Abstract
Background
The aim of this study was to investigate the benefit of nephron sparing surgery (NSS) compared with extirpative nephrectomy in different tumor stages of renal cell carcinoma.
Material/Methods
We reviewed the Surveillance, Epidemiology and End Results (SEER) database for NSS and extirpative nephrectomy in localized (stages T1–2N0M0) renal cell carcinoma diagnosed after 2004. We used the variable screening function of the SEER database to identified 55,947 cases that met inclusion and exclusion criteria for survival analysis. Overall mortality and cancer-specific mortality were the primary index outcomes. Stratification analysis was done by T stage subgroups. We also performed survival analysis using propensity score analysis, and changed the survival model to the competing-risk model for cancer-specific mortality analysis.
Results
Overall, NSS significantly decreased the risk of overall mortality (HR 0.717, 0.668–0.769) and cancer-specific mortality (HR 0.604, 0.525–0.694) when compared to extirpative nephrectomy. In subgroup analysis, NSS had a lower overall mortality risk and cancer-specific mortality compared to extirpative nephrectomy only for T1a stage renal cell carcinoma (HR 0.654, 0.599–0.714, p<0.01 and HR 0.554, 0.458–0.670, p<0.01, respectively), but not for T1b or T2 stage. The propensity score analysis, which included standardized mortality ratio weight adjustment, showed the same results. Additionally, for cancer-specific mortality, a competing-risk model gave the exactly same outcome.
Conclusions
Compared to extirpative nephrectomy, NSS provided superior overall survival and cancer-specific survival for localized renal cell carcinoma only in T1a stage, not in T1b or T2 stage. NSS should be recommended when the surgery is possible. Further prospective study is needed to confirm this result.
MeSH Keywords: Carcinoma, Renal Cell; Nephrectomy; Organ Sparing Treatments; Survival Analysis
Background
In recent years, the diagnosis of early stage renal cell carcinoma (RCC) has increased largely due to the wide utilization of imaging examinations including CT, ultrasonography, and MRI [1]. For many years, extirpative nephrectomy was the standard treatment for RCC. However, with increased awareness of the risk for chronic kidney disease after extirpative nephrectomy, there has been increased support for treatments that preserve renal function [2]. Partial nephrectomy has gradually replaced extirpative nephrectomy over the past decade, especially for T1 stage RCC. In addition, nephron sparing surgery (NSS) has been recommended for T1 stage RCC in several guidelines [3,4], although this recommendation is based on retrospective studies and database analysis. While there is no doubt that NSS has a surgical advantage for small RCC as a minimally invasive surgery, the benefit of NSS on oncologic outcomes is still controversial. It remains unclear whether NSS has superior or equivalent survival outcomes to extirpative nephrectomy for localized RCC. In this study, we aimed to investigate the benefit of NSS compared to extirpative nephrectomy in different RCC stages using a relatively current study population.
Material and Methods
Data source and patient population
We used the Surveillance, Epidemiology and End Results (SEER) database to identify RCC patients as the study population. Maintained by the National Cancer Institute, the SEER program covers nearly 26% of the US population [5] and has relatively complete survival data. Based on the AJCC (American Joint Committee on Cancer) 6th edition TNM stage classification, we selected a relatively current study population: patients diagnosed between 2004 and 2013 (AJCC 6th edition stage system has been recorded since 2004). Only patients with RCC indicated as the first tumor were included. The inclusion and exclusion criteria are presented in Figure 1. Inclusion criteria included: 1) cancer primary site limited to the kidney (International Classification of Diseases for Oncology, Third Edition (ICD-O-3) code C64.9, and site record B ICD-O-3, kidney); 2) tumor stage limited to T1a, T1b, and T2 by AJCC Stage, 6th Edition and no lymph node or metastasis (T1a, T1b, 2N0M0); 3) cancer diagnosis years limited to 2004–2013; 4) age at diagnosis 18 years or older. Exclusion criteria included: 1) not the first tumor; 2) patients with bilateral RCC; 3) surgery cannot be classified by index surgery type (NSS or extirpative nephrectomy). We ultimately identified 55,947 cases for analysis. Because no patient identifying information was used in this study, it was considered exempt from the requirement for ethical approval by the Institutional Review Board.
Figure 1.

The flowchart of data screening.
Covariates and outcomes
Demographic characteristics included age, gender, race, and marital status. Marital status was recorded as never married, ever married, and married. Cancer-related covariates included histology type, T stage, tumor size, grade, laterality, multi-incident cancer during the life (sequence number), and two treatment-related variables: surgery type and regional lymph node dissection. Histology was categorized as clear cell RCC (ccRCC), chromophobe type, papillary adenocarcinoma, RCC (unspecified), and other types. Regional lymph node dissection was recoded as yes or no lymph node removed, regardless of the number of lymph nodes removed. Our index variable of surgery type was re-assembled as NSS and extirpative nephrectomy. NSS included local tumor destruction or excision, and partial nephrectomy (surgery of primary site code 10–15, 20–27, 30). Extirpative nephrectomy included any entire kidney resection surgery with or without other procedures (surgery of primary site code 40, 50). Follow-up and vital statistics were identified in the SEER database. The latest follow-up cutoff date was December 31, 2013. The endpoint included overall mortality and cancer-specific mortality.
Statistical analysis
Baseline characteristics between surgery types were compared using t-test for continuous variables and chi-square test for factor variables. Survival differences between two surgery types were compared using Kaplan-Meier estimation and log-rank test. Univariable and multivariable Cox proportional hazard regression were performed to identify the prognostic factors. We also used propensity score analysis (including propensity score adjustment and weighting) to confirm the risks. Additionally, a competing-risk model was used to analysis cause-specific mortality risk [6] as supplemental data. All statistics were performed using the R statistical package (Version 3.2.5, R Foundation for Statistical Computing, Vienna, Austria; www.r-project.org) and Stata version 14.1 (StataCorp LP, College Station, TX, USA). A two-sided p<0.05 was considered statistical significant.
Results
Patient characteristics
Table 1 lists the basic demographic and tumor characteristic of the included 55,947 patients by surgery type. The mean age of patients treated with NSS was significantly younger than for patients treated with extirpative nephrectomy (58.6±12.7 versus 60.1±12.6 years, p<0.01). Other variable distributions also differed between the surgery types. For the entire cohort and for T1a stage stratification, almost every single variable was significantly different between NSS and extirpative nephrectomy except for tumor laterality. For T1b stage, there was no difference in tumor laterality, sequence number, or marital status (p=0.055, p=0.417, and p=0.133, respectively). For T2 stage, no difference was observed in age, gender, tumor size, tumor grade, laterality, or marital status. The number of events was distinct between two surgery types in the different cohorts. For the entire cohort, 5,087 patients (15.28%) in the extirpative nephrectomy group and 1,676 patients (7.40%) in the NSS group died during the follow-up (p<0.01). For NSS, the death rate was 6.65%, 10.33%, and 14.26% for patients with T1a, T1b, and T2 stage, respectively. The death rate for patients undergoing extirpative nephrectomy was 14.04%, 15.22%, and 17.66% for T1a, T1b, and T2 stage RCC, respectively.
Table 1.
Baseline descriptive characteristics of 55947 patients with localized renal cell carcinoma (T1–2N0M0) treated by nephrectomy or nephron sparing surgery.
| Variable | Whole cohort | T1a Stage | T1b Stage | T2 Stage | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| NE | NSS | P | NE | NSS | P | NE | NSS | P | NE | NSS | P | |
| Number of patients | 33292 | 22655 | 13309 | 18623 | 12705 | 3513 | 7278 | 519 | ||||
|
| ||||||||||||
| Age (continuous) | 60.1 (12.6) | 58.6 (12.7) | <0.01 | 60.1 (12.6) | 58.4 (12.8) | <0.01 | 60.7 (12.5) | 59.6 (12.6) | <0.01 | 58.9 (12.5) | 59.1 (13.0) | 0.81 |
|
| ||||||||||||
| Gender | ||||||||||||
| Female | 13161 | 8613 | <0.01 | 5572 | 7255 | <0.01 | 4970 | 1186 | <0.01 | 2619 | 172 | 0.192 |
| Male | 20131 | 14042 | 7737 | 11368 | 7735 | 2327 | 4659 | 347 | ||||
|
| ||||||||||||
| Race | ||||||||||||
| Black | 4091 | 2584 | 0.010 | 1765 | 2018 | <0.01 | 1416 | 476 | <0.01 | 910 | 90 | 0.005 |
| White | 27026 | 18492 | 10727 | 15303 | 10421 | 2797 | 5878 | 392 | ||||
| Other | 1943 | 1346 | 728 | 1105 | 777 | 209 | 438 | 32 | ||||
|
| ||||||||||||
| Histology | ||||||||||||
| ccRCC | 19627 | 12505 | <0.01 | 7938 | 10456 | <0.01 | 7751 | 1842 | <0.01 | 3938 | 207 | <0.01 |
| Chromophobe type | 2182 | 1420 | 650 | 1099 | 750 | 265 | 782 | 56 | ||||
| Papillary adenocar | 3629 | 3663 | 1562 | 2875 | 1223 | 671 | 844 | 117 | ||||
| RCC unspecified | 6106 | 4069 | 2444 | 3391 | 2374 | 570 | 1288 | 108 | ||||
| Other types | 1748 | 998 | 715 | 802 | 607 | 165 | 426 | 31 | ||||
|
| ||||||||||||
| Tumor size (cm) | 5.4 (3.4) | 3.1 (2.0) | <0.01 | 2.9 (0.8) | 2.5 (0.8) | <0.01 | 5.5 (0.8) | 5.1 (0.8) | <0.01 | 10.0 (4.3) | 10.3 (7.6) | 0.39 |
|
| ||||||||||||
| Grade | ||||||||||||
| Grade I | 3971 | 3609 | <0.01 | 2099 | 3211 | <0.01 | 1353 | 352 | <0.01 | 519 | 46 | 0.195 |
| Grade II | 16417 | 11034 | 6951 | 9210 | 6310 | 1621 | 3156 | 203 | ||||
| Grade III | 7690 | 3658 | 2469 | 2633 | 3047 | 876 | 2174 | 149 | ||||
| Grade IV | 1210 | 253 | 239 | 156 | 497 | 71 | 474 | 26 | ||||
|
| ||||||||||||
| Laterality | ||||||||||||
| Left | 16315 | 10997 | 0.283 | 6368 | 9045 | 0.204 | 6295 | 1676 | 0.055 | 3652 | 276 | 0.190 |
| Right | 16970 | 11652 | 6937 | 9573 | 6410 | 1836 | 3623 | 243 | ||||
|
| ||||||||||||
| RLN removed | ||||||||||||
| No | 29867 | 22104 | <0.01 | 12560 | 18266 | <0.01 | 11515 | 3360 | <0.01 | 5792 | 478 | <0.01 |
| Yes | 3378 | 514 | 729 | 331 | 1175 | 143 | 1474 | 40 | ||||
|
| ||||||||||||
| Sequence number | ||||||||||||
| One primary only | 29250 | 20045 | 0.026 | 11635 | 16540 | <0.01 | 11153 | 3066 | 0.417 | 6462 | 439 | 0.004 |
| 1st of 2 or more primaries | 4042 | 2610 | 1674 | 2083 | 1552 | 447 | 816 | 80 | ||||
|
| ||||||||||||
| Marital status | ||||||||||||
| Never married | 4881 | 3413 | <0.01 | 1881 | 2794 | <0.01 | 1843 | 534 | 0.133 | 1157 | 85 | 0.384 |
| Ever married | 6325 | 3789 | 2588 | 3057 | 2463 | 632 | 1274 | 100 | ||||
| Married | 20605 | 14336 | 8269 | 11841 | 7818 | 2192 | 4518 | 303 | ||||
|
| ||||||||||||
| Vital status | ||||||||||||
| Alive | 28205 | 20979 | <0.01 | 11441 | 17384 | <0.01 | 10771 | 3150 | <0.01 | 5993 | 445 | 0.049 |
| Dead | 5087 (0.1528) | 1676 (0.0740) | 1868 (0.1404) | 1239 (0.0665) | 1934 (0.1522) | 363 (0.1033) | 1285 (0.1766) | 74 (0.1426) | ||||
NE – nephrectomy; ccRCC – clear cell renal cell carcinoma; Papillary adenocar – Papillary adenocarcinoma; RLN – regional lymph nodes. Sequence number: The sequence of renal cell carcinoma of all reportable tumors which occur over the lifetime of a patient. Ever married consisted Divorced, Separated and Widowed. Missing values were omitted for some variables.
Survival outcomes
Table 2 shows the overall survival and cancer-specific survival outcomes of the entire cohort by using univariable and multivariable analysis. Both univariable and multivariable results showed that NSS was significantly associated with a lower risk of overall mortality and cancer-specific mortality (hazard ratio (HR) 0.717, 0.668–0.769 and HR 0.604, 0.525–0.694, both p<0.01). Additionally, in multivariable analysis, age, gender, race, histology, T stage, grade, sequence number, and marital status were significantly associated with overall mortality. Age, gender, histology, T stage, grade, and marital status were associated with cancer-specific mortality. Corresponding predictive ability (C-index) was 0.7163 and 0.7756, respectively.
Table 2.
Univariable and multivariable Cox regression analysis for prediction of overall mortality and cancer specific mortality.
| Variables | Overall mortality | Cancer specific mortality | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Univariable | Multivariable | Univariable | Multivariable | |||||
|
| ||||||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Surgery | ||||||||
| NE | Reference | <0.01 | Reference | <0.01 | Reference | <0.01 | Reference | <0.01 |
| NSS | 0.607 (0.574–0.643) | 0.717 (0.668–0.769) | 0.357 (0.319–0.399) | 0.604 (0.525–0.694) | ||||
|
| ||||||||
| Age (continuous) | 1.057 (1.054–1.059) | <0.01 | 1.054 (1.051–1.057) | <0.01 | 1.046 (1.042–1.049) | <0.01 | 1.041 (1.037–1.046) | <0.01 |
|
| ||||||||
| Gender | ||||||||
| Female | Reference | <0.01 | Reference | <0.01 | Reference | 0.006 | Reference | 0.004 |
| Male | 1.143 (1.086–1.201) | 1.323 (1.247–1.404) | 1.128 (1.035–1.228) | 1.158 (1.049–1.278) | ||||
|
| ||||||||
| Race | ||||||||
| Black | Reference | <0.01 <0.01 |
Reference | <0.01 <0.01 |
Reference | 0.497 0.617 |
Reference | 0.262 0.896 |
| White | 0.815 (0.759–0.875) | 0.785 (0.723–0.852) | 0.957 (0.842–1.087) | 0.920 (0.795–1.065) | ||||
| Other | 0.681 (0.598–0.776) | 0.704 (0.609–0.813) | 0.947 (0.767–1.171) | 0.985 (0.780–1.242) | ||||
|
| ||||||||
| Histology | ||||||||
| ccRCC | Reference | <0.01 0.005 0.001 <0.01 |
Reference | <0.01 0.691 0.804 <0.01 |
Reference | <0.01 0.669 0.034 <0.01 |
Reference | <0.01 0.452 0.733 <0.01 |
| Chromophobe type | 0.573 (0.502–0.654) | 0.538 (0.457–0.634) | 0.435 (0.336–0.563) | 0.349 (0.252–0.483) | ||||
| Papillary adenocarcinoma | 1.112 (1.032–1.197) | 0.983 (0.902–1.071) | 0.971 (0.850–1.110) | 0.943 (0.809–1.099) | ||||
| RCC unspecified | 1.107 (1.042–1.177) | 0.991 (0.924–1.063) | 1.119 (1.009–2.242) | 1.021 (0.907–1.148) | ||||
| Other types | 1.560 (1.421–1.713) | 1.236 (1.112–1.374) | 2.089 (1.815–2.404) | 1.592 (1.363–1.860) | ||||
|
| ||||||||
| T stage | ||||||||
| T1a | Reference | <0.01 <0.01 |
Reference | <0.01 <0.01 |
Reference | <0.01 <0.01 |
Reference | <0.01 <0.01 |
| T1b | 1.457 (1.380–1.539) | 1.227 (1.145–1.316) | 2.329 (2.104–2.579) | 1.742 (1.541–1.969) | ||||
| T2 | 1.797 (1.684–1.916) | 1.528 (1.367–1.707) | 4.610 (4.159–5.109) | 2.997 (2.566–3.501) | ||||
|
| ||||||||
| Tumor size (cm) | 1.004 (1.003–1.004) | <0.01 | 1.000 (0.999–1.001) | 0.809 | 1.005 (1.005–1.006) | <0.01 | 1.001 (1.000–1.003) | 0.026 |
|
| ||||||||
| Grade | ||||||||
| Grade I | Reference | 0.476 <0.01 <0.01 |
Reference | 0.007 0.005 <0.01 |
Reference | 0.055 <0.01 <0.01 |
Reference | 0.777 <0.01 <0.01 |
| Grade II | 0.973 (0.901–1.050) | 0.898 (0.830–0.971) | 1.160 (0.997–1.350) | 0.978 (0.838–1.142) | ||||
| Grade III | 1.379 (1.269–1.499) | 1.133 (1.038–1.236) | 2.466 (2.113–2.878) | 1.719 (1.464–2.017) | ||||
| Grade IV | 2.788 (2.471–3.146) | 2.055 (1.811–2.332) | 7.434 (6.188–8.932) | 4.049 (3.340–4.908) | ||||
|
| ||||||||
| Laterality | ||||||||
| Left | Reference | 0.716 | Reference | 0.573 | Reference | 0.420 | Reference | 0.113 |
| Right | 0.991 (0.944–1.040) | 1.016 (0.963–1.071) | 1.034 (0.953–1.123) | 1.076 (0.983–1.178) | ||||
|
| ||||||||
| RLN removed | ||||||||
| No | Reference | <0.01 | Reference | 0.241 | Reference | <0.01 | Reference | 0.086 |
| Yes | 1.187 (1.089–1.293) | 1.060 (0.962–1.167) | 1.737 (1.531–1.970) | 1.132 (0.983–1.303) | ||||
|
| ||||||||
| Sequence number | ||||||||
| One primary only | Reference | <0.01 | Reference | <0.01 | Reference | 0.007 | Reference | 0.866 |
| 1st of 2 or more primaries | 1.715 (1.619–1.818) | 1.389 (1.302–1.483) | 1.165 (1.042–1.303) | 0.989 (0.875–1.119) | ||||
|
| ||||||||
| Marital status | ||||||||
| Never married | Reference | <0.01 0.003 |
Reference | 0.856 <0.01 |
Reference | <0.01 0.059 |
Reference | 0.050 0.133 |
| Ever married | 1.616 (1.491–1.752) | 1.009 (0.920–1.106) | 1.780 (1.537–2.062) | 1.180 (1.000–1.392) | ||||
| Married | 0.894 (0.831–0.963) | 0.674 (0.621–0.732) | 1.137 (0.995–1.300) | 0.894 (0.772–1.035) | ||||
|
| ||||||||
| C-index | 0.7163 | 0.7756 | ||||||
NE – nephrectomy; NSS – nephron sparing surgery; HR – hazard ratio; C-index – Harrell’s concordance index; RLN – regional lymph nodes. Sequence number: The sequence of renal cell carcinoma of all reportable tumors which occur over the lifetime of a patient. Ever married consisted Divorced, Separated and Widowed. Observations with missing values were omitted during statistical process.
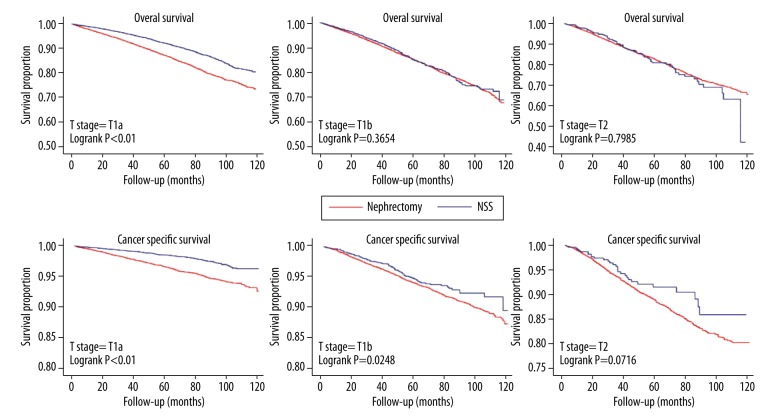
We further analyzed the impact of surgery type on survival outcomes in different T stage stratification including T1a, T1b, and T2 stage. The Kaplan-Meier curve and log-rank test for overall survival and cancer-specific survival in different subgroup are shown in Figure 2. Compared to extirpative nephrectomy, NSS significantly decreased the overall mortality and cancer-specific mortality in the T1a stage subgroup (both p<0.01). NSS decreased cancer specific mortality in the T1b stage subgroup (p=0.0248) but not overall mortality (p=0.3654). No significant difference was observed for overall or cancer-specific mortality in the T2 stage subgroup. In multivariable analysis, NSS significantly decreased the risk of overall mortality (HR: 0.654, 95% confidence interval (CI): 0.599–0.714, p<0.01) and cancer-specific mortality (HR: 0.554, 95% CI: 0.458–0.670, p<0.01) in the T1a stage subgroup. No overall survival benefit for NSS was observed in the T1b stage subgroup (HR: 0.939, 95% CI: 0.821–1.073, p=0.354) or the T2 stage subgroup (HR: 0.949, 95% CI: 0.723–1.244, p=0.703). Similarly, no cancer-specific survival benefit was observed in the T1b stage subgroup (p = 0.073) or the T2 stage subgroup (p=0.145). Propensity score analysis, including propensity score adjustment and standardized mortality ratio weight, were used as the alternative analysis. The results were consistent with conventional multivariable analysis (Table 3). Additionally, we changed the survival analysis model by using a competing-risk model and the results were not altered (Table 4). This result showed the stability of our outcomes.
Figure 2.
The Kaplan-Meier curve for overall survival and cancer specific survival in different treatment subgroups.
Table 3.
Multivariable Cox proportional analysis of two surgery type for overall mortality and cancer specific mortality stratified by T stage.
| Outcomes | Methods | Variables | T1a | T1b | T2 |
|---|---|---|---|---|---|
| Overall mortality | Multivariable* | Nephrectomy | Reference | Reference | Reference |
| NSS | 0.654 (0.599–0.714) | 0.939 (0.821–1.073) | 0.949 (0.723–1.244) | ||
| P<0.01 | P=0.354 | P=0.703 | |||
|
| |||||
| PS adjusted | Nephrectomy | Reference | Reference | Reference | |
| NSS | 0.694 (0.636–0.759) | 0.950 (0.831–1.086) | 1.008 (0.769–1.322) | ||
| P<0.01 | P=0.450 | P=0.952 | |||
|
| |||||
| SMRW | Nephrectomy | Reference | Reference | Reference | |
| NSS | 0.672 (0.614–0.734) | 1.041 (0.922–1.175) | 1.035 (0.813–1.317) | ||
| P<0.01 | P=0.515 | P=0.782 | |||
|
| |||||
| Cancer specific mortality | Multivariable* | Nephrectomy | Reference | Reference | Reference |
| NSS | 0.554 (0.458–0.670) | 0.803 (0.632–1.021) | 0.741 (0.495–1.109) | ||
| P<0.01 | P=0.073 | P=0.145 | |||
|
| |||||
| PS adjusted | Nephrectomy | Reference | Reference | Reference | |
| NSS | 0.591 (0.488–0.716) | 0.811 (0.639–1.030) | 0.804 (0.538–1.202) | ||
| P<0.01 | P=0.086 | P=0.288 | |||
|
| |||||
| SMRW | Nephrectomy | Reference | Reference | Reference | |
| NSS | 0.516 (0.423–0.629) | 0.949 (0.770–1.170) | 0.747 (0.517–1.080) | ||
| P<0.01 | P=0.624 | P=0.121 | |||
Adjusted for age, gender, race, histology, tumor size, grade, laterality, RLN removed, sequence number and marital status.
PS – propensity score; SMRW – standardized mortality ratio weight; NSS – nephron sparing surgery. Cancer specific mortality was calculated using Fine and Gray model.
Table 4.
Multivariable regression of cancer specific mortality (competing risk model) stratified by T stage.
| Outcomes | Methods | Variables | T1a | T1b | T2 |
|---|---|---|---|---|---|
| Cancer specific mortality | Multivariable* | Nephrectomy | Reference | Reference | Reference |
| NSS | 0.565 (0.461–0.691) | 0.792 (0.625–1.004) | 0.739 (0.490–1.116) | ||
| P<0.01 | P=0.054 | P=0.150 | |||
|
| |||||
| PS adjusted | Nephrectomy | Reference | Reference | Reference | |
| NSS | 0.595 (0.485–0.729) | 0.800 (0.632–1.012) | 0.791 (0.530–1.181) | ||
| P<0.01 | P=0.062 | P=0.251 | |||
|
| |||||
| SMRW | Nephrectomy | Reference | Reference | Reference | |
| NSS | 0.522 (0.428–0.636) | 0.935 (0.759–1.153) | 0.732 (0.507–1.057) | ||
| P<0.01 | P=0.532 | P=0.096 | |||
Adjusted for age, gender, race, histology, tumor size, grade, laterality, RLN removed, sequence number and marital status.
PS – propensity score; SMRW – standardized mortality ratio weight; NSS – nephron sparing surgery. Cancer specific mortality was calculated using Fine and Gray model.
Discussion
In this study, our results showed the benefit in oncology outcomes for NSS were only significant in the T1a stage subgroup but not in the T1b stage or T2 stage subgroups. These findings were consistent with previous study reports. In T1b stage RCC, no difference between partial nephrectomy and radical nephrectomy with regards to cancer-specific or other cause-specific mortality was founded [7]. Moreover, Kopp et al. [8] concluded there was no significant survival difference between partial nephrectomy and radical nephrectomy in clinical T2 stage RCC. However, there is still considerable controversial regarding the benefit of partial nephrectomy compared to radical nephrectomy in localized RCC patients. Almost all the studies in favor of partial nephrectomy were based on observational cohorts with possible selection bias. Other studies have not found a difference between partial nephrectomy and radical nephrectomy in different populations. A meta-analysis by Kim et al. concluded there was a benefit for partial nephrectomy in treatment of localized renal tumors [9].
There were some limitations to the SEER database as used in our study. Some baseline confounders were not included in our analysis due to their absence in the database; such as diabetes mellitus [10,11], American Society of Anesthesiology (ASA) classification status for RCC [12], Charlson Comorbidity Index (CCI) [13], basic performance status [14] or other comorbidities that have been demonstrated to be prognostic predictors. Another important confounder absent in database was the tumor location in the kidney, which could also affect the selection of partial nephrectomy or radical nephrectomy; and which has been reported to be highly correlated with tumor recurrence [15]. Tumor location also plays a key role in the RENAL nephrometry score system, which has been shown to be a strong prognostic predictor [8]. Similarly, preoperative GFR has been demonstrated to significantly affect renal function decrease [16]; and it has been reported that the benefit of partial nephrectomy only exists in patients with good preoperative eGFR [17].
Tan [18] reviewed various experimental or observational studies and found that there was a large potential bias with regards to surgery selection; and that one intrinsic property of observational studies was that high-risk patients were prone to receive radical nephrectomy. Shuch et al. [19] concluded that the SEER-Medicare dataset was biased involving unmeasured confounders based on the use of two matched control groups. Observed confounders could be adjusted by using various statistical models like conventional multi-regression and propensity score analysis (adjustment, weighting, or matching). Similar to outcomes to our study, Tan et al. [20] found that by using instrument variable analysis, partial nephrectomy was associated with a survival benefit for T1a stage patients. Instrument variable analysis can somewhat control the unobserved confounders and generate pseudo-randomization. The key point of instrument variable analysis is to find a proper instrument variable that must significantly affect the treatment selection and simultaneously should not be associated with the health outcome or with other measured or unmeasured confounders. This results in a relatively unbiased estimation close to randomized controlled trial outcomes [21]. Well-performed randomized controlled trials could sufficiently reduce bias. To date, there has been only one randomized trial comparing the oncologic outcome of NSS and radical nephrectomy [22]. This trial had many defects and the trial was prematurely terminated before reaching adequate sample size. Despite decades long study period used in the trial, and numerous participating institutions, there was only an average of one patient per year per institution required for study inclusion. In addition, during the study period, a rapid development of surgical technique occurred, including the use of laparoscopic and robotic-assisted laparoscopic techniques; in addition, warm ischemia time, kidney cortex preservation and minimally invasive conceptions were introduced. These limitations make the trial results difficult to interrupt and translate into current practice.
Based on current literature, partial nephrectomy improves the overall survival of T1a stage tumors mainly by decreasing the reduction of renal function and subsequent cardiovascular events. Yiu et al. [23] demonstrated, by using a small cohort that radical nephrectomy increased the risk of new-onset chronic kidney disease but achieved equivalent overall survival in T1 stage patients. This increased risk was supported by various studies [16,24–29]. On the other hand, partial nephrectomy had a lower cardiovascular event risk compared to radical nephrectomy [30]. Huang et al. [31] demonstrated that partial nephrectomy could decrease subsequent cardiovascular-related deaths in older patients with 4 cm or smaller tumors; and it possibly contributed to the overall survival benefit.
Our results also showed a benefit in cancer-specific survival for NSS. This may seem counterintuitive as extirpative nephrectomy is thought to provide better oncologic control. Radical nephrectomy, as a standard treatment for resectable RCC, has been used for decades, as partial nephrectomy may possibly result in positive surgical margins or residual tumor that could adversely affect prognosis [15]. Thus, the benefit of partial nephrectomy goes against conventional logic, although other studies [9] have shown similar results to our study. In Tan’s instrument variable analysis, partial nephrectomy had no significant cancer-specific mortality difference compared to radical nephrectomy (HR, 0.82; 95% CI, 0.19–3.49) [20]. However this result had limitations. For example, the study population was older patients, and as our results showed, age had a significant effect on the prognosis. Additionally, there were 257 cancer-specific death events in the Tan study (with only 37 cancer-specific events for the partial nephrectomy cohort), which possibly lacks statistical power to define significance. As a rule of thumb, to achieve sufficient multivariable regression analysis, the number of events should reach above 20-fold the number of covariates. Our study, using a more current population (2004–2013), had a relatively large number of death events including cancer-specific deaths. The number in each T stage strata exceeded 500, and was therefore more likely to result in statistical significant findings. Targeted therapy could also significantly affect RCC survival. It has been reported previously that overall survival has been significantly improved in the era of targeted therapy compared to the era of pre-targeted therapy [32]. Our study avoided this confounder by using a current population cohort mainly within the targeted therapy era. Nevertheless, current evidence cannot provide a firm conclusion regarding partial nephrectomy and decreasing cancer-specific mortality.
In summary, our results should be interpreted with caution, especially with regards to the benefit of NSS on cancer-specific mortality. Although to date no strong evidence has shown that NSS increases the risk of any cause mortality, the benefit of NSS is still controversial. We suggest that NSS should be considered as an alternative, when possible, for small renal mass RCC. The indications for performing NSS or extirpative nephrectomy need further study and discussion.
Conclusions
Compared with extirpative nephrectomy, nephron sparing surgery provided superior overall survival and cancer-specific survival for localized renal cell carcinoma only in T1a stage, not in T1b or T2 stage. Nephron sparing surgery should be recommended when the surgery is possible. Further prospective study is needed to confirm this result.
Footnotes
Source of support: Departmental sources
References
- 1.Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: A need to reassess treatment effect. J Natl Cancer Inst. 2006;98(18):1331–34. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 2.Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: A retrospective cohort study. Lancet Oncol. 2006;7(9):735–40. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182(4):1271–79. doi: 10.1016/j.juro.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67(5):913–24. doi: 10.1016/j.eururo.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Giordano SH, Kuo Y, Duan Z, et al. Limits of observational data in determining outcomes from cancer therapy. Cancer. 2008;112(11):2456–66. doi: 10.1002/cncr.23452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 7.Meskawi M, Becker A, Bianchi M, et al. Partial and radical nephrectomy provide comparable long-term cancer control for T1b renal cell carcinoma. Int J Urol. 2014;21(2):122–28. doi: 10.1111/iju.12204. [DOI] [PubMed] [Google Scholar]
- 8.Kopp RP, Mehrazin R, Palazzi KL, et al. Survival outcomes after radical and partial nephrectomy for clinical T2 renal tumours categorised by R.E.N.A.L. nephrometry score. BJU Int. 2014;114(5):708–18. doi: 10.1111/bju.12580. [DOI] [PubMed] [Google Scholar]
- 9.Kim SP, Thompson RH, Boorjian SA, et al. Comparative effectiveness for survival and renal function of partial and radical nephrectomy for localized renal tumors: A systematic review and meta-analysis. J Urol. 2012;188(1):51–57. doi: 10.1016/j.juro.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Vavallo A, Simone S, Lucarelli G, et al. Pre-existing type 2 diabetes mellitus is an independent risk factor for mortality and progression in patients with renal cell carcinoma. Medicine (Baltimore) 2014;93(27):e183. doi: 10.1097/MD.0000000000000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee H, Kwak C, Kim HH, et al. Diabetes mellitus as an independent predictor of survival of patients surgically treated for renal cell carcinoma: A propensity score matching study. J Urol. 2015;194(6):1554–60. doi: 10.1016/j.juro.2015.05.097. [DOI] [PubMed] [Google Scholar]
- 12.de Cassio Zequi S, de Campos EC, Guimaraes GC, et al. The use of the American Society of Anesthesiology Classification as a prognostic factor in patients with renal cell carcinoma. Urol Int. 2010;84(1):67–72. doi: 10.1159/000273469. [DOI] [PubMed] [Google Scholar]
- 13.Patel HD, Kates M, Pierorazio PM, et al. Comorbidities and causes of death in the management of localized T1a kidney cancer. Int J Urol. 2014;21(11):1086–92. doi: 10.1111/iju.12527. [DOI] [PubMed] [Google Scholar]
- 14.Shvarts O, Lam JS, Kim HL, et al. Eastern Cooperative Oncology Group performance status predicts bone metastasis in patients presenting with renal cell carcinoma: implication for preoperative bone scans. J Urol. 2004;(172):867–70. doi: 10.1097/01.ju.0000135803.91207.b0. [DOI] [PubMed] [Google Scholar]
- 15.Shim M, Song C, Park S, et al. Hilar location is an independent prognostic factor for recurrence in T1 renal cell carcinoma after nephrectomy. Ann Surg Oncol. 2015;22(1):344–50. doi: 10.1245/s10434-014-4153-0. [DOI] [PubMed] [Google Scholar]
- 16.Pignot G, Bigot P, Bernhard JC, et al. Nephron-sparing surgery is superior to radical nephrectomy in preserving renal function benefit even when expanding indications beyond the traditional 4-cm cutoff. Urol Oncol. 2014;32(7):1024–30. doi: 10.1016/j.urolonc.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Roos FC, Steffens S, Junker K, et al. Survival advantage of partial over radical nephrectomy in patients presenting with localized renal cell carcinoma. BMC Cancer. 2014;14:372. doi: 10.1186/1471-2407-14-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan HJ. Survival benefit of partial nephrectomy: Reconciling experimental and observational data. Urol Oncol. 2015;33(12):505.e21–24. doi: 10.1016/j.urolonc.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Shuch B, Hanley J, Lai J, et al. Overall survival advantage with partial nephrectomy: a bias of observational data? Cancer. 2013;119(16):2981–89. doi: 10.1002/cncr.28141. [DOI] [PubMed] [Google Scholar]
- 20.Tan HJ, Norton EC, Ye Z, et al. Long-term survival following partial vs. radical nephrectomy among older patients with early-stage kidney cancer. JAMA. 2012;307(15):1629–35. doi: 10.1001/jama.2012.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hadley J, Yabroff KR, Barrett MJ, et al. Comparative effectiveness of prostate cancer treatments: Evaluating statistical adjustments for confounding in observational data. J Natl Cancer Inst. 2010;102(23):1780–93. doi: 10.1093/jnci/djq393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. 2011;59(4):543–52. doi: 10.1016/j.eururo.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 23.Lai TC, Ma WK, Yiu MK. Partial nephrectomy for T1 renal cancer can achieve an equivalent oncological outcome to radical nephrectomy with better renal preservation: the way to go. Hong Kong Med J. 2016;22(1):39–45. doi: 10.12809/hkmj144482. [DOI] [PubMed] [Google Scholar]
- 24.Kaushik D, Kim SP, Childs MA, et al. Overall survival and development of stage IV chronic kidney disease in patients undergoing partial and radical nephrectomy for benign renal tumors. Eur Urol. 2013;64(4):600–6. doi: 10.1016/j.eururo.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 25.Thompson RH. Partial versus radical nephrectomy: The debate regarding renal function ends while the survival controversy continues. Eur Urol. 2014;65(2):378–79. doi: 10.1016/j.eururo.2013.07.036. discussion 379–80. [DOI] [PubMed] [Google Scholar]
- 26.Chung JS, Son NH, Lee SE, et al. Overall survival and renal function after partial and radical nephrectomy among older patients with localised renal cell carcinoma: A propensity-matched multicentre study. Eur J Cancer. 2015;51(4):489–97. doi: 10.1016/j.ejca.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Mashni JW, Assel M, Maschino A, et al. New chronic kidney disease and overall survival after nephrectomy for small renal cortical tumors. Urology. 2015;86(6):1137–43. doi: 10.1016/j.urology.2015.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yap SA, Finelli A, Urbach DR, et al. Partial nephrectomy for the treatment of renal cell carcinoma (RCC) and the risk of end-stage renal disease (ESRD) BJU Int. 2015;115(6):897–906. doi: 10.1111/bju.12883. [DOI] [PubMed] [Google Scholar]
- 29.Jang HA, Kim JW, Byun SS, et al. Oncologic and functional outcomes after partial nephrectomy versus radical nephrectomy in T1b renal cell carcinoma: A multicenter, matched case-control study in Korean patients. Cancer Res Treat. 2016;48(2):612–20. doi: 10.4143/crt.2014.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Capitanio U, Terrone C, Antonelli A, et al. Nephron-sparing techniques independently decrease the risk of cardiovascular events relative to radical nephrectomy in patients with a T1a-T1b renal mass and normal preoperative renal function. Eur Urol. 2015;67(4):683–89. doi: 10.1016/j.eururo.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 31.Huang WC, Elkin EB, Levey AS, et al. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors – is there a difference in mortality and cardiovascular outcomes? J Urol. 2009;181(1):55–61. doi: 10.1016/j.juro.2008.09.017. discussion 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaishampayan U, Vankayala H, Vigneau FD, et al. The effect of targeted therapy on overall survival in advanced renal cancer: A study of the national surveillance epidemiology and end results registry database. Clin Genitourin Cancer. 2014;12(2):124–29. doi: 10.1016/j.clgc.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]