n = 20, p = 0.0105), HV phenotype (n = 18, p = 0.0494), rmpA (n = 19, p = 0.035), rmpA2 (n = 19, p = 0.0007) and aerobactin gene (n = 19, p = 0.0059), in comparison to isolates with lower grade serum resistance (grades 1–4).
Mouse lethality experiments
LD50 > 106 (low virulence) was found in 10 of 13 post-craniotomy isolates, but in 7 of 20 primary meningitis isolates (p = 0.0324). The prevalence of virulence factors were significantly higher in isolates with LD50 < 106 (high virulence) than those with low virulence (LD50 > 106), including mean bacterial size >2 μm (16/16 vs. 8/17, p = 0.0009), HV phenotype (16/16 vs. 6/17, p < 0.0001), rmpA (16/16 vs. 7/17, p = 0.0003), rmpA2 (14/16 vs. 6/17, p = 0.0039), aerobactin gene (16/16 vs. 6/17, p < 0.0001), virulent K serotypes (16/16 vs. 8/17, p = 0.0009) and high-grade serum resistance (15/16 vs. 8/17, p = 0.0066).
PFGE
Two isolates were nontypeable. The remaining 31 isolates were classified into 19 major pulsotypes (A~S types) by using the Dice coefficient and UPGMA algorithm (Fig. 1). Among them, 8 pulsotypes with a similarity coefficient of more than 80% included 5 isolates in pulsotype C (C1-C5), 3 isolates in pulsotype P (P1-P2) and 2 isolates each in pulsotype E (E1-E2), pulsotype H (H1-H2), pulsotype I (I1-I2), pulsotype J (J1-J2), pulsotype N (N1-N2) and pulsotype R (R1-R2). In general, no major single clone (indistinguishable or >95% similarity) was broadly disseminated among the K. pneumoniae strains that caused meningitis in Taiwan.
Figure 1.
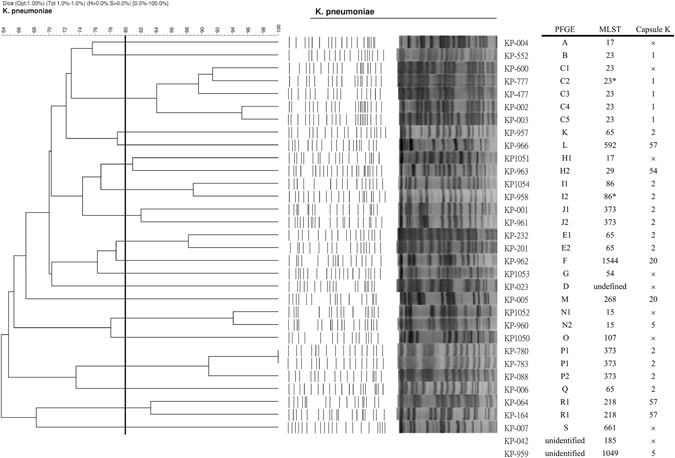
Fingerprinting profiles (A to S) of XbaI-digested genomic DNA using the Dice coefficient and UPGMA for Klebsiella pneumoniae cerebrospinal fluid isolates collected in Taiwan. Eight pulsotypes (C, E, H, I, J, N, P, and R) show a Dice similarity coefficient of more than 80%. Corresponding MLST (multilocus sequence typing) and capsule K serotype of each isolate were presented. *One nucleotide change; x, unidentified, non-virulent capsule K types, i.e., not for K1, K2, K5, K20, K54, K57 types.
MLST
From all isolates performing MLST, 6 strains were ST23 (including one ST23-like variant), 5 strains were ST373, 4 strains were ST65, 2 strains were ST86 (including one ST86-like variant), ST15, ST17 and ST218 respectively. Others were one each for ST29, ST54, ST107, ST185, ST268, ST592, ST661, ST1049, ST1544 and unidentified ST (strain KP-023).
The association between genotyping, sequence type and K capsule serotypes of K. pneumoniae meningitis isolates were summarized in Fig. 1. Notably, the 5 primary meningitis isolates of pulsotype C (C1-C5) were capsule serotype K1, including ST23 in 4 strains and ST 23-like in 1 strain. Five ST373 strains belonged to pulsotypes J and P. Isolates of ST373, ST65 and ST86 belonged to capsule serotype K2. Two isolates of ST218 and 1 isolate of ST29 belonged to capsule serotype K57 and K54 respectively.
Demographic data and clinical outcomes
The 33 K. pneumoniae isolates from CSF basically of primary meningitis or post-craniotomy meningitis were collected from 1999 to 2013. However, detailed clinical data could be retrieved for the 12 patients from 2009 to 2013 (Table 4). The reasons of craniotomy were mainly to remove hematoma of various causes. Five of six patients in this group underwent craniectomy and two patients developed brain abscess (Fig. 2). The main comorbidities of patients with primary meningitis were diabetes mellitus and alcoholic liver cirrhosis (Table 4). Two patients in this group developed brain abscess and two patients developed ventriculitis (Fig. 2). Only one patient died during the hospital episode. There were no statistically significant differences in the demographic data, CSF profiles and clinical outcomes between the two groups of K. pneumoniae meningitis.
Table 4.
Clinical profiles and outcome of Klebsiella pneumoniae isolates from CSF of post-craniotomy and primary meningitis.
| Straina | Age (yr) | Sex | Comorbidity | Ventilator use | Sugarb (mg/dl) | CSF profiles | Outcome | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RBC (per μl) | WBC (per μl) | Neutrophil (%) | Lymphocyte (%) | Sugar (mg/dl) | Protein (mg/dL) | |||||||
| Post-craniotomy meningitis | ||||||||||||
| KP1050 | 56 | M | Head injury | Nil | 149 | 5 | 18 | 72 | 28 | 54 | 108.0 | Survived |
| KP1051 | 23 | M | SAH | Nil | Nil | 1030 | 133 | 60 | 40 | 57 | 57.5 | Survived |
| KP1052 | 59 | M | ICH | Yes | Nil | 1250 | 2016 | 92 | 8 | 23 | 873.5 | Survived |
| KP1053 | 61 | M | AVM rupture | Yes | Nil | 200 | 75900 | 100 | 0 | <1 | 592 | Survived |
| KP1054 | 51 | F | AVM rupture | Nil | Nil | 110 | 1177 | 72 | 28 | <1 | 206.7 | Survived |
| KP-960 | 56 | M | Head injury | Nil | Nil | 0 | 6 | 50 | 50 | 1 | 412.0 | Survived |
| Primary meningitis | ||||||||||||
| KP-958 | 48 | M | DM, ALC | Nil | 342 | 760 | 1632 | 97 | 3 | 118 | >500 | Survived |
| KP-959 | 62 | M | ALC | Yes | Nil | 24500 | 94600 | 65 | 35 | 1 | >500 | Died |
| KP-961 | 55 | M | DM | Nil | 489 | 576 | 12672 | 97 | 3 | 219 | 325 | Survived |
| KP-962 | 68 | F | DM | Nil | 405 | 30 | 372 | 71 | 29 | 205 | 173.3 | Survived |
| KP-963 | 50 | M | DM | Nil | 430 | 190 | 5712 | 97 | 3 | 241 | >500 | Survived |
| KP-966 | 62 | M | ALC | Yes | 157 | 27000 | 62700 | 72 | 28 | <1 | >500 | Survived |
| p | 0.390 | 1.000 | NA | 1.000 | NA | 0.176 | 0.522 | 0.345 | 0.345 | NA | NA | 1.000 |
Note CSF, cerebrospinal fluid; M, male; F, female; SAH, subarachnoid hemorrhage related to a brain aneurysm; ICH, intracerebral hemorrhage; AVM, arteriovenous malformation; DM, diabetes mellitus; ALC, alcoholic liver cirrhosis; RBC, red blood cell count (normal, <5/μl); WBC, white blood cell count (normal, <5/μl); NA, not applicable; afrom Chi-Mei Medical Center in Tainan City, southern Taiwan, 2009–2013 (n = 12); brandom blood sugar obtained on the day of CSF collection.
Figure 2.
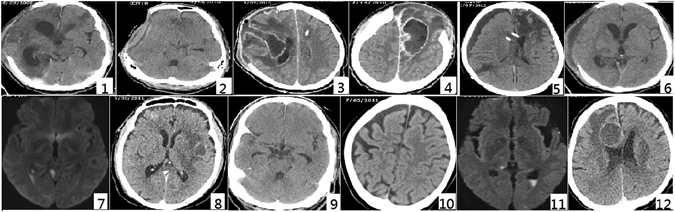
Brain imaging studies of patients with Klebsiella pneumoniae meningitis from post-craniotomy meningitis groups (no. 1–6) and primary meningitis group (no. 7–12). No. 1, strain KP1050, hydrocephalus (computed tomography, CT); No. 2, strain KP1051, brain edema with compression effect (CT); No. 3, strain KP1052, brain abscess (CT); No. 4, strain KP1053, brain abscess (CT); No. 5, strain KP1054, subdural effusion (CT); No. 6, strain KP-960, subdural effusion (CT); No. 7, strain KP-958, ventriculitis (Magnetic Resonance Imaging, MRI); No. 8, strain KP-959, brain abscess (CT); No. 9, strain KP-961, hydrocephalus; No. 10, KP-962, essentially intact in brain parenchyma (CT); No. 11, strain KP-963, ventriculitis (MRI); and No. 12, strain KP-966, brain abscess (CT).
Discussion
The study investigated the antibiotic resistance profiles, β-lactamase genes, various hypervirulence determinants, mouse lethality, as well as the epidemiology of genomic macrorestriction patterns and sequence types of 33 K. pneumoniae isolates that were capable of invading the central nervous system to cause primary meningitis (n = 20) or post-craniotomy meningitis (n = 13). Generally consistent with our hypothesis, primary meningitis isolates were more susceptible to third- and fourth-generation cephalosporins, were more hypervirulent (hvKP) and had higher mouse lethality capability than post-craniotomy meningitis isolates (mostly cKP). Exceptionally, serum resistance did not reach statistically significant difference between groups, suggesting that high-grade serum resistance (n = 23) is the major common virulence determinant of K. pneumoniae isolates with capability to invade the central nervous system, regardless of primary or post-craniotomy meningitis.
The post-craniotomy isolates had significantly higher carriage rate of ESBL/AmpC genes (69.2%) than primary meningitis isolates did (0%), suggesting that it is appropriate for meropenem but not for broad-spectrum cephalosporin in the empirical therapy of post-craniotomy meningitis. Chang et al. reported data of 49 adult K. pneumoniae meningitis cases (36 spontaneous meningitis and 13 post-neurosurgical meningitis), collected over a period of 11 years (2000–2010) in Taiwan28. Data on antibiotic resistance of these isolates also showed none of 36 spontaneous meningitis isolates but only 2 of 13 (15.4%) post- neurosurgical meningitis isolates were resistant to third- and fourth-generation cephalosporins and one of the strains was ESBL-producing28. Thus, it is important to monitor the trend of antibiotic resistance in K. pneumoniae CSF strains for the empirical antibiotic strategy of meningitis.
With regard to specific virulence factors, primary meningitis isolates were more virulent than post-craniotomy isolates in term of the expression of larger bacterial size, HV phenotype, rmpA, rmpA2, aerobactin gene, virulent capsule K serotypes (K1, K2, K5, K20, K54, and K57), and high mouse lethality (LD50 < 106). Capsular K2 serotype was the most common serotype observed (33.3%). Most hvKP isolates were significantly associated with the above-mentioned virulence factors and exhibited high-grade serum resistance as well as high mouse lethality. Furthermore, aerobactin is one of the siderophore systems, which mediate acquisition of iron to help virulent bacteria to overcome iron starvation, while bacteria invade and proliferate in the human systems14, 24, 25. In general, the current data support our initial hypothesis. The presence of these virulent genes and characters of primary meningitis hvKP isolates might assist in their capability to invade CSF space through intact central nervous system barrier to cause infection, whereas the less virulent cKP might still be able to cross the CSF space through disruption of central nervous system barrier, probably with the aid of the mechanism of serum resistance. We found that high-grade serum resistance occurred in 7 of 13 (53.8%) post-craniotomy isolates and in 16 of 20 (80%) in primary meningitis isolates, without reaching significant difference (p = 0.139). We acknowledge that high serum resistance is the major common virulence determinant of hvKP and cKP isolates contributing to invade the central nervous system, regardless of primary or post-craniotomy status.
With regard to molecular epidemiology, no major genomic clone or sequence type broadly existed among the 33 K. pneumoniae CSF isolates. However, there were 5 primary meningitis isolates of pulsotype C (C1-C5) belonging to ST 23 or ST23-like sequence type. The ST23 has been recognized as the most prevalent MLST type of capsule serotype K1 K. pneumoniae isolates from liver abscess in Taiwan29. The ST65, ST86, ST373 and ST375 (not detected in the study) have been the major clones associated with K2 K. pneumoniae liver abscesses in Taiwan29, 30. In addition, ST65, ST65-like and ST86-like MLST types were most predominant among K2 serotype isolates of the community-acquired infection cases from Singapore, Hong Kong and China31, 32.
ST218 has been reported in capsule serotype K57 liver abscess isolates in Taiwan30. ST29 K54 ESBL-producing K. pneumoniae with HV phenotype was detected in Hunan, China33. We previously reported on a mycotic aneurysm caused by hypermucoviscous ST29 K54 K. pneumoniae in Taiwan34. Furthermore, the ST29 was the predominant ST of carbapenem-resistant K. pneumoniae isolates in Saudi Arabia35. The ST15, ST17, ST54, and ST107 isolates producing ESBL, DHA-1, KPC-2 or NDM-1metallo-β-lactamase have been found in Taiwan, China and other various geographic areas in the world36–42. Importantly, 5 of 9 ESBL/AmpC producers in our current study belonged to these international clones, which often exhibited high-grade serum resistance. In addition to our recent report on the bacteremic isolates43, we continuously highlight the importance of monitoring the emergence of hypervirulence in the ESBL/AmpC-producing K. pneumoniae, particularly in the CSF isolates.
Although the K. pneumoniae isolates had different virulence mechanisms between the groups of primary and post-craniotomy meningitis, they did not result in significantly different clinical features and outcomes among limited patients between groups. The reasons could be explained by the effectiveness of BSC and meropenem against the isolates causing primary and post-craniotomy meningitis respectively. However, continuous monitoring for the emerging resistance profiles of K. pneumoniae between different groups of meningitis is clinically important.
Conclusion
Primary K. pneumoniae meningitis isolates had hypervirulence profiles of virulent capsule K serotypes, larger capsule size, HV phenotype, rmpA, rmpA2, aerobactin gene, high serum resistance and high capability of mouse lethality. Post-craniotomy K. pneumoniae meningitis isolates had relatively low virulence profiles and exhibited low mouse lethality, but still had high serum resistance which supported their capabilities to invade the central nervous system. The MLST international clones (ST15, ST17, ST54, and ST107) have been found in the post-craniotomy meningitis isolates in Taiwan, which could produce ESBL/AmpC β-lactamases. Therefore, physicians should be aware the emerging trend of antibiotic resistance in the empirical treatment for K. pneumoniae, particularly in post-craniotomy meningitis.
Acknowledgements
This work was supported by the National Science Council of Taiwan (NSC-100-2314-B-384-002) and Chi-Mei Medical Center Research Foundation (CMFHT10202, CMFHR10412, and CMFHR10508). We thank Fu-Der Wang, Taipei Veterans General Hospital and Wen-Chien Ko, National Cheng-Kung University Hospital, for kindly providing the isolates used in this study.
Author Contributions
W.-L.Y. contributed to study design, data interpretation and reviewing the manuscript. Y.-H.K. and M.-F.L. contributed to manuscript drafting. C.-C.C., Y.-C.Y. and H.-J.T. contributed to data acquisition, experiments and analysis. Y.-C.C. contributed to the supervision of this investigation. All of the authors were involved in writing the manuscript, critically revising it for intellectual content and approving the final version submitted for publication.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yu, W. L. & Chuang, Y. C. Clinical features, diagnosis, and treatment of Klebsiella pneumoniae infection. In Calderwood, S. B., Bloom, A. (Eds) UpToDate (2015).
- 2.Yu, W. L., Chuang, Y. C. Invasive liver abscess syndrome caused by Klebsiella pneumoniae. In Calderwood, S. B., Bloom, A. (Eds) UpToDate (2015).
- 3.Chan KS, et al. Pyogenic liver abscess caused by Klebsiella pneumoniae: analysis of the clinical characteristics and outcomes of 84 patients. Chin Med J (Engl) 2007;120:136–139. [PubMed] [Google Scholar]
- 4.Ku YH, Chuang YC, Yu WL. Clinical spectrum and molecular characteristics of Klebsiella pneumoniae causing community-acquired extrahepatic abscess. J Microbiol Immunol Infect. 2008;41:311–317. [PubMed] [Google Scholar]
- 5.Lu CH, Chang WN, Wu HS. Klebsiella pneumoniae meningitis: analysis on clinical features of thirty-two adult patients. Chin Med J (Taipei) 1997;60:296–302. [PubMed] [Google Scholar]
- 6.Fang CT, et al. Microbiologic features of adult community-acquired bacterial meningitis in Taiwan. J Formos Med Assoc. 2000;99:300–304. [PubMed] [Google Scholar]
- 7.Lu CH, Chang WN, Chang HW. Adult bacterial meningitis in southern Taiwan: epidemiologic trend and prognostic factors. J Neurol Sci. 2000;182:36–44. doi: 10.1016/S0022-510X(00)00445-7. [DOI] [PubMed] [Google Scholar]
- 8.Lu CH, et al. Community-acquired bacterial meningitis in adults: the epidemiology, timing of appropriate antimicrobial therapy, and prognostic factors. Clin Neurol Neurosurg. 2002;104:352–358. doi: 10.1016/S0303-8467(02)00052-5. [DOI] [PubMed] [Google Scholar]
- 9.Lee LH, et al. Adult Streptococcus pneumoniae meningitis in southern Taiwan: epidemiologic trends and prognostic factors. J Clin Neurosci. 2005;12:32–35. doi: 10.1016/j.jocn.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 10.Chang WN, et al. Changing epidemiology of adult bacterial meningitis in southern Taiwan: a hospital-based study. Infection. 2008;36:15–22. doi: 10.1007/s15010-007-7009-8. [DOI] [PubMed] [Google Scholar]
- 11.Hsu CL, et al. Management of severe community-acquired septic meningitis in adults: from emergency department to intensive care unit. J Formos Med Assoc. 2009;108:112–118. doi: 10.1016/S0929-6646(09)60041-3. [DOI] [PubMed] [Google Scholar]
- 12.Huang CR, et al. Community-acquired spontaneous bacterial meningitis in adult diabetic patients: an analysis of clinical characteristics and prognostic factors. Infection. 2002;30:346–350. doi: 10.1007/s15010-002-3010-4. [DOI] [PubMed] [Google Scholar]
- 13.Tang LM, Chen ST, Hsu WC, Chen CM. Klebsiella meningitis in Taiwan: an overview. Epidemiol Infect. 1997;119:135–142. doi: 10.1017/S0950268897007930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russo TA, et al. Hypervirulent K. pneumoniae secretes more and more active iron-acquisition molecules than “classical” K. pneumoniae thereby enhancing its virulence. PLoS One. 2011;6:e26734. doi: 10.1371/journal.pone.0026734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chuang YC, et al. Can the rmpA gene predict metastatic meningitis among patients with primary Klebsiella pneumoniae liver abscess? J. Infect. 2013;67:166–168. doi: 10.1016/j.jinf.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Clinical Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing. MIC testing. Informational supplement M100-S22. Wayne (PA): CLSI (2012).
- 17.Jarlier V, Nicolas MH, Fournier G, Philippon A. Extended broad-spectrum β-lactamases conferring transferable resistance to newer β-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev Infect Dis. 1988;10:867–878. doi: 10.1093/clinids/10.4.867. [DOI] [PubMed] [Google Scholar]
- 18.Tang HJ, Ku YH, Lee MF, Chuang YC, Yu WL. In vitro activity of imipenem and colistin against a carbapenem-resistant Klebsiella pneumoniae isolate coproducing SHV-31, CMY-2, and DHA-1. Biomed Res Int. 2015;2015:568079. doi: 10.1155/2015/568079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang CT, et al. Klebsiella pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin Infect Dis. 2007;45:284–293. doi: 10.1086/519262. [DOI] [PubMed] [Google Scholar]
- 20.Yu WL, et al. Polymerase chain reaction analysis for detecting capsule serotypes K1 and K2 of Klebsiella pneumoniae causing abscesses of the liver and other sites. J Infect Dis. 2007;195:1235–1236. doi: 10.1086/512686. [DOI] [PubMed] [Google Scholar]
- 21.Yu WL, et al. Association between rmpA and magA genes and clinical syndromes caused by Klebsiella pneumoniae in Taiwan. Clin Infect Dis. 2006;42:1351–1358. doi: 10.1086/503420. [DOI] [PubMed] [Google Scholar]
- 22.Yu WL, et al. Comparison of prevalence of virulence factors for Klebsiella pneumoniae liver abscesses between isolates with capsular K1/K2 and non-K1/K2 serotypes. Diagn Microbiol Infect Dis. 2008;62:1–6. doi: 10.1016/j.diagmicrobio.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Yu WL, Lee MF, Tang HJ, Chang MC, Chuang YC. Low prevalence of rmpA and high tendency of rmpA mutation correspond to low virulence of extended spectrum β-lactamase-producing Klebsiella pneumoniae isolates. Virulence. 2015;6:162–172. doi: 10.1080/21505594.2015.1016703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu VL, et al. Virulence characteristics of Klebsiella and clinical manifestations of K. pneumoniae bloodstream infections. Emerg Infect Dis. 2007;13:986–993. doi: 10.3201/eid1307.070187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Podschun R, Sievers D, Fischer A, Ullmann U. Serotypes, hemagglutinins, siderophore synthesis, and serum resistance of Klebsiella isolates causing human urinary tract infections. J Infect Dis. 1993;168:1415–1421. doi: 10.1093/infdis/168.6.1415. [DOI] [PubMed] [Google Scholar]
- 26.Pfaller MA, et al. Comparative evaluation of an automated ribotyping system versus pulsed-field gel electrophoresis for epidemiological typing of clinical isolates of Escherichia coli and Pseudomonas aeruginosa from patients with recurrent gram-negative bacteremia. Diagn Microbiol Infect Dis. 1996;25:1–8. doi: 10.1016/0732-8893(96)00082-X. [DOI] [PubMed] [Google Scholar]
- 27.Diancourt L, Passet V, Verhoef J, Grimont PA, Brisse S. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J Clin Microbiol. 2005;43:4178–4182. doi: 10.1128/JCM.43.8.4178-4182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang WN, Huang CR, Lu CH, Chien CC. Adult Klebsiella pneumoniae meningitis in Taiwan: an overview. Acta Neurol Taiwan. 2012;21:87–96. [PubMed] [Google Scholar]
- 29.Siu LK, et al. Molecular typing and virulence analysis of serotype K1 Klebsiella pneumoniae strains isolated from liver abscess patients and stool samples from noninfectious subjects in Hong Kong, Singapore, and Taiwan. J Clin Microbiol. 2011;49:3761–375. doi: 10.1128/JCM.00977-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao CH, Huang YT, Chang CY, Hsu HS, Hsueh PR. Capsular serotypes and multilocus sequence types of bacteremic Klebsiella pneumoniae isolates associated with different types of infections. Eur J Clin Microbiol Infect Dis. 2014;33:365–369. doi: 10.1007/s10096-013-1964-z. [DOI] [PubMed] [Google Scholar]
- 31.Lin JC, et al. Genotypes and virulence in serotype K2 Klebsiella pneumoniae from liver abscess and non-infectious carriers in Hong Kong, Singapore and Taiwan. Gut Pathog. 2014;6:21. doi: 10.1186/1757-4749-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao J, et al. Multilocus sequence types and virulence determinants of hypermucoviscosity-positive Klebsiella pneumoniae isolated from community-acquired infection cases in Harbin, North China. Jpn J Infect Dis. 2016;69:357–360. doi: 10.7883/yoken.JJID.2015.321. [DOI] [PubMed] [Google Scholar]
- 33.Yan Q, Zhou M, Zou M, Liu WE. Hypervirulent Klebsiella pneumoniae induced ventilator-associated pneumonia in mechanically ventilated patients in China. Eur J Clin Microbiol Infect Dis. 2016;35:387–396. doi: 10.1007/s10096-015-2551-2. [DOI] [PubMed] [Google Scholar]
- 34.Chuang YC, Lee MF, Yu WL. Mycotic aneurysm caused by hypermucoviscous Klebsiella pneumoniae serotype K54 with sequence type 29: an emerging threat. Infection. 2013;41:1041–1044. doi: 10.1007/s15010-013-0447-6. [DOI] [PubMed] [Google Scholar]
- 35.Uz Zaman T, et al. Multi-drug carbapenem-resistant Klebsiella pneumoniae infection carrying the OXA-48 gene and showing variations in outer membrane protein 36 causing an outbreak in a tertiary care hospital in Riyadh, Saudi Arabia. Int J Infect Dis. 2014;28:186–192. doi: 10.1016/j.ijid.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 36.Löhr IH, et al. Persistence of a pKPN3-like CTX-M-15-encoding IncFIIK plasmid in a Klebsiella pneumoniae ST17 host during two years of intestinal colonization. PLoS One. 2015;10:e0116516. doi: 10.1371/journal.pone.0116516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, et al. Novel NDM-9 metallo-β-lactamase identified from a ST107 Klebsiella pneumoniae strain isolated in China. Int J Antimicrob Agent. 2014;44:90–91. doi: 10.1016/j.ijantimicag.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 38.Rodrigues C, Machado E, Ramos H, Peixe L, Novais Â. Expansion of ESBL-producing Klebsiella pneumoniae in hospitalized patients: a successful story of international clones (ST15, ST147, ST336) and epidemic plasmids (IncR, IncFIIK) Int J Med Microbiol. 2014;304:1100–1108. doi: 10.1016/j.ijmm.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Lee MY, et al. High prevalence of CTX-M-15-producing Klebsiella pneumoniae isolates in Asian countries: diverse clones and clonal dissemination. Int J Antimicrob Agents. 2011;38:160–163. doi: 10.1016/j.ijantimicag.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 40.Yan JJ, et al. Allocation of Klebsiella pneumoniae bloodstream isolates into four distinct groups by ompK36 typing in a Taiwanese university hospital. J Clin Microbiol. 2015;53:3256–3263. doi: 10.1128/JCM.01152-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu L, et al. Emergence of blaNDM-1 among Klebsiella pneumoniae ST15 and novel ST1031 clinical isolates in China. Diagn Microbiol Infect Dis. 2013;75:373–376. doi: 10.1016/j.diagmicrobio.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 42.Markovska R, et al. Clonal dissemination of multilocus sequence type ST15 KPC-2-producing Klebsiella pneumoniae in Bulgaria. APMIS. 2015;123:887–894. doi: 10.1111/apm.12433. [DOI] [PubMed] [Google Scholar]
- 43.Yu WL, et al. Impacts of hypervirulence determinants on clinical features and outcomes of bacteremia caused by extended-spectrum β-Lactamase-producing Klebsiella pneumoniae. Microb Drug Resist. 2017;23:376–383. doi: 10.1089/mdr.2016.0018. [DOI] [PubMed] [Google Scholar]


