Abstract
Background
Pulmonary vein antrum isolation (PVAI) under sedation has proven to be a useful strategy for catheter ablation of atrial fibrillation (AF).
Methods
To evaluate the clinical benefits of respiratory management using supraglottic airways (SGAs) under deep sedation while monitoring the bispectral (BIS) index during the PVAI and the durations from admission to the catheterization room to starting the radiofrequency energy delivery (Time α), and from starting the radiofrequency energy delivery to completion of the PVAI (Time β), X-ray time, frequency of dislocations of the three-dimensional maps (D3DM), procedure-related complications, and proportion of an AF-free rate 15 months after the PVAI (PAFFR) in patients who received deep sedation without SGAs (Group A: n=48) and those with SGAs (Group B: n=51) were evaluated.
Results
There were no significant differences in patient characteristics, Time α (77±3 versus 78±2 min; p=0.816), complications of cardiac tamponade (2% versus 2%; p=0.966), or PAFFR (81% versus 88%; p=0.313) between the two groups. However, the Time β (84±4 versus 67±3; p=0.001), X-ray time (53±2 versus 34±2; p<0.001), and minor complications of nasal bleeding (25% versus 0%; p=0.001) were significantly shorter and lower in Group B than in Group A, in accordance with a reduction in the hypoxia (15% versus 0%; p=0.007) and D3DM (31% versus 8%; p=0.003).
Conclusions
These results may demonstrate the clinical benefits of deep sedation with SGAs while monitoring the BIS index without any hypoxia during PVAI in patients with AF.
Abbreviations: PVAI, pulmonary vein antrum isolation; RFCA, radiofrequency catheter ablation; AF, atrial fibrillation; SGA, supraglottic airway; BIS, bispectral; LA, left atrium; BMI, body mass index
Keywords: Catheter ablation, Atrial fibrillation, Deep sedation, Supraglottic airways, Bispectral index
1. Introduction
Pulmonary vein (PV) antrum isolation (PVAI) has proven to be a useful strategy for radiofrequency catheter ablation (RFCA) of atrial fibrillation (AF) worldwide [1], [2], [3]. Many kinds of sedation or anesthesia during PVAI including simple minimal sedation, deep sedation, and general anesthesia, have generally been applied in these procedures [4], [5], [6], [7], [8]. However, patient movements because of a procedure-related incidence of pain and/or hypoxia associated with sedation or anesthesia often occur during PVAI, and procedural delays during PVAI often unexpectedly occur. Moreover, patient movements can often and easily cause a dislocation of the three-dimensional (3D) map, which can also lead to procedural delays during the PVAI procedure, especially with the use of the EnSiteTM system (St. Jude Medical, St. Paul, MN, USA), rather than the CARTOTM system (Biosense Webster Ltd., Tokyo, Japan), in real clinical practice. During anesthesia, supraglottic airways (SGAs) have been introduced over the last 10 years, and are widely used for the majority of general anesthetics because of their efficacy and safety [9]. Moreover, it has been reported that the bispectral index (BIS) could accurately detect deep levels of sedation [10]. Thus, in this study, we evaluated the clinical benefit of respiratory management using SGAs under deep sedation while monitoring the BIS index with a BIS monitoring system (A-3100CTM, NIHON KODEN, Tokyo, Japan) during RFCA of AF using the EnSite NavX/VelocityTM Cardiac Mapping System (St. Jude Medical, St. Paul, MN, USA).
2. Material and methods
2.1. Baseline clinical characteristics of the patient groups (Table 1)
Table 1.
Patient characteristics.
| Group A | Group B | p Value | |
|---|---|---|---|
| Number of patients | 48 | 51 | |
| Male | 32 (67%) | 37 (73%) | 0.530 |
| Age (years) | 61±2 | 64±2 | 0.341 |
| Body surface area (m2) | 1.65±0.03 | 1.62±0.02 | 0.459 |
| Body mass index (kg/m2) | 21.4±0.6 | 22.8±0.6 | 0.066 |
| CHADS2 score | 1.3±0.1 | 1.7±0.2 | 0.081 |
| LVEF (%) | 62±1 | 62±1 | 0.796 |
| Volume of LA and PVs | |||
| LA diameter by echocardiography (mm) | 38±1 | 37±1 | 0.625 |
| LA volume index by echocardiography (ml/m2) | 32±2 | 32±1 | 0.829 |
| Number of PVs | 4.1±0.1 | 4.0±0.1 | 0.536 |
| Type of atrial fibrillation | |||
| Paroxysmal | 39 (81%) | 42 (82%) | 0.888 |
| Persistent | 9 (19%) | 9 (18%) | 0.888 |
| Type of ablation procedures | |||
| PVAI alone | 42 (88%) | 44 (86%) | 0.858 |
| PVAI+SVCI | 4 (8%) | 2 (4%) | 0.368 |
| PVAI+CTI | 2 (4%) | 5 (10%) | 0.264 |
LVEF=left ventricular ejection fraction, LA=left atrium, CT=computed tomography, PVs=pulmonary veins, PVAI=pulmonary vein antrum isolation, SVCI=superior vena cava isolation, CTI=cavo tricuspid isthmus ablation.
This study was approved by the institutional review committee and ethics review board of our hospitals. From January 2014 to September 2015, 99 consecutive patients (69 males and 30 females, with a mean age of 63±2 years) with non-valvular paroxysmal or persistent AF underwent an initial session of RFCA using the EnSite NavX/VelocityTM Cardiac Mapping System (St. Jude Medical, St. Paul, MN, USA) at our hospitals. AF was classified according to the HRS/EHRA/ECAS 2012 Consensus Statement on Catheter and Surgical Ablation of AF [11]. Patients with long lasting AF, hemodialysis, RFCA using CARTOTM (Biosense Webster Ltd., Tokyo, Japan), and/or a re-session of RFCA for AF, were excluded from this study. All included patients had their history recorded, and underwent a physical examination, laboratory analysis, chest radiogram, 12-lead electrocardiogram, and echocardiography (SONOS 2500, Hewlett-Packard, San Diego, CA, USA) within at least one month before admission. CHADS2 score [12], chamber size, and volume of the left atrium (LA) by echocardiography, and anatomy and size of the PVs and LA by computed tomography (Aquilion 64 and Aquilion ONE TSX-301A, TOSHIBA, Tokyo, Japan), were also evaluated prior to the RFCA.
2.2. Procedure for RFCA of AF (Fig. 1)
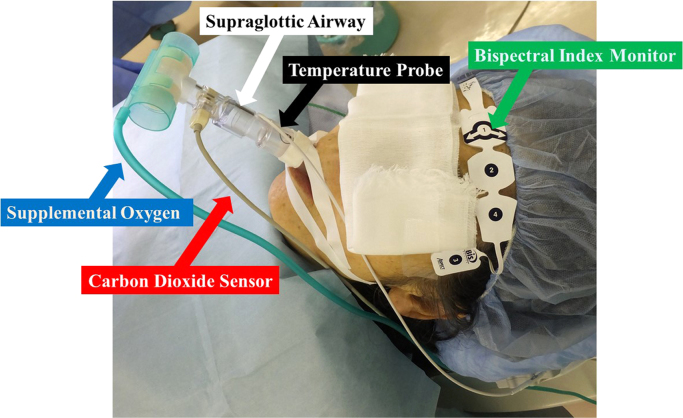
Fig. 1.
In a patient with a supraglottic airway (SGA) (white arrow), in who deep sedation was monitored by a bispectral index monitor (green arrow) pasted on the front of the forehead, radiofrequency catheter ablation of atrial fibrillation was performed under an intravenous administration of propofol and dexmedetomidine. Supplemental oxygen (blue arrow) was routinely used with a flow rate of 5–10 l per minute via an SGA with spontaneous breathing to maintain the peripheral oxygen saturation at more than 95%. The exhaling carbon dioxide sensor (red arrow) was attached to the SGA. A temperature probe to monitor the esophageal temperature (black arrow) was inserted through the nostril of the patient through a side hole of the SGA.
All patients were effectively anticoagulated for at least one month before the procedure. The procedures were performed following transesophageal echocardiography, to rule out any LA thrombi. All patients gave their informed consent. RFCA was performed as described previously [[1], [3], [4]]. In brief, deep sedation without (Group A) or with SGAs (i-gelTM, Intersurgical Ltd., Wokingham, Berkshire, UK) (Group B) was performed for the RFCA of AF under an intravenous administration of propofol and dexmedetomidine. The initial i-gelTM (Intersurgical Ltd., Wokingham, Berkshire, UK) size chosen was based on the manufacturer׳s recommendations with respect to patient body weight [13]. A temperature probe (SensiThermTM, St. Jude Medical, St. Paul, MN, USA) for monitoring the esophageal temperature was inserted through a nostril in those patients without an SGA or a side hole of the SGA with an SGA under deep sedation, and placed between the level of the left superior and inferior pulmonary veins in both groups. Femoral arterial access was routinely acquired for continuous blood pressure and heart rate monitoring. A 100 unit per kilogram administration of heparin was administered following the transseptal puncture, and heparinized saline was additionally infused to maintain the activated clotting time at 300–350 s. After an open irrigated 3.5-mm-tip ablation catheter (FlexAbilityTM, St. Jude Medical, St. Paul, MN, USA) and circular mapping catheter (OptimaTM, St. Jude Medical, St. Paul, MN, USA) were positioned in the LA after a double transseptal puncture, the LA was reconstructed by an EnSiteTM system (St. Jude Medical, St. Paul, MN, USA). Then, a circumferential PVAI was performed under electroanatomic guidance with a 3D mapping system. The generator was set to a maximal temperature of 45 °C, maximum power of 35 W, and irrigation rate of 13 ml per minute. When ablating the posterior wall in front of the esophagus, a maximum power of 20–25 W was used to avoid esophageal damage from the high energy supplied, and when the temperature exceeded 39 °C, the energy supply was discontinued. Each application of radiofrequency energy was delivered for about 30–90 s while dragging, with the goal of a ≥70% decrease in the electrogram amplitude at the local site [14]. The completion of the PVAI procedure was defined as the achievement of bidirectional conduction block between the LA and PVs under the administration of isoproterenol and adenosine. In patients with persistent AF, when AF persisted after the PVAI, internal electrical conversion was performed in order to recover sinus rhythm. If firing from the superior vena cava (SVC) was induced under an intravenous administration of isoproterenol after recovering sinus rhythm, an SVC isolation was additionally performed. Further, if common atrial flutter was previously documented or induced by programmed stimulation after the PVAI, an additional cavo-tricuspid isthmus (CTI) line ablation was performed. There was no potential for confounding data between the centers and operators, because the procedures for the RFCA of AF were performed by electrophysiologists who were trained in advanced RFCA, and the cases were assisted by four-to-five staff members who were well-trained in catheterization laboratory practices at our three hospitals.
2.3. Respiratory management during the PVAI
Respiratory management during the RFCA was performed under an intravenous administration of propofol (bolus dose of 1 mg/kg and maintenance dose of 2 g/kg to 5 g/kg per hour) and dexmedetomidin (bolus dose of 1 μg/kg and maintenance dose of 0.2 μg/kg to 0.7 μg/kg per hour). In all patients, it was attempted to maintain the BIS index level between 40 and 60 [10] using a bispectral index monitor pasted on the front of the forehead (Fig. 2). If necessary, an intravenous bolus administration of pentazocine (15–30 mg) was used when patient movements resulting from an incidence of pain during the RFCA occurred. If pentazocine was not effective enough, or the BIS level was over 60, an intravenous bolus administration of propofol at a dose of 1 mg/kg was added. The peripheral oxygen saturation was monitored continuously, and supplemental oxygen was routinely used with a flow rate of 5–10 l per minute via a mask in the patients in Group A, or via an SGA in Group B with spontaneous breathing to maintain the peripheral oxygen saturation at more than 95%. When the peripheral oxygen saturation fell to less than 95%, even though supplemental oxygen with a flow rate of 10 l per minute via a mask or SGA during the procedures was used, an oropharyngeal airway was inserted into the mouth. To monitor the exhaling carbon dioxide concentration, a CO2 Sensor (TG-980P TM, NIHON KODEN, Tokyo, Japan) was attached to the SGA during the procedure. If it was still not adequate, mechanical respiratory support with non-invasive positive pressure ventilation (NIPPV) or endotracheal intubation was used. Hypotension was defined as a systolic blood pressure of 90 mmHg or less. If the hypotension did not improve with an intravenous administration of supplementary fluids, an intravenous administration of dopamine (maintenance dose of 5 μg/kg/min) was used.
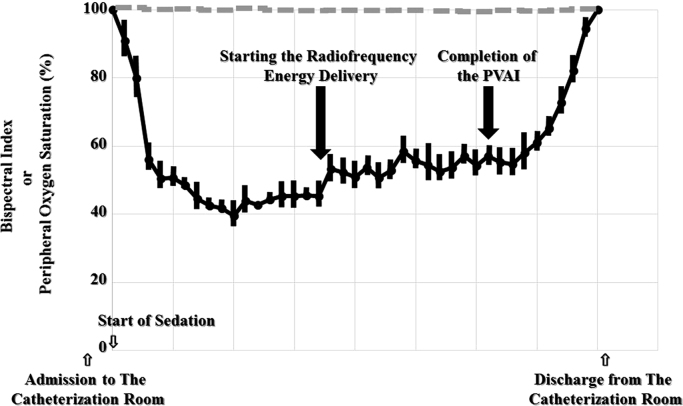
Fig. 2.
Monitoring the bispectral index (full line) and peripheral oxygen saturation (dotted line) under deep sedation using a supraglottic airway (Group A) during radiofrequency catheter ablation (RFCA) of atrial fibrillation using EnSiteTM (St. Jude Medical, St. Paul, MN, USA). RFCA could be steadily performed by monitoring the BIS index without any hypoxia. PVAI=pulmonary vein antrum isolation.
2.4. Evaluation of the procedure time and frequency of dislocations of the 3D maps
The X-ray time; the duration (in minutes) from admission to the catheterization room to starting the radiofrequency energy delivery, defined as Time α; and the duration from starting the radiofrequency energy delivery to the completion of the PVAI, defined as Time β, were evaluated. The X-ray dose could not be evaluated, because there were five new and old X-ray machines, single- or bi-plane, used in our three hospitals. Therefore, the X-ray dose could not be compared, due to the high X-ray dose with the old machines and the low dose with the new ones. Further, the frequency of the dislocations of the 3D maps, defined as a case in which the ablation procedure had to be delayed more than at least one minute associated with a more than 5 mm shift of the catheter position or ablation tags, was also evaluated.
2.5. Complications associated with the procedures and adverse events associated with insertion of SGAs
Persistent and/or transient complications associated with the procedures, including any new PV stenosis, phrenic nerve palsy, cerebrovascular accidents, death, cardiac tamponade, pericardial effusions, vascular complications, and any bleeding, during or within the 48 h after the procedures, were evaluated. Adverse events associated with the insertion of the SGA, including laryngeal spasms, transient nerve damage, hematomas, or vagal responses, were also evaluated.
2.6. Patient care and follow-up
Following the procedure, anticoagulation therapy was continued for at least three months after the RFCA in all patients. All patients underwent monthly follow-ups for at least six months after the RFCA. The anti-arrhythmic agents were withdrawn in all patients three months after the RFCA. The proportion of AF-free rates were evaluated during the 15-month follow-up period. Documented episodes of AF of >30 s after the three-month blanking period were identified as recurrences of AF. If AF recurred after the three-month blanking period, the anti-arrhythmic agent(s) were re-administered.
2.7. Statistical analysis
Numerical results are expressed in the text as the mean±standard deviation. Paired data were compared by Student׳s t-tests. The comparison of the proportion of AF-free patients between in Groups A and B was performed using the Kaplan–Meier survival analysis with a log-rank test. All analyses were performed with SAS version 9.2 software (SAS Institute, Cary, NC, USA). A p-value of <0.05 was considered to indicate statistical significance.
3. Results
3.1. Patient characteristics (Table 1)
There were 48 and 51 patients in Groups A and B, respectively. There was no statistical difference in the sex (67% versus 73% of male; p=0.530); age (61±2 versus 64±2 years; p=0.341); body surface area (1.65±0.03 versus 1.62±0.02 m2; p=0.459); left ventricular ejection fraction (62±1 versus 62±1%; p=0.796); LA diameter (38±1 versus 37±1 mm; p=0.625); LA volume index by echocardiography (32±2 versus 32±1 ml/m2; p=0.829); number of PVs (4.1±0.1 versus 4.0±0.1; p=0.536); type of AF (81% versus 82% of paroxysmal AF; p=0.888 and 19% versus 18% of persistent AF; p=0.888); or type of RFCA procedures (88% versus 86% of PVAI alone; p=0.858, 8% versus 4% of PVAI+SVCI; p=0.368, and 2% versus 5% of PVAI+CTI; p=0.264) observed between the two groups. The body mass index (BMI) (21.4±0.6 versus 22.8±0.6 kg/m2; p=0.066) and CHADS2 score (1.3±0.1 versus 1.7±0.2; p=0.081) tended to be higher and larger in Group B, but ultimately there was no statistically significant difference between the two groups.
3.2. Deep sedation, respiratory management, hypotension, and the BIS index (Table 2 and Fig. 2)
Table 2.
Hypoxia, hypotension, procedure time, dislocation of the 3D maps, and complications associated with the procedures.
| Group A | Group B | p Value | |
|---|---|---|---|
| Hypoxia | |||
| Hypoxia under deep sedation | 7 (15%) | 0 (0%) | 0.007 |
| Respiratory support with an oropharyngeal airway | 7 (15%) | 1 (2%) | 0.025 |
| Mechanical respiratory support | 2 (4%) | 0 (0%) | 0.159 |
| Non-invasive positive pressure ventilations | 1 (2%) | 0 (0%) | 0.322 |
| Endotracheal intubation | 1 (2%) | 0 (0%) | 0.322 |
| Hypotension | |||
| Hypotension under deep sedation | 13 (27%) | 11 (22%) | 0.528 |
| Catecholamine use for hypotension | 0 (0%) | 1 (2%) | 0.322 |
| Procedure time and dislocation of the 3D maps | |||
| Time α (min) | 77±3 | 78±2 | 0.816 |
| Time β (min) | 84±4 | 67±3 | 0.001 |
| X-ray time (min) | 53±2 | 34±2 | <0.001 |
| Dislocation of the 3D maps | 15 (31%) | 4 (8%) | 0.003 |
| Complications associated with the procedures | |||
| Cardiac tamponade | 1 (2%) | 1 (2%) | 0.966 |
| Nasal bleeding | 12 (25%) | 0 (0%) | <0.001 |
| Other complications associated with the procedures | 0 (0%) | 0 (0%) | NA |
| Adverse events with insertion of oropharyngeal airways | 1 (2%) | NA | NA |
| Adverse events with insertion of SGAs | NA | 0 (0%) | NA |
Time α=duration from admission to the catheterization room to starting the radiofrequency energy delivery, Time β=duration from starting the radiofrequency energy delivery to completion of the pulmonary vein antrum isolation, NA=not applicable. SGAs= supraglottic airways.
The insertion success rate of the SGA, i-gelTM (Intersurgical Ltd., Wokingham, Berkshire, UK), in Group B was 98% (50 of 51), and only one patient had an airway leak with the SGA. In that patient, a mask was used instead of an SGA for respiratory management. The proportion of respiratory support with an oropharyngeal airway because of hypoxia in Group A was significantly larger than that in Group B (15% versus 0%; p=0.007). Because respiratory support with an oropharyngeal airway was not effective in two of seven patients in Group A, mechanical respiratory support with NIPPV (one of seven) or endotracheal intubation (one of seven) was needed instead. All patients in Group B were controlled between a BIS index level of about 40–60 [10] without any hypoxia (Fig. 2). The prevalence of hypotension was similar between the two groups (27% versus 22%; p=0.528). The exhaling carbon dioxide concentration was within normal range in all patients during the procedure (data not shown). The hypotension in all those patients, except for one, steadily improved with an intravenous administration of supplementary fluids. However, one patient in Group B needed an intravenous administration of dopamine. There were no statistical differences in the total doses of propofol, dexmedetomidin, and pentazocine during the procedure between the two groups (data not shown). The BMI in the patients that needed oropharyngeal airways or mechanical respiratory support during the PVAI statistically was significantly larger than that in those who did not (22.1±1.1 versus 25.3±1.1 kg/m2; p=0.019).
3.3. Procedure time (Table 2)
There were no significant differences in the Time α (77±3 versus 78±2 min; p=0.816) between the two groups. However, both the Time β (84±4 versus 67±3 min; p=0.001) and X-ray time (53±2 versus 34±2 min; p<0.001), probably associated with the X-ray dose, were significantly shorter in Group B than Group A, in accordance with a reduction in the frequency of dislocations of the 3D maps (31% versus 8%; p=0.003). The dislocations of the 3D maps were thought to be caused by patient movements resulting from incidences of pain and difficulty in obtaining patient cooperation associated with deep sedation, contributing to the discontinuation of the RFCA procedures.
3.4. Hypoxia, dislocations of the 3D maps, and the procedure time (Table 3)
Table 3.
Procedure time and dislocation of the 3D maps.
| Dislocation of the 3D maps | Yes (n=19) | No (n=80) | p Value |
|---|---|---|---|
| Time α (min) | 78±3 | 78±2 | 0.881 |
| Time β (min) | 88±7 | 71±3 | 0.047 |
| X-ray time (min) | 48±2 | 41±2 | 0.013 |
Time α=duration from admission to the catheterization room to starting the radiofrequency energy delivery, Time β=duration from starting the radiofrequency energy delivery to completion of the pulmonary vein antrum isolation.
There were 19 and 80 out of 99 patients with or without dislocations of the 3D maps during the PVAI, respectively. There were no significant differences in the Time α (78±3 versus 78±2 min; p=0.881) between those with or without dislocations of the 3D maps. However, both the Time β (88±7 versus 71±3 min; p=0.047) and X-ray time (48±2 versus 41±2 min; p=0.013) were significantly shorter without dislocations of the 3D maps during the PVAI than with it.
3.5. Complications associated with the procedures (Table 2)
There were no significant differences in the complications involving cardiac tamponade (2% versus 2%; p=0.966) between the two groups. However, minor complications involving nasal bleeding were significantly fewer in number in Group B than Group A (25% versus 0%; p<0.001). No other complications associated with the procedures were observed in either group (both 0%, respectively). Further, in Group B, there were no adverse events associated with the insertion of the SGAs, including laryngeal spasms, transient nerve damage, hematomas, and vagal responses. On the other hand, in Group A, there was one adverse event of oral minor bleeding associated with the insertion of the oropharyngeal airway.
3.6. Proportion of AF-free rates (Fig. 3)
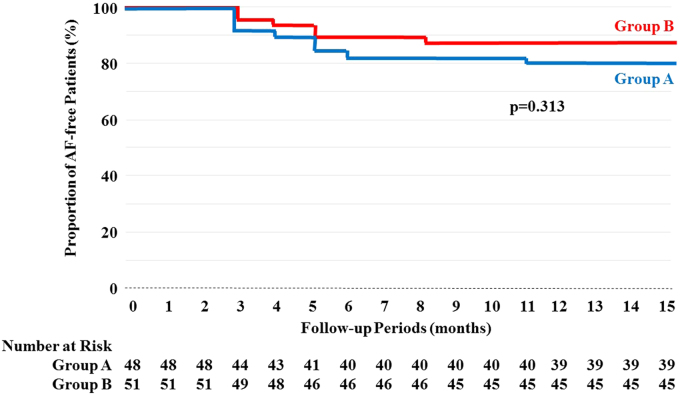
Fig. 3.
Atrial fibrillation-free Kaplan–Meier curves after the blanking period between the two groups. This analysis, completed with a log-rank test, revealed that there were no statistically significant differences in the proportion of the AF-free rates (p=0.313) between the groups with deep sedation with supraglottic airways (SGAs) (red line) (88%, 45 of 51) and those without SGAs (blue line) (81%, 39 of 48) during the latter three months of the 15-month follow-up period, as the initial three-month blanking period was excluded from consideration.
The Kaplan–Meier survival analysis with a log-rank test revealed that there were no statistically significant differences in the proportion of AF-free rates (p=0.313) between Group B (88%, 45 of 51) and Group A (81%, 39 of 48) during the latter three months of the 15-month follow-up period, as the initial three-month blanking period was excluded (Fig. 3).
4. Discussion
4.1. The new findings and important points of this manuscript
The duration of the procedure and X-ray times, which probably contributed to a reduced X-ray dose, could be significantly shortened in patients with deep sedation using SGAs while monitoring the BIS index in accordance with a reduction in the frequency of dislocations of the 3D mapping.
Minor complications associated with nasal bleeding could be more significantly decreased in patients with SGAs than in those without.
However, there were no statistically significant differences in the proportion of AF-free rates between the two groups during the latter three months of the 15-month follow-up period.
The BMI in the patients that needed respiratory support was significantly greater than that in those who did not, indicating that deep sedation using SGAs during the PVAI may be more recommended and effective in obese patients.
Our study demonstrated that RFCA could be steadily performed under deep sedation using SGAs while monitoring the BIS index, and rendered the procedure safer and more comfortable for those patients undergoing long procedures.
4.2. Efficacy of respiratory management using SGAs during the PVAI (Table 2)
It has been reported that endotracheal intubation is not necessary in patients with deep sedation for RFCA of AF [6]. However, that report did not clearly demonstrate the level of deep sedation according to a known scoring system, such as the Ramsey Sedation Score [15], Richmond Agitation–Sedation Scale [16], or BIS index. In our study, all patients were controlled between a Ramsey Sedation Score level of 4–5 [15], a Richmond Agitation–Sedation Scale of −4 to −5 [16], and a BIS index of 40–60. There were seven, one, and one patient(s) who required respiratory support with an oropharyngeal airway, NIPPV, or endotracheal intubation, respectively, in the patients with deep sedation without SGAs (Group A), probably due to deep sedation and/or obesity-induced hypoxia. These findings may indicate that our sedation might have been a deeper sedation than that in the trial [6]. There was only one patient in which an oropharyngeal airway was used in the patients with deep sedation with SGAs (Group B), because of airway leaks of the SGA. The SGA, i-gelTM (Intersurgical Ltd., Wokingham, Berkshire, UK), features a non-inflatable cuff and the possibility to introduce an esophageal catheter. Its successful use during anesthesia has been proven in some randomized control trials [[17], [18]]. Thus, the present study could reveal that deep sedation while monitoring the BIS index with SGAs during the PVAI significantly maintained a stable respiratory condition without any depression of the oxygen saturation. One of the probable mechanism(s) of deep sedation-induced hypoxia is obesity-associated glossoptosis contributing to obstructive apnea and hypopnea. It has been reported that sleep apnea syndrome (SAS) is complicated by other diseases including AF, heart failure, hypertension, diabetes mellitus, obesity, all of which are risk factors for AF [19]. Although the interactions between AF and SAS were not evaluated in this study, sedation-induced hypoxia might be associated with obesity and obstructive apnea. Actually, the BMI of the patients that needed oropharyngeal airways or mechanical respiratory support was significantly larger than that in those that did not (22.1±1.1 versus 25.3±1.1 kg/m2; p=0.019), and there were no adverse events observed during the insertion of the SGAs, indicating that deep sedation with SGAs during PVAI may be recommended and effective, especially in obese patients.
4.3. Hypoxia, dislocations of the 3D maps, and the procedure time (Table 2, Table 3)
Once hypoxia and/or dislocations of the 3D maps occur, it takes time until the peripheral oxygen saturation and/or 3D maps normalize. Thus, it may be important to know how to reduce the hypoxia and dislocations of the 3D maps during the PVAI, in order to steadily and successfully perform the RFCA, shorten the procedure time, and render the procedure safer and more comfortable for the patients. In this study, the frequency of dislocations of the 3D maps due to patient movements resulting from incidences of pain and/or difficulties in obtaining patient cooperation associated with deep sedation in Group A, were interestingly and significantly larger than that in Group B (31% versus 8%; p=0.003), in spite of both groups undergoing the same level of deep sedation. Hence, it has been reported that hypoxia increases pain sensitivity [20], and that this may be one of the underlying mechanism(s) for the higher frequency of dislocations of the 3D maps associated with the incidence of pain in Group A than in Group B. Owing to a reduction in the frequency of dislocations of the 3D maps, the RFCA procedure for AF might be possible to perform steadily, and this could render the procedure safer and more comfortable for patients undergoing long procedures. As a result, both the Time β (84±4 versus 67±3 min; p=0.001) and X-ray time (53±2 versus 34±2 min; p<0.001) could be significantly shortened.
4.4. Complications associated with the procedures (Table 2)
Neither major complications associated with the procedures other than cardiac tamponade, nor adverse events associated with the insertion of the SGAs, were observed in either group. However, although perceived to be a minor complication, nasal bleeding was significantly more frequent in occurrence in Group A than Group B (25% versus 0%; p<0.001). Because the activated clotting time was maintained at 300–350 s during the PVAI, it was comparably easy for bleeding to occur in that situation. Hence, a temperature probe to monitor the esophageal temperature (Fig. 1) was inserted through the side hole of the SGA when an SGA was used (Group B), and therefore the nasal and/or intraoral bleeding could be significantly reduced.
4.5. Proportion of an AF-free rate (Fig. 3)
The identified proportion of an AF-free rate revealed that there were no statistically significant differences in the proportion of the AF-free rate (p=0.313) between in Group B (88%, 45 of 51) and in Group A (81%, 39 of 48). The collection of supporting data from a longer follow-up period for these patients may be needed in order to determine the effects of deep sedation in those AF patients in which an SGA was used during the PVAI on the proportion of an AF-free rate.
5. Limitations of the study
This study was not a randomized clinical trial. Although our study was a multi-center trial, it was limited by a relatively short follow-up period (15 months), and a relatively small number of involved patients. Because of the short follow-up period, our study still could not demonstrate the long-term clinical benefits. Whether our results can safely be extrapolated to the inclusion of a larger number of patients, and a longer follow-up period for these patients, should be determined in further studies. Recent advancement in new technologies including the cryoballoon- [2] and hot balloon [21]-based ablation systems has facilitated a stable lesion creation of PVs. However, currently they can be used only in limited facilities in some countries, including Japan. When they become commercially available all over the world, they may improve current respiratory support systems and help lead to the development of new ones, as well as alternative anesthesia methods.
6. Conclusions
These results may demonstrate the clinical benefits, efficacy, feasibility, and safety of deep sedation with SGAs while monitoring the BIS index without any hypoxia during the PVAI in patients with AF.
Funding sources
None.
Conflict of interest
All authors declare no conflicts of interest related to this study.
Acknowledgements
We thank Mrs. Kensuke Kawasaki and Shu Takata for their technical assistance with the electrophysiological study in the cardiac catheterization laboratory, and Mr. John Martin for his linguistic assistance with this paper.
References
- 1.Haissaguerre M., Jais P., Shah D.C. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 2.Luik A., Radzewitz A., Kieser M. Cryoballoon versus open irrigated radiofrequency ablation in patients with paroxysmal atrial fibrillation: the prospective, randomized, controlled, noninferiority FreezeAF study. Circulation. 2015;132:1311–1319. doi: 10.1161/CIRCULATIONAHA.115.016871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi A., Iesaka Y., Takahashi Y. Electrical connections between pulmonary veins: implication for ostial ablation of pulmonary veins in patients with paroxysmal atrial fibrillation. Circulation. 2002;105:2998–3003. doi: 10.1161/01.cir.0000019585.91146.ab. [DOI] [PubMed] [Google Scholar]
- 4.Ichihara N., Miyazaki S., Taniguchi H. Simple minimal sedation for catheter ablation of atrial fibrillation. Circ J. 2015;79:346–350. doi: 10.1253/circj.CJ-14-1106. [DOI] [PubMed] [Google Scholar]
- 5.Lu F., Lin J., Benditt D.G. Conscious sedation and anesthesia in the cardiac electrophysiology laboratory. J Cardiovasc Electrophysiol. 2013;24:237–245. doi: 10.1111/jce.12001. [DOI] [PubMed] [Google Scholar]
- 6.Kottkamp H., Hindricks G., Eitel C. Deep sedation for catheter ablation of atrial fibrillation: a prospective study in 650 consecutive patients. J Cardiovasc Electrophysiol. 2011;22:1339–1343. doi: 10.1111/j.1540-8167.2011.02120.x. [DOI] [PubMed] [Google Scholar]
- 7.Salukhe T.V., Willems S., Drewitz I. Propofol sedation administered by cardiologists without assisted ventilation for long cardiac interventions: an assessment of 1000 consecutive patients undergoing atrial fibrillation ablation. Europace. 2012;14:325–330. doi: 10.1093/europace/eur328. [DOI] [PubMed] [Google Scholar]
- 8.Wutzler A., Rolf S., Huemer M. Safety aspects of deep sedation during catheter ablation of atrial fibrillation. Pacing Clin Electrophysiol. 2012;35:38–43. doi: 10.1111/j.1540-8159.2011.03260.x. [DOI] [PubMed] [Google Scholar]
- 9.Bamgbade O.A., Macnab W.R., Khalaf W.M. Evaluation of the i-gel airway in 300 patients. Eur J Anaesthesiol. 2008;25(10):865–866. doi: 10.1017/S0265021508004511. [DOI] [PubMed] [Google Scholar]
- 10.Wang ZH, Chen H, Yang YL, et al. Bispectral index can reliably detect deep sedation in mechanically ventilated patients: a prospective multicenter validation study. Anesth Analg; 2016. Epub 2016 Dec 22. [DOI] [PubMed]
- 11.Calkins H, Kuck KH, Cappato R, et al. h/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm; 2012;9:632–96.e21; 2012. [DOI] [PubMed]
- 12.Gage B.F., Waterman A.D., Shannon W. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. J Am Med Assoc. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 13.Theiler L., Gutzmann M., Kleine-Brueggeney M. i-gel supraglottic airway in clinical practice: a prospective observational multicentre study. Br J Anaesth. 2012;109:990–995. doi: 10.1093/bja/aes309. [DOI] [PubMed] [Google Scholar]
- 14.Kumagai K., Sakamoto T., Nakamura K. Pre-procedural prediction of termination of persistent atrial fibrillation by catheter ablation as an indicator of reverse remodeling of the left atrium. Circ J. 2013;77:1416–1423. doi: 10.1253/circj.cj-12-0934. [DOI] [PubMed] [Google Scholar]
- 15.American College of Critical Care Medicine of the Society of Critical Care Medicine, American Society of Health-System Pharmacists, American College of Chest Physicians Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Am J Health Syst Pharm. 2002;59:150–178. doi: 10.1093/ajhp/59.2.150. [DOI] [PubMed] [Google Scholar]
- 16.Riessen R. Comparison of the RAMSAY score and the Richmond Agitation Sedation Score for the measurement of sedation depth. Crit Care. 2012;16:326. [Google Scholar]
- 17.Richardson P.B., Krishnan S., Janakiraman C. Extubation after anaesthesia: a randomised comparison of three techniques. Acta Clin Croat. 2012;51:529–536. [PubMed] [Google Scholar]
- 18.Theiler L.G., Kleine-Brueggeney M., Kaiser D. Crossover comparison of the laryngeal mask supreme and the i-gel in simulated difficult airway scenario in anesthetized patients. Anesthesiology. 2009;111:55–62. doi: 10.1097/ALN.0b013e3181a4c6b9. [DOI] [PubMed] [Google Scholar]
- 19.Miller J.D., Aronis K.N., Chrispin J. Obesity, exercise, obstructive sleep apnea, and modifiable atherosclerotic cardiovascular disease risk factors in atrial fibrillation. J Am Coll Cardiol. 2015;66:2899–2906. doi: 10.1016/j.jacc.2015.10.047. [DOI] [PubMed] [Google Scholar]
- 20.Ristoiu V., Shibasaki K., Uchida K. Hypoxia-induced sensitization of transient receptor potential vanilloid 1 involves activation of hypoxia-inducible factor-1 alpha and PKC. Pain. 2011;152:936–945. doi: 10.1016/j.pain.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi Y., Sohara H., Takeda H. Long-term results of radiofrequency hot balloon ablation in patients with paroxysmal atrial fibrillation: safety and rhythm outcomes. J Cardiovasc Electrophysiol. 2015;26:1298–1306. doi: 10.1111/jce.12820. [DOI] [PubMed] [Google Scholar]