Abstract
Background
The therapeutic goals of atrial fibrillation (AF) patients are to reduce symptoms and prevent severe complications associated with AF. This study compared the efficacy of flecainide versus pilsicainide in reducing the frequency of AF and improving quality of life (QOL) in symptomatic paroxysmal AF patients without structural heart disease.
Methods
The Atrial Fibrillation and Quality Of Life (AF-QOL) study was a prospective, multicenter, randomized, open-label crossover study that compared flecainide and pilsicainide as antiarrhythmic drug therapy. Patients were randomized to receive 3 months of treatment with flecainide twice daily or pilsicainide 3 times daily. Each treatment consisted of a dose-finding phase (weeks 1–4) and an efficacy phase (weeks 5–12). Forty-three patients completed the trial. The main outcome was the number of days with documented AF episodes using a patient-operated electrocardiogram. QOL questionnaires (SF-36 and AF-specific QOL scores) were also completed.
Results
The median (range) AF frequencies (days/8 weeks) were 2 (0–50) in the flecainide treatment group and 1 (0–54) in the pilsicainide treatment group (no significant between-group difference). No significant difference in the first recurrence of AF during the efficacy phase was noted between flecainide and pilsicainide treatments. The frequency and severity scores of AF-related symptoms improved from baseline to the end of the treatment periods. No significant differences in SF-36 or AF-related QOL scores were noted between the treatment groups.
Conclusions
This study found no difference in AF frequency or QOL between symptomatic paroxysmal AF patients who received flecainide or pilsicainide.
Keywords: atrial fibrillation, Flecainide, Pilsicainide, Quality of life, Symptomatic
1. Introduction
Atrial fibrillation (AF) is the most clinically prevalent tachyarrhythmia, and symptomatic AF impairs quality of life (QOL) [1], [2], [3], [4]. The therapeutic goals of AF patients are to reduce symptoms and prevent severe complications associated with AF [1]. Antiarrhythmic drugs modestly prevent AF recurrence but may only be clinically successful when AF-related symptoms, rather than AF recurrence rates, are reduced [1]. Therefore, the efficacy of antiarrhythmic drugs for AF-related symptoms in terms of QOL and AF recurrence in symptomatic AF patients without structural heart disease should be reconsidered.
Flecainide is a class Ic antiarrhythmic drug that exhibits a high affinity for open-state sodium (Na+) channels with slow onset and offset kinetics. Flecainide also blocks the rapid component of the delayed rectifier current [5]. Flecainide is typically administered twice daily because it is metabolized in the liver and has a half-life of approximately 14 h [5]. Flecainide is used worldwide to reduce AF-related symptoms and to provide sustained restoration of sinus rhythm [6]. Flecainide is recommended for long-term rhythm control in AF patients who do not have structural heart disease, based on the guidelines of the European Society of Cardiology [1], [3].
Pilsicainide hydrochloride is another class Ic antiarrhythmic drug that exhibits a pure Na+ channel-blocking action with slow kinetic recovery. Pilsicainide is generally administered 3 times daily because it has a half-life of approximately 5 h, and it is primarily excreted in urine [r7]. This drug was developed and is commonly used in Japan [8]. However, there is no evidence to clearly demonstrate that pilsicainide reduces the recurrence of AF and AF-related symptoms.
The Japanese guideline for pharmacotherapy of atrial fibrillation (Japanese Circulation Society 2013) recommends pilsicainide and flecainide as first-line drugs for paroxysmal AF without structural heart disease [9]. There is insufficient information to determine whether flecainide is superior to pilsicainide for reducing AF recurrence and AF-related symptoms and improving QOL, even though both drugs are class Ic antiarrhythmic agents. This study compared the efficacy of flecainide and pilsicainide in reducing AF frequency and improving QOL in symptomatic paroxysmal AF patients without structural heart disease.
2. Materials and methods
2.1. Patients
Patients with a history of symptomatic paroxysmal AF without structural heart disease who met the following criteria were included in this study: paroxysmal AF with a frequency of 2 or more symptomatic episodes per month; AF previously demonstrated on electrocardiogram (ECG) or Holter recordings when the patient complained of symptoms; presence of sinus rhythm before the start of the study; aged between 20 and 75 years; and written informed consent provided to participate in the study.
The following exclusion criteria were used: patients with obvious structural heart disease; syncope or transient ischemic attack associated with AF; history of cerebral vascular accidents associated with the occurrence of AF; resting heart rate of less than 40 beats per minute; sick sinus syndrome; PR interval of 0.28 s or more; second- or third-degree atrioventricular block; implantation of pacemaker or implantable cardioverter defibrillator; AF caused by reversible non-cardiac disease such as hyperthyroidism; requirement for on-going therapy with other antiarrhythmic drugs; significant serious non-cardiac diseases such as hepatic, renal, hematological, or lung diseases; pregnancy or the possibility of pregnancy and breast feeding; or a judgement by the attending physician that patient participation would be inappropriate.
Previously administered antiarrhythmic drugs were discontinued for at least 1 week (except at least 3 months for amiodarone) before entry into the study. The use of rate-control drugs such as beta-blockers, verapamil, diltiazem, and digoxin were permitted if the dose was not altered during the study.
2.2. Study design
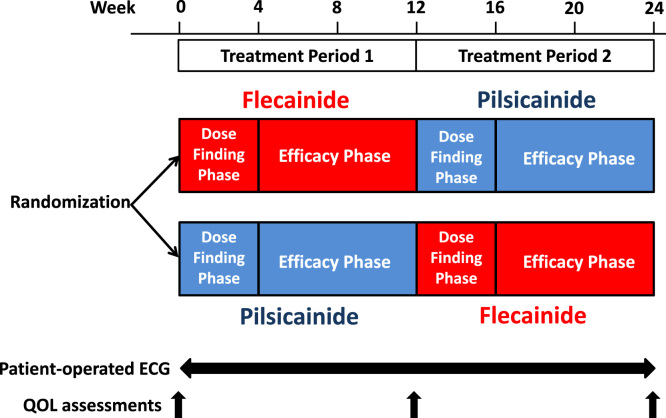
The Atrial Fibrillation and Quality Of Life (AF-QOL) study was a prospective, multicenter, randomized, open-label crossover comparison of flecainide and pilsicainide. Patients were randomized to receive flecainide twice daily during treatment period 1 followed by pilsicainide 3 times daily during treatment period 2 (flecainide-pilsicainide) or the reverse treatment (pilsicainide-flecainide). Participants were randomly assigned following a 3-factor randomized block design to 1 of 2 treatments using a web-based registration system (Medi-Skette Corporation, Tokyo, Japan). Each treatment consisted of a dose-finding phase (weeks 1–4) and an efficacy phase (weeks 5–12) (Fig. 1).
Fig. 1.
Study design.
Qualified patients entered a 4-week dose-finding phase to determine the optimal individual drug dose. Each patient initially received 50 mg of flecainide twice daily or 25 mg of pilsicainide 3 times daily. Each physician determined the optimal dose based on patient complaints, AF-related symptoms, and tolerance. The doses were increased to 100 mg of flecainide twice daily or up to 75 mg of pilsicainide 3 times daily if the efficacy was considered insufficient and the limiting side effects were absent. No efficacy evaluations were performed during this phase. Each patient continued their fixed optimal dose of flecainide or pilsicainide during weeks 5 to 12 of the efficacy phase.
Baseline data, including a QOL assessment using the Japanese AF-specific QOL questionnaire, the Japanese Society of Electrocardiology Atrial Fibrillation Quality of Life Questionnaire (AFQLQ) [10], [11], and the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) Japanese version [12], [13], were obtained from each patient prior to entering the study. Each patient was provided with a patient-operated, leadless, single-channel ECG recorder (Omron Heart Scan 801w, Tokyo, Japan) that included a lightweight, handheld ECG recording system with a liquid crystal display and digital storage capacity for offline digital analysis [14]. Each patient was also requested to perform 30-second ECG recordings at least once daily at a predetermined time and when they experienced any arrhythmia-related symptoms during the study. All recorded ECGs were digitally stored on a memory card, and 2 experienced cardiologists determined the occurrences of AF in a blinded fashion.
Each patient documented the consumption of flecainide or pilsicainide during the study. Patient recordings were maintained daily in patient diaries. Patient history, including diary entries, could be reviewed at the end of each treatment period. Adverse events were recorded. Physical examinations, 12-lead ECG, routine laboratory tests, and QOL assessments were also performed.
The protocol was approved by the institutional review boards of Tokyo Women׳s Medical University (approval number: 2188, approval date: April 30, 2011, re-approval number: 130513, re-approval date: June 4, 2013) and the other participating hospitals. This study was registered in UMIN Clinical Trials Registry (UMIN-CTR: UMIN 000006090).
2.3. Endpoints
The primary endpoint was the difference in the frequency (days/8 weeks) of symptomatic and asymptomatic AF recorded on a patient-operated ECG recorder between weeks 4 and 12 (i.e., the efficacy period). The secondary endpoints included the time to first recurrence of AF during each treatment period (between weeks 4 and 12) and QOL, as assessed using the AFQLQ and SF-36 questionnaire.
The AFQLQ is a self-reported measure of AF-related symptoms and QOL that comprises 26 questions in 3 subsets: 1) frequency of 6 symptoms associated with AF (palpitations, pulse deficit, irregular pulse, shortness of breath, dizziness, and chest discomfort) rated from 0 to 4, with 0 being “3 times or more a week”, 1 being “1 or 2 times a week”, 2 being “1 or 2 times a month”, 3 being “less than 1 time a month”, or 4 being “none”; 2) severity of symptoms rated from 0 to 3, with 0 being”extreme”, 1 being “moderate”, 2 being “mild”, or 4 being “none”; and 3) anxiety and limitation of daily activities related to AF and AF treatment rated from 0 to 4 (always to never). Questionnaire items were summed to generate the frequency, severity, anxiety, and limitation of daily activity subset scores, which ranged from 0 to 24, 0 to 18, and 0 to 56, respectively, with higher scores indicating less symptomatology [10], [11].
The SF-36 is a self-reported measure of health-related QOL comprising 36 questions that assess 8 specific QOL health status dimensions. The SF-36 also provides 2 important summary measures: the Physical Component Summary (PCS) and the Mental Component Summary (MCS) scores. Items are summed to obtain total scores, and each 8 subscale score from 0 to 100 is assessed, with higher scores indicating more desirable health states [12], [13].
2.4. Statistical analysis
We assumed that the rate of preventive effect (first recurrence) of AF at 3 months after the initiation of the antiarrhythmic drug would be 83% in patients treated with flecainide and 68% in patients with pilsicainide using routine ECG based on a clinical trial (flecainide) and a single-center report (pilsicainide) [15], [16]. We estimated that a sample size of 40 patients would be needed for this crossover study to obtain a two-sided alpha level of 0.05 and a beta level of 0.80. We enrolled 65 patients assuming that more than 25% of the patients would withdraw from the study before completing both treatments based on a previous crossover study of flecainide and placebo [17].
Data comparing flecainide with pilsicainide were examined for a period effect and treatment–period interaction using a two-sample t-test (Welch t-test). Data are presented as the means±standard deviations (SDs), medians (ranges), or as the number of patients. The number of days with documented AF episodes was compared between flecainide and pilsicainide treatments using the Wilcoxon signed-rank test. Categorical variables were subjected to chi-square analysis. The time to the first documented recurrence of AF during each treatment period was analyzed using the Kaplan-Meier method and compared using the log-rank test. The magnitude of the QOL score change was compared between the AFQLQ, SF-36 PCS, and SF-36 MCS scores using one-way analysis of variance (ANOVA). Confidence intervals were calculated for differences in parameters of AFQLQ and SF-36 between the mean values of 2 treatments. Changes in the AFQLQ scores during treatment period 1 and 2 were assessed by AF recurrence status (no recurrence, 1−9 days/8 weeks and ≥10 days/8 weeks) and compared using one-way ANOVA. A p-value <0.05 was considered significant. Data analyses were performed using SPSS statistical software (version 11.01, SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Study patients
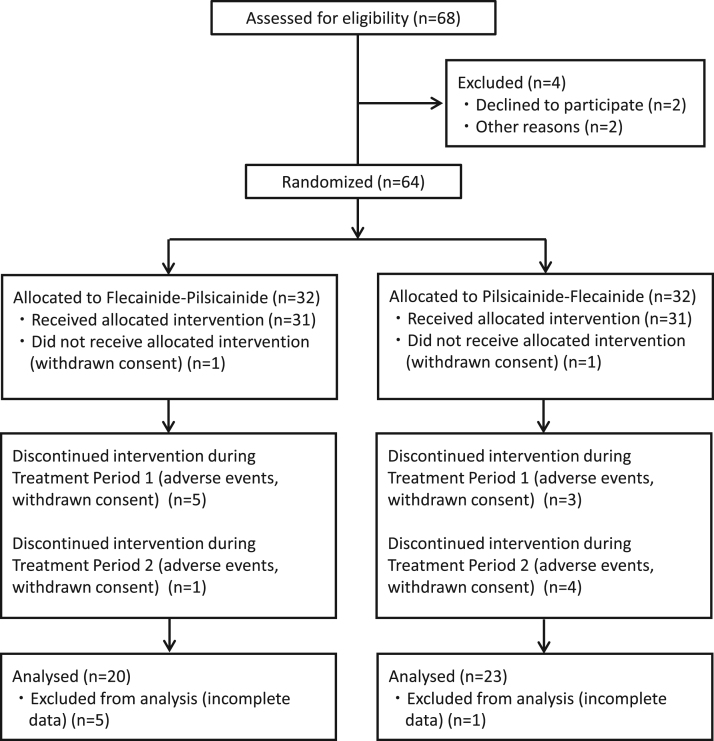
A total of 64 patients from 13 participating centers qualified for the study and provided written informed consent. Patients were recruited from August 2011 to September 2013. Fifteen patients did not complete the 2 crossover treatments. A total of 43 patients were available for efficacy evaluation. The median patient age was 64 years, and 25 (58%) of the efficacy-evaluable patients were male. Twenty-two of the 43 patients had received antiarrhythmic drug therapy prior to study entry, and 10 patients used heart rate-control drugs such as beta-blockers or non-dihydropyridine calcium channel blockers. Twenty patients were enrolled in the flecainide-pilsicainide arm, and 23 patients were in the pilsicainide-flecainide arm (Fig. 2). No significant differences in clinical characteristics were noted between the efficacy-evaluable and efficacy-excluded patients, except concomitant hypertension (34/43 versus 10/21, p=0.01). There were no differences in the clinical characteristics of the efficacy-evaluated patients between the flecainide-pilsicainide and pilsicainide-flecainide arms (Table 1). The final daily dose of flecainide administered during the efficacy phase was 100 mg in 37 patients, 150 mg in 2 patients, and 200 mg in 4 patients. The final daily dose of pilsicainide administered during the efficacy phase was 75 mg in 10 patients, 100 mg in 5 patients, 150 mg in 27 patients, and 200 mg in 1 patient.
Fig. 2.
Patient flow chart.
Table 1.
Baseline characteristics of patients.
| Efficacy-evaluable | Efficacy-excluded | ||
|---|---|---|---|
| patients |
patients | ||
| Flecainide | Pilsicainide | ||
| ―Pilsicainide | ―Flecainide | ||
| Number | 20 | 23 | 21 |
| Age, years | 64 (54–73) | 63 (44–75) | 64 (38–74) |
| Male | 11 (58%) | 14 (58%) | 15 (71%) |
| Height, cm | 163±9 | 164±11 | 166±8 |
| Body weight, kg | 67±11 | 66±14 | 64±9 |
| Hypertension | 18 (90%) | 16 (69%) | 10 (48%) |
| Diabetes mellitus | 3 (15%) | 8 (34%) | 2 (10%) |
| History of TIA/stroke | 1 (1%) | 1 (1%) | 1 (5%) |
| CHADS2 score | |||
| 0 | 2 | 6 | 10 |
| 1 | 14 | 8 | 8 |
| 2 | 3 | 6 | 2 |
| 3 | 1 | 3 | 1 |
| ≥ 4 | 0 | 0 | 0 |
| Concomitant medications | |||
| Antiplatelet drugs | 2 (10%) | 4 (17%) | 3 (14%) |
| Anticoagulant drugs | 16 (80%) | 15 (65%) | 11 (52%) |
| Antihypertensive drugs | 7 (35%) | 8 (35%) | 4 (19%) |
| Antidiabetic drugs | 3 (15%) | 3 (13%) | 2 (10%) |
| Lipid-lowering drugs | 12 (60%) | 12 (52%) | 8 (38%) |
The values are expressed as n (%), median (range), or mean±SD.
CHADS2 score = cardiac failure, hypertension, age ≥75 years, diabetes, previous stroke or TIA (doubled).
TIA, transient ischemic attack.
3.2. Primary endpoint: frequency of AF
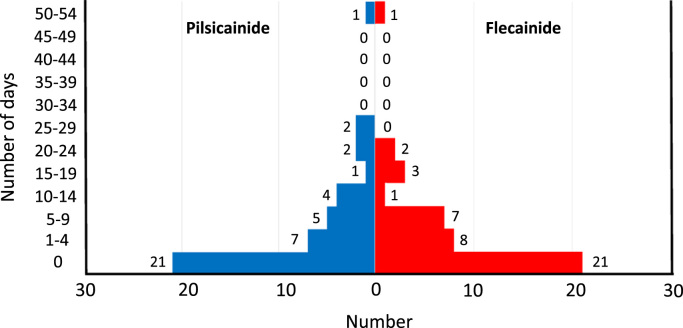
Twenty-six patients experienced AF episodes during the efficacy phases of the study. Eighteen (69%) of these patients experienced AF episodes during both efficacy phases. Fig. 3 shows the distributions of the frequency of AF (days/8 weeks) for both treatments. The distributions for flecainide and pilsicainide treatments were very similar. The median AF frequencies were 2 (range 0–50) days/8 weeks for flecainide treatment and 1 (range 0–54) day/8 weeks for pilsicainide treatment. These values were not significantly different.
Fig. 3.
Distribution of the primary study end point (number of days with documented atrial fibrillation episodes) in patients who received flecainide or pilsicainide.
3.3. Secondary endpoints
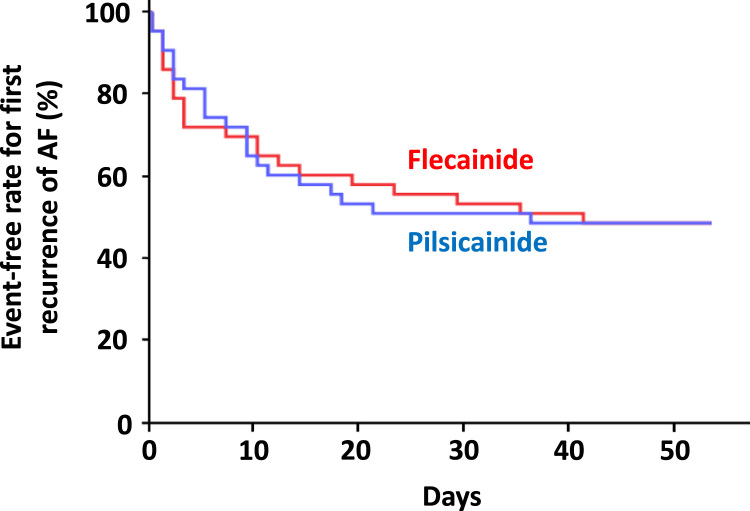
No significant difference in the first recurrence of AF during the efficacy phase was noted between the flecainide and pilsicainide treatments (Fig. 4). Table 2 summarizes the QOL assessment as the secondary endpoint in this study. No significant differences in these endpoints were noted between treatments. No difference in the adherence rate of flecainide or pilsicainide was noted during each treatment phase (median 99.1% versus 97.0%).
Fig. 4.
Cumulative event-free rate for the first recurrence of atrial fibrillation (AF) during each treatment period (between weeks 4 and 12).
Table 2.
Differences in quality of life assessments between flecainide and pilsicainide treatments.
| Flecainide | Pilsicainide | Flecainide-Pilsicainide | |
|---|---|---|---|
| AFQLQ | |||
| AFQLQ1 | 16.6±5.3 | 16.3±5.9 | −0.3 (−0.9–1.6) |
| AFQLQ2 | 12.4±4.0 | 12.6±4.1 | −0.2 (−1.3–0.9) |
| AFQLQ3 | 42.5±9.2 | 43.3±8.5 | −0.8 (−2.9–1.4) |
| SF-36 | |||
| PCS | 47.7±10.2 | 48.1±11.4 | −0.4 (−9.8–6.2) |
| MCS | 48.0±10.4 | 48.7±10.2 | −0.7 (−13.3–6.1) |
| Subscales | |||
| Physical functioning | 48.1±9.4 | 48.4±12.5 | −0.3 (−2.4–1.7) |
| Role physical | 47.6±10.7 | 46.9±12.5 | 0.6 (−2.0–3.3) |
| Bodily pain | 48.6±9.9 | 49.9±11.5 | −1.3 (−4.1–1.6) |
| General health | 44.3±9.9 | 44.9±9.3 | −0.5 (−2.7–1.7) |
| Vitality | 50.0±10.2 | 50.3±10.2 | −0.4 (−3.0–2.3) |
| Social functioning | 47.2±11.7 | 50.5±10.3 | −3.3 (−6.6–0.0) |
| Role emotional | 49.0±9.4 | 47.9±10.6 | 1.1 (−2.2–4.4) |
| Mental health | 49.3±9.4 | 49.8±9.2 | −0.5 (−3.2–2.2) |
The values are expressed as the mean±SD or median (95% confidence interval).
AFQLQ, Atrial Fibrillation Quality of Life Questionnaire; AFQLQ1, Frequency of occurrence of 6 symptoms (palpitations, pulse deficit, irregular pulse, shortness of breath, dizziness, and chest discomfort); AFQLQ2, The severity of these symptoms; AFQLQ3, Anxiety and limitation of daily activities related to AF and AF treatment; MCS, Mental component summary; PCS, Physical component summary.
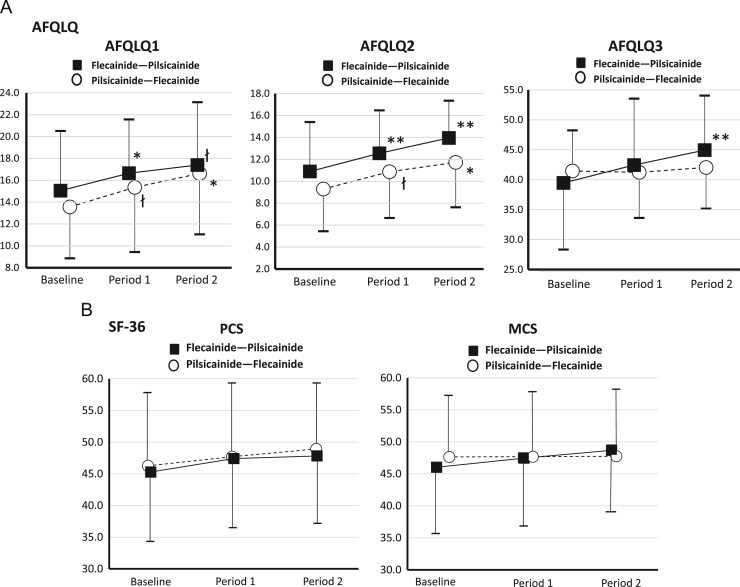
The AFQLQ frequency and severity of AF-related symptom scores (AFQLQ1 and 2), but not anxiety and limitation of daily activities related to AF and AF treatment (AFQLQ3), improved from baseline to the end of the treatment periods (Fig. 5A). When changes in the AFQLQ scores were assessed by AF recurrence status, patients who experienced no or less frequent AF recurrence tended to show improvement in AFQLQ1 and 2 scores (Table 3). However, the SF-36 scores did not change significantly from baseline to the end of the treatment periods (Fig. 5B).
Fig. 5.
Quality of life scores as assessed using the Atrial Fibrillation Quality of Life Questionnaire (AFQLQ) (A) and Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) (B) at specified times during the study. Mean±SD values are provided for each phase of the study. łp<0.10, *p<0.05, **p<0.01 versus baseline. AFQLQ 1, Frequency of occurrence of 6 symptoms (palpitations, pulse deficit, irregular pulse, shortness of breath, dizziness, and chest discomfort); AFQLQ 2, Severity of these symptoms; AFQLQ 3, Anxiety and limitation of daily activities related to AF and AF treatment; PCS, Physical component summary; MCS, Mental component summary.
Table 3.
Change in the Atrial Fibrillation Quality of Life Questionnaire (AFQLQ) scores by atrial fibrillation recurrence status.
| Treatment Period 1 |
|||||||
|---|---|---|---|---|---|---|---|
| No recurrence (n=19) |
Recurrence (1−9 days/8 weeks) (n=14) |
Recurrence (≥10 days/8 weeks) (n=10) |
|||||
| Baseline | Change | Baseline | Change | Baseline | Change | p-value | |
| AFQLQ1 | 15.9±4.2 | 3.1±5.0 [3.0] | 10.0±4.7 | 3.6±3.5 [4.0] | 15.9±4.5 | −1.7±3.6 [1.0] | 0.014 |
| AFQLQ2 | 9.7±4.9 | 3.4±4.0 [2.0] | 8.7±2.0 | 1.2±2.1 [1.0] | 13.2±3.1 | −0.2±2.7 [0.0] | 0.029 |
| AFQLQ3 | 37.9±10.2 | 1.4±8.5 [1.0] | 40.2±6.4 | 4.2±7.0 [3.0] | 46.9±6.6 | −1.2±3.5 [0.5] | 0.264 |
| Treatment Period 2 |
|||||||
| No recurrence (n=23) |
Recurrence (1–9 days/8 weeks) (n=13) |
Recurrence (≥10 days/8 weeks) (n=7) |
|||||
| Baseline | Change | Baseline | Change | Baseline | Change | p-value | |
| AFQLQ1 | 14.6±5.2 | 4.4±6.6 [4.0] | 13.3±4.4 | 1.7±5.7 [2.0] | 14.7±5.7 | −0.1±3.1 [1.0] | 0.186 |
| AFQLQ2 | 9.7±4.9 | 4.9±5.0 [4.0] | 10.3±3.3 | 0.6±3.6 [1.0] | 12.3±3.6 | 0.4±2.7 [0.0] | 0.016 |
| AFQLQ3 | 39.0±9.6 | 4.1±6.6 [4.0] | 42.4±6.7 | −0.9±6.2 [1.0] | 44.0±10.1 | −5.1±8.3 [3.0] | 0.414 |
The values are expressed as the mean±SD, [median].
AFQLQ 1, Frequency of occurrence of 6 symptoms (palpitations, pulse deficit, irregular pulse, shortness of breath, dizziness, and chest discomfort); AFQLQ 2, The severity of these symptoms; AFQLQ 3, Anxiety and limitation of daily activities related to AF and AF treatment;
3.4. Adverse events
Four patients discontinued the study because of adverse effects. One patient developed sinus bradycardia (45 bpm) with dizziness on exertion during treatment period 2 (flecainide). One patient experienced syncope during treatment period 1 (flecainide) and developed sinus arrest (10 seconds), which was noted using ECG monitoring after admission and drug discontinuation. This patient received a pacemaker. One patient experienced xerocheilia during treatment period 1 (pilsicainide), and 1 patient experienced stomatitis during treatment period 1 (pilsicainide).
4. Discussion
Our study revealed the following findings. 1) There was no significant difference in the AF frequency between flecainide and pilsicainide treatments. 2) There was no significant difference in the first documented recurrence of AF during the efficacy phase between the treatments. 3) AFQLQ AF-related symptoms and severity scores improved from baseline to the end of the treatment periods. 4) There were no significant differences in QOL scores between the treatments.
4.1. AF recurrence
Previous reports demonstrated that flecainide significantly reduced the number of AF recurrences in paroxysmal AF patients [6]. However, few studies have reported direct head-to-head comparisons of flecainide and other antiarrhythmic drugs using crossover and randomized clinical trials to assess the preventive effect on AF recurrence in patients with paroxysmal AF [6]. Seventeen of the 43 efficacy-evaluable patients experienced no recurrence of AF during treatment with flecainide and pilsicainide. There was no difference in the distribution of the frequency of days with AF episodes between these drugs with similar pharmacological mechanisms. Our results also revealed no difference in the time to first recurrence of AF between flecainide and pilsicainide treatments. However, 10 patients used beta-blockers or non-dihydropyridine calcium channel blockers. These patients may not have experienced arrhythmia-related symptoms when AF occurred because these rate-control drugs reduced the ventricular rate. Therefore, the recurrence of AF may be underestimated in these patients.
4.2. QOL
A QOL assessment has been included in several studies of patients with AF. QOL was used to assess specific interventions, such as pacemaker implantation, pharmacological therapy and catheter ablation. Rate- and rhythm-control drugs improved QOL, but no significant difference was noted between rate and rhythm strategies [18], [19]. Alliot et al. noted the lack of evidence of the relationship between the achievement of sinus rhythm and QOL [18]. Our results revealed that the antiarrhythmic drugs flecainide and pilsicainide improved AF-related symptoms and symptom severity (AFQLQ1 and 2) from baseline to the end of the treatment period. Because patients who experienced either no or less frequent AF recurrence tended to show improvement in AFQLQ1 and 2 scores, the effect of flecainide and pilsicainide on AF-related symptoms and symptom severity might be related to the suppression of AF recurrence. However, anxiety and limitation of daily activities related to AF and AF treatment (AFQLQ3) did not change from baseline to the end of treatment periods for either drug. This item included questions regarding specific treatments such as the side effects of the drug, food restriction due to drug-food interaction (especially warfarin), electrical cardioversion, hospitalization due to AF treatment, and adherence to medication [10], [11]. Therefore, the majority of the questions in the AFQLQ3 item for paroxysmal AF patients without structural heart disease might not be affected only by intervention with pilsicainide or flecainide. However, we did not identify a difference in these measurements (AFQLQ1 and 2) upon comparing the drugs.
Comparisons of antiarrhythmic drugs in the treatment of AF have been traditionally evaluated based on the pharmacologic action, not the pharmacokinetic characteristics, of the drug. Pharmacokinetic profiles may partially contribute to medication adherence via dosing frequency or concentration-dependent effects. Dosing frequency also affects the adherence to medication [20]. A recent report demonstrated that frequent daily dosing was an independent risk factor for non-adherence to cardiovascular drugs in Japanese patients with AF [21]. A twice daily dosage of flecainide provides a superior dosing frequency to pilsicainide (3 times daily), which should improve adherence to the medication. However, no difference in adherence rate between flecainide and pilsicainide was observed during each treatment phase. This cross-over study did not find a relationship between dosing frequency and medication adherence. Each patient in this prospective interventional study documented the consumption of both drugs in patient diaries that were specific for each drug, which is unlike a real-world setting. This requirement may have improved adherence to both drugs.
AF studies that assess QOL typically use health- and AF-related QOL. The SF-36, a generic health-related QOL instrument, is widely used as a measure of outcome in cohort studies and clinical trials. The Japanese version was validated in Japanese populations [10], [11] and is applicable across Japanese patients with several diseases including cardiovascular disease [22], [23], [24], [25], [26], [27]. AFQLQ, an AF-specific QOL questionnaire, was developed in Japan and validated for Japanese AF patients [10], [11]. AFQLQ has been used to assess outcomes in clinical trials for Japanese AF patients [8], [28]. To assess QOL, both generic and disease-specific assessments are required because both tools can detect subtle clinical changes in patients with cardiovascular disease [29], [30]. Therefore, the Japanese version SF-36 and AFQLQ have been used as QOL assessment tools and measures of outcomes in clinical studies for Japanese patients with AF [8], [28], [31]; both were used in this study.
However, this methodology seems limited for the clarification of the differences in the effects of specific antiarrhythmic drugs on QOL in AF patients. A crossover study on the beta-blockers sotalol and atenolol in the treatment of symptomatic paroxysmal AF revealed no difference in the frequency of AF episodes based on Holter monitoring, health-related QOL, and AF-related symptoms between the two drugs [32]. Moreover, the Japanese AF-specific QOL questionnaire AFQLQ contains no items related to the dosing frequency of antiarrhythmic drugs, but the dosing frequency may affect QOL. Therefore, we could not methodologically detect any effect that different dosing frequencies of the drugs had on QOL based on the questionnaires used in this study.
4.3. Doses of study drugs
This study used a relatively low dose of flecainide or pilsicainide. Recent guidelines recommend that safety, rather than efficacy, considerations should primarily guide the selection and use of antiarrhythmic drugs for AF [1], [3], [4]. Therefore, investigators tend to select a low dose for safety reasons. Four patients discontinued the study due to adverse effects, including cardiac and non-cardiac events, during both treatments. The number of these incidences were not high in the present study compared with those in previous clinical trials [17], [33].
4.4. Limitations
There were some limitations in this study. First, the number of subjects was small. Our estimated power might be insufficient to detect differences in efficacy between the drugs because our results demonstrated that the AF recurrence rate was approximately 50% in both groups. This study used a relatively low dose for treatment, which may have affected the results. Our results are limited in their generalizability to the management of Japanese patients with AF. Second, the efficacy-evaluation period was only 8 weeks in this study, which may be insufficient to assess the effect of a single treatment. Third, approximately 30% of the patients who entered the study discontinued the study. A previous crossover study revealed that more than 25% of patients discontinued the study [17], [32]. It is difficult to complete two treatment phases using fixed doses of antiarrhythmic drugs for 6 months in symptomatic AF patients in the catheter ablation era. Half of the patients who discontinued the study subsequently received catheter ablation. Fourth, a study design that compares the efficacy of two treatments typically includes a control comparison. However, we did not establish a control phase before each treatment period. Therefore, we could not assess either treatment effect in comparison with a control.
5. Conclusions
This prospective crossover study demonstrated no clear difference in the AF frequency or QOL between symptomatic paroxysmal AF patients treated with flecainide or pilsicainide. However, an improvement in AF-related symptoms was noted from baseline following treatment with flecainide or pilsicainide.
Funding
This study was supported by the Japan Heart Foundation [12100027].
Disclosures
The conflict of interest disclosure statements are provided below: Shiga T has received research funding and speaker׳s honoraria from Eisai and Daiichi-Sankyo. Yoshioka K has received research funding and speaker׳s honoraria from Daiichi-Sankyo. Watanabe E and Matsumoto N have received speaker׳s honoraria from Daiichi-Sankyo. Kusano K and Komatsu T have received speaker׳s honoraria from Eisai and Daiichi-Sankyo. Ikeda T and Yamashita T have received research funding from Daiichi-Sankyo and speaker׳s honoraria from Eisai and Daiichi-Sankyo. Takahashi N has received research funding from Eisai and Daiichi-Sankyo and speaker׳s honoraria from Daiichi-Sankyo. The other co-authors have no disclosures.
Acknowledgements
We thank the study investigators, healthcare staff, and patients who participated in this study.
Appendix
The following investigators participated in the AF-QOL study:
Takashi Komatsu, Division of Cardioangiology, Nephrology and Endocrinology, Department of Internal Medicine, Iwate Medical University, Morioka, Japan.
Keiko Fukushima, Shinya Fujii, and Masahiro Yagi, Division of Cardiology, Sendai Cardiovascular Center, Sendai, Japan.
Yasuo Okumura and Atsushi Hirayama, Division of Cardiology, Department of Medicine, Nihon University School of Medicine, Tokyo, Japan.
Atsushi Suzuki, Ryo Koyanagi, Tsuyoshi Suzuki, and Tsuyoshi Shiga, Department of Cardiology, Tokyo Women׳s Medical University, Tokyo, Japan.
Hisako Omori, Department of Medicine, Tokyo Women׳s Medical University Medical Center East, Tokyo, Japan.
Noriyuki Fujino and Takanori Ikeda, Department of Cardiovascular Medicine, Toho University Faculty of Medicine, Tokyo, Japan.
Naoki Matsumoto, Department of Pharmacology, St. Marianna University School of Medicine, Kawasaki, Japan.
Mari Amino, Koichiro Yoshioka, Department of Cardiovascular Medicine, Tokai University School of Medicine, Isehara, Japan.
Hiroto Harigaya, Yoshihiro Sobue, and Eiichi Watanabe, Department of Cardiology, Fujita Health University School of Medicine, Toyoake, Japan.
Kengo Kusano, Department of Cardiovascular Medicine, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan.
Naohiko Takahashi, Department of Cardiology and Clinical Examination, Faculty of Medicine, Oita University, Yufu, Japan.
Tatsushi Shinzato, Chikara Oshiro, and Hisashi Yoshida, Shonan Hospital, Okinawa, Japan.
Steering Committee: Hagiwara N, Koretsune Y, Ikeda T, Yamashita T, Shiga T, Koyanagi R, Takahashi N, Watanabe E, Komatsu T, Okuyama Y, Yoshioka K, Kusano K, Matsumoto N, Shizuta S, and Komiya N.
Protocol Committee: Yamashita T, Suzuki T, and Shiga T.
Ethical Review Board: Saikawa T.
Safety Monitoring Committee: Kobayashi Y and Murakawa Y.
Case Examination Committee: Yamashita T, Otsuka M, Suzuki A, and Shiga T.
Data management: Kuwajima Y (Medi-Skette Corporation), Sato S, and Sato Y (Intelligent Clinical Research and Innovation Center, Tokyo Women׳s Medical University Hospital).
Secretariat: Shimizu K and Kobayakawa N (Intelligent Clinical Research and Innovation Center, Tokyo Women׳s Medical University Hospital).
References
- 1.Camm A.J., Kirchhof P., Lip G.Y. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 2.Thrall G., Lane D., Carroll D. Quality of life in patients with atrial fibrillation: a systematic review. Am J Med. 2006;119(448):e1–e19. doi: 10.1016/j.amjmed.2005.10.057. [DOI] [PubMed] [Google Scholar]
- 3.Kirchhof P., Benussi S., Kotecha D. ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS: the Task Force for the management of atrial fibrillation of the European Society of Cardiology (ESC)Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC Endorsed by the European Stroke Organisation (ESO) Eur Heart J. 2016;2016(37):2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 4.January C.T., Wann L.S., Alpert J.S. AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American heart association task force on practice guidelines and the heart rhythm society. J Am Coll Cardiol. 2014;2014(64):e1–e76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 5.Holmes B., Heel R.C. Flecainide. A preliminary review of its pharmacodynamics properties and therapeutic efficacy. Drugs. 1985;29:1–33. doi: 10.2165/00003495-198529010-00001. [DOI] [PubMed] [Google Scholar]
- 6.Aliot E., Capucci A., Crijns H.J. Twenty-five years in the making: flecainide is safe and effective for the management of atrial fibrillation. Europace. 2011;13:161–173. doi: 10.1093/europace/euq382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plosker G.L. Pilsicainide. Drugs. 2010;70:455–467. doi: 10.2165/11204960-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Ogawa S., Yamashita T., Yamazaki T. Optimal treatment strategy for patients with paroxysmal atrial fibrillation: j-rhythm Study. Circ J. 2009;73:242–248. doi: 10.1253/circj.cj-08-0608. [DOI] [PubMed] [Google Scholar]
- 9.JCS Joint Working Group Guidelines for pharmacotherapy of atrial fibrillation (JCS 2013): digest version. Circ J. 2014;78:1997–2021. doi: 10.1253/circj.cj-66-0092. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita T., Kumagai K., Koretsune Y. A new method for evaluating quality of life specific to patients with atrial fibrillation: atrial fibrillation quality of life questionnaire (AFQLQ) Jpn J Electrocardiol. 2003;23:332–343. [in Japanese] [Google Scholar]
- 11.Yamashita T., Komatsu T., Kumagai K. Internal consistency and reproducibility of Atrial Fibrillation Quality of Life Questionnaire (AFQLQ) Jpn J Electrocardiol. 2005;25:488–494. [in Japanese] [Google Scholar]
- 12.Fukuhara S., Bito S., Green J. Translation, adaptation, and validation of the SF-36 Health Survey for use in Japan. J Clin Epidemiol. 1998;51:1037–1044. doi: 10.1016/s0895-4356(98)00095-x. [DOI] [PubMed] [Google Scholar]
- 13.Fukuhara S., Ware J.E., Kosinski M. Psychometric and clinical tests of validity of the Japanese SF-36 health survey. J Clin Epidemiol. 1998;51:1045–1053. doi: 10.1016/s0895-4356(98)00096-1. [DOI] [PubMed] [Google Scholar]
- 14.Kaleschke G., Hoffmann B., Drewitz I. Prospective, multicentre validation of a simple, patient-operated electrocardiographic system for the detection of arrhythmias and electrocardiographic changes. Europace. 2009;11:1362–1368. doi: 10.1093/europace/eup262. [DOI] [PubMed] [Google Scholar]
- 15.Chimienti M., Cullen M.T., Jr, Casadei G. Safety of flecainide versus propafenone for the long-term management of symptomatic paroxysmal supraventricular tachyarrhythmia. Report from the Flecainide and Propafenone Italian Study (FAPIS) group. Eur Heart J. 1995;16:1943–1951. doi: 10.1093/oxfordjournals.eurheartj.a060852. [DOI] [PubMed] [Google Scholar]
- 16.Komatsu T., Sato Y., Tachibana H. Randomized crossover study of the long-term effects of pilsicainide and cibenzoline in preventing recurrence of symptomatic paroxysmal atrial fibrillation-Influence of the duration of arrhythmia before therapy- Circ J. 2006;70:667–672. doi: 10.1253/circj.70.667. [DOI] [PubMed] [Google Scholar]
- 17.Andesrson J.L., Gilbert E.M., Allpert B.L. Prevention of symptomatic recurrences of paroxysmal atrial fibrillation in patients initially tolerating antiarrhythmic therapy. A multicenter, double-blind, crossover study of flecainide and placebo with transtelephonic monitoring. Flecainide Supraventricular Tachycardia Study Group. Circulation. 1989;80:1557–1570. doi: 10.1161/01.cir.80.6.1557. [DOI] [PubMed] [Google Scholar]
- 18.Aliot E., Botto G.L., Crijns H.J. Quality of life in patients with atrial fibrillation: how to assess it and how to improve it. Europace. 2014;16:787–796. doi: 10.1093/europace/eut369. [DOI] [PubMed] [Google Scholar]
- 19.Ha A.C., Breithardt G., Camm A.J. Health-related quality of life in patients with atrial fibrillation treated with rhythm control versus rate control: insights from a prospective international registry (Registry on Cardiac Rhythm Disorders Assessing the Control of Atrial Fibrillation: record-af) Circ Cardiovasc Qual Outcomes. 2014;7:896–904. doi: 10.1161/HCQ.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 20.Coleman C.I., Roberts M.S., Sobieraj D.M. Effect of dosing frequency on chronic cardiovascular disease medication adherence. Curr Med Res Opin. 2012;28:669–680. doi: 10.1185/03007995.2012.677419. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki T, Shiga T, Omori H. , et al. Adherence to medication and characteristics of Japanese patients with non-valvular atrial fibrillation. J Cardiol (in press); 2017. [DOI] [PubMed]
- 22.Seki E., Watanabe Y., Sunayama S. Effects of phase III cardiac rehabilitation programs on health-related quality of life in elderly patients with coronary artery disease: juntendo Cardiac Rehabilitation Program (J-CARP) Circ J. 2003;67:73–77. doi: 10.1253/circj.67.73. [DOI] [PubMed] [Google Scholar]
- 23.Tada H., Naito S., Kurosaki K. Segmental pulmonary vein isolation for paroxysmal atrial fibrillation improves quality of life and clinical outcomes. Circ J. 2003;67:861–865. doi: 10.1253/circj.67.861. [DOI] [PubMed] [Google Scholar]
- 24.Nishiyama S., Momomura S., Ishiwata S. Health-related quality of life in Japanese patients with ischemic heart disease: a multicenter cooperative investigation assessed using SF-36. J Cardiol. 2005;46:211–220. [in Japanese] [PubMed] [Google Scholar]
- 25.Okano Y., Tamura K., Kuji T. Effects of angiotensin II receptor blockers on relationships between 24-hour blood pressure, autonomic function, and health-related QOL. Clin Exp Hypertens. 2009;31:250–258. doi: 10.1080/10641960902822500. [DOI] [PubMed] [Google Scholar]
- 26.Shinohara Y., OASIS Study Group Factors affecting health-related quality of life assessed with the SF-36v2 health survey in outpatients with chronic-stage ischemic stroke in Japan--cross-sectional analysis of the OASIS study. Cerebrovasc Dis. 2010;29:361–371. doi: 10.1159/000281834. [DOI] [PubMed] [Google Scholar]
- 27.Izawa K.P., Watanabe S., Oka K. Age-related differences in physiologic and psychosocial outcomes after cardiac rehabilitation. Am J Phys Med Rehabil. 2010;89:24–33. doi: 10.1097/PHM.0b013e3181c5607d. [DOI] [PubMed] [Google Scholar]
- 28.Yamashita T., Inoue H., Okumura K. Randomized trial of angiotensin II-receptor blocker vs. dihydropiridine calcium channel blocker in the treatment of paroxysmal atrial fibrillation with hypertension (J-RHYTHM II Study) Europace. 2011;13:473–479. doi: 10.1093/europace/euq439. [DOI] [PubMed] [Google Scholar]
- 29.Spertus J.A., Winder J.A., Dewhurst T.A. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 30.Brown N., Melville M., Gray D. Quality of life four years after acute myocardial infarction: short form 36 scores compared with a normal population. Heart. 1999;81:352–358. doi: 10.1136/hrt.81.4.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsuneda T., Yamashita T., Fukunami M. Rate control and quality of life in patients with permanent atrial fibrillation: the quality of life and atrial fibrillation (QOLAF) study. Circ J. 2006;70:965–970. doi: 10.1253/circj.70.965. [DOI] [PubMed] [Google Scholar]
- 32.Steeds R.P., Birchall A.S., Smith M. An open label, randomised, crossover study comparing sotalol and atenolol in the treatment of symptomatic paroxysmal atrial fibrillation. Heart. 1999;82:170–175. doi: 10.1136/hrt.82.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aliot E., Denjoy I. Comparison of the safety and efficacy of flecainide versus propafenone in hospital out-patients with symptomatic paroxysmal atrial fibrillation/flutter. The Flecainide AF French Study Group. Am J Cardiol. 1996;77:66A–71A. doi: 10.1016/s0002-9149(97)89120-5. [DOI] [PubMed] [Google Scholar]