Abstract
Atrial fibrillation (AF) is the most common sustained arrhythmia, causing a 2-fold increase in mortality and a 5-fold increase in stroke. The Asian population is rapidly aging, and in 2050, the estimated population with AF will reach 72 million, of whom 2.9 million may suffer from AF-associated stroke. Therefore, stroke prevention in AF is an urgent issue in Asia. Many innovative advances in the management of AF-associated stroke have emerged recently, including new scoring systems for predicting stroke and bleeding risks, the development of non-vitamin K antagonist oral anticoagulants (NOACs), knowledge of their special benefits in Asians, and new techniques. The Asia Pacific Heart Rhythm Society (APHRS) aimed to update the available information, and appointed the Practice Guideline sub-committee to write a consensus statement regarding stroke prevention in AF. The Practice Guidelines sub-committee members comprehensively reviewed updated information on stroke prevention in AF, emphasizing data on NOACs from the Asia Pacific region, and summarized them in this 2017 Consensus of the Asia Pacific Heart Rhythm Society on Stroke Prevention in AF. This consensus includes details of the updated recommendations, along with their background and rationale, focusing on data from the Asia Pacific region. We hope this consensus can be a practical tool for cardiologists, neurologists, geriatricians, and general practitioners in this region. We fully realize that there are gaps, unaddressed questions, and many areas of uncertainty and debate in the current knowledge of AF, and the physician׳s decision remains the most important factor in the management of AF.
Keywords: Anticoagulation, Atrial fibrillation, Non-vitamin K antagonist oral anticoagulants, Vitamin K antagonist, Stroke
External reviewers
Professor Gregory YH Lip.
University of Birmingham Institute for Cardiovascular Sciences, City Hospital, Birmingham, England; and Aalborg Thrombosis Research Unit, Department of Clinical Medicine, Faculty of Health, Aalborg University, Aalborg, Denmark.
email: g.y.h.lip@bham.ac.uk.
1. Epidemiology of atrial fibrillation-associated stroke in Asia
Stroke and systemic thromboembolism are the most clinically important complications observed in patients with atrial fibrillation (AF) [1]. Stroke caused by AF is defined as cardioembolic stroke, and once it occurs, it often results in death (up to 20%) or disability (approximately 60%) [2], [3], [4]. Therefore, appropriate thromboprophylaxis is mandatory.
Cardioembolic stroke occurs most commonly in the elderly, especially the oldest-old AF patients [5], [6]. The Asian population is rapidly aging, and in 2050, the estimated population with AF will reach 72 million, of whom 2.9 million may suffer from AF-associated stroke [7]. Thus, stroke prevention in AF is an urgent healthcare and public-health concern in Asia.
The incidence of AF-associated stroke has been extensively investigated worldwide. Overall, the incidence of stroke in patients with AF is 4–5-fold higher than that in patients without AF [8], [9]. Importantly, the incidence varies significantly with patients’ clinical characteristics and risk factors, the more common ones being included in risk scores such as the CHA2DS2-VASc (Congestive heart failure, Hypertension, Age ≥ 75 [doubled], Diabetes, Stroke [doubled]-Vascular disease, Age 65–74, Sex category [female]) score [10]. Other risk factors may include reduced renal function or chronic kidney disease (CKD) [11], [12], and low body weight [13].
In addition, the incidence of stroke is also significantly affected by the use of oral anticoagulation therapy (OAC) and the quality of anticoagulation control. The overall annual incidences of ischemic stroke reported in Asia ranged from 1.3% in Japanese AF patients (n=3588; mean age, 68.1 ±13.5 years; mean CHA2DS2-VASc score, 2.4) from three prospective registries (Shinken Database, J-RHYTHM Registry, and Fushimi AF Registry) [14] to 10.4% in hospitalized Chinese AF patients (n=3333; mean age, 79.5±9.2 years; mean CHA2DS2-VASc score, 3.8) from the Queen Mary Hospital, Hong Kong [15]. Of the 186,570 AF patients without OAC selected from the National Health Insurance Research Database (NHIRD) in Taiwan, 23,723 (12.7%) experienced ischemic stroke during the follow-up period of 3.4 years (3.7%/year) [16]. Even higher incidences of AF-associated stroke, ranging from 13.0% to 15.4% for 1-to-3–year periods, were reported in the Far East and Southeast Asia [17]. The reported incidence of AF-associated stroke varies substantially due to clinical setting (hospitalized versus community), unrecorded use of OAC at follow-up, different methods of analysis, and diverse clinical characteristics of AF patients in different regions.
Is the prevalence of AF-associated stroke in Asia higher or lower when compared with that reported from Western countries? The annual incidence of ischemic stroke in non-anticoagulated AF patients in the United States was 2.1%, with the incidence increasing from 0.57% in patients < 65 years, to 1.41% in those between 65 and 74 years, to 2.58% in those between 75 and 84 years, and even further to 4.42% in those >85 years of age [18]. Although no direct comparisons have been made in non-anticoagulated AF patients, recent global clinical trials may offer some insights [19], [20], [21], [22]. For example, a sub-analysis of the RE-LY trial [23] comparing rates of ischemic and hemorrhagic stroke events between Asians and non-Asians demonstrated the absolute rate of ischemic stroke was numerically higher in Asians than in non-Asians in all treatment groups (2.05%/year versus 1.14%/year in the dabigatran 110 mg group, 1.12%/year versus 0.81%/year in the dabigatran 150 mg group, and 2.02%/year versus 0.98%/year in the warfarin group) [23]. The rates of hemorrhagic stroke in the warfarin group were significantly higher in Asians than in non-Asians (hazard ratio, 2.4; 95% confidence interval, 1.3–4.7) [23]. Similarly, the sub-analysis of the ROCKET AF trial [24] comparing event rates between East Asians (not including Japan) and non-East-Asians revealed the absolute rate of ischemic stroke was numerically higher in East Asia compared to non-East Asia (2.24/100 patient-years versus 1.60/100 patient-years in the warfarin group), and the rate of hemorrhagic stroke similarly higher in East Asia (1.24/100 patient-years versus 0.39/100 patient-years) [24]. The sub-analysis of the ENGAGE AF trial [25] comparing East Asians and non-East-Asians revealed the absolute rates of ischemic stroke were numerically higher in East Asia than in non-East Asia (1.31/100 patient-years versus 0.89/100 patient-years in the warfarin group), with the rate of hemorrhagic stroke also higher in East Asia (1.23/100 patient-years versus 0.41/100 patient-years) [25]. Similar trends were also demonstrated in the ARISTOTLE trial [26]. Thus, Asian AF patients are more prone to suffer from ischemic stroke compared with non-Asians, even with anticoagulation. In addition, Asian patients are more prone to hemorrhagic stroke, as previously reported [27].
2. Stroke risk scores
2.1. CHADS2 versus CHA2DS2-VASc scores
The risk of AF-associated stroke is not homogeneous and depends on patients’ ages and comorbidities, which have been used to formulate clinical scores to aid risk stratification. The CHADS2 (congestive heart failure, hypertension, age ≥ 75 [doubled], diabetes mellitus, and prior stroke or transient ischemic attack [TIA]) score has been commonly used to guide antithrombotic therapies for AF patients since its original validation in 2001 (Table 1) [28]. In 2010, the CHA2DS2-VASc was developed [10], and has been confirmed to be superior to the CHADS2 score in identifying truly low-risk patients (Table 1) [29], [30], [31]. The CHA2DS2-VASc score is recommended by the European Society of Cardiology (ESC) [32], American College of Cardiology/American Heart Association (ACC/AHA) [33], and the National Institute for Health and Care Excellence (NICE) for stroke risk stratification in AF (http://guidance.nice.org.uk/CG/Wave0/638).
Table 1.
Calculations of the CHADS2 and CHA2DS2-VASc score.
| CHADS2 | CHA2DS2-VASc | |
|---|---|---|
| Congestive heart failure | 1 | 1 |
| Hypertension | 1 | 1 |
| Age ≥ 75 y | 1 | 2 |
| Diabetes mellitus | 1 | 1 |
| Previous Stroke/TIA | 2 | 2 |
| Vascular disease (prior MI, PAD, or aortic plaque) | – | 1 |
| Age 65–74 y | – | 1 |
| Sex category(i.e., female sex) | – | 1 |
| Maximum score | 6 | 9 |
CHADS2, congestive heart failure, hypertension, age ≥ 75 [doubled], diabetes mellitus, and prior stroke or transient ischemic attack; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥ 75 [doubled], diabetes, stroke [doubled]-vascular disease, age 65–74, sex category [female]; MI, myocardial infarction; PAD, peripheral artery disease; TIA, transient ischemic attack
The diagnostic accuracy of CHADS2 and CHA2DS2-VASc scores was compared among 186,570 AF patients in Taiwan, who did not receive anti-platelet agents or OAC [34]. The CHA2DS2-VASc score outperformed CHADS2 score in predicting ischemic stroke. More importantly, the stroke risk in patients with a CHADS2 score of 0 was not low; the annual stroke rate ranged from 1.15% (CHA2DS2-VASc score=0) to 4.47% (CHA2DS2-VASc score=3). This is consistent with data from the Danish nationwide cohort study, where patients with a CHADS2 score of 0 had a stroke rate as high as 3.2%/year when further stratified by the CHA2DS2-VASc score [35]. The annual risk of ischemic stroke for Asian AF patients stratified by CHADS2 and CHA2DS2-VASc scores is shown in Table 2. Additionally, the CHA2DS2-VASc score has been demonstrated to be better than the ATRIA (anticoagulation and risk factors in atrial fibrillation) score for the prediction of ischemic stroke for Asian AF patients [16]. Based on the current evidence in Asians, use of the CHA2DS2-VASc score is recommended for stroke risk stratification in Asian AF patients.
Table 2.
Annual risk of ischemic stroke for AF patients stratified by CHA2DS2-VASc score in Taiwan AF cohort [8] .
| Scores | Incidence (per 100 person-years) |
|---|---|
| CHADS2score | |
| 0 | 1.80 |
| 1 | 3.08 |
| 2 | 4.49 |
| 3 | 5.33 |
| 4 | 4.86 |
| 5 | 5.80 |
| 6 | 7.10 |
| CHA2DS2-VASc score | |
| 0 | 1.15 |
| 1 | 2.11 |
| 2 | 3.39 |
| 3 | 3.89 |
| 4 | 4.61 |
| 5 | 5.12 |
| 6 | 5.18 |
| 7 | 6.22 |
| 8 | 7.98 |
| 9 | 10.50 |
CHADS2, congestive heart failure, hypertension, age ≥ 75 [doubled], diabetes mellitus, and prior stroke or transient ischemic attack; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥ 75 [doubled], diabetes, stroke [doubled]-vascular disease, age 65–74, sex category [female];
2.2. Should Asian AF patients with one stroke risk factor be treated?
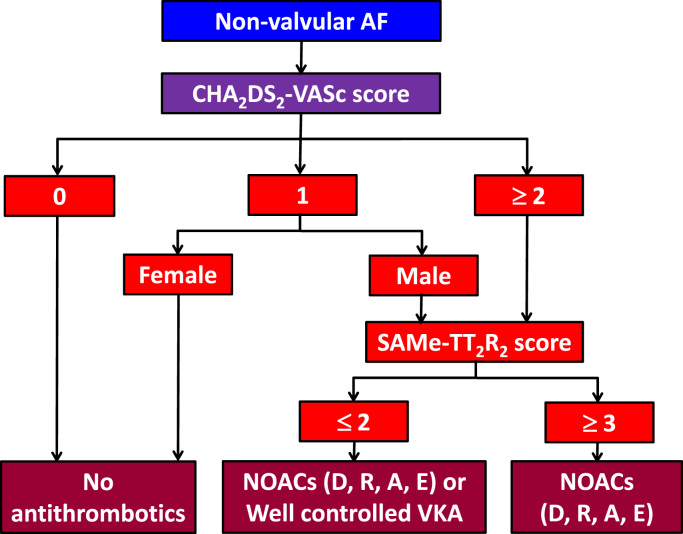
In a recent registry study from Taiwan which enrolled 12,935 AF males with a CHA2DS2-VASc score of 1 and 7900 AF females with a CHA2DS2-VASc score of 2 (i.e., one non-sex stroke risk factor) [36], AF males with a CHA2DS2-VASc score of 1 had an annual stroke rate ranging between 1.96% and 3.50%, depending on the specific covariate composing the score. For AF female patients with one additional stroke risk factor (CHA2DS2-VASc score of 2), the annual stroke rate ranged from 1.91% to 3.34%. The annual risk of ischemic stroke for these patients, left untreated, exceeds the treatment threshold for the initiation of OAC (1.7%/year for warfarin and 0.9%/year for non-vitamin K antagonist oral anticoagulants [NOACs]) [37]. Therefore, we recommend that OAC should be considered for Asian AF patients with one additional risk factor beyond sex: i.e., CHA2DS2-VASc score of 1 for males and 2 for females. This recommendation is similar to the 2016 ESC AF guidelines [38].
Given that the CHA2DS2-VASc score is best at identifying “low-risk” patients, and the benefits of stroke prevention are evident with ≥ 1 non-sex stroke risk factors, the initial step should be to identify low-risk patients (i.e., CHA2DS2-VASc score 0 in males, 1 in females) who do not need antithrombotic therapy – rather than focus on identifying high-risk patients. Thus, the default should be to offer stroke prevention (i.e., OAC) to all patients with AF, unless they can be categorized as “low-risk.”
2.3. Do Asian AF patients have a lower age threshold for stroke?
The risk of ischemic stroke for Asian AF patients is higher than that of non-Asians [39]. Previous studies of NOACs showed that Asian AF patients treated with NOACs had a higher risk of ischemic stroke than non-Asians, despite similar CHADS2 and CHA2DS2-VASc scores [7]. Although the detailed mechanism(s) behind this remained unknown, a recent study from Taiwan has demonstrated that the risk of ischemic stroke may start to rise from age 50 upwards [40]. For these Chinese patients aged 50–64 years, the annual stroke risk was 1.78%, which exceeds the treatment threshold for OAC use for stroke prevention [40]. A similar age threshold (i.e., 50 years) for an increased risk of ischemic stroke was also observed in a study from Hong Kong [41].
A modified CHA2DS2-VASc score, mCHA2DS2-VASc, which assigned one point for patients aged 50–74 years, outperformed CHA2DS2-VASc score for stroke risk stratification for Chinese AF patients, with a higher C-index (0.71 versus 0.69, DeLong test P<0.0001) and an improved net reclassification index [42]. Most importantly, for patients with a CHA2DS2-VASc score of 0 (males) or 1 (females), having a mCHA2DS2-VASc score of 1 (males) or 2 (females) due to the resetting of the age threshold, the use of warfarin was associated with a positive net clinical benefit when balancing the benefit of ischemic stroke reduction against the risk of intracranial hemorrhage (ICH) [42]. Whether the mCHA2DS2-VASc score could be used to guide stroke-prevention strategies for Asian AF patients needs to be confirmed via further studies.
Recommendations
-
•
The CHA2DS2-VASc score is recommended for the prediction of stroke risk in Asian patients with non-valvular AF.
-
•
The initial step is to identify low-risk patients (i.e., without any stroke risk factors; CHA2DS2-VASc score = 0 for males or 1 for females), where no antithrombotic therapy is recommended.
-
•
For patients with at least one stroke risk factor (beyond female sex alone), i.e., CHA2DS2-VASc score ≥ 1 for males or ≥ 2 for females, OAC should be considered, and NOACs are recommended over VKA.
3. Bleeding risk assessment
The stroke risk reduction with OAC should be balanced against the increased risk of bleeding, especially ICH. Several scoring systems have been proposed to estimate the risk of bleeding in AF, such as the HEMORR2HAGES, HAS-BLED, ATRIA, ORBIT, and ABC-bleeding scores [43], [44], [45], [46], [47]. Bleeding scores have been subject to inappropriate use – they should be used to “flag up” the patients at risk for bleeding for more regular review and follow-up, and importantly, to address reversible bleeding-risk factors [48].
The HAS-BLED (Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile International Normalized Ratio (INR), Elderly, Drugs/alcohol concomitantly) score has been proposed as a simple clinical score to predict clinically relevant bleeding in AF patients (Table 3). A HAS-BLED score≥3 indicates a high risk of bleeding, and previous studies have demonstrated that the HAS-BLED score performed better than other bleeding scores [49], [50]. In warfarin users, HAS-BLED would significantly outperform the ATRIA and ORBIT scores that do not consider “labile INR” as a risk factor [51], [52]. Also, the HAS-BLED score has been validated in AF patients on no antithrombotic therapy, aspirin, warfarin and non-warfarin anticoagulants (and thus, is applicable to every step of the AF patient treatment pathway), as well as being validated in Asian AF patients [53]. A high HAS-BLED score should not be used to exclude patients from OAC therapy but allows clinicians to address the correctable risk factors for bleeding, such as uncontrolled hypertension, labile INRs (for a warfarin user) and concomitant use of aspirin, NSAIDs or alcohol excess/abuse.
Table 3.
Calculation of the HAS-BLED score.
| Clinical characteristics | Definition | Score |
|---|---|---|
| Hypertension | SBP>160 mmHg | 1 |
| Abnormal renal and liver function (1 score each) | Renal: dialysis, transplantation, or creatinine ≥ 2.3 mg/dL | 1 or 2 |
| Liver: chronic hepatitis, cirrhosis, bilirubin > 2 ULN, with ALT>3 ULN | ||
| Stroke | Previous history, particularly lacunar | 1 |
| Bleeding tendency or predisposition | Recent bleed, anemia, etc. | 1 |
| Labile INRs | Unstable/high INR, or TTR <60% | 1 |
| Elderly | Age>65 y, extreme frailty | 1 |
| Drugs or alcohol (1 score each) | Drugs: concomitant antiplatelet, or NSAID use | 1 or 2 |
| Alcohol excess | ||
| Maximum score | 9 |
ALT: alanine transaminase; Cr: creatinine; INR: international normalized ratio; NSAID: non-steroidal anti-inflammatory drugs; TTR: time in therapeutic range; ULN: upper limit of normal
Recommendations
-
•
The HAS-BLED score is recommended for the prediction of bleeding risk in Asian patients with non-valvular AF.
-
•
A HAS-BLED score ≥ 3 suggests a high risk of bleeding, but does not preclude the use of OAC. Such patients should have regular review and follow-up of the modifiable bleeding-risk factors (uncontrolled hypertension, labile INRs [for a warfarin user] and concomitant use of aspirin, NSAIDs or alcohol excess/abuse).
4. Role of aspirin
There is no evidence for the effectiveness of aspirin in stroke prevention in AF in Asia. In a Japanese trial, aspirin was no better than placebo in low-risk patients [54]. In a recent Hong Kong cohort study, aspirin showed a non-significant reduction in ischemic strokes, compared with no therapy [55]. OAC is more effective than aspirin for stroke prevention in AF, and the net clinical benefit is positive for OAC versus no treatment or aspirin, but neutral or negative for aspirin versus no antithrombotic therapy, even with a single stroke risk factor [55], [56], [57], [58].
The risks of ischemic stroke and ICH in a real-world cohort of Chinese AF patients were reported recently from Hong Kong [15]. The incidence of ischemic stroke on aspirin was higher than that on dabigatran (110 mg) (7.95%/year vs 2.24%/year). The incidence of ICH was lower in dabigatran (110 mg) users than in those on aspirin (0.32%/year vs 0.80%/year) [15]. In the AVERROES trial, the risk of stroke was significantly lower in the apixaban group than in aspirin group (relative risk reduction 45%, P<0.001), with a similar risk of major bleeds [59]. The risk of ICH was numerically lower in the apixaban group [59]. The totality of these data suggest that there is no role for aspirin in stroke prevention in Asians.
Nonetheless, the use of aspirin is highly prevalent in many Asian countries [60], [61]. In the REgistry on cardiac rhythm disORDers (RecordAF-Asia Pacific [AP]) registry, a prospective observational survey of the management of patients with recently diagnosed AF in eight Asian-Pacific countries, aspirin was more commonly used than VKAs (56–66% vs. 35–47%) [62]. A recent study using Taiwan׳s NHIRD between 2001 and 2008 showed that the percentage of AF patients who received warfarin, aspirin, or no treatment in Taiwan was 16%, 62% and 22%, respectively [63]. In Phase I of the Global Registry on Long-Term Oral Antithrombotic Treatment in Patients with Atrial Fibrillation (GLORIA-AF) registry, 49.6% of Chinese AF patients received aspirin alone [64]. This is reaffirmed in recent real-life data from Hong Kong, where 61% of patients received aspirin [65].
This continued use of aspirin may be partly explained by the misconception that aspirin is associated with a lower risk of bleeding. In addition, there are several issues specific to the Asia-Pacific region. First, INR control is generally poor in the Asia-Pacific region [66]. In a recent study from Hong-Kong, for example, the median time in therapeutic range (TTR) was 38.8% [15]. This may be due to limited access to anticoagulation clinics or to the interaction of VKAs with food or herbal drugs, which are commonly used in this region. Second, Asians treated with VKAs are at higher risk of ICH [7], [67]. which may discourage physicians from prescribing VKAs. Third, the financial burden of using NOACs needs to be considered. Because there are no data showing benefit of aspirin, this consensus statement does not recommend the use of aspirin solely for stroke prevention in AF patients.
Recommendations
-
•
Aspirin is not recommended solely for stroke prevention in AF.
5. Role of vitamin K antagonists (VKAs)
VKAs have been the mainstay of treatment for stroke prevention in AF for more than half a century. In a meta-analysis of 6 randomized control trials (RCTs) involving 2900 patients with non-valvular AF, VKA therapy with a target INR between 2.0 and 3.0 reduced the risk of stroke by 64% and mortality by 26%, compared with placebo or no therapy [68]. Although VKA therapy doubles the risk of ICH, the absolute risk increases by only 0.2%/year.
Despite this, VKA therapy has long been grossly underutilized in Asia-Pacific countries, with a utilization rate typically around 15–20% [69], [70], [71]. In addition to various limitations related to the narrow therapeutic window and the wide assortment of drug-food interactions, the higher baseline risk of ICH [72], [73], [74] and poorer TTR [75], [76] observed in Asian populations both undermine the benefits of VKA therapy.
Recently, the NOACs, including dabigatran, rivaroxaban, apixaban, and edoxaban have been shown in large RCTs to be at least as effective as VKA in stroke prevention, but with a consistently lower risk of ICH [19], [20], [21], [22]. NOACs are regarded as the preferred agents for stroke prevention in non-valvular AF [32], [33], [39], and have been used extensively over the past few years. However, VKAs would remain a viable option in certain clinical scenarios.
5.1. Role of VKAs in chronic kidney disease
AF and CKD commonly co-exist. The prevalence of AF increases with reduced glomerular filtration rate (GFR) [12], [77], [78], [79], [80]. Patients with CKD can be categorized according to GFR (Table 4). While long-term OAC can effectively reduce ischemic stroke risk in general AF patients, whether this can be extended to severe CKD patients remains inconclusive.
Table 4.
Staging of chronic kidney disease.
| CKD stage | GFR level (mL/min/1.73 m2) |
|---|---|
| Stage 1 | ≥90 |
| Stage 2 | 60–89 |
| Stage 3 | 30–59 |
| Stage 4 | 15–29 |
| Stage 5 | <15 |
CKD: chronic kidney disease; GFR: glomerular filtration rate.
Among patients with AF and a GFR of 30 mL/min or above, i.e., mild to moderate renal impairment or Stage 1 to 3 CKD, both VKA [81] and all 4 of the NOACs have been shown to be as effective in stroke reduction as in the general AF population [32], [33], [58]. AF patients with GFR < 25–30 mL/min were excluded from all pivotal NOAC trials.
Previous observational studies of AF patients with end-stage CKD on hemodialysis therapy have reported conflicting results on the net clinical benefits [82] and harms [83], [84], [85], [86].
For example, the Dialysis Outcomes and Practice Patterns Study (DOPPS), an international, observational study of hemodialysis practices and outcomes, demonstrated that hemodialysis patients with AF receiving VKA therapy had higher stroke risks compared to non-VKA users [87], [88]. In a North American study consisting of 1671 hemodialysis patients with AF, VKA therapy was associated with a nearly 2-fold increase in stroke [83]. Furthermore, the risk of hemorrhagic stroke among hemodialysis patients with AF on VKA also increases substantially [84], [89].
As a result, clinical guidelines from Kidney Disease: Improving Global Outcomes (KDIGO) no longer recommend warfarin therapy for stroke prevention in AF among dialysis patients [90]. It is plausible that frequent heparinization during hemodialysis, reduced levels of protein C, protein S and antithrombin III [91], [92], [93], [94], [95], as well as the fluctuations in blood pressure in hemodialysis patients with AF might diminish the overall benefit of warfarin due to a higher thrombotic and bleeding risk. Nonetheless, good-quality anticoagulation control with high TTR may mitigate risks [96].
In contrast to developed countries, peritoneal dialysis instead of hemodialysis is the primary mode of renal replacement therapy in many Asian countries or regions. Despite the paucity of clinical data, VKA therapy appears to have a net clinical benefit in terms of ischemic stroke, ICH, and mortality among AF patients on peritoneal dialysis [97], [98]. Until further RCTs to evaluate the net clinical benefit of OAC in dialysis patients with AF become available, the choice of long-term OAC should be highly individualized.
5.2. Role of VKAs in valvular heart disease
The efficacy of VKA therapy in stroke prevention in patients with underlying valvular heart diseases, particularly chronic rheumatic heart disease and prosthetic heart valves, has long been established. Although in the pivotal studies of NOACs, patients with moderate or severe mitral stenosis or mechanical heart-valve prosthesis were excluded, patients with other mitral or aortic valvular disease were allowed to participate [99], [100], [101], [102]. The safety and efficacy of NOACs do not appear to be different with respect to the valvular status of individual patients, including those with bioprosthetic valves [103]. In a recent meta-analysis of 4 phase III AF trials comprising 13,585 patients with and 58,098 without valvular heart diseases, high-dose NOACs provided similar efficacy and safety [104].
To date, there has been only one randomized controlled study comparing warfarin and NOAC in patients with mechanical heart-valve prosthesis, which was prematurely terminated because of excessive strokes and bleeding with dabigatran [105]. Mechanistically, mechanical heart-valve prosthesis induces sufficient thrombin generation via the intrinsic pathway, overwhelming the clinically relevant concentration of dabigatran [106].
Taken collectively, VKAs remain the only drugs for patients with moderate or severe mitral stenosis and patients with mechanical heart-valve prosthesis.
5.3. Identifying patients likely to do well on VKA with good anticoagulation control: the SAMe-TT2R2 score
Anticoagulation control with warfarin is influenced by many demographic and clinical factors. The more common factors have been used to formulate the SAMe-TT2R2 (Sex [female], Age [less than 60], Medical history [more than two comorbidities], Treatment [interacting medications, e.g., amiodarone], Tobacco use [doubled], Race [doubled]) score, which may help in predicting whether a patient is likely to have a good anticoagulation control if VKA is used [107], [108]. A SAMe-TT2R2 score 0–2 predicts a good response to VKA (i.e., high TTR>65%), while a SAMe-TT2R2 score>2 suggests that the patient is less likely to achieve a good TTR on VKA, thus flagging up the patient for additional education/counseling or more regular INR checks and clinic reviews, or for use of a NOAC [107], [108].
The SAMe-TT2R2 score has been validated in two Asian populations where a score of 0–2 predicted a TTR≥70% (Hong Kong cohort) or a TTR ≥60% (Singapore cohort), and a score of≥ 3 predicted a TTR<70% (Hong Kong cohort) or a TTR<60% (Singapore cohort) [109], [110]. Therefore, the SAMe-TT2R2 score can be used to help predict the performance of VKA in Asians by identifying those patients likely to achieve good or poor TTR, and to assist in decision-making in the selection of OAC (i.e., VKA or NOAC).
Recommendations
-
•
VKAs have a role in stroke prevention in patients with stage 4, and possibly stage 5, CKD.
-
•
VKA remains the only drug for stroke prevention in patients with moderate or severe mitral stenosis and those with mechanical-valve prosthesis.
-
•
The SAMe-TT2R2 score may help predict whether a patient is likely to have good anticoagulation control if a VKA is used. A SAMe-TT2R2 score 0–2 predicts a good response to VKA, while a SAMe-TT2R2 score >2 flags up the patient for additional education/counseling or more regular INR checks and clinic reviews, or use of a NOAC.
6. Role of non-vitamin K antagonist oral anticoagulants (NOACs)
NOACs have revolutionized the approach to stroke prevention in AF [111], [112]. There are now four NOACs available: one oral direct thrombin inhibitor (dabigatran); and three oral factor Xa inhibitors (rivaroxaban, apixaban, and edoxaban). The pharmacokinetic data of the four NOACs are shown in Table 5 [32], [113], [114], [115].
Table 5.
Pharmacokinetic characteristics of NOACs.
| Dabigatran | Rivaroxaban | Apixaban | Edoxaban | |
|---|---|---|---|---|
| Absorption with food | No effect | +39% | No effect | +(6–22%) |
| Intake with food recommended | No | Mandatory | No | No |
| Renal clearance | 80% | 35% | 27% | 50% |
| Bioavailability | 6% | 80% | 60% | 62% |
| CYP metabolism | None | 66% | 15% | <4% |
| Transporter | P-glycoprotein | P-glycoprotein | P-glycoprotein | P-glycoprotein |
| Hours to Cmax | 3 | 2–4 | 3 | 1–2 |
| Half-life, hours | 12–17 | 5–13 | 9–14 | 10–14 |
Compared with warfarin, all NOACs have more predictable pharmacokinetics and fewer drug-drug interactions, allowing fixed dosing without the need for regular monitoring of anticoagulation status [116].
6.1. Major randomized clinical trials
The efficacy and safety of the four NOACs have been tested in four major RCTs: the RE-LY trial, the ROCKET AF trial, the ARISTOTLE trial, and the ENGAGE AF trial [19], [20], [21], [22]. The primary efficacy endpoints were stroke plus systemic embolization events (SEEs). In general, NOACs showed non-inferiority in primary efficacy endpoints when compared with dose-adjusted warfarin with target INR of 2.0–3.0, except dabigatran 150 mg and apixaban 5 mg, which showed superiority to warfarin. Most NOACs showed a decreased risk of major bleeding compared with warfarin, except dabigatran 150 mg and rivaroxaban 20 mg. A pre-specified meta-analysis comprising these 4 major RCTs of NOACs also demonstrated a favorable risk-benefit profile, with significant reductions in stroke, ICH, and mortality, and with similar major bleeding as for warfarin, but increased gastrointestinal bleeding [112].
One should be careful to integrate this information into patient care in Asia, as Asians are prone to bleeding with warfarin use. Therefore, a more detailed examination of the subsets of Asians from these RCTs is important.
6.2. Asian sub-analyses of major RCTs
Among 71,783 participants in the four major RCTs of NOACs [19], [20], [21], [22], 7650 patients were from Asia, mostly East Asian countries. The Asian sub-analyses of all these RCTs have been published [23], [24], [26], [117]. The efficacy endpoints (stroke/SEEs, ischemic stroke, hemorrhagic stroke, myocardial infarction, all-cause mortality, and cardiovascular mortality) and safety endpoints (major bleeding, ICH, gastrointestinal bleeding, and bleeding due to any cause) of NOACs versus warfarin in the 4 RCTs are summarized in Table 6 [58]. These data suggest great advantages of using NOACs for stroke prevention in AF patients in Asia.
Table 6.
| Stroke/SEE | Ischemic stroke | Hemorrhagic stroke | Myocardial infarction | All-cause death | CV death | Major bleeding | Intracranial hemorrhage | GI bleeding | Bleeding due to any cause | |
|---|---|---|---|---|---|---|---|---|---|---|
| Dabigatrana 150 mg | V | V | V | NR | V | V | V | |||
| Dabigatrana 110 mg | V | NR | V | V | V | |||||
| Rivaroxabanb | V | NR | ||||||||
| Apixabanc | V | NR | V | V | NR | V | ||||
| Edoxaband 60 mg | V | V | V | V | V | V | ||||
| Edoxaband 30 mg | V | V | V | V |
CV: cardiovascular; GI: gastrointestinal; NOACs: non-vitamin K antagonist oral anticoagulants; NR: not reported; SEE: systemic embolization events; V: P value less than 0.05 when compared with warfarin.
Modified from Lip et al. [58] with permission
China, Japan, South Korea, Taiwan, Hong Kong, Philippines, Singapore, Malaysia, Thailand, India.
China, South Korea, Taiwan, Hong Kong.
China, Japan, South Korea, Taiwan, Hong Kong, Philippines, Singapore, Malaysia.
China, Japan, South Korea, Taiwan.
6.3. Meta-analysis of NOACs in Asia
In a recent meta-analysis, the differences in efficacy and safety outcomes of NOACs in Asian patients were compared with those in non-Asian patients [118]. The 5 RCTs included the studies RE-LY, ROCKET AF, J-ROCKET AF, ARISTOTLE, and ENGAGE AF, comprising 8928 Asian patients (5250 with NOACs and 3678 with VKAs) and 64,033 non-Asian patients (37,800 with NOACs and 26,233 with VKAs) [19], [20], [21], [22], [23], [24], [25], [26], [119], [120]. There were 2 separate analyses: a meta-analysis for standard-dose NOACs (dabigatran 150 mg, edoxaban 60 mg, rivaroxaban 20 mg, and apixaban 5 mg); and a meta-analysis for low-dose NOACs (dabigatran 110 mg, edoxaban 30 mg, and rivaroxaban 15 mg) (Table 7) [118].
Table 7.
Odds ratios of NOACs vs warfarin in meta-analysis.
| Standard dose NOACs |
Low dose NOACs |
|||||
|---|---|---|---|---|---|---|
| Asian | Non-Asian | Interaction P value | Asian | Non-Asian | Interaction P value | |
| Stroke/SEE | 0.65 | 0.85 | 0.045 | 0.93 | 1.07 | 0.353 |
| (0.52–0.83) | (0.77–0.93) | (0.71–1.21) | (0.93–1.24) | |||
| Ischemic stroke | 0.89 | 0.95 | 0.673 | 1.06 | 1.29 | 0.504 |
| (0.67–1.17) | (0.84–1.06) | (0.68–1.65) | (0.88–1.90) | |||
| Myocardial infarction | 0.97 | 0.98 | 0.977 | 0.92 | 1.28 | 0.352 |
| (0.59–1.58) | (0.82–1.12) | (0.48–1.79) | (1.06–1.55) | |||
| All-cause mortality | 0.80 | 0.91 | 0.219 | 0.89 | 0.88) | 0.934 |
| (0.65–0.98) | (0.86–0.97) | (0.70–1.15) | (0.81–0.96 | |||
| Major bleeding | 0.57 | 0.89 | 0.004 | 0.52 | 0.64 | 0.579 |
| (0.44–0.74) | (0.76–1.04) | (0.32–0.86) | (0.38–1.09) | |||
| Intra-cranial hemorrhage | 0.33 | 0.52 | 0.059 | 0.28 | 0.32 | 0.661 |
| (0.22–0.50) | (0.42–0.64) | (0.16–0.49) | (0.24–0.44) | |||
| Hemorrhagic stroke | 0.32 | 0.56 | 0.046 | 0.35 | 0.34 | 0.944 |
| (0.19–0.52) | (0.44–0.70) | (0.18–0.68) | (0.23–0.50) | |||
| GI bleeding | 0.79 | 1.44 | 0.041 | 0.67 | 0.87 | 0.460 |
| (0.48–1.32) | (1.12–1.85) | (0.39–1.15) | (0.56–1.35) | |||
GI: gastrointestinal; NOACs: non-vitamin K antagonist oral anticoagulants; SEE: systemic embolization event
Standard-dose NOACs significantly reduced stroke/SEE in both Asian and non-Asian patients, and the effect size of this reduction was greater in Asian patients than in non-Asians (P interaction=0.045) (Table 7). All-cause mortality was also reduced in Asian and non-Asian patients, but heterogeneity was not significant (P interaction=0.219) [118]. In Asians, standard-dose NOACs significantly reduced major bleeding, ICH, and hemorrhagic stroke, compared with warfarin. There was no increase in GI bleeding. In non-Asians, standard-dose NOACs significantly reduced ICH and hemorrhagic stroke, with an increase in GI bleeding, compared with warfarin. Heterogeneity was evident in major bleeding, hemorrhagic stroke, and GI bleeding among Asians versus non-Asians [118].
The efficacy of low-dose NOACs in stroke/SEE, ischemic stroke, myocardial infarction and all-cause mortality, was generally similar to that of warfarin in both Asians and non-Asians, except for an increase in myocardial infarction and a decrease in all-cause mortality among NOAC users in non-Asians (Table 7). No significant heterogeneity could be found between Asians and non-Asians. The main benefits of low-dose NOACs were in the safety aspects, because major bleeding, ICH, and hemorrhagic stroke significantly reduced in Asians while ICH and hemorrhagic stroke significantly reduced in non-Asians, compared with warfarin. Both Asians and non-Asians showed a numerically lower risk of GI bleeding, compared with warfarin. No heterogeneity was shown between Asians and non-Asians [118].
In another meta-analysis of 3155 Asian patients with NOACs in the RE-LY and ENGAGE AF trials, efficacy and safety with standard-dose versus low-dose NOACs were compared [121]. Risks of stroke/SEE and ischemic stroke significantly reduced with standard-dose versus low-dose NOACs (RR 0.62, 95% CI 0.45–0.85; and RR 0.55, 95% CI 0.38–0.79, respectively). Rates of major, intracranial, and life-threatening bleeding with the two dosing regimens were broadly similar (RR 1.31, 95% CI 0.74–2.33; RR 1.54, 95% CI 0.72–3.30; and RR 1.49, 95% CI 0.87–2.55, respectively). Therefore, standard-dose NOACs represent a more appealing therapeutic option than low-dose NOACs in Asians, with a significant reduction in ischemic stroke without an excess of major bleeding [121]. Nevertheless, label- or guideline-adherent NOAC dosing offers a balance of efficacy and safety outcomes, compared to warfarin [122].
Recommendations
-
•For Asian patients with non-valvular AF, standard-dose NOACs (dabigatran 150 mg bid, rivaroxaban 20 mg od, apixaban 5 mg bid, or edoxaban 60 mg od) are the default doses of choice for stroke prevention unless label guidance recommends low-dose regimens as follows:
-
■For dabigatran, the 110mg bid dose is recommended in the elderly (age > 75), in patients with a high bleeding risk (HAS-BLED≥3) or in patients receiving interacting drugs (e.g. verapamil)
-
■For rivaroxaban, the 15 mg od dose is recommended where the Cockroft-Gault creatinine clearance (CrCl) is 30–49 mL/min.
-
■For apixaban, 2.5 mg bid is used in patients with two or more of the following criteria: age ≥ 80 years, body weight ≤ 60 kg, or serum creatinine ≥ 1.5 mg/dL.
-
■For edoxaban, the 30-mg od dose is recommended in patients with any one of the following criteria: eGFR of 30 to 50 mL/min, a body weight ≤ 60 kg, or the concomitant use of verapamil or quinidine (potent P-glycoprotein [P-gp] inhibitors).
-
■
-
•Low-dose NOACs (rivaroxaban 15 mg od, apixaban 2.5 mg bid, edoxaban 30 mg od) should be used with caution in severe CKD (CrCl 15–30 mL/min) according to drug labels.
-
■Dabigatran should not be used in patients with CrCl < 30 mL/min.
-
■
7. Role of left atrial appendage closure and excision
7.1. Rationale and techniques for LAA closure
The left atrial appendage (LAA) is thought to be the most important site of thrombus formation leading to ischemic stroke in AF patients. Occlusion or excision of the LAA has been proposed as a way to reduce thromboembolic events [123]. However, not all strokes in AF patients are cardio-embolic or due to AF, and the LAA is probably not the only left atrial region where thrombi can potentially originate. Even after removal or closure of the LAA, antithrombotic therapy may still be needed [124].
Surgical excision or ligation of the LAA is often performed as a concomitant procedure during open heart surgery. Less invasive techniques using epicardial or trans-septal approaches have been developed to occlude the LAA [125], [126], [127]. These techniques are aimed at providing an alternative for AF patients at high risk for stroke but with contraindications for chronic OAC use.
Devices for trans-septal LAA occlusion include the WATCHMAN device (Boston Scientific, Natick, MA, USA) and the Amplatzer Cardiac Plug (St. Jude Medical, Minneapolis, MN, USA). The WATCHMAN device is deployed percutaneously via a transseptal puncture and has a permeable, polyester fabric membrane that covers a self-expanding nitinol cage with barbs to anchor the device in the LAA [128]. The Amplatzer Cardiac Plug consists of a proximal disc and a distal lobe with hooks to anchor the device in the LAA. It does not require anticoagulation [127].
An alternative strategy is to tie off the LAA using an epicardial suture device, referred to as the LARIAT device (SentreHEART, Redwood City, CA, USA) [129]. More recently, the US FDA alerted against its off-label use for LAA closure, as its safety and effectiveness for this indication has not been established. (http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm454501.htm; date last accessed, June 26, 2016).
7.2. Percutaneous closure of LAA
The most data exist for the WATCHMAN device and early studies suggest non-inferiority to warfarin for the composite endpoint of stroke, SEE, and cardiovascular death; however, early adverse events occurred more frequently in the intervention arm (5.5–7.4%), including pericardial bleeding [126], [130]. A prospective randomized open study, the PROTECT AF study, found that, compared to warfarin therapy, the WATCHMAN device reduced the combined outcome of stroke, SEE, and cardiovascular death, and was also superior in terms of cardiovascular and all-cause mortality [131]. In contrast, the PREVAIL study, which included patients with higher risk of stroke based on CHADS2 scores, failed to show non-inferiority to warfarin for its primary endpoint (composite of stroke, SEE and cardiovascular or unexplained death) but did so for the second co-primary endpoint of stroke or SEE>7 days after randomization [132]. Device-related complications reduced with increasing operator experience.
For patients with a contraindication for warfarin, the data are more limited. In the ASAP registry study, patients with a WATCHMAN device implanted had a lower rate of stroke and SEE, and it was lower than would be expected for the level of risk as predicted by their CHADS2 scores (1.7% versus 7.3%) [128]. This was at the expense of an 8.7% rate of serious procedural or device-related complications, and in comparison with historical CHADS2 score event rates.
Less data are available for the Amplatzer devices, with earlier studies mostly being retrospective, nonrandomized case series that also included devices that were not dedicated to LAA occlusion [127], [133], [134]. In the larger series, procedure-related complications occurred in around 5% of patients, but the lack of a control group precludes any comparisons with pharmacological treatment.
While the use of percutaneous approaches for LAA closure is feasible, there are still sparse data for most. The exception is the WATCHMAN device, for which there is some trial evidence of its non-inferiority (and even superiority in one trial) to warfarin. This does come at the cost of peri-procedural and device-related complications and significant financial outlay. Of note, none of the trials included significant numbers of Asian patients, and thus its utility in our patient population is uncertain. Nonetheless, it may be reasonable to consider the use of this device in patients at high risk of stroke or SEE from AF who have significant contraindications for any OAC therapy.
7.3. Surgical occlusion or excision of LAA
There is no conclusive evidence that surgical LAA excision or occlusion reduces stroke risk in AF patients [124]. Retrospective or observational studies in different patient populations have shown inconsistent results for surgical LAA excision or occlusion [135]. This could be partly due to low rates of successful closure using current techniques, though this appears to improve with experience [136], [137]. A large randomized trial of left atrial ligation in patients undergoing cardiac surgery (LAAOS III) is underway, and may provide some evidence for surgical closure of the LAA [138].
Randomized studies to date have been small and did not show any benefit of LAA occlusion at the time of coronary-artery bypass surgery in patients at risk of stroke. In the LAAOS study, 52 out of 77 patients were randomized to receive LAA occlusion; 2 of them suffered a perioperative thromboembolic event [137]. After 13±7 months, no additional patients had stroke. Similarly, the LAAOS II study, which was conducted to assess the feasibility of a larger trial (i.e., LAAOS III) also found no significant reduction in embolic events in the LAA-occlusion arm but concluded that concomitant LAA occlusion was safe and feasible [139]. On follow-up, surgical excision resulted in a higher rate of LAA closure (73%) than either suture occlusion (23%) or stapler occlusion (0%) [136].
Surgical techniques and devices for occluding the LAA are still being developed. The most widely used device, the AtriClip (Atricure, West Chester, OH, USA), consists of 2 parallel, straight, rigid titanium tubes and 2 nitinol springs with a knit-braided polyester fabric [140]. Early non-randomized studies of its use during open heart surgery have demonstrated high success rates and good short-term durability (98.4%) based on imaging studies [141].
Surgical LAA closure may be conceptually reasonable, but there is a paucity of evidence for its efficacy and safety. In the absence of stronger clinical data, especially in the form of RCTs, recommendations can only be based on expert consensus. Importantly, there is no evidence for its use in place of OAC for patients who could otherwise be treated with the latter. It does appear safe in patients who are undergoing open heart surgery for other indications and may be considered in these patients.
Recommendations
-
•
Interventional percutaneous LAA closure with the WATCHMAN device may be considered in patients with non-valvular AF who have high risk of stroke, but major contraindications to OAC therapy.
-
•
Surgical excision of the LAA may be considered in patients undergoing concomitant cardiac surgery.
8. Practical use of NOACs
8.1. Drug-drug and drug-food interaction
The use of VKAs is complicated by their unpredictable and variable performance, which is due to several factors. These include numerous drug-food and drug-drug interactions [142], [143], [144]. Many herbs used as foods, and also medicinal supplements, can interact with VKAs [145]. There is evidence that bleeding rates are higher on warfarin and NOACs seem preferable for stroke prevention in Asians [7], [146]. One of the main advantages of NOACs has been a predictable onset and offset of action, and fewer drug-drug interactions as compared to warfarin [143]. As NOAC usage is increasing, various drug-drug interactions are coming to light (Table 8) [144], [147].
Table 8.
Drug-drug interactions of NOACs.
| Mechanism | Dabigatran | Rivaroxaban | Apixaban | Edoxaban | |
|---|---|---|---|---|---|
| Rifampicin | P-gp, CYP3A4 | Contraindication | Contraindication | Contraindication | Use with caution |
| HIV protease inhibitor | P-gp, CYP3A4 | Contraindication | Contraindication | Contraindication | Contraindication |
| Itraconazole | P-gp, CYP3A4 | Contraindication | Contraindication | Contraindication | 50% dose |
| Ketoconazole | |||||
| Carbamazepine | P-gp, CYP3A4 | Contraindication | Contraindication | Contraindication | Contraindication |
| Phenobarbital | |||||
| Phenytoin | |||||
| Amiodarone | P-gp | 50% dose | 50% dose | 50% dose | 50% dose |
| If ≥ 75 years | If ≥ 75 years | If ≥ 75 years | If ≥ 75 years | ||
| Verapamil | P-gp | 50% dose | No data | No data | No data |
| Dronedarone | P-gp, CYP3A4 | Contraindication | No data | No data | 50% dose |
CYP: cytochrome P450; HIV; human immunodeficiency virus; P-gp: P-glycoprotein.
The action of NOACs can be influenced at various stages in their absorption, metabolism, and elimination. These drugs have variable renal excretion, hepatic metabolism, and re-secretion into the gut via a P-gp transporter. The cytochrome P450 (CYP 450) enzyme system is responsible for hepatic clearance of NOACs. All these elimination pathways could be points of interaction with food or drugs.
Dabigatran is predominantly (80%) eliminated by renal excretion, and not affected by CYP 450 enzyme modulators. Clinicians prescribing anticoagulants should have accurate knowledge of various modes of elimination of each drug and their possible modification by various drugs. When a patient on NOACs develops a thrombotic or bleeding complication, co-medications should be carefully reviewed for a possibility of drug-drug interactions.
There is a significant re-secretion of NOACs into the intestine via the transporter P-gp, which may be involved to some extent in renal excretion [148]. Inhibitors of P-gp may therefore result in higher plasma levels and consequently, increased anticoagulant activity [149]. Common P-gp inhibitors used in AF patients include verapamil, dronedarone, amiodarone, and quinidine.
Rivaroxaban and apixaban are mainly metabolized by CYP3A4 [150]. Any concomitant medication that modulates CYP3A4 may therefore affect plasma concentrations and effects, and should be evaluated [151].
8.1.1. Interaction with rate- and rhythm-control drugs
Patients with AF requiring anticoagulation are frequently co-administered various rate- or rhythm-controlling drugs. Several of these agents can interact with anticoagulants (Table 8). In particular, amiodarone and verapamil have been shown to increase the bioavailability of dabigatran [152]. In one study, a single 120-mg dose of verapamil 1 h before dabigatran increased its plasma concentration (AUC) and peak serum concentration (Cmax) by 143% and 179% respectively [153]. The effect was minimized if verapamil was given 2 h before dabigatran [153].
8.1.2. Interaction with antifungals and antibiotics
Antifungal agents like ketoconazole, itraconazole, and posaconazole are very strong inhibitors of P-gp, and are contraindicated for use with NOACs (Table 8) [150]. Among antifungals, fluconazole was found to have least effect on rivaroxaban and can be used with caution [150]. One study found a 2-fold increase in apixaban exposure with co-administration of ketoconazole [154].
Clarithromycin has been shown to increase the bioavailability of dabigatran from 6.5% to 10.1%, while the Cmax and AUC increased by 60.2% and 49.1%, respectively [155]. Being a strong inhibitor of both CYP3A4 and P-gp, clarithromycin can also increase plasma levels of rivaroxaban and lead to bleeding [156]. Erythromycin may significantly increase the activity of rivaroxaban by about 34% [150]. Ritonavir, an antiretroviral agent, increased the activity of rivaroxaban by 158% [150]. Rifampin has been shown to reduce exposure to edoxaban while increasing exposure to its active metabolites M4 and M6 without significantly changing aPTT [157].
8.1.3. Interaction with miscellaneous drugs
Several antiepileptic drugs including carbamazepine, phenytoin, valproic acid, levetiracetam, and topiramate are inducers of P-gp, and may affect the anticoagulant activity of NOACs. Naproxen has been shown to increase the activity of apixaban [158], and can increase bleeding time in patients on rivaroxaban[159] and edoxaban [160]. Co-administration of pantoprazole caused moderate reduction in dabigatran absorption [161]. Antacids have no effect on apixaban, rivaroxaban, and edoxaban, while they may reduce bioavailability of dabigatran by 12–28% without affecting its efficacy [152]. (http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000829/WC500041059.pdf. Accessed: 30-Sep-2016).
8.1.4. Interaction with foods
One of the main factors for unpopularity of VKAs has been their strong predilection for interaction with various foods rich in vitamin K. Unlike VKAs, NOACs are not significantly affected by food intake, with the exception of rivaroxaban, which has better absorption and near 100% bioavailability when taken with food. Therefore, it is strongly recommended that rivaroxaban is taken with food at dinner time. Apixaban and edoxaban are not affected by food intake [115], [162].
Recommendations
-
•
NOACs should not be combined with potent P-gp inducers, such as rifampicin, carbamazepine, phenobarbital, and phenytoin.
-
•
NOACs should not be combined with potent P-gp inhibitors, such as HIV protease inhibitors, itraconazole, and ketoconazole. The only exception is edoxaban: a 50% reduction of edoxaban dose can be combined with itraconazole and ketoconazole.
-
•
When combined with amiodarone, a lower dose NOAC is considered in the elderly.
-
•
A 50% reduction in dabigatran dose is needed when combined with verapamil; while data for other NOACs are scarce.
-
•
Dabigatran should not be combined with dronedarone.
-
•
A 50% reduction in the edoxaban dose is needed when combined with dronedarone, while data for rivaroxaban and apixaban are lacking.
8.2. How to switch?
Switching between oral anticoagulants is often required in the clinical setting. In both the ROCKET AF and ARISTOTLE trials, the risk of stroke increased after the end of the trials in patients in whom the study NOAC was switched to open warfarin [163], [164]. This was explained by a delay in achieving a therapeutic INR after switching. The timing of the interruption and initiation of the drugs must be chosen to minimize any gap in the therapeutic anticoagulation status.
When a VKA is switched to a NOAC, the NOAC should be started once the INR is approximately 2.0 or less. When a NOAC is switched to a VKA, VKA should be combined with parenteral heparin or combined with NOAC until the INR approaches 2.0. Since factor Xa inhibitors affect the INR, the INR should be measured 24 h after the last NOAC intake.
Recently, the Dresden NOAC registry from Germany reported the risk associated with switching from a VKA to a NOAC [165]. At 30 days of follow-up from switching, major cardiovascular events and major bleeding events occurred in 0.8% and 0.3%, respectively. In general, switching between oral anticoagulants can be performed safely with careful monitoring of the INR.
Recommendations
-
•
When a VKA is switched to a NOAC, the NOAC should be started once the INR is approximately 2.0 or less.
-
•
When a NOAC is switched to a VKA, VKA should be combined with parenteral heparin or combined with a NOAC until the INR is approximately 2.0.
8.3. Patients with chronic kidney disease
The prevalence of CKD, similar to that of AF, is increasing worldwide, including in the Asia-Pacific (AP) region. The staging of CKD is shown in Table 4. All individuals with a GFR < 60 mL/min for 3 months are classified as having CKD, irrespective of the presence or absence of kidney damage. End-stage renal disease (ESRD) is defined as a CrCl of less than 15 mL/minute/1.73 m2.
CKD can affect up to 10% of the adult population, especially elderly people, and carries a high risk for AF. Up to 30% of patients with AF have some renal dysfunction; hence, it is important to screen for renal dysfunction in AF patients.
Higher incidence of AF has been reported even in patients with early renal dysfunction [78], [166]. The prevalence of AF in patients with impaired kidney function or on dialysis is considerably higher than in the general population, with estimates that about one in 5–6 patients on hemodialysis has AF [80], [87], [167]. In an Asian study, the incidence rates of AF were 12.1, 7.3, and 5.0 per 1000 person-years for ESRD, CKD, and control patients, respectively [168]. Among patients with ESRD, age, hypertension, heart failure, coronary artery disease, peripheral arterial occlusive disease, and chronic obstructive pulmonary disease were significant risk factors for new-onset AF.
Further, AF and CKD have an unhealthy relationship: AF predisposes people to CKD and CKD increases the risk of AF [77], [169]. CKD in adult patients with incident AF is independently associated with increased risk of developing ESRD [170].
The estimated CrCl (eCrCl) and the estimated GFR (eGFR) measure slightly different things. In the context of NOAC treatment, CrCl is best assessed by the Cockcroft-Gault (CG) method [171], as this method was used in the NOAC trials.
8.3.1. Stroke and bleeding in patients with CKD
CKD increases stroke risk in patients with AF, as well as the risk of major bleeds [172]. Renal impairment (CrCl<60 mL/min) doubles the risk of stroke [173]. It has even been proposed to add renal function to the CHADS2 scoring system as a manner to improve prediction of stroke [11], [174]. In patients on NOAC, impaired renal function was shown to be an independent risk factor for stroke in a sub-study of the ROCKET AF trial [11]. However, in other “real-world” studies, renal impairment was not an independent predictor of ischemic stroke or thromboembolism in AF and did not significantly improve the predictive ability of the CHADS2 or CHA2DS2-VASc scores [173], [175].
In a Japanese study, as renal function declined below an eGFR of 60 mL/min, stroke risk increased regardless of whether AF was also present. The hazard ratios for stroke were 1.9 and 3.1 in patients with eGFR of 40–70 mL/min and < 40 mL/min, respectively, as compared to those with an eGFR>70 mL/min [176].
The risk of ischemic stroke has also been reported to be higher in patients with CKD and ESRD [87], [172], [177]. Hemodialysis patients typically have multiple comorbidities, and AF would be expected to increase the risk of ischemic stroke at least as much as in patients without renal failure. New-onset AF in hemodialysis patients adversely affected the outcomes in a retrospective cohort study obtained from the Taiwanese NHIRD [178]. Compared to the control group, the patients with new-onset AF had higher risks of ischemic stroke (HR, 1.27), all-cause mortality (HR, 1.59), in-hospital cardiovascular death (HR, 1.83), myocardial infarction (HR, 1.33), and heart failure (HR, 1.9). After adjustment for in-hospital deaths, AF was associated with a higher risk of heart failure (HR, 1.56) and in-hospital cardiovascular death (HR, 1.65), but not stroke or myocardial infarction [178].
Renal dysfunction is a risk marker not only for stroke but also for death, myocardial infarction, and bleeding. Renal impairment (CrCl < 60 mL/min) especially ESRD, increases the risk of major bleeding by almost 60% in anticoagulated patients with AF [173]. In addition, warfarin may also promote vascular calcification in the CKD patient.
8.3.2. VKA in CKD patients
The extent to which CKD increases the risk of thromboembolism in patients with nonvalvular AF and the benefits of anticoagulation in this group remain unclear. Only warfarin was associated with a decreased risk of stroke or SEE among patients with CKD, whereas both warfarin and aspirin were associated with an increased risk of bleeding [172]. High-risk patients with AF (CHA2DS2-VASc ≥ 2) and renal failure still derive a net benefit from anticoagulation with warfarin, especially if quality of anticoagulation is good [96], [179]. In a meta-analysis, the presence of CKD in patients with AF was associated with a 50% increase in risk of thromboembolism, which can be effectively decreased with appropriate antithrombotic therapy [180].
8.3.2.1. Patients with non-ESRD
There is evidence from certain AF studies suggesting the use of OAC in patients with mild to moderate CKD provides similar, or potentially even greater, benefit compared to its use in the general population. In a post hoc analysis of patients with CKD stage III in the Stroke Prevention in Atrial Fibrillation III trial, warfarin use markedly decreased ischemic stroke and/or systemic venous thromboembolism (VTE) by 76% (95% CI, 42–90; P < 0.001) compared to aspirin plus low, fixed doses of warfarin [81].
In patients with both CKD and AF one year after discharge from an acute myocardial infarction in the SWEDEHEART registry, Carrero et al. showed that warfarin was associated with lower risk of the composite endpoint including death, repeat myocardial infarction, or ischemic stroke [181]. Bleeding risk did not increase. The lower event rate for the primary outcome was observed across all strata of eGFR and primarily driven by mortality events, while the risk of bleeding was not significantly higher in patients treated with warfarin in any CKD stratum [181].
8.3.2.2. Patients with ESRD on dialysis
AF patients with severe renal impairment or on dialysis have been excluded from large RCTs evaluating antithrombotic therapy in AF. Therefore, the optimal approach to anticoagulation in patients with non-valvular AF who have severe renal disease or are on dialysis is controversial. Although warfarin is indicated to prevent ischemic strokes in most patients with AF, evidence supporting its use in hemodialysis patients is limited.
The systematic use of any oral anticoagulant or acetylsalicylic acid has not been demonstrated to be beneficial for AF patients who are hemodialysis-dependent. Several observational studies have raised concerns about the use of warfarin in dialysis patients with non-valvular AF [84], [87], [182]. Warfarin use in patients with AF and CKD was not associated with significant reductions in stroke risk or mortality in patients with AF on chronic hemodialysis, but might actually have contributed to greater bleeding risk. In a population-based, retrospective, cohort study of 1626 AF patients on dialysis from Montreal, Canada, no reduction in stroke risk was found with warfarin, even after adjusting for multiple factors (HR, 1.14; 95% CI, 0.78–1.67), but a significantly higher risk of bleeding on warfarin (HR, 1.44; 95% CI, 1.13–1.85) was observed [89]. Similar findings were reported by Winkelmayer et al. [84] In the Dialysis Outcomes and Practice Patterns Study (DOPPS), Wizemann et al. demonstrated that warfarin use in AF patients > 75 years of age (n=1107) was associated with a 2.2-fold higher risk for the composite stroke/death outcome, but in the two groups under age of 75, no difference with warfarin use was observed [87].
There is a possibility that the benefit of warfarin in these patients may be outweighed by its risks, and therefore, RCTs are needed [183], [184]. The current Canadian AF guidelines do not advocate any oral anticoagulation in dialyzed patients, because of an unproven benefit for stroke prevention and a high bleeding risk with warfarin [185], [186]. In Europe, many centers routinely anticoagulate these patients with warfarin, but aiming for a TTR > 65–70%. Indeed, Olesen et al. demonstrated a favorable analysis for the use of warfarin in 901 patients with ESRD in a large observational study from the National Danish Registry from 1997–2008 evaluating patients with AF at hospital discharge; this showed a 56% reduction in risk for the composite stroke/death outcome compared to no antithrombotic therapy (HR 0.44; 95% CI, 0.26–0.74) [172]. The use of warfarin in ESRD patients must thus be individualized, weighing the risks versus the benefits.
One meta-analysis on the use of warfarin for AF showed that it may have an unfavorable risk/benefit ratio in patients with ESRD but not in those with non-ESRD. Thirteen publications from 11 cohorts (6 retrospective and 5 prospective), including > 48,500 total patients with > 11,600 warfarin users, were included in the meta-analysis [187]. In patients with AF and non-ESRD, warfarin resulted in a lower risk of ischemic stroke/thromboembolism (HR, 0.70; 95% CI, 0.54–0.89; P = .004) and mortality (HR, 0.65; 95% CI, 0.59–0.72; P < .00001), but had no effect on major bleeding (HR, 1.15; 95% CI, 0.88–1.49; P = .31). In patients with AF and ESRD, warfarin had no effect on the risks of stroke (HR, 1.12; 95% CI, 0.69–1.82; P = .65) or mortality (HR, 0.96; 95% CI, 0.81–1.13; P = .60), but increased the risks of major bleeding (HR, 1.30; 95% CI, 1.08–1.56; P = .005) [187]. As mentioned, this would be highly dependent on the quality of anticoagulation control, and the net clinical benefit may still be positive where the TTR is >70%.
8.3.3. NOACs
NOACs have a more stable dose response than warfarin but are dependent on renal clearance. Used correctly, NOACs are at least as safe as well-controlled warfarin. Similar to warfarin, they can cause bleeding, especially if used in excessive doses, in patients at higher risk for bleeds, and in patients with reduced kidney function.
8.3.3.1. Renal clearance of NOACs
Clearance of NOACs from the body is dependent on renal function, which should be assessed regularly (Table 5). Dabigatran is an oral direct thrombin inhibitor that is 80% renally cleared, and thus has a potential to cause more bleeding in patients with reduced renal function. Of the oral factor Xa drugs, rivaroxaban has 35% renal clearance while apixaban has 27% renal clearance and edoxaban has 50% renal excretion. Apixaban has the lowest renal clearance and is potentially safer in patients with renal impairment. The renal clearance of warfarin is <1% and hence it may be safest pharmacokinetically for patients with severe CKD [186].
8.3.3.2. NOACs in patients with mild-to-moderate CKD
Subgroup analyses of RE-LY, ARISTOTLE, ROCKET AF, and ENGAGE AF have demonstrated that all four NOACs produced comparable results in the primary efficacy endpoints (stroke and SEE) and the primary safety endpoint (major bleeding) across different stages of renal function (Table 9) [188], [189], [190], [191]. The only exception was found in the ARISTOTLE trial [189]. Apixaban seems to produce less bleeding compared to warfarin in patients with an eGFR≤50 mL/min than in those with a higher eGFR (P for interaction 0.030).
Table 9.
Efficacy and safety of NOACs in patients with CKD.
| Primary efficacy endpoint (%/y)a | Primary safety endpoint (%/y)b | |||||||
|---|---|---|---|---|---|---|---|---|
| RE-LY | ||||||||
| eGFR (mL/min) | <50 | 50 to<80 | ≥80 | P (int) | <50 | 50 to <80 | ≥80 | P (int) |
| Dabi 150 | 1.53 | 1.25 | 0.71 | 5.50 | 3.35 | 2.04 | ||
| Dabi 110 | 2.32 | 1.69 | 0.88 | 5.45 | 2.84 | 1.48 | ||
| Warfarin | 2.70 | 1.83 | 1.05 | 5.49 | 3.70 | 2.43 | ||
| HR (95% CI) | ||||||||
| Dabi 150/W | 0.56 | 0.68 | 0.67 | 0.7522 | 1.01 | 0.91 | 0.84 | 0.6393 |
| (0.37–0.85) | (0.50–0.92) | (0.42–1.09) | (0.79–1.30) | (0.75–1.11) | (0.62–1.13) | |||
| Dabi 110/W | 0.85 | 0.93 | 0.84 | 0.9108 | 0.99 | 0.76 | 0.61 | 0.0607 |
| (0.59–1.24) | (0.70–1.23) | (0.54–1.32) | (0.77–1.28) | (0.62–0.94) | (0.44–0.84 | |||
| ROCKET AF | ||||||||
| CrCl (mL/min) | 30–49 | ≥50 | 30–49 | ≥50 | ||||
| Riva 15 | 2.32 | 17.82 | ||||||
| Riva 20 | 1.57 | 14.24 | ||||||
| Warfarin | 2.77 | 2.00 | 18.28 | 13.67 | ||||
| HR (95% CI) | ||||||||
| Riva/W | 0.84 (0.57–1.23) | 0.78 | 0.76 | 0.98 | 1.04 | 0.4496 | ||
| (0.63–0.98) | (0.84–1.14) | (0.96–1.13) | ||||||
| ARISTOTLE | ||||||||
| eGFR (mL/min) | ≤50 | >50–80 | >80 | ≤50 | >50–80 | >80 | ||
| Api 5 | 2.11 | 1.24 | 0.99 | 3.21 | 2.45 | 1.46 | ||
| Warfarin | 2.67 | 1.69 | 1.12 | 6.44 | 3.21 | 1.84 | ||
| HR (95% CI) | ||||||||
| Api/W | 0.79 | 0.74 | 0.88 | 0.705 | 0.50 | 0.77 | 0.80 | 0.030 |
| (0.55–1.14) | (0.56–0.97) | (0.64–1.22) | (0.38–0.66) | (0.62–0.94) | (0.61–1.04) | |||
| ENGAGE AF | ||||||||
| CrCl (mL/min) | 30–50 | >50 | 30–50 | >50 | ||||
| HDER 60/30 | 2.3 | 1.4 | 4.0 | 2.5 | ||||
| Warfarin | 2.7 | 1.6 | 5.3 | 3.1 | ||||
| HR | ||||||||
| HDER/W | 0.87 | 0.87 | 0.94 | 0.76 | 0.82 | 0.62 | ||
| (0.65–1.18) | (0.72–1.04) | (0.58–0.98) | (0.71–0.95) | |||||
Api: apixaban; CI: confidence interval; CKD: chronic kidney disease; CrCl: creatinine clearance; Dabi: dabigatran; eGFR: estimated glomerular filtration rate; HDER: high-dose edoxaban regimen; HR: hazard ratio: Int: interaction; NOACs: non-vitamin K antagonists; Riva: rivaroxaban; W: warfarin
Stroke and systemic embolization events;
Major bleeding.
8.3.3.3. Meta-analysis of NOAC in CKD
There have been several meta-analyses addressing the efficacy of NOACs in relation to VKAs in patients with mild or moderate CKD [192], [193]. The data are very consistent across studies, showing that in patients with mild or moderate CKD (eGFR 30–79 mL/min), all NOACs are associated with decreased rates of thromboembolism compared with warfarin. Among patients with mild CKD (defined as an eGFR of between 50 and 79 mL/min), major bleeding was also significantly reduced in patients receiving NOACs; however, in patients with moderate CKD (eGFR 30–49 mL/min) the overall bleeding rate was similar to that of warfarin [192], [193].
8.3.3.4. Can we use NOACs in patients with CrCl<30 mL/min?
The major RCTs of NOACs have excluded patients with CrCl<30 mL/min, except for an apixaban trial, which excluded patients with CrCl<25 mL/min. European and American dosing recommendations state that apixaban and rivaroxaban can be administered to patients with an eCrCl>15 mL/min. However, the evidence for this recommendation comes from pharmacokinetic studies in a limited number of patients. Because of limited experience with NOACs at this level of renal dysfunction, the Canadian guidelines have recommended that VKAs are generally the preferred agent for patients with an eCrCl of 15–30 mL/min [185].
The US Food and Drug Administration has approved dabigatran 75 mg bid for patients with an eGFR of 15–30 mL/min, and apixaban (5.0 mg bid) for ESRD with hemodialysis, based on their pharmacokinetic and pharmacodynamic modelling profiles. Dabigatran is considered a less-than-ideal choice because of its risk of increasing bleeding when CrCl drops below 50 mL/min. Drug levels may substantially fluctuate with dialysis treatment, particularly because dialysis clears 50% to 60% of the drug [194]. We do not recommend the use of NOACs in patients with ESRD with an eCrCl < 15 mL/min or in patients on dialysis, until there are clinical data to confirm their safety and efficacy [186].
Recommendations
-
•
In the context of NOAC treatment, CrCl is best assessed by the Cockcroft-Gault method, as this was used in the NOAC trials.
-
•
For patients with moderate CKD, i.e., eCrCl 30–49 mL/min, NOACs are preferred over VKA in stroke prevention in Asians, due to a lower risk of ICH.
-
•
Standard-dose NOACs should not be used in patients with severe CKD, i.e., eCrCl < 30 mL/min (<25 mL/min for apixaban).
-
•Low-dose NOACs (rivaroxaban 15 mg od, apixaban 2.5 mg bid, edoxaban 30 mg od) should be used with caution in severe CKD (CrCl 15–30 mL/min) according to drug labels.
-
■Dabigatran should not be used in patients with CrCl < 30 mL/min.
-
■
-
•
In patients with ESRD or dialysis, NOACs are contraindicated. Although VKA with good-quality anticoagulation control (TTR > 70%) might be useful, the data are lacking.
8.4. Patients with coronary heart disease
Patients with AF may have concurrent coronary heart disease (CHD), either in a stable form or with acute coronary syndrome (ACS). The use of OAC and dual antiplatelet therapy (DAPT) may increase the bleeding risk. The use of glycoprotein IIb/IIIa inhibitors is not recommended due to a potentially higher risk of bleeding. In stabilized patients, OAC can be restarted after parenteral anticoagulation is stopped. It is reasonable to restart the NOAC that the patient was taking before the ACS or elective procedure [113]. The same principle applies for AF patients after coronary bypass grafting. The use of ticagrelor or prasugrel as part of the triple therapy regimen is not recommended, given that their bleeding risk when associated with NOACs is unknown [195]. (Triple therapy stands for low-dose aspirin (75–100 mg/d), clopidogrel 75 mg/d, and an OAC; dual therapy means clopidogrel 75 mg/d and an OAC.).
For long-term management of AF patients after revascularization and/or ACS, a management algorithm has been suggested to reduce the risk of bleeding while protecting against coronary events (Fig. 1). The bleeding risk can be defined by the HAS-BLED score [44]. Those with a HAS-BLED score of 0–2 have a low bleeding risk; while a HAS-BLED score ≥ 3 suggests a high bleeding risk. There is no indication that the advantages of NOACs over VKAs are not preserved in AF patients with CHD, especially in Asians [39], [113].
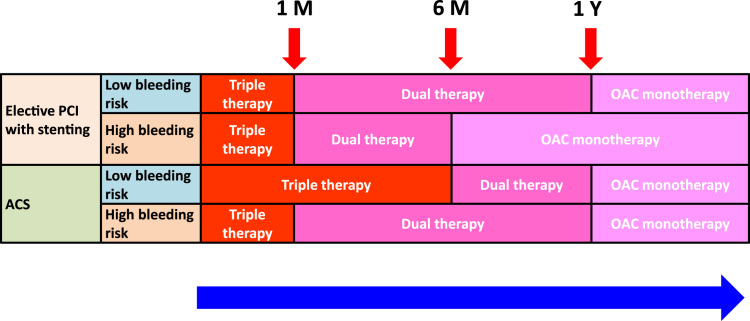
Fig. 1.
Flow chart for the long-term management of patients with atrial fibrillation and acute coronary syndrome/percutaneous intervention. ACS, acute coronary syndrome; M, month; OAC, oral anticoagulant; PCI, percutaneous intervention; Y, year.
For all stable CHD patients with AF, the rule of thumb is to use anticoagulation as monotherapy and to discontinue any antiplatelet agents at 1 year after patient presentation with ACS, except for those with a very high risk of coronary events and an acceptably low bleeding risk [39], [113].
In the recent PIONEER AF-PCI trial, 2124 participants with non-valvular AF who had undergone PCI with stenting were randomly assigned to receive low-dose rivaroxaban (15 mg od) plus a P2Y12 inhibitor for 12 months (Group 1), very-low-dose rivaroxaban (2.5 mg bid) plus DAPT for 1, 6, or 12 months (Group 2), or standard therapy with a dose-adjusted VKA (once daily) plus DAPT for 1, 6, or 12 months (Group 3) [196]. The primary safety outcome was clinically significant bleeding (a composite of major bleeding or minor bleeding according to Thrombolysis in Myocardial Infarction [TIMI] criteria, or bleeding requiring medical attention). The study did not have enough power to examine the difference in major CV events (death from cardiovascular causes, myocardial infarction, or stroke) [197]. The rates of clinically significant bleeding were lower in the two groups receiving rivaroxaban than in the group receiving standard therapy, but there was no significant difference in major bleeding [196]. Compared with Group 3, the rates of cardiovascular death were 29% higher in Group 1 and 19% higher in Group 2, though these differences did not reach significance. One should interpret this trial cautiously in that the rates of ischemic stroke were numerically higher in Group 1 and Group 2, compared to Group 3 (Group 1 versus Group 3, HR 3.28, CI 0.68–15.78; Group 2 versus Group 3, HR 2.87, CI 0.58–14.23) [196], though these results, again, did not reach statistical significance.
It is still too early to use only single antiplatelet therapy plus OAC to replace the conventional triple-therapy regimen (i.e., DAPT plus OAC) in this clinical setting (Fig. 1). Again, NOACs are preferred over VKAs in Asians [58], and no data suggest that one NOAC is better than another [39].
Recommendations
-
•
The use of glycoprotein IIb/IIIa inhibitors is not recommended due to a potentially higher risk of bleeding.
-
•
The use of ticagrelor or prasugrel as part of the triple-therapy regimen is not recommended, given that their bleeding risk when used with NOACs is unknown.
-
•
For patients receiving elective PCI, triple therapy should be used for 1 month, followed by dual therapy up to 1 year (or up to 6 months for patients with high bleeding risk).
-
•
For patients with ACS, triple therapy should be used for 6 months, and followed by dual therapy up to 1 year (or for 1 month, followed by dual therapy up to 1 year in patients with high bleeding risk).
-
•
For all stable CHD patients with AF, the recommendation is to use anticoagulation as monotherapy and to discontinue any antiplatelet agents at 1 year after presentation with ACS, except for those with a very high risk of coronary events and an acceptably low bleeding risk.
8.5. Patients with a history of stroke
Prior stroke or transient ischemic attack (TIA) (history of stroke/TIA) is a powerful independent predictor of subsequent stroke, with a relative risk between 2.2 and 2.5 [198], [199]. In a Japanese pooled analysis of three registries of 5,188 person-years, it was demonstrated via multivariate Cox regression analysis that the HR for subsequent recurrence was 3.25 (CI, 1.86–5.67) [14]. The prevalence of a history of stroke/TIA in AF patients was high in Asians (18.8% in China and 22.1% in Southeast Asia) compared with those in other regions (13.8% overall) [66].
Asian AF patients with prior stroke/TIA have strong indications for OAC, unless there are contraindications or inappropriate conditions for OAC. When prescribing OAC to patients with prior stroke/TIA, these patients are also at significantly higher risk for ICH during OAC than those without prior stroke/TIA [200], [201], [202], [203], [204], [205].
8.5.1. Patients with a history of ischemic stroke
The efficacy and safety profiles of NOACs between the patient groups with and without prior stroke/TIA were consistent [200], [201], [202], [203], indicating that NOACs can be used safely even in patients with prior stroke/TIA. There was no interaction in the efficacy and safety between patients with and without prior stroke [24]. Warfarin was shown to be associated with a numerically increased risk of major bleeding in the Asian patients compared with the non-Asians [24], [25], [26], and most importantly, it increased the incidence of ICH by 1.5–3.9 times in Asian patients [67].
Use of NOACs is associated with significantly lower risk of major bleeding than warfarin in Asian or East Asian AF patients, except for rivaroxaban [24], [25], [26]. Dabigatran 150 mg reduced stroke and SEE more effectively than warfarin [23], while other NOAC regimens, including dabigatran 110 mg, rivaroxaban 20 mg, apixaban 5 mg, and edoxaban 60 mg, had similar efficacy compared with warfarin [24], [25], [26]. Thus, NOACs seem the best option for stroke prevention when treating Asian patients with prior stroke/TIA.
The NOAC trials generally excluded patients within first 7–14 days after acute ischemic stroke. However, the risk of recurrent stroke is highest in the early phase after the first stroke/TIA [206]. The 2016 ESC Guidelines for the Management of Atrial Fibrillation proposed to initiate OAC between 1 and 12 days after an ischemic stroke [38], depending on stroke severity, using the so-called “1–3-6–12 day rule” as follows: (i) in patients with TIA, NOAC can be initiated at day 1; (ii) after mild stroke (National Institute of Health Stroke Scale (NIHSS) < 8), NOAC can be initiated after 3 days, or after ICH is excluded by imaging modality (CT or MRI); (iii) in moderate stroke (NIHSS 8–16), NOAC can be initiated after 5–7 days; and (iv) in severe stroke (NIHSS > 16) after 12–14 days [113]. This is based on no trial evidence, and is simply expert opinion.
8.5.2. Patients with a history of intracranial hemorrhage
Patients with a history of ICH have higher recurrence rates of ICH when OAC is taken. Several studies have shown that in patients with a history of ICH, OAC treatment was associated with a significant reduction in ischemic stroke/all-cause mortality rates in comparison with no treatment [207], [208].
In a recent report from the Taiwan NHIRD, warfarin use was found to be possibly beneficial for AF patients with prior ICH having a CHA2DS2-VASc score ≥ 6 [209]. Whether the use of NOACs can lower the threshold for treatment deserves further study [209]. One should know that, in all the RCTs of NOACs, a history of spontaneous ICH was an exclusion criteria. Therefore, the decision to use OAC should be individualized.
In patients with high cardioembolic risk, CHA2DS2-VASc score ≥ 6 for instance, and a low ICH risk, OAC can be started after 4–8 weeks; the CHA2DS2-VASc score threshold may be lower for NOACs. For patients with low cardioembolic risk and high ICH risk, the use of a NOAC might be omitted [38].
Recommendations
-
•
NOACs are preferred over VKA in patients with a history of ischemic stroke/TIA.
-
•
After an acute episode of ischemic stroke/TIA, NOACs can be initiated based on the “1–3-6–12 day rule”: In patients with TIA, NOAC can be initiated at day 1. In patients with mild stroke (NIHSS < 8), NOAC can be initiated after 3 days. In patients with moderate stroke (NIHSS 8–16), NOAC can be initiated after 5–7 days, and in severe stroke (NIHSS > 16) after 12–14 days.
-
•
For patients with a history of ICH, the decision to use OAC should be individualized. In patients with high cardioembolic risk, CHA2DS2-VASc score ≥ 6 for instance, and a low ICH risk, NOAC can be started after 4–8 weeks. For patients with low cardioembolic risk and high ICH risk, the use of a NOAC might be omitted.
8.6. Peri-operative use of NOACs
Surgical interventions or invasive procedures require careful planning and temporary discontinuation of anticoagulants. Both patient characteristics (kidney function, age, history of bleeding complications, concomitant medication) and surgical factors should be taken into account when deciding the timing of discontinuing and restarting the anticoagulants [113], [210], [211], [212], [213]. Clinicians must always weigh the risks of thrombosis associated with discontinuing antithrombotic medications against the risks of potential bleeding inherent to the contemplated procedure [214].
8.6.1. Assessment of bleeding and stroke risk
Assessment of bleeding risk should include an understanding of the patient׳s comorbidities and the invasive nature of contemplated procedure. Active cancer, thrombocytopenia, and a history of bleeding are associated with an increased risk of perioperative bleeding [215]. Formal bleeding-risk assessment is essential, and can be done by using the well-validated HAS-BLED score [44].
All patients with AF receiving anticoagulants should be assessed using the CHA2DS2-VASc score before surgery, and patients with higher scores should be considered for minimal duration of anticoagulation discontinuation, especially if they are on NOACs [211], [216]. The duration of preoperative discontinuation and re-initiation of NOAC therapy should be determined on the basis of the pharmacokinetic properties of each agent [217], [218], [219], [220], [221], [222].
8.6.2. Do we need a bridging strategy?
Since VKAs have a slower onset and offset of action, bridging with low molecular weight heparin (LMWH) or unfractionated heparin (UFH) has often been used in AF patients with higher thromboembolic risk [223]. However, bridging therapy is not always necessary in NOAC-treated patients since the predictable waning of the anticoagulation effect allows properly timed short-term cessation and re-initiation of NOAC therapy.
The BRIDGE trial has shown that in VKA-treated patients, bridging with LMWH has no benefit regarding thromboembolism but is instead an inferior strategy, since it can lead to a higher incidence of major bleeding [224]. In a meta-analysis of AF patients with intermediate CHADS2 scores who were anticoagulated with warfarin and required temporary interruption of warfarin for an elective surgery or procedure, periprocedural bridging with UFH or LMWH was associated with a higher rate of major bleeding with no significant difference in mortality or stroke [225].
Heparin bridging is generally not necessary for NOACs, because their half-lives are similar to those of LMWH. Bleeding and thromboembolic outcomes in the periprocedural period using NOACs versus warfarin have been investigated in the RE-LY, ROCKET AF, and ARISTOTLE trials [226], [227], [228]. In these studies, NOACs and warfarin were generally interrupted. There were no statistically significant differences between the dabigatran, rivaroxaban, or apixaban groups and their respective warfarin groups with respect to bleeding or thromboembolic complications.
In the Perioperative Dabigatran Study, a Canadian multicenter prospective study of perioperative management, 541 adult patients receiving dabigatran for any indication (AF, 97%) underwent an invasive procedure requiring NOAC interruption [229]. The outcomes of the study included major and minor bleeding, thromboembolism, and death, and the results suggested that interruption of dabigatran without bridging is safe [229]. Observational analyses from the Dresden NOAC Registry (76% rivaroxaban, 24% dabigatran) suggested no difference in bleeding or thromboembolic complications in the periprocedural period between rivaroxaban and dabigatran [230]. Heparin bridging did not reduce cardiovascular events but led to significantly higher rates of major bleeding [230].
8.6.3. Peri-operative strategy
When the intervention carries minimal bleeding risk and/or when adequate local hemostasis is possible, as with some dental procedures or interventions for cataract or glaucoma, the procedure can be scheduled 24 h after the last dose, and then, the drug can be restarted 6 h later. Stoppage of NOACs depending on renal function for patients undergoing procedures with minor or major bleeding risk is shown in Table 10.
Table 10.
Last intake of NOACs before elective surgical intervention.
| Dabigatran |
Rivaroxaban, Apixaban, Edoxaban |
|||
|---|---|---|---|---|
| Low bleeding risk | High bleeding risk | Low bleeding risk | High bleeding risk | |
| CrCl ≥ 80 mL/min | ≥24 h | ≥48 h | ≥24 h | ≥48 h |
| CrCl 50–79 mL/min | ≥24 h | ≥48 h | ≥24 h | ≥48 h |
| CrCl 30–49 mL/min | ≥48 h | ≥96 h | ≥24 h | ≥48 h |
Whenever a patient on anticoagulation needs surgery, the anticoagulant should be discontinued. If surgery cannot be delayed, reversal of the anticoagulant may be considered. Idarucizumab, a specific antibody for dabigatran, was tested in a phase III, (REVERSE-AD) trial in patients with acute life-threatening bleeding and those requiring urgent surgical intervention [231]. Idarucizumab showed a rapid and near-maximal reversal of the anticoagulant effects of dabigatran [231]. No reversal agents are currently available for clinical use to reverse the Xa inhibitors. Therefore, in emergency situations, off-label administration of prothrombin complex concentrate (PCC) or recombinant factor VII can be used in circumstances of life-threatening bleeding [232], [233].
There are no data suggesting how to resume NOACs after surgical procedures. For procedures with immediate and complete hemostasis, the NOACs can be resumed 6–8 h after the intervention. In general, NOACs can be restarted 24 h after procedures with low bleeding risk, and may be restarted 48–72 h after procedures with high bleeding risk [113], [222], [234].
Recommendations
-
•
Bridging with LMWH or UFH is not necessary for VKA-treated patients undergoing planned surgical intervention.
-
•
Bridging with LMWH or UFH is not necessary for NOAC-treated patients undergoing planned surgical intervention.
-
•
Stoppage of NOACs depends on renal function and the risk of bleeding of different surgical procedures.
-
•
For patients receiving rivaroxaban, apixaban, and edoxaban, NOACs should be withheld for 24 h for procedures with low bleeding risk, or for 48 h for procedures with high bleeding risk, irrespective of renal function.
-
•
For patients with an eCrCl ≥ 50 mL/min receiving dabigatran, the drug should be withheld for 24 h for procedures with low bleeding risk, or for 48 h for procedures with high bleeding risk.
-
•
For patients with an eCrCl = 30–49 mL/min receiving dabigatran, the drug should be withheld for 48 h for procedures with low bleeding risk, or for 96 h for procedures with high bleeding risk.
-
•
NOACs can be restarted 24 h after procedures with low bleeding risk, and may be restarted 48–72 h after procedures with high bleeding risk.
8.7. Patients undergoing cardioversion
Thromboembolism after cardioversion is relatively uncommon overall (<1% within 30 days) but is a potentially devastating complication [235], [236]. Observational studies suggest that thromboembolic risk after cardioversion is highest in the first 72 h and that the majority of events occur within 10 days [235], [237]. These thromboembolic events are thought to be due to the embolization of pre-existing thrombi at the time of cardioversion or from the formation and subsequent migration of thrombi that formed while atrial function was still depressed after cardioversion.
8.7.1. AF duration ≥ 48 h or of unknown duration
Anticoagulation with warfarin for at least 3 weeks before and continuing for at least 4 weeks after cardioversion has been shown to be associated with a low risk of thromboembolism [238], [239]. Furthermore, the addition of trans-esophageal echocardiography (TEE) screening before cardioversion in patients who had adequate anticoagulation prior to cardioversion did not appear to reduce that risk; however, it did find left atrial thrombus in 7.7%, despite anticoagulation [239]. As for NOACs, subgroup studies of patients on dabigatran (RE-LY), rivaroxaban (ROCKET AF), and apixaban (ARISTOTLE) demonstrated comparable event rates to patients on warfarin, lending support to their use in patients with non-valvular AF [240], [241], [242]. While these studies all showed low event rates after cardioversion, they were not sufficiently powered to demonstrate superiority over warfarin.
A recent randomized study of rivaroxaban versus VKA also showed similar event rates between the 2 treatment arms, but a reduction in time to cardioversion in the rivaroxaban arm [243]. The ENSURE-AF trial was the largest prospective RCT of anticoagulation for cardioversion of patients with non-valvular AF [244]. A total of 2199 patients were randomly assigned to receive edoxaban or enoxaparin-warfarin. Both the primary efficacy endpoint (stroke/SEE, myocardial infarction, and cardiovascular mortality) and the primary safety endpoint (major and clinically relevant non-major bleeding) did not differ significantly between the 2 treatment groups [244].
In patients who have not had 3 weeks of anticoagulation before cardioversion, TEE guidance can be used to reduce the risk of thromboembolism [245], [246]. If no thrombus is observed (including in the LAA), cardioversion can proceed with UFH or LMWH coverage and anticoagulation should then be continued for at least 4 weeks. This expedited anticoagulation strategy appears to be safe in observational studies [247]. If a thrombus is observed on TEE, the cardioversion should be deferred and the patient commenced on at least 4 weeks of anticoagulation. A repeat TEE to ensure thrombus resolution should be considered before another cardioversion attempt, as thrombi may not always resolve with anticoagulation [248]. If a thrombus persists on the repeat TEE, then an alternative strategy such as rate control with appropriate anticoagulation should be considered. The same principles can be applied to NOACs.
8.7.2. AF duration <48 h
For patients who have been in AF for less than 48 h, it is common practice to perform cardioversion without TEE or antecedent anticoagulation, but there are no RCTs to support this. These recommendations are based on observational studies that found a low risk of thromboembolic events in patients who either spontaneously reverted or were cardioverted without having been anticoagulated [235], [249]. It is recommended that these patients may be cardioverted early with UFH or LMWH coverage. However, it should be noted that some patients, such as those with diabetes, heart failure or significant valvular disease, may be at higher risk [235].
8.7.3. Emergent cardioversion
For patients with AF who require emergency cardioversion because of hemodynamic instability (angina, myocardial infarction, shock, or pulmonary edema), initiation of anticoagulation should not delay interventions to stabilize the patient. Again, there are no RCTs that have tested optimal anticoagulation strategies in such patients, but it may be reasonable to administer UFH or LMWH prior to cardioversion unless contraindicated. If the AF or atrial flutter has been present for more than 48 h or if its duration is unclear, oral anticoagulation is recommended for at least 4 weeks after emergency cardioversion. If warfarin is used, bridging with UFH or LMWH is indicated until the INR is therapeutic. For patients with AF and thromboembolic risk factors (CHA2DS2-VASc ≥ 1 for males, or ≥2 for females), long-term anticoagulation with an oral anticoagulant is recommended.
8.7.4. Atrial flutter
There is less data on the thromboembolic risk of cardioverting atrial flutter, but it is probably similar to AF and has been shown to be associated with thrombi and episodes of AF [238]. It is recommended that the anticoagulation management strategy for cardioversion of atrial flutter should hence be the same as for AF.
Recommendations
-
•
For patients with AF or atrial flutter of 48 h duration or longer, or when the duration of AF is unknown, anticoagulation with warfarin (INR 2.0 to 3.0) or NOAC is recommended for at least 3 weeks before and 4 weeks after cardioversion, regardless of the CHA2DS2-VASc score and the method (electrical or pharmacological) used.
-
•
For patients with AF or atrial flutter of 48 h duration or longer, or when the duration of AF is unknown, and who have not been anticoagulated for the preceding 3 weeks, it is reasonable to perform TEE before cardioversion and to proceed with cardioversion if no LA thrombus is observed, including in the LAA, provided that anticoagulation is achieved before TEE with therapeutic warfarin (INR 2.0–3.0) or NOAC, and maintained after cardioversion for at least 4 weeks.
-
•
For patients undergoing a TEE-guided strategy in whom a thrombus is identified, VKA (INR 2.0–3.0) or NOAC is recommended for at least 4 weeks, followed by a repeat TEE to ensure thrombus resolution.
-
•
If thrombus resolution is evident on repeat TEE, cardioversion should be performed, and OAC should be considered for at least 4 weeks, or lifelong if risk factors are present (i.e., CHA2DS2-VASc ≥ 1 in males or ≥ 2 in females).
-
•
For patients with AF or atrial flutter of more than 48 h duration or unknown duration that requires immediate cardioversion for hemodynamic instability, anticoagulation with UFH or LMWH should be initiated as soon as possible and anticoagulation should be continued for at least 4 weeks after cardioversion unless contraindicated.
-
•
For patients with AF or atrial flutter of less than 48 h duration and with high risk of stroke, anticoagulation with UFH or LMWH is recommended as soon as possible before cardioversion, followed by long-term anticoagulation therapy.
-
•
Following cardioversion for AF or flutter of any duration, long-term anticoagulation therapy should be considered in patients with a CHA2DS2-VASc ≥ 1 in males or ≥ 2 in females.
8.8. Peri-ablation procedure
Catheter ablation of AF is associated with a potential risk of SEE not only due to the introduction and manipulation of catheters and long sheaths within the atrium, but also the endocardial damage produced by ablations [250]. Recent studies show that the incidence of SEE with AF ablation was approximately 1–5% [251], [252].
Several studies have assessed the safety and efficacy of NOACs compared to warfarin during the periprocedural period of AF ablation. The results of these studies have demonstrated that NOACs, such as dabigatran, could be a safe and effective alternative to warfarin during the periprocedural period of AF ablation [253], [254]. Antithrombotic strategies for the prevention of SEE should be specified for 3 different stages, that is, pre-ablation, during-ablation, and post-ablation stages [250].
8.8.1. Pre-Ablation
For patients who have had AF for more than 48 h, or for an unknown duration, systemic anticoagulation with NOACs or VKAs for at least 3 weeks is recommended. For patients being treated with VKAs, the ablation should be performed without interruption of VKAs.
Several non-randomized studies have shown that uninterrupted VKA use is associated with a lower risk of thromboembolic and hemorrhagic complications compared to interrupted VKAs [251], [255], [256].
The VENTURE AF trial showed similar event rates in patients on uninterrupted rivaroxaban compared with uninterrupted VKA [257]. In the recent RE-CIRCUIT trial, 704 patients who were scheduled for catheter ablation of AF were randomly assigned to receive either dabigatran (150 mg bid) or warfarin (target INR, 2.0 to 3.0) [258]. Ablation was performed after 4 to 8 weeks of uninterrupted anticoagulation, which was continued during ablation and for 8 weeks afterwards. The incidence of major bleeding events (primary endpoint) during and up to 8 weeks after ablation was significantly lower with dabigatran than with warfarin (1.6% vs. 6.9%, absolute risk difference, –5.3 percentage points; 95% CI −8.4 to −2.2; P<0.001). The relative risk reduction versus warfarin was 77.2% (HR 0.22, 95% CI, 0.08–0.59). Dabigatran was associated with fewer periprocedural pericardial tamponades and groin hematomas than warfarin. One thromboembolic event occurred in the warfarin group. These findings confirmed that uninterrupted dabigatran was associated with fewer bleeding complications than uninterrupted warfarin, while maintaining antithrombotic efficacy [258].
A TEE should be performed for patients who do not receive appropriate systemic anticoagulants for 3 weeks or for whom the duration of treatment is uncertain before the intervention [259]. Several studies suggested that even adequate anticoagulation could not exclude the possibility of the presence of left atrial thrombi or sludge in the LAA [260], [261], [262]. TEE can be performed on the day of, or up to 24 h before, the ablation to rule out the presence of atrial thrombi. Once a left atrial thrombus is detected, ablation should be delayed; it may be reconsidered after a 3-month treatment of systemic anticoagulants. However, TEE may not be necessary in patients with paroxysmal AF and full anticoagulation for >3 weeks and with a low risk of thromboembolism such as CHA2DS2-VASc 0 for males and 1 for females.
For patients in sinus rhythm with a CHA2DS2-VASc score of 0 for males or 1 for females, NOACs can be initiated on the day of the ablation procedure and continued post-ablation [250]. Although prospective and randomized controlled trials are lacking, there is a trend toward the initiation of the antithrombotic treatments before ablation, even for patients who present with sinus rhythm.
8.8.2. During Ablation
Heparin should be administered prior to or immediately after trans-septal puncture, and the dose should be adjusted to achieve and maintain an activated clotting time (ACT) of 300 to 400 seconds [259]. An intravenous loading dose of 5,000–15,000 units (or 90–200 U/kg) of heparin should be administered at the beginning of the procedure [250]. Previous studies showed that patients on NOAC required a larger dose of heparin and took longer time to reach the target ACT level than those on a VKA [257], [263]. After the loading dose of heparin, continuous heparin infusion at an initial rate of 1000–1500 U/kg/h can be started depending on the levels of ACT. The target ACT level should be achieved and maintained by administering heparin ranging between 2,500 and 7,500 U intermittently. The ACT level should be checked every 10–15 min before the therapeutic anticoagulation is achieved and, then every 15–30 min for the rest of the procedure [259]. Uninterrupted VKAs or NOACs influence the ACT and the time needed to reach the target ACT level [250], [254], [264].
All sheaths should be continuously flushed with heparinized saline solution, with a suggested dose of 2000 units per 250 mL [265], [266]. Heparin infusion can be discontinued once all catheters are removed from the left atrium. To decrease the risk of bleeding of the puncture site, ACT should be less than 250 s before the sheath is removed, otherwise protamine should be used to reverse the anticoagulation effect of heparin.
8.8.3. Post-ablation
The atria are vulnerable to the formation of thrombi after ablation, and therefore adequate post-ablation anticoagulation is crucial to prevent SEE. Systemic oral anticoagulants should be continued for at least 2 months after ablation [259]. Recently, a cohort study demonstrated that most thromboembolic events occurred within 4 weeks post-ablation, and the thromboembolic risk beyond 3 months after ablation was relatively low compared with a matched non-ablated AF cohort [267]. For patients who discontinue VKAs or with a suboptimal INR at the time of ablation, LMWH should be administered 4–6 h after AF ablation, along with the re-initiation of VKAs once hemostasis has been achieved. The LMWH should be maintained until a therapeutic INR level (2.0–3.0) has been achieved [250]. Because of the increased risk of bleeding on full-dose LMWH (1 mg/kg bid), a dosage reduction of LMWH (0.5 mg/kg) should be considered [259]. In patients who continue VKAs, the use of LMWH may be avoided and the INR should be maintained between 2.0 and 3.0 [259].
In patients on NOACs, the next dose should be resumed within 3–4 h once hemostasis is achieved [268]. Decisions regarding the use of systemic anticoagulants for longer than two months following ablation should be made based on the patient׳s risk for stroke [259]. Long-term anticoagulation is recommended for patients at a high risk of stroke (CHA2DS2-VASc score ≥ 1 in males or ≥ 2 in females). Continuous ECG monitoring should be considered to detect asymptomatic AF in patients who discontinue systemic anticoagulants [259]. Recently, several studies have demonstrated that noninvasive ambulatory ECG monitoring can significantly improve the detection of AF [269], [270]. In patients who are at an increased risk of stroke, noninvasive ambulatory ECG monitoring may be necessary.
Recommendations
-
•
NOACs can be safe and effective alternatives to warfarin during the periprocedural period of AF ablation.
-
•
For patients who have had AF for more than 48 h or an unknown duration, systemic anticoagulation with NOACs or VKAs for at least 3 weeks before any ablation procedure is recommended.
-
•
For patients treated with VKAs or NOACs, the ablation should be performed without interruption of VKAs or NOACs.
-
•
A TEE should be performed for patients who do not receive appropriate systemic anticoagulants for 3 weeks, or for whom the duration of treatment is uncertain before the intervention.
-
•
TEE can be performed on the day of, or up to 24 h before, the ablation to rule out the presence of atrial thrombi.
-
•
TEE may not be necessary in patients with paroxysmal AF and full anticoagulation for > 3 weeks and with a low risk of thromboembolism such as when CHA2DS2-VASc = 0 for males and = 1 for females.
-
•
For patients in sinus rhythm with a CHA2DS2-VASc score = 0 for males or = 1 for females, NOACs can be initiated on the day of the ablation procedure and continued post-ablation.
-
•
Heparin should be administered prior to, or immediately after, trans-septal puncture and the dose should be adjusted to achieve and maintain an ACT of 300 to 400 seconds.
-
•
Systemic oral anticoagulants should be continued for at least 2 months after ablation, and for longer in the presence of risk factors, irrespective of the apparent success of rhythm control.
-
•
For patients who discontinue VKAs or who have a suboptimal INR at the time of ablation, LMWH should be administered 4–6 h after AF ablation along with the re-initiation of VKAs once hemostasis has been achieved.
-
•
In patients on NOACs, the next dose should be resumed within 3–4 h once hemostasis is achieved.
-
•
Long-term anticoagulation is recommended for patients at a high risk of stroke (CHA2DS2-VASc score ≥1 in males or ≥2 in females).
8.9. Management of bleeding complications
The management strategy for bleeding in patients on OAC therapy depends primarily on the severity of bleeding, and the types of OAC used. Generally, bleeding events are broadly classified into mild, moderate, and severe bleeding. Mild bleeding events include epistaxis, small bruises, and bleeding after minor trauma; moderate bleeding events include gross hematuria, spontaneous large bruises, and any bleeding requiring transfusion but no hemodynamic compromise; and severe bleeding events refer to potentially life-threatening bleeding, such as bleeding into critical sites (ICH and retroperitoneal bleeding), or bleeding leading to hemodynamic instability.
General measures for active bleeding in patients on OAC include: mechanical compression if the bleeding site is accessible; hemodynamic assessment; clarification of the types and last dose of OAC; and blood tests for complete blood count, liver and kidney function, and basic coagulation tests. In cases of mild bleeding which stops spontaneously, no intervention is needed after ascertaining either the dosage and timing of the drug (for patients taking NOACs) or therapeutic level of INR (for those on VKA). If bleeding persists or recurs, the next dose of NOAC can be delayed or omitted. Among patients on VKA, VKA can be omitted until INR < 2.
In the setting of moderate bleeding, OAC should be withheld immediately and standard supportive measurements must be started. These include fluid replacement to maintain urine output, blood transfusion, and additional hemodynamic support. For patients on VKA, intravenous vitamin K1 (1–10 mg) may be considered [271]. Activated charcoal can reduce further absorption of NOAC if the drug has been administered within 2–4 h. Regardless of the types of OAC, specific diagnostic procedures and therapeutic interventions to control bleeding, such as endoscopy, should be considered.
In case of severe or life-threatening bleeding, in addition to the aforementioned measures, treatments to reverse anticoagulation effects should be considered. For patients on VKA, fresh frozen plasma may restore coagulation more rapidly than vitamin K.
For patients on dabigatran, idarucizumab should be considered [231]. When idarucizumab is not available, hemodialysis accelerates the clearance of dabigatran from the body. On the contrary, andexanet Alfa, a recombinant modified human factor Xa decoy protein, has also been demonstrated to be effective in reversing the anticoagulation effect of various factor Xa inhibitors; but it has yet to be approved by regulatory bodies. Unlike dabigatran, hemodialysis is in general not effective in the removal of factor Xa inhibitors out of the body. For patients on factor Xa inhibitors, PCC or activated prothrombin complex (aPCC) concentrates can be considered in severe or life-threatening bleeding with a starting dose of 25 U/kg and can be repeated if clinically indicated.
Recommendations
-
•
In case of mild bleeding which stops spontaneously, no intervention is needed after ascertaining the dosage and timing of drug for patients taking NOACs.
-
•
In the setting of moderate bleeding, OAC should be withheld immediately and standard supportive measurements must be started.
-
•
In case of severe or life-threatening bleeding, treatments to reverse anticoagulation effects should be considered, such as idarucizumab in patients receiving dabigatran.
-
•
When idarucizumab is not available, hemodialysis accelerates the clearance of dabigatran from the body.
-
•
For patients on factor Xa inhibitors, PCC or aPCC concentrates can be considered in severe or life-threatening bleeding.
8.10. Reversal agents
Specific reversal agents for NOACs are now available. Idarucizumab is a monoclonal antibody fragment and binds dabigatran with an affinity that is 350 times as high as that observed with thrombin [272]. In the recent RE-VERSE AD study, the efficacy and safety of idarucizumab was tested in dabigatran-treated patients who had serious bleeding or required urgent procedures [231]. In the recent interim analysis of the first 90 patients, idarucizumab completely reversed the anticoagulant effect of dabigatran within minutes [231]. Immediately after the administration of idarucizumab, the concentration of unbound dabigatran was reduced to a level at or near the lower limit of quantification in all but 1 patient [231]. The US FDA has granted accelerated approval to idarucizumab (Praxbind®) to rapidly reverse the effects of dabigatran.
Andexanet alfa (andexanet) is a specific reversal agent for both direct and indirect factor Xa inhibitors [273]. Andexanet is a recombinant modified human factor Xa decoy protein that is catalytically inactive but that retains the ability to bind factor Xa inhibitors in the active site with high affinity and a 1:1 stoichiometric ratio. In a recently published clinical trial, andexanet reversed the anticoagulant activity of apixaban and rivaroxaban in healthy older participants within minutes after administration and for the duration of infusion, without clinical evidence of toxic effects [274]. In the ANNEXA-4 trial, an initial bolus and subsequent 2-h infusion of andexanet substantially reduced anti-factor Xa activity in patients with acute major bleeding associated with factor Xa inhibitors, with effective hemostasis occurring in 79% [275]. However, the US FDA has delayed approval of andexanet. Aripazine (ciraparantag, PER 977) is a small molecule that interacts with anticoagulants through non-covalent hydrogen bonding and electrostatic interactions. This agent appears to inhibit nearly all anticoagulants with the exception of vitamin K antagonists and argatroban [276]. Clinical trials are awaited to confirm its efficacy and safety in AF patients.
Recommendations
-
•
Idarucizumab, a specific reversal agent for dabigatran, is indicated in patients with serious bleeding or requiring urgent procedures.
9. Management algorithm
CHA2DS2-VASc score has outperformed other scoring systems in predicting AF-associated stroke in Asians [34], [36]; therefore, the APHRS consensus on stroke prevention in AF recommends the use of CHA2DS2-VASc scores in the prediction of stroke risk. A management algorithm is shown in Fig. 2.
Fig. 2.
Management algorithm for stroke prevention in Asian patients with non-valvular atrial fibrillation. A, apixaban; AF, atrial fibrillation; CHA2DS2-VASc, Congestive heart failure, Hypertension, Age ≥75 [doubled], Diabetes, Stroke [doubled]-Vascular disease, Age 65–74, Sex category [female]; D, dabigatran; E, edoxaban; NOAC, non-vitamin K antagonist oral anticoagulant; SAMe-TT2R2,Sex female, Age less than 60, Medical history [more than two comorbidities], Treatment [interacting medications, eg. amiodarone], Tobacco use [doubled], Race [doubled]; R, rivaroxaban; VKA, vitamin K antagonist.
The first step is to identify those patients with low risk (i.e. CHA2DS2-VASc score 0 in males, 1 in females); no antithrombotic agent is recommended for them. The second step is offer stroke prevention to those with ≥ 1 additional stroke risk factors. The third step is to use the SAMe-TT2R2 score to identify patients who have a possibility of doing well with VKA (SAMe-TT2R2 score, 0–2) or those patients who are unlikely to achieve a good TTR by taking VKA (SAMe-TT2R2 score ≥3), so a NOAC should be used initially, without subjecting the patient to a “trial of warfarin” period.
No head-to-head RCT has tested the superiority of one NOAC versus another, and therefore, one can choose any NOAC, based on available evidence.
Conflict of interest
Chern-En Chiang has been on the speakers’ bureau for Astrazeneca, Bayer, Boehringer Ingelheim, Chugai, Daiichi-Sankyo, GSK, MSD, Novartis, Pfizer, Roche, Sanofi-aventis, Servier, Tanabe, Takeda, and TTY.
Ken Okumura has received remuneration from Boehringer Ingelheim, Daiichi-Sankyo, Medtronic and Johnson & Johnson.
Shu Zhang has been an advisory board member of Boston Scientific, an investigator for Boston Scientific, and an investigator for Medtronic.
Tze-Fan Chao has declared no conflict of interest related to this paper.
Chung-Wah Siu has declared no conflict of interest related to this paper.
Toon Wei Lim has received research funding from Bayer, Biotronik, Boehringer Ingelheim, Boston Scientific, Medtronic, and Pfizer. He has been on the advisory board of Bayer, Boehringer Ingelheim, and Pfizer. He has received travel support & honoraria from Bayer, Biotronik, Boehringer Ingelheim, Boston Scientific, Medtronic, Pfizer, and St. Jude Medical.
Anil Saxena worked as consultant for and attended advisory board meetings of Boehringer-Ingelheim, Bayer Pharma, and Pfizer.
Yoshihide Takahashi has received speaker fees from Biosense Webster.
Wee Siong Teo has received research grants from Pfizer.
Acknowledgment
This work was supported, in part, by grants from the Ministry of Health and Welfare (MOHW106-TDU-B-211–113001), and from the Ministry of Science and Technology (MOST 105–2314-B-075-037), and intramural grants from the Taipei Veterans General Hospital (V106C-064).
References
- 1.Freedman B., Potpara T.S., Lip G.Y.H. Stroke prevention in atrial fibrillation. Lancet. 2016;388:806–817. doi: 10.1016/S0140-6736(16)31257-0. [DOI] [PubMed] [Google Scholar]
- 2.Gladstone D.J., Bui E., Fang J. Potentially preventable strokes in high-risk patients with atrial fibrillation who are not adequately anticoagulated. Stroke. 2009;40:235–240. doi: 10.1161/STROKEAHA.108.516344. [DOI] [PubMed] [Google Scholar]
- 3.Tomita H., Hagii J., Metoki N. Impact of sex difference on severity and functional outcome in patients with cardioembolic stroke. J Stroke Cerebrovasc Dis. 2015;24:2613–2618. doi: 10.1016/j.jstrokecerebrovasdis.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 4.Perera K.S., Vanassche T., Bosch J. Global survey of the frequency of atrial fibrillation–associated stroke. Embolic stroke of undetermined source global registry. Stroke. 2016;47:2197–2202. doi: 10.1161/STROKEAHA.116.013378. [DOI] [PubMed] [Google Scholar]
- 5.Yamashita Y., Hamatani Y., Esato M. Clinical characteristics and outcomes in extreme elderly (Age ≥ 85 Years) Japanese patients with atrial fibrillation: the Fushimi AF registry. Chest. 2016;149:401–412. doi: 10.1378/chest.15-1095. [DOI] [PubMed] [Google Scholar]
- 6.Leyden J.M., Kleinig T.J., Newbury J. Adelaide stroke incidence study: declining stroke rates but many preventable cardioembolic strokes. Stroke. 2013;44:1226–1231. doi: 10.1161/STROKEAHA.113.675140. [DOI] [PubMed] [Google Scholar]
- 7.Chiang C.E., Wang K.L., Lip G.Y. Stroke prevention in atrial fibrillation: an Asian perspective. Thromb Haemost. 2014;111:789–797. doi: 10.1160/TH13-11-0948. [DOI] [PubMed] [Google Scholar]
- 8.Wolf P.A., Abbott R.D., Kannel W.B. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 9.Chien K.L., Su T.C., Hsu H.C. Atrial fibrillation prevalence, incidence and risk of stroke and all-cause death among Chinese. Int J Cardiol. 2010;139:173–180. doi: 10.1016/j.ijcard.2008.10.045. [DOI] [PubMed] [Google Scholar]
- 10.Lip G.Y., Nieuwlaat R., Pisters R. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 11.Piccini J.P., Stevens S.R., Chang Y. Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: validation of the R(2)CHADS(2) index in the ROCKET AF (rivaroxaban once-daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation) and ATRIA (AnTicoagulation and risk factors in atrial fibrillation) study cohorts. Circulation. 2013;127:224–232. doi: 10.1161/CIRCULATIONAHA.112.107128. [DOI] [PubMed] [Google Scholar]
- 12.Lau Y.C., Proietti M., Guiducci E. Atrial fibrillation and thromboembolism in patients with chronic kidney disease. J Am Coll Cardiol. 2016;68:1452–1464. doi: 10.1016/j.jacc.2016.06.057. [DOI] [PubMed] [Google Scholar]
- 13.Hamatani Y., Ogawa H., Uozumi R. Low body weight is associated with the incidence of stroke in atrial fibrillation patients- insight from the Fushimi AF registry. Circ J. 2015;79:1009–1017. doi: 10.1253/circj.CJ-14-1245. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki S., Yamashita T., Okumura K. Incidence of ischemic stroke in Japanese patients with atrial fibrillation not receiving anticoagulation therapy-pooled analysis of the shinken database, J-RHYTHM registry, and Fushimi AF registry. Circ J. 2015;79:432–438. doi: 10.1253/circj.CJ-14-1131. [DOI] [PubMed] [Google Scholar]
- 15.Ho C.-W., Ho M.-H., Chan P.-H. Ischemic stroke and intracranial hemorrhage with aspirin, dabigatran, and warfarin: impact of quality of anticoagulation control. Stroke. 2015;46:23–30. doi: 10.1161/STROKEAHA.114.006476. [DOI] [PubMed] [Google Scholar]
- 16.Chao T.F., Liu C.J., Wang K.L. Using the CHA2DS2-VASc score for refining stroke risk stratification in ׳low-risk׳ Asian patients with atrial fibrillation. J Am Coll Cardiol. 2014;64:1658–1665. doi: 10.1016/j.jacc.2014.06.1203. [DOI] [PubMed] [Google Scholar]
- 17.Guo Y., Lip G.Y., Apostolakis S. The unmet need of stroke prevention in atrial fibrillation in the far East and South East Asia. Malays J Med Sci. 2012;19:1–7. [PMC free article] [PubMed] [Google Scholar]
- 18.Singer D.E., Chang Y., Borowsky L.H. A new risk scheme to predict ischemic stroke and other thromboembolism in atrial fibrillation: the ATRIA study stroke risk score. J Am Heart Assoc. 2013;2:e000250. doi: 10.1161/JAHA.113.000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connolly S.J., Ezekowitz M.D., Yusuf S. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 20.Patel M.R., Mahaffey K.W., Garg J. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 21.Granger C.B., Alexander J.H., McMurray J.J. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 22.Giugliano R.P., Ruff C.T., Braunwald E. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 23.Hori M., Connolly S.J., Zhu J. Dabigatran versus warfarin: effects on ischemic and hemorrhagic strokes and bleeding in Asians and Non-Asians with atrial fibrillation. Stroke. 2013;44:1891–1896. doi: 10.1161/STROKEAHA.113.000990. [DOI] [PubMed] [Google Scholar]
- 24.Wong K.S., Hu D.Y., Oomman A. Rivaroxaban for stroke prevention in East Asian patients from the ROCKET AF trial. Stroke. 2014;45:1739–1747. doi: 10.1161/STROKEAHA.113.002968. [DOI] [PubMed] [Google Scholar]
- 25.Yamashita T., Koretsune Y., Yang Y. Edoxaban vs. warfarin in East Asian patients with atrial fibrillation-an ENGAGE AF-TIMI 48 subanalysis. Circ J. 2016;80:860–869. doi: 10.1253/circj.CJ-15-1082. [DOI] [PubMed] [Google Scholar]
- 26.Goto S., Zhu J., Liu L. Efficacy and safety of apixaban compared with warfarin for stroke prevention in patients with atrial fibrillation from East Asia: a subanalysis of the apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) Trial. Am Heart J. 2014;168:303–309. doi: 10.1016/j.ahj.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Shen A.Y., Yao J.F., Brar S.S. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol. 2007;50:309–315. doi: 10.1016/j.jacc.2007.01.098. [DOI] [PubMed] [Google Scholar]
- 28.Gage B.F., Waterman A.D., Shannon W. Validation of clinical classification schemes for predicting stroke: results from the national registry of atrial fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 29.Olesen J.B., Lip G.Y., Hansen M.L. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124. doi: 10.1136/bmj.d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chao T.-F., Lin Y.-J., Tsao H.-M. CHADS2 and CHA2DS2-VASc scores in the prediction of clinical outcomes in patients with atrial fibrillation after catheter ablation. J Am Coll Cardiol. 2011;58:2380–2385. doi: 10.1016/j.jacc.2011.08.045. [DOI] [PubMed] [Google Scholar]
- 31.Potpara T.S., Polovina M.M., Licina M.M. Reliable identification of "truly low" thromboembolic risk in patients initially diagnosed with “lone” atrial fibrillation: the Belgrade atrial fibrillation study. Circ Arrhythm Electrophysiol. 2012;5:319–326. doi: 10.1161/CIRCEP.111.966713. [DOI] [PubMed] [Google Scholar]
- 32.Camm A.J., Lip G.Y., De Caterina R. focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the American College of Cardiology. Eur Heart J. 2012;2012(33):2719–2747. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 33.January C.T., Wann L.S., Alpert J.S. AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;2014(130):2071–2104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 34.Chao T.-F., Liu C.-J., Tuan T.-C. Comparisons of CHADS2 and CHA2DS2-VASc scores for stroke risk stratification in atrial fibrillation: which scoring system should be used for Asians? Heart Rhythm. 2016;13:46–53. doi: 10.1016/j.hrthm.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 35.Olesen J.B., Torp-Pedersen C., Hansen M.L. The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0-1: a nationwide cohort study. Thromb Haemost. 2012;107:1172–1179. doi: 10.1160/TH12-03-0175. [DOI] [PubMed] [Google Scholar]
- 36.Chao T.-F., Liu C.-J., Wang K.-L. Should atrial fibrillation patients with 1 additional risk factor of the CHA2DS2-VASc score (Beyond Sex) receive oral anticoagulation? J Am Coll Cardiol. 2015;65:635–642. doi: 10.1016/j.jacc.2014.11.046. [DOI] [PubMed] [Google Scholar]
- 37.Eckman M.H., Singer D.E., Rosand J. Moving the tipping point. Circ Cardiovasc Qual Outcomes. 2011;4:14–21. doi: 10.1161/CIRCOUTCOMES.110.958108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirchhof P., Benussi S., Kotecha D. ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;2016(18):1609–1678. doi: 10.1093/europace/euw295. [DOI] [PubMed] [Google Scholar]
- 39.Chiang C.-E., Wu T.-J., Ueng K.-C. Guidelines of the Taiwan Heart Rhythm Society and the Taiwan Society of Cardiology for the management of atrial fibrillation. J Formos Med Assoc. 2016;2016(115):893–952. doi: 10.1016/j.jfma.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 40.Chao T.-F., Wang K.-L., Liu C.-J. Age threshold for increased stroke risk among patients with atrial fibrillation: a nationwide cohort study From Taiwan. J Am Coll Cardiol. 2015;66:1339–1347. doi: 10.1016/j.jacc.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 41.Chan P.-H., Lau C.-P., Tse H.-F. CHA2DS2-VASc recalibration with an additional age category (50-64 Years) enhances stroke risk stratification in Chinese patients with atrial fibrillation. Can J Cardiol. 2016;32:1381–1387. doi: 10.1016/j.cjca.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 42.Chao T.F., Lip G.Y., Liu C.J. Validation of a Modified CHA2DS2-VASc score for stroke risk stratification in Asian patients with atrial fibrillation: a nationwide cohort study. Stroke. 2016;47:2462–2469. doi: 10.1161/STROKEAHA.116.013880. [DOI] [PubMed] [Google Scholar]
- 43.Gage B.F., Yan Y., Milligan P.E. Clinical classification schemes for predicting hemorrhage: results from the national registry of atrial fibrillation (NRAF) Am Heart J. 2006;151:713–719. doi: 10.1016/j.ahj.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 44.Pisters R., Lane D.A., Nieuwlaat R. A novel user-friendly score (HAS-BLED) To assess 1-year risk of major bleeding in patients with atrial fibrillation. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 45.Fang M.C., Go A.S., Chang Y. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) study. J Am Coll Cardiol. 2011;58:395–401. doi: 10.1016/j.jacc.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O׳Brien E.C., Simon D.N., Thomas L.E. The ORBIT bleeding score: a simple bedside score to assess bleeding risk in atrial fibrillation. Eur Heart J. 2015;36:3258–3264. doi: 10.1093/eurheartj/ehv476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hijazi Z., Oldgren J., Lindbäck J. The novel biomarker-based ABC (age, biomarkers, clinical history)-bleeding risk score for patients with atrial fibrillation: a derivation and validation study. Lancet. 2016;387:2302–2311. doi: 10.1016/S0140-6736(16)00741-8. [DOI] [PubMed] [Google Scholar]
- 48.Lip G.Y.H., Lane D.A. Bleeding risk assessment in atrial fibrillation: observations on the use and misuse of bleeding risk scores. J Thromb Haemost. 2016;14:1711–1714. doi: 10.1111/jth.13386. [DOI] [PubMed] [Google Scholar]
- 49.Friberg L., Rosenqvist M., Lip G.Y.H. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500–1510. doi: 10.1093/eurheartj/ehr488. [DOI] [PubMed] [Google Scholar]
- 50.Apostolakis S., Lane D.A., Guo Y. Performance of the HEMORR2HAGES, ATRIA, and HAS-BLED bleeding risk–prediction scores in patients with atrial fibrillation undergoing anticoagulation: the AMADEUS (Evaluating the use of SR34006 compared to warfarin or acenocoumarol in patients with atrial fibrillation) Study. J Am Coll Cardiol. 2012;60:861–867. doi: 10.1016/j.jacc.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 51.Proietti M., Senoo K., Lane D.A. Major bleeding in patients with non-valvular atrial fibrillation: impact of time in therapeutic range on contemporary bleeding risk scores. Sci Rep. 2016;6:24376. doi: 10.1038/srep24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Senoo K., Proietti M., Lane D.A. Evaluation of the HAS-BLED, ATRIA, and ORBIT bleeding risk scores in patients with atrial fibrillation taking warfarin. Am J Med. 2016;129:600–607. doi: 10.1016/j.amjmed.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 53.Naganuma M., Shiga T., Sato K. Clinical outcome in Japanese elderly patients with non-valvular atrial fibrillation taking warfarin: a single-center observational study. Thromb Res. 2012;130:21–26. doi: 10.1016/j.thromres.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 54.Sato H., Ishikawa K., Kitabatake A. Low-dose aspirin for prevention of stroke in low-risk patients with atrial fibrillation: japan atrial fibrillation stroke trial. Stroke. 2006;37:447–451. doi: 10.1161/01.STR.0000198839.61112.ee. [DOI] [PubMed] [Google Scholar]
- 55.Siu C.W., Lip G.Y., Lam K.F. Risk of stroke and intracranial hemorrhage in 9727 Chinese with atrial fibrillation in Hong Kong. Heart Rhythm. 2014;11:1401–1408. doi: 10.1016/j.hrthm.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 56.Lip G.Y.H., Skjøth F., Rasmussen L.H. Net clinical benefit for oral anticoagulation, aspirin, or no therapy in nonvalvular atrial fibrillation patients with 1 additional risk factor of the CHA2DS2-VASc score (Beyond Sex) J Am Coll Cardiol. 2015;66:488–490. doi: 10.1016/j.jacc.2015.05.044. [DOI] [PubMed] [Google Scholar]
- 57.Senoo K., Lau Y.C., Dzeshka M. Efficacy and safety of non-vitamin K antagonist oral anticoagulants vs. warfarin in Japanese patients with atrial fibrillation-meta-analysis. Circ J. 2015;79:339–345. doi: 10.1253/circj.CJ-14-1042. [DOI] [PubMed] [Google Scholar]
- 58.Lip G.Y., Wang K.L., Chiang C.E. Non-vitamin K antagonist oral anticoagulants (NOACs) for stroke prevention in Asian patients with atrial fibrillation: time for a reappraisal. Int J Cardiol. 2015;180:246–254. doi: 10.1016/j.ijcard.2014.11.182. [DOI] [PubMed] [Google Scholar]
- 59.Connolly S.J., Eikelboom J., Joyner C. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–817. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- 60.Guo Y., Pisters R., Apostolakis S. Stroke risk and suboptimal thromboprophylaxis in Chinese patients with atrial fibrillation: would the novel oral anticoagulants have an impact? Int J Cardiol. 2013;168:515–522. doi: 10.1016/j.ijcard.2012.09.187. [DOI] [PubMed] [Google Scholar]
- 61.Gamra H., Murin J., Chiang C.E. Use of antithrombotics in atrial fibrillation in Africa, Europe, Asia and South America: insights from the International RealiseAF Survey. Arch Cardiovasc Dis. 2014;107:77–87. doi: 10.1016/j.acvd.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 62.Amerena J., Chen S.A., Sriratanasathavorn C. Insights into management of atrial fibrillation in Asia Pacific gained from baseline data from REgistry on cardiac rhythm disORDers (RecordAF-Asia Pacific [AP]) registry. Am J Cardiol. 2012;109:378–382. doi: 10.1016/j.amjcard.2011.08.046. [DOI] [PubMed] [Google Scholar]
- 63.Chang C.H., Yang Y.H., Chen J.H. Cost-effectiveness of dabigatran etexilate for the prevention of stroke and systemic embolism in atrial fibrillation in Taiwan. Thromb Res. 2014;133:782–789. doi: 10.1016/j.thromres.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 64.Huisman M.V., Ma C.S., Diener H.-C. Antithrombotic therapy use in patients with atrial fibrillation before the era of non-vitamin K antagonist oral anticoagulants: the global registry on long-term oral antithrombotic treatment in patients with atrial fibrillation (GLORIA-AF)phase I cohort. Europace. 2016;18:1308–1318. doi: 10.1093/europace/euw073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chan E.W., Lau W.C.Y., Siu C.W. Effect of suboptimal anticoagulation treatment with antiplatelet therapy and warfarin on clinical outcomes in patients with nonvalvular atrial fibrillation: a population-wide cohort study. Heart Rhythm. 2016;13:1581–1588. doi: 10.1016/j.hrthm.2016.03.049. [DOI] [PubMed] [Google Scholar]
- 66.Oldgren J., Healey J.S., Ezekowitz M. Variations in cause and management of atrial fibrillation in a prospective registry of 15,400 emergency department patients in 46 countries: the RE-LY atrial fibrillation registry. Circulation. 2014;129:1568–1576. doi: 10.1161/CIRCULATIONAHA.113.005451. [DOI] [PubMed] [Google Scholar]
- 67.Yasaka M., Lip G.Y.H. Impact of non-vitamin K antagonist oral anticoagulants on intracranial bleeding in Asian patients with non-valvular atrial fibrillation. Circ J. 2014;78:2367–2372. doi: 10.1253/circj.cj-14-0720. [DOI] [PubMed] [Google Scholar]
- 68.Hart R.G., Pearce L.A., Aguilar M.I. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 69.Lin L.J., Cheng M.H., Lee C.H. Compliance with antithrombotic prescribing guidelines for patients with atrial fibrillation-A nationwide descriptive study in Taiwan. Clin Ther. 2008;30:1726–1736. doi: 10.1016/j.clinthera.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 70.Siu C.W., Tse H.F. Net clinical benefit of warfarin therapy in elderly Chinese patients with atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7:300–306. doi: 10.1161/CIRCEP.113.000858. [DOI] [PubMed] [Google Scholar]
- 71.Li S.Y., Zhao X.Q., Wang C.X. One-year clinical prediction in Chinese ischemic stroke patients using the CHADS2 and CHA2DS2-VASc scores: the China National Stroke Registry. CNS Neurosci Ther. 2012;18:988–993. doi: 10.1111/cns.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang L.-F., Yang J., Hong Z. Proportion of different subtypes of stroke in China. Stroke. 2003;34:2091–2096. doi: 10.1161/01.STR.0000087149.42294.8C. [DOI] [PubMed] [Google Scholar]
- 73.Huang C.Y., Chan F.L., Yu Y.L. Cerebrovascular disease in Hong Kong Chinese. Stroke. 1990;21:230–235. doi: 10.1161/01.str.21.2.230. [DOI] [PubMed] [Google Scholar]
- 74.Yang Q.D., Niu Q., Zhou Y.H. Incidence of cerebral hemorrhage in the Changsha community. Cerebrovasc Dis. 2004;17:303–313. doi: 10.1159/000077341. [DOI] [PubMed] [Google Scholar]
- 75.Wallentin L., Yusuf S., Ezekowitz M.D. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet. 2010;376:975–983. doi: 10.1016/S0140-6736(10)61194-4. [DOI] [PubMed] [Google Scholar]
- 76.Singer D.E., Hellkamp A.S., Piccini J.P. Impact of global geographic region on time in therapeutic range on warfarin anticoagulant therapy: data from the ROCKET AF clinical trial. J Am Heart Assoc. 2013;2:e000067. doi: 10.1161/JAHA.112.000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baber U., Howard V.J., Halperin J.L. Association of chronic kidney disease with atrial fibrillation among adults in the United States: reasons for geographic and racial differences in stroke (REGARDS) study. Circ Arrhythm Electrophysiol. 2011;4:26–32. doi: 10.1161/CIRCEP.110.957100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alonso A., Lopez F.L., Matsushita K. Chronic kidney disease is associated with the incidence of atrial fibrillation: the atherosclerosis risk in communities (ARIC) study. Circulation. 2011;123:2946–2953. doi: 10.1161/CIRCULATIONAHA.111.020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ananthapanyasut W., Napan S., Rudolph E.H. Prevalence of atrial fibrillation and its predictors in nondialysis patients with chronic kidney disease. Clin J Am Soci Nephrol. 2010;5:173–181. doi: 10.2215/CJN.03170509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Soliman E.Z., Prineas R.J., Go A.S. Chronic kidney disease and prevalent atrial fibrillation: the chronic renal insufficiency cohort (CRIC) Am Heart J. 2010;159:1102–1107. doi: 10.1016/j.ahj.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hart R.G., Pearce L.A., Asinger R.W. Warfarin in atrial fibrillation patients with moderate chronic kidney disease. Clin J Am Soci Nephrol. 2011;6:2599–2604. doi: 10.2215/CJN.02400311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abbott K.C., Trespalacios F.C., Taylor A.J. Atrial fibrillation in chronic dialysis patients in the United states: risk factors for hospitalization and mortality. BMC Nephrol. 2003;4:1. doi: 10.1186/1471-2369-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chan K.E., Lazarus J.M., Thadhani R. Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J Am Soc Nephrol. 2009;20:2223–2233. doi: 10.1681/ASN.2009030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Winkelmayer W.C., Liu J., Setoguchi S. Effectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillation. Clin J Am Soc Nephrol. 2011;6:2662–2668. doi: 10.2215/CJN.04550511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Elliott M.J., Zimmerman D., Holden R.M. Warfarin anticoagulation in hemodialysis patients: a systematic review of bleeding rates. Am J Kidney Dis. 2007;50:433–440. doi: 10.1053/j.ajkd.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 86.Biggers J.A., Remmers A.R., Jr, Glassford D.M. The risk of anticoagulation in hemodialysis patients. Nephron. 1977;18:109–113. doi: 10.1159/000180784. [DOI] [PubMed] [Google Scholar]
- 87.Wizemann V., Tong L., Satayathum S. Atrial fibrillation in hemodialysis patients: clinical features and associations with anticoagulant therapy. Kidney Int. 2010;77:1098–1106. doi: 10.1038/ki.2009.477. [DOI] [PubMed] [Google Scholar]
- 88.Tobe S.W., Clase C.M., Gao P. Cardiovascular and renal outcomes with telmisartan, ramipril, or both in people at high renal risk: results from the ONTARGET and TRANSCEND studies. Circulation. 2011;123:1098–1107. doi: 10.1161/CIRCULATIONAHA.110.964171. [DOI] [PubMed] [Google Scholar]
- 89.Shah M., Avgil Tsadok M., Jackevicius C.A. Warfarin use and the risk for stroke and bleeding in patients with atrial fibrillation undergoing dialysis. Circulation. 2014;129:1196–1203. doi: 10.1161/CIRCULATIONAHA.113.004777. [DOI] [PubMed] [Google Scholar]
- 90.Herzog C.A., Asinger R.W., Berger A.K. Cardiovascular disease in chronic kidney disease. A clinical update from kidney disease: improving global outcomes (KDIGO) Kidney Int. 2011;80:572–586. doi: 10.1038/ki.2011.223. [DOI] [PubMed] [Google Scholar]
- 91.Lai K.N., Yin J.A., Yuen P.M. Effect of hemodialysis on protein C, protein S, and antithrombin III levels. Am J Kidney Dis. 1991;17:38–42. doi: 10.1016/s0272-6386(12)80248-4. [DOI] [PubMed] [Google Scholar]
- 92.Kim S.B., Yang W.S., Lee S.K. Effect of increasing serum albumin on haemostatic factors synthesized in the liver in CAPD patients. Nephrol Dial Transplant. 1998;13:2053–2058. doi: 10.1093/ndt/13.8.2053. [DOI] [PubMed] [Google Scholar]
- 93.Clyne N., Egberg N., Lins L.-E. Effects of hemodialysis and longterm erythropoietin treatment on protein C, and on free and total protein S. Thromb Res. 1995;80:161–168. doi: 10.1016/0049-3848(95)00162-k. [DOI] [PubMed] [Google Scholar]
- 94.Lai K.N., Yin J.A., Yuen P.M. Protein C, protein S, and antithrombin III levels in patients on continuous ambulatory peritoneal dialysis and hemodialysis. Nephron. 1990;56:271–276. doi: 10.1159/000186153. [DOI] [PubMed] [Google Scholar]
- 95.Bertoli S.V., di Belgiojoso G.B., Trezzi M. Reduced blood levels of coagulation inhibitors in chronic hemodialysis compared with CAPD. Adv Perit Dial. 1995;11:127–130. [PubMed] [Google Scholar]
- 96.Friberg L., Benson L., Lip G.Y.H. Balancing stroke and bleeding risks in patients with atrial fibrillation and renal failure: the Swedish Atrial Fibrillation Cohort study. Eur Heart J. 2014;36:297–306. doi: 10.1093/eurheartj/ehu139. [DOI] [PubMed] [Google Scholar]
- 97.Chan P.H., Huang D., Yip P.S. Ischaemic stroke in patients with atrial fibrillation with chronic kidney disease undergoing peritoneal dialysis. Europace. 2016;18:665–671. doi: 10.1093/europace/euv289. [DOI] [PubMed] [Google Scholar]
- 98.Chan P.H., Siu C.W. Clinical benefit of warfarin in dialysis patients with atrial fibrillation. J Am Coll Cardiol. 2015;66:1310–1311. doi: 10.1016/j.jacc.2015.03.601. [DOI] [PubMed] [Google Scholar]
- 99.Ezekowitz M.D., Nagarakanti R., Noack H. Comparison of dabigatran and warfarin in patients with atrial fibrillation and valvular heart disease: the RE-LY trial (randomized evaluation of long-term anticoagulant therapy) Circulation. 2016;134:589–598. doi: 10.1161/CIRCULATIONAHA.115.020950. [DOI] [PubMed] [Google Scholar]
- 100.Avezum A., Lopes R.D., Schulte P.J. Apixaban in comparison with warfarin in patients with atrial fibrillation and valvular heart disease: findings from the apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) trial. Circulation. 2015;132:624–632. doi: 10.1161/CIRCULATIONAHA.114.014807. [DOI] [PubMed] [Google Scholar]
- 101.Breithardt G., Baumgartner H., Berkowitz S.D. Clinical characteristics and outcomes with rivaroxaban vs. warfarin in patients with non-valvular atrial fibrillation but underlying native mitral and aortic valve disease participating in the ROCKET AF trial. Eur Heart J. 2014;35:3377–3385. doi: 10.1093/eurheartj/ehu305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.De Caterina R., Renda G., Carnicelli A.P. Valvular heart disease patients on edoxaban or warfarin in the ENGAGE AF-TIMI 48 Trial. J Am Coll Cardiol. 2017;69:1372–1382. doi: 10.1016/j.jacc.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 103.Carnicelli A.P., De Caterina R., Halperin J.L. Edoxaban for the prevention of thromboembolism in patients with atrial fibrillation and bioprosthetic valves. Circulation. 2017;135:1273–1275. doi: 10.1161/CIRCULATIONAHA.116.026714. [DOI] [PubMed] [Google Scholar]
- 104.Renda G., Ricci F., Giugliano R.P. Non–Vitamin K antagonist oral anticoagulants in patients with atrial fibrillation and valvular heart disease. J Am Coll Cardiol. 2017;69:1363–1371. doi: 10.1016/j.jacc.2016.12.038. [DOI] [PubMed] [Google Scholar]
- 105.Eikelboom J.W., Connolly S.J., Brueckmann M. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206–1214. doi: 10.1056/NEJMoa1300615. [DOI] [PubMed] [Google Scholar]
- 106.Jaffer I.H., Stafford A.R., Fredenburgh J.C. Dabigatran is less effective than warfarin at attenuating mechanical heart valve-induced thrombin generation. J Am Heart Assoc. 2015;4:e002322. doi: 10.1161/JAHA.115.002322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Apostolakis S., Sullivan R.M., Olshansky B. Factors affecting quality of anticoagulation control among patients with atrial fibrillation on warfarin: the SAMe-TT2R2 score. Chest. 2013;144:1555–1563. doi: 10.1378/chest.13-0054. [DOI] [PubMed] [Google Scholar]
- 108.Larsen T.B., Lip G.Y.H. Warfarin or novel oral anticoagulants for atrial fibrillation? Lancet. 2014;383:931–933. doi: 10.1016/S0140-6736(13)62376-4. [DOI] [PubMed] [Google Scholar]
- 109.Chan P.H., Hai J.J., Chan E.W. Use of the SAMe-TT2R2 score to predict good anticoagulation control with warfarin in Chinese patients with atrial fibrillation: relationship to ischemic stroke incidence. PLoS One. 2016;11:e0150674. doi: 10.1371/journal.pone.0150674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bernaitis N., Ching C.K., Chen L. The sex, age, medical history, treatment, tobacco use, race risk (SAMe TT2R2) score predicts warfarin control in a Singaporean population. J Stroke Cerebrovasc Dis. 2017;26:64–69. doi: 10.1016/j.jstrokecerebrovasdis.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 111.Husted S., De Caterina R., Andreotti F. Non-vitamin K antagonist oral anticoagulants (NOACs): no longer new or novel. Thromb Haemost. 2014;111:781–782. doi: 10.1160/TH14-03-0228. [DOI] [PubMed] [Google Scholar]
- 112.Ruff C.T., Giugliano R.P., Braunwald E. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 113.Heidbuchel H., Verhamme P., Alings M. Updated European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2015;17:1467–1507. doi: 10.1093/europace/euv309. [DOI] [PubMed] [Google Scholar]
- 114.Eriksson B.I., Quinlan D.J., Weitz J.I. Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor xa inhibitors in development. Clin Pharmacokinet. 2009;48:1–22. doi: 10.2165/0003088-200948010-00001. [DOI] [PubMed] [Google Scholar]
- 115.Lip G.Y.H., Agnelli G. Edoxaban: a focused review of its clinical pharmacology. Eur Heart J. 2014;35:1844–1855. doi: 10.1093/eurheartj/ehu181. [DOI] [PubMed] [Google Scholar]
- 116.De Caterina R., Husted S., Wallentin L. New oral anticoagulants in atrial fibrillation and acute coronary syndromes: esc working group on thrombosis-task force on anticoagulants in heart disease position paper. J Am Coll Cardiol. 2012;59:1413–1425. doi: 10.1016/j.jacc.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 117.Shimada Y.J., Yamashita T., Koretsune Y. Effects of regional differences in asia on efficacy and safety of edoxaban compared with warfarin-insights from the ENGAGE AF-TIMI 48 trial. Circ J. 2015;79:2560–2567. doi: 10.1253/circj.CJ-15-0574. [DOI] [PubMed] [Google Scholar]
- 118.Wang K.L., Lip G.Y., Lin S.J. Non-Vitamin K antagonist oral anticoagulants for stroke prevention in asian patients with nonvalvular atrial fibrillation: meta-analysis. Stroke. 2015;46:2555–2561. doi: 10.1161/STROKEAHA.115.009947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Koretsune Y., Yamashita T., Yang Y. Edoxaban versus warfarin in East-Asian (Including Japanese) patients with atrial fibrillation―an ENGAGE AF-TIMI 48 Sub-analysis. Circ J. 2014 [78:I-484] [Google Scholar]
- 120.Hori M., Matsumoto M., Tanahashi N. Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation-the J-ROCKET AF study. Circ J. 2012;76:2104–2111. doi: 10.1253/circj.cj-12-0454. [DOI] [PubMed] [Google Scholar]
- 121.Wang K.-L., Giugliano R.P., Goto S. Standard dose versus low dose non–vitamin K antagonist oral anticoagulants in Asian patients with atrial fibrillation: a meta-analysis of contemporary randomized controlled trials. Heart Rhythm. 2016;13:2340–2347. doi: 10.1016/j.hrthm.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 122.Lip G.Y.H., Clemens A., Noack H. Patient outcomes using the European label for dabigatran. A post-hoc analysis from the RE-LY database. Thromb Haemost. 2014;111:933–942. doi: 10.1160/TH13-09-0734. [DOI] [PubMed] [Google Scholar]
- 123.Blackshear J.L., Odell J.A. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61:755–759. doi: 10.1016/0003-4975(95)00887-X. [DOI] [PubMed] [Google Scholar]
- 124.Whitlock R.P., Healey J.S., Connolly S.J. Left atrial appendage occlusion does not eliminate the need for warfarin. Circulation. 2009;120:1927–1932. doi: 10.1161/CIRCULATIONAHA.108.844779. [DOI] [PubMed] [Google Scholar]
- 125.Bayard Y.L., Omran H., Neuzil P. PLAATO (Percutaneous left atrial appendage transcatheter occlusion) for prevention of cardioembolic stroke in non-anticoagulation eligible atrial fibrillation patients: results from the European PLAATO study. EuroIntervention. 2010;6:220–226. [PubMed] [Google Scholar]
- 126.Holmes D.R., Reddy V.Y., Turi Z.G. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374:534–542. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]
- 127.Park J.W., Bethencourt A., Sievert H. Left atrial appendage closure with Amplatzer cardiac plug in atrial fibrillation: initial European experience. Catheter Cardiovasc Interv. 2011;77:700–706. doi: 10.1002/ccd.22764. [DOI] [PubMed] [Google Scholar]
- 128.Reddy V.Y., Möbius-Winkler S., Miller M.A. Left atrial appendage closure with the watchman device in patients with a contraindication for oral anticoagulation: the ASAP Study (ASA plavix feasibility study with watchman left atrial appendage closure technology) J Am Coll Cardiol. 2013;61:2551–2556. doi: 10.1016/j.jacc.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 129.Bartus K., Han F.T., Bednarek J. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol. 2013;62:108–118. doi: 10.1016/j.jacc.2012.06.046. [DOI] [PubMed] [Google Scholar]
- 130.Reddy V.Y., Doshi S.K., Sievert H. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3-year Follow-up of the PROTECT AF (Watchman left atrial appendage system for embolic protection in patients with atrial fibrillation) trial. Circulation. 2013;127:720–729. doi: 10.1161/CIRCULATIONAHA.112.114389. [DOI] [PubMed] [Google Scholar]
- 131.Reddy V.Y., Sievert H., Halperin J. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312:1988–1998. doi: 10.1001/jama.2014.15192. [DOI] [PubMed] [Google Scholar]
- 132.Holmes D.R., Jr, Kar S., Price M.J. Prospective randomized evaluation of the watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Colle Cardiol. 2014;64:1–12. doi: 10.1016/j.jacc.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 133.Nietlispach F., Gloekler S., Krause R. Amplatzer left atrial appendage occlusion: single center 10-year experience. Catheter Cardiovasc Interv. 2013;82:283–289. doi: 10.1002/ccd.24872. [DOI] [PubMed] [Google Scholar]
- 134.López Mínguez J.R., Nogales Asensio J.M., Gragera J.E. Two-year clinical outcome from the Iberian registry patients after left atrial appendage closure. Heart. 2015;101:877–883. doi: 10.1136/heartjnl-2014-306332. [DOI] [PubMed] [Google Scholar]
- 135.Dawson A.G., Asopa S., Dunning J. Should patients undergoing cardiac surgery with atrial fibrillation have left atrial appendage exclusion? Interact Cardiovasc Thorac Surg. 2010;10:306–311. doi: 10.1510/icvts.2009.227991. [DOI] [PubMed] [Google Scholar]
- 136.Kanderian A.S., Gillinov A.M., Pettersson G.B. Success of surgical left atrial appendage closure: assessment by transesophageal echocardiography. J Am Coll Cardiol. 2008;52:924–929. doi: 10.1016/j.jacc.2008.03.067. [DOI] [PubMed] [Google Scholar]
- 137.Healey J.S., Crystal E., Lamy A. Left atrial appendage occlusion study (LAAOS): results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. Am Heart J. 2005;150:288–293. doi: 10.1016/j.ahj.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 138.Whitlock R., Healey J., Vincent J. Rationale and design of the left atrial appendage occlusion study (LAAOS) III. Ann Cardiothorac Surg. 2014;3:45–54. doi: 10.3978/j.issn.2225-319X.2013.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Whitlock R.P., Vincent J., Blackall M.H. Left atrial appendage occlusion study II (LAAOS II) Can J Cardiol. 2013;29:1443–1447. doi: 10.1016/j.cjca.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 140.Fumoto H., Gillinov A.M., Ootaki Y. A novel device for left atrial appendage exclusion: the third-generation atrial exclusion device. J Thorac Cardiovasc Surg. 2008;136:1019–1027. doi: 10.1016/j.jtcvs.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 141.Ailawadi G., Gerdisch M.W., Harvey R.L. Exclusion of the left atrial appendage with a novel device: early results of a multicenter trial. J Thorac Cardiovasc Surg. 2011;142:1002–1009. doi: 10.1016/j.jtcvs.2011.07.052. [DOI] [PubMed] [Google Scholar]
- 142.Holbrook A.M., Pereira J.A., Labiris R. Systematic overview of warfarin and its drug and food interactions. Arch Intern Medi. 2005;165:1095–1106. doi: 10.1001/archinte.165.10.1095. [DOI] [PubMed] [Google Scholar]
- 143.Shameem R., Ansell J. Disadvantages of VKA and requirements for novel anticoagulants. Best Pract Res Clin Haematol. 2013;26:103–114. doi: 10.1016/j.beha.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 144.Fitzgerald J.L., Howes L.G. Drug Interactions of direct-acting oral anticoagulants. Drug Saf. 2016;39:841–845. doi: 10.1007/s40264-016-0443-8. [DOI] [PubMed] [Google Scholar]
- 145.Leite P.M., Martins M.A.P., Castilho R.O. Review on mechanisms and interactions in concomitant use of herbs and warfarin therapy. Biomed Pharmacother. 2016;83:14–21. doi: 10.1016/j.biopha.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 146.Levy J.H., Spyropoulos A.C., Samama C.M. Direct oral anticoagulants: new drugs and new concepts. JACC: Cardiovasc Interv. 2014;7:1333–1351. doi: 10.1016/j.jcin.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 147.Voukalis C., Lip G.Y.H., Shantsila E. Drug-drug interactions of non-vitamin K oral anticoagulants. Expert Opin Drug Metab Toxicol. 2016;12:1445–1461. doi: 10.1080/17425255.2016.1225037. [DOI] [PubMed] [Google Scholar]
- 148.Gnoth M.J., Buetehorn U., Muenster U. In vitro and in vivo P-glycoprotein transport characteristics of rivaroxaban. J Pharmacol Exp Ther. 2011;338:372–380. doi: 10.1124/jpet.111.180240. [DOI] [PubMed] [Google Scholar]
- 149.Stöllberger C., Finsterer J. Relevance of P-glycoprotein in stroke prevention with dabigatran, rivaroxaban, and apixaban. Herz. 2015;40:140–145. doi: 10.1007/s00059-014-4188-9. [DOI] [PubMed] [Google Scholar]
- 150.Mueck W., Kubitza D., Becka M. Co-administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol. 2013;76:455–466. doi: 10.1111/bcp.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Denisov I.G., Grinkova Y.V., Baylon J.L. Mechanism of drug–drug interactions mediated by human cytochrome P450 CYP3A4 monomer. Biochemistry. 2015;54:2227–2239. doi: 10.1021/acs.biochem.5b00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liesenfeld K.H., Lehr T., Dansirikul C. Population pharmacokinetic analysis of the oral thrombin inhibitor dabigatran etexilate in patients with non-valvular atrial fibrillation from the RE-LY trial. J Thromb Haemost. 2011;9:2168–2175. doi: 10.1111/j.1538-7836.2011.04498.x. [DOI] [PubMed] [Google Scholar]
- 153.Härtter S., Sennewald R., Nehmiz G. Oral bioavailability of dabigatran etexilate (Pradaxa®) after co-medication with verapamil in healthy subjects. Br J Clin Pharmacol. 2013;75:1053–1062. doi: 10.1111/j.1365-2125.2012.04453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Frost C.E., Byon W., Song Y. Effect of ketoconazole and diltiazem on the pharmacokinetics of apixaban, an oral direct factor Xa inhibitor. Br J Clin Pharmacol. 2015;79:838–846. doi: 10.1111/bcp.12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Delavenne X., Ollier E., Basset T. A semi-mechanistic absorption model to evaluate drug–drug interaction with dabigatran: application with clarithromycin. Br J Clin Pharmacol. 2013;76:107–113. doi: 10.1111/bcp.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fralick M., Juurlink D.N., Marras T. Bleeding associated with coadministration of rivaroxaban and clarithromycin. CMAJ. 2016;188:669–672. doi: 10.1503/cmaj.150580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mendell J., Chen S., He L. The Effect of Rifampin on the Pharmacokinetics of Edoxaban in Healthy Adults. Clin Drug Investig. 2015;35:447–453. doi: 10.1007/s40261-015-0298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Frost C., Shenker A., Gandhi M.D. Evaluation of the effect of naproxen on the pharmacokinetics and pharmacodynamics of apixaban. Br J Clin Pharmacol. 2014;78:877–885. doi: 10.1111/bcp.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kubitza D., Becka M., Mueck W. Rivaroxaban (BAY 59-7939) – an oral, direct Factor Xa inhibitor – has no clinically relevant interaction with naproxen. Br J Clin Pharmacol. 2007;63:469–476. doi: 10.1111/j.1365-2125.2006.02776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mendell J., Lee F., Chen S. The effects of the antiplatelet agents, aspirin and naproxen, on pharmacokinetics and pharmacodynamics of the anticoagulant edoxaban, a direct factor Xa inhibitor. J Cardiovasc Pharmacol. 2013;62:212–221. doi: 10.1097/FJC.0b013e3182970991. [DOI] [PubMed] [Google Scholar]
- 161.Stangier J., Eriksson B.I., Dahl O.E. Pharmacokinetic profile of the oral direct thrombin inhibitor dabigatran etexilate in healthy volunteers and patients undergoing total hip replacement. J Clin Pharmacol. 2005;45:555–565. doi: 10.1177/0091270005274550. [DOI] [PubMed] [Google Scholar]
- 162.Song Y., Chang M., Suzuki A. Evaluation of crushed tablet for oral administration and the effect of food on apixaban pharmacokinetics in healthy adults. Clin Ther. 2016;38:1674–1685. doi: 10.1016/j.clinthera.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 163.Patel M.R., Hellkamp A.S., Lokhnygina Y. Outcomes of discontinuing rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: analysis from the ROCKET AF trial (Rivaroxaban once-daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation) J Am Coll Cardiol. 2013;61:651–658. doi: 10.1016/j.jacc.2012.09.057. [DOI] [PubMed] [Google Scholar]
- 164.Eikelboom J.W., Vanassche T., Connolly S.J. Switching patients from blinded study drug to warfarin at the end of the ENGAGE AF–TIMI 48 trial: setting a new standard. J Am Coll Cardiol. 2014;64:585–587. doi: 10.1016/j.jacc.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 165.Beyer-Westendorf J., Gelbricht V., Förster K. Safety of switching from vitamin K antagonists to dabigatran or rivaroxaban in daily care – results from the Dresden NOAC registry. Br J Clin Pharmacol. 2014;78:908–917. doi: 10.1111/bcp.12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.McManus D.D., Corteville D.C.M., Shlipak M.G. Relation of kidney function and albuminuria with atrial fibrillation (from the heart and soul study) Am J Cardiol. 2009;104:1551–1555. doi: 10.1016/j.amjcard.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Winkelmayer W.C., Liu J., Patrick A.R. Prevalence of atrial fibrillation and warfarin use in older patients receiving hemodialysis. J Nephrol. 2012;25:341–353. doi: 10.5301/jn.5000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Liao J.N., Chao T.F., Liu C.J. Incidence and risk factors for new-onset atrial fibrillation among patients with end-stage renal disease undergoing renal replacement therapy. Kidney Int. 2015;87:1209–1215. doi: 10.1038/ki.2014.393. [DOI] [PubMed] [Google Scholar]
- 169.Winkelmayer W.C., Turakhia M.P. Warfarin treatment in patients with atrial fibrillation and advanced chronic kidney disease: sins of omission or commission? JAMA. 2014;311:913–915. doi: 10.1001/jama.2014.1781. [DOI] [PubMed] [Google Scholar]
- 170.Bansal N., Fan D., Hsu C.Y. Incident atrial fibrillation and risk of end-stage renal disease in adults with chronic kidney disease. Circulation. 2013;127:569–574. doi: 10.1161/CIRCULATIONAHA.112.123992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cockcroft D.W., Gault M.H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 172.Olesen J.B., Lip G.Y.H., Kamper A.-L. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012;367:625–635. doi: 10.1056/NEJMoa1105594. [DOI] [PubMed] [Google Scholar]
- 173.Apostolakis S., Guo Y., Lane D.A. Renal function and outcomes in anticoagulated patients with non-valvular atrial fibrillation: the AMADEUS trial. Eur Heart J. 2013;34:3572–3579. doi: 10.1093/eurheartj/eht328. [DOI] [PubMed] [Google Scholar]
- 174.Camm A.J., Savelieva I. “R” for “renal” and for “risk”: refining risk stratification for stroke in atrial fibrillation. Circulation. 2013;127:169–171. doi: 10.1161/CIRCULATIONAHA.112.147512. [DOI] [PubMed] [Google Scholar]
- 175.Banerjee A., Fauchier L., Vourc׳h P. Renal impairment and ischemic stroke risk assessment in patients with atrial fibrillation: the loire valley atrial fibrillation project. J Am Coll Cardiol. 2013;61:2079–2087. doi: 10.1016/j.jacc.2013.02.035. [DOI] [PubMed] [Google Scholar]
- 176.Nakayama M., Metoki H., Terawaki H. Kidney dysfunction as a risk factor for first symptomatic stroke events in a general Japanese population—the Ohasama study. Nephrol Dial Transplant. 2007;22:1910–1915. doi: 10.1093/ndt/gfm051. [DOI] [PubMed] [Google Scholar]
- 177.Wiesholzer M., Harm F., Tomasec G. Incidence of stroke among chronic hemodialysis patients with nonrheumatic atrial fibrillation. Am J Nephrol. 2001;21:35–39. doi: 10.1159/000046216. [DOI] [PubMed] [Google Scholar]
- 178.Shih C.J., Ou S.M., Chao P.W. Risks of death and stroke in patients undergoing hemodialysis with new-onset atrial fibrillation: a competing-risk analysis of a nationwide cohort. Circulation. 2016;133:265–272. doi: 10.1161/CIRCULATIONAHA.115.018294. [DOI] [PubMed] [Google Scholar]
- 179.Bonde A.N., Lip G.Y.H., Kamper A.-L. Net clinical benefit of antithrombotic therapy in patients with atrial fibrillation and chronic kidney disease: a nationwide observational cohort study. J Am Coll Cardiol. 2014;64:2471–2482. doi: 10.1016/j.jacc.2014.09.051. [DOI] [PubMed] [Google Scholar]
- 180.Providência R., Marijon E., Boveda S. Meta-analysis of the influence of chronic kidney disease on the risk of thromboembolism among patients with nonvalvular atrial fibrillation. Am J Cardiol. 2014;114:646–653. doi: 10.1016/j.amjcard.2014.05.048. [DOI] [PubMed] [Google Scholar]
- 181.Carrero J., Evans M., Szummer K. Warfarin, kidney dysfunction, and outcomes following acute myocardial infarction in patients with atrial fibrillation. JAMA. 2014;311:919–928. doi: 10.1001/jama.2014.1334. [DOI] [PubMed] [Google Scholar]
- 182.Chan K.E., Lazarus J.M., Thadhani R. Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J Am Soc Nephrol. 2009;20:2223–2233. doi: 10.1681/ASN.2009030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Garg L., Chen C., Haines D.E. Atrial fibrillation and chronic kidney disease requiring hemodialysis — does warfarin therapy improve the risks of this lethal combination? Int J Cardiol. 2016;222:47–50. doi: 10.1016/j.ijcard.2016.07.118. [DOI] [PubMed] [Google Scholar]
- 184.Shen J.I., Montez-Rath M.E., Lenihan C.R. Outcomes after warfarin initiation in a cohort of hemodialysis patients with newly diagnosed atrial fibrillation. Am J Kidney Dis. 2015;66:677–688. doi: 10.1053/j.ajkd.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Macle L., Cairns J.A., Andrade J.G. The 2014 atrial fibrillation guidelines companion: a practical approach to the use of the Canadian cardiovascular society guidelines. Can J Cardiol. 2015;31:1207–1218. doi: 10.1016/j.cjca.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 186.Witt C.T., Healey J.S. Oral anticoagulant use in patients with chronic kidney disease: how to choose, and the importance of empiric human data. Can J Cardiol. 2014;30:853–854. doi: 10.1016/j.cjca.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 187.Dahal K., Kunwar S., Rijal J. Stroke, major bleeding, and mortality outcomes in warfarin users with atrial fibrillation and chronic kidney disease: a meta-analysis of observational studies. Chest. 2016;149:951–959. doi: 10.1378/chest.15-1719. [DOI] [PubMed] [Google Scholar]
- 188.Hijazi Z., Hohnloser S.H., Oldgren J. Efficacy and safety of dabigatran compared with warfarin in relation to baseline renal function in patients with atrial fibrillation: a RE-LY (Randomized evaluation of long-term anticoagulation therapy) trial analysis. Circulation. 2014;129:961–970. doi: 10.1161/CIRCULATIONAHA.113.003628. [DOI] [PubMed] [Google Scholar]
- 189.Hohnloser S.H., Hijazi Z., Thomas L. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J. 2012;33:2821–2830. doi: 10.1093/eurheartj/ehs274. [DOI] [PubMed] [Google Scholar]
- 190.Fox K.A.A., Piccini J.P., Wojdyla D. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J. 2011;32:2387–2394. doi: 10.1093/eurheartj/ehr342. [DOI] [PubMed] [Google Scholar]
- 191.Bohula E.A., Giugliano R.P., Ruff C.T. Impact of renal function on outcomes with edoxaban in the ENGAGE AF-TIMI 48 trial. Circulation. 2016;134:24–36. doi: 10.1161/CIRCULATIONAHA.116.022361. [DOI] [PubMed] [Google Scholar]
- 192.Sardar P., Chatterjee S., Herzog E. Novel oral anticoagulants in patients with renal insufficiency: a meta-analysis of randomized trials. Can J Cardiol. 2014;30:888–897. doi: 10.1016/j.cjca.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 193.Nielsen P.B., Lane D.A., Rasmussen L.H. Renal function and non-vitamin K oral anticoagulants in comparison with warfarin on safety and efficacy outcomes in atrial fibrillation patients: a systemic review and meta-regression analysis. Clin Res Cardiol. 2015;104:418–429. doi: 10.1007/s00392-014-0797-9. [DOI] [PubMed] [Google Scholar]
- 194.Chan K.E., Giugliano R.P., Patel M.R. Nonvitamin K anticoagulant agents in patients with advanced chronic kidney disease or on dialysis with AF. J Am Coll Cardiol. 2016;67:2888–2899. doi: 10.1016/j.jacc.2016.02.082. [DOI] [PubMed] [Google Scholar]
- 195.Lip G.Y., Windecker S., Huber K. Management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous coronary or valve interventions: a joint consensus document of the European Society of Cardiology Working Group on Thrombosis, European Heart Rhythm Association (EHRA), European Association of Percutaneous Cardiovascular Interventions (EAPCI) and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS) and Asia-Pacific Heart Rhythm Society (APHRS) Eur Heart J. 2014;35:3155–3179. doi: 10.1093/eurheartj/ehu298. [DOI] [PubMed] [Google Scholar]
- 196.Gibson C.M., Mehran R., Bode C. Prevention of Bleeding in Patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375:2423–2434. doi: 10.1056/NEJMoa1611594. [DOI] [PubMed] [Google Scholar]
- 197.Bhatt D.L.O. PIONEERs! the beginning of the end of full-dose triple therapy with warfarin? Circulation. 2017;135:334–337. doi: 10.1161/CIRCULATIONAHA.116.025923. [DOI] [PubMed] [Google Scholar]
- 198.Atrial Fibrillation Investigators Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154:1449–1457. [PubMed] [Google Scholar]
- 199.Stroke Risk in Atrial Fibrillation Working Group Independent predictors of stroke in patients with atrial fibrillation: a systematic review. Neurology. 2007;69:546–554. doi: 10.1212/01.wnl.0000267275.68538.8d. [DOI] [PubMed] [Google Scholar]
- 200.Diener H.-C., Connolly S.J., Ezekowitz M.D. Dabigatran compared with warfarin in patients with atrial fibrillation and previous transient ischaemic attack or stroke: a subgroup analysis of the RE-LY trial. Lancet Neurol. 2010;9:1157–1163. doi: 10.1016/S1474-4422(10)70274-X. [DOI] [PubMed] [Google Scholar]
- 201.Hankey G.J., Patel M.R., Stevens S.R. Rivaroxaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of ROCKET AF. Lancet Neurol. 2012;11:315–322. doi: 10.1016/S1474-4422(12)70042-X. [DOI] [PubMed] [Google Scholar]
- 202.Easton J.D., Lopes R.D., Bahit M.C. Apixaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of the ARISTOTLE trial. Lancet Neurol. 2012;11:503–511. doi: 10.1016/S1474-4422(12)70092-3. [DOI] [PubMed] [Google Scholar]
- 203.Rost N.S., Giugliano R.P., Ruff C.T. Outcomes with edoxaban versus warfarin in patients with previous cerebrovascular events. Findings from ENGAGE AF-TIMI 48 (effective anticoagulation with factor Xa next generation in atrial fibrillation-thrombolysis in myocardial infarction 48) Stroke. 2016;47:2075–2082. doi: 10.1161/STROKEAHA.116.013540. [DOI] [PubMed] [Google Scholar]
- 204.Hart R.G., Diener H.C., Yang S. Intracranial hemorrhage in atrial fibrillation patients during anticoagulation with warfarin or dabigatran: the RE-LY trial. Stroke. 2012;43:1511–1517. doi: 10.1161/STROKEAHA.112.650614. [DOI] [PubMed] [Google Scholar]
- 205.Hankey G.J., Stevens S.R., Piccini J.P. Intracranial hemorrhage among patients with atrial fibrillation anticoagulated with warfarin or rivaroxaban: the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation. Stroke. 2014;45:1304–1312. doi: 10.1161/STROKEAHA.113.004506. [DOI] [PubMed] [Google Scholar]
- 206.Giles M.F., Rothwell P.M. Risk of stroke early after transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol. 2007;6:1063–1072. doi: 10.1016/S1474-4422(07)70274-0. [DOI] [PubMed] [Google Scholar]
- 207.Nielsen P.B., Larsen T.B., Skjøth F. Restarting anticoagulant treatment after intracranial hemorrhage in patients with atrial fibrillation and the impact on recurrent stroke, mortality, and bleeding: a nationwide cohort study. Circulation. 2015;132:517–525. doi: 10.1161/CIRCULATIONAHA.115.015735. [DOI] [PubMed] [Google Scholar]
- 208.Kuramatsu J.B., Gerner S.T., Schellinger P.D. ANticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA. 2015;313:824–836. doi: 10.1001/jama.2015.0846. [DOI] [PubMed] [Google Scholar]
- 209.Chao T.F., Liu C.J., Liao J.N. Use of oral anticoagulants for stroke prevention in patients with atrial fibrillation who have a history of intracranial hemorrhage. Circulation. 2016;133:1540–1547. doi: 10.1161/CIRCULATIONAHA.115.019794. [DOI] [PubMed] [Google Scholar]
- 210.Tafur A., Douketis J.D. Perioperative anticoagulant management in patients with atrial fibrillation: practical implications of recent clinical trials. Pol Arch Med Wewn. 2015;125:666–671. doi: 10.20452/pamw.3049. [DOI] [PubMed] [Google Scholar]
- 211.Kannan A., Poongkunran C., Shenoy S. Perioperative management of anticoagulation-review of latest evidence. Am J Ther. 2016;23:e474–e484. doi: 10.1097/MJT.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 212.Mar P.L., Familtsev D., Ezekowitz M.D. Periprocedural management of anticoagulation in patients taking novel oral anticoagulants: review of the literature and recommendations for specific populations and procedures. Int J Cardiol. 2016;202:578–585. doi: 10.1016/j.ijcard.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 213.Spyropoulos A.C., Al-Badri A., Sherwood M.W. Periprocedural management of patients receiving a vitamin K antagonist or a direct oral anticoagulant requiring an elective procedure or surgery. J Thromb Haemost. 2016;14:875–885. doi: 10.1111/jth.13305. [DOI] [PubMed] [Google Scholar]
- 214.Oprea A.D., Noto C.J., Halaszynski T.M. Risk stratification, perioperative and periprocedural management of the patient receiving anticoagulant therapy. J Clin Anesth. 2016;34:586–599. doi: 10.1016/j.jclinane.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 215.Tafur A.J., McBane R., Wysokinski W.E. Predictors of major bleeding in peri-procedural anticoagulation management. J Thromb Haemost. 2012;10:261–267. doi: 10.1111/j.1538-7836.2011.04572.x. [DOI] [PubMed] [Google Scholar]
- 216.Douketis J.D., Spyropoulos A.C., Spencer F.A. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141:e326S–e3250. doi: 10.1378/chest.11-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Tran H., Joseph J., Young L. New oral anticoagulants: a practical guide on prescription, laboratory testing and peri-procedural/bleeding management. Intern Med J. 2014;44:525–536. doi: 10.1111/imj.12448. [DOI] [PubMed] [Google Scholar]
- 218.Kubitza D., Becka M., Wensing G. Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939—an oral, direct Factor Xa inhibitor—after multiple dosing in healthy male subjects. Eur J Clin Pharmacol. 2005;61:873–880. doi: 10.1007/s00228-005-0043-5. [DOI] [PubMed] [Google Scholar]
- 219.Levy J.H., Faraoni D., Spring J.L. Managing new oral anticoagulants in the perioperative and intensive care unit setting. Anesthesiology. 2013;118:1466–1474. doi: 10.1097/ALN.0b013e318289bcba. [DOI] [PubMed] [Google Scholar]
- 220.Spyropoulos A.C., Douketis J.D., Gerotziafas G. Periprocedural antithrombotic and bridging therapy: recommendations for standardized reporting in patients with arterial indications for chronic oral anticoagulant therapy. J Thromb Haemost. 2012;10:692–694. doi: 10.1111/j.1538-7836.2012.04630.x. [DOI] [PubMed] [Google Scholar]
- 221.Stangier J., Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost. 2009;15(Suppl 1):9S–16S. doi: 10.1177/1076029609343004. [DOI] [PubMed] [Google Scholar]
- 222.Spyropoulos A.C., Douketis J.D. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood. 2012;120:2954–2962. doi: 10.1182/blood-2012-06-415943. [DOI] [PubMed] [Google Scholar]
- 223.Tseng E., Crowther M.A., Hillis C.M. Bridging anticoagulation for interruption of warfarin in a patient with atrial fibrillation. CMAJ. 2016;188:361–362. doi: 10.1503/cmaj.150867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Douketis J.D., Spyropoulos A.C., Kaatz S. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823–833. doi: 10.1056/NEJMoa1501035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ayoub K., Nairooz R., Almomani A. Perioperative heparin bridging in atrial fibrillation patients requiring temporary interruption of anticoagulation: evidence from meta-analysis. J Stroke Cerebrovasc Dis. 2016;25:2215–2221. doi: 10.1016/j.jstrokecerebrovasdis.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 226.Healey J.S., Eikelboom J., Douketis J. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the randomized evaluation of long-term anticoagulation therapy (RE-LY) randomized trial. Circulation. 2012;126:343–348. doi: 10.1161/CIRCULATIONAHA.111.090464. [DOI] [PubMed] [Google Scholar]
- 227.Sherwood M.W., Douketis J.D., Patel M.R. Outcomes of temporary interruption of rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: results from the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET AF) Circulation. 2014;129:1850–1859. doi: 10.1161/CIRCULATIONAHA.113.005754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Garcia D., Alexander J.H., Wallentin L. Management and clinical outcomes in patients treated with apixaban vs warfarin undergoing procedures. Blood. 2014;124:3692–3698. doi: 10.1182/blood-2014-08-595496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Schulman S., Carrier M., Lee A.Y.Y. Perioperative management of dabigatran: a prospective cohort study. Circulation. 2015;132:167–173. doi: 10.1161/CIRCULATIONAHA.115.015688. [DOI] [PubMed] [Google Scholar]
- 230.Beyer-Westendorf J., Gelbricht V., Förster K. Peri-interventional management of novel oral anticoagulants in daily care: results from the prospective Dresden NOAC registry. Eur Heart J. 2014;35:1888–1896. doi: 10.1093/eurheartj/eht557. [DOI] [PubMed] [Google Scholar]
- 231.Pollack C.V., Reilly P.A., Eikelboom J. Idarucizumab for dabigatran reversal. N Engl J Med. 2015;373:511–520. doi: 10.1056/NEJMoa1502000. [DOI] [PubMed] [Google Scholar]
- 232.Daniels P.R. Peri-procedural management of patients taking oral anticoagulants. BMJ. 2015;351:h2391. doi: 10.1136/bmj.h2391. [DOI] [PubMed] [Google Scholar]
- 233.Crowther M., Crowther M.A. Antidotes for novel oral anticoagulants. current status and future potential. Arterioscler Thromb Vasc Biol. 2015;35:1736–1745. doi: 10.1161/ATVBAHA.114.303402. [DOI] [PubMed] [Google Scholar]
- 234.Heidbuchel H., Verhamme P., Alings M. European Heart Rhythm Association practical guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2013;15:625–651. doi: 10.1093/europace/eut083. [DOI] [PubMed] [Google Scholar]
- 235.Airaksinen K.E.J., Grönberg T., Nuotio I. Thromboembolic complications after cardioversion of acute atrial fibrillation: the FinCV (Finnish CardioVersion) study. J Am Coll Cardiol. 2013;62:1187–1192. doi: 10.1016/j.jacc.2013.04.089. [DOI] [PubMed] [Google Scholar]
- 236.Gentile F., Elhendy A., Khandheria B.K. Safety of electrical cardioversion in patients with atrial fibrillation. Mayo Clin Proc. 2002;77:897–904. doi: 10.4065/77.9.897. [DOI] [PubMed] [Google Scholar]
- 237.Berger M., Schweitzer P. Timing of thromboembolic events after electrical cardioversion of atrial fibrillation or flutter: a retrospective analysis. Am J Cardiol. 1998;82:1545–1547. doi: 10.1016/s0002-9149(98)00704-8. [DOI] [PubMed] [Google Scholar]
- 238.Gallagher M.M., Hennessy B.J., Edvardsson N. Embolic complications of direct current cardioversion of atrial arrhythmias: association with low intensity of anticoagulation at the time of cardioversion. J Am Coll Cardiol. 2002;40:926–933. doi: 10.1016/s0735-1097(02)02052-1. [DOI] [PubMed] [Google Scholar]
- 239.Seidl K., Rameken M., Drögemüller A. Embolic events in patients with atrial fibrillation and effective anticoagulation: value of transesophageal echocardiography to guide direct-current cardioversion: final results of the Ludwigshafen observational cardioversion study. J Am Coll Cardiol. 2002;39:1436–1442. doi: 10.1016/s0735-1097(02)01785-0. [DOI] [PubMed] [Google Scholar]
- 240.Nagarakanti R., Ezekowitz M.D., Oldgren J. Dabigatran versus warfarin in patients with atrial fibrillation: an analysis of patients undergoing cardioversion. Circulation. 2011;123:131–136. doi: 10.1161/CIRCULATIONAHA.110.977546. [DOI] [PubMed] [Google Scholar]
- 241.Piccini J.P., Stevens S.R., Lokhnygina Y. Outcomes after cardioversion and atrial fibrillation ablation in patients treated with rivaroxaban and warfarin in the ROCKET AF trial. J Am Coll Cardiol. 2013;61:1998–2006. doi: 10.1016/j.jacc.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 242.Flaker G., Lopes R.D., Al-Khatib S.M. Efficacy and safety of apixaban in patients after cardioversion for atrial fibrillation: insights from the ARISTOTLE trial (apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation) J Am Coll Cardiol. 2014;63:1082–1087. doi: 10.1016/j.jacc.2013.09.062. [DOI] [PubMed] [Google Scholar]
- 243.Cappato R., Ezekowitz M.D., Klein A.L. Rivaroxaban vs. vitamin K antagonists for cardioversion in atrial fibrillation. Eur Heart J. 2014;35:3346–3355. doi: 10.1093/eurheartj/ehu367. [DOI] [PubMed] [Google Scholar]
- 244.Goette A., Merino J.L., Ezekowitz M.D. Edoxaban versus enoxaparin–warfarin in patients undergoing cardioversion of atrial fibrillation (ENSURE-AF): a randomised, open-label, phase 3b trial. Lancet. 2016;388:1995–2003. doi: 10.1016/S0140-6736(16)31474-X. [DOI] [PubMed] [Google Scholar]
- 245.Klein A.L., Grimm R.A., Murray R.D. Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N Engl J Med. 2001;344:1411–1420. doi: 10.1056/NEJM200105103441901. [DOI] [PubMed] [Google Scholar]
- 246.Weigner M.J., Thomas L.R., Patel U. Early cardioversion of atrial fibrillation facilitated by transesophageal echocardiography: short-term safety and impact on maintenance of sinus rhythm at 1 year. Am J Med. 2001;110:694–702. doi: 10.1016/s0002-9343(01)00716-1. [DOI] [PubMed] [Google Scholar]
- 247.Wu L.A., Chandrasekaran K., Friedman P.A. Safety of expedited anticoagulation in patients undergoing transesophageal echocardiographic-guided cardioversion. Am J Med. 2006;119:142–146. doi: 10.1016/j.amjmed.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 248.Jaber W.A., Prior D.L., Thamilarasan M. Efficacy of anticoagulation in resolving left atrial and left atrial appendage thrombi: a transesophageal echocardiographic study. Am Heart J. 2000;140:150–156. doi: 10.1067/mhj.2000.106648. [DOI] [PubMed] [Google Scholar]
- 249.Weigner M.J., Caulfield T.A., Danias P.G. RIsk for clinical thromboembolism associated with conversion to sinus rhythm in patients with atrial fibrillation lasting less than 48 hours. Ann Intern Med. 1997;126:615–620. doi: 10.7326/0003-4819-126-8-199704150-00005. [DOI] [PubMed] [Google Scholar]
- 250.Sticherling C., Marin F., Birnie D. Antithrombotic management in patients undergoing electrophysiological procedures: a European Heart Rhythm Association (EHRA) position document endorsed by the ESC working group thrombosis, heart rhythm society (HRS), and Asia Pacific heart rhythm society (APHRS) Europace. 2015;17:1197–1214. doi: 10.1093/europace/euv190. [DOI] [PubMed] [Google Scholar]
- 251.Di Biase L., Burkhardt J.D., Mohanty P. Periprocedural stroke and management of major bleeding complications in patients undergoing catheter ablation of atrial fibrillation. Impact Periprocedural Ther Int Norm Ratio Circ. 2010;121:2550–2556. doi: 10.1161/CIRCULATIONAHA.109.921320. [DOI] [PubMed] [Google Scholar]
- 252.Santangeli P., Di Biase L., Horton R. Ablation of atrial fibrillation under therapeutic warfarin reduces periprocedural complications: evidence from a meta-analysis. Circ Arrhythm Electrophysiol. 2012;5:302–311. doi: 10.1161/CIRCEP.111.964916. [DOI] [PubMed] [Google Scholar]
- 253.Kim J.-S., She F., Jongnarangsin K. Dabigatran vs warfarin for radiofrequency catheter ablation of atrial fibrillation. Heart Rhythm. 2013;10:483–489. doi: 10.1016/j.hrthm.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 254.Bassiouny M., Saliba W., Rickard J. Use of dabigatran for periprocedural anticoagulation in patients undergoing catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol. 2013;6:460–466. doi: 10.1161/CIRCEP.113.000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Wazni O.M., Beheiry S., Fahmy T. Atrial fibrillation ablation in patients with therapeutic international normalized ratio. Comp Strateg Anticoagulation Manag Periprocedural Period Circ. 2007;116:2531–2534. doi: 10.1161/CIRCULATIONAHA.107.727784. [DOI] [PubMed] [Google Scholar]
- 256.Schmidt M., Segerson N.M., Marschang H. Atrial fibrillation ablation in patients with therapeutic international normalized ratios. Pacing Clin Electrophysiol. 2009;32:995–999. doi: 10.1111/j.1540-8159.2009.02429.x. [DOI] [PubMed] [Google Scholar]
- 257.Cappato R., Marchlinski F.E., Hohnloser S.H. Uninterrupted rivaroxaban vs. uninterrupted vitamin K antagonists for catheter ablation in non-valvular atrial fibrillation. Eur Heart J. 2015;36:1805–1811. doi: 10.1093/eurheartj/ehv177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Calkins H., Willems S., Gerstenfeld E.P. Uninterrupted dabigatran versus warfarin for ablation in atrial fibrillation. N Engl J Med. 2017;376:1627–1636. doi: 10.1056/NEJMoa1701005. [DOI] [PubMed] [Google Scholar]
- 259.Calkins H., Kuck K.H., Cappato R. 2012 h/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the heart rhythm society (HRS) task force on catheter and surgical ablation of atrial fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and theHeart Rhythm Society. Heart Rhythm. 2012;9:632–696. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 260.Frühauf M., Eitel C., Bollmann A. Should transesophageal echocardiography be done in all patients who underwent catheter ablation of atrial fibrillation? A case report and review of the literature. Clin Res Cardiol. 2010;99:125–128. doi: 10.1007/s00392-009-0091-4. [DOI] [PubMed] [Google Scholar]
- 261.Puwanant S., Varr B.C., Shrestha K. Role of the CHADS2Score in the evaluation of thromboembolic risk in patients with atrial fibrillation undergoing transesophageal echocardiography before pulmonary vein isolation. J Am Coll Cardiol. 2009;54:2032–2039. doi: 10.1016/j.jacc.2009.07.037. [DOI] [PubMed] [Google Scholar]
- 262.Scherr D., Sharma K., Dalal D. Incidence and predictors of periprocedural cerebrovascular accident in patients undergoing catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2009;20:1357–1363. doi: 10.1111/j.1540-8167.2009.01540.x. [DOI] [PubMed] [Google Scholar]
- 263.Armbruster H.L., Lindsley J.P., Moranville M.P. Safety of novel oral anticoagulants compared with uninterrupted warfarin for catheter ablation of atrial fibrillation. Ann Pharmacother. 2015;49:278–284. doi: 10.1177/1060028014563950. [DOI] [PubMed] [Google Scholar]
- 264.May M.A., Gruel Y., Fauchier L. Letter by May et al. regarding article, “Use of dabigatran for periprocedural anticoagulation in patients undergoing catheter ablation for atrial fibrillation” by Bassiouny et al. Circ Arrhythm Electrophysiol. 2013;6:e65. doi: 10.1161/CIRCEP.113.000515. [DOI] [PubMed] [Google Scholar]
- 265.Di Biase L., Gaita F., Toso E. Does periprocedural anticoagulation management of atrial fibrillation affect the prevalence of silent thromboembolic lesion detected by diffusion cerebral magnetic resonance imaging in patients undergoing radiofrequency atrial fibrillation ablation with open irrigated catheters? Results from a prospective multicenter study. Heart Rhythm. 2014;11:791–798. doi: 10.1016/j.hrthm.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 266.Cauchemez B., Extramiana F., Cauchemez S. High-flow perfusion of sheaths for prevention of thromboembolic complications during complex catheter ablation in the left atrium. J Cardiovasc Electrophysiol. 2004;15:276–283. doi: 10.1046/j.1540-8167.2004.03401.x. [DOI] [PubMed] [Google Scholar]
- 267.Karasoy D., Gislason G.H., Hansen J. Oral anticoagulation therapy after radiofrequency ablation of atrial fibrillation and the risk of thromboembolism and serious bleeding: long-term follow-up in nationwide cohort of Denmark. Eur Heart J. 2015;36:307–315. doi: 10.1093/eurheartj/ehu421. [DOI] [PubMed] [Google Scholar]
- 268.Lakkireddy D., Reddy Y.M., Di Biase L. Feasibility and safety of dabigatran versus warfarin for periprocedural anticoagulation in patients undergoing radiofrequency ablation for atrial fibrillation: results from a multicenter prospective registry. J Am Coll Cardiol. 2012;59:1168–1174. doi: 10.1016/j.jacc.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 269.Gladstone D.J., Spring M., Dorian P. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467–2477. doi: 10.1056/NEJMoa1311376. [DOI] [PubMed] [Google Scholar]
- 270.Svennberg E., Engdahl J., Al-Khalili F. Mass screening for untreated atrial fibrillation: the STROKESTOP study. Circulation. 2015;131:2176–2184. doi: 10.1161/CIRCULATIONAHA.114.014343. [DOI] [PubMed] [Google Scholar]
- 271.Yiu K.-H., Siu C.-W., Jim M.-H. Comparison of the efficacy and safety profiles of intravenous vitamin K and fresh frozen plasma as treatment of warfarin-related over-anticoagulation in patients with mechanical heart valves. Am J Cardiol. 2006;97:409–411. doi: 10.1016/j.amjcard.2005.08.062. [DOI] [PubMed] [Google Scholar]
- 272.Schiele F., van Ryn J., Canada K. A specific antidote for dabigatran: functional and structural characterization. Blood. 2013;121:3554–3562. doi: 10.1182/blood-2012-11-468207. [DOI] [PubMed] [Google Scholar]
- 273.Lu G., DeGuzman F.R., Hollenbach S.J. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med. 2013;19:446–451. doi: 10.1038/nm.3102. [DOI] [PubMed] [Google Scholar]
- 274.Siegal D.M., Curnutte J.T., Connolly S.J. Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl J Med. 2015;373:2413–2424. doi: 10.1056/NEJMoa1510991. [DOI] [PubMed] [Google Scholar]
- 275.Connolly S.J., Milling T.J.J., Eikelboom J.W. Andexanet alfa for acute major bleeding associated with factor Xa inhibitors. N Engl J Med. 2016;375:1131–1141. doi: 10.1056/NEJMoa1607887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Greinacher A., Thiele T., Selleng K. Reversal of anticoagulants: an overview of current developments. Thromb Haemost. 2015;113:931–942. doi: 10.1160/TH14-11-0982. [DOI] [PubMed] [Google Scholar]